Abstract
Background
Increasing attention has been paid to the potential relationship between gut and lung. The bacterial dysbiosis in respiratory tract and intestinal tract is related to inflammatory response and the progress of lung diseases, and the pulmonary diseases could be improved by regulating the intestinal microbiome. This study aims to generate the knowledge map to identify major the research hotspots and frontier areas in the field of gut–lung axis.
Materials and methods
Publications related to the gut–lung axis from 2011 to 2021 were identified from the Web of Science Core Collection. CiteSpace 5.7.R2 software was used to analyze the publication years, journals, countries, institutions, and authors. Reference co-citation network has been plotted, and the keywords were used to analyze the research hotspots and trends.
Results
A total of 3315 publications were retrieved and the number of publications per year increased over time. Our results showed that Plos One (91 articles) was the most active journal and The United States (1035 articles) published the most articles. We also observed the leading institution was the University of Michigan (48 articles) and Huffnagle Gary B, Dickson Robert P and Hansbro Philip M, who have made outstanding contributions in this field.
Conclusion
The Inflammation, Infection and Disease were the hotspots, and the regulation of intestinal flora to improve the efficacy of immunotherapy in lung cancer was the research frontier. The research has implications for researchers engaged in gut–lung axis and its associated fields.
Keywords: Bibliometric, Knowledge map, Gut–lung axis, Inflammation
Introduction
With the development of microbial analysis technology and bioinformatics, microbial research has greatly expanded its scope. The gut, a critical immune organ, harbors a flora of microorganisms [1]. Increasingly, there is a mounting evidence to suggest the regulation of intestinal flora and its metabolites on distal organs, which in turn affects the occurrence and development of diseases [2–4]. Concepts such as “gut–brain axis”, “gut–liver axis”, “gut–lung axis” were developed to illustrate the relationship between organs. The gut–lung axis is bidirectional and is the crosstalk between the respiratory and digestive system [5]. According to the theories of traditional Chinese medicine, lung and intestines are in a closely related organ system [6]. The intestines and lung are homologous structurally from a histological embryological point of view [7]. Studies have found the destruction of intestinal integrity in patients with chronic obstructive pulmonary disease (COPD) [8]. Patients with lung cancer have gastrointestinal dysmotility as well [9]. Studies have shown that respiratory symptoms and pulmonary function changed in patients with intestinal bowel disease (IBD) and intestinal bowel syndrome (IBS) even without acute or chronic respiratory diseases [10, 11]. Gastroesophageal reflux disease would cause respiratory symptoms and aggravate the existing respiratory diseases [12]. Researchers have also found that lipopolysaccharide (LPS) atomization and high-calorie diet synergistically promoted the pulmonary inflammatory process in rat, that is relevant to the change in gut microbiota [13]. The above studies have confirmed the close relationship between intestines and lung, especially in the pathological states involving inflammation.
Bibliometrics is used to evaluate the information of literature, and the database Web of Science Core Collection (WoSCC) is often used in the bibliometric analysis. CiteSpace, a software developed by Chaomei Chen [14], has become a key tool for bibliometric analysis in recent years. It is applied to generate visual knowledge map to explore the knowledge domain [15]. Based on WoSCC, Xiaoquan Huang performed a global bibliometric analysis from 1998 to 2018 and evaluated the emerging trends in the field of gastrointestinal microbiology, whose study has found that the new therapeutic targets in intestinal microflora would be the focus of future research [16]. However, there is no bibliometric analysis in the field of the gut–lung axis.
To analyze the research situation and trends concerning the gut–lung axis within the past 10 years, CiteSpace 5.7.R2 was applied in this study, which aims to identify the key authors, institutions, countries, important journals, research focuses and emerging trends in this field.
Results
Distribution of articles by publication years
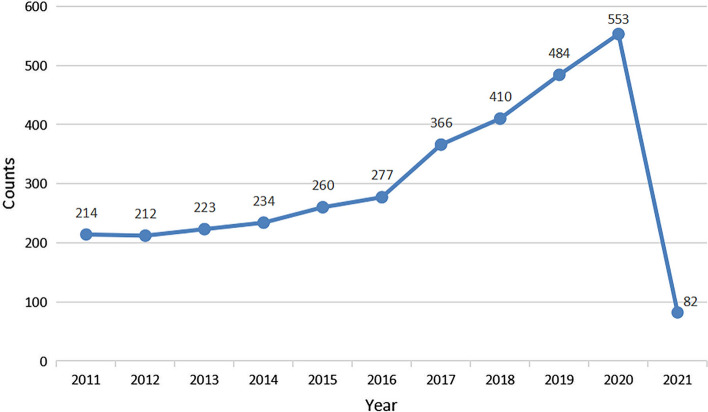
From 2011 to 2021, 3,315 articles were published. There was an increasing trend for a quantity of research publications on gut–lung axis, from 214 in 2011 to 553 in 2020 (Fig. 1), which indicates an increasing interest in this field in recent years.
Fig. 1.
Trend of publications in the field of gut–lung axis from 2011 to 2021
Funding source
The top 10 major funding sources are shown in Table 1. United States Department of Health and Human Services, National Institutes of Health, National Natural Science Foundation of China mainly funded this field. The United States and China contributed the most fundings in this field.
Table 1.
Top 10 funding sources
| Ranking | Funding source | Country/region | Frequency |
|---|---|---|---|
| 1 | United States Department of Health and Human Services | United States | 591 |
| 2 | National Institutes of Health | United States | 588 |
| 3 | National Natural Science Foundation of China | China | 300 |
| 4 | European Commission | Europe | 186 |
| 5 | National Heart, Lung, and Blood Institute | United States | 159 |
| 6 | National Institute of Allergy and Infectious Disease | United States | 152 |
| 7 | National Cancer Institution | United States | 129 |
| 8 | National Institute of Diabetes and Digestive and Kidney Diseases | United States | 103 |
| 9 | Ministry of Education, Culture, Sports, Science and Technology | Japan | 89 |
| 10 | Japan Society for the Promotion of Science | Japan | 84 |
Journal analysis
The top 10 journals are listed by the number of publication in Table 2, which have published 361 articles in total and accounts for about 11% of the total number of publications. Plos One has published 91 articles, followed by Frontiers in Immunology, 73 articles. The impact factor (IF) of the 10 journals ranged from 2.192 to 7.561. The top 10 cited journals are listed in Table 3. Plos One was the most active journal (1586 citations), followed by Proceedings of the National Academy of Sciences of the United States of America (1,409 citations). In addition, articles in top journals such as New England Journal of Medicine, Lancet, Nature and Science were widely cited in the field of the gut–lung axis.
Table 2.
Top 10 most publication journals
| Ranking | Journal | Frequency | IF |
|---|---|---|---|
| 1 | Plos One | 91 | 3.24 |
| 2 | Frontiers in Immunology | 73 | 7.561 |
| 3 | Scientific Reports | 45 | 4.379 |
| 4 | Frontiers in Microbiology | 36 | 5.640 |
| 5 | International Journal of Molecular Sciences | 25 | 5.923 |
| 6 | Oncotarget | 20 | 5.168* |
| 7 | Mucosal Immunology | 19 | 7.313 |
| 8 | World Journal of Gastroenterology | 18 | 5.742 |
| 9 | Journal of Immunology | 17 | 5.422 |
| 10 | Journal of Surgical Research | 17 | 2.192 |
IF, impact factors in 2020. *, the impact factors of Oncotarget in 2016.
Table 3.
Top 10 most cited journals
| Ranking | Journals | Citation times | IF |
|---|---|---|---|
| 1 | Plos One | 1586 | 3.24 |
| 2 | Proceedings of the National Academy of Sciences of the United States of America | 1409 | 11.205 |
| 3 | Nature | 1364 | 49.962 |
| 4 | Science | 1184 | 47.728 |
| 5 | New England Journal of Medicine | 1044 | 91.245 |
| 6 | Cell | 890 | 41.582 |
| 7 | American Journal of Respiratory and Critical Care Medicine | 878 | 21.405 |
| 8 | Nature Medicine | 848 | 53.44 |
| 9 | Journal of Immunology | 833 | 5.422 |
| 10 | Lancet | 810 | 79.321 |
IF, impact factors in 2020
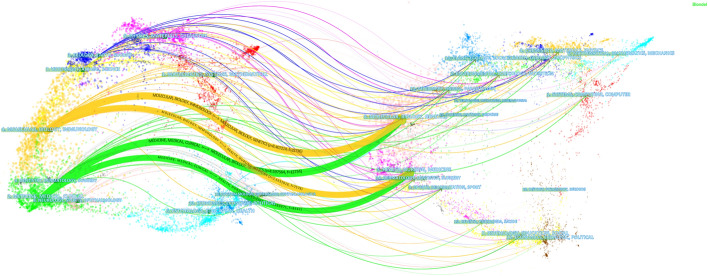
A dual-map overlay graph of journals is shown in Fig. 2 to clarify the relationship between journals [17]. There are four main citation paths, two orange and two green. The orange paths indicate that the articles published in Molecular/Biology/Immunology journals often cite what was published in Molecular/Biology/Genetic and Health/Nursing/Medicine. The green paths indicate the articles published in Medicine/Medical/Clinical cite articles published in Molecular/Biology/Genetics and Health/Nursing/Medicine. The articles were published in the journals of medicine, health, molecule, gene, biology, immunity, nursing and other fields. All of the analysis above would provide a reference for the researchers in the field of the gut–lung axis.
Fig. 2.
The dual-map overlay of gut–lung axis research. The dual-map overlay of journals represents the subject distribution of journals, with the left side of the graph representing citing journals and the right cited journals. The colored lines represent the citation relationship between articles in citing and in cited journals
Country analysis
The top 10 countries are listed in Table 4. The United States published the most articles (1035 articles), which accounted for nearly 1/3 of the total amount and surpassed China (554 articles) and Germany (255 articles). The country co-occurrence map is shown in Fig. 3A with 59 nodes and 65 links. It could be seen from Fig. 3B and C that the main cooperation countries of the United States were Thailand and Uganda in this field with the link strength of 0.12. The main cooperation country of China was Pakistan with a link strength of 0.2. The United States and China were the main research forces, however, their cooperation was not close in this field.
Table 4.
Top 10 most publication countries
| Ranking | Country | Frequency |
|---|---|---|
| 1 | United States of America | 1035 |
| 2 | The People's Republic of China | 554 |
| 3 | The Federal Republic of Germany | 255 |
| 4 | The Republic of Italy | 203 |
| 5 | Japan | 190 |
| 6 | The United Kingdom of Great Britain and Northern Ireland | 176 |
| 7 | The Republic of France | 165 |
| 8 | Commonwealth of Australia | 153 |
| 9 | Canada | 147 |
| 10 | The Federative Republic of Brazil | 123 |
Fig. 3.
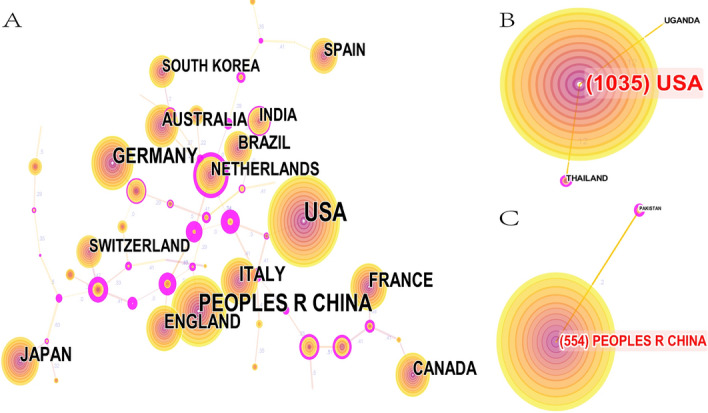
Co-occurrence analysis of countries. A Country co-occurrence map. B Cooperation network of the United States. C Cooperation network of China. The size of the node represents the number of publications. The link between nodes represents the existence of cooperation. The thickness of the lines represents the closeness of cooperation, and the thicker the lines are, the closer the cooperation is
Institution analysis
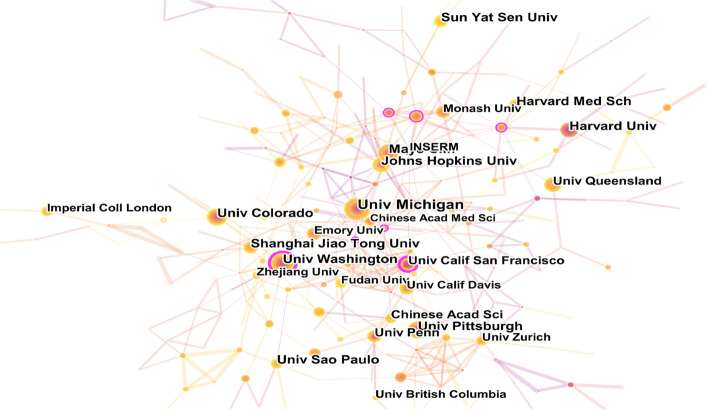
The institution co-occurrence map is shown in Fig. 4 with 357 nodes and 443 links. The top 10 institutions in the number of publications in this field are listed in Table 5. The University of Michigan was the most productive one (48 articles), followed by the University of Washington (32 articles) and Colorado State University (32 articles). Most are located in the United States among the top 10 institutions, and the support of American institutions has been an important factor for the dominance of the United States in this field.
Fig. 4.
Co-occurrence analysis of institutions. The size of the node represents the number of publications, the link between nodes represents the cooperation between institutions, and the thickness of the lines represents the degree of cooperation
Table 5.
Top 10 most publication institutions
| Ranking | Institution | Country/region | Frequency |
|---|---|---|---|
| 1 | The University of Michigan | United States | 48 |
| 2 | University of Washington | United States | 32 |
| 3 | Colorado State University | United States | 32 |
| 4 | Shanghai Jiao Tong University | China | 30 |
| 5 | Harvard University | United States | 30 |
| 6 | Johns Hopkins University | United States | 30 |
| 7 | Mayo Clinic | United States | 30 |
| 8 | Harvard Medical School | United States | 29 |
| 9 | Sun Yat-Sen University | China | 29 |
| 10 | University of Pittsburgh | United States | 29 |
Author analysis
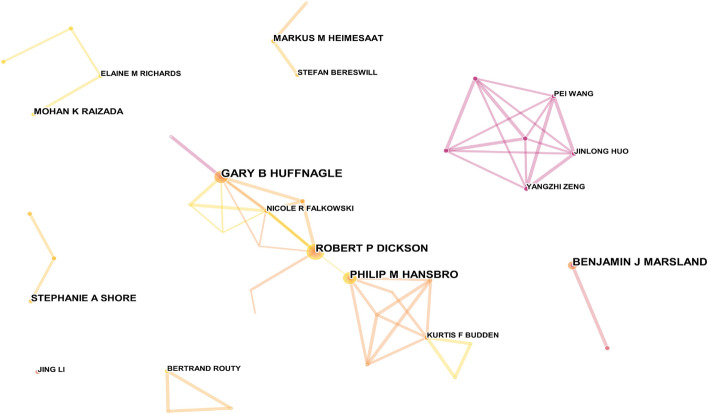
The author co-occurrence map is shown in Fig. 5, with 430 nodes and 658 links. The top 5 most productive authors and their affiliated institutions are shown in Table 6. The top 3 authors, Huffnagle Gary B, Dickson Robert P and Hansbro Philip M, have formed the largest cooperative network among many small scattered research groups. Dickson Robert P and Huffnagel Gary B were both from the University of Michigan Medical School.
Fig. 5.
Co-occurrence analysis of authors. The size of the node represents the number of publications, the link between nodes represents the cooperation between authors and the thickness of the link represents the degree of cooperation
Table 6.
Top 5 most publication authors
| Ranking | Frequency | Author | Country | Institution |
|---|---|---|---|---|
| 1 | 13 | Huffnagle Gary B | United States | University of Michigan Medical School |
| 2 | 12 | Dickson Robert P | United States | University of Michigan Medical School |
| 3 | 11 | Hansbro Philip M | Australia | The University of Newcastle |
| 4 | 11 | Marsland Benjamin J | Australia | Central Clinical School, Monash University |
| 5 | 8 | Shore Stephanie A | United States | Harvard University |
Keyword analysis
The top 40 keywords with the most occurrences are shown in Table 7. It is shown that the most frequent keywords were “gut microbiota” (362), “inflammation” (280), “disease” (239) and “infection” (238). The keyword co-occurrence map is shown in Fig. 6 with 197 nodes and 283 lines. It is shown in Fig. 6 that “gut microbiota” often occurs together with “bacteria”, “supplement” and “pathology”. “Inflammation” often occurs together with “intestinal barrier”, “lung microbiome”, “tissue”, “epithelial cell” and “lipopolysaccharide”. “Infection” often occurs together with “diversity”, “Escherichia Coli” and “epidemiology”. “Disease” often occurs together with “protection”, “metabolite”, “expression”, “allergic asthma” and “exposure”.
Table 7.
Top 40 keywords with the highest frequency of occurrence
| Ranking | Frequency | Year of first occurrence | Keyword |
|---|---|---|---|
| 1 | 362 | 2013 | Gut microbiota |
| 2 | 280 | 2011 | Inflammation |
| 3 | 239 | 2011 | Disease |
| 4 | 238 | 2011 | Infection |
| 5 | 226 | 2011 | Expression |
| 6 | 201 | 2013 | Microbiota |
| 7 | 195 | 2011 | Lung |
| 8 | 190 | 2014 | Microbiome |
| 9 | 179 | 2011 | Lung cancer |
| 10 | 167 | 2011 | Cancer |
| 11 | 154 | 2011 | Cell |
| 12 | 144 | 2011 | Asthma |
| 13 | 115 | 2015 | Intestinal microbiota |
| 14 | 106 | 2013 | Bacteria |
| 15 | 106 | 2011 | Mice |
| 16 | 105 | 2011 | In vitro |
| 17 | 100 | 2013 | Cystic fibrosis |
| 18 | 100 | 2012 | Activation |
| 19 | 99 | 2013 | T cell |
| 20 | 88 | 2011 | Mechanism |
| 21 | 86 | 2011 | Colorectal cancer |
| 22 | 85 | 2013 | Risk |
| 23 | 84 | 2011 | Identification |
| 24 | 83 | 2012 | Gut |
| 25 | 78 | 2011 | Immune response |
| 26 | 74 | 2015 | Immunity |
| 27 | 73 | 2011 | Pathogenesis |
| 28 | 73 | 2011 | Diagnosis |
| 29 | 72 | 2013 | Diversity |
| 30 | 70 | 2011 | Model |
| 31 | 67 | 2013 | Probiotics |
| 32 | 65 | 2011 | Oxidative stress |
| 33 | 62 | 2013 | Cell lung cancer |
| 34 | 62 | 2014 | Children |
| 35 | 58 | 2016 | Health |
| 36 | 58 | 2018 | Immunotherapy |
| 37 | 56 | 2015 | Biomarker |
| 38 | 55 | 2011 | Therapy |
| 39 | 54 | 2013 | Metabolism |
| 40 | 53 | 2011 | Obstructive Pulmonary disease |
Fig. 6.
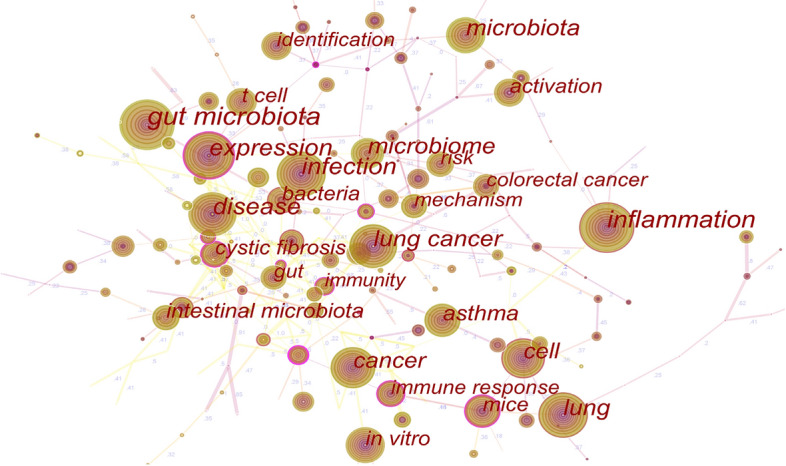
Co-occurrence analysis of keywords. The node size represents the number of occurrences. The larger the nodes are, the more frequent the keyword occurs. The co-occurrence keywords are linked with the lines, their thickness represents the degree of connection
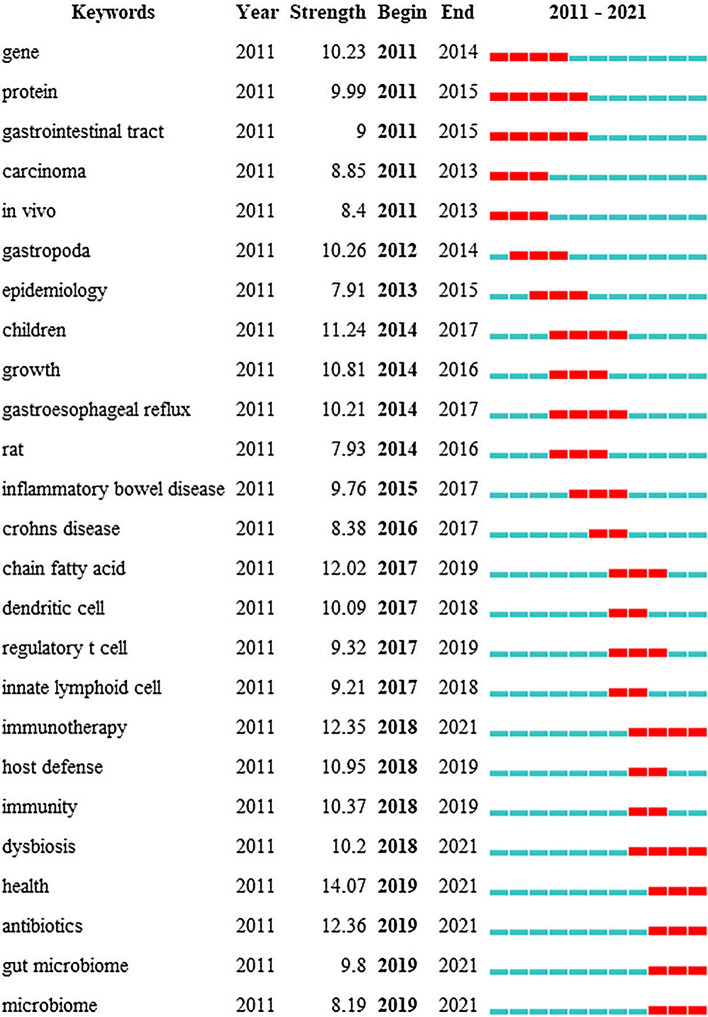
The top 25 keywords with burst impact are shown in Fig. 7. “Gene”, “protein”, “gastrointestinal tract”, “carcinoma”, “in vivo” and “gastropoda” were the keywords that had the earliest burst impact. “Epidemiology”, “children”, “growth”, “gastroesophageal reference”, “rat”, “inflammatory bowel disease” and “Crohn’s disease” had burst impact during 2014–2016. From 2017 to 2019, the keywords with burst impact were “chain fast acid”, “dental cell”, “regulatory T cell”, “internal lymphoid cell”, “immunity”, “host defense”, “immunity” and “dysbiosis.” The bursts with the most recent onset were “immunity”, “dysbiosis”, “health”, “antibiotics”, “gut microbiome” and “microbiome”, which indicated the forefront in the field of the gut–lung axis. The keyword with the highest strength was “health”, with a score of 14.07, followed by “antibiotics” and “immunotherapy”, with the score of 12.36 and 12.35, respectively.
Fig. 7.
Top 25 keywords in burst impact. The blue and white squares in each row on the right side of the figure correspond to the year of hotspot. Red squares represent the year of hotspot, and blue squares represent non-hotspot year. The recent successive red squares represent the research hotspots in recent years
References analysis
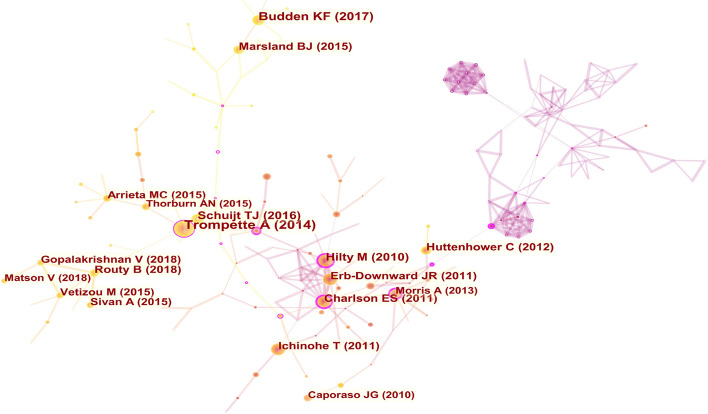
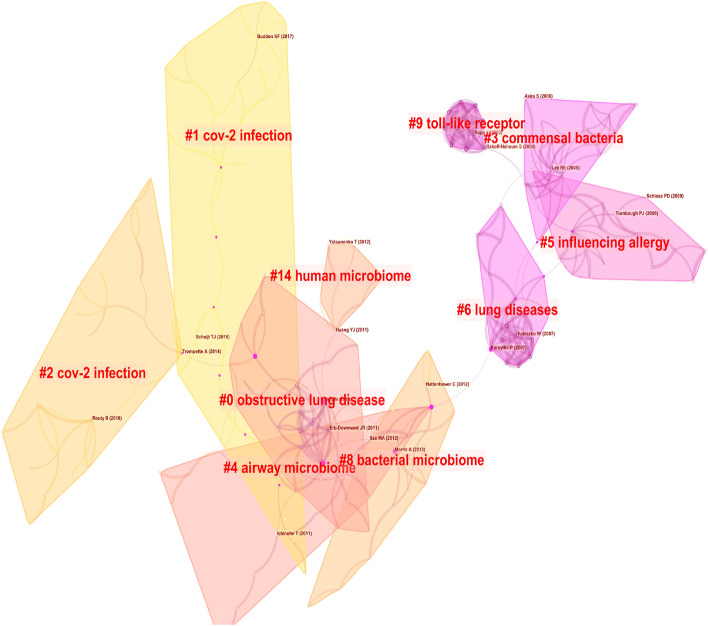
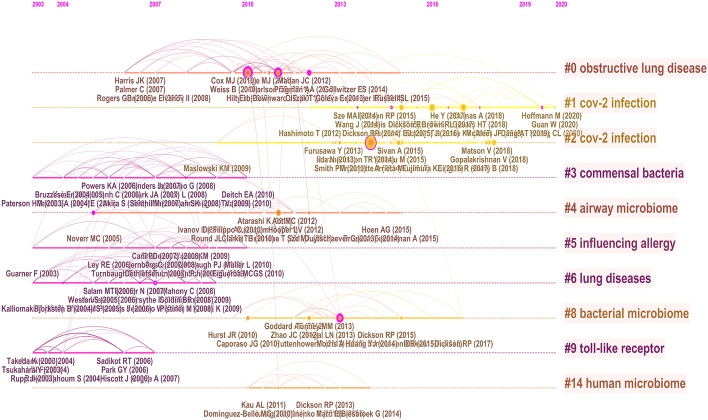
The knowledge map of the co-occurrence references is shown in Fig. 8 with 313 nodes and 632 links. The nine largest clusters were presented by cluster analysis (Fig. 9), including #0 obstructive lung disease, #1 cov-2 infection, #2 cov-2 infection, #3 commensal bacteria, #4 airway microbiome, #5 influencing allergy, #6 lung diseases, #9 toll-like receptor, #14 human microbiome. It is shown in Fig. 10 that cov-2 infection has attracted much attention in recent years.
Fig. 8.
Co-occurrence analysis of references. The node size represents the citation frequency of the cited references, and the node with purple circle represents the key references. The larger purple circle indicates that the reference is more important
Fig. 9.
The cluster map of co-cited references. The cited references are clustered, each clustered box represents a category
Fig. 10.
Timeline map of clustering of co-cited references. The results of clusters are shown at the right side, the warm color indicates a more recent cluster, and the cold color indicates an earlier one
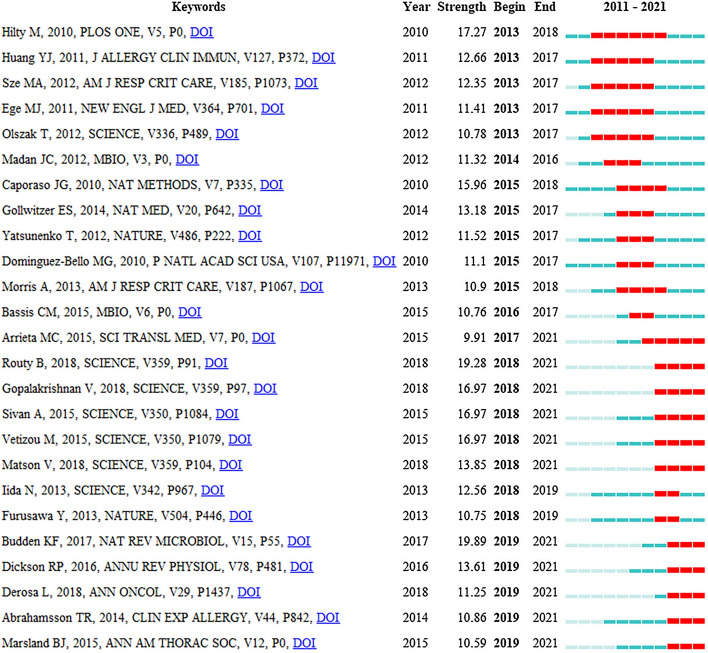
The top 10 cited references in this field are listed in Tables 8 and 9, which could be often considered fundamental in gut–lung axis research. The articles published by Trompette A in Nature Medicine had the highest number of citations (191), and the articles published by Hilty M had the highest centrality of score of 0.35. High centrality is often regarded as turning points or key points in a field [18].
Table 8.
Top 10 most cited references
| Authors | Frequency | Year of publication | Journal | Title | Focus |
|---|---|---|---|---|---|
| Trompette A [19] | 191 | 2014 | Nature Medicine | Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis | Fiber diet, bacterial metabolites, allergic airway disease |
| Budden KF [20] | 122 | 2017 | Nature Reviews Microbiology | Emerging pathogenic links between microbiota and the gut–lung axis | Gut–lung axis |
| Hilty M [21] | 101 | 2010 | Plos One | Disordered microbial communities in asthmatic airways | Dysbacteriosis of respiratory tract, asthmatic airways |
| Tim J Schuijt [22] | 94 | 2016 | Gut | The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia | Bacterial pneumonia |
| Erb-Downward JR [23] | 88 | 2011 | Plos One | Analysis of the lung microbiome in the “healthy” smoker and in COPD | Pulmonary microorganism, chronic obstructive pulmonary disease |
| Emily S Charlson [24] | 86 | 2011 | American Journal of Respiratory and Critical Care Medicine | Topographical continuity of bacterial populations in the healthy human respiratory tract | Distribution of respiratory tract microbiome |
| Takeshi Ichinohe [25] | 86 | 2011 | Proceedings of the national academy of sciences of the united states of America | Microbiota regulates immune defense against respiratory tract influenza A virus infection | Immunity after influenza virus infection |
| Bertrand Routy [26] | 84 | 2018 | Science | Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors | Intestinal flora, immune checkpoint inhibitors |
| Human Microbiome Project Consortium [27] | 82 | 2012 | Nature | Structure, function and diversity of the healthy human microbiome | Structure, function and diversity of microbiome |
| Benjamin J Marsland [28] | 77 | 2015 | Annals of the American Thoracic Society | The Gut–Lung Axis in Respiratory Disease | Intestinal flora, Respiratory diseases |
Table 9.
Top 10 references ranked by centrality
| Author | Frequency | Year of publication | Journal | Title | Focus |
|---|---|---|---|---|---|
| Hilty M [21] | 0.35 | 2010 | Plos One | Disordered microbial communities in asthmatic airways | Dysbacteriosis of respiratory tract, asthmatic airways |
| Mairi C Noverr [29] | 0.34 | 2005 | Infection and Immunity | Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13 | Antibiotic therapy, allergic airway, interleukin-13 |
| Alison Morris [30] | 0.32 | 2013 | American Journal of Respiratory and Critical Care Medicine | Comparison of the respiratory microbiome in healthy nonsmokers and smokers | Differences of respiratory tract microbiome |
| Paul Forsythe [31] | 0.32 | 2007 | American Journal of Respiratory and Critical Care Medicine | Oral Treatment with Live Lactobacillus reuteri Inhibits the Allergic Airway Response in Mice | Probiotics, allergic airway |
| Emily S Charlson [24] | 0.22 | 2011 | American Journal of Respiratory and Critical Care Medicine | Topographical continuity of bacterial populations in the healthy human respiratory tract | Distribution of microorganisms in lung |
| Torsten Olszak [32] | 0.21 | 2012 | Science | Microbial exposure during early life has persistent effects on natural killer T cell function | Microbial exposure during early life, natural killer T cells |
| Christine M Bassis [33] | 0.18 | 2015 | mBio | Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals | Source of respiratory tract microorganism |
| Trompette A [19] | 0.17 | 2014 | Nature Medicine | Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis | Fiber diet, bacterial metabolites, allergic airway |
| Jian Wang [34] | 016 | 2014 | Journal of Experimental Medicine | Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation | Influenza virus infection, Th17, intestinal immune injury |
| Rebecca L Brown [35] | 0.15 | 2017 | Rebecca L Brown | The microbiota protects against respiratory infection via GM-CSF signaling | Microbiota, GM-CSF signal, respiratory tract infection |
Twenty-five references with burst impact are shown in Fig. 11. Eleven articles have been highly cited in recent 4 years, and 5 of them were published in the journal Science [26, 36–39], which were about the significance of microbiota in tumor immunotherapy. Researchers have found that programmed cell death protein-1 (PD-1) and programmed cell death protein ligand-1 (PD-L1) have significantly improved the survival rate of non-small cell lung cancer (NSCLC) [40], while antibiotic therapy led to an imbalance of intestinal flora, which affected the anti-tumor efficacy of immune checkpoint inhibitors (ICI). However the therapeutic effect of ICI was restored after manipulating the microbiota [26, 37, 38]. The studies above have provided a reference for clinicians in the application of ICI. The intervention of intestinal flora in improving the efficacy of lung cancer immunotherapy was a new frontier hotspot, which was consistent with the results of keyword burst detection.
Fig. 11.
Top 25 cited references with burst impact. The blue and white squares in each row on the right side of the figure correspond to the year. The red squares represent that the references were highly cited in a short period and the recent successive red squares represent that the references were highly cited in recent years
Discussion
Increasing attention has been paid to the field of gut–lung axis in recent years. Multiple intestinal diseases would result in respiratory symptoms and changes in the respiratory flora. Intestinal microbiota disorders are also present in patients with respiratory diseases. The antibiotic application may lead to disturbances in intestinal flora.
This bibliometric study aims to analyze the current status and trend of articles in the field of gut–lung axis in recent 10 years by using CiteSpace 5.7.R2. The results have demonstrated the important authors, core teams, active journals, research focuses and research development trends in this field.
The number of publications in this field has been increasing, which indicates a broad research prospect in the field of the gut–lung axis. The top 10 journals accounted for 1/9 of the total studies, suggesting that the articles were widely distributed among various journals. The greatest number of articles on the gut–lung axis were published on Plos One and were cited the most frequently. As the most active journal in this field, Plos One has a certain influence in the field of the gut–lung axis.
The analysis of the collaboration network map has indicated that the United States was the leading research force in the field of the gut–lung axis. Besides the high participation of American Institutions, the large amount of the United States’ financial support for the field may be another important factor for the United States to be dominant in this field. Although the United States and China have produced the largest number of research articles, their cooperation is not close and strengthening the cooperation between which in this field may contribute to more ground-breaking results. Among the three key authors in this field, both Dickson Robert P and Huffnagle Gary B came from the University of Michigan Medical School who focused on the field of respiratory and critical medicine. Researchers have found that the lung microbiome is rich in gut-associated bacteria in patients with sepsis and acute respiratory distress syndrome (ARDS) [41]. The key characteristics of the lung microbiome (bacterial load and enrichment of gut-associated bacteria) were correlated with ARDS, which could predict the prognosis of critical patients [42, 43]. The hyperoxia would cause changes in the microbiota of the lung and intestines and would lead to lung injury [44]. Hansbro Philip M has studied widely and achieved some research results in the effect of diet on mucosal immunity [45], short-chain fatty acids (SCFAs), and the role of inflammasomes in regulating intestinal and pulmonary inflammation [46, 47], and 146 bacterial species were found to differ between the patients with COPD and normal individuals by examining the fecal microbiome [48].
Keyword analysis has identified 3 research focuses in the field of the gut–lung axis: Inflammation, Infection and Disease. (1) Inflammation: The gut flora and its metabolites are critical in maintaining normal mucosal immune function [49]. The mucosal barrier is rich in Group 2 initial lymphocytes that can migrate from the gut to the lung to participate in the inflammatory process [50, 51]. Respiratory immune response belongs to the category of the mucosal immune response. The commensal microflora would contribute to activating human immune cells after bacteriae, viruses or other pathogenic microorganisms infection [22, 25, 52, 53]. In addition, SCFAs, the metabolites of the intestinal flora, plays a significant role in preventing airway allergic reaction and inhibiting airway inflammation [19, 54, 55]. (2) Infection: When there is pulmonary bacterial infection, the intestinal flora would increase host defense through toll-like receptor and nod-like receptor signaling [52, 56]. After influenza A virus infection, the intestinal flora is involved in the activation of inflammasomes, contributing to dendritic cell migration [25]. Moreover, Bifidobacterium could regulate Th1/Th2 immune response and enhance the disease resistance of mice [57]. (3) Disease: The three most related ones are pulmonary obstructive disease, cystic fibrosis and lung cancer. ① The occurrence of asthma is closely related to the early intestinal flora imbalance in children, which is associated with early life antibiotic exposure and the severity of asthma has a dose-dependent correlation with antibiotics [58–61]. Appropriate probiotic supplementation was beneficial to the treatment of asthma [56, 62]. Additionally, patients with obstructive pulmonary disease had a higher risk of IBD and IBS [63, 64]. Moreover, IBD also increases the mortality of patients with COPD and asthma [65]. ② Cystic fibrosis is an autosomal recessive genetic disease, mainly characterized by respiratory and gastrointestinal symptoms, which reflects the correlation between lung and gut. Compared with healthy subjects, Faecalibacterium, Roseburia and Bifidobacterium decreased in the intestinal tract in patients with cystic fibrosis. However, breastfeeding or probiotic application was beneficial to the recovery of intestinal flora structure, which could reduce the deterioration of the pulmonary condition and the number of hospitalizations [66, 67]. ③ The abundance of Firmicutes and Proteobacteria is relatively low in patients with lung cancer while relatively high in Bacteroidetes and Fusobacteria [68]. Lipopolysaccharide produced by G-bacilli in the gut could induce inflammatory response and lung metastasis of melanoma, while the changes in the intestinal flora could prevent this process [69]. Furthermore, diversities of intestinal flora are also crucial in the immunotherapy in lung cancer [26, 37, 38, 70].
The references analysis has revealed the important references in the field of the gut–lung axis in the past 10 years. The references listed in Tables 8 and 9 would provide an important reference for the study in this field. Additionally, the timeline of references analysis (Fig. 8C) suggests that CoV-2 infection has attracted much attention in this field in recent years. Studies have found that intestinal flora imbalance could lead to the destruction of the intestinal barrier, which may contribute to SARS-CoV-2 transferring from the lung to the intestines through the circulatory and lymphatic system, leading to secondary infection and multiple organ failure [53, 71, 72]. The use of probiotics could significantly improve fever, cough, diarrhea and other clinical symptoms of COVID-19 patients and reduce the risk of respiratory failure [73, 74], which provides a new direction for the treatment of COVID-19. Moreover, burst detection demonstrates that immunotherapy, antibiotics, dysbiosis, health, gut microbiome and microbiome are new research directions in the field of the gut–lung axis. Antibiotics could lead to dysbacteriosis, affecting the efficacy of tumor immunotherapy. It is a frontier field of the improvement of immunotherapy efficacy in lung cancer by modulating intestinal flora.
Conclusion
Based on the results of CiteSpace, this study has identified the important journals, countries and collaborators in the field of the gut–lung axis. According to the keywords, references and burst detection, the research focuses and frontier hotspots of the gut–lung axis were determined. In addition, new therapeutic targets in gut microbiota have great potential in treating pulmonary diseases.
This study retrieved publications from the WoSCC with the limitation of language (English) and literature type (article and review), which may not be sufficient in the representation of all the current research on the gut–lung axis. However, this study has covered the majority of articles in the field of the gut–lung axis in recent 10 years, which could reflect the overall status and trends in this field.
Methods
Search strategy
WoSCC has a wide range of selective literature and its data analysis format meets the requirements of CiteSpace software. We reviewed papers published in the past 10 years on WoSCC on March 26, 2021. The retrieval strategies are as follows:
The language was “English”, the document types included “article” and “review”. “Procedures paper”, “book chapter”, “data paper”, “early access” and “retracted publication” were excluded; publication time was from 2011/01/01 to 2021/3/26. 3315 articles (including 2469 articles and 846 reviews) were screened out. The “fully recorded and cited references” of these documents were extracted into CiteSpace 5.7.R2 in “plain text” format to identify the main countries, institutions, authors, keywords and references.
Parameter settings
The parameters of CiteSpace 5.7.R2 were set as follows:
Time slicing: each year as a time slice from 2011 to 2021.
Term source: title, abstract, author keywords, and keywords plus.
Node types: author, institution, country, keywords, reference.
Top Nperslice: “Top Nperslice = 50” for author, institution, country and keyword node type, “Top Nperslice = 25” for reference node type.
Pruning options: Pathfinder, pruning the merged network. The information of country, author, institution, keywords and references were analyzed visually.
Acknowledgements
We thank Xuan Wang for her help in language polishing.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- IBD
Intestinal bowel disease
- IBS
Intestinal bowel syndrome
- LPS
Lipopolysaccharide
- WoSCC
Web of Science Core Collection
- PD-L1
Programmed cell death protein ligand-1
- PD-1
Programmed cell death protein-1
- ICI
Immune checkpoint inhibitors
- SCFAs
Short-chain fatty acids
- ARDS
Acute respiratory distress syndrome
Authors' contributions
XG and TL designed the research. ZW, C Bai and TH collected the data. CL, XM, HY processed the data. ZW and CB wrote the paper. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China, Grant Number 81874421 and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine, Grant Number ZYYCXTD-C-202006.
Availability of data and materials
All the data used to support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors declare that they consent for publication.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhendong Wang and Chen Bai contributed equally to this work
Contributor Information
Tiegang Liu, Email: liutiegang2009@163.com.
Xiaohong Gu, Email: guxiaohong1962@163.com.
References
- 1.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol. 2018;48:39–49. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durack J, Lynch SV. The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20–40. doi: 10.1084/jem.20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang HX, Wang YP. Gut Microbiota-brain Axis Chin Med J (Engl). 2016;129:2373–2380. doi: 10.4103/0366-6999.190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripathi A, Debelius J, Brenner DA, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y, Wen Q, Yao F, et al. Gut-lung axis: The microbial contributions and clinical implications. Crit Rev Microbiol. 2017;43:81–95. doi: 10.1080/1040841X.2016.1176988. [DOI] [PubMed] [Google Scholar]
- 6.Liu P, Wang P, Tian DZ, et al. Study on Traditional Chinese Medicine theory of lung being connected with large intestine. J Tradit Chin Med. 2012;32:482–487. doi: 10.1016/S0254-6272(13)60059-X. [DOI] [PubMed] [Google Scholar]
- 7.Wang YJ, Zhou D, Feng Y, Chen G, Li N. T-UCRs with digestive and respiratory diseases. Bioorg Med Chem Lett. 2020;30:16–127306. doi: 10.1016/j.bmcl.2020.127306. [DOI] [PubMed] [Google Scholar]
- 8.Rutten EPA, Lenaerts K, Buurman WA, Wouters EFM. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145:245–252. doi: 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- 9.Lipowska A, Micic D, Cavallo A, McDonald E. Autoimmune Gastrointestinal Dysmotility Due to Small Cell Lung Cancer. Am J Gastroenterol. 2016;111:S981–S981. doi: 10.14309/00000434-201610001-02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazar A, Atis S, Konca K, et al. Respiratory symptoms and pulmonary functional changes in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96:1511–1516. doi: 10.1111/j.1572-0241.2001.03748.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Liu JS, Peng SH, et al. Gut-lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J Gastroenterol. 2013;19:6794–6804. doi: 10.3748/wjg.v19.i40.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrosimov VN, Ponomareva IB, Nizov AA, Solodun MV. On respiratory manifestations of gastroesophageal reflux disease. Ter Arkh. 2018;90:131–136. doi: 10.26442/terarkh2018908131-136. [DOI] [PubMed] [Google Scholar]
- 13.Bai C, Liu T, Xu J, et al. Effect of High Calorie Diet on Intestinal Flora in LPS-Induced Pneumonia Rats. Sci Rep. 2020;10:1701–1701. doi: 10.1038/s41598-020-58632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CM, Hu ZG, Liu SB, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12:593–608. doi: 10.1517/14712598.2012.674507. [DOI] [PubMed] [Google Scholar]
- 15.Chen CM. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc Natl Acad Sci USA. 2004;101:5303–5310. doi: 10.1073/pnas.0307513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang XQ, Fan XWW, Ying J, Chen SY. Emerging trends and research foci in gastrointestinal microbiome. J Transl Med. 2019;17:67. doi: 10.1186/s12967-019-1810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Leydesdorff L. Patterns of connections and movements in dual-map overlays: A new method of publication portfolio analysis. J Assoc Inf Sci Technol. 2014;65:334–351. doi: 10.1002/asi.22968. [DOI] [Google Scholar]
- 18.Chaomei SMBC. CiteSpace II: visualization and knowledge discovery in bibliographic databases. In: AMIA Annual Symposium proceedings. 2005 [PMC free article] [PubMed]
- 19.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 20.Budden KF, Gellatly SL, Wood DLA, et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Revs Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 21.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5:1. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun TJ, Lu JM, Sheng BP, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:475–483. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the "healthy" smoker and in COPD. PLoS ONE. 2011;6:2–e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184:957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 27.Huttenhower C, Gevers D, Knight R, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsland BJ, Trompette A, Gollwitzer ES. The Gut-lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12:S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 29.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun. 2005;73:30–38. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris A, Beck JM, Schloss PD, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007;175:561–569. doi: 10.1164/rccm.200606-821OC. [DOI] [PubMed] [Google Scholar]
- 32.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer t cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassis CM, Erb-Downward JR, Dickson RP, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio. 2015;6:2–e00037. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Li FQ, Wei HM, et al. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211:2683–2683. doi: 10.1084/jem.2014062511242014c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM-CSF signaling. Nat Commun. 2017;8:1–1512. doi: 10.1038/s41467-016-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia L, Liu Y, Wang Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist. 2019;24:S31–S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickson RP, Singer BH, Newstead MW, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1:10–16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickson RP, Schultz MJ, van der Poll T, et al. Lung microbiota predict clinical outcomes in critically ill patients. Am J Respir Crit Care Med. 2020;201:555–563. doi: 10.1164/rccm.201907-1487OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4:59–72. doi: 10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashley SL, Sjoding MW, Popova AP, et al. Lung and gut microbiota are altered by hyperoxia and contribute to oxygen-induced lung injury in mice. Sci Transl Med. 2020;12:556. doi: 10.1126/scitranslmed.aau9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alemao CA, Budden KF, Gomez HM, et al. Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy. 2021;76:714–734. doi: 10.1111/all.14548. [DOI] [PubMed] [Google Scholar]
- 46.Donovan C, Liu G, Shen S, et al. The role of the microbiome and the NLRP3 inflammasome in the gut and lung. J Leukoc Biol. 2020;108:925–935. doi: 10.1002/JLB.3MR0720-472RR. [DOI] [PubMed] [Google Scholar]
- 47.Rufting S, Xenaki D, Malouf M, et al. Short-chain fatty acids increase TNF alpha-induced inflammation in primary human lung mesenchymal cells through the activation of p38 MAPK. Am J Physiol-Lung Cell Molecu Physiol. 2019;316:L157–L174. doi: 10.1152/ajplung.00306.2018. [DOI] [PubMed] [Google Scholar]
- 48.Bowerman KL, Rehman SF, Vaughan A, et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat Commun. 2020;11:1–5886. doi: 10.1038/s41467-020-19701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youn YJ, Maureen G, Ozorio DSV, Anujit S, Ian MD. Gut microbiota and immune system interactions. Microorganisms. 2020;8:10–1587. doi: 10.3390/microorganisms8101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mjosberg J, Rao A. Lung inflammation originating in the gut. Science. 2018;359:36–37. doi: 10.1126/science.aar4301. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y, Mao K, Chen X, et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359:114–119. doi: 10.1126/science.aam5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L-W, Chen P-H, Hsu C-M. Commensal Microflora Contribute to Host Defense Against Escherichia Coli Pneumonia Through Toll-Like Receptors. Shock. 2011;36:67–75. doi: 10.1097/SHK.0b013e3182184ee7. [DOI] [PubMed] [Google Scholar]
- 53.Yu H, Jianhui W, Fang L, Yuan S. Main Clinical Features of COVID-19 and Potential Prognostic and Therapeutic Value of the Microbiota in SARS-CoV-2 Infections. Front Microbiol. 2020;11:1302. doi: 10.3389/fmicb.2020.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isabel H, Baines KJ, Berthan BS, et al. Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in Asthma. Nutrients. 2017;9:1–57. doi: 10.3390/nu9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomoda K, Kubo K, Dairiki K, et al. Whey peptide-based enteral diet attenuated elastase-induced emphysema with increase in short chain fatty acids in mice. BMC Pulm Med. 2015;15:1–7. doi: 10.1186/s12890-015-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immunity. 2014;82:4596–4606. doi: 10.1128/IAI.01636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahooti M, Abdolalipour E, Salehzadeh A, et al. Immunomodulatory and prophylactic effects of Bifidobacterium bifidum probiotic strain on influenza infection in mice. World J Microbiol Biotechnol. 2019;35:6–91. doi: 10.1007/s11274-019-2667-0. [DOI] [PubMed] [Google Scholar]
- 58.Abrahamsson TR, Jakobsson HE, Andersson AF, et al. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 59.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 60.Alhasan MM, Cait AM, Heimesaat MM, et al. Antibiotic use during pregnancy increases offspring asthma severity in a dose-dependent manner. Allergy. 2020;75:1979–1990. doi: 10.1111/all.14234. [DOI] [PubMed] [Google Scholar]
- 61.Loewen K, Monchka B, Mahmud SM, Jong G, Azad MB. Prenatal antibiotic exposure and childhood asthma: a population-based study. Eur Respir J. 2018;52:1702070. doi: 10.1183/13993003.02070-2017. [DOI] [PubMed] [Google Scholar]
- 62.Sagar S, Morgan ME, Chen S, et al. Bifidobacterium breve and Lactobacillus rhamnosus treatment is as effective as budesonide at reducing inflammation in a murine model for chronic asthma. Respir Res. 2014;15:1–46. doi: 10.1186/1465-9921-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ekbom A, Brandt L, Granath F, Lofdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn's disease in a population suffering from COPD. Lung. 2008;186:167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 64.Roussos A, Koursarakos P, Patsopoulos D, Gerogianni I, Philippou N. Increased prevalence of irritable bowel syndrome in patients with bronchial asthma. Respir Med. 2003;97:75–79. doi: 10.1053/rmed.2001.1409. [DOI] [PubMed] [Google Scholar]
- 65.Vutcovici M, Bitton A, Ernst P, et al. Inflammatory bowel disease and risk of mortality in COPD. Eur Respir J. 2016;47:1357–1364. doi: 10.1183/13993003.01945-2015. [DOI] [PubMed] [Google Scholar]
- 66.Burke DG, Fouhy F, Harrison MJ, et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol. 2017;17:1–58. doi: 10.1186/s12866-016-0921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu AG, Lu J, Meng LA, et al. Associations between gut microbial colonization in early life and respiratory outcomes in cystic fibrosis. J Pediatrics. 2015;167:138–147. doi: 10.1016/j.jpeds.2015.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei-Quan Z, Shu-Kang Z, Jun-Wen L, et al. Alterations of fecal bacterial communities in patients with lung cancer. Am J Transl Res. 2018;10:3171–3185. [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu M, Huang K, Liu Y, et al. Modulation of intestinal microbiota by glycyrrhizic acid prevents high-fat diet-enhanced pre-metastatic niche formation and metastasis. Mucosal Immunol. 2019;12:945–957. doi: 10.1038/s41385-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 70.Gui Q-F, Lu H-F, Zhang C-X, Xu Z-R, Yang Y-H. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res. 2015;14:5642–5651. doi: 10.4238/2015.May.25.16. [DOI] [PubMed] [Google Scholar]
- 71.Busra A, Belma A. Gut-lung axis and dysbiosis in COVID-19. Turk J Biol. 2020;44:265–272. doi: 10.3906/biy-2005-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dhar D, Mohanty A. Gut microbiota and Covid-19-possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Udds DA, Maryam M, Muhammed W, et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed Pharmacother. 2021;133:110947. doi: 10.1016/j.biopha.2020.110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gabriella DE, Giancarlo C, Massimiliano M, et al. Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front Med. 2020;7:389. doi: 10.3389/fmed.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used to support the findings of this study are available from the corresponding author upon reasonable request.