To the Editor:
Current availability of various vaccination platforms against SARS-CοV-2 generates optimism toward the development of robust herd immunity and end of the pandemic. For particular populations however, such as the hematopoietic cell transplant (HCT) recipients who represent a highly vulnerable population to COVID-19 with dismal prognosis and mortality higher than 20% [1–3], there is an urgent need for a prompt and effective protection.
Notwithstanding the prioritization of HCT recipients to COVID-19 vaccination, limited information is available on whether and to what extent, they could mount functional immune responses as they were generally excluded from vaccination trials [4]. Humoral responses post SARS-CοV-2 vaccination have been studied in solid organ transplant (SOT) [5, 6] or HCT [7] recipients, patients with hematologic malignancies [8–12] or/and after CAR T-cell therapy [13]. Nevertheless, information on both the B- and T-cell components of the adaptive immunity in terms of neutralizing antibody viral inhibition and interferon-γ secreting SARS-CoV-2-specific T-cells, after vaccination of HCT recipients is lacking. To gain insights in the adaptive immune responses post-HCT, we studied neutralizing antibody and T-cellular immune responses to SARS-CoV-2 vaccination of HCT recipients.
In our Institutional Review Board approved study, SARS-CoV-2 unexposed, adult HCT recipients scheduled to receive two doses of BNT162b2, were prospectively enrolled after providing written consent. Unexposed, fully vaccinated, health-care professionals served as control. Unexposed individuals were those reporting no close contact with known case, or/and none of the typical COVID-19 symptoms or/and negative testing since the pandemic initiation.
Blood samples were collected on day 1 and day 22 before the first and second BNT162b2 dose, respectively, and on day 50 post second dose. Neutralizing antibodies against SARS-CoV-2 (CoV-2-NAbs) were measured in serum using an FDA-approved methodology (ELISA, cPass™; GenScript) [14] where CoV-2-NAbs ≥30% are considered positive and ≥50% as providing clinically relevant viral inhibition [15].
T-cell responses were measured as previously described [16]. Briefly, peripheral blood mononuclear cells (PBMCs) were pulsed with spike pepmixes and interferon-gamma secretion was measured by Elispot and counted as Spot-forming cells (SFCs) on Eli.Scan Elispot scanner (A.EL.VIS) using the Eli.Analyse software V6.2.SFC. SARS-CoV-2 spike-specific T cells (spike-STs) were expressed as SFCs per number of input cells. Response was considered positive, if SFCs were ≥30 per 5 × 105 PBMCs.
Statistics were performed with GraphPad Prism. Descriptive statistics used median (range) values. Continuous variables were analyzed using one-way ANOVA with Bonferroni’s correction or Kruskal–Wallis for multiple comparisons. Mann–Whitney or 2-tailed Student’s t test were used for two group comparisons. Correlations between continuous variables were assessed using the Pearson’s or Spearman’s correlation coefficients. P-values ≤0.05 were considered significant.
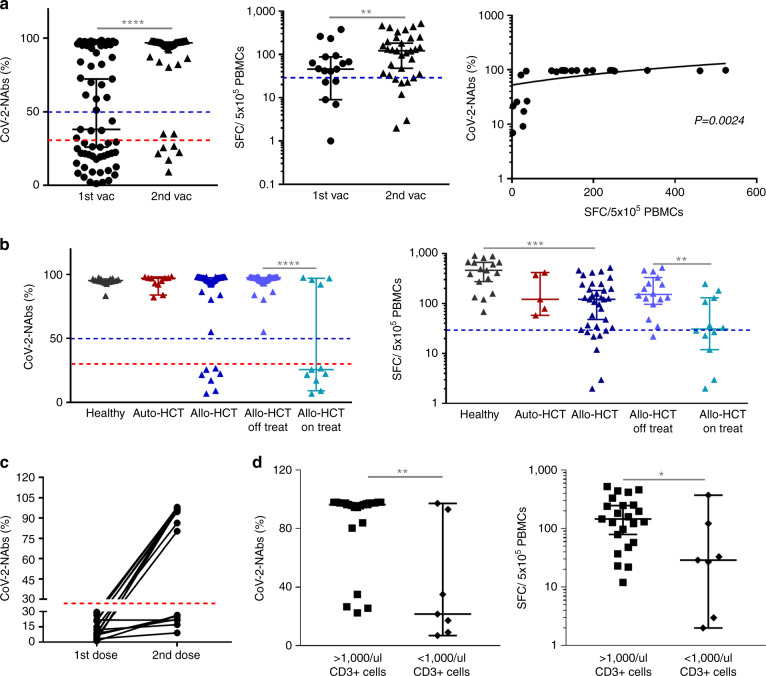
Sixty-three eligible HCT patients (49 allo-, 14 auto-HCT) of median age 49 (21–71) years, at 2.8 (0.17–31) and 2.1 years (1.25–8) post allo- and auto-HCT respectively, who were vaccinated with the Pfizer-BioNTech were enrolled (Supplementary Table 1). No clinically significant adverse event related to SARS-CoV-2-vaccination was reported. CoV-2-NAb responses were studied in all patients, while T-cell responses were measured in 36/63 vaccinated patients (31 allo-HCT/5 auto-HCT). As control cohort, 17 unexposed, health-care vaccinees of median age 57 years (29–68) and without any known underlying disease, were included. CoV-2-NAbs and spike-STs were barely detectable before vaccination but a discernable activity was observed after the first dose, reaching highly protective levels after the second dose in 88% and 79% of all tested patients (p < 0.0001, p = 0.002, respectively; Fig. 1A and Supplementary Table 2). Notably, CoV-2-NAbs strongly correlated with T-cell responses in the tested patients (Pearson r = 0.6009; p = 0.0024; Fig. 1A).
Fig. 1. Humoral and T cell responses in HCT recipients.
A Humoral and T cell responses after the second vaccination dose as compared to the first dose in HCT recipients. The red and blue dotted lines in the left graph indicate the 30% and 50% threshold above which CoV-2-NAbs are considered positive or highly protective, respectively. The blue dotted line in the middle graph indicates the threshold above which T cell response was considered positive. Median with 95% CI is shown. ****p < 0.0001, **p = 0.002. B CoV-2-NAbs and spike-STs after the second dose in healthy subjects, auto- and allo-HCT recipients, as well as the allo-HCT subgroups, on and off immunosuppression. The red and blue dotted lines in the left graph indicate the 30% and 50% threshold above which CoV-2-NAbs are considered positive and have been associated with clinically relevant viral inhibition, respectively. The blue dotted line in the right graph indicates the threshold above which T cell response was considered positive. Median with 95% CI is shown. ****p < 0.0001, ***p = 0.0005, **p = 0.0044. C Humoral protective immune responses after the 1st and 2nd vaccination dose in allo-HCT patients. Each line represents a single allo-HCT patient. The red dotted line indicates the 30% threshold above which CoV-2-NAbs are considered positive. D Association of circulating CD3+ cells <1000/μl with neutralization capacity and levels of spike-STs. Median with 95% CI is shown. **p = 0.0029, *p = 0.0292. HCT hematopoietic cell transplantation, Auto-HCT autologous HCT, Allo-HCT allogeneic HCT, CoV-2-NAbs neutralizing antibodies against SARS-CoV-2, spike-STs SARS-CoV-2 spike-specific T cells.
All auto-HCT patients (including 2 patients receiving maintenance immunotherapy post-autoHCT) showed protective B- and T-cell responses, similar to healthy subjects, however, the long interval post transplantation (>1.25 year) may have generated an unintended bias toward elevated immune responses (Fig. 1B). Post complete vaccination, neutralizing inhibition was similar among allo-HCT recipients, auto-HCT patients and healthy volunteers whereas circulating spike-STs were significantly lower – yet within protective levels -, in allo-HCT patients over healthy individuals (p < 0.0001) (Fig. 1B and Supplementary Table 2). This difference in spike-STs over the rather uniform CoV-2-Nabs levels across groups, practically reflects the different assay read-outs (SFCs/number of input cells vs % viral inhibition) and the broad dynamic range (2-906 SFCs/5 × 105 PBMCs) of the entire spike-ST pool (Supplementary Table 2), rather than critical differences in protective adaptive immunity across cohorts.
Allo-HCT patients developed frequent (85% and 75%) and high (97% CoV-2-NΑb inhibition and 125 SFCs/5 × 105 PBMCs-Supplementary Table 2) B- and T-cell responses post-second vaccination. Interestingly, early T-cellular protective immune responses were reached in 62% of allo-HCT pts after the 1st dose, whereas only 46% developed neutralizing humoral capacity at that time, in line with the detection of S-reactive CD4+ and CD8+ T-cells as early as Day7 and Day10 after the first BNT1622 dose [17]. The effect of the second dose was profound in this cohort where 69% of patients who had failed to mount any functional humoral response with the 1st dose, achieved protective immunity after the 2nd dose (Fig. 1C). This underscores the importance of second immunization in vulnerable patients who may remain otherwise, sub-optimally protected while serve as a viral reservoir for reactivations and novel mutations.
Importantly, a significant proportion of allo-HCT recipients under active immunosuppression failed to reach protective levels of CoV-2-NAbs and spike-STs, demonstrating lower immunogenicity to vaccination, compared to patients off-treatment (Fig. 1B, p < 0.0001, p = 0.0044 respectively) whose adaptive immune responses were similar to auto-HCT recipients and healthy subjects. Only 36% and 50% of patients on systemic immunosuppression reached protective (>50%) CoV-2-NAb inhibition and spike-ST ( ≥ 30 SFCs/5 × 105 PBMCs) levels, compared to 100% and 93% of immunosuppression-free patients, respectively (Supplementary Table 2). One patient under immunosuppression who mounted marginal adaptive immune responses post-second vaccination (CoV-2-NAbs: 40%, CoV-2-STs: 33 SFC/5 × 105 PBMCs), succumbed to COVID-19 infection 40 days later. As others have shown, circulating CD3+ cells <1000/μl were associated with impaired neutralization capacity [7], but also with suboptimal spike-ST levels (Fig. 1D; p = 0.003, p = 0.03 respectively].
The majority of available literature on SARS-CoV-2-vaccination immune responses in immunocompromised patients relies on the B-cell component of adaptive immunity and in particular, serological responses rather than functional neutralizing capacity [7, 9–11, 18]. T-cellular immune responses however, are an indispensable component of protection, especially early post-vaccination [17], in the absence yet of optimal CoV-2-Nabs [19], while unlike the relatively short-lived humoral response, T-cell immunity against SARS-CοV-2 may be heightened and long-lasting [20, 21].
In transplantation, compound B- and T-cell immune responses post SARS-CοV-2-vaccination have been only studied in SOT patients demonstrating poor immune reactivity [5]. Our study provides insights in the whole spectrum of adaptive immune responses in terms of functional immune protection of HCT patients following SARS-CοV-2 vaccination. Limitations of the study include the wide range of post-HCT interval, lack of matching between controls and cases and the relatively small sample size of control group. Results from trials investigating the immunogenicity of vaccines in this vulnerable population, such as the NCT04723706, will solidify the vaccination effect in establishing protective immunity in HCT patients.
In conclusion, active immunosuppression emerged as the major determinant of poor/suboptimal adaptive responses. Immunosuppression-free HCT patients may elicit powerful humoral neutralizing and T-cell responses, whereas it seems highly unlikely that those on systemic immunosuppression, could be protected by full vaccination. Limited data from patients receiving a third dose show humoral responses in almost half of the allo-HCT subjects who failed to respond after two doses [22]. The current, yet dynamically formulated, guidance in HCT recipients, recommends a fourth vaccine dose for those within the first 2 years post-HCT or under systemic immunosuppression [23], however, further studies are needed to find robust immune correlates and evaluate additional vaccine doses or alternative therapeutic platforms, such as adoptive immunotherapy with convalescent-donor CoV-2-STs [16, 24, 25].
Supplementary information
Acknowledgements
EG is supported by the ASH Global Research Award.
Author contributions
EG, AP, EY, IS, AA designed the study. EG, AP and EY analyzed data and wrote the manuscript. TT, FS, EEK, MG, AP, AK, FC collected samples and performed laboratory analysis. GK and IB collected samples and clinical data. DC, DS, EY, IS, AA edited and approved the final draft.
Funding
This study was in part funded by an investigator-driven grant from Prefecture of Macedonia.
Data availability
Data are available upon request.
Competing interests
EG has consulted for Amyndas, Alexion, Omeros, and Sanofi Pharmaceuticals. EY has consulted for BlueBirdBio and Vertex/CrispRTherapeutics. Remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Eleni Gavriilaki, Anastasia Papadopoulou.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-022-01675-w.
References
- 1.Sharma A, Bhatt NS, Martin AS, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah GL, DeWolf S, Lee YJ, Tamari R, Dahi PB, Lavery JA, et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest. 2020;130:6656–67. doi: 10.1172/JCI141777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, Martínez-Fernández JR, Crespo M, Gayoso J, et al. COVID-19 in transplant recipients: The Spanish experience. Am J Transpl. 2021;21:1825–37. doi: 10.1111/ajt.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub-Hohenbleicher H, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131:e150175. doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–6. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redjoul R, Bouter ALE, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398:298–9. doi: 10.1016/S0140-6736(21)01594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv 2021. 10.1101/2021.04.06.21254949. [DOI] [PMC free article] [PubMed]
- 9.Jurgens EM, Ketas TJ, Zhao Z, Satlin MJ, Small CB, Sukhu A et al. Serologic response to mRNA COVID-19 vaccination in lymphoma patients. Am J Hematol 2021. 10.1002/AJH.26322. [DOI] [PMC free article] [PubMed]
- 10.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11:138. doi: 10.1038/s41408-021-00530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terpos E, Trougakos IP, Gavriatopoulou M, Papassotiriou I, Sklirou AD, Ntanasis-Stathopoulos I, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137:3674–6. doi: 10.1182/blood.2021011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bird S, Panopoulou A, Shea RL, Tsui M, Saso R, Sud A, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8:e389–e392. doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhakal B, Abedin S, Fenske T, Chhabra S, Ledeboer N, Hari P, et al. Response to SARS-CoV-2 vaccinationin patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138:1278–81. [DOI] [PMC free article] [PubMed]
- 14.Tan CW, Chia WN, Qin X, Liu P, Chen MI-C, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38:1073–8. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 15.Walsh EE, Robert W, Frenck J, Falsey AR, Kitchin N, Absalon J, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–50. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papayanni P-G, Chasiotis D, Koukoulias K, Georgakopoulou A, Iatrou A, Gavriilaki E et al. Vaccinated and convalescent donor–derived severe acute respiratory syndrome coronavirus 2–specific T cells as adoptive immunotherapy for high-risk coronavirus disease 2019 patients. Clin Infect Dis. 2021;73:2073–82. [DOI] [PMC free article] [PubMed]
- 17.Kalimuddin S, Tham CYL, Qui M, Alwis R, de, Sim JXY, Lim JME, et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Medicine. 2021;2:682. doi: 10.1016/j.medj.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamez A-C, Pradier A, Giannotti F, Petitpas A, Urdiola MF, Vu D-L, et al. Antibody responses to SARS-CoV2 vaccination in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2021;56:3094–6. [DOI] [PMC free article] [PubMed]
- 19.McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2020;590:630–4. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Yang X, Zhong J, Zhou Y, Tang Z, Zhou H, et al. Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection. Nat Commun. 2021;12:1724. doi: 10.1038/s41467-021-22036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Heal Eur. 2021;10:100208. [DOI] [PMC free article] [PubMed]
- 22.Redjoul R, Le Bouter A, Parinet V, Fourati S, Maury S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8:e681–83. doi: 10.1016/S2352-3026(21)00274-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19 Vaccines for Moderately or Severely Immunocompromised People | CDC. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Accessed 8 Feb 2022).
- 24.Keller MD, Harris KM, Jensen-Wachspress MA, Kankate V, Lang H, Lazarski CA, et al. SARS-CoV-2 specific T-cells are rapidly expanded for therapeutic use and target conserved regions of membrane protein. Blood. 2020. 10.1182/blood.2020008488. [DOI] [PMC free article] [PubMed]
- 25.Martits‐Chalangari K, Spak CW, Askar M, Killian A, Fisher TL, Atillasoy E, et al. ALVR109, an off-the-shelf partially HLA matched SARS-CoV-2-specific T cell therapy, to treat refractory severe COVID-19 pneumonia in a heart transplant patient: Case report. Am J Transplant. 2021. 10.1111/AJT.16927. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.