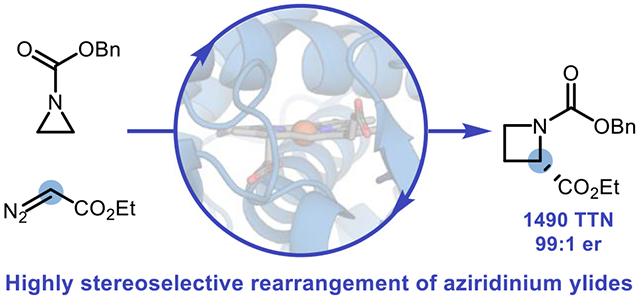
Abstract
We report enantioselective one-carbon ring expansion of aziridines to make azetidines as a new-to-nature activity of engineered ‘carbene transferase’ enzymes. A laboratory-evolved variant of cytochrome P450BM3, P411-AzetS, not only exerts unparalleled stereocontrol (99:1 er) over a [1,2]-Stevens rearrangement, but also overrides the inherent reactivity of aziridinium ylides, cheletropic extrusion of olefins, to perform a [1,2]-Stevens rearrangement. By controlling the fate of the highly reactive aziridinium ylide intermediates, these evolvable biocatalysts promote a transformation which cannot currently be performed using other catalyst classes.
Graphical Abstract
Ring-size manipulation has emerged as a powerful strategy to convert readily available cyclic structures into ring-expanded or ring-contracted compounds that are more difficult to synthesize using conventional means.1 In particular, “cut and sew” strategies relying on transition-metal catalyzed oxidative addition across C–C bonds are useful approaches for insertion of carbon monoxide or two-carbon fragments such as olefins and alkynes to effect one- or two-carbon ring expansions, respectively.2 For nitrogen-containing heterocycles, one possible strategy for ring expansion is to induce a [1,2]-Stevens rearrangement by formation of an ammonium ylide, resulting in one-carbon ring expansion.3 Pioneering works by Hata, West, and Couty demonstrated this approach for 4- to 5-membered ring expansions, wherein treatment of an azetidine with a diazo compound in the presence of a copper catalyst provided facile access to the corresponding pyrrolidine.4 Conceptually, carbene transfer followed by an intramolecular [1,2]-Stevens rearrangement complements “cut and sew” reactions for non-carbonylative, one-carbon homologation of nitrogen-containing compounds. Given the prevalence of nitrogen heterocycles across numerous sectors of the chemical industry, especially pharmaceuticals,5 extending these methodologies to other saturated N-heterocycles would represent a new approach for the synthesis of important chiral amine building blocks.
Despite their promising properties,6 azetidines are underrepresented relative to closely related nitrogen-containing heterocycles: this is due to a lack of robust synthetic methods to access these species,7–8 especially using asymmetric catalysis.9–10 Application of a ring-expansion strategy for the asymmetric, one-carbon homologation of readily prepared aziridines via carbene insertion would be an attractive new entry toward the enantioselective synthesis of azetidines (Figure 1). However, this approach comes with two major selectivity challenges. The first is the innate reactivity of the intermediate aziridinium ylides, which undergo highly favorable cheletropic extrusion of olefins in many contexts.11 Schomaker and others have demonstrated that these reactive intermediates can be harnessed in [2,3]-Stevens rearrangements and other ring-opening reactions.12 However, we are unaware of any examples of a one-carbon ring expansion of aziridines through a [1,2]-Stevens rearrangement strategy. Secondly, the diradical mechanism of the [1,2]-Stevens rearrangement13 has made it a challenging reaction class for asymmetric catalysis: few asymmetric variations have been reported.14 Enantiopure quaternary ammonium salts can undergo [1,2]-Stevens rearrangements with N-to-C chirality transfer;15 however, escape of the radical pair from the solvent cage is often competitive with radical recombination,16 and erosion of enantiopurity is often observed. General strategies for stereocontrol over these rearrangements are an unmet challenge facing the field of asymmetric catalysis.
Figure 1:
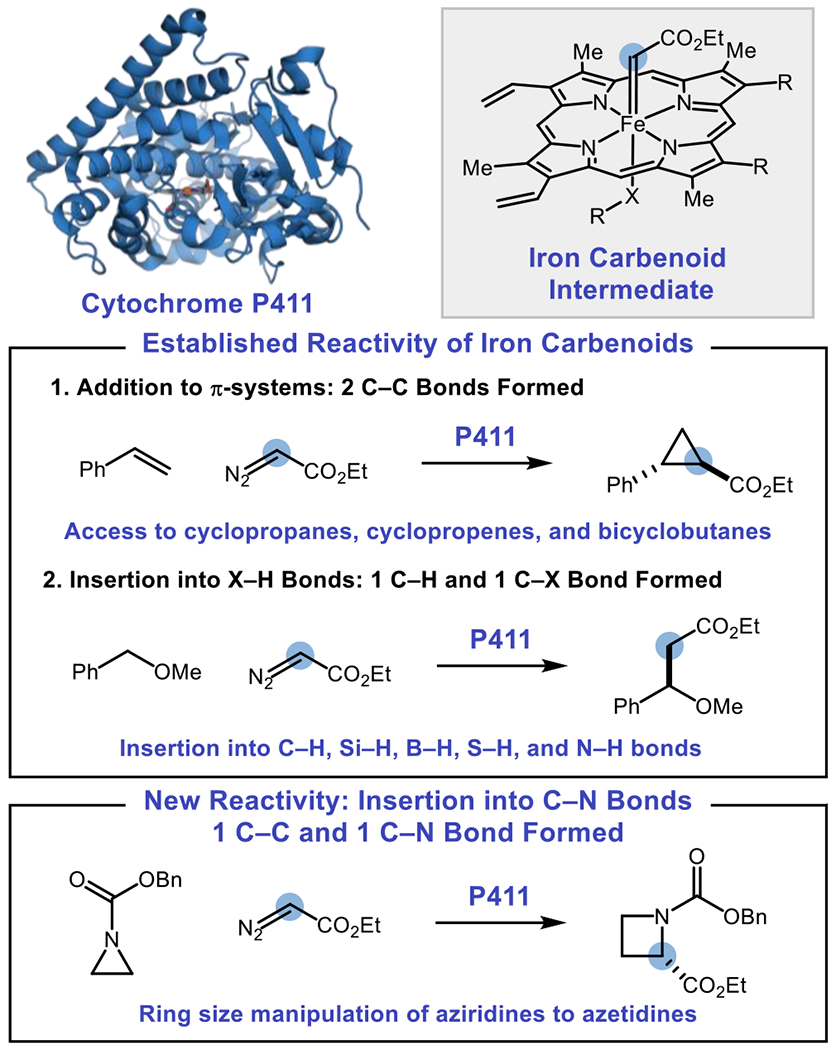
Classification of enzyme-mediated carbene transfer reactions for various bond disconnections.
The joint selectivity challenges presented by the asymmetric one-carbon ring expansion of aziridines into azetidines requires a potential catalyst not only to select for the [1,2]-Stevens rearrangement in preference to cheletropic extrusion of olefins, but also to exert enantiocontrol over potential radical intermediates. Nature utilizes ring-size manipulation in the biosynthesis of natural products, with common strategies for biocatalytic ring expansion including oxidative ring expansions17 and carbocation rearrangements.18 Furthermore, enzymes derived from cytochrome P450BM3, such as cytochromes P411, and other hemoproteins have emerged as powerful catalysts for carbene transfer reactions,19 and formation of strained rings such as cyclopropanes and cyclopropenes with excellent stereoselectivities has been reported.20 The most common reactions of enzymatic iron carbenoid intermediates are additions across π-systems19–20 or X–H bond insertions:21–22 biocatalytic C–N bond insertion through Stevens rearrangements of any kind have yet to be reported. We envisioned that a carbene transfer enzyme could potentially achieve the requisite chemo- and stereoselection necessary to perform this challenging reaction (Figure 1).
We initiated our studies by screening a panel of hemoproteins for the model reaction of benzyl aziridine-1-carboxylate 1 with ethyl diazoacetate (EDA) as a carbene precursor to provide enantioenriched azetidine 2 (Table 1) in suspensions of Escherichia coli (E. coli) whole cells. We were delighted to find that a variant of P411BM3-CIS23 with the additional mutations P248T, I263G, and L437F (“Parent F2”), provided the product with 3.6% yield, 73 total turnover numbers (TTNs), and 90:10 er favoring the (S)-enantiomer (Entry 1). Parent F2 is derived from hemoproteins originally engineered for the cyclopropanation of heteroatom-substituted olefins24 and is 17 mutations away from its wild-type progenitor, cytochrome P450BM3 from Bacillus megaterium, which natively catalyzes the oxidation of long-chain fatty acids.25 Control experiments revealed that hemin is unable to catalyze this reaction (see SI for details). Further control reactions indicated that the observed formation of the ring-opened hydrolysis product of 1 is not an enzyme-dependent process. No other aziridine-derived byproducts (e.g., cheletropic extrusion products11, carbene insertion into the benzylic C–H21c, or α-amino C–H bonds of the substrate21e) were identified, including a second ring expansion to form the corresponding pyrrolidine.4 Further experiments demonstrated that neither 2 nor the unsubstituted benzyl azetidine-1-carboxylate underwent ring expansion under the disclosed conditions. Chemoselectivity for aziridine ring expansion over azetidine ring expansion in this system can be attributed to the increased pyramidalization at nitrogen observed for acylaziridines and related compounds, which increases their N-nucleophilicity relative to less strained amides.26
Table 1:
Lineage and Reaction Optimizationa
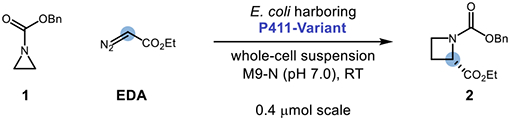
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Variant | Mutations Relative to Prior Generation | TTN | Yield (%) | e.r. |
|
| |||||
| 1 | Parent F2 | None | 73 | 3.6 | 90:10 |
| 2 | F2.1 | G263Y | 70 | 3.5 | 75:25 |
| 3 | F2.2 | T327V | 126 | 6.3 | 56:44 |
| 4 | F2.3 | A330T | 193 | 9.6 | 59:41 |
| 5 | F2.4 | H266P | 394 | 19.7 | 62:38 |
| 6 | F2.5 | M177Q | 699 | 34.9 | 94:6 |
| 7 | F2.6 | T436G | 945 | 47.3 | 93:7 |
| 8 | F2.7 | L233F | 997 | 49.8 | 94:6 |
| 9 | F2.8 | T149M | 1040 | 52.0 | 99:1 |
| 10 | F2.9 | R47Q | 1190 | 59.7 | 99:1 |
| 11 | P411-AzetS | M118K | 1200 | 59.9 | 99:1 |
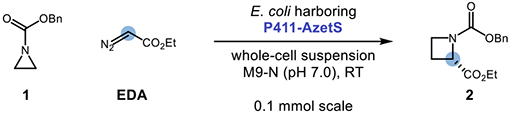
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Change from Conditions Above | TTN | Yield (%) | e.r. | |
|
| |||||
| 12 | None | 1580 | 79.1 | 99:1 | |
| 13 | 20 mM [1]; 30 mM [EDA] | 2200 | 55.0 | 99:1 | |
| 14 | Lysate | 1090 | 54.4 | 99:1 | |
| 15 | Lysate; 20 mM [1]; 30 mM [EDA] | 1570 | 39.3 | 99:1 | |
| 16 | 4 °C | 1610 | 80.2 | 99:1 | |
| 17 | Lysate; 4 °C | 1380 | 68.7 | 99:1 | |
Reactions were performed on the designated scale and run for 16 h with 10 mM of 1, 15 mM of EDA, and 5 μM of protein. TTN and yields were determined via GC analysis of crude reaction mixtures relative to an internal standard and represent the average of three experiments. The enantiomeric ratio (er] of the product was determined by chiral GC.
Encouraged by this promising initial activity and high enantioselectivity, we chose Parent F2 as a starting point for directed evolution to improve enzyme performance using iterative site-saturation mutagenesis (SSM) of residues located in the heme domain (Entries 2–11), screening for improved azetidine yield by gas chromatography. Sites were selected for mutagenesis based on success in previous directed evolution campaigns of P450BM3 as well as prior knowledge of residues responsible for substrate binding and catalysis in the heme domain of this protein scaffold.17a Ten beneficial mutations were identified during this campaign, resulting in a more efficient ‘azetidine synthase’ (P411-AzetS) with a net improvement of 16-fold in TTN and improved enantioselection (99:1 er). With P411-AzetS in hand, we next examined the impact of varying the reaction conditions on the product yield (Entries 12–17). Notably, increasing the scale from 4 μmol to 100 μmol resulted in an increase in the reaction yield. When the concentrations of 1 and EDA were doubled to 20 mM and 30 mM, respectively, a decrease in reaction yield was observed (although TTN increased). The ring expansion reaction also proceeded in clarified cell lysate, albeit with decreased yields when compared to analogous reactions performed with whole-cell suspensions. Lastly, decreasing the reaction temperature from 22 to 4 °C did not have a meaningful impact on the reaction yields when run in whole-cell suspensions.
Next, we sought to examine the substrate scope of this reaction and whether or not the new selectivities we observed could be extended to other substrates (Scheme 1). When this reaction was run at 0.5-mmol scale, azetidine 2 could be formed in 75% yield, 1490 TTN, 67% isolated yield, and 99:1 er. Other aromatic groups could be used in lieu of a phenyl group with uniformly high enantioselection observed in all cases. Notably, a thiophene-bearing aziridine could undergo chemoselective ring expansion to azetidine 3 with no observed cyclopropanation byproducts. This selectivity is notable not only because thiophenes are known to react with EDA-derived metal carbenoids under mild conditions,27 but also because Parent F2 was originally engineered to perform cyclopropanation of heteroatom-substituted olefins.24 Fluorine substituents were also tolerated on the arene ring at the para, meta, and ortho positions to furnish fluorinated products 4–6. In addition to EDA, other diazoacetate compounds could participate in one-carbon ring expansion with at least 99:1 er (7–8). When methyl diazoacetate was used as the carbene precursor to yield 9, a notable decrease in er (81:19) was observed. One hypothesis for this decrease in enantiopurity is that the smaller aliphatic chain allows for greater conformational freedom of the iron porphyrin carbene intermediate or the putative diradical intermediate. This explanation is consistent with prior work on enzyme-mediated carbene transfer reactions using perfluoroalkyl-stabilized diazo compounds as carbene precursors, where the substrate chain length has a profound influence on the absolute stereochemical configuration of the reaction product.21e The reaction could also be scaled up from 0.5-mmol scale to 10-mmol scale to furnish 2 in 1220 TTN, 61% yield, and 99:1 er with an isolated yield of 1.44 g (55% isolated yield), demonstrating that gram-scale production of enantioenriched azetidines is viable using this platform and that extension of this activity could be a powerful tool for the asymmetric synthesis of chiral heterocycles.
Scheme 1:
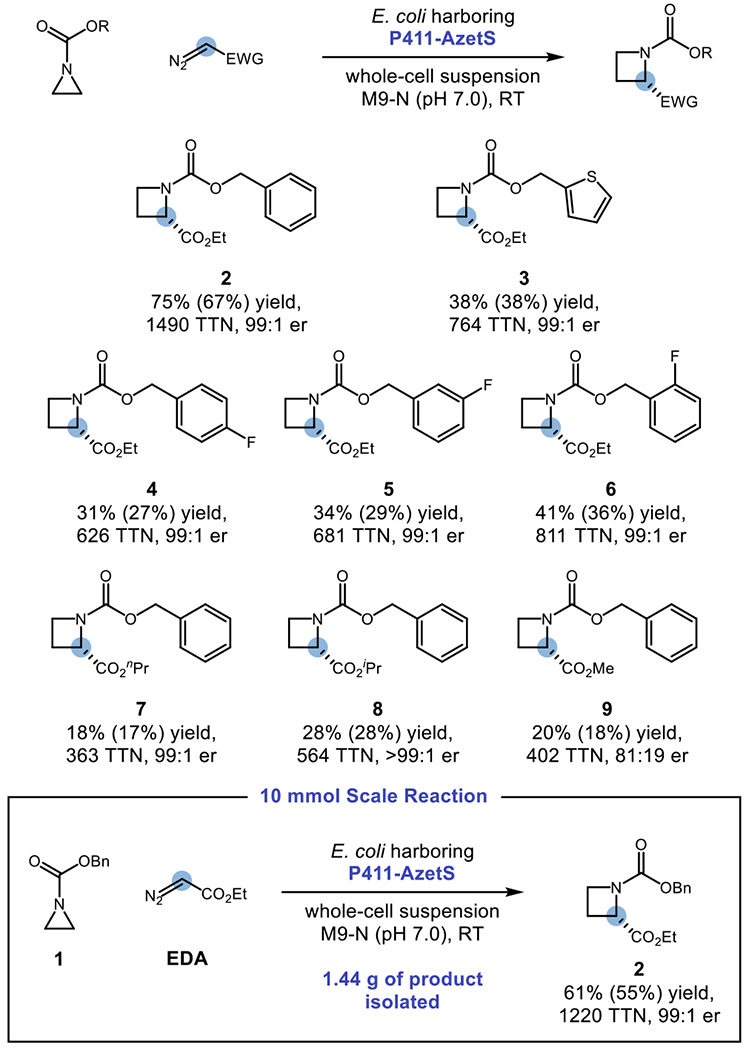
Substrate Scopea
a Reactions were performed on 0.5-mmol scale unless otherwise specified. Analytical yields and TTN were determined by GC-FID. Yields for isolated and purified material are designated in parentheses. The er was determined by Chiral GC. For 0.5-mmol scale reactions, all numbers reported represent the average of two trials. For the 10-mmol scale reaction, the reported numbers represent one run.
The current P411-AzetS lineage performs poorly with other substrate classes. Aziridine substrates with substituents on the carbon backbone of the ring were unable to undergo ring expansion due to their pronounced capacity for ring opening by hydrolysis relative to unsubstituted aziridine rings. This limitation also prevented N-alkyl or N-aryl aziridines from serving as viable substrates. Other classes of nitrogen protecting groups (e.g., amides and sulfonamides) demonstrated poor activity; one explanation is that the decreased N-nucleophilicity of these species hinders their ability to form aziridinium ylides. Finally, other carbamate-protecting groups (e.g., -Boc, -Alloc, and -CO2Me) did not form the desired products, suggesting that the arene may be necessary for proper substrate binding with this lineage of enzymes. With respect to the diazo coupling partner, diazoacetates were uniquely effective: when other diazo coupling partners were subjected to the reaction conditions, only unreacted diazo starting materials or dimerization products were recovered. Efforts to expand the observed, unprecedented reactivity and selectivity to the synthesis of other classes of azetidines are ongoing.
A hypothetical mechanism for the one-carbon ring expansion of aziridines is shown in Figure 2. The reaction of a hemoprotein with a suitable carbene precursor forms an electrophilic iron carbenoid intermediate, which could be trapped by a sufficiently nucleophilic aziridine. Ammonium ylides are commonly proposed as intermediates in hemoprotein-catalyzed N–H insertion reactions,22 and Schomaker has reported numerous examples where carbamate-protected aziridines react with metal-carbenoid electrophiles to form aziridinium ylides.11,12c–f At the present time, it is not clear whether this intermediate would exist as a “free” or metal-bound ylide, although computational analysis of enzymatic N–H insertion reactions suggests that ammonium ylide intermediates react after dissociation from the iron center.22f Finally, the aziridinium ylide could undergo the desired [1,2]-Stevens rearrangement preferentially over cheletropic extrusion of ethylene, liberating the desired product and regenerating the hemoprotein. We envisioned that the active site of an enzyme could mimic solvent caging effects, which are known to exert selectivity over radical recombination in [1,2]-Stevens rearrangements, to achieve asymmetric induction during ring expansion.15–16 Such effects may also explain why free hemin is unable to catalyze the reaction in the absence of the specific confinement provided by the active sites of this enzyme lineage. Additionally, hemoproteins demonstrate high stereoselectivity in radical reactions, both in their native activity28 as well as in new-to-nature activity cultivated through protein engineering,29 lending further support to this hypothesis.
Figure 2:
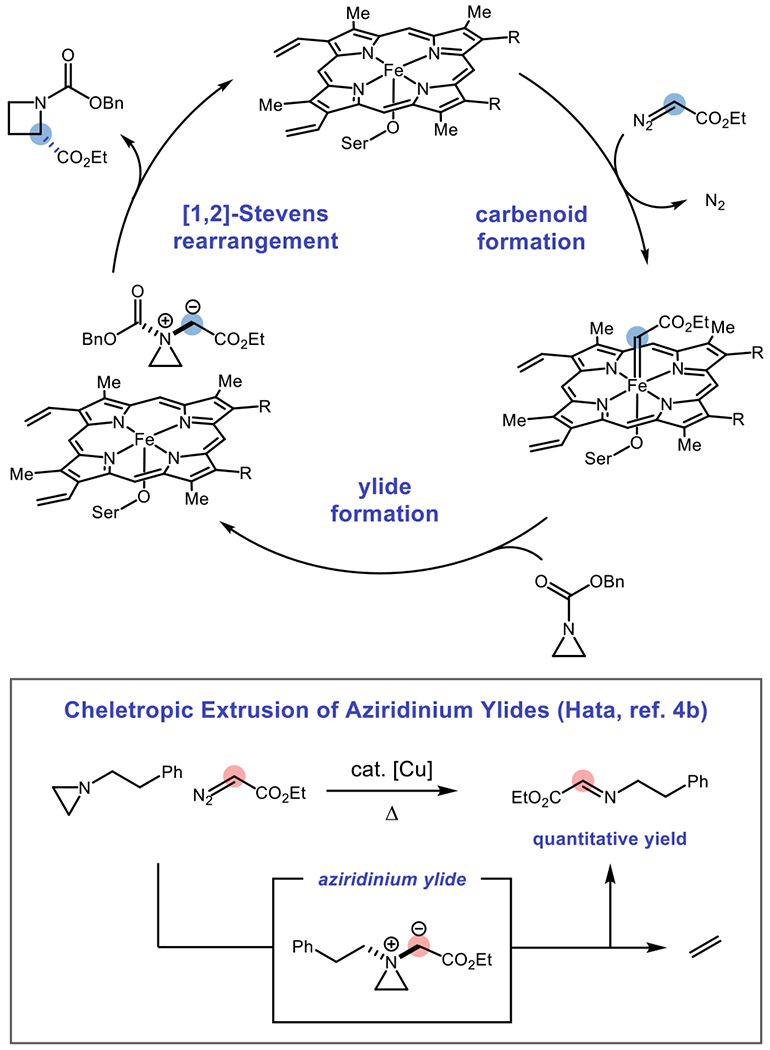
Possible catalytic cycle for one-carbon ring expansion of aziridines to furnish chiral azetidines, with cheletropic extrusion of ethylene as a possible side reaction. The ammonium ylide may also remain iron-bound.
In summary, we have demonstrated unprecedented hemoprotein-catalyzed [1,2]-Stevens rearrangement in the context of a one-carbon ring expansion of aziridines to azetidines. This system not only represents a rare example of a highly enantioselective [1,2]-Stevens rearrangement of ammonium ylides, but also exhibits unprecedented selectivity for the [1,2]-Stevens rearrangement of aziridinium ylides over cheletropic extrusion of ethylene. We are optimistic that observed selectivities can be extended to other types of [1,2]-Stevens rearrangements, providing the grounds for future work in this area toward the synthesis of enantioenriched heterocycles and other chiral amines.
Supplementary Material
ACKNOWLEDGMENT
Research was sponsored by the U.S. Army Research Office and accomplished under contracts W911NF-19-D-0001 and W911NF-19-2-0026 for the Institute for Collaborative Biotechnologies. D.C.M. was supported by a Ruth Kirschstein NIH Postdoctoral Fellowship (F32GM128247). The authors wish to thank Dr. Sabine Brinkmann-Chen, Dr. Nathaniel Goldberg, and Dr. Nicholas Porter for assistance preparing the manuscript.
Footnotes
The Supporting Information is available free of charge at: DOI: “https://pubs.acs.org/doi/10.1021/jacs.2c00251”.
General information and protocols, detailed description of experimental methods, characterization data for all compounds, NMR spectra of all products, and chiral GC traces of all products. (SI_1)
Full nucleotide and amino-acid sequences for all reported enzyme variants. (SI_2)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Zhao K; Yamashita K; Carpenter JE; Sherwood TC; Ewing WR; Cheng PTW; Knowles RR Catalytic Ring Expansions of Cyclic Alcohols Enabled by Proton-Coupled Electron Transfer. J. Am. Chem. Soc 2019, 141, 8752. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dherange BD; Kelly PQ; Liles JP; Sigman MS; Levin MD Carbon Atom Insertion into Pyrroles and Indoles Promoted by Chlorodiazirines. J. Am. Chem. Soc 2021, 143, 11337. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kennedy SH; Dherange BD; Berger KJ; Levin MD Skeletal editing through direct nitrogen deletion of secondary amines. Nature, 2021, 593, 223. [DOI] [PubMed] [Google Scholar]; (d) Donald JR; Unsworth WP Ring-Expansion Reactions in the Synthesis of Macrocycles and Medium-Sized Rings. Chem. -Eur. J 2017, 23, 8780. [DOI] [PubMed] [Google Scholar]; (e) Dowd P; Zhang W Free radical-mediated ring expansion and related annulations. Chem. Rev 1993, 93, 2091. [Google Scholar]
- (2).For reviews, see:; (a) Chen P-H; Billett BA; Tsukamoto T; Dong G “Cut and Sew” Transformations via Transition-Metal-Catalyzed Carbon–Carbon Bond Activation. ACS Catal. 2017, 7, 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xu T; Dermenci A; Dong G Transition metal-catalyzed C-C bond activation of four-membered cyclic ketones. Top. Curr. Chem 2014, 346, 233. [DOI] [PubMed] [Google Scholar]; (c) Gao Y; Fu X-F; Yu Z-X Transition Metal-Catalyzed Cycloadditions of Cyclopropanes for the Synthesis of Carbocycles: C–C Activation in Cyclopropanes. Top. Curr. Chem 2014, 346, 195. [DOI] [PubMed] [Google Scholar]; (d) Xia Y; Dong G Temporary or removable directing groups enable activation of unstrained C–C bonds. Nat. Rev. Chem 2020, 4, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Jun C-H Transition metal-catalyzed carbon–carbon bond activation. Chem. Soc. Rev 2004, 33, 610. [DOI] [PubMed] [Google Scholar]; (f) Chen F; Wang T; Jiao N Recent Advances in Transition-Metal-Catalyzed Functionalization of Unstrained Carbon–Carbon Bonds. Chem. Rev 2014, 114, 8613. [DOI] [PubMed] [Google Scholar]; (g) Souillart L; Cramer N Catalytic C–C Bond Activations via Oxidative Addition to Transition Metals. Chem. Rev 2015, 115, 9410. [DOI] [PubMed] [Google Scholar]
- (3).For representative examples, see:; (a) Tayama E Ring-Substitution, Enlargement, and Contraction by Base-Induced Rearrangements of N-Heterocyclic Ammonium Salts. Heterocycles, 2016, 92, 793. [Google Scholar]; (b) Wittig G; Tenhaeff H; Schoch W; Koenig G Einige Synthesen über Ylide. Liebigs Ann. Chem 1951, 572, 1. [Google Scholar]; (c) Chicharro R; de Castro S; Reino J; Arán VJ Synthesis of Tri- and Tetracyclic Condensed Quinoxalin-2-ones Fused Across the C-3–N-4 Bond. Eur. J. Org. Chem 2003, 2003, 2314. [Google Scholar]; (d) Pedrosa R; Andrés C; Delgado M Stereocontrolled Ring Enlargement by Diastereoselective Stevens Rearrangement in Chiral 1,3-Oxazolidinium Salts. A Novel Entry to Enantiopure Morpholines. Synlett, 2000, 6, 893. [Google Scholar]; (e) Harthong S; Bach R; Besnard C; Guénée L; Lacour J Ring-Expansion Reactions of Binaphthyl Azepines and Ferrocenophanes through Metal-Catalyzed [1,2]-Stevens Rearrangements. Synthesis, 2013, 45, 2070. [Google Scholar]; (f) Vanecko JA; West FG A Novel, Stereoselective Silyl-Directed Stevens [1,2]-Shift of Ammonium Ylides. Org. Lett 2002, 4, 2813. [DOI] [PubMed] [Google Scholar]; (g) Hanessian S; Mauduit M Highly Diastereoselective Intramolecular [1,2]-Stevens Rearrangements—Asymmetric Syntheses of Functionalized Isopavines as Morphinomimetics. Angew. Chem. Int. Ed 2001, 40, 3810. [DOI] [PubMed] [Google Scholar]; (h) Liou J-P; Cheng C-Y Total synthesis of (±)-desoxycodeine-D: a novel route to the morphine skeleton. Tetrahedron Letters, 2000, 41, 915. [Google Scholar]; (i) Sharma A; Besnard C; Guénée L; Lacour J Asymmetric synthesis of ethano-Tröger bases using CuTC-catalyzed diazo decomposition reactions. Org. Biomol. Chem 2012, 10, 966. [DOI] [PubMed] [Google Scholar]; (j) Vanecko JA; Wan H; West FG Recent advances in the Stevens rearrangement of ammonium ylides. Application to the synthesis of alkaloid natural products. Tetrahedron, 2006, 62, 1043. [Google Scholar]; (k) Kowalkowska A; Jończyk A [1,2] Stevens sigmatropic rearrangement of pyrrolidinium ylides—simple synthesis of 3-aryl-2-cyano-1-methylpiperidines. Tetrahedron, 2015, 71, 9630. [Google Scholar]; (1) Lahm G; Pacheco JCO; Opatz T Rearrangements of Nitrile-Stabilized Ammonium Ylides. Synthesis, 2014, 46, 2413. [Google Scholar]; (m) Empel C; Jana S; Koenigs RM Advances in [1,2]-Sigmatropic Rearrangements of Onium Ylides via Carbene Transfer Reactions. Synthesis, 2021, 53, 4567. [Google Scholar]
- (4).(a) Hata Y; Watanable M Fragmentation reaction of aziridinium ylids. Tetrahedron Letters, 1972, 13, 3827. [Google Scholar]; (b) Hata Y; Watanabe M Fragmentation reaction of aziridinium ylids. II. Tetrahedron Letters, 1972, 13, 4659. [Google Scholar]; (c) Bott TM; Vanecko JA; West FG One-Carbon Ring Expansion of Azetidines via Ammonium Ylide [1,2]-Shifts: A Simple Route to Substituted Pyrrolidines.J. Org. Chem 2009, 74, 2832. [DOI] [PubMed] [Google Scholar]; (d) Drouillat B; d’Aboville E; Bourdreux F; Couty F Synthesis of 2-Phenyl- and 2,2-Diarylpyrrolidines through Stevens Rearrangement Performed on Azetidinium Ions. Eur. J. Org. Chem 2014, 2014, 1103. [Google Scholar]; (e) Couty F; Durrat F; Evano G; Prim D Synthesis and reactivity of enantiomerically pure N-alkyl-2-alkenyl azetidinium salts. Tetrahedron Letters, 2004, 45, 7525. [Google Scholar]
- (5).Approximately 59% of all small-molecule drugs contain at least one nitrogen-containing heterocycle:; Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257. [DOI] [PubMed] [Google Scholar]
- (6).(a) St. Jean DJ; Fotsch C Mitigating Heterocycle Metabolism in Drug Discovery. J. Med. Chem 2012, 55, 6002. [DOI] [PubMed] [Google Scholar]; (b) Wang DX; Booth H; Lerner-Marmarosh N; Osdene TS; Abood LG Structure–activity relationships for nicotine analogs comparing competition for [3H]nicotine binding and psychotropic potency. Drug Dev. Res 1998, 45, 10. [Google Scholar]
- (7).(a) Brandi A; Cicchi S; Cordero FM Novel Syntheses of Azetidines and Azetidinones. Chem. Rev 2008, 108, 3988. [DOI] [PubMed] [Google Scholar]; (b) Mehra V; Lumb I; Anand A; Kumar V Recent advances in syn-thetic facets of immensely reactive azetidines. RSC Adv. 2017, 7, 45763. [Google Scholar]
- (8).For recent photochemical [2+2] strategies to access azetidines:; (a) Becker MR; Richardson AD; Schindler CS Functionalized azetidines via visible light-enabled aza Paternò-Büchi reactions. Nat. Commun 2019, 10, 5095. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Becker MR; Wearing ER; Schindler CS Synthesis of azetidines via visible-light-mediated intermolecular [2+2] photocycloadditions. Nat. Chem 2020, 12, 898. [DOI] [PubMed] [Google Scholar]; (c) Richardson AD; Becker MR; Schindler CS Synthesis of azetidines by aza Paternò–Büchi reactions. Chem. Sci 2020, 11, 7553. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sakamoto R; Inada T; Sakura S; Maruoka K [2 + 2] Photocycloadditions between the Carbon–Nitrogen Double Bonds of Imines and Carbon–Carbon Double Bonds. Org. Lett 2016, 18, 6252. [DOI] [PubMed] [Google Scholar]; (e) Flores D; Neville M; Schmidt V Intermolecular 2+2 Imine-Olefin Photocycloadditions Enabled by Cu(I)-Alkene MLCT. June 28 2021, ChemRxiv, DOI: 10.26434/chemrxiv-2021-t45sg (accessed 2022-02-22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).For selected examples, see:; (a) Malik S; Nadir UK A Facile Synthesis of 1-Arenesulfonylazetidines through Reaction of 1-Arenesulfonylaziridines with Dimethylsulfoxonium Methylide Generated under Microwave Irradiation. Synlett, 2008, 1, 108. [Google Scholar]; (b) Han J-Q; Zhang H-H; Xu P-F; Luo Y-C Lewis Acid and (Hypo)iodite Relay Catalysis Allows a Strategy for the Synthesis of Polysubstituted Azetidines and Tetrahydroquinolines. Org. Lett 2016, 18, 5212. [DOI] [PubMed] [Google Scholar]; (c) He G; Zhao Y; Zhang S; Lu C; Chen G Highly Efficient Syntheses of Azetidines, Pyrrolidines, and Indolines via Palladium Catalyzed Intramolecular Amination of C(sp3)–H and C(sp2)–H Bonds at γ and δ Positions. J. Am. Chem. Soc 2012, 134, 3. [DOI] [PubMed] [Google Scholar]; (d) Zhang H-H; Luo Y-C; Wang H-P; Chen W; Xu P-F TiCl4 Pro-moted Formal [3 + 3] Cycloaddition of Cyclopropane 1,1-Diesters with Azides: Synthesis of Highly Functionalized Triazinines and Azetidines. Org. Lett 2016, 16, 4896. [DOI] [PubMed] [Google Scholar]; (e) Lowe JT; Lee MD; Akella LB; Davoine E; Donckele EJ; Durak L; Duvall JR; Gerard B; Holson EB; Joliton A; Kesavan S; Lemercier BC; Liu H; Marié J-C; Mulrooney CA; Muncipinto G; Welzel-O’Shea M; Panko LM; Rowley A; Suh B-C; Thomas M; Wanger FF; Wei J; Foley MA; Marcaurelle LA Synthesis and Profiling of a Diverse Collection of Azetidine-Based Scaffolds for the Development of CNS-Focused Lead-like Libraries. J. Org. Chem 2012, 77, 7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).For synthetic sequences leading to enantioenriched aziridines, see:; (a) Kapoor R; Chawla R; Singh S; Yadav LDS Organocatalytic Asymmetric Synthesis of 1,2,4-Trisubstituted Azetidines by Reductive Cyclization of Aza-Michael Adducts of Enones. Synlett, 2012, 23, 1321. [Google Scholar]; (b) Hanessian S; Bernstein N; Yang RY; Maguire R Asymmetric synthesis of L-azetidine-2-carboxylic acid and 3-substituted congeners--conformationally constrained analogs of phenylalanine, naphthylalanine, and leucine. Bioorg. Med. Chem. Lett 1999, 17, 1437. [DOI] [PubMed] [Google Scholar]; (c) Marichev KO; Wang K; Dong K; Greco N; Massey LA; Deng Y; Arman H; Doyle MP Synthesis of Chiral Tetrasubstituted Azetidines from Donor–Acceptor Azetines via Asymmetric Copper(I)-Catalyzed Imido-Ylide [3+1]-Cycloaddition with Metallo-Enolcarbenes. Angew. Chem. Int. Ed 2019, 58, 16188. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Singh GS Advances in synthesis and chemistry of azetidines. In Advances in Heterocyclic Chemistry. Academic Press, 2001; pp 1–74. [Google Scholar]; (e) Ma X; Zhao H; Binayeva M; Ralph G; Diane M; Zhao S; Wang C-Y; Biscoe MR A General Approach to Stereospecific Cross-Coupling Reactions of Nitrogen-Containing Stereocenters. Chem, 2020, 6, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Dequina HJ; Schomaker JM Aziridinium Ylides: Underused Intermediates for Complex Amine Synthesis. Trends in Chemistry, 2020, 2, 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Clark JS; Hodgson PB; Goldsmith MD; Blake AJ; Cooke PA; Street LJ Rearrangement of ammonium ylides produced by intramolecular reaction of catalytically generated metal carbenoids. Part 2. Stereoselective synthesis of bicyclic amines. J. Chem. Soc. Perkin Trans 1, 2001, 3325. [Google Scholar]; (b) Rowlands GJ; Barnes WK Studies on the [2,3]-Stevens rearrangement of aziridinium ions. Tetrahedron Letters, 2004, 45, 5347. [Google Scholar]; (c) Nicastri KA; Zappia SA; Pratt JC; Duncan JM; Guzei IA; Fernández I; Schomaker JM Tunable Aziridinium Ylide Reactivity: Noncovalent Interactions Enable Divergent Product Outcomes. ACS Catal. 2022, 12, 1572. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schmid SC; Guzei IA; Fernández I; Schomaker JM Ring Expansion of Bicyclic Methyleneaziridines via Concerted, Near-Barrierless [2,3]-Stevens Rearrangements of Aziridinium Ylides. ACS Catal. 2018, 8, 7907. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Schmid SC; Guzei IA; Schomaker JM A Stereoselective [3+1] Ring Expansion for the Synthesis of Highly Substituted Methylene Azetidines. Angew. Chem. Int. Ed 2017, 56, 12229. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Eshon J; Nicastri KA; Schmid SC; Raskopf WT; Guzei IA; Fernández I; Schomaker JM Intermolecular [3+3] ring expansion of aziridines to dehydropiperidines through the intermediacy of aziridinium ylides. Nat. Commun 2020, 11, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Bach R; Harthong S; Lacour J Nitrogen- and Sulfur-Based Stevens and Related Rearrangements. Comprehensive Organic Synthesis II, 2014, 3, 992. [Google Scholar]; (b) Lepley AR; Becker RH; Giumanini AG Benzyne addition to N,N-dimethylbenzylamine. J. Org. Chem 1971, 36, 1222. [Google Scholar]
- (14).(a) Qu J-P; Xu Z-H; Zhou J; Cao C-L; Sun X-L; Dai L-X; Tang Y Ligand-Accelerated Asymmetric [1,2]-Stevens Rearrangment of Sulfur Ylides via Decomposition of Diazomalonates Catalyzed by Chiral Bisoxazoline/Copper Complex. Adv. Synth. Catal 2009, 351, 308. [Google Scholar]; (b) Tomooka K; Sakamaki J; Harada M; Wada R Enantioselective [1,2]-Stevens Rearrangement Using Sugar-Derived Alkoxides as Chiral Promoters. Synlett, 2008, 5, 683. [Google Scholar]; (c) Hong F-L; Shi C-Y; Hong P; Zhai T-Y Zhu X-Q Lu X Ye L-W Copper-Catalyzed Asymmetric Diyne Cyclization via [1,2]-Stevens-Type Rearrangement for the Synthesis of Chiral Chromeno[3,4-c]pyrroles. Angew. Chem. Int. Ed 2022, 61, e202115554. [DOI] [PubMed] [Google Scholar]
- (15).(a) Tayama E; Nanbara S; Nakai T Asymmetric [1,2] Stevens Rearrangement of (S)-N-Benzylic Proline-derived Ammo-nium Salts under Biphasic Conditions. Chem. Lett 2006, 35, 478. [Google Scholar]; (b) Gonçalves-Farbos M-H; Vial L; Lacour J Enantioselective [1,2]-Stevens rearrangement of quaternary ammonium salts. A mechanistic evaluation. Chem. Commun 2008, 829–831. [DOI] [PubMed] [Google Scholar]; (c) Palombi L The first electro-induced asymmetric Stevens rearrangement of (S)- and (R)-N-benzyl proline-derived ammonium salts. Catalysis Communications, 2011, 12, 485. [Google Scholar]; (d) Glaeske KW; West FG Chirality Transfer from Carbon to Nitrogen to Carbon via Cyclic Ammonium Ylides. Org. Lett 1999, 1, 31. [Google Scholar]; (e) Vial L; Gonçalves M-H; Morgantini P-Y; Weber J; Bernardinelli G; Lacour J Unusual Regio- and Enantioselective [1,2]-Stevens Rearrangement of a Spirobi[dibenzazepinium] Cation. Synlett, 2004, 9, 1565. [Google Scholar]
- (16).(a) Woodward JR Radical Pairs in Solution. Prog. React. Kinet. Mec 2002, 27, 165. [Google Scholar]; (b) Franck J; Rainbowitsch E Some remarks about free radicals and the photochemistry of solutions. Trans. Faraday Soc 1934, 30, 120. [Google Scholar]; (c) Braden DA; Parrack EE; Tyler DR Solvent cage effects. I. Effect of radical mass and size on radical cage pair recombination efficiency. II. Is geminate recombination of polar radicals sensitive to solvent polarity? Coord. Chem. Rev 2001, 211, 279. [Google Scholar]
- (17).For reviews and representative examples, see:; (a) Whitehouse CJC; Bell SG; Wong L-L P450BM3 (CYP102A1): connecting the dots. Chem. Soc. Rev. 2011, 41, 1218. [DOI] [PubMed] [Google Scholar]; (b) Thiel D; Doknić D; Deska J Enzymatic aerobic ring rearrangement of optically active furylcarbinols. Nat. Commun 2014, 5, 5278. [DOI] [PubMed] [Google Scholar]; (c) Tang M-C; Zou Y; Watanabe K; Walsh CT; Tang Y Oxidative Cyclization in Natural Product Biosynthesis. Chem. Rev 2017, 117, 5226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) BatErdene U; Kanayama D; Tan D; Turner WC; Houk KN; Ohashi M; Tang Y Iterative Catalysis in the Biosynthesis of Mitochondrial Complex II Inhibitors Harzianopyridone and Atpenin B. J. Am. Chem. Soc 2000, 142, 8550. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Fürst MJL; Gran-Scheuch A; Aalbers FS; Fraaije MW Baeyer–Villiger Monooxygenases: Tunable Oxidative Biocatalysts. ACS Catal. 2019, 9, 11207. [Google Scholar]; (f) Leisch H; Morley K; Lau PCK Baeyer–Villiger Monooxygenases: More Than Just Green Chemistry. Chem. Rev 2011, 111, 4165. [DOI] [PubMed] [Google Scholar]; (g) Deska J; Thiel D; Gianolio E The Achmatowicz Rearrangement – Oxidative Ring Expansion of Furfuryl Alcohols. Synthesis, 2015, 47, 3435. [Google Scholar]
- (18).For reviews and representative examples, see:; (a) Christianson DW Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev 2017, 117, 11570. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hoshino T; Kouda M; Abe T; Ohashi S New Cyclization Mechanism for Squalene: a Ring-expansion Step for the Five-membered C-ring Intermediate in Hopene Biosynthesis. Biosci. Biotechnol. Biochem 1999, 63, 2038. [DOI] [PubMed] [Google Scholar]; (c) Xu M; Jia M; Hong YJ; Yin X; Tantillo DJ; Proteau PJ; Peters RJ Premutilin Synthase: Ring Rearrangement by a Class II Diterpene Cyclase. Org. Lett 2018, 20, 1200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Quan Z; Dickschat JS Biosynthetic Gene Cluster for Asperterpenols A and B and the Cyclization Mechanism of Asperterpenol A Synthase. Org. Lett 2020, 22, 7552. [DOI] [PubMed] [Google Scholar]; (e) Xu R; Fazio GC; Matsuda SPT On the origins of triterpenoid skeletal diversity. Phytochemistry, 2004, 65, 261. [DOI] [PubMed] [Google Scholar]; (f) Rudolf JD; Chang C-Y Terpene synthases in disguise: enzymology, structure, and opportunities of non-canonical terpene synthases. Nat. Prod. Rep 2020, 37, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Dickschat JS Bacterial Diterpene Biosynthesis. Angew. Chem. Int. Ed 2019, 58, 15964. [DOI] [PubMed] [Google Scholar]
- (19).(a) Brandenberg OF; Fasan R; Arnold FH Exploiting and engineering hemoproteins for abiological carbene and nitrene transfer reactions. Curr. Opin. Biotechnol 2017, 47, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang Y; Arnold FH Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer. Acc. Chem. Res 2021, 54, 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu Z; Arnold FH New-to-nature chemistry from old protein machinery: carbene and nitrene transferases. Curr. Opin. Biotechnol 2021, 69, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dunham NP; Arnold FH Nature’s Machinery, Repurposed: Expanding the Repertoire of Iron-Dependent Oxygenases. ACS Catal. 2020, 10, 12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Chen KC; Arnold FH Engineering Cytochrome P450s for Enantioselective Cyclopropenation of Internal Alkynes. J. Am. Chem. Soc 2020, 142, 6891. [DOI] [PubMed] [Google Scholar]; (b) Chen KC; Huang X; Kan SBJ; Zhang RK; Arnold FH Enzymatic construction of highly strained carbocycles. Science, 2018, 360, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Kan SBJ; Lewis RD; Chen K; Arnold FH Directed evolution of cytochrome c for carbon–silicon bond formation: Bringing silicon to life. Science, 2016, 354, 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kan SBJ; Huang X; Gumulya Y; Chen K; Arnold FH Genetically programmed chiral organoborane synthesis. Nature, 2017, 552, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang RK; Chen K; Huang X; Wohlschlager L; Renata H; Arnold FH Enzymatic assembly of carbon–carbon bonds via iron-catalysed sp3 C–H functionalization. Nature, 2019, 565, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chen K; Zhang S-Q; Brandenberg OF; Hong X; Arnold FH Alternate Heme Ligation Steers Activity and Selectivity in Engineered Cytochrome P450-Catalyzed Carbene-Transfer Reactions. J. Am. Chem. Soc 2018, 140, 16402. [DOI] [PubMed] [Google Scholar]; (e) Zhang J; Huang X; Zhang RK; Arnold FH Enantiodivergent α-Amino C–H Fluoroalkylation Catalyzed by Engineered Cytochrome P450s. J. Am. Chem. Soc 2019, 141, 9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).(a) Wang ZJ; Peck NE; Renata H; Arnold FH Cytochrome P450-catalyzed insertion of carbenoids into N–H bonds. Chem. Sci 2014, 5, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Steck V; Carminati DM; Johnson NR; Fasan R Enantioselective Synthesis of Chiral Amines via Biocatalytic Carbene N–H Insertion. ACS Catal. 2020, 10, 10967. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sreenilayam G; Fasan R Myoglobin-catalyzed intermolecular carbene N–H insertion with arylamine substrates. Chem. Commun 2015, 15, 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sreenilayam G; Moore EJ Steck V; Fasan R Metal Substitution Modulates the Reactivity and Extends the Reaction Scope of Myoglobin Carbene Transfer Catalysts. Adv. Synth. Catal 2017, 359, 2076. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Steck V; Sreenilayam G; Fasan R Selective Functionalization of Aliphatic Amines via Myoglobin-Catalyzed Carbene N–H Insertion. Synlett, 2020, 31, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Liu Z; Calvó-Tusell C; Zhou AZ; Chen K; Garcia-Borràs M; Arnold FH Dual-Function Enzyme Catalysis for Enantioselective Carbon–Nitrogen Bond Formation. Nature Chemistry, 2021, 13, 1166. [DOI] [PubMed] [Google Scholar]
- (23).Coelho PS; Wang ZJ; Ener ME; Baril SA; Kannan A; Arnold FH; Brustad EM A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Nat. Chem. Biol 2013, 9, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Brandenberg OF; Prier CK; Chen K; Knight AM; Wu Z; Arnold FH Stereoselective Enzymatic Synthesis of Heteroatom-Substituted Cyclopropanes. ACS Catal. 2018, 8, 2629. [Google Scholar]
- (25).Narhi LO; Fulco AJ Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem 1986, 261, 7160. [PubMed] [Google Scholar]
- (26).(a) Ohwada T; Okamoto I; Shudo K; Yamaguchi K Intrinsic pyramidal nitrogen of N-sulfonamides. Tetrahedron Letters, 1998, 39, 7877. [Google Scholar]; (b) Ferraris D; Drury III WJ; Cox C; Lectka T “Orthogonal” Lewis Acids: Catalyzed Ring Opening and Rearrangement of Acylaziridines. J. Org. Chem 1998, 63, 4568. [Google Scholar]; (c) Cho SJ; Cui C; Lee JY; Park JK; Suh SB; Park J; Kim BH; Kim KS N-Protonation vs O-Protonation in Strained Amides: Ab Initio Study. J. Org. Chem 1997, 62, 4068. [Google Scholar]
- (27).Waser M; Moher ED; Borders SSK; Hansen MM; Hoard DW; Laurila ME; LeTourneau ME; Miller RD; Phillips ML; Sullivan KA; Ward JA; Xie C; Bye CA; Leitner TJ; Herzog-Krimbacher B; Kordian M; Müllner M Process Development for a Key Synthetic Intermediate of LY2140023, a Clinical Candidate for the Treatment of Schizophrenia. Org. Process Res. Dev 2011, 15, 1266. [Google Scholar]
- (28).Ortiz de Montellano PR Hydrocarbon Hydroxylation by Cytochrome P450 Enzymes. Chem. Rev 2010, 110, 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Yang Y; Cho I; Qi X; Liu P; Arnold FH An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp3)–H bonds. Nat. Chem 2019, 11, 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


