Abstract
Ehlers–Danlos syndrome (EDS) is a group of related connective tissue disorders consisting of 13 subtypes, each with its own unique phenotypic and genetic variation. The overlap of symptoms and multitude of EDS variations makes it difficult for patients to achieve a diagnosis early in the course of their disease. The most common form, hypermobile type EDS (hEDS) and its variant, hypermobile spectrum disorder (HSD), are correlated with rheumatologic and inflammatory conditions. Evidence is still needed to determine the pathophysiology of hEDS; however, the association among these conditions and their prevalence in hEDS/HSD may be explained through consideration of persistent chronic inflammation contributing to a disruption of the connective tissue. Aberrant mast cell activation has been shown to play a role in disruption of connective tissue integrity through activity of its mediators including histamine and tryptase which affects multiple organ systems resulting in mast cell activation disorders (MCAD). The overlap of findings associated with MCAD and the immune-mediated and rheumatologic conditions in patients with hEDS/HSD may provide an explanation for the relationship among these conditions and the presence of chronic inflammatory processes in these patients. It is clear that a multidisciplinary approach is required for the treatment of patients with EDS. However, it is also important for clinicians to consider the summarized symptoms and MCAD-associated characteristics in patients with multiple complaints as possible manifestations of connective tissue disorders, in order to potentially aid in establishing an early diagnosis of EDS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12026-022-09280-1.
Keywords: Mast cell activation disorder, Mast cell, Joint hypermobility, Ehlers–danlos syndrome, Primary immunodeficiency disease, Allergy, Histamine, Tryptase, Immunology, Rheumatic disease, Musculoskeletal disease
Introduction
Ehlers–Danlos syndromes (EDS), a heterogenous group of connective tissue disorders (CTD), are characterized by joint hypermobility, skin hyperextensibility, and tissue fragility [1]. Of the 13 subtypes of EDS that have been described, hypermobile EDS (hEDS) is the most common, and as opposed to the twelve other EDS subtypes, hEDS lacks confirmatory laboratory/molecular tests [2]. hEDS and the hEDS variant, hypermobility spectrum disorder (HSD) are characteristically defined by the combination of the following three criteria:
Generalized joint hypermobility (Beighton score)
Combination of any two or more features including systemic manifestations, family history, or musculoskeletal complications and
The absence of exclusion criteria (Supplemental Tables 1,2,3) [1].
Common systemic features of hEDS include rheumatological manifestations, such as joint instability, arthralgia, myalgia, soft tissue injuries, and arthritis [3]. Additionally, recent studies have reported an increase in the prevalence of immune-mediated disorders such as rhinitis, asthma, urticaria, celiac disease, functional gastrointestinal disorders, as well as neuropathies in the hEDS/HSD population [4–16]. The relationship between immune-mediated disorders and hEDS/HSD is poorly understood. A potential link between hEDS/HSD and immune-mediated disorders may be explained by recurrent or chronic release of mast cell (MC)-derived factors. MCs have shown to contribute to barrier function and homeostasis within the connective tissue of multiple organ systems and are native to the epithelium of the gastrointestinal, urogenital and respiratory tracts, among others [17, 18]. While MCs are normally under tight regulation, aberrant immune activation of these cells results in mast cell activation disorders (MCAD) [17, 18]. Due to the intertwined relationship between MC and connective tissue, shared clinical features between MCAD and hEDS/HSD have been described in the literature [9, 19–21]. To that end, the objective of this review is to identify shared clinical features between EDS and MCAD- or MC-related conditions to establish a potential relationship. As both of these disorders are systemic conditions with multifactorial manifestations, understanding and recognizing the relationship between the two conditions may allow providers to identify and treat these cohorts of patients sooner.
Mast cells and mast cell activation disorders
MCs develop in the bone marrow and continue to mature at their site of residence in the connective tissue through the interaction of Kit tyrosine kinase receptor on MC surfaces with stem cell factor [18, 22]. While the exact mechanism of action of MC is out of the scope of this review, it is important to note that MC express a multitude of receptors such as high-affinity IgE and low-affinity IgG receptors, complement receptors, and toll-like receptors [17, 18, 22–24]. Upon the binding of these various receptors to their target, MCs release preformed granule mediators such as histamine, protease, as well as lipid mediators such as prostaglandins and leukotrienes [17, 18, 23, 24]. These mediators are able to carry out a variety of responses such as vasodilation, interactions with nerves, and facilitation of cellular chemotaxis [17, 23, 24].
While normally under tight molecular control, aberrant release of these preformed granules may result in MCAD. MCAD is characterized by recurrent episodes of the release of MC mediators which may occur in any organ system [18, 25]. Due to the various locations and receptors of MC, signs and symptoms of MCAD are diverse and may include irritable bowel symptoms; recurrent flushing, urticaria, and pruritus; cardiac arrhythmias and episodic hypotension; neuropsychiatric disorders; genitourinary conditions; rheumatological manifestations; and anaphylaxis [17, 26]. Mast cell activation syndrome (MCAS), a subtype of MCAD, should be considered in patients who experience recurrent or chronic episodes of MC activation in two or more organ systems, respond to treatment that combats MC-derived mediators or prevents MC degranulation, and have symptoms consistent with increased validated MC-derived mediators [27].
Irregular MC-mediated release may result from intrinsic defects including mutations in the c-kit gene or associated signaling pathways as well as multiplication of a MC specific protease subunit encoding alpha tryptase [17, 18]. Interestingly, factors external to the MC account for most MCAD [17]. In addition to the allergen-specific IgE driven diseases, there are immunologic and nonimmunologic syndromes that can drive MC activation. Recurrent or chronic release of MC-derived factors, including proinflammatory molecules, growth factors, and proteases, has been linked by several studies to heritable CTD like hEDS and HSD [9, 19, 21, 28]. The potential connection between hEDS/HSD and MC-related disorders may be explained by the presence of MC mediators, especially tryptase and histamine which have been found to promote proliferation of fibroblasts and production of collagen [28]. In 2014, it was found that familial hypertryptasemia may be associated with MCAS and was described in 9 families with an autosomal dominant inheritance pattern of elevated basal serum tryptase levels and MCAS. symptoms [22]. Furthermore, in 2016, the same group identified germline mutations in the gene that encodes alpha-tryptase, TPSAB1 that led to increases in basal serum tryptase levels in 35 families presenting with MCAS-related complaints [29]. Of the total 96 patients identified with this mutation, 28% had joint hypermobility, a twofold increase when compared to incidence in the general population, thus indicating a possible correlation between hypermobile syndromes and MC-related disorders [29]. Another potential link may be explained in the reported triad of postural tachycardia syndrome (POTS), EDS, and MCAD, in which 46% of the population with the genetic mutation in TPSAB1 exhibited orthostatic intolerance [29]. However, the connection among these three conditions has been debated in the literature [30].
MCAD-related disorders and hEDS/HSD
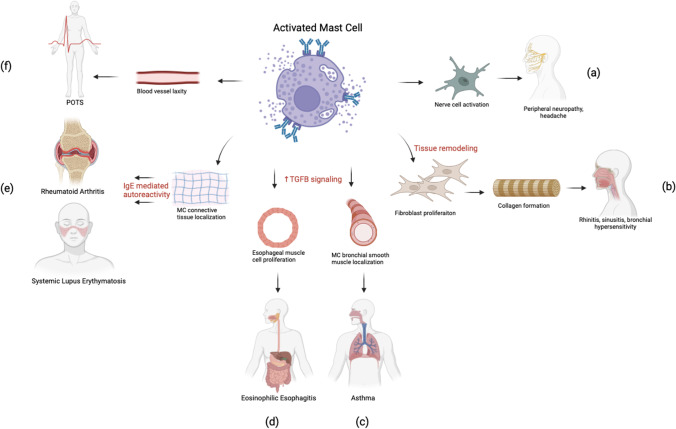
An association between EDS/HSD and MC-related disorders has been highlighted in the literature through prevalent findings of MC-related diseases in EDS/HSD patients (Fig. 1; Table 1) [9, 19–21]. A carefully obtained history in patients with the following described conditions and symptoms supporting MCAD- or MC-related etiology should be obtained in order to establish a potential EDS/HSD diagnosis.
Fig. 1.
Aberrant mast cell (MC) activation can produce inflammatory changes at the connective tissue level leading to dysfunction in multiple organ systems. In peripheral nerves, MC can localize to epineurium, perineurium, and endoneurium and release mediators which may activate nociceptors producing symptoms such as peripheral neuropathy and headache (a). MC-mediated upregulation of cytokines can lead to dysfunctional fibroblast proliferation in nasal and bronchial tissue leading to rhinitis and sinusitis (b). MC-induced TGFB upregulation and localization within bronchial smooth muscle cells can cause modification of matrix proteins of bronchial parenchyma contributing to tissue damage and Asthma (c). MC-induced TGFB upregulation in esophageal tissue can cause proliferation and smooth muscle contraction contributing to eosinophilic esophagitis symptoms (d). When MCs are localized to connective tissues, they can cause microenvironmental changes to the extracellular matrix, inducing IgE medicated autoreactivity and contributing to rheumatologic conditions such as rheumatoid arthritis and systemic lupus erythematosus (e). MC-induced changes may contribute to laxity within blood vessels, causing pooling of the blood in extremities, contributing to postural tachycardia syndrome (POTS) in which patients experience increase of heart rate with standing, a finding commonly associated with hEDS (f)
Table 1.
MCAD-related disorders in hEDS/HSD patients
| Disorder | First author | Study type | Participants | Findings |
|---|---|---|---|---|
| Mast cell activation disorder | Szalewski (2019) | Retrospective case series | > 2 million patient charts at a university hospital | Significant increase in prevalence of idiopathic urticaria in EDS patients compared to random co-occurrence in the general population |
| Song (2020) | Retrospective case series | 98 EDS patients at a physical medicine and rehabilitation clinic | 24% EDS patients had MCAS diagnosis | |
| Cheung (2015) | Retrospective case series | 15 patients with diagnosis of POTS and EDS | 66% of patients with POTS and EDS had symptoms suggestive of a mast cell disorder | |
| Luzgina (2011) | Prospective case–control | 12 patients with phenotypical signs of connective tissue disorders and 16 patients without these signs | Patients with signs of connective tissue disorder had a higher density of chymase positive mast cells in their undamaged skin | |
| Headaches | Malhotra (2020) | Retrospective case series | 140 patients with hypermobility disorders | 66% of patients with hypermobility disorders reported headache or neck pain; of those, migraine (83%) was the most common headache type |
| Rombaut (2010) | Retrospective case series | 72 hEDS patients compared to 69 patients with fibromyalgia and 65 patients with rheumatoid arthritis | 32% hEDS patients reported headache, 76% FM reported headache, 1.6% RA patients reported headache | |
| Maeland (2011) | Questionnaire | 250 EDS patients | 48% EDS patients reported headache | |
| Puledda (2015) | Prospective case–control study | 33 HSD/hEDS patients with migraines matched with 66 migraine controls | HSD/hEDS patients had significantly more frequent headache symptoms and earlier age of onset compared to controls with migraines | |
| Peripheral neuropathies | Cazzato (2016) | Prospective case series | 20 adults with HSD/hEDS | 19 out of 20 patients reported neuropathic pain. All 20 patients had a decrease in intraepidermal nerve density |
| Voermans (2009) | Prospective case series | 40 EDS patients | 85% had mild-to-moderate muscle weakness, 60% had reduction in vibration sense, 13% had axonal polyneuropathy | |
| Benistan (2019) | Prospective case series | 37 hEDS patients | 97% reported severe chronic pain with the most common type of pain being neuropathic | |
| Urticaria | Szalewski (2019) | Retrospective case series | > 2 million patient charts at a university hospital | Significant increase in prevalence of idiopathic urticaria in EDS patients compared to random co-occurrence in the general population |
| Greiwe (2018) | Case report | EDS patient | Pt. with EDS, MCAS, POTS presents with inducible urticaria | |
| Sachinvala (2018) | Case report | EDS patient | EDS patient with history of adrenergic urticaria successfully treated with omalizumab | |
| Sinusitis | Ayres (1985) | Retrospective case series | 20 EDS patients | 25% EDS patients had recurrent sinusitis |
| Zhang (2019) | Case Report | hEDS patient | hEDS patient with severe sinusitis | |
| Fouda (2000) | Prospective case–control | 30 HSD patients with sinusitis and 10 controls with sinusitis | Tissue biopsies from middle meatus of patients with HSD showed increased density and abundant amount of collagen fibrils compared to controls on histology | |
| Asthma | Morgan (2007) | Retrospective case series | 126 HSD patients, 162 EDS patients, 221 healthy controls | Significant increased prevalence of asthmatic symptoms and atopy in HSD/EDS patients compared to controls |
| Al-Rawi (2012) | Prospective case–control | 100 patients with asthma and 100 controls | Joint hypermobility was found in 70.1% of patients with asthma and 29.9% of controls | |
| Gastrointestinal disorders: Eosinophilic esophagitis | Traif (1992) | Case report | EDS patient | EDS patient with eosinophilic gastroenteritis |
| Abonia (2013) | Retrospective case series | 42 patients with eosinophilic esophagitis with a connective tissue disorder | Eightfold risk of eosinophilic esophagitis in patients with connective tissue disorder when compared to the general population | |
| Gastrointestinal disorders: Celiac disease | Danese (2011) | Prospective case series | 31 hEDS patients | 16% of hEDS patients diagnosed with celiac disease |
| Fikree (2015) | Prospective case–control | 13 patients with primary diagnosis of celiac disease | 30.8% prevalence of hEDS found in patients with celiac disease | |
| Gastrointestinal disorders: Inflammatory bowel disease | Vounotrypidis (2009) | Prospective case–control | 83 patients with irritable bowel disease and 67 healthy controls | Prevalence of hEDS found to be 12.2% in Crohn’s disease and 3.6% in ulcerative colitis |
| Fikree (2015) | Prospective case control | 25 patients with Crohn’s disease and 38 patients with ulcerative colitis | 32% prevalence of HSD/EDS found in patients with Crohn’s Disease and 21% prevalence found in ulcerative colitis | |
| Castori (2010) | Prospective case series | 21 hEDS patients | Symptoms of IBD presented as abdominal pain in 61.9% of patients and constipation/diarrhea in 33.3% of patients | |
| Urogynecologic conditions | Castori (2012) | Case series | 82 post-puberal women with joint hypermobility | Dysmenorrhea (82.9%), menorrhagia (53.7%), irregular menses (46.3%), and vulvodynia (31.7%) |
| Sorokin (1994) | Questionnaire | 68 women with EDS | Recurrent anovulation (41.3%), recurrent vaginal infection (53%), abnormal cytologic smears (19%), irregular menses (28%), endometriosis (15.8%), vaginal dryness (25%) | |
| Hurst (2014) | Questionnaire | 775 reproductive age women with EDS | 44.1% infertility, 32.8% intermenstrual bleeding,; 32.9% heavy menstrual bleeding, 92.5% dysmenorrhea and 77% dyspareunia | |
| Hugon-Rodin (2016) | Questionnaire | 386 women with hEDS | 76% menorrhagia, 72% dysmenorrhea, 43% dyspareunia | |
| Rheumatologic conditions | Ozlece (2015) | Case report | EDS patient | MS diagnosis was established in a patient with past history of EDS |
| Vilsaar (2008) | Case series | 4 MS patients | EDS diagnosis in 4 MS patients | |
| Sachinvala (2018) | Case study | EDS patient | MS diagnosis in patient with EDS | |
| Rodgers (2017) | Retrospective Case series | 379 hEDS patients | 97 patients (25.5%) found to have at least one rheumatologic condition | |
| Branch (1978) | Case report | EDS patient | EDS patient with systemic lupus erythematosus and myasthenia gravis | |
| Asherson (2006) | Case report | EDS patient | EDS patient with systemic lupus erythematous | |
| Wallman (2014) | Retrospective case series | 109 patients suffering from autonomic dysfunction with at least one POTS symptom | 18% of patients with POTS diagnosis met criteria for EDS | |
| Shaw (2019) | Questionnaire | 4385 patients with POTS | 25% of participants had diagnosis of EDS with 9% having a diagnosis of MCAS |
Summary of studies demonstrating MCAD-related disorders seen in hEDS/HSD patients. Findings divided into: headache, peripheral neuropathies, urticaria, sinusitis, asthma, eosinophilic esophagitis, celiac disease, inflammatory bowel disease, urogynecologic conditions, rheumatologic conditions. Studies were obtained from literature review
Headaches
Headache has been shown to be prevalent in hEDS/HSD patients when compared to controls [20, 31–34]. In a study by Song et al., 67% of hEDS patients reported symptoms of headache [20]. While the relationship between headaches and hEDS/HSD patients found in this study may be due to multifactorial etiologies associated with hEDS/HSD such as craniocervical junction instability, temporomandibular joint instability, and dysautonomia, MC-related diseases should be a consideration [20, 31–34]. Mediators of MC may activate the trigeminal pathway which, when prolonged, can cause intracranial headaches [35]. This may explain why Song et al. found that 95% of patients with both hEDS and MCAD also had headaches [20].
Peripheral neuropathy
Peripheral neuropathies have been associated with EDS/HSD [13–15]. In a study done in 2016, 95% of EDS patients reported neuropathic pain and 100% of patients showed a decrease in intraepidermal nerve fiber density [14]. In a later study, it was identified that of the 37 hEDS patients studied, 97% reported chronic pain, with neuropathic pain being the most common complaint [13]. Causes of peripheral neuropathy in hEDS/HSD have been explained by presence of ligament and capsular laxity resulting in abnormal pressure on peripheral nerves, or genetic factors involving deficiency of TNXB or collagen I, II, and V in the connective tissue of peripheral nerves [15, 36]. Nevertheless, MC-related disorders as an etiology of peripheral neuropathy in EDS patients cannot be ruled out. In peripheral nerves, MCs are located in the epineurium, perineurium, and endoneurium [37]. Chronic degranulation may result in nerve injury [37, 38]. Furthermore, MCs have been implicated in the activation of nociceptors in nerve endings [37, 38]. Future studies establishing this association between MC-related disorders, peripheral neuropathies, and hEDS/HSD are warranted.
Urticaria/flushing/angioedema
Urticaria is a finding prevalent in patients with EDS when compared to the general population [9, 10]. In addition to basophils, MCs are one of the most commonly implicated inflammatory cells involved in urticaria [39]. Elevated levels of proinflammatory cytokines such as IL-33 promote the adhesion, maturation, and degranulation of MCs in chronic urticaria [40]. While previous studies by Greiwe and Szalewski et al. have established a connection between EDS and urticaria, the direct cause of this association has not been well established and it is therefore not unreasonable that this relationship could be explained by MC-related disorders.
Rhinitis/Sinusitis
EDS/HSD has been associated with rhinitis and/or sinusitis [4–6]. A previous study found an anatomic variation with increased density and quantity of collagen fibrils in EDS patients with sinusitis as compared to control patients with sinusitis [5]. MCs play a role in upregulation of chemokines/cytokines in fibroblasts and epithelial cells as well as expression of matrix metalloproteinases, which interact with extracellular matrix proteins and thus may play a role in the nasal and bronchial hyperresponsiveness and tissue remodeling found in EDS patients with sinusitis [41].
Asthma
Localization of MCs within the bronchial smooth muscle bundles in patients with asthma indicates an important role of MCs in the pathophysiology of this disease [42]. Unsurprisingly, studies have pointed to a relationship between asthma and hEDS/HSD. In one study, a questionnaire and clinical assessment was performed on 509 subjects (221 healthy controls, 126 HSD, 162 EDS). Asthma was significantly more prevalent among patients with EDS/HSD when compared to the control group (HSD: OR 2.7, 95% CI 1.4–4.1, p = 0.002; EDS: OR 3.1, 95% CI 1.8–5.2, p < 0.001) [7]. A smaller study followed 200 patients to determine prevalence of joint hypermobility in patients with asthma (100 asthma patients, 100 matched healthy controls). It was found that joint hypermobility was present in 70.1% of patients with asthma compared to only 29.9% in healthy controls [8]. Morgan et al. suggest that modifications of the matrix proteins in the lung parenchyma as a result of hEDS/HSD may alter the biomechanics, repair and remodeling responses following tissue damage leading to the development of asthma [7]. In patients with MC-related disorders, chronic tissue damage could be exacerbated by the aberrant release of MCs, increasing the likelihood of development of asthma. Though not a well-established connection, the relationship between asthma, MC-related disorders, and hEDS/HSD is plausible.
Gastrointestinal disorders
Several studies have demonstrated hEDS/HSD patients with eosinophilic esophagitis (EoE) [43, 44]. In a study by Abonia et al., the rate of EoE present in a hospital-based cohort of patients with CTD, including hEDS/HSD, was analyzed. It was found that there is an eightfold increase of risk of EoE in patients with CTD, and these patients may be at an increased risk for diffuse extraesophageal gastrointestinal diseases when compared to EoE patients without CTD [43]. While previously studies have focused on the role of eosinophils in the pathogenesis of EoE, recent studies have suggested that MCs have a role in the clinical manifestation of the disease [45]. Animal and cellular studies have shown that MCs can cause esophageal muscle cells to proliferate into a more contractile phenotype and that the mediators released by MCs can activate smooth muscle contraction, thus causing esophageal abnormalities [45]. Furthermore, Abonia et al. noted that TGFB signaling is often enhanced in patients with connective tissue diseases and that elevated TGFB levels in esophageal tissue of EoE patients are localized in both eosinophils and MC, thus explaining this relationship [43].
Recent studies have also shown key involvement of the innate immune system in the pathogenesis of celiac disease, evidenced by the increase in number of MC during the progression of this condition [11]. Celiac disease is an autoimmune disease involving the activation of ingested gluten by the enzyme tissue transglutaminase and its subsequent recognition by CD4 + T cells as a pathogen in the small intestine at the level of the epithelium [12]. Frossi et al. showed that activation of the innate immune system happens first before the induction of the gluten-specific T cell response. In addition to gluten exposure, genetic factors such as HLA-DQ2 and DQ8 haplotypes and environmental factors also play a role in the development of celiac disease [12]. A combination of these factors contributes to epithelial insult and leads to subsequent clinical manifestations such as diarrhea, anemia, and abdominal pain among others. Danese et al. found over a tenfold increase in prevalence of celiac disease in patients with hEDS/HSD. Another small study found that hEDS was likely to be diagnosed in 30% of patients who presented to a GI clinic with a new diagnosis of celiac disease [46]. Patients with celiac disease have been shown to have increased antibody titers against collagen types I, III, V, and VI, potentially explaining this interrelationship between hEDS/HSD and celiac disease [47].
Elevated numbers of MC have been observed in patients with inflammatory bowel disease (IBD) as well [48]. Ulcerative colitis and Crohn’s disease have also been implicated in the literature to affect patients with EDS. One study analyzing patients from a hospital in Greece found that the prevalence of hEDS in Crohn’s disease was 12.2% and the prevalence of hEDS in ulcerative colitis was 3.6% [49]. Fikree also described a high prevalence of patients with EDS/HSD with Crohn disease and ulcerative colitis (32% and 21%, respectively) [46]. While the association of IBD and MC-related conditions is important to consider, another potential explanation between the association of hEDS/HSD and IBD may be due to the close proximity of connective tissue components to the muscularis propria and myenteric plexus in the GI tract, and thus, aberrant connective tissue dysfunction may be a possible cause for gastrointestinal diseases [50]. Other stem cell studies have shown that collagen is necessary for enteric neural progenitor cells to differentiate into neurons and glia cells, thus supporting the notion that the connective tissue is involved in the development of the enteric nervous system [51].
Urogynecologic conditions
Patients with hEDS have been identified via a questionnaire as having menstrual disturbances such as menorrhagia (32.9–76%) [52–55]. This may be due to increased MC activation due to the release of heparin which will contribute to increased prevalence of not only menorrhagia, but also dyspareunia and vaginitis [56]. It has been previously reported that diphenhydramine administered as a vaginal douche successfully reduced dysfunctional uterine bleeding in patients with MCAS not responsive to oral antihistamine treatment [57]. While these authors do not recommend this form of treatment, it does provide insight into the potential MC etiology of menorrhagia.
An additional survey of 1225 women with EDS found that 44.1% self-reported infertility [54]. Infertility could potentially be the result of anovulation, pelvic pain, and vaginal dryness. Pelvic pain such as interstitial cystitis and vulvodynia in particular may be associated with MCAS. In a study that looked at vulvar biopsies in patients with vulvodynia, > 60 MC/mm^2 (range, 40–120 MC) were found in subepithelial distribution surrounding the vestibular glands [57]. Majority were in a degranulated or activated state, which can lead to nociceptive and neuropathic pain over time [57].
Rheumatologic conditions
Kolkhir et al. discovered similarities between the pathogenesis of systemic lupus erythematosus (SLE) and chronic spontaneous urticaria, which both involve IgE-mediated autoreactivity brought on by MC [58]. Key involvement of MCs in multiple sclerosis (MS) and rheumatoid arthritis (RA) also has been demonstrated in separate studies [59, 60]. Multiple studies have found an association between MS and EDS [61–63]. In a study done in 2008, it was found that the prevalence of EDS was up to 11 times more frequent in patients with MS than in the general population [63]. Potential hypothesis elucidating the association of the two conditions implicates abnormalities of extracellular matrix proteins such as collagen in the venular walls of glial cells within the blood brain barrier [63]. These abnormalities or microenvironmental changes within the extracellular matrix may result in activation of metalloproteinases that can cause altered migration of immune cells in the central nervous system, thus resulting in MS [63]. Lastly, SLE and RA have been described in separate cases in the literature as being comorbid conditions to patients with EDS [64–66]. A retrospective analysis of patients with hEDS showed an association between hEDS and various rheumatologic conditions [65]. Patients with hEDS who had associated joint pain, joint laxity and arthralgia who received comprehensive rheumatologic evaluation were likely to be diagnosed with at least one rheumatologic condition [65]. Although the findings of this analysis suggest an association between rheumatologic conditions such as psoriasis, ankylosing spondylitis, and rheumatoid arthritis and hEDS, the mechanism underlying the association between one genetic disorder leading to increased risk of multiple rheumatologic conditions with varying etiologies is not yet established [65].
Postural tachycardia syndrome
POTS is defined as a multifactorial syndrome in which patients experience a recurrent increase in heart rate on standing without orthostatic hypotension [67]. It has been reported that hEDS is the most common disorder associated with POTS [68]. In one study done on the POTS population, it was found that 18% met the criteria for EDS [69]. Patients with hEDS may have vascular laxity which could lead to the pooling of blood in the lower extremities thus leading to POTS, which could be a potential explanation for this relationship [67]. Another potential explanation, however, could be the relationship between MC-related disorders, EDS, and POTS [28, 67]. Shibao et al. described a group of patients with POTS who also experienced MC-related symptoms such as flushing, shortness of breath, headache, lightheadedness, excessive diuresis, and gastrointestinal symptoms [70]. When comparing this group to patients with those without MC-related symptoms and POTS as well as normal controls, it was found that this subgroup of patients had higher levels of urine methylhistamine [70]. Cheung et al. found that in a questionnaire given to patients with a diagnosis of both POTS and EDS, 66% participants reported MC symptoms [19]. In another questionnaire study, of the 4835 patients who were diagnosed with POTS, 25% were found to have a diagnosis of EDS and 9% were found to have been diagnosed with MCAS [71]. Because all three disorders are based on clinical diagnosis rather than quantitative studies, further work needs to be done to determine to what extent the association among this triad of conditions exists [30].
COVID-19
Recent studies highlighted overlapping symptoms between patients with previous COVID-19 and patients with EDS- or MC-related disorders. Similar to EDS- and MC-related disorders, COVID-19 is a multisystem disorder associated with extrapulmonary manifestations including thrombosis, arrhythmias, gastrointestinal symptoms, dermatologic, and neurologic complications [72]. Carfi et al. explored persistent symptoms in 87.4% of 143 patients after acute COVID-19 infection, in which the most commonly reported symptoms were fatigue, dyspnea, joint pain, and chest pain [73]. These symptoms have been commonly reported in patients with EDS/MC-related disorders due to the many associated conditions and complications as noted above. It is unclear at the moment whether COVID-19 symptoms are worsened by coexisting EDS, and risk of complications from COVID-19 differ based on the patient’s presenting risk factors and other medical conditions not related to EDS. Theoharides published a study in 2020, exploring the role of MC in COVID-19 infection and found that MC may serve as hosts for SARS-CoV-2 by expressing angiotensin converting enzyme 2, an important receptor for SARS-CoV-2 [74]. Following stimulation of MC by SARS-CoV-2, MCs are able to rapidly secrete preformed granules as well as cytokines and chemokines 6–24 h later. MC-derived vasoactive mediators are then able to infiltrate multiple organs, including crossing the blood brain barrier leading to “COVID Brain Fog.”[72] Interestingly, pharmacotherapy used in MC-related disorder patients has been found to be effective in mitigating the severity of the viral illness and preventing increase in post-COVID-19 chronic illnesses, bringing to light the importance of MC-targeted treatment in management of COVID-19 patients and maintenance of antihistamine therapy in MC-related disorder patients with COVID-19 infection [75–77]. Valent et al. also recommended that patients with mastocytosis receive vaccination for SARS-CoV-2 as a majority has been found to tolerate the vaccine well with the exception of few reports of adverse reactions [75]. Although there have been no published studies indicating whether EDS patients can undergo COVID-19 vaccination, those with a known history of anaphylaxis or severe reactions to vaccines should discuss risks and benefits of receiving the vaccine with their primary care doctor.
Treatment
There is no cure for MC-related disorders; therefore, MCAD and related conditions should be treated based on symptoms (Table 2). Therapy should always include targeted therapy at controlling MC mediator production and release such as antihistamines, omalizumab, or leukotriene antagonists [28]. In general, the comorbidity of hEDS/HSD is not known to affect the approach to treatment of MC-related conditions; however, corticosteroids should be avoided [28]. Patients should reduce exposure to any triggers as patients may have physical sensitivities (temperature, ultraviolet radiation, etc.), antigenic sensitivities (pollen, mold, etc.), and food intolerances [28]. Desensitization therapy can also be considered. An in-depth review of treatment modalities is beyond the scope of this review.
Table 2.
MCAD treatments
| Allergens |
1. Avoidant measures (diet, environment) 2. Medications: histamine blockade 3. Desensitization (immunotherapy) 4. Traditional Chinese herbal therapy 5. Omalizumab 6. Anti-interleukin monoclonal antibodies |
| Autoimmune disorders |
1. Anti-inflammatory agents 2. Immune globulin supplement 3. Traditional Chinese herbal therapy |
| Primary immune deficiency |
1. Prophylactic antibodies 2. Immune globulin supplement |
| Connective tissue disorders |
1. Physical therapy 2. Surgery 3. Vagal stimulation 4. Acupuncture 5. Oxygen therapy |
There is no specific treatment regimen for MCAD disorders. Rather, treatment is supportive treatment of symptoms. Table adapted from Seneviratne et al
Discussion
The purpose of this review was to demonstrate the critical role that MC play in EDS by highlighting close associations of EDS with various MC-associated conditions and other immunologic disorders. Over the years, it has been purported that EDS has affected approximately 255 million people worldwide [2]. Given the association of EDS with many different disorders as noted above, this finding reinforces the need for physicians from different specialties to closely communicate with each other to manage a patient with EDS. hEDS is the most common subtype that is found to be associated with these disorders, most likely due to a higher number of individuals diagnosed with this subtype compared to others. A recent abstract studied the prevalence of MCAS in hEDS patients and showed that nearly 1 in 3 patients with MCAS had comorbid diagnosis of hEDS in a sample size of 37,665 patients diagnosed with either MCAS or hEDS or both [76]. However, despite this close relationship, both hEDS and MCAS have no specific genetic marker yet discovered. Therefore, it is important for clinicians to diagnose and manage these conditions independently as a direct causation has not been found between hEDS and MCAS and unnecessary diagnostic testing and procedures can potentially harm patients with either disorder.
MCs are associated closely with the epithelium, contributing to its homeostasis by responding to and repairing tissue injury [74, 78–80]. MCs display an array of receptors which recognize molecules derived from tissue injury or inflammation through direct Toll-like receptors and indirect immunoglobulin receptors. Upon co-engagement of receptors that recognize alarmins and pathogens, MCs release mediators that result in innate and adaptive immune responses, regulate blood flow, and coordinate tissue repair [78]. Due to the disrupted collagen in patients with hEDS, where these MC reside, MCs act aberrantly, thus resulting in the syndrome of symptoms and disorders discussed in this review.
Relatedly, both EDS and MCAS have been redefined several times due to their wide range of systemic manifestations and subsequent need to categorize the condition into different subtypes based on specific clinical features. The 2017 EDS classification provided stricter criteria for EDS diagnosis and also illuminated the difference between hEDS and HSD. Kohn and Chang in 2020 stated that the classification of MCAD is still being refined today; however, in the article, they simplified the definitions of different types of MCAS, and proposed that primary MCAS due to mastocytosis is to be called mastocytosis, which in part would support the idea that concurrent MCAS in patients with hEDS is related simply to mastocytosis [30]. Defining specific criteria for these conditions have alleviated some of the concerns in misdiagnosing either condition as both rely on the symptoms for diagnosis.
Above, we note studies that showed that a significant percentage of individuals with a genetic mutation in TPSAB1 had MCAS, EDS, or POTS, or a combination of the three. Weiler et al. emphasized that these types of findings do not mean that one condition is a cause of another and that clinicians should be wary about diagnosing and treating the three conditions as a group [18]. Kohn and Chang also emphasized that overlapping or shared symptoms cannot be the basis of establishing an association between hEDS and MCAD and that there must be a common pathologic mechanism that exists to define such a relationship [30]. As shown above, the symptoms experienced by patients with hEDS, MCAD, or both are nonspecific and not unique to any particular condition or EDS subtype. This idea that an association between two conditions cannot be created based on shared symptoms alone is also reflected when exploring the relationship between hEDS and any MC-related disorders noted above. Therefore, more studies need to be done with patients that are diagnosed with EDS and MCAD using the most recent criteria published. It is clear from our review, however, that MCs are a crucial component of the pathogenesis of EDS as there is increasing prevalence of MC-related disorders in EDS patients. As hEDS is the only EDS subtype without a genetic etiology, our findings highlight the need to discover the genetic marker of hEDS so that the exact mechanisms of how MCs are involved in hEDS/HSD can be further elucidated.
Limitations
There are several limitations in this review. First, most of the studies that we mentioned had small sample sizes, as we made an effort to focus on studies that included patients diagnosed with the most recent EDS criteria. As more individuals are being diagnosed with EDS using this criteria, our hope is that there will be many studies published soon with larger sample sizes. In addition, the patient population explored in these studies were mostly women; therefore, the findings noted in our review may not be entirely attributable to male EDS patients. Lastly, as noted above, the associations made between EDS- and MC-related conditions are based on studies that do not establish a direct causation and focus on overlapping symptoms. As genetic etiology is currently unknown for the most common type of EDS, hEDS, there is no paper that provides sufficient evidence for the relationship of hEDS with its associated conditions. Most of the studies also focused on hEDS only, which is a limitation in itself as the findings may not apply to other EDS subtypes.
Conclusion
This study demonstrates an association between the features of MCAD- or MC-related conditions and hEDS/HSD based on the current literature. While no current evidence exists that directly links the two conditions, this paper demonstrates that these multifactorial conditions have many overlapping features that should be further explored. Recognizing the shared clinical features of these conditions should allow for earlier diagnosis and treatment of patients with hEDS/HSD and MC-related conditions.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- EDS
Ehlers–Danlos Syndrome
- HSD
Hypermobile spectrum disorder
- hEDS
Hypermobile Ehlers–Danlos syndrome
- MC
Mast cells
- MCAD
Mast cell activation disorder
- MCAS
Mast cell activation syndrome
- IgE
Immunoglobulin E
- POTS
Postural tachycardia syndrome
- EoE
Eosinophilic esophagitis
- CTD
Connective tissue disorders
- IBD
Inflammatory bowel disease
- SLE
Systemic lupus erythematosus
- MS
Multiple sclerosis
- RA
Rheumatoid arthritis
Authors contributions
A.MO., D.C., and S.U. performed the literature review and wrote the manuscript with the consultation of A.MA and B.R.
Data availability
No additional data are available.
Declarations
Ethical approval
Not required.
Conflicts of interest
The authors have no conflicts of interest to report.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:8–26. doi: 10.1002/ajmg.c31552. [DOI] [PubMed] [Google Scholar]
- 2.Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, et al. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type: clinical description and natural history. Am J Med Genet C Semin Med Genet. 2017;175:48–69. doi: 10.1002/ajmg.c.31538. [DOI] [PubMed] [Google Scholar]
- 3.Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A. A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet. 2017;175:148–157. doi: 10.1002/ajmg.c.31539. [DOI] [PubMed] [Google Scholar]
- 4.Ayres JG, Pope FM, Reidy JF, Clark TJ. Abnormalities of the lungs and thoracic cage in the Ehlers-Danlos syndrome. Thorax. 1985;40:300–305. doi: 10.1136/thx.40.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouda EE, Badry FAE, Aboul-Khair MM. Collagen changes in cases of hypermobility syndrome (HMS) with rhino-sinusitis: a light and ultrastructural microscopic study [abstract] J Allergy Clin Immunol. 2000 doi: 10.1016/S0091-6749(00)91374-5. [DOI] [Google Scholar]
- 6.Zhang W, Windsor K, Jones R, Taunton DO. Hypermobile type Ehlers-Danlos syndrome associated with hypogammaglobulinemia and fibromyalgia: a case-based review on new classification, diagnosis, and multidisciplinary management. Clin Case Rep. 2019;7:680–685. doi: 10.1002/ccr3.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan AW, Pearson SB, Davies S, Gooi HC, Bird HA. Asthma and airways collapse in two heritable disorders of connective tissue. Ann Rheum Dis. 2007;66:1369–73. doi: 10.1136/ard.2006.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Rawi ZS, Gorial FI, Menshad AD. Joint hypermobility and joint hypermobility syndrome in Iraqi patients with asthma. Int J Mod Biol Med. 2012;2:39–45. [Google Scholar]
- 9.Szalewski RJ, Davis BP. Ehlers-Danlos Syndrome is associated with Idiopathic Urticaria-a retrospective study [abstract]. J Allergy Clin Immunol. 2019;143. 10.1016/j.jaci.2018.12.204.
- 10.Greiwe J. An index case of a rare form of inducible urticaria successfully treated with omalizumab [abstract] Ann Allergy Asthma Immunol. 2018;121:S84. doi: 10.1016/j.anai.2018.09.273. [DOI] [Google Scholar]
- 11.Frossi B, Carli MD, Calabro A. Coeliac disease and mast cells. Int J Mol Sci. 2019;20:3400. doi: 10.3390/ijms20143400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMcp1113994. [DOI] [PubMed] [Google Scholar]
- 13.Benistan K, Martinez V. Pain in hypermobile Ehlers-Danlos syndrome: new insights using new criteria. Am J Med Genet A. 2019;179:1226–1234. doi: 10.1002/ajmg.a.61175. [DOI] [PubMed] [Google Scholar]
- 14.Cazzato D, Castori M, Lombardi R, Caravello F, Bella ED, Petrucci A, et al. Small fiber neuropahy is a common feature of Ehlers-Danlos syndromes. Neurology. 2016;87:155–9. doi: 10.1212/WNL.0000000000002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voermans NC, Alfen NV, Pillen S, Lammens M, Schalkwijk J, Zwarts MJ, et al. Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol. 2009;65:687–97. doi: 10.1002/ana.21643. [DOI] [PubMed] [Google Scholar]
- 16.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65(1):155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton MJ. Nonclonal Mast Cell Activation Syndrome: A Growing Body of Evidence. Immunol Allergy Clin North Am. 2018;38(3):469–481. doi: 10.1016/j.iac.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiler CR, Austen KF, Akin C, Barkoff MS, Bernstein JA, Bonadonna P, Butterfield JH, Carter M, Fox CC, Maitland A, Pongdee T, Mustafa SS, Ravi A, Tobin MC, Vliagoftis H, Schwartz LB. AAAAI Mast Cell Disorders Committee Work Group Report: Mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144(4):883–896. doi: 10.1016/j.jaci.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Cheung I, Vadas P. A new disease cluster: Mast Cell Activation Syndrome, Postural Orthostatic Tachycardia Syndrome, and Ehlers-Danlos Syndrome [abstract] J Allergy Clin Immunol. 2015;135:AB65. doi: 10.1016/j.jaci.2014.12.1146. [DOI] [Google Scholar]
- 20.Song B, Yeh P, Harrell J. Systemic manifestations of Ehlers-Danlos syndrome. Proc (Bayl Univ Med Cent). 2020;34:49–53. doi: 10.1080/08998280.2020.1805714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luzgina NG, Potapova OV, Shkurupiy VA. Structural and functional peculiarities of mast cells in undifferentiated connective tissue dysplasia. Bull Exp Biol Med. 2011;150:676–8. doi: 10.1007/s10517-011-1220-4. [DOI] [PubMed] [Google Scholar]
- 22.Lyons JL, Sun G, Stone KD, Nelson C, Wisch L, O’Brien M, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471–4. doi: 10.1016/j.jaci.2013.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douaiher J, Succar J, Lancerotto L, Gurish MF, Orgill DP, et al. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv Immunol. 2014;122:211–252. doi: 10.1016/B978-0-12-800267-4.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dvorak AM, McLeod RS, Onderdonk A, Monahan-Earley RA, Cullen JB, et al. Ultrastructural evidence for piecemeal and anaphylactic degranulation of human gut mucosal mast cells in vivo. Int Arch Allergy Immunol. 1992;99:74–83. doi: 10.1159/000236338. [DOI] [PubMed] [Google Scholar]
- 25.Theoharides TC, Valent P, Akin C. Mast Cells, Mastocytosis, and Related Disorders. N Engl J Med. 2015;373(19):1884–1886. doi: 10.1056/NEJMc1510021. [DOI] [PubMed] [Google Scholar]
- 26.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–25. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akin C. Mast cell activation disorders. J Allergy Clin Immunol Prac. 2014;2:252–257. doi: 10.1016/j.jaip.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Seneviratne SL, Maitland A, Afrin L. Mast cell disorders in Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2017;175:226–236. doi: 10.1002/ajmg.c.31555. [DOI] [PubMed] [Google Scholar]
- 29.Lyons JL, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564–1569. doi: 10.1038/ng.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohn A, Chang C. The relationship between Hypermobile Ehlers-Danlos Syndrome (hEDS), Postural Orthostatic Tachycardia Syndrome (POTS), and Mast Cell Activation Syndrome (MCAS) Clin Rev Allergy Immunol. 2020;58:273–297. doi: 10.1007/s12016-019-08755-8. [DOI] [PubMed] [Google Scholar]
- 31.Maeland S, Assmus J, Berglund B. Subjective health complaints in individuals with Ehlers-Danlos syndrome: a questionnaire study. Int J Nurs Stud. 2011;48:720–4. doi: 10.1016/j.ijnurstu.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra A, Pace A, Maya TR, Colman R, Gelb BD, Mehta L, et al. Headaches in hypermobility syndromes: a pain in the neck? Am J Med Genet A. 2020;182:2902–2908. doi: 10.1002/ajmg.a.61873. [DOI] [PubMed] [Google Scholar]
- 33.Puledda F, Vigiano A, Celletti C, Petolicchio B, Toscano M, Vicenzini E, et al. A study of migraine characteristics in joint hypermobility syndrome a.k.a. Ehlers-Danlos syndrome, hypermobility type. Neurol Sci. 2015;36:1417–24. doi: 10.1007/s10072-015-2173-6. [DOI] [PubMed] [Google Scholar]
- 34.Rombaut L, Malfait F, Paepe AD, Rimbaut S, Verbruggen G, Wandele ID, et al. Impairment and impact of pain in female patients with Ehlers-Danlos syndrome: a comparative study with fibromyalgia and rheumatoid arthritis. Arthritis Rheum. 2011;63:1979–87. doi: 10.1002/art.30337. [DOI] [PubMed] [Google Scholar]
- 35.Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–76. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castori M, Voermans NC. Neurological manifestations of Ehlers-Danlos syndrome(s): a review. Iran J Neurol. 2014;13:190–208. [PMC free article] [PubMed] [Google Scholar]
- 37.Jaggi AS, Kaur G, Bali A, Singh N. Pharmacological investigations on mast cell stabilizer and histamine receptor antagonist in vincristine-induced neuropathic pain. Naunyn-Schmiedeberg’s Arch Pharmacolo. 2017;390:1087–1096. doi: 10.1007/s00210-017-1426-8. [DOI] [PubMed] [Google Scholar]
- 38.Aich A, Afrin LB, Gupta K. Mast-cell mediated mechanisms of nociception. Int J Mol Sci. 2015;16:290968–329092. doi: 10.3390/ijms161226151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anita C, Baquerizo K, Korman A, Bernstein JA, Alikhan A. Urticaria: a comprehensive review: epidemiology, diagnosis, and work-up. J Am Acad Dermatol. 2018;79:599–614. doi: 10.1016/j.jaad.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Church MK, Kolkhir P, Metz M, Maurer M. The role and relevance of mast cells in urticaria. Immunol Rev. 2018;282:232–247. doi: 10.1111/imr.12632. [DOI] [PubMed] [Google Scholar]
- 41.Pawankar R. Mast cells in allergic airway disease and chronic rhinosinusitis. Chem Immunol Allergy. 2005;87:111–120. doi: 10.1159/000087639. [DOI] [PubMed] [Google Scholar]
- 42.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–84. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 43.Abonia JP, Wen T, Stucke EM, Grotjan T, Griffith MS, Kemme KA, et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. 2014;132:378–86. doi: 10.1016/j.jaci.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Traif I, Jewell L, Thomson ABR. Eosinophilic gastroenteritis in a patient with Ehlers-Danlos syndrome-a rare combination. Can J Gastroenterol Hepatol. 1992;6. 10.1155/1992/563541.
- 45.Nelson M, Zhang X, Pan Z, Spechler SJ, Souza RF. Mast cell effects on esphageal smooth muscle and their potential role in eosinophilic esophagitis and achalasia. Am J Physiol Gastrointest Liver Physiol. 2021;320:G319–G327. doi: 10.1152/ajpgi.00290.2020. [DOI] [PubMed] [Google Scholar]
- 46.Fikree A, Aktar R, Grahame R, Hakim AJ, Morris JK, Knowles CH, et al. Functional gastrointestinal disorders are associated with joint hypermobility syndrome in secondary care: a case-control study. Neurogastroenterol Motil. 2015;27:569–79. doi: 10.1111/nmo.12535. [DOI] [PubMed] [Google Scholar]
- 47.Danese C, Castori M, Celletti C, Amato S, Russo CL, Grammatico P, et al. Screening for celiac disease in the joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type. Am J Med Genet A. 2011;155A:2314–6. doi: 10.1002/ajmg.a.34134. [DOI] [PubMed] [Google Scholar]
- 48.He SH, Xie H, Fu YL. Inhibition of tryptase release from human colon mast cells by histamine receptor antagonists. Asian P J Allergy Immunol. 2005;23:35–39. [PubMed] [Google Scholar]
- 49.Vounotrypidis P, Efremidou E, Zezos P, Pitiakoudis M, Maltezos E, Lyratzopoulos N, et al. Prevalence of joint hypermobility and patterns of articular manifestations in patients with inflammatory bowel disease. Gastroenterol Res Pract. 2009;2009:924138. doi: 10.1155/2009/924138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregersen H, Kassab G. Biomechanics of the gastrointestinal tract. Neurogastroenterol Motil. 1996;8:277–97. doi: 10.1111/j.1365-2982.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 51.Raghavan S, Gilmont RR, Bitar KN. Neuroglial differentiation of adult enteric neuronal progenitor cells as a function of extracellular matrix composition. Biomaterial. 2013;34:6649–58. doi: 10.1016/j.biomaterials.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castori M, Morlino S, Dordoni C, Celletti C, Camerota F, Ritelli M, et al. Gynecologic and obstetric implications of the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome hypermobility type) in 82 Italian patients. Am J Med Genet A. 2012;158A:2176–82. doi: 10.1002/ajmg.a.35506. [DOI] [PubMed] [Google Scholar]
- 53.Hugon-Rodin J, Lebegue G, Becourt S, Hamonet C, Gompel A. Gynecologic symptoms and the influence on reproductive life in 386 women with hypermobility type ehlers-danlos syndrome: a cohort study. Orphanet J Rare Dis. 2016;11:124. doi: 10.1186/s13023-016-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurst BS, Lange SS, Kullstam SM, Usadi RS, Matthews ML, Marshburn PB, et al. Obstetric and gynecologic challenges in women with Ehlers-Danlos syndrome. Obstet Gynecol. 2014;123:506–513. doi: 10.1097/AOG.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 55.Sorokin Y, Johnson MP, Rogowski N, Richardson DA, Evans MI. Obstetric and gynecologic dysfunction in the Ehlers-Danlos syndrome. J Reprod Med. 1994;39:281–4. [PubMed] [Google Scholar]
- 56.Tailor V, Khullar V. Gynaecological considerations in POTS. In: Gall N, Kavi LL, Lobo MD (eds) Postural Tachycardia Syndrome. Springer, Cham; 2021. 10.1007/978-3-030-54165-1_18.
- 57.Afrin LB, Dempsey TT, Rosenthal LS, Dorff SR. Successful mast-cell-targeted treatment of chronic dyspareunia, vaginitis, and dysfunctional uterine bleeding. J Obstet Gynaecol. 2019;29:664–669. doi: 10.1080/0144615.2018.1550475. [DOI] [PubMed] [Google Scholar]
- 58.Kolkhir PV, Olisova OY, Kochergin NG, Sulaimanov SA. Chronic urticaria: diagnostic approach practiced by medical specialists and general practitioners of Russia. Russ J Skin Vener Dis. 2015;18:45–51. doi: 10.17816/dv36963. [DOI] [Google Scholar]
- 59.Conti P, Shaik-Dasthagirisaheb Mast cell serotonin immunoregulatory effects impacting on neuronal function: implications for neurodegenerative and psychiatric disorders. Neurotox Res. 2015;28:147–53. doi: 10.1007/s12640-015-9533-0. [DOI] [PubMed] [Google Scholar]
- 60.Min HK, Kim K, Lee S, Kim H. Roles of mast cells in rheumatoid arthritis. Korean J Intern Med. 2020;35:12–24. doi: 10.3904/kjim.2019.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozlece HK, Ilik F, Huseyinoglu N. Coexistence of Ehlers-Danlos syndrome and multiple sclerosis. Iran J Neurol. 2015;14(2):116–117. [PMC free article] [PubMed] [Google Scholar]
- 62.Sachinvala ND, Stergiou A, Haines DE. Remitting long-standing major depression in a multiple sclerosis patient with several concurrent conditions. Neuropsychiatr Dis Treat. 2018;14:2545–2550. doi: 10.2147/NDT.S168282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vilisaar J, Harikrishnan S, Suri M, Constantinescu CS. Ehlers-Danlos syndrome and multiple sclerosis: a possible association. Mult Scler. 2008;14:567–70. doi: 10.1177/135245850783187. [DOI] [PubMed] [Google Scholar]
- 64.Branch CE, Jr, Swift TR. Systemic lupus erythematous, myasthenia gravis, and Ehlers-Danlos Syndrome. Ann Neurol. 1978;4:374–5. doi: 10.1002/ana.410040414. [DOI] [PubMed] [Google Scholar]
- 65.Rodgers KR, Gui J, Dinulos MBP, Chou RC. Ehlers-Danlos syndrome hypermobility type is associated with rheumatic diseases. Sci Rep. 2017;7:39636. doi: 10.1038/srep39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asherson RA, Bosman C, Tikly M, Spiro F, Pope FM. Ehlers-Danlos syndrome type IV in a young man. J Rheumatol. 2006;33:2091–6. [PubMed] [Google Scholar]
- 67.Bonamichi-Santos R, Yoshimi-Kanamori K, Giavina-Bianchi P, Aun MV. Association of Postural Tachycardia Syndrome and Ehlers-Danlos Syndrome with Mast Cell Activation Disorders. Immunol Allergy Clin North Am. 2018;38(3):497–504. 10.1016/j.iac.2018.04.004. [DOI] [PubMed]
- 68.Mathias CJ, Low DA, Iodice V, Owens AP, Kirbis M, Grahame R. Postural tachycardia syndrome – current experiences and concepts. Nat Rev Neurol. 2011;8:22–34. doi: 10.1038/nrneurol.2011.187. [DOI] [PubMed] [Google Scholar]
- 69.Wallman D, Weinberg J, Hohler AD. Ehlers-Danlos Syndrome and Postural Tachycardia Syndrome: a relationship study. J Neurol Sci. 2014;340:99–102. doi: 10.1016/j.jns.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Shibao C, Arzubiaga C, Roberts LJ, 2nd, Raj S, Black B, Harris P, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45:385–90. doi: 10.1161/01.HYP.0000158259.68614.40. [DOI] [PubMed] [Google Scholar]
- 71.Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, et al. The face of postural tachycardia syndrome – insights from a large cross-sectional online community-based survey. J Intern Med. 2019;286(4):438–448. doi: 10.1111/joim.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theoharides TC, Conti PJ. COVID-19 and Multisystem inflammatory syndrome, or is it Mast Cell Activation Syndrome? Biol Regul Homeostat Agents. 2020;34(5):1633–1636. doi: 10.23812/20-EDIT3. [DOI] [PubMed] [Google Scholar]
- 73.Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theoharides TC. Potential association of mast cells with coronavirus disease. Ann Allergy Asthma Immunol. 2021;126(3):217–218. doi: 10.1016/j.anai.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valent P, Akin C, Bonadonna P, et al. Risk and management of patients with mastocytosis and MCAS in the SARS-CoV-2 (COVID-19) pandemic: expert opinions. J Allergy Clin Immunol. 2020;146(2):300–306. doi: 10.1916/j.jaci.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Afrin LB, Ackerley MB, Bluestein LS, et al. Diagnosis of mast cell activation syndrome: a global “consensus-2”. Diagnosis (Berl). 2020:dex-2020–0005.10.1515/dx-2020-0005. https://www.ncbi.nlm.org/pubmed/32324159. [DOI] [PubMed]
- 77.Mathias K, Mantha A, Mathias L, et al. POS1366 The Relationship of Mast Cell Activation Syndrome and Hypermobile Ehlers-Danlos Syndrome in Hospitalized Patients in the United States. Ann Rheum Dis. 2021;80:965. doi: 10.1136/annrheumdis-2020-219825. [DOI] [PubMed] [Google Scholar]
- 78.Brock I, Prendergast W, Maitland A. Mast cell activation disease and immunoglobulin deficiency in patients with hypermobile Ehlers-Danlos syndrome/hypermobility spectrum disorder. Am J Med Genet. 2021:1–9. 10.1002/ajmg.c.31940. [DOI] [PubMed]
- 79.Tete G, D’orto B, Ferrante L, Pollizzi E, Cattoni FJ. Role of mast cells in oral inflammation. Biol Regul Homeost Agents. 2021;35:65–70. doi: 10.23812/21-4supp1-6. [DOI] [PubMed] [Google Scholar]
- 80.Yang C, Chen N, Tang XL, Qian XH, Cai CP. Immunomodulatory effects of IL-33 and IL-25 in an ovalbumin-induced allergic rhinitis mouse model. Biol Regul Homeost Agents. 2021;35(2):571–581. doi: 10.23812/20-615-A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data are available.