Abstract
The field of exercise physiology has enjoyed tremendous growth in the past 40 years. With its foundations in the natural sciences, it is an interdisciplinary field that is highly relevant to human performance and health. The focus of this review is on highlighting new approaches, knowledge, and opportunities that have emerged in exercise physiology over the last four decades. Key among these is the adoption of advanced technologies by exercise physiologists to address fundamental research questions, and the expansion of research topics to range from molecular to organismal, and population scales in order to clarify the underlying mechanisms and impact of physiological responses to exercise in health and disease. Collectively, these advances have ensured the position of the field as a partner in generating new knowledge across many scientific and health disciplines.
Keywords: bioenergetics, disease, fatigue, genetics, health, metabolism, muscle, performance, physical activity
The past 40 years have been a period of explosive growth in knowledge and application for the field of exercise physiology. This growth is reflected by the volume of research performed and the expansion of exercise physiology principles into interdisciplinary and translational research. Figure 1 illustrates the increase in publications in exercise physiology since 1980, as well as its representation in highly regarded scientific and clinical journals such as the Proceedings of the National Academy of Sciences and the Journal of the American Medical Association, respectively. While the number of publications in all scientific fields likely follows a similar growth pattern over the same period, it is notable that “exercise physiology” has shown strong growth overall and in its breadth of applications in scientific and clinical journals that are not strictly focused on exercise.
Figure 1 —
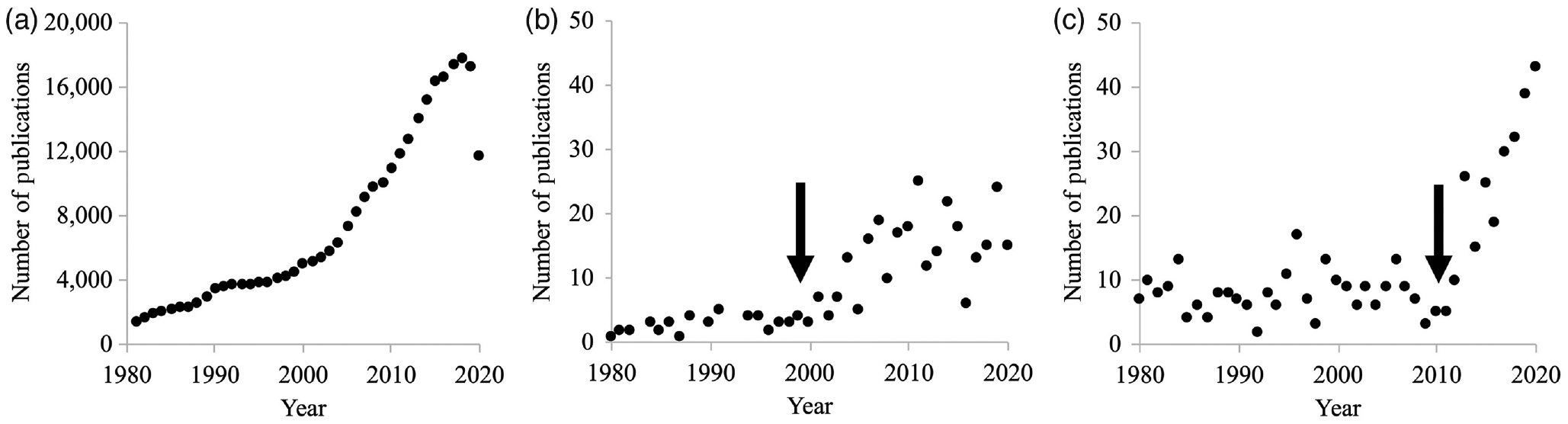
Number of publications including the term “exercise physiology” indexed in PubMed between 1980 and 2020. (a) Total number of “exercise physiology” publications indexed in PubMed, (b) number of “exercise physiology” publications in Proceedings of the National Academy of Sciences indexed in PubMed, and (c) number of publications in the Journal of the American Medical Association indexed in PubMed. Arrows indicate apparent inflection points beyond which the rate of publications increased in representative basic and clinical science journals.
Where did we leave off? 40 years ago, critical questions in the field were largely focused on understanding how energy is generated to support exercise. At that time, these questions concentrated on the kinetics of oxygen consumption and fuel selection during various exercise protocols, as well as the interplay between “aerobic” and “anaerobic” metabolism (Faulkner & White, 1981). The drive to understand the limits to human physical performance was strong, and these studies helped establish the critical features of exceptional human performance as well as the boundaries within which subsequent research has applied these same approaches and concepts to understanding the mechanisms that support human health and function in a range of populations.
The discipline of exercise physiology has both pushed and been pushed by technological advances (of course!). Our ability to identify molecular factors associated with specific responses and adaptations to exercise in addition to being able to visualize protein interactions, understand cellular biochemistry, and investigate in vivo structure and function are but a few examples of how technological developments have enhanced our ability to ask increasingly sophisticated questions related to physiology and the response to exercise.
From the beginning, exercise physiology has been an integrative science; understanding the physiological responses to acute or chronic exercise in the whole organism requires an acknowledgement of the influence of variables such as the mechanical, neural, environmental, psychological, hormonal, diurnal, and nutritional factors that affect our exercise responses. This necessary integration has become even more apparent as we join with researchers in other disciplines within kinesiology (e.g., biomechanics, neuroscience), the STEM fields, and clinical researchers in the health professions (medicine and public health). Indeed, Faulkner and White (1981) discussed the importance of placing graduate students into high-caliber postdoctoral positions in basic sciences in order to increase the rigor and impact of exercise physiology studies. A quick evaluation of current fundings by the National Institutes of Health (NIH) suggests that this advice has been followed: there are currently 26 funded individual and institutional training grants and another 477 research grants that include the search term “exercise physiology.” The number of funded NIH grants grows to over 47,000 when the word “exercise” is in the search term. While clearly not a systematic analysis, these numbers provide some indication of the extent to which exercise physiology has made its way into mainstream biomedical research in the past 40 years.
It must be noted that, until relatively recently, a substantial limitation to existing studies in exercise physiology has been the nearly exclusive use of healthy, young, White males as study participants. Indeed, even studies using animal models were historically performed only on young male animals. As a result of this limitation, there are significant knowledge gaps regarding the generalizability of the results of these earlier studies to most of the population; those who are non-White, nonmale, or >65 years of age. In recent years, biomedical researchers have begun to address this problem, in part through requirements by agencies that fund research to be inclusive of persons from underrepresented groups, including racial and ethnic minorities, women, children, and older adults. Given the clear disparities in health outcomes in various portions of the population, studies that are inclusive and diverse are essential to advancing the field.
The intent of this review is to highlight some of the new methods, knowledge, and opportunities that have been developed in the field of exercise physiology in the last 40 years. The examples discussed are meant to provide an understanding of the breadth and depth of scientific endeavor in the field. Indeed, a clear theme that has emerged is the position of exercise physiology at the intersection of research from multiple fields that are related to human performance and health. While a full discussion of the many important discoveries in our field is beyond the scope of this review, readers are directed to the excellent journal Exercise and Sport Sciences Reviews. Published by the American College of Sports Medicine and under the direction of Editor-in-Chief Roger Enoka, this quarterly journal is an outstanding resource for those interested in learning about innovative, timely, and high-impact research in physiology and exercise science.
New Tools and Approaches
In the last 40 years, technology and innovation have advanced the field of exercise physiology in remarkable ways. Imaging techniques have evolved to allow visualization of molecular, cellular, and physiological phenomena. An explosion in wearable sensor technology has allowed researchers to collect rich data sets of physical activity and metabolic variables both inside and outside of the laboratory. Genetic advances created the opportunity for exercise physiologists to delve into genomic, epigenomic, transcriptomic, and metabolomic mechanisms that underpin physiological responses to exercise. The quantity of data that can be obtained has created opportunities for the use of advanced analytical techniques in the field, including meta-analysis and computational modeling, to create and interpret powerful data reflecting a wide range of physiological variables and responses.
Visualizing Physiological Processes
Classical electron and light microscopy techniques have been used by exercise physiologists to understand the form of skeletal muscle since the 1950s (Porter & Palade, 1957). As early as the 1960s, light microscopy was being used to show changes in the arrangements of myofilaments during muscular contraction as evidence for the “sliding filament theory” of skeletal muscle contraction (Huxley, 1969). In the many years since, advances in single-molecule approaches and X-ray crystallography led to the eventual development of the “swinging lever-arm model” to explain actomyosin interactions during muscle contraction (Geeves et al., 2005; Spudich, 2001). In fact, the current depth of understanding of actomyosin interactions in the generation of force, velocity, and power is an excellent example of the multidisciplinary nature of exercise physiology, as experts from classical molecular biology, genetics, biophysics, biochemistry, and physiology have all contributed to the current understanding of skeletal muscle contraction at the molecular level.
At the cellular level, advances in biotechnology have allowed for the novel identification and visualization of cells and proteins involved in the response to exercise. For example, exercise physiologists routinely use antibodies to label proteins on either the cell surface or within the cytosol of cells. Antibodies can be fluorescently conjugated for visualization via microscopy or flow cytometry. These labeled proteins provide information about the identity of cells within a tissue in innumerable contexts; including by providing information about the junctions that form between cells or about the types of cells that become activated to promote adaptations to exercise. Specifically, significant work has been done to understand skeletal muscle responses to exercise and disease (Tidball, 2011). Much of this work has been fueled by advances in visualizing cells that infiltrate damaged, injured, or diseased skeletal muscle. Satellite cells, in addition to vascular, perivascular, and immune cells, have been implicated in the regenerative response to skeletal muscle damage and disease (Hyldahl et al., 2013). Understanding the complexities of skeletal muscle repair and regeneration is another prime example of scientists from across disciplines, including immunology, cardiovascular biology, molecular and cellular biology, and physiology, pursuing research questions alongside exercise physiologists.
At the physiological level, imaging techniques, including magnetic resonance imaging and spectroscopy (MRS), near-infrared spectroscopy (NIRS), and positron emission tomography (PET), have informed exercise physiologists about the structure and metabolic function of skeletal muscle, cardiac muscle, and the brain before, during, and after exercise. Magnetic resonance imaging and spectroscopy use a static magnetic field and radiofrequency pulses to encode metabolite-specific information that produces anatomical images or biochemical information (energy compounds, blood flow, etc.), respectively. MRS can be used to detect a variety of lipids and metabolites, such as glycogen, adenosine triphosphate (ATP), phosphocreatine, inorganic phosphate, deoxymyoglobin, and acetylcarnitine in human skeletal muscle (Kushmerick, 1986); (see below). As MRS technology has improved through advances in magnet capabilities, electronics, and data computing, we can now gather even richer biochemical information and resolve the concentrations of metabolites that were previously undetectable, including nicotinamide adenine dinucleotide, and various phosphomonoesters and phosphodiesters (Meyerspeer et al., 2021). Furthermore, ATP flux can be calculated through oxidative and nonoxidative metabolic pathways (Boska, 1991; Kemp et al., 1993). This information is used to understand metabolic responses to exercise across the lifespan and the healthspan, including understanding how aging and disease affect energy metabolism (Fitzgerald et al., 2016; Krumpolec et al., 2020).
Other imaging techniques that have been important in the last 40 years are NIRS and PET. NIRS is used in exercise physiology for assessing tissue oxygenation noninvasively using optical sensors (Britton Chance et al., 1988; Hamaoka et al., 2011). Advances in NIRS have provided the flexibility to assess tissue oxygenation using portable systems for a wide variety of experimental conditions (Ryan et al., 2013; Shiga et al., 1995). In the future, NIRS will continue to prove useful in the field of exercise physiology due to its potential to be integrated into clothing or other wearable sensors, thus providing continuous data both in the laboratory and in free-living settings.
In contrast to the relative portability of NIRS, PET produces images of metabolic processes and blood flow by detecting the decay of isotope tracers. These tracers are available for amino acids, water, lactate, and glucose, to name a few substances important in exercise metabolism. In exercise physiology, PET is most commonly used to detect glucose metabolism using a fluoridated analog of glucose. When combined with computed tomography or magnetic resonance imaging, this technique can be used to evaluate both the amount and anatomical location of active glucose metabolism (Rudroff et al., 2015). Another advantage of PET is that it can be used to monitor the amount and spatial distribution of muscle activity that occurred during various exercise conditions in a free-living environment (Rudroff et al., 2015).
Wearables
Advances in wearable technologies have greatly expanded the capabilities of exercise scientists to understand human metabolism in health and disease. By the mid-1990s, exercise physiologists were using physical activity monitors to objectively determine the physical activity behaviors of adults and children (Matthews & Freedson, 1995; Melanson et al., 1996). What started as bulky monitors are now sleek and sophisticated research grade and consumer devices that can be worn on the body or integrated into our everyday devices, like watches and cell phones. This allows exercise physiologists to quantify type, duration, and intensity of activity that occurs outside of the lab in a free-living setting. This information about physical activity behaviors compliments and enhances the understanding of physiological responses to exercise by providing context about participants’ physical activity and sedentary behaviors (Wright et al., 2017).
In addition to providing extensive data about physical activity, there are wearable technologies that are designed to sample sweat, tears, saliva, and interstitial fluid to monitor a variety of metabolic analytes, including lactate, glucose, sodium, potassium, and hydrogen ions (Gao et al., 2018). These technologies can be applied to understanding the physiological response to exercise in wartime fighters, firefighters, athletes, and clinical populations. Perhaps the most utilized wearable technology for the monitoring of metabolites is the continuous glucose monitor. Continuous glucose monitors measure glucose in the interstitial fluid at regular intervals and transmit the information to a receiver for storage and analysis. An advantage of continuous glucose monitors is that researchers can use them to understand glucose metabolism in free-living situations during the late postexercise and nocturnal periods. Continuous glucose monitoring has also been used in athletes with and without diabetes to determine metabolic responses to exercise and carbohydrate requirements during racing (Ishihara et al., 2020; Ortega et al., 2020). In the future, we expect that continuous glucose monitoring will continue to be important in understanding the glucose response to exercise in people with and without diabetes. Future glucose sensors will be paired with sensors for other analytes to provide an even richer understanding of the metabolic effects of physical activity. This information will be useful for researchers, coaches, and medical professionals to aid in the optimization of performance and health for exercising people.
Genetic Advances
The fields of cellular and molecular biology have naturally integrated with exercise physiology in the investigation of the mechanisms of physiological phenomena. In the late 1980s, the Federation of American Societies for Experimental Biology held a symposium to introduce molecular biology to physiologists (Chien & Gargus, 1987); and in 1988, Booth published the first review highlighting the importance of molecular biology specifically to exercise physiologists (Booth, 1988). Since then, exercise physiologists have utilized genomic, epigenetic, transcriptomic, and metabolomic approaches as they have become available to examine the mechanisms of physiological responses to exercise, as well as the mechanisms for exercise-induced health benefits (Gomes et al., 2020; Neufer et al., 2015).
Genomics is the study of the genome, which encompasses all genes encoded by deoxyribonucleic acid. Exercise physiologists have utilized genomics tools to understand the genetic underpinnings of many facets of exercise, including the genetic basis of physical activity behavior, athletic performance, cardiorespiratory fitness, body composition, metabolism, and hemodynamic traits (Sarzynski et al., 2016). Genome-wide association studies are one of the most common genomic tools that exercise physiologists have utilized. Using Genome-wide association studies, genetic variants can be identified and associated with a specific outcome, such as a propensity to engage in leisure-time physical activity (Aasdahl et al., 2021) or the trainability of cardiorespiratory fitness (Williams et al., 2017). Genomic data provide key information about the expression of protein-encoding genes that transduce the physiological effects of exercise, but genomics does not provide a complete picture of the molecular mechanisms at play in the cell.
Following the boom of genomics research in the field of exercise physiology in the early 2000s and 2010s, epigenetic research has more recently exploded to gain a more detailed view of the molecular basis of exercise physiology. Epigenetics provide information about the transcriptional regulation of genes, including modifications that control and alter gene expression without changing the underlying deoxyribonucleic acid (McGee & Hargreaves, 2019). In this way, exercise physiologists can use epigenetics to understand not only a predisposition to a certain attribute, as with genetics, but also can assess an epigenetic response to a stimulus, such as exercise. In the field of exercise physiology, there is interest in the epigenetic regulation of skeletal muscle adaptations to exercise, metabolism, and inflammatory processes (Jacques et al. 2019), with much left to explore.
Transcriptomics and metabolomics are even newer fields than epigenomics, and they stand to further extend our knowledge of the molecular underpinnings of exercise physiology. Transcriptomics is the study of ribonucleic acid products in the cell. Transcriptomic signatures may be used by exercise physiologists to identify novel cell populations that are important for exercise adaptations in health and disease (Cho & Doles, 2017). On the other hand, metabolomics examines how the genome, epigenome, and transcriptome interact to produce the downstream metabolites that affect physiological responses to exercise. This branch of “omics” uses a systematic approach to quantify all metabolites in a sample, including lipids, carbohydrates, nucleotides, and small molecules. For example, exercise physiologists can utilize metabolomics to elucidate the time course of metabolic responses to exercise, which has public health implications for understanding the different health outcomes associated with lifelong exercise compared with acute training programs (Gomes et al., 2020; Kelly et al., 2020). Transcriptomics and metabolomics will likely remain hot topics in exercise physiology, especially as a means to identify cells and biomarkers that might characterize individual responses to training, nutrition, or timing of exercise.
Analytical Approaches
As technological advances have pushed the field of exercise physiology, analytical advances have also been important to generating and interpreting new knowledge. Increases in computing power have made modeling physiological phenomena a useful tool to exercise physiologists. Computational modeling provides exercise physiologists with an alternative to human and animal studies while also generating unique information that experimental research alone cannot produce. Importantly, models also serve to guide hypothesis-generation for future experimental research. There are endless applications for computational modeling in exercise physiology. For example, computational models can be used to understand the mechanisms of muscle fatigue in aging (Callahan et al., 2016), muscle energetics during locomotion (Umberger & Rubenson, 2011), and injury risk in various types of athletes (Hadid et al., 2018).
Statistical analyses also have advanced our ability to understand the totality of research in a given area of investigation. The sheer volume of exercise physiology publications has necessitated the need for rigorous, quantitative synthesis of research results to supplement narrative research reviews. Meta-analysis in exercise physiology started to gain momentum in the early 1990s and is widely utilized today. In one of the earliest meta-analyses in the field of exercise physiology, Lokey et al. (1991) evaluated the effect of physical activity on pregnancy outcomes. At the time, the American College of Obstetricians and Gynecologists recommended that pregnant women engage in at least 3 days per week of exercise at a heart rate of up to 140 beats per minute for <15 min. Narrative reviews had thus far been unable to resolve whether additional intensity, duration, and modes of exercise were also safe during pregnancy. The results of the meta-analysis indicated that longer exercise bouts as well as modes of exercise including jogging and weight bearing were not unsafe for pregnant women (Lokey et al., 1991). While exercise during pregnancy is currently widely accepted as safe, meta-analysis is still being used to understand how exercise affects pregnancy complications, including gestational diabetes (Ming et al., 2018). Meta-analyses have also shown that exercise reduces the rates of falls in older adults (Sherrington et al., 2019), may be useful for the treatment of depression (Stanton & Reaburn, 2014), and can improve physical functioning and fatigue symptoms in breast cancer patients (Juvet et al., 2017).
New Knowledge
A complete listing of the key new concepts in exercise physiology is beyond the scope of this review, but several exciting areas of research are highlighted in this section. Fortunately, the creation of the internet and associated search engines, databases, and electronicallyavailable primary research reports and materials allow us to explore these and other topics extensively.
In 1977, M. Joan Dawson and colleagues reported the first use of MRS for the study of muscle bioenergetics—the production and use of ATP to power muscular contractions—during contractions in excised whole frog and toad muscles (Dawson et al., 1977). Shortly after that, research groups from the University of Pennsylvania and Oxford University became the first to use noninvasive MRS techniques to study cellular bioenergetics in vivo in resting and exercising human muscle (Chance et al., 1980; Chance et al., 1981), thus laying the groundwork for the rich explorations into bioenergetics that have continued since that time. In the late 1980s, Ron Meyer and others (Arnold et al., 1984; Meyer, 1988) built on the work of Doris Taylor et al. (1983) to develop the phosphocreatine recovery protocol as a robust means of quantifying muscle oxidative capacity in vivo. This approach has been used to determine the effects of exercise training, aging, and disease on muscle mitochondrial function in a variety of muscle groups. The use of MRS to quantify ATP flux directly from the creatine kinase reaction, as well as through glycolysis and oxidative phosphorylation (Boska, 1991), have led to a number of discoveries related to the interactions between oxidative and nonoxidative energy production under various conditions (Lanza et al., 2006). The role of glycogen stores and perfusion have also been used to answer important questions about substrate-level metabolism and the impact of oxygen delivery on muscle function (Brillault-Salvat et al., 1997).
Also beginning in the 1980s, George Brooks and colleagues began a relentless pursuit of clarifying the details of lactate metabolism in resting (Donovan & Brooks, 1983) and exercising (Stanley et al., 1988) skeletal muscle, demonstrating in the process that lactate production is indeed driven not by anoxia in muscle cells, but rather in response to increases in energy demand that are met through higher glycolytic flux. This work led to Brooks’ development of the lactate shuttle hypothesis which posits that lactate is shuttled from sites of production in the cytosol of working muscle to other regions within the cell and other tissues (e.g., heart, brain, liver, skeletal muscle) that then use that lactate for energy and gluconeogenesis (Brooks, 1991). This concept has been important to interpreting the “anaerobic” or “lactate” threshold in that the shuttling and use of lactate as fuel in various tissues renders invalid the concept that the lactate threshold (rapid increase in the accumulation of blood lactate as the rate of production exceeds the clearance rate) occurs due to insufficient oxygen in exercising muscle (Poole et al., 2021). The on-going interest in this topic remains clear in the literature.
The 1990s saw an increased interest in understanding the mechanisms of muscle fatigue, defined as the fall of force in response to muscular contractions. Building on the work of Brenda Bigland-Ritchie and others in the 1980s (Bigland-Ritchie, 1981; Cooke & Pate, 1985; Edwards et al., 1977), investigators explored the neural (Enoka & Stuart, 1992) and metabolic (Miller et al., 1987) bases of muscle fatigue in vivo. Careful work at the cellular level provided insight about the intracellular sources of muscle fatigue, including the deleterious effects of changes in calcium kinetics, and accumulation of inorganic phosphate and hydrogen ions on contractile function on muscle force and velocity (Lännergren & Westerblad, 1991). Subsequently, the roles of inorganic phosphate and acidosis on contractile failure and fatigue were clearly demonstrated at the molecular (Debold et al., 2008), cellular (Fitts, 2008; Knuth et al., 2006) and in vivo, whole-muscle levels (Lanza et al., 2006).
Another new concept that emerged in the 1990s and was expanded upon in the 2000s was based on the demonstration of substantial positive adaptations to aerobic energy metabolism in response to “high-intensity interval training” (Perry et al., 2008; Sperlich et al., 2010). Until that time, it was generally considered that “aerobic” adaptations such as increased capillary and mitochondrial density, maximal rates of oxidative phosphorylation, and maximal oxygen consumption during whole-body exercise required months of long-duration (several approximately 60-min exercise bouts per week) “endurance” training. This approach was sometimes termed “long, slow distance” training. However, studies by several investigators showed that, in fact, substantial modifications to the structures that support oxidative metabolism, as well as peak values of oxygen consumption, can be made with 6–8 short (approximately 30 s), maximal bursts of exercise repeated following brief (approximately 4 min) periods of recovery. Remarkably, significant improvements in these key variables could be elicited in as little as 6 training sessions carried out over 2 weeks (Larsen et al., 2014). The results of these studies have sparked a revolution in training programs for people of all ages whose goals range from general fitness to high-level athletic performance.
During this same time, an appreciation by exercise physiologists developed for the important role of bone metabolism in health and the role of exercise in maintaining bone mass. At the time, it was well understood that bone remodeling followed the dictates of Wolffe’s Law, that is, that bone density and strength will adapt based on the loads imposed on it, in much the same way that muscles do. However, additional factors emerged as important to changes in bone health. The effects of hormonal status, energy balance and type of exercise training, in populations ranging from postmenopausal women to highly-trained male cyclists, were the focus of a series of studies by Wendy Kohrt and colleagues (e.g., Barry & Kohrt, 2007; Gozansky et al., 2005). Overall, physical inactivity, menopause and older age, and negative energy balance can all be detrimental to bone health. Nontraditional forms of exercise or loading interventions have also been examined. T’ai Chi is a form of exercise that may have beneficial effects on bone physiology (Zhou et al., 2021), although the most efficacious protocols have not yet been determined. Likewise, the use of whole-body vibration as a treatment for osteoporosis also seems promising (Verschueren et al., 2003). On-going research in the area of exercise and optimal bone health will be an important focus in exercise physiology in the years to come.
In the 2000s, the term “metabolic flexibility” was introduced to describe the interplay between fat and carbohydrate as substrates for energy metabolism in response to fasting or feeding (Goodpaster & Kelley, 2002; Hood et al., 2006). Exercise physiologists have used exercise as a probe for understanding the etiology and reversibility of metabolic inflexibility, which is a problem most evident in the context of obesity and insulin resistance. The associations between metabolic inflexibility—generally measured as insulin resistance or impaired fatty acid metabolism—and mitochondrial function have been established in a number of studies (Dubé et al., 2014; Stephenson & Hawley, 2014). Notably, Meex et al. (2010) showed that exercise training can reverse this problem in part through an improvement in mitochondrial capacity.
Finally, a remarkable new area of research opening up in the last 10 years is focused on understanding how the gut microbiome influences disease, and the mechanisms by which exercise might modulate these effects (O’Sullivan et al., 2015). In addition to innovative work in humans (cf. Cook et al., 2016), studies using animal models are addressing these questions, as well. For example, recent research paradigms have imposed changes in the microbiome that appear to reverse the impaired vascular function typically associated with aging (Brunt et al., 2019). A report by Welly et al. (2016) evaluated how exercise compared with negative energy balance in improving the gut microbiome and markers of metabolic health in obesity prone rats, and found exercise to have significant positive effects. Certainly, this will be an area of intense investigation in the future.
These are but a glimpse into some of the exciting research that has taken place in exercise physiology over the past 40 years. No doubt the breadth and depth of knowledge in this field will continue to grow as investigators around the world probe further into understanding the mechanisms and limitations of maximal human performance and optimal health. The next section highlights a few areas of scientific inquiry that are certain to provide continued opportunities for exploration by exercise physiologists.
Current and Future Opportunities
A survey of the contributions that researchers in exercise physiology have made in the past 40 years reveals the extent to which this field has been integrated with other STEM and health disciplines. This recognition of and respect for exercise physiology by researchers in fields well beyond its own are a testament to its impact on science and public health. Indeed, as various disciplines become increasingly interwoven, it will be important to ensure that exercise physiology retains recognition as a field of endeavor in its own right! A few of the areas that we can expect to have continued opportunities for important discoveries in the future are highlighted below.
Perhaps one of the best examples of the extent to which exercise physiology has been assimilated into the health sciences is the concept of “Exercise is Medicine,” a term that appears to have first been introduced into the scientific literature by Douglas Hoffman (1993). The basic concept is that physical activity and exercise promote beneficial physiological adaptations that support health and well-being and, as such, can be prescribed to mitigate inactivity-related disease. In this way, following appropriate assessment, the core principles of exercise physiology are used to design therapeutic treatments, in much the same way as pharmaceutical interventions are typically used. A seminal study that paved the way for this concept was that reported by Steven Blair et al. (1989), which identified the association between low fitness level and high mortality, particularly in men. A very active period of research related to exercise as an intervention for good health has followed (Kraus et al., 2019; Nicolucci et al., 2011). In 2007, the “Exercise is Medicine” concept was formalized into a program jointly sponsored by the American College of Sports Medicine and the American Medical Association. This program has grown to become a Global Health Initiative that endorses the approach of using evidence-based information to create personalized physical activity and exercise prescriptions to optimize individual and population health. Clearly, the importance of fitness and physical activity in the promotion of good health has been a major contribution of exercise physiology over the past 40 years.
Large-scale longitudinal studies designed to understand or mitigate societal problems such as aging-related declines in physical function (Ferrucci, 2008; Pahor et al., 2014) or the impact of lifestyle interventions like exercise on the management and progression of Type 2 diabetes (Johansen et al., 2017) are but two examples of the importance of understanding on a population scale what the interactions are between disease and exercise or physical inactivity. As the critical variables in these associations are identified, smaller-scale mechanistic studies can be designed to pursue a deeper understanding of the problem and develop evidence-based interventions to correct them. In addition, while these large studies are generally associative and therefore cannot assign causality, technological advances are allowing mechanistic measures to be added to these studies over time. In combination with new methods for managing and interpreting “big data,” opportunities for high-impact discoveries from these types of studies are expected to grow rapidly in the coming years.
Exercise physiologists are typically part of a large team of investigators in projects such those mentioned above, which might also include clinicians, epidemiologists, statisticians, and data management experts. In fact, a parallel development in recent years is the concept of applying “Team Science” approaches to research, which recognize that the potential contributions of these large, interdisciplinary projects to generating new scientific knowledge is far more than the sum of its parts (Cooke & Hilton, 2015). One principle of Team Science is that laboratories both large and small can combine to produce exciting new work, as can teamwork between laboratories focused on very different aspects or scales of a given problem (Bennett & Gadlin, 2012). It will be exciting to see where this powerful approach takes the field of exercise physiology in the future.
Another acknowledgment of the important role of exercise physiology in human health and performance is an initiative launched in 2016 by the NIH titled Molecular Transducers of Physical Activity in Humans (NIH, 2020). In many ways, this initiative was the culmination of work by Frank Booth and others who worked tirelessly in the early 2000’s to promote the need for research designed to understand and apply the molecular basis for how physical inactivity exacerbates disease in humans (Booth et al., 2000). The goal of this innovative and ambitious project, which is supported by NIH’s Common Fund and managed by a consortium of institutions and investigators, is to identify the precise mechanisms by which physical activity and exercise evoke their positive effects on human health (NIH, 2020; Neufer et al., 2015). This is a prospective, large-scale intervention study in humans in which participants complete a 12 week, supervised exercise training study (Sanford et al., 2020). Parallel studies are being performed in animal models to investigate mechanisms that are not feasible to obtain in humans. Approximately 2,600 males and females of all ages, races, and ethnic backgrounds are being studied. This is the largest and most expensive exercise training program to date that will examine basic mechanisms of physiological adaptations and their associated impact on physical function and health.
An exciting opportunity that will continue to grow in coming years is that of investigations related to the exercise physiology of microgravity and long-duration space flight. The goal of landing people on Mars as well as the expansion of space exploration into the private sector and by many countries means that greater numbers of people are being exposed to prolonged periods of time in zero gravity. Understanding the consequences of these missions on various human physiological systems—including those systems that support exercise—will be critical to ensuring the health of future astronauts. It will be exciting to follow this line of research as it builds upon the classic early studies in this area, which began in the mid-1960s. In addition to studies of the impact of space flight on humans (Grigoriev, 1983) and small animals (Mondon et al., 1983), bedrest has been used as a surrogate for the unloading associated with microgravity, thus enabling a wider range of studies than are possible only with spaceflight (Greenleaf, 1989; Katkovsky & Pomyotov, 1976). Collectively, profound effects on cardiovascular, respiratory, neuromuscular, and bone physiology have been observed in response to zero or micro-gravity (Edgerton & Roy, 1994). Exercise countermeasures to the deleterious effects of space were introduced in the 1970s (Klein et al., 1977). The on-going challenge will be to develop countermeasures that are feasible and effective in preventing the negative effects of prolonged missions in space. As with other aspects of technological advances driven by space exploration, we can expect the new knowledge generated about exercise physiology in zero- and micro-gravity to have carry-over effects into the health and performance capabilities of the general population.
Additional on-going and emerging opportunities for scientific investigation involving exercise physiology include understanding the multiple roles (e.g., energy production, signaling) of mitochondria in supporting health and performance (Glancy et al., 2015), understanding how aging affects the neuromuscular system (Hepple & Rice, 2016), determining the mechanisms by which exercise supports brain health and function (Tyndall et al., 2018), the interplay between engineered solutions to limb loss and enhancing mobility performance and resistance to fatigue (Grimmer et al., 2019), and the limits of human performance in extreme conditions (Burnley & Jones, 2018; Sullivan-Kwantes et al., 2020). Fundamental questions related to how the sex hormones influence and are influenced by exercise performance will be important to address in order to optimize performance and health throughout the lifespan in all humans.
Conclusion
Over the past 40 years, concepts and approaches in exercise physiology have become woven into the larger fabric of scientific investigation into human health and performance, from molecules to populations. The investigations by exercise physiologists drive the development of new tools and techniques to answer increasingly novel and impactful research questions. Exercise physiologists also embrace the newly developed methodologies from other biomedical disciplines. Technological advances will continue to be key for exercise physiologists in their pursuit to uncover a deeper understanding of the physiological basis of human health, performance, and disease.
The evolution of the field has moved it toward a position of centrality in the natural sciences. Notably, however, this centrality means there is also risk of a loss of identity as these topics are absorbed into other disciplines. This risk can be mitigated by the continued rigorous training of exercise physiologists in our colleges and universities. A solid foundation in biochemistry, physics, molecular biology, and computer sciences will be increasingly important for future exercise physiologists who want to contribute to generating new knowledge about how humans and other animals respond to acute and chronic exercise. Likewise, training students to be critical components of scientific teams will allow them to flourish in the realm of integrative research and thought. There is clearly a need for research and application of holistic, physiological studies that can place “basic” studies in the context of the entire organism and how it moves in its environment. The future is bright, and the opportunities are immense for exercise physiologists in the next 40 years.
Acknowledgment
Financial support was provided by NIH R01 AG058607 (K.L. Hayes).
References
- Aasdahl L, Nilsen TIL, Meisingset I, Nordstoga AL, Evensen KAI, Paulsen J, … Skarpsno ES (2021). Genetic variants related to physical activity or sedentary behaviour: A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 18(1). 10.1186/s12966-020-01077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DL, Matthews PM, & Radda GK (1984). Metabolic recovery after exercise and the assessment of mitochondrial function in Vivo in human skeletal muscle by means of 31P NMR. Magnetic Resonance in Medicine, 1(3), 307–315. 10.1002/mrm.1910010303 [DOI] [PubMed] [Google Scholar]
- Barry DW, & Kohrt WM (2007). BMD decreases over the course of a year in competitive male cyclists. Journal of Bone and Mineral Research, 23(4), 484–491. 10.1359/jbmr.071203 [DOI] [PubMed] [Google Scholar]
- Bennett LM, & Gadlin H (2012). Collaboration and team science. Journal of Investigative Medicine, 60(5), 768–775. 10.2310/jim.0b013e318250871d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B (1981). EMG/force relations and fatigue of human voluntary contractions. Exercise and Sport Sciences Reviews, 9(1), 75–117. 10.1249/00003677-198101000-00002. [DOI] [PubMed] [Google Scholar]
- Blair SN, Hohl HWI, Paffenbarger RS Jr., Clark DG, Cooper KH, & Gibbons LW (1989). Physical fitness and all-cause mortality. A prospective study of healthy men and women. The Journal of the American Medical Association, 262(17), 2395–2401. 10.1001/jama.1989.03430170057028 [DOI] [PubMed] [Google Scholar]
- Booth FW (1988). Perspectives on molecular and cellular exercise physiology. Journal of Applied Physiology, 65(4), 1461–1471. 10.1152/jappl.1988.65.4.1461 [DOI] [PubMed] [Google Scholar]
- Booth FW, Gordon SE, Carlson CJ, & Hamilton MT (2000). Waging war on modern chronic diseases: primary prevention through exercise biology. Journal of Applied Physiology, 88(2), 774–787. 10.1152/jappl.2000.88.2.774 [DOI] [PubMed] [Google Scholar]
- Boska M (1991). Estimating the ATP cost of force production in the human gastrocnemius/soleus muscle group using 31P MRS and 1HMRI. NMR in Biomedicine, 4(4), 173–181. 10.1002/nbm.1940040404 [DOI] [PubMed] [Google Scholar]
- Brillault-Salvat C, Giacomini E, Jouvensal L, Bloch C, Wary G, & Carlier PG (1997). Simultaneous determination of muscle perfusion and oxygenation by interleaved NMR plethysmography and deoxymyoglobin spectroscopy. NMR in Biomedicine, 10(7), 315–323. [DOI] [PubMed] [Google Scholar]
- Brooks GA (1991). Current concepts in lactate exchange. Medicine & Science in Sports & Exercise, 23(8), 895–906. [PubMed] [Google Scholar]
- Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM,Gonzalez A, … Seals DR (2019). Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. The Journal of Physiology, 597(9), 2361–2378. 10.1113/JP277336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnley M, & Jones AM (2018). Power–duration relationship: Physiology, fatigue, and the limits of human performance. European Journal of Sport Science, 18(1), 1–12. 10.1080/17461391.2016.1249524 [DOI] [PubMed] [Google Scholar]
- Callahan DM, Umberger BR, & Kent JA (2016). Mechanisms of in vivo muscle fatigue in humans: investigating age-related fatigue resistance with a computational model. The Journal of Physiology, 594(12), 3407–3421. 10.1113/JP271400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Eleff S, & Leigh JS (1980). Noninvasive, nondestructive approaches to cell bioenergetics. Proceedings of the National Academy of Sciences, 77(12), 7430–7434. 10.1073/pnas.77.12.7430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Eleff S, Leigh JS, Sokolow D, & Sapega A (1981). Mitochondrial regulation of phosphocreatine/inorganic phosphate ratios in exercising human muscle: A gated 31P NMR study. Proceedings of the National Academy of Sciences, 78(11), 6714–6718. 10.1073/pnas.78.11.6714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Nioka S, Kent J, McCully K, Fountain M, Greenfeld R, & Holtom G (1988). Time-resolved spectroscopy of hemoglobin and myoglobin in resting and ischemic muscle. Analytical Biochemistry, 174(2), 698–707. 10.1016/0003-2697(88)90076-0 [DOI] [PubMed] [Google Scholar]
- Chien S, & Gargus JJ (1987). Molecular biology in physiology. The FASEB Journal, 1(2), 97–102. 10.1096/fasebj.1.2.2886391 [DOI] [PubMed] [Google Scholar]
- Cho DS, & Doles JD (2017). Single cell transcriptome analysis of muscle satellite cells reveals widespread transcriptional heterogeneity. Gene, 636, 54–63. 10.1016/j.gene.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MD, Allen JM, Pence BD, Wallig MA, Gaskins HR, White BA, & Woods JA (2016). Exercise and gut immune function: Evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunology & Cell Biology, 94(2), 158–163. 10.1038/icb.2015.108 [DOI] [PubMed] [Google Scholar]
- Cooke NJ, & Hilton ML (Eds.). (2015). Enhancing the effectiveness of team science. National Academies Press. 10.17226/19007 [DOI] [PubMed] [Google Scholar]
- Cooke R, & Pate E (1985). The effects of ADP and phosphate on the contraction of muscle fibers. Biophysical Journal, 48(5), 789–798. 10.1016/S0006-3495(85)83837-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, & Wilkie DR (1977). Contraction and recovery of living muscles studied by 31P nuclear magnetic resonance. The Journal of Physiology, 267(3), 703–735. 10.1113/jphysiol.1977.sp011835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debold EP, Beck SE, & Warshaw DM (2008). Effect of low pH on single skeletal muscle myosin mechanics and kinetics. American Journal of Physiology-Cell Physiology, 295(1), C173–C179. 10.1152/ajpcell.00172.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan CM, & Brooks GA (1983). Endurance training affects lactate clearance, not lactate production. American Journal of Physiology-Endocrinology and Metabolism, 244(1), E83–E92. 10.1152/ajpendo.1983.244.1.E83 [DOI] [PubMed] [Google Scholar]
- Dubé JJ, Coen PM, DiStefano G, Chacon AC, Helbling NL, Desimone ME, Stafanovic-Racic M, … Goodpaster BH (2014). Effects of acute lipid overload on skeletal muscle insulin resistance, metabolic flexibility, and mitochondrial performance. American Journal of Physiology-Endocrinology and Metabolism, 307(12), E1117–E1124. 10.1152/ajpendo.00257.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, & Roy RR (1994). Neuromuscular adaptation to actual and simulated weightlessness. Advances in Space Biology and Medicine, 4, 33–67 10.1016/s1569-2574(08)60134-3 [DOI] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA, & Merton PA (1977). Fatigue of long duration in human skeletal muscle after exercise. The Journal of Physiology, 272(3), 769–778. 10.1113/jphysiol.1977.sp012072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, & Stuart DG (1992). Neurobiology of muscle fatigue. Journal of Applied Physiology, 72(5), 1631–1648. 10.1152/jappl.1992.72.5.1631 [DOI] [PubMed] [Google Scholar]
- Faulkner JA, & White TP (1981). Current and future topics in exercise physiology. In Brooks GA (Ed.), Perspectives on the academic discipline of physical education: A tribute to G. Lawrence Rarick (pp. 74–96). Human Kinetics Publishers. [Google Scholar]
- Ferrucci L (2008). The Baltimore Longitudinal Study of Aging (BLSA): A 50-year-long journey and plans for the future. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63(12), 1416–1419. 10.1093/gerona/63.12.1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts RH (2008). The cross-bridge cycle and skeletal muscle fatigue. Journal of Applied Physiology, 104(2), 551–558. 10.1152/japplphysiol.01200.2007 [DOI] [PubMed] [Google Scholar]
- Fitzgerald LF, Christie AD, & Kent JA (2016). Heterogeneous effects of old age on human muscle oxidative capacity in vivo: a systematic review and meta-analysis. Applied Physiology, Nutrition, and Metabolism, 41(11), 1137–1145. 10.1139/apnm-2016-0195 [DOI] [PubMed] [Google Scholar]
- Gao W, Brooks GA, & Klonoff DC (2018). Wearable physiological systems and technologies for metabolic monitoring. Journal of Applied Physiology, 124(3), 548–556. 10.1152/japplphysiol.00407.2017 [DOI] [PubMed] [Google Scholar]
- Geeves MA, Fedorov R, & Manstein DJ (2005). Molecular mechanism of actomyosin-based motility. Cellular and Molecular Life Sciences, 62(13), 1462–1477. 10.1007/s00018-005-5015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glancy B, Hartnell LM, Malide D, Yu Z-X, Combs CA, Connelly PS, Subramaniam S, & Balaban RS (2015). Mitochondrial reticulum for cellular energy distribution in muscle. Nature, 523(7562), 617–620. 10.1038/nature14614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes C, Almeida JA, Franco OL, & Petriz B (2020). Omics and the molecular exercise physiology. Advanced Clinical Chemistry, 96, 55–84. 10.1016/bs.acc.2019.11.003 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, & Kelley DE (2002). Skeletal muscle triglyceride:Marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Current Diabetes Reports, 2(3), 216–222. 10.1007/s11892-002-0086-2 [DOI] [PubMed] [Google Scholar]
- Gozansky WS, van Pelt RE, Jankowski CM, Schwartz RS, & Kohrt WM (2005). Protection of bone mass by estrogens and raloxifene during exercise-induced weight loss. The Journal of Clinical Endocrinology & Metabolism, 90(1), 52–59. https://doi/.org/10.1210/jc.2004-0275 [DOI] [PubMed] [Google Scholar]
- Greenleaf JE (1989). Energy and thermal regulation during bed rest and spaceflight. Journal of Applied Physiology, 67(2), 507–516. 10.1152/jappl.1989.67.2.507 [DOI] [PubMed] [Google Scholar]
- Grigoriev AI (1983). Correction of changes in fluid-electrolyte metabolism in manned space flights. Aviation, Space, and Environmental Medicine, 54(4), 318–323. [PubMed] [Google Scholar]
- Grimmer M, Riener R, Walsh CJ, & Seyfarth A (2019). Mobility related physical and functional losses due to aging and disease—A motivation for lower limb exoskeletons. Journal of NeuroEngineering and Rehabilitation, 16(1), Article 2. 10.1186/s12984-018-0458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadid A, Epstein Y, Shabshin N, & Gefen A (2018). Biomechanical model for stress fracture–related factors in athletes and soldiers. Medicine & Science in Sports & Exercise, 50(9), 1827–1836. 10.1249/MSS.0000000000001628 [DOI] [PubMed] [Google Scholar]
- Hamaoka T, McCully KK, Niwayama M, & Chance B (2011). The use of muscle near-infrared spectroscopy in sport, health and medical sciences: recent developments. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 369(1955), 4591–4604. 10.1098/rsta.2011.0298 [DOI] [PubMed] [Google Scholar]
- Hepple RT, & Rice CL (2016). Innervation and neuromuscular control in ageing skeletal muscle. The Journal of Physiology, 594(8), 1965–1978. 10.1113/JP270561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DF (1993). Arthritis and exercise. Primary Care, 20(4), 895–910. [PubMed] [Google Scholar]
- Hood DA, Irrcher I, Ljubicic V, & Joseph A-M (2006). Coordination of metabolic plasticity in skeletal muscle. Journal of Experimental Biology, 209(12), 2265–2275. 10.1242/jeb.02182 [DOI] [PubMed] [Google Scholar]
- Huxley HE (1969). The mechanism of muscular contraction. Science 164(3886), 1356–1366. 10.1126/science.164.3886.1356 [DOI] [PubMed] [Google Scholar]
- Hyldahl RD, Schwartz LM, & Clarkson PM (2013). NF-KB activity functions in primary pericytes in a cell- and non-cell-autonomous manner to affect myotube formation. Muscle & Nerve, 47(4), 522–531. 10.1002/mus.23640 [DOI] [PubMed] [Google Scholar]
- Ishihara K, Uchiyama N, Kizaki S, Mori E, Nonaka T, & Oneda H (2020). Application of continuous glucose monitoring for assessment of individual carbohydrate requirement during ultramarathon race. Nutrients, 12(4), 1121. 10.3390/nu12041121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques M, Hiam D, Craig J, Barrès R, Eynon N, & Voisin S (2019). Epigenetic changes in healthy human skeletal muscle following exercise—A systematic review. Epigenetics, 14(7), 633–648. 10.1080/15592294.2019.1614416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen MY, MacDonald CS, Hansen KB, Karstoft K, Christensen R, Pedersen M, … Ried-Larsen M (2017). Effect of an intensive lifestyle intervention on glycemic control in patients with type 2 diabetes. JAMA, 318(7), 637–646. 10.1001/jama.2017.10169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvet LK, Thune I, Elvsaas IKØ, Fors EA, Lundgren S, Bertheussen G, … Oldervoll LM (2017). The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. The Breast, 33, 166–177. 10.1016/j.breast.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Katkovsky BS, & Pomyotov YD (1976). Cardiac output during physical exercises following real and simulated space flight. Life Science and Space Research, 14, 301–305. [PubMed] [Google Scholar]
- Kelly RS, Kelly MP, & Kelly P (2020). Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease, 1866(12), Article 165936. 10.1016/j.bbadis.2020.165936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp GJ, Taylor DJ, Styles P, & Radda GK (1993). The production, buffering and efflux of protons in human skeletal muscle during exercise and recovery. NMR in Biomedicine, 6(1), 73–83. 10.1002/nbm.1940060112 [DOI] [PubMed] [Google Scholar]
- Klein KE, Wegmann HM, Collier SR, & Kuklinksi P (1977). Athletic endurance training—advantage for space flight? The significance of physical fitness for selection and training of Spacelab crews. Aviation, Space, and Environmental Medicine, 48(3), 215–222. [PubMed] [Google Scholar]
- Knuth ST, Dave H, Peters JR, & Fitts RH (2006). Low cell pH depresses peak power in rat skeletal muscle fibres at both 30°C and 15°C: Implications for muscle fatigue. The Journal of Physiology, 575(3), 887–899. 10.1113/jphysiol.2006.106732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WE, Powell KE, Haskell WL, Janz KF, Campbell WW, Jakicic JM, … Piercy KL (2019). Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Medicine & Science in Sports & Exercise, 51(6), 1270–1281. 10.1249/mss.0000000000001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumpolec P, Klepochová R, Just I, Tušek Jelenc M, Frollo I, Ukropec J, … Valkovič L (2020). Multinuclear MRS at 7T uncovers exercise driven differences in skeletal muscle energy metabolism between young and seniors. Frontiers in Physiology, 11, Article 644. 10.3389/fphys.2020.00644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick MJ (1986). Spectroscopic applications of magnetic resonance to biomedical problems. Cardiovascular and Interventional Radiology, 8(5–6), 382–389. 10.1007/bf02552375 [DOI] [PubMed] [Google Scholar]
- Lännergren J, & Westerblad H (1991). Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. The Journal of Physiology, 434(1), 307–322. 10.1113/jphysiol.1991.sp018471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Befroy DE, & Kent-Braun JA (2006). In vivo ATP production during free-flow and ischaemic muscle contractions in humans. The Journal of Physiology, 577(1), 353–367. 10.1113/jphysiol.2006.114249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RG, Maynard L, & Kent JA (2014). High-intensity interval training alters ATP pathway flux during maximal muscle contractions in humans. Acta Physiologica, 211(1), 147–160. 10.1111/apha.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokey EA, Tran ZV, Wells CL, Myers BC, & Tran AC (1991). Effects of physical exercise on pregnancy outcomes: A meta-analytic review. Medicine & Science in Sports & Exercise, 23(11), 1234–1239. 10.1249/00005768-199111000-00006 [DOI] [PubMed] [Google Scholar]
- Matthews CE, & Freedson PS (1995). Field trial of a three-dimensional activity monitor: comparison with self report. Medicine & Science in Sports & Exercise, 27(7), 1071–1078. 10.1249/00005768-199507000-00017 [DOI] [PubMed] [Google Scholar]
- McGee SL, & Hargreaves M (2019). Epigenetics and Exercise. Trends in Endocrinology & Metabolism, 30(9), 636–645. 10.1016/j.tem.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Meex RCR, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, … Hesselink MKC (2010). Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes, 59(3), 572–579. 10.2337/db09-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson EL, Freedson PS, & Blair S (1996). Physical activity assessment: A review of methods. Critical Reviews in Food Science and Nutrition, 36(5), 385–396. 10.1080/10408399609527732 [DOI] [PubMed] [Google Scholar]
- Meyer RA (1988). A linear model of muscle respiration explains monoexponential phosphocreatine changes. American Journal of Physiology-Cell Physiology, 254(4), C548–C553. 10.1152/ajpcell.1988.254.4.C548 [DOI] [PubMed] [Google Scholar]
- Meyerspeer M, Boesch C, Cameron D, Dezortová M, Forbes SC, Heerschap A, … Willis D (2021). 31P magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR in Biomedicine, 34(5), Article 4246. 10.1002/nbm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RG, Giannini D, Milner-Brown HS, Layzer RB, Koretsky AP, Hooper D, & Weiner MW (1987). Effects of fatiguing exercise on high-energy phosphates, force, and EMG: Evidence for three phases of recovery. Muscle & Nerve, 10(9), 810–821. 10.1002/mus.880100906 [DOI] [PubMed] [Google Scholar]
- Ming W-K, Ding W, Zhang CJP, Zhong L, Long Y, Li Z, … Wang Z (2018). The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis. BMC Pregnancy and Childbirth, 18(1), Article 440. 10.1186/s12884-018-2068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondon CE, Dolkas CB, & Reaven GM (1983). Effect of confinement in small space flight size cages on insulin sensitivity of exercise-trained rats. Aviation, Space, and Environmental Medicine, 54(10), 919–922. [PubMed] [Google Scholar]
- National Institutes of Health. (2020). Molecular transducers of physical activity in humans. https://commonfund.nih.gov/moleculartransducers
- Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, … Laughlin MR (2015). Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metabolism, 22(1), 4–11. 10.1016/j.cmet.2015.05.011 [DOI] [PubMed] [Google Scholar]
- Nicolucci A, Balducci S, Cardelli P, Zanuso S, & Pugliese G (2011). Improvement of quality of life with supervised exercise training in subjects with type 2 diabetes mellitus. JAMA Internal Medicine, 171(21), 1951–1953. 10.1001/archinternmed.2011.561 [DOI] [PubMed] [Google Scholar]
- Ortega JF, Morales-Palomo F, Ramirez-Jimenez M, Moreno-Cabañas A, & Mora-Rodríguez R (2020). Exercise improves metformin 72-h glucose control by reducing the frequency of hyperglycemic peaks. Acta Diabetologica, 57(6), 715–723. 10.1007/s00592-020-01488-7 [DOI] [PubMed] [Google Scholar]
- O’Sullivan O, Cronin O, Clarke SF, Murphy EF, Molloy MG, Shanahan F, & Cotter PD (2015). Exercise and the microbiota. Gut Microbes, 6(2), 131–136. 10.1080/19490976.2015.1011875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, … Williamson JD (2014). Effect of structured physical activity on prevention of major mobility disability in older adults. JAMA, 311(23), 2387–2396. 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CGR, Heigenhauser GJF, Bonen A, & Spriet LL (2008). High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Applied Physiology, Nutrition, and Metabolism, 33(6), 1112–1123. 10.1139/h08-097 [DOI] [PubMed] [Google Scholar]
- Poole DC, Rossiter HB, Brooks GA, & Gladden LB (2021). The anaerobic threshold: 50+ years of controversy. The Journal of Physiology, 599(3), 737–767. 10.1113/jp279963 [DOI] [PubMed] [Google Scholar]
- Porter KR, & Palade GE (1957). Studies on the endoplasmic reticulum. The Journal of Biophysical and Biochemical Cytology, 3(2), 269–300. 10.1083/jcb.3.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudroff T, Kindred JH, & Kalliokoski KK (2015). [18F]-FDG positron emission tomography—An established clinical tool opening a new window into exercise physiology. Journal of Applied Physiology, 118(10), 1181–1190. 10.1152/japplphysiol.01070.2014 [DOI] [PubMed] [Google Scholar]
- Ryan TE, Brizendine JT, & McCully KK (2013). A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near-infrared spectroscopy. Journal of Applied Physiology, 114(2), 230–237. 10.1152/japplphysiol.01043.2012 [DOI] [PubMed] [Google Scholar]
- Sanford JA, Nogiec CD, Lindholm ME, Adkins JN, Amar D, Dasari S, … Rivas MA (2020). Molecular Transducers of Physical Activity Consortium (MoTrPAC): Mapping the dynamic responses to exercise. Cell, 181(7), 1464–1474. 10.1016/j.cell.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzynski MA, Loos RJF, Lucia A, Perusse L, Roth SM, Wolfarth B, … Bouchard C (2016). Advances in exercise, fitness, and performance genomics in 2015. Medicine & Science in Sports & Exercise, 48(10), 1906–1916. 10.1249/mss.0000000000000982 [DOI] [PubMed] [Google Scholar]
- Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, … Lamb SE (2019). Exercise for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews. 1, CD012424. 10.1002/14651858.cd012424.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga T, Tanabe K, Nakase Y, Shida T, & Chance B (1995). Development of a portable tissue oximeter using near infra-red spectroscopy. Medical & Biological Engineering & Computing, 33(4), 622–626. 10.1007/bf02522525 [DOI] [PubMed] [Google Scholar]
- Sperlich B, Zinner C, Heilemann I, Kjendlie P-L, Holmberg H-C, & Mester J (2010). High-intensity interval training improves VO2peak, maximal lactate accumulation, time trial and competition performance in 9–11-year-old swimmers. European Journal of Applied Physiology, 110(5), 1029–1036. 10.1007/s00421-010-1586-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JA (2001). The myosin swinging cross-bridge model. Nature Reviews Molecular Cell Biology, 2(5), 387–392. 10.1038/35073086 [DOI] [PubMed] [Google Scholar]
- Stanley WC, Wisneski JA, Gertz EW, Neese RA, & Brooks GA (1988). Glucose and lactate interrelations during moderate-intensity exercise in humans. Metabolism, 37(9), 850–858. 10.1016/0026-0495(88)90119-9 [DOI] [PubMed] [Google Scholar]
- Stanton R, & Reaburn P (2014). Exercise and the treatment of depression: A review of the exercise program variables. Journal of Science and Medicine in Sport, 17(2), 177–182. 10.1016/j.jsams.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Stephenson EJ, & Hawley JA (2014). Mitochondrial function in metabolic health: A genetic and environmental tug of war. Biochimica et Biophysica Acta (BBA)—General Subjects, 1840(4), 1285–1294. 10.1016/j.bbagen.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Sullivan-Kwantes W, Haman F, Kingma BRM, Martini S, Gautier-Wong E, Chen KY, & Friedl KE (2020). Human performance research for military operations in extreme cold environments. Journal of Science and Medicine in Sport. Advance online publication. 10.1016/j.jsams.2020.11.010 [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Bore PJ, Styles P, Gadian DG, & Radda GK (1983). Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Molecular Biology and Medicine, 1(1), 77–94. [PubMed] [Google Scholar]
- Tidball JG (2011). Mechanisms of muscle injury, repair, and regeneration. Comprehensive Physiology, 1(4), 2029–2062. 10.1002/cphy.c100092 [DOI] [PubMed] [Google Scholar]
- Tyndall AV, Clark CM, Anderson TJ, Hogan DB, Hill MD, Longman RS, & Poulin MJ (2018). Protective effects of exercise on cognition and brain health in older adults. Exercise and Sport Sciences Reviews, 46(4), 215–223. 10.1249/jes.0000000000000161 [DOI] [PubMed] [Google Scholar]
- Umberger BR, & Rubenson J (2011). Understanding muscle energetics in locomotion. Exercise and Sport Sciences Reviews, 39(2), 59–67. 10.1097/JES.0b013e31820d7bc5 [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, & Boonen S (2003). Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. Journal of Bone and Mineral Research, 19(3), 352–359. 10.1359/JBMR.0301245 [DOI] [PubMed] [Google Scholar]
- Welly RJ, Liu T-W, Zido TM, Rowles JL, Park Y-M, Smith TN, Swanson KS, Padilla J, & Viera-Potter VJ (2016). Comparison of diet versus exercise on metabolic function and gut microbiota in obese rats. Medicine & Science in Sports & Exercise, 48(9), 1688–1698. 10.1249/mss.0000000000000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CJ, Williams MG, Eynon N, Ashton KJ, Little JP, Wisloff U, & Coombes JS (2017). Genes to predict VO2max trainability: a systematic review. BMC Genomics, 18(S8), Article 831. 10.1186/s12864-017-4192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SP, Hall Brown TS, Collier SR, & Sandberg K (2017). How consumer physical activity monitors could transform human physiology research. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 312(3), R358–R367. 10.1152/ajpregu.00349.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhao Z-H, Fan X-H, Li W-H, & Chen Z (2021). Different training durations and frequencies of Tai Chi for bone mineral density improvement: A systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine, 2021, Article 6665642. 10.1155/2021/6665642 [DOI] [PMC free article] [PubMed] [Google Scholar]


