Background:
The “1-3-6-12-day rule” for starting direct oral anticoagulants (DOACs) in patients with nonvalvular atrial fibrillation after acute ischemic stroke or transient ischemic attack recommends timings that may be later than used in clinical practice. We investigated more practical optimal timing of DOAC initiation according to stroke severity.
Methods:
The combined data of prospective registries in Japan, Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-nonvalvular atrial fibrillation (September 2011 to March 2014) and RELAXED (February 2014 to April 2016) were used. Patients were divided into transient ischemic attack and 3 stroke subgroups by the National Institutes of Health Stroke Scale score: mild (0–7), moderate (8–15), and severe (≥16). The early treatment group was defined as patients starting DOACs earlier than the median initiation day in each subgroup. Outcomes included a composite of recurrent stroke or systemic embolism, ischemic stroke, and severe bleeding within 90 days. Six European prospective registries were used for validation.
Results:
In the 1797 derivation cohort patients, DOACs were started at median 2 days after transient ischemic attack and 3, 4, and 5 days after mild, moderate, and severe strokes, respectively. Stroke or systemic embolism was less common in Early Group (n=785)—initiating DOACS within 1, 2, 3, and 4 days, respectively—than Late Group (n=1012) (1.9% versus 3.9%; adjusted hazard ratio, 0.50 [95% CI, 0.27–0.89]), as was ischemic stroke (1.7% versus 3.2%, 0.54 [0.27–0.999]). Major bleeding was similarly common in the 2 groups (0.8% versus 1.0%). On validation, both ischemic stroke (2.4% versus 2.2%) and intracranial hemorrhage (0.2% versus 0.6%) were similarly common in Early (n=547) and Late (n=1483) Groups defined using derivation data.
Conclusions:
In Japanese and European populations, early DOAC initiation within 1, 2, 3, or 4 days according to stroke severity seemed to be feasible to decrease the risk of recurrent stroke or systemic embolism and no increase in major bleeding. These findings support ongoing randomized trials to better establish the optimal timing of DOAC initiation.
Keywords: acute ischemic stroke, anticoagulation, atrial fibrillation, cardioembolism, stroke prevention
Anticoagulation with direct oral anticoagulants (DOACs) started early after acute ischemic stroke (IS) or transient ischemic attack (TIA) related to nonvalvular atrial fibrillation (NVAF) has been found to be associated with a decreased risk of poor clinical outcomes compared with warfarin in practical clinical settings, mainly attributed to lower risks of intracranial hemorrhage (ICH).1–3 However, the optimal timing for initiating DOACs after IS has remained unclear. Ongoing randomized trials on early versus late DOAC initiation have not published their final results.4 A recent randomized trial comparing early apixaban initiation with late warfarin initiation, involving 91 patients, showed the safety of early apixaban.5
The “1-3-6-12-day rule” is a known consensus opinion with graded increase in delay of anticoagulation between 1 and 12 days after onset of IS/TIA according to neurological severity and reasonable from the perspective that the timing should vary according to the severity.6 However, with increasing observational data suggesting that earlier initiation of DOACs might be safe, the timings of 1-3-6-12 days might be somewhat later than currently used in a real-world practical setting. In the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement (SAMURAI)-NVAF registry including patients with acute IS/TIA of any neurological severity, we found that the risks for stroke, systemic embolism, major bleeding, and death were comparable whether DOACs were started within 3 days or from 4 days or later after the onset.7 As the 1-3-6-12-day rule set different timings according to neurological severity, we would be better to reanalyze the data by separating patients with different neurological severity. We hypothesized that the earlier days for starting DOACs were more practical for patients with any categories of neurological severity.
We aimed to propose a new optimal timing for initiation of DOACs according to severity of IS/TIA using 2 registries in Japan. The appropriateness of the timing was also externally validated using multiple registries from countries outside Japan.
Methods
Data Availability
De-identified individual participant data (IPD) of the derivation cohorts are available from the principal investigator of SAMURAI-NVAF (Toyoda) and RELAXED (Recurrent Embolism Lessened by rivaroxaban, an Anti-Xa agent, of Early Dosing for Acute Ischemic Stroke and Transient Ischemic Attack With Atrial Fibrillation: Minematsu) on reasonable request.
Study Population
The combined data from 2 prospective, multicenter, observational studies on NVAF patients with acute IS/TIA (SAMURAI-NVAF and RELAXED) were used. The design and main outcomes of the studies have been described elsewhere.8–12 The committee members of the studies are listed in Table S1. The study protocol and associated documents were reviewed and approved by the Institutional Review Boards of each participating study center in both studies. Patients or their relatives provided written, informed consent according to ethical regulations.
Patients with NVAF who visited the hospital clinic within 7 days (within 48 hours in RELAXED) of the onset of IS/TIA were enrolled between September 2011 and March 2014 in SAMURAI-NVAF and between February 2014 and April 2016 in RELAXED. Inclusion and exclusion criteria of the studies are listed in Table S2. From the 2 registries, only patients who took DOACs after the onset of IS/TIA were enrolled in the present study. Patient eligibility for anticoagulant therapy and the choice of agents were determined by each investigator in SAMURAI-NVAF, and dabigatran, rivaroxaban, and apixaban were available during the study period. In contrast, all patients started or resumed rivaroxaban within 30 days after IS/TIA in RELAXED. Note that the official daily dose of rivaroxaban in Japan is 15 or 10 mg based on the results of a domestic trial,13 lower than that in other countries. NVAF was diagnosed on 12-lead ECG or 24-hour or longer monitoring for AF detection during acute hospitalization or from previous medical documents.
Distribution of symptomatic infarcts did not affect inclusion in SAMURAI-NVAF, whereas infarcts in the middle cerebral artery area demonstrated by diffusion-weighted imaging or TIA showing symptoms corresponding to this area were mandatory in RELAXED. Infarct size was measured locally if the longest diameter exceeded 15 mm in SAMURAI-NVAF and centrally measured and automatically quantified in RELAXED. In the present study, infarcts were regarded as small if the longest diameter was ≤15 mm in SAMURAI-NVAF and if the volume was ≤1.7 cm3 in RELAXED, corresponding to the volume of a sphere with a diameter of 15 mm.
Patients were divided into 4 subgroups by neurological severity according to the previous European guidelines as follows: TIA as focal neurological symptoms disappearing within 24 hours; mild IS with a National Institutes of Health Stroke Scale (NIHSS) score of 0 to 7; moderate IS as a score of 8 to 15; and severe IS as a score of 16 or more.6 The NIHSS scores were assessed by expert stroke neurologists/neurosurgeons. The early treatment group (Early Group) was defined as patients who started DOACs earlier than the median day of DOAC initiation in each severity subgroup, and the late treatment group (Late Group) as those starting DOACs at the median day or later.
For external validation, IPD from 3 multicenter prospective cohort studies, including Early Recurrence and Cerebral Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation (RAF14 and RAF-NOAC15) from 29 centers in Europe and Asia and CROMIS-2 (Clinical Relevance of Microbleeds in Stroke) study16,17 from 79 centers in the United Kingdom and one in the Netherlands, and 3 single-center prospective cohort studies from Basel,18 Verona,19 and Erlangen20 were used (Table S1); all of these were used for a series of pooled IPD analyses on early DOAC initiation together with SAMURAI-NVAF.1,21,22 Inclusion and exclusion criteria of the studies are listed in Table S2. The definitions of the 4 subgroups by stroke severity and the 2 groups by the timing of initiating DOACs were identical with those of the derivation cohort; that is, the cutoff days between Early and Late Groups were the median day of initiating DOACs for the derivation cohort.
Outcome Events and Follow-Up Period
The primary efficacy outcome of the derivation cohort was a composite of stroke (ischemic or hemorrhagic) and systemic embolism (an acute vascular occlusion of the extremities or any organ and must be documented by angiography, etc) within 90 days after the index IS/TIA.23 The secondary efficacy outcomes were IS and death within 90 days. The primary safety outcome was major bleeding defined by the International Society on Thrombosis and Hemostasis within 90 days.24 The secondary safety outcome was any ICH (except for cerebral microbleeds) within 90 days. Events were assessed by medical record verification or self-report at the hospital clinic (or by telephone survey for patients with aftereffects too severe to allow a visit to the clinic).
In the validation cohort, ischemic stroke, death, and ICH within 90 days were the outcome events of interest, because data on systemic embolism and major bleeding were not available for all of the participating studies.
Statistical Analysis
Continuous variables are summarized as medians (interquartile range [IQR]) and categorical variables as frequencies and percentages. Intergroup comparisons were performed using Pearson chi-squared test for categorical variables and Mann-Whitney U test for continuous variables. The major outcomes of the derivation and validation cohorts are presented as Kaplan-Meier curves. The relationship between the groups (Early or Late) and outcomes was examined by multivariable Cox proportional hazards model analysis with adjustment for the following prespecified covariates: sex, age, history of diabetes, stroke, or TIA, and infarct size (small or larger). The analysis results are presented as hazard ratios (HRs) with 95% CIs. In the validation analysis, the same adjusting factors were used except for infarct size, because its data were lacking. Missing data were not imputed. P<0.05 was considered significant in the adjusted analyses. All statistical analyses were performed using JMP version13.1.0; JMP software (SASInstitute, Cary, NC).
Results
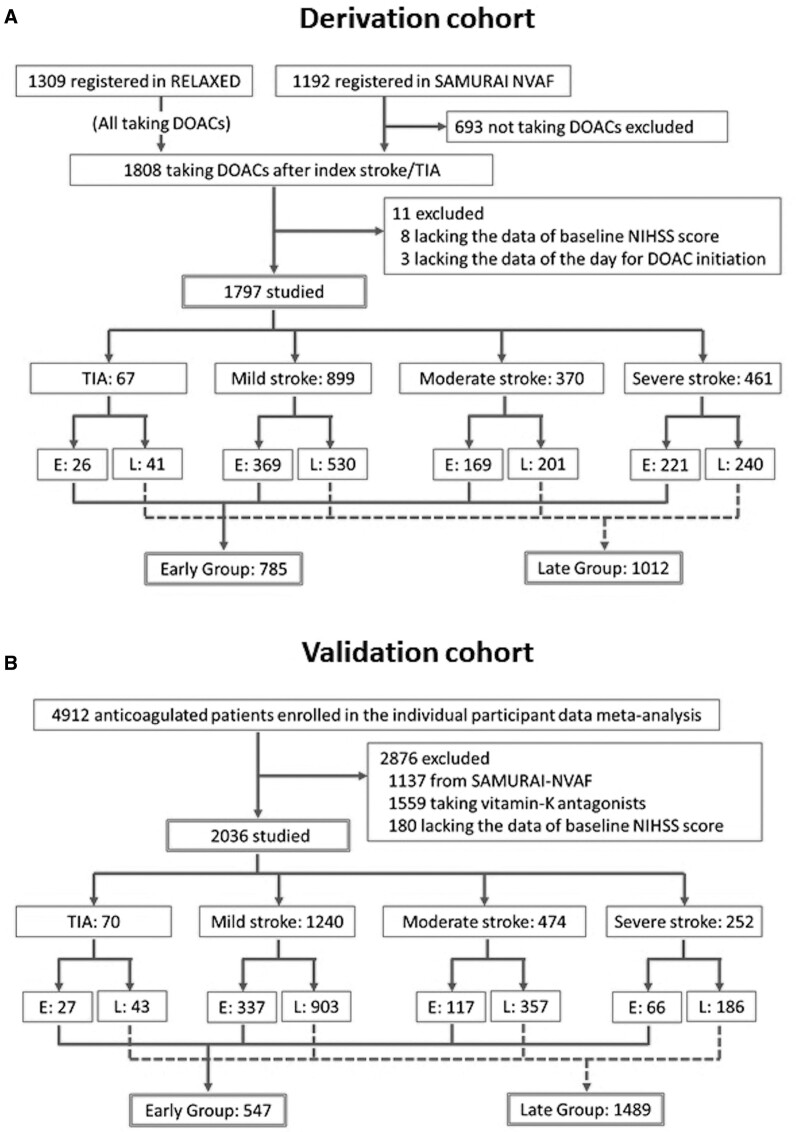
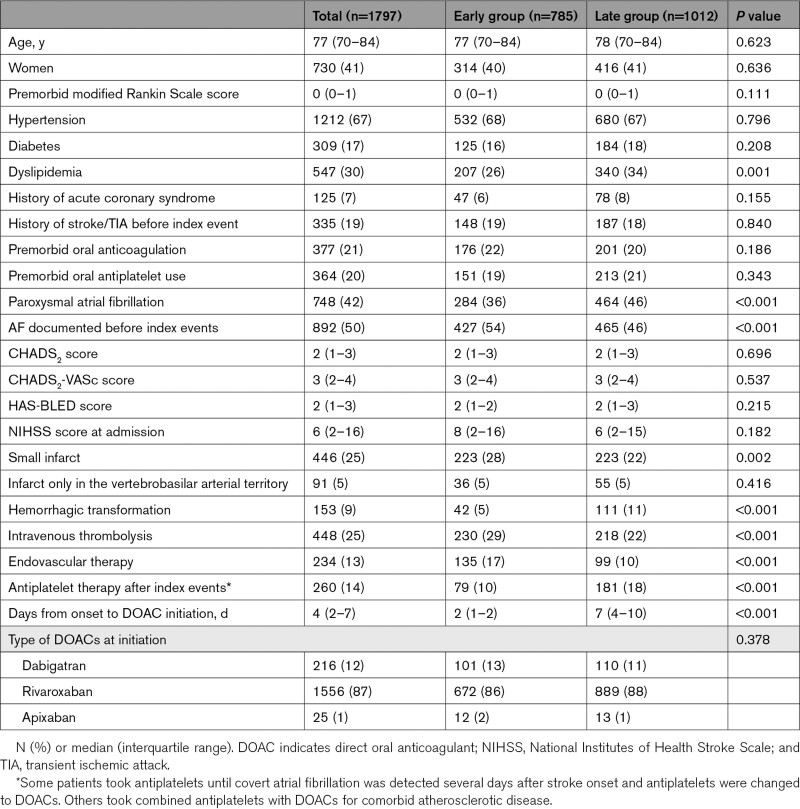
Of the 1192 patients in SAMURAI-NVAF and the 1309 in RELAXED, 1808 started or resumed DOACs after the index IS/TIA (Figure 1A). Of these, 11 patients lacking the essential data were excluded. Finally, 1797 patients were studied (median age of 77 years, 730 women). Table 1 shows the patients’ baseline characteristics. Of these, age and the NIHSS scores were used for the scatter plots to ascertain the similarlity of distribution of the characteristics between the derivation and validation cohorts; their distribution was similar (Figure S1). Sixty-seven patients had TIA, 899 had mild IS, 370 had moderate IS, and 461 had severe IS. Patients’ characteristics of these four subgroups are shown in Table S3.
Figure 1.
Study profile. A, The derivation cohort, (B) the validation cohort. DOAC indicates direct oral anticoagulant; NIHSS, National Institutes of Health Stroke Scale; and TIA, transient ischemic attack.
Table 1.
Baseline Characteristics of Patients in the Derivation Cohort
DOACs were initiated at a median of 4 days (IQR, 2–7 days) after the index IS/TIA overall, 2 days (1–5 days) after TIA, 3 days (2–7 days) after mild IS, 4 days (2–7 days) after moderate IS, and 5 days (2–9 days) after severe IS. Thus, a day before each median day (1, 2, 3, and 4 days, respectively) was the cutoff to separate Early and Late Groups. Twenty-six TIA patients starting DOACs within 1 day, 369 mild IS patients within 2 days, 169 moderate IS patients within 3 days, and 221 severe IS patients within 4 days belonged to Early Group (785 in total) and the remaining 1012 to Late Group. The median delay from onset to DOAC initiation was 2 days (IQR, 1–2 days) in Early Group and 7 days (4–10 days) in Late Group. Dyslipidemia, paroxysmal AF, AF detected after the index event, hemorrhagic transformation, and use of antiplatelet agents after the index event were less common, and small infarcts and performance of intravenous thrombolysis and mechanical thrombectomy were more common in Early Group than Late Group (Table 1).
Analysis of the Derivation Cohort
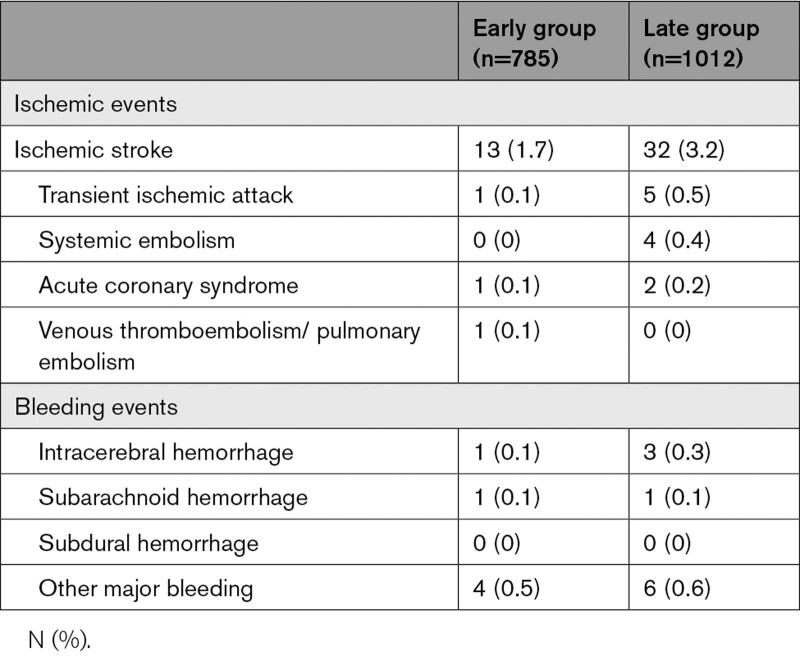
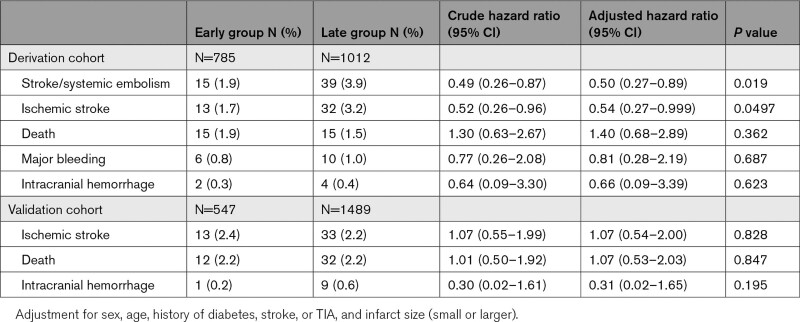
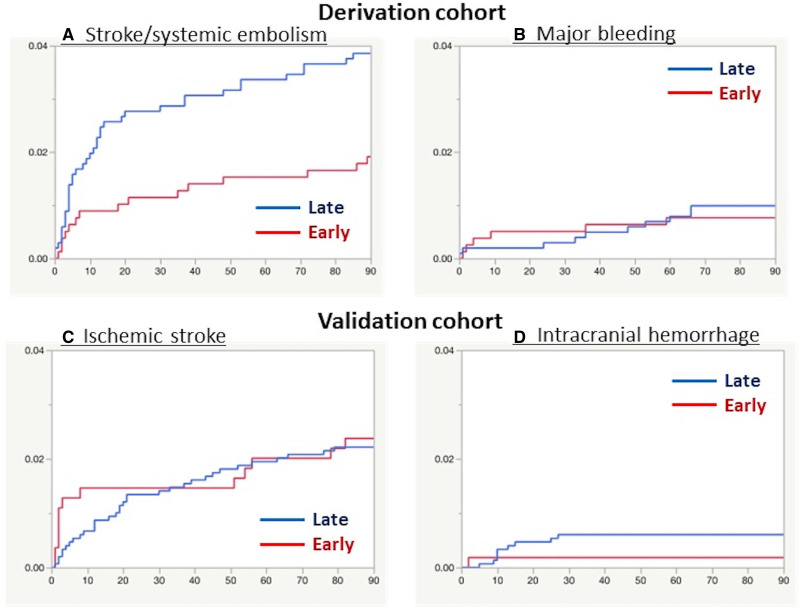
The numbers of ischemic and bleeding events are shown in Table 2. The primary efficacy outcome of stroke/systemic embolism occurred in 15 patients (1.9%) of Early Group and 39 (3.9%) of Late Group (adjusted hazard ratio, 0.50 [95% CI, 0.27–0.89]; Table 3 and Figure 2A). IS occurred in 13 (1.7%) and 32 (3.2%) patients, respectively (0.54 [0.27–0.999]). Fifteen (1.9%) and 15 (1.5%) died, respectively (1.41 [0.68–2.89]). Major bleeding occurred in 6 (0.8%) and 10 (1.0%), respectively (0.81 [0.28–2.19]; Figure 2B). ICH occurred in 2 (0.3%) and 4 (0.4%) patients, respectively (0.66 [0.09–3.39]).
Table 2.
Types of Events in the Derivation Cohort
Table 3.
Efficacy and Safety Outcomes
Figure 2.
Kaplan-Meier analysis of outcomes. The Kaplan-Meier curves for the time to the first event of stroke or systemic embolism (A) and major bleeding (B) for the derivation cohort and that of ischemic stroke (C) and intracranial hemorrhage (D) in the validation cohort are shown. NIHSS indicates National Institutes of Health Stroke Scale.
Outcomes in the 4 subgroups by neurological severity are listed in Table S4. Stroke/systemic embolism occurred in one patient with TIA (0% in Early Group and 2.4% in Late Group), 33 patients with mild IS (2.7% and 4.3%, respectively), 5 patients with moderate IS (0.6% and 2.0%, respectively), and 15 patients with severe IS (1.8% and 4.6%, respectively). Major bleeding occurred in one patient with TIA (0% and 2.4%, respectively), 5 patients with mild IS (0% and 0.9%, respectively), 4 patients with moderate IS (1.8% and 0.5%, respectively), and 6 patients with severe IS (1.4% and 1.3%, respectively).
External Validation
Of the 4912 patients who took oral anticoagulants and were enrolled in the IPD meta-analysis,1 1137 recruited from SAMURAI-NVAF, 1559 who took vitamin-K antagonists, and 180 lacking baseline NIHSS data were excluded. Finally, 2036 patients were studied (median age 78 years, 1039 women, Figure 1B). Patients’ characteristics are shown in Table S5. Both the CHADS2-VASc (median 5 versus 3) and HAS-BLED score (median 3 versus 2) were high relative to the derivation cohort.
Of the 2036 patients, 70 had TIA, 1240 had mild IS, 474 had moderate IS, and 252 had severe IS. DOACs were initiated at a median of 6 days (IQR, 3–12 days) after the index IS/TIA overall, 2 days (IQR, 1–3 days) after TIA, 5 days (IQR, 2–10 days) after mild IS, 8 days (IQR, 4–14 days) after moderate IS, and 8 days (IQR, 4–14 days) after severe IS. Using the same cutoff days between Early and Late Groups as the derivation cohort, 27, 337, 117, and 66 patients in the 4 subgroups, respectively, were assigned to Early Group (547 in total). The remaining 1489 patients belonged to Late Group. The median delay from onset to DOAC initiation was 2 days (IQR, 1–2 days) in Early Group and 8 days (5–14 days) in Late Group.
IS occurred in 13 patients (2.4%) of Early Group and 33 (2.2%) of Late Group (adjusted HR, 1.07 [95% CI, 0.54–2.00]; Figure 2C). Twelve (2.2%) and 32 (2.2%) patients, respectively, died (1.07 [0.53–2.03]). ICH occurred in 1 (0.2%) and 9 (0.6%) patients, respectively (0.31 [0.02–1.65]; Figure 2D). Outcomes are shown by neurological severity in the 4 subgroups in Table S6.
Discussion
In the present study using a combined database from 2 Japanese registries, the optimal timing for initiation of DOACs after NVAF-associated IS/TIA was determined according to neurological severity. The major new finding was that graded increase in delay of anticoagulation between 1 and 4 days after the index IS/TIA according to neurological severity, that is, within 1 day after TIA, within 2 days after mild IS, within 3 days after moderate IS, and within 4 days after severe IS (the so-called 1-2-3-4-day rule) was associated with better efficacy and similar safety compared with later DOAC initiation in Japanese population. The similar safety and similar efficacy of the rule was ascertained by external validation using known European registries.
In the 4 known randomized controlled trials comparing each DOAC and warfarin in NVAF, patients within 14 days after IS/TIA onset (within 7 days in ARISTOTLE) were excluded from the trial participants.25–28 In the 1-3-6-12 day rule, anticoagulation was recommended to start 12 days after onset of severe IS (NIHSS score ≥16) after imaging evaluation of hemorrhagic transformation on the same day.6 The median initiation days after severe IS for both the present derivation (5 days) and validation (8 days) cohorts were considerably earlier than 12 days in the rule. Thus, many Japanese and European researchers seemed to judge that DOAC initiation within 5 to 8 days after severe IS was generally safe. Infarct size is an established predictor for hemorrhagic transformation.14,29,30 In the patients with severe IS from SAMURAI-NVAF, 12% had a small infarct and 56% had a medium-sized infarct that was defined as smaller than one-third of the territory of the middle, anterior, or posterior cerebral artery or cerebellar hemisphere.8 The median infarct size of the severe IS patients from RELAXED was 34.6 cm3.12 For such patients with small to medium-sized infarcts, 12 days would be too long to refrain from anticoagulation fearful of ICH. Figure 2A shows that occurrence of stroke and systemic embolism was particularly common in the initial 1 to 2 weeks. In addition, DOACs are short acting, and their power can be soon decreased after ceasing medication when hemorrhagic transformation occurs. A recent trial set 7 to 9 days for starting apixaban after onset of a medium-sized IS and excluded a large IS from the trial.5 These guides seem to be safe to minimize the risk of ICH but could increase recurrent embolic events within the initial days after IS. Several observational studies and their pooled analyses reported earlier days of DOAC initiation around 7 days or earlier.1–4,7 There are 4 ongoing randomized trials to determine the appropriate timing of DOAC initiation after stroke (TIMING, OPTIMAS, ELAN, and START; URL: https://www.clinicaltrials.gov; Unique identifier: NCT02961348, NCT03759938, NCT03148457, NCT03021928); the former 2 set the day of early DOAC initiation as 4 days or less regardless of neurological severity, and the latter 2 set different times of early DOAC initiation by severity: at 6 days in ELAN and 6 to 21 days in START for severe IS.4 The present setting of comparing events after severe IS before and after 4 days was even earlier than ELAN and START.
This was a nonrandomized study, and patients’ characteristics varied to some extent between Early and Late Groups. A lower percentage of AF documented before the index events in Late Group was a natural reason for the delayed DOAC initiation. It is interesting that Early Group more frequently had patients undergoing acute reperfusion therapies. Post-therapeutic ICH must have been carefully excluded before starting early DOACs since it is an essential complication of reperfusion therapies. One should also note that patients with very high admission NIHSS scores and those with huge size of infarcts were rarely included in either Early or Late Group, since patients with very severe stroke for whom poststroke anticoagulation was regarded to be risky were not enrolled. In addition, warfarin tended to be chosen more frequently than DOACs for severely disturbed patients partly because of economic problems. In SAMURAI-NVAF, 65% of the patients with the discharge modified Rankin Scale score of 3 to 4 and 91% of the patients with the score of 5 who required anticoagulation were treated with warfarin.8
Both the risks of stroke/systemic embolism and IS were halved in Early Group compared with Late Group presumably mainly because of prevention of recurrent thromboembolism within the initial days. In addition, high-risk patients for both ischemia and bleeding that caused us to hesitate in giving early anticoagulation might more commonly have belonged to Late Group, although CHADS2-VASc and HAS-BLED scores were similar between the groups. Mortality was similar between the groups; in SAMURAI-NVAF the leading cause of death was not bleeding or cardiovascular disease but infection.9 Major bleeding occurred in 0.8% to 1.0% and ICH occurred in 0.3% to 0.4% over 90 days in both groups. The prevalence was not as high as compared with a meta-analysis of patients with previous IS/TIA, mainly chronic, from the 3 major randomized trials, where major bleeding occurred in 5.4% and ICH in 0.9% of patients receiving DOACs during median 1.8 to 2.0 years.31
External validation using a large European population with an almost even choice among dabigatran, rivaroxaban, and apixaban resolved the limitation of the derivation cohort to some extent that was composed only of Japanese patients with more frequent use of rivaroxaban than other DOACs. Overall, 27% (547/2036) of the validation patients started DOACs within the 1-2-3-4-day cutoff, and this early group showed a low prevalence of ICH (0.2% versus 0.7% in Late Group). Although both the derivation and validation population were nonrandomized, the present results show that ICH within 3 months after onset in patients with IS/TIA was rare in case of early initiation of DOACs chosen by expert stroke physicians based on their judgment that the early use was not risky.
Some factors listed in the 1-3-6-12 day rule as favoring early or delayed initiation of oral anticoagulation, including cardiac thrombi on ultrasound, necessity for major surgical intervention, hemorrhagic transformation, neurological instability, ageing, and uncontrol of blood pressure were not assessed here,6 because the present purpose was to propose an optimal timing for initiation of DOACs simply only using NIHSS scores. In the real clinical setting, it is essential to determine the timing by adding these factors in consideration. Findings from brain CT or MRI and those from echocardiography would be especially important. Since infarct size is an established predictor for hemorrhagic transformation as stated above,14,29,30 it would be better to determine the timing for starting DOACs based on the combined information of NIHSS scores and infarct size. Anticoagulation should be avoided if parenchymal hemorrhage is identified. In this study, hemorragic transformation was less common in Early Group than Late Group. In SAMURAI-NVAF, DOACs were initiated in median 3 days after onset of small-size infarction (the longest diameter ≤15 mm), 4 days after onset of medium-size infarction, and 6 days after onset of large-size infarction (larger than one-third of the arterial territory).8 In RELAXED, DOACs were initiated in median 2.9 days after onset of small-size infarction (<4.0 cm3), 2.9 days after onset of medium-size infarction (4.0–22.4 cm3), and 5.8 days after onset of large-size infarction (≥22.5 cm3).12 Intracardiac thrombi detected on ultrasound were associated with the risk of recurrent ischemic stroke overall (adjusted HR, 2.35 [95% CI, 1.07–5.16]) in the pooled IPD from the East-Asian Ischemic Stroke Patients with Atrial Fibrillation (EAST-AF) registry and SAMURAI-NVAF.32 Thus, one should consider earlier initiation of anticoagulation than usual once the thrombi was detected. Other echocardiographic findings such as left ventricular dysfunction would be potential factors to consider earlier initiation of DOACs. However, further review will be essential to start DOACs earlier than the 1-2-3-4-day cutoff for patients with thrombi since this cutoff is so early.
The present study has important limitations in addition to the above ones. First, and crucially, all of the studies used for derivation or validation were nonrandomized. Only the patients for whom considered clinically safe were enrolled. The choice of anticoagulants depended on the discretion of the physicians in charge except for RELAXED. Second, the present 1-2-3-4-day rule cannot be applied to patients with very severe stroke for whom poststroke anticoagulation seems to be risky, since such patients were not enrolled in the derivation or validation cohort. Third, low doses of rivaroxaban approved in Japan (15 or 10 mg daily) might affect the prevalence of events although the approval was based on the unique pharmacokinetics in Japanese subjects showing higher rivaroxaban exposure than Whites when using the same dosage.13,33 Fourth, data on edoxaban, another DOAC, were scarce. Other limitations inherent in SAMURAI-NVAF or RELAXED, including low prevalence of events and the effect of combined therapy such as acute reperfusion therapy, heparin-bridging, and concomitant use of antiplatelets, have been described in detail elsewhere.8–10,12
Early anticoagulation after stroke has been a longstanding question needing a balance between the benefit of preventing early recurrent thromboembolism and the risk of triggering ICH. Of note, warfarin even showed a paradoxical increase in the risk of IS in the first 7 days of use probably because of a transient hypercoagulable state caused by deactivation of protein C and protein S.34 DOACs have the major potential advantage of lower intracranial bleeding risk. The present “1-2-3-4-day rule” seem to be feasible in the real-world clinical setting by careful exclusion of patients with factors favoring delayed initiation of anticoagulation such as huge infarcts, hemorrhagic transformation of infarcts, and uncontrolled hypertension.6 However, given the potential for bias and confounding inherent in observational hospital-based studies, data from ongoing randomized trials will be essential to guide any change in clinical practice or guidelines.4
Article Information
Acknowledgments
We thank Dr David J. Seiffge (University Hospital Basel and University of Basel) for his constructive comments.
Sources of Funding
SAMURAI-NVAF was supported in part by a Grant-in-aid (H23-Junkanki-Ippan-010) from the Ministry of Health, Labour and Welfare, Japan, Grants from the Japan Agency for Medical Research and Development (AMED: JP21lk0201094h and JP21lk0201109h), and an Intramural Research Fund (H29-1-1) for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center. RELAXED was planned by the Japan Cardiovascular Research Foundation and funded by Bayer Yakuhin Ltd. (contract No.: 017926). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosures
Dr Toyoda reports personal fees from Daiichi-Sankyo, Bayer Yakuhin, Bristol-Myers-Squibb (BMS), and Takeda. Dr Minematsu reports personal fees from Bayer, Pfizer, and consulting fees from BMS. Dr Yasaka reports personal fees from Daiichi-Sankyo, Bayer Yakuhin, BMS, Boehringer Ingelheim, and CSL Behring. Dr Paciaroni reports personal fees from Sanofi-Aventis, Boehringer Ingelheim, Bayer, BMS, Daiichi-Sankyo, and Pfizer. Dr Werring reports personal fees from Bayer, Alnylam, NovoNordisk, and Portola. Dr Yamagami reports personal fees from Bayer Yakuhin, Stryker, and Daiichi-Sankyo, and research grant from BMS. Dr Nagao reports personal fees from Bayer, Nippon Boehringer Ingelheim, Daiichi-Sankyo, Phizer, and BMS. Yoshimura reports personal fees from Boehringer-Ingelheim, Daiichi-Sankyo, Bayer, Termo, Medtronic, Kaneka Medics, Johnson & Johnson Health Care Systems Inc, Stryker, and BMS. Engelter reports personal fees from Bayer, Pfizer, and grants from Daiichi-Sankyo. Dr Cappellari reports consulting fees from Boehringer-Ingelheim and Pfizer-BMS, and advisory board from Daiichi-Sankyo. Dr Kitazono reports grants from Takeda, Chugai, Daiichi-Sankyo, Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical, Bayer Yakuhin, Boehringer Ingelheim, Torii Pharmaceutical, Otsuka Pharmaceutical, and Taisho Pharma. The other collaborators report no disclosures. Dr Koga reports personal fees from Ono Pharmaceutical, Daiichi-Sankyo, Bayer, and grants from Takeda Pharmaceutical, Daiichi-Sankyo, Nippon Boehringer Ingelheim, Shionogi, Astellas Pharma. The other authors report no conflicts.
Supplemental Material
Tables S1–S6
Figure S1
STROBE reporting guidelines
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CROMIS
- Clinical Relevance of Microbleeds in Stroke study
- DOAC
- direct oral anticoagulant
- ICH
- intracranial hemorrhage
- IPD
- individual participant data
- IQR
- interquartile range
- IS
- ischemic stroke
- NIHSS
- National Institutes of Health Stroke Scale
- NVAF
- nonvalvular atrial fibrillation
- RELAXED
- Recurrent Embolism Lessened by rivaroxaban, an Anti-Xa agent, of Early Dosing for Acute Ischemic Stroke and Transient Ischemic Attack With Atrial Fibrillation
- SAMURAI
- Stroke Acute Management with Urgent Risk-Factor Assessment and Improvement
- TIA
- transient ischemic attack
A list of all SAMURAI, RELAXED, RAF, RAF-NOAC, CROMIS-2, NOACISP LONGTERM, Erlangen registry and Verona registry Investigators is given in the Supplemental Material.
This manuscript was sent to Theresa A. Jones, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.036695.
For Sources of Funding and Disclosures, see page 1548.
Contributor Information
Shunsuke Kimura, Email: kimura.shunsuke@ncvc.go.jp.
Sohei Yoshimura, Email: s-yoshi@hyo-med.ac.jp.
Kazuo Minematsu, Email: kminemat@ncvc.go.jp.
Masahiro Yasaka, Email: yasakamasahiro@gmail.com.
Maurizio Paciaroni, Email: maurizio.paciaroni@unipg.it.
David J. Werring, Email: d.werring@ucl.ac.uk.
Hiroshi Yamagami, Email: yamagami.hiroshi@ncvc.go.jp.
Takehiko Nagao, Email: longtail@nms.ac.jp.
Shinichi Yoshimura, Email: s-yoshi@hyo-med.ac.jp.
Alexandros Polymeris, Email: alexandros.polymeris@usb.ch.
Annaelle Zietz, Email: annaellevalerie.zietz@usb.ch.
Stefan T. Engelter, Email: stefan.engelter@usb.ch.
Bernd Kallmünzer, Email: bernd.kallmuenzer@uk-erlangen.de.
Manuel Cappellari, Email: manuel_cappellari@libero.it.
Tetsuya Chiba, Email: chiba.tetsuya@ncvc.go.jp.
Takeshi Yoshimoto, Email: yoshimototakeshi1982@ncvc.go.jp.
Masayuki Shiozawa, Email: m.sio@ncvc.go.jp.
Takanari Kitazono, Email: kitazono@intmed2.med.kyushu-u.ac.jp.
Masatoshi Koga, Email: koga@ncvc.go.jp.
References
- 1.Seiffge DJ, Paciaroni M, Wilson D, Koga M, Macha K, Cappellari M, Schaedelin S, Shakeshaft C, Takagi M, Tsivgoulis G, et al. ; CROMIS-2, RAF, RAF-DOAC, SAMURAI, NOACISP LONGTERM, Erlangen and Verona registry collaborators. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol. 2019;85:823–834. doi: 10.1002/ana.25489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masotti L, Grifoni E, Dei A, Vannucchi V, Moroni F, Panigada G, Spolveri S, Landini G. Direct oral anticoagulants in the early phase of non valvular atrial fibrillation-related acute ischemic stroke: focus on real life studies. J Thromb Thrombolysis. 2019;47:292–300. doi: 10.1007/s11239-018-1775-2 [DOI] [PubMed] [Google Scholar]
- 3.Xian Y, Xu H, O’Brien EC, Shah S, Thomas L, Pencina MJ, Fonarow GC, Olson DM, Schwamm LH, Bhatt DL, et al. Clinical effectiveness of direct oral anticoagulants vs warfarin in older patients with atrial fibrillation and ischemic stroke: findings from the Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) Study. JAMA Neurol. 2019;76:1192–1202. doi: 10.1001/jamaneurol.2019.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, Milling TJ, Engelter ST, Fischer U, Norrving B. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. 2019;18:117–126. doi: 10.1016/S1474-4422(18)30356-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labovitz AJ, Rose DZ, Fradley MG, Meriwether JN, Renati S, Martin R, Kasprowicz T, Murtagh R, Kip K, Beckie TM, et al. ; AREST Investigators. Early apixaban use following stroke in patients with atrial fibrillation: results of the AREST Trial. Stroke. 2021;52:1164–1171. doi: 10.1161/STROKEAHA.120.030042 [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 7.Mizoguchi T, Tanaka K, Toyoda K, Yoshimura S, Itabashi R, Takagi M, Todo K, Shiozawa M, Yagita Y, Yoshimoto T, et al. ; SAMURAI Study Investigators. Early initiation of direct oral anticoagulants after onset of stroke and short- and long-term outcomes of patients with nonvalvular Atrial Fibrillation. Stroke. 2020;51:883–891. doi: 10.1161/STROKEAHA.119.028118 [DOI] [PubMed] [Google Scholar]
- 8.Toyoda K, Arihiro S, Todo K, Yamagami H, Kimura K, Furui E, Terasaki T, Shiokawa Y, Kamiyama K, Takizawa S, et al. ; SAMURAI Study Investigators. Trends in oral anticoagulant choice for acute stroke patients with nonvalvular atrial fibrillation in Japan: the SAMURAI-NVAF study. Int J Stroke. 2015;10:836–842. doi: 10.1111/ijs.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arihiro S, Todo K, Koga M, Furui E, Kinoshita N, Kimura K, Yamagami H, Terasaki T, Yoshimura S, Shiokawa Y, et al. ; SAMURAI Study Investigators. Three-month risk-benefit profile of anticoagulation after stroke with atrial fibrillation: The SAMURAI-Nonvalvular Atrial Fibrillation (NVAF) study. Int J Stroke. 2016;11:565–574. doi: 10.1177/1747493016632239 [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura S, Koga M, Sato S, Todo K, Yamagami H, Kumamoto M, Itabashi R, Terasaki T, Kimura K, Yagita Y, et al. ; SAMURAI Study Investigators. Two-Year outcomes of anticoagulation for acute ischemic stroke with nonvalvular atrial fibrillation□- SAMURAI-NVAF Study. Circ J. 2018;82:1935–1942. doi: 10.1253/circj.CJ-18-0067 [DOI] [PubMed] [Google Scholar]
- 11.Yasaka M, Minematsu K, Toyoda K, Yamagami H, Yoshimura S, Nagao T, Mori E, Hirano T, Hamasaki T, Yamaguchi T. Design and Rationale of the RELAXED (Recurrent Embolism Lessened by rivaroxaban, an Anti-Xa agent, of Early Dosing for acute ischemic stroke and transient ischemic attack with atrial fibrillation) Study. J Stroke Cerebrovasc Dis. 2016;25:1342–1348. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.035 [DOI] [PubMed] [Google Scholar]
- 12.Yasaka M, Minematsu K, Toyoda K, Mori E, Hirano T, Hamasaki T, Yamagami H, Nagao T, Yoshimura S, Uchiyama S; RELAXED study group. Rivaroxaban administration after acute ischemic stroke: The RELAXED study. PLoS One. 2019;14:e0212354. doi: 10.1371/journal.pone.0212354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, Izumi T, Koretsune Y, Kajikawa M, Kato M, et al. ; J-ROCKET AF study investigators. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454 [DOI] [PubMed] [Google Scholar]
- 14.Paciaroni M, Agnelli G, Falocci N, Caso V, Becattini C, Marcheselli S, Rueckert C, Pezzini A, Poli L, Padovani A, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: The RAF Study. Stroke. 2015;46:2175–2182. doi: 10.1161/STROKEAHA.115.008891 [DOI] [PubMed] [Google Scholar]
- 15.Paciaroni M, Agnelli G, Falocci N, Tsivgoulis G, Vadikolias K, Liantinioti C, Chondrogianni M, Bovi P, Carletti M, Cappellari M, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with Non-Vitamin-K Oral Anticoagulants (RAF-NOACs) Study. J Am Heart Assoc. 2017;6:e007034. doi: 10.1161/JAHA.117.007034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charidimou A, Wilson D, Shakeshaft C, Ambler G, White M, Cohen H, Yousry T, Al-Shahi Salman R, Lip G, Houlden H, et al. The Clinical Relevance of Microbleeds in Stroke study (CROMIS-2): rationale, design, and methods. Int J Stroke. 2015;10 (Suppl A100):155–161. doi: 10.1111/ijs.12569 [DOI] [PubMed] [Google Scholar]
- 17.Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R, Lip GYH, Cohen H, Banerjee G, Houlden H, et al. ; CROMIS-2 collaborators. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol. 2018;17:539–547. doi: 10.1016/S1474-4422(18)30145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiffge DJ, Traenka C, Polymeris A, Hert L, Peters N, Lyrer P, Engelter ST, Bonati LH, De Marchis GM. Early start of DOAC after ischemic stroke: Risk of intracranial hemorrhage and recurrent events. Neurology. 2016;87:1856–1862. doi: 10.1212/WNL.0000000000003283 [DOI] [PubMed] [Google Scholar]
- 19.Cappellari M, Carletti M, Danese A, Bovi P. Early introduction of direct oral anticoagulants in cardioembolic stroke patients with non-valvular atrial fibrillation. J Thromb Thrombolysis. 2016;42:393–398. doi: 10.1007/s11239-016-1393-9 [DOI] [PubMed] [Google Scholar]
- 20.Macha K, Volbers B, Bobinger T, Kurka N, Breuer L, Huttner HB, Schwab S, Köhrmann M. Early initiation of anticoagulation with direct oral anticoagulants in patients after transient ischemic attack or ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:2317–2321. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.031 [DOI] [PubMed] [Google Scholar]
- 21.Seiffge DJ, De Marchis GM, Koga M, Paciaroni M, Wilson D, Cappellari M, Macha Md K, Tsivgoulis G, Ambler G, Arihiro S, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. 2020;87:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappellari M, Seiffge DJ, Koga M, Paciaroni M, Forlivesi S, Turcato G, Bovi P, Yoshimura S, Tanaka K, Shiozawa M, et al. ; SAMURAI-NVAF, RAF-NOAC, NOACISP LONG-TERM, and Verona Study Groups. A nomogram to predict unfavourable outcome in patients receiving oral anticoagulants for atrial fibrillation after stroke. Eur Stroke J. 2020;5:384–393. doi: 10.1177/2396987320945840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezekowitz MD, Connolly S, Parekh A, Reilly PA, Varrone J, Wang S, Oldgren J, Themeles E, Wallentin L, Yusuf S. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805.e1–10, 810.e1. doi: 10.1016/j.ahj.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 24.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 25.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. ; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 26.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. ; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 27.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, et al. ; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 28.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, et al. ; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 29.Lodder J. CT-detected hemorrhagic infarction; relation with the size of the infarct, and the presence of midline shift. Acta Neurol Scand. 1984;70:329–335. doi: 10.1111/j.1600-0404.1984.tb00833.x [DOI] [PubMed] [Google Scholar]
- 30.Okada Y, Yamaguchi T, Minematsu K, Miyashita T, Sawada T, Sadoshima S, Fujishima M, Omae T. Hemorrhagic transformation in cerebral embolism. Stroke. 1989;20:598–603. doi: 10.1161/01.str.20.5.598 [DOI] [PubMed] [Google Scholar]
- 31.Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298–3304. doi: 10.1161/STROKEAHA.112.673558 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Koga M, Lee KJ, Kim BJ, Mizoguchi T, Park EL, Lee J, Yoshimura S, Cha JK, Lee BC, et al. ; CRCS-K Investigators, the SAMURAI Study Investigators. Transesophageal echocardiography in ischemic stroke with atrial fibrillation. J Am Heart Assoc. 2021;10:e022242. doi: 10.1161/JAHA.121.022242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaneko M, Tanigawa T, Hashizume K, Kajikawa M, Tajiri M, Mueck W. Confirmation of model-based dose selection for a Japanese phase III study of rivaroxaban in non-valvular atrial fibrillation patients. Drug Metab Pharmacokinet. 2013;28:321–331. doi: 10.2133/dmpk.dmpk-12-rg-109 [DOI] [PubMed] [Google Scholar]
- 34.Azoulay L, Dell’Aniello S, Simon TA, Renoux C, Suissa S. Initiation of warfarin in patients with atrial fibrillation: early effects on ischaemic strokes. Eur Heart J. 2014;35:1881–1887. doi: 10.1093/eurheartj/eht499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data (IPD) of the derivation cohorts are available from the principal investigator of SAMURAI-NVAF (Toyoda) and RELAXED (Recurrent Embolism Lessened by rivaroxaban, an Anti-Xa agent, of Early Dosing for Acute Ischemic Stroke and Transient Ischemic Attack With Atrial Fibrillation: Minematsu) on reasonable request.