Abstract
South Asians (SAs) account for a quarter of the world’s population and are one of the fastest-growing immigrant groups in the United States (US). South Asian Immigrants (SAIs) are disproportionately more at risk of developing cardiovascular disease (CVD) than other ethnic/racial groups. Atherosclerosis is a chronic inflammatory disorder and is the major cause of CVD. Traditional CVD risk factors, though important, do not fully explain the elevated risk of CVD in SAIs. High-density lipoproteins (HDLs) are heterogeneous lipoproteins that modify their composition and functionality depending on physiological or pathological conditions. With its cholesterol efflux, anti-inflammatory, and antioxidant functions, HDL is traditionally considered a protective factor for CVD. However, its functions can be compromised under pathological conditions, such as chronic inflammation, making it dysfunctional (Dys-HDL). SAIs have a high prevalence of type 2 diabetes and metabolic syndrome, which may further promote Dys-HDL. This review explores the potential association between Dys-HDL and CVD in SAIs and presents current literature discussing the role of Dys-HDL in CVD.
Keywords: South Asians, South Asian Immigrants, Cardiovascular disease, Dysfunctional HDL, Dyslipidemia
Introduction
South Asians (SAs) account for a quarter of the world’s population and represent the largest non-white ethnic group in the United Kingdom and Canada, constituting approximately 5% of each country’s total population [1–3]. South Asian Immigrants (SAIs) are individuals originating from the Indian subcontinent (India, Pakistan, Bangladesh, Nepal, the Maldives, Bhutan, and Sri Lanka) [4]. In the United States (US), the 3.5 million SAIs are the second-largest immigrant group, representing 8.2% of the migrant population [5]. The SA American community grew nearly 40% from 2010 to 2017 [6]. Additionally, there was a 72% increase in undocumented SA Indian Americans between 2010 and 2017 [7]. With the rapidly increasing SAI population in the Western world, there is an exigent need to closely explore the health issues and monitor disease prevalence.
The death toll due to cardiovascular disease (CVD), including coronary artery disease (CAD), remains high, even in nations with advanced medical care systems. CVD is the leading cause of mortality in both developed and developing countries and remains a common cause of mortality in the US during the COVID-19 pandemic [8, 9]. Unfortunately, SAs have higher rates of CVD and experience adverse effects related to CVD at younger ages (< 40 years) compared to other ethnic groups [10–14]. According to the INTERHEART study, deaths due to acute myocardial infarction (AMI) in SAs occur 5–10 years earlier than in western populations. Also, SA men encountering AMI were 5.6 years younger than women [13]. Studies examining CVD prevalence in SAs in the US have found a three times higher risk for this group than the national average [15]. A Norwegian study comparing CVD risk factors between SAIs, ethnic Norwegians, and other immigrants indicated the highest risk of CVD belonged to SAIs [16]. In an analysis of 10.4 million US deaths, the mortality burden due to CVD as determined by proportional mortality ratio (PMR) was the highest among Asian Indian men and women [17]. Another study conducted in California also showed the highest PMR among Indian men and women compared to five other ethnic groups [18]. The INTERHEART study showed traditional risk factors account for 75% of CVD [19]. Although hypertension, age, obesity, type 2 diabetes (T2D), and dyslipidemia may partly explain CVD burden, current studies do not explain why SAIs experience increased prevalence and worse outcomes than other populations. Even after adjustment for traditional risk factors, SAs are associated with an increased risk of CVD outcomes [20]. Thus, there is an urgent need to explore additional ethnicity-specific factors further to explain disease prevalence.
High-density lipoprotein (HDL) has been known for its protective effects against CVD and is traditionally considered a marker for risk prediction [21–23]. The CVD protection by HDL is through several functions, including cholesterol efflux, anti-inflammatory, antioxidant, antithrombotic, and antiapoptotic properties [24, 25]. However, HDL may lose its anti-atherosclerotic properties and instead develop pro-inflammatory functions, leading to the formation of dysfunctional HDL (Dys-HDL) [26–28]. Thus, HDL quantity and functionality are not necessarily related, which calls for further exploration of the role of HDL functionality in contributing to CVD [22]. Because one-third of SAs have low levels of HDL (< 40 mg/dL) and are prone to developing CVD, more research needs to be done within this population to understand other possible differences in HDL, such as size, anti-inflammatory function, and genetic variability [14] [29].
Additionally, risk scoring methods, traditionally used to assess CVD risk in Caucasians, do not accurately estimate risk in SAIs. These scoring methods are likely to underestimate or overestimate the SAI population’s scores [30]. Furthermore, current treatment recommendations for CVD, such as medication and exercise, tend to follow western guidelines [29]. According to general guidelines, the standard low-density lipoprotein (LDL) lowering therapies may not always improve outcomes in this ethnic group as these levels may be “normal” [31]. Thus, the current management of CVD risk remains unclear in SAIs. In this review, we discuss the potential role of Dys-HDL in explaining the high prevalence of CVD in SAIs and explore existing literature discussing this possible association.
Roles of HDL in Protecting Against CVD
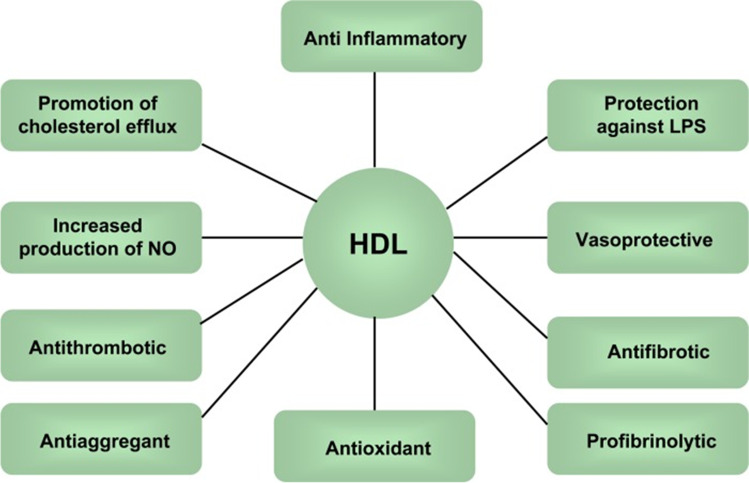
HDL has traditionally been shown to have CVD protective effects [23, 32, 33] (Fig. 1). HDL has a reverse cholesterol efflux function, which allows the particle to maintain cholesterol homeostasis. The process of cholesterol efflux is when excess cholesterol is transferred from cells, mainly macrophages, to HDL particles, which helps promote atherosclerotic plaque regression [34]. HDL carries out this function primarily through the ATP Binding Cassette Transporter A1 (ABCA1), which promotes plaque regression and reduces plaque stability [35–37] (Fig. 2). Furthermore, HDL impedes oxidative changes in LDL, preventing LDL-induced monocyte adherence and eventual plaque formation [23]. HDL particles also have a vasodilatory effect on vascular endothelium in hypercholesterolemic men [36]. This vasodilatory effect is explained by the ability of HDL particles to induce the phosphorylation of endothelial nitric oxide synthase (eNOS) and thus stimulate subsequent nitric oxide production. In addition to cholesterol efflux and anti-oxidative effects, HDL particles also have anti-inflammatory effects that decrease adhesion molecules on endothelial cells. Specifically, HDL may lower the expression of vascular cell adhesion molecule 1 (VCAM-1) in plaques. These particles may also inhibit chemokines’ expression, promoting monocyte infiltration through direct disturbance of transcription factors [23]. These anti-atherogenic effects on the molecular level may contribute to clinical health outcomes and CVD risk.
Fig. 1.
Protective effects of HDL against CVD (Abbreviations: HDL: high-density lipoprotein; NO: nitric oxide; LPS: lipopolysaccharide; figure derived from [38]) [38]
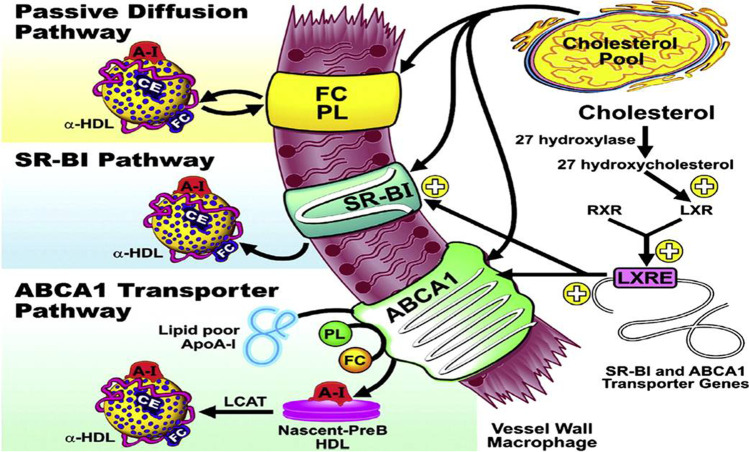
Fig. 2.
Schematic diagram of the pathways involved with HDL-mediated cholesterol efflux: passive diffusion of FC from the macrophage with subsequent esterification by LCAT; transport of cholesterol to HDL particles via the SR-BI transporter on the vessel surface; ABCA1 transporter on the macrophages which mobilizes free cholesterol and phospholipids to lipid-poor ApoA1; cholesterol from macrophages and other tissues are stored in mature particles known as alpha-HDL; LXRs are nuclear receptors induced in response to elevated levels of cholesterol; Excess cellular cholesterol can be converted to 27 hydroxycholesterol via the 27 hydroxylase enzyme, which binds to LXR (after dimerizing to RXR); LXR-RXR dimer can then bind the LXRE promoter element and increase the expression of the ABCA1 and SR-BI transporters; the presence of PL enables HDL to solubilize and transport unesterified FC released from cells (Abbreviations: SR-BI: Scavenger Receptor Class B Type I, ABCA1: Adenosine Triphosphate-Binding Cassette Transporter A1; FC: free cholesterol; LCAT: Lecithin Cholesterol Acyltransferase; LXR: Liver X Receptor; RXR: Retinoic X Receptor; LXRE: LXR Response Element; PL: phospholipid; figure derived from [39]) [39–41]
Pathophysiology of Dys-HDL
Although HDL has been linked with decreased CVD risk, merely increasing HDL levels may not significantly reduce CVD events. For instance, niacin significantly increases HDL by 20–25% but fails to demonstrate any long-term benefit for the prevention of CVD events in randomized control trials [42]. Failure of similar therapies that increase HDL levels called for further research into the molecular pathways that affect HDL functionality [32]. Lipid peroxidation products such as myeloperoxidase or malondialdehyde may impair HDL cholesterol efflux capacity and lead to Dys-HDL formation, which becomes ineffective as an anti-inflammatory agent and appears to be pro-inflammatory [21, 27]. The pro-inflammatory effects may be explained by altered protein composition, containing increased levels of serum amyloid A and ceruloplasmin, proteins known to be active in acute-phase inflammatory reactions [26]. This points to the possibility that the intrinsic anti-atherosclerotic abilities of the HDL particles that are present may be more important than the sheer amount of HDL in the body.
Another possible theory for HDL to become dysfunctional is the alteration of ApoA1. ApoA1 is the main protein responsible for maintaining the antioxidant function of HDL [43]. Dodani et al. identified six novel Single Nucleotide Polymorphisms (SNPs) of APOA1 in SAIs with sub-clinical CAD: three were correlated with metabolic syndrome (MS) and one with low HDL levels [11, 44] (see the “Genetics” section). These polymorphisms may relate to Dys-HDL in SAIs, predisposing them to CAD risk [43]. Dys-HDL inhibits HDL-associated antioxidant enzymes, thus blocking ApoA1’s effects of promoting cholesterol efflux. As a result, this increases the formation of LDL-derived oxidized lipids [23]. A small cross-sectional study between healthy subjects and stable CAD patients indicated that HDL in healthy patients inhibited endothelial superoxide production, increased eNOS mechanistic pathways, and promoted nitric oxide production for endothelial repair. These characteristics were absent in CAD patients [21]. In addition to functional changes, structural properties may also play a role in causing Dys-HDL.
HDL size may contribute to CVD risk as larger HDL particles are associated with cardioprotective effects, whereas smaller HDL particles are linked to decreased cardiac protection [45, 46]. A clinical trial measuring lipid parameters was conducted between Asian Indian men and Caucasian men from the Framingham Offspring Study. Results indicated that despite HDL concentrations being similar between the two groups, there were increased concentrations of small HDL particles compared to large HDL particles in Asian Indian men [47]. Thus, HDL size may have a direct effect on lipid metabolism. Larger HDL particles, particularly a subfraction of HDL known as HDL2b, are more efficient in reverse cholesterol transport [48]. Studies show that levels of HDL2b are lower among Asian Indians compared to non-Asian Indians, suggesting impaired reverse cholesterol transport in SAs [48]. Inefficient cholesterol efflux due to small HDL size may potentially explain why SAs experience a weaker association between CVD and HDL levels compared to other ethnicities.
Dys-HDL in SAIs
The number of clinical studies exploring the association between Dys-HDL and CVD in SAIs is limited. One cross-sectional study used the HDL inflammatory index (HII) to look at 30 SAIs and determined that 50% had Dys-HDL (HII ≥ 1.00) [28, 49]. The study also used Carotid Intima-Media Thickness (CIMT) as a surrogate for atherosclerotic disease and found 41.4% of subjects had evidence of sub-clinical CAD (CIMT ≥ 0.80 mm). Notably, the participants selected did not have clinical CAD but were considered high risk. Among the subjects, 42.9% of SAIs with ≥ 40 mg/dL of HDL had Dys-HDL compared to 7.1% with ≤ 40 mg/dL [28]. Another study conducted on 130 first-generation SAIs showed that Dys-HDL, as determined by the HII, was significantly correlated with CIMT after adjusting for age, hypertension, and family history of CVD [11]. Thus, these studies further demonstrate that HDL levels alone may not be the best predictor for atherosclerotic disease and could be used in conjunction with functionality to better predict future risk [11, 50].
The functionality of HDL particles may differ among SAs compared to other ethnic groups. One study compared HDL functions in SAIs to Caucasian subjects in three different age groups (neonates, adolescents, adults). After subjecting the adult group to a high-calorie diet, the anti-oxidative capacity of HDL was impaired in SAs compared to their Caucasian counterparts [51]. In addition, anti-inflammatory capacity was impaired among SA neonates and was affected by an 8-day low-calorie diet in adult SAs only. Cholesterol efflux capacity was similar between both ethnicities, but HDL’s ability to prevent LDL oxidation was impaired in adult SAI males compared to Caucasian males [51]. These results suggest that even if the HDL’s cholesterol efflux capacity is unaffected, certain ethnic groups, such as SAIs, may have impairment in other HDL functions, which can lead to disease progression. This concept of Dys-HDL may explain the higher risk of CVD seen in this ethnic group, and thus, it may be a significant risk factor to consider in clinical therapy for these patients. However, studies with larger sample sizes are needed to better delineate the causes of Dys-HDL to demonstrate its clinical utility.
Role of Metabolic Syndrome in Dys-HDL
The underlying factors that lead to CVD also contribute to the high prevalence of metabolic disorders such as MS and T2D [44, 52, 53]. For instance, determinants of T2D and MS include insulin resistance and clustering of other pro-atherogenic factors due to lifestyle shifts, urbanization, diet, and possibly genetics. A randomized population-based study of SAIs in the US showed a T2D prevalence of 17.4%, which was significantly higher when compared to Hispanic whites (7.8%) and non-Hispanic blacks (13%) [54]. A study has also indicated high glucose intolerance and decreased beta-cell function compared to other ethnic groups [55]. In a sample of 129 adult SAIs in the US, there was an MS prevalence of ~ 29.7% (≥ 35 years old), which represents a significant proportion of the population [44]. At the same body mass index, SAs have a more pronounced atherogenic lipoprotein profile and dysglycemia compared to Caucasians [29, 56, 57]. Unfortunately, the coexistence of T2D, MS, and dyslipidemia likely worsens the CVD risk, as many of the risk factors overlap between these conditions [58].
Recent literature suggests that hyperglycemia and insulin resistance seen in T2D may play a role in Dys-HDL. T2D leads to significant oxidative stress, which reduces the antioxidative capacity of HDL particles. Loss of suppressive effects of insulin on lipolysis leads to the buildup of free fatty acids, which promotes the secretion of VLDL from the liver resulting in hypertriglyceridemia [59]. Triglycerides are transported to both HDL and LDL through cholesteryl ester transfer proteins (CETP) resulting in greater clearance of triglyceride enriched HDL [59]. Insulin resistance also causes ApoA1 to undergo conformational change through non-enzymatic glycation, forming unstable particles [26, 60, 61]. This instability also contributes to rapid HDL clearance from the circulation, leading to an overall decrease in HDL.
Genetics
The genetic sequence variation of SAs is poorly understood and limits the development of a comprehensive map for genes involved in metabolism. Whole-genome sequencing conducted in 2013 on 168 SAs indicated variations in genes related to core metabolic properties, including lipid metabolism, insulin signaling, and T2D [62]. One of these genes encodes a protein product called Apolipoprotein H, which is a component of several circulating lipoproteins. Variations in this gene are involved in LDL and triglyceride metabolism [63]. Other genes include insulin growth factors (IGF1 and IGF2) and LYN genes, which play a role in insulin receptor activation and glucose utilization, respectively [53]. These genes, which initially may be protective, become deleterious in changing environments, such as migration to westernized societies [62]. Additionally, a 25-base pair deletion in MYBPC3, a gene encoding cardiac myosin binding protein C, is unique to only 4% of SAs and indicates a sevenfold increase in cardiomyopathy [64]. Thus, genetic variations in SAIs are an important factor in disease prevalence.
Several studies indicate the crucial role that the APOA1 gene may play in CVD. A small cross-sectional study on SAIs explored six SNPs located on non-coding regions of the APOA1 gene. Results indicated that three (G2, G3, and G5) of the six APOA1 SNPs were strongly associated with MS, a primary risk factor for T2D and CVD. However, less is known about which of the three interferes with normal HDL function [11]. DNA sequencing on seven SNPs on the APOA1 gene from 94 SAIs showed that several polymorphisms are common among this ethnic group and almost always occur together. Specifically, SNPs T655C, T756C, and T1001C were found to be different from those reported in European Caucasians. This genetic variability of the APOA1 gene may contribute to decreased levels of HDL, which may explain why SAs are disproportionately affected [43]. However, further exploration is needed to delineate the association between genetic factors and Dys-HDL.
Conclusion
The SAI population is unique in that it faces a disproportionately higher risk for CVD and requires special attention. Although traditional risk factors, such as hypertension, obesity, diabetes, age, and metabolic derangements, may contribute to CVD development, these parameters do not fully explain the excess CVD risk in this specific population. Dyslipidemia, characterized by elevated triglycerides, low HDL, and high LDL, is conventionally used to monitor CVD health in clinical practice. However, there is a need to also look at lipid functionality. We need to understand nontraditional risk factors extensively and develop new methods to accurately measure and estimate CVD risk in this population. The traditional understanding of HDL as “good cholesterol” must be revisited as recent literature suggests quantity and function are independent, and simply raising HDL levels may not lower CVD risk. Understanding the underlying causes of CVD in SAIs remains a topic of interest to ensure culturally appropriate medical health services are delivered.
This review suggests that Dys-HDL may contribute to CVD in SAIs as its pro-inflammatory effects render HDL’s antioxidant properties inactive, thereby causing a cascade of events that ultimately impair normal HDL function. Although the pathophysiology of Dys-HDL is described in the literature, more work is needed to study its role among specific ethnic groups, specifically SAIs. Thus far, clinical trials involving Dys-HDL and SAIs are limited and include a small sample size. Dys-HDL’s use as a prognostic biomarker may be warranted to predict future disease and yield better patient outcomes. Further research with larger sample sizes is needed to strengthen the power of unconventional risk factors such as Dys-HDL in predicting CVD in vulnerable populations. Additionally, the SAI group is so diverse that the sample needs to be carefully selected to reflect this heterogeneous population. Understanding the implication of Dys-HDL in SAIs may lead to improved diagnostic and therapeutic approaches to CVD.
Author Contribution
Rohan Dod and Aishwarya Rajendran contributed to performing the literature search, drafting the manuscript, and making draft revisions. Mayuri Kathrotia prepared and made manuscript draft revisions. Amanda Clarke edited and formatted the manuscript. Sunita Dodani contributed to the conception of the review, provided expertise in cardiovascular disease, and made critical revisions to the manuscript. All authors have read and agreed with the content of the manuscript.
Declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.A demographic snapshot of South Asians in the United States. SAALT. 2015. http://saalt.org/wp-content/uploads/2016/01/Demographic-Snapshot-updated_Dec-2015.pdf. Accessed 21 Oct 2021.
- 2.Ethnicity and national identity in England and Wales. UK Office for National Statistics. 2011. https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11#background-notes. Accessed 21 Oct 2021.
- 3.Statistics Canada. Immigration and ethnocultural diversity in Canada: National Household Survey. 2011. http://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-010-x/99-010-x2011001-eng.cfm#a3. Accessed 21 Oct 2021.
- 4.Central Intelligence Agency. The World Fact Book. 2017. https://www.cia.gov/library/publications/the-world-factbook/geos/in.html. Accessed 22 Oct 2021.
- 5.Key findings about U.S. immigrants. Pew Research Center. 2020. https://www.pewresearch.org/fact-tank/2020/08/20/key-findings-about-u-s-immigrants/. Accessed 22 Oct 2021.
- 6.Demographic snapshot of South Asians in the United States. SAALT. 2019. https://saalt.org/wp-content/uploads/2019/04/SAALT-Demographic-Snapshot-2019.pdf. Accessed 23 Oct 2021.
- 7.Warren R. US undocumented population continued to fall from 2016 to 2017 and visa overstays significantly exceeded illegal crossings for the seventh consecutive year. Journal on Migration and Human Security. 2019;7(1):19–22. doi: 10.1177/2331502419830339. [DOI] [Google Scholar]
- 8.Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. doi: 10.1016/s0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pareek M, Bangash MN, Pareek N, Pan D, Sze S, Minhas JS, et al. Ethnicity and COVID-19: an urgent public health research priority. Lancet (London, England) 2020;395(10234):1421–1422. doi: 10.1016/S0140-6736(20)30922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volgman AS, Palaniappan LS, Aggarwal NT, Gupta M, Khandelwal A, Krishnan AV, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation. 2018;138(1):e1–e34. doi: 10.1161/CIR.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 11.Dodani S, Henkhaus R, Dong L, Butler MG. Apo lipoprotein A1 gene polymorphisms predict cardio-metabolic risk in South Asian immigrants. Dis Markers. 2012;32(1):9–19. doi: 10.3233/DMA-2012-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed ST, Rehman H, Akeroyd JM, Alam M, Shah T, Kalra A, et al. Premature coronary heart disease in South Asians: burden and determinants. Curr Atheroscler Rep. 2018;20(1):6. doi: 10.1007/s11883-018-0706-1. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet. 2004;364(9438):937–952. doi: 10.1016/s0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 14.Karthikeyan G, Teo KK, Islam S, McQueen MJ, Pais P, Wang X, et al. Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: an analysis from the INTERHEART Study. J Am Coll Cardiol. 2009;53(3):244–253. doi: 10.1016/j.jacc.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Ardeshna DR, Bob-Manuel T, Nanda A, Sharma A, Skelton WPt, Skelton M, et al. Asian-Indians: a review of coronary artery disease in this understudied cohort in the United States. Ann Transl Med. 2018;6(1):12. doi: 10.21037/atm.2017.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabanal KS, Selmer RM, Igland J, Tell GS, Meyer HE. Ethnic inequalities in acute myocardial infarction and stroke rates in Norway 1994–2009: a nationwide cohort study (CVDNOR) BMC Public Health. 2015;15:1073. doi: 10.1186/s12889-015-2412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jose PO, Frank ATH, Kapphahn KI, Goldstein BA, Eggleston K, Hastings KG, et al. Cardiovascular disease mortality in Asian Americans. J Am Coll Cardiol. 2014;64(23):2486–2494. doi: 10.1016/j.jacc.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palaniappan L, Wang Y, Fortmann SP. Coronary heart disease mortality for six ethnic groups in California, 1990–2000. Ann Epidemiol. 2004;14(7):499–506. doi: 10.1016/j.annepidem.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Vilahur G, Badimon JJ, Bugiardini R, Badimon L. Perspectives: the burden of cardiovascular risk factors and coronary heart disease in Europe and worldwide. European Heart Journal Supplements. 2014;16(suppl_A):A7–A11. doi: 10.1093/eurheartj/sut003. [DOI] [Google Scholar]
- 20.Pursnani S, Merchant M. South Asian ethnicity as a risk factor for coronary heart disease. Atherosclerosis. 2020;315:126–130. doi: 10.1016/j.atherosclerosis.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121(7):2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovingh GK, Rader DJ, Hegele RA. HDL re-examined. Curr Opin Lipidol. 2015;26(2):127–132. doi: 10.1097/MOL.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 23.Annema W, von Eckardstein A. High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ J. 2013;77(10):2432–48. doi: 10.1253/circj.cj-13-1025. [DOI] [PubMed] [Google Scholar]
- 24.Salazar J, Olivar LC, Ramos E, Chávez-Castillo M, Rojas J, Bermúdez V. Dysfunctional high-density lipoprotein: an innovative target for proteomics and lipidomics. Cholesterol. 2015;2015:296417. doi: 10.1155/2015/296417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serban C, Muntean D, Mikhailids DP, Toth PP, Banach M. Dysfunctional HDL: the journey from savior to slayer. Clinical Lipidology. 2014;9(1):49–59. doi: 10.2217/clp.13.83. [DOI] [Google Scholar]
- 26.Rosenson RS, Brewer HB, Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13(1):48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodani S, Dong L, Guirgis FW, Reddy ST. Carotid intima media thickness and low high-density lipoprotein (HDL) in South Asian immigrants: could dysfunctional HDL be the missing link? Arch Med Sci. 2014;10(5):870–879. doi: 10.5114/aoms.2014.46208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodani S, Kaur R, Reddy S, Reed GL, Navab M, George V. Can dysfunctional HDL explain high coronary artery disease risk in South Asians? Int J Cardiol. 2008;129(1):125–132. doi: 10.1016/j.ijcard.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Bilen O, Kamal A, Virani SS. Lipoprotein abnormalities in South Asians and its association with cardiovascular disease: current state and future directions. World J Cardiol. 2016;8(3):247–257. doi: 10.4330/wjc.v8.i3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Findlay SG, Kasliwal RR, Bansal M, Tarique A, Zaman A. A comparison of cardiovascular risk scores in native and migrant South Asian populations. SSM Popul Health. 2020;11:100594. doi: 10.1016/j.ssmph.2020.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saeed A, Virani SS, Mulukutla S, Chow CK. Dyslipidemia and cardiovascular disease prevention in south asians: a review and discussion of causes, challenges and management strategies. Curr Diabetes Rev. 2021 doi: 10.2174/1573399817999210112192419. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt A, Rohatgi A. HDL cholesterol efflux capacity: cardiovascular risk factor and potential therapeutic target. Curr Atheroscler Rep. 2016;18(1):2. doi: 10.1007/s11883-015-0554-1. [DOI] [PubMed] [Google Scholar]
- 33.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. New Engl J Med. 2007;357(13):1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 34.Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport. Circ Res. 2019;124(10):1505–1518. doi: 10.1161/CIRCRESAHA.119.312617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenson RS, Brewer HB, Jr, Ansell B, Barter P, Chapman MJ, Heinecke JW, et al. Translation of high-density lipoprotein function into clinical practice: current prospects and future challenges. Circulation. 2013;128(11):1256–1267. doi: 10.1161/CIRCULATIONAHA.113.000962. [DOI] [PubMed] [Google Scholar]
- 36.Kontush A. HDL-mediated mechanisms of protection in cardiovascular disease. Cardiovasc Res. 2014;103(3):341–349. doi: 10.1093/cvr/cvu147. [DOI] [PubMed] [Google Scholar]
- 37.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7(5):365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Namiri-Kalantari R, Gao F, Chattopadhyay A, Wheeler AA, Navab KD, Farias-Eisner R, et al. The dual nature of HDL: anti-inflammatory and pro-inflammatory. BioFactors. 2015;41(3):153–159. doi: 10.1002/biof.1205. [DOI] [PubMed] [Google Scholar]
- 39.Dodani S, Grice DG, Joshi S. Is HDL function as important as HDL quantity in the coronary artery disease risk assessment? J Clin Lipidol. 2009;3(2):70–77. doi: 10.1016/j.jacl.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Wang B, Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol. 2018;14(8):452–463. doi: 10.1038/s41574-018-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289(35):24020–24029. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, et al. Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. New Engl J Med. 2011;365(24):2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 43.Henkhaus RS, Dodani S, Manzardo AM, Butler MG. APOA1 gene polymorphisms in the South Asian immigrant population in the United States. Indian J Hum Genet. 2011;17(3):194–200. doi: 10.4103/0971-6866.92103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dodani S, Henkhaus R, Wick J, Vacek J, Gupta K, Dong L, et al. Metabolic syndrome in South Asian immigrants: more than low HDL requiring aggressive management. Lipids Health Dis. 2011;10:45. doi: 10.1186/1476-511X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol. 2015;6:218. doi: 10.3389/fphar.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makshood M, Post WS, Kanaya AM. Lipids in South Asians: epidemiology and management. Curr Cardiovasc Risk Rep. 2019;13(8):24. doi: 10.1007/s12170-019-0618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhalodkar NC, Blum S, Rana T, Bhalodkar A, Kitchappa R, Kim KS, et al. Comparison of levels of large and small high-density lipoprotein cholesterol in Asian Indian men compared with Caucasian men in the Framingham Offspring Study. Am J Cardiol. 2004;94(12):1561–1563. doi: 10.1016/j.amjcard.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 48.Superko HR, Enas EA, Kotha P, Bhat NK, Garrett B. High-density lipoprotein subclass distribution in individuals of Asian Indian descent: the National Asian Indian Heart Disease Project. Prev Cardiol. 2005;8(2):81–86. doi: 10.1111/j.1520-037x.2005.3766.x. [DOI] [PubMed] [Google Scholar]
- 49.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42(8):1308–1317. doi: 10.1016/S0022-2275(20)31582-0. [DOI] [PubMed] [Google Scholar]
- 50.Dodani S. Excess coronary artery disease risk in South Asian immigrants: can dysfunctional high-density lipoprotein explain increased risk? Vasc Health Risk Manag. 2008;4(5):953–961. doi: 10.2147/vhrm.s2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakker LEH, Boon MR, Annema W, Dikkers A, van Eyk HJ, Verhoeven A, et al. HDL functionality in South Asians as compared to white Caucasians. Nutr Metab Cardiovas. 2016;26(8):697–705. doi: 10.1016/j.numecd.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Khan NA, Wang H, Anand S, Jin Y, Campbell NR, Pilote L, et al. Ethnicity and sex affect diabetes incidence and outcomes. Diabetes Care. 2011;34(1):96–101. doi: 10.2337/dc10-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frank AT, Zhao B, Jose PO, Azar KM, Fortmann SP, Palaniappan LP. Racial/ethnic differences in dyslipidemia patterns. Circulation. 2014;129(5):570–579. doi: 10.1161/circulationaha.113.005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Misra R, Patel T, Kotha P, Raji A, Ganda O, Banerji M, et al. Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: results from a national study. J Diabetes Complications. 2010;24(3):145–153. doi: 10.1016/j.jdiacomp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Kanaya AM, Herrington D, Vittinghoff E, Ewing SK, Liu K, Blaha MJ, et al. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care. 2014;37(6):1621–8. doi: 10.2337/dc13-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Araneta MR, Kanaya AM, Hsu WC, Chang HK, Grandinetti A, Boyko EJ, et al. Optimum BMI cut points to screen Asian Americans for type 2 diabetes. Diabetes Care. 2015;38(5):814–820. doi: 10.2337/dc14-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel SA, Shivashankar R, Ali MK, Anjana RM, Deepa M, Kapoor D, et al. Is the "South Asian phenotype" unique to South Asians?: comparing cardiometabolic risk factors in the CARRS and NHANES studies. Glob Heart. 2016;11(1):89–96 e3. doi: 10.1016/j.gheart.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unnikrishnan R, Gupta PK, Mohan V. Diabetes in South Asians: phenotype, clinical presentation, and natural history. Curr Diab Rep. 2018;18(6):30. doi: 10.1007/s11892-018-1002-8. [DOI] [PubMed] [Google Scholar]
- 59.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown BE, Nobecourt E, Zeng J, Jenkins AJ, Rye K-A, Davies MJ. Apolipoprotein A-I glycation by glucose and reactive aldehydes alters phospholipid affinity but not cholesterol export from lipid-laden macrophages. PloS one. 2013;8(5):e65430–e. doi: 10.1371/journal.pone.0065430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kashyap SR, Osme A, Ilchenko S, Golizeh M, Lee K, Wang S, et al. Glycation reduces the stability of ApoAI and increases HDL dysfunction in diet-controlled type 2 diabetes. J Clin Endocrinol Metab. 2017;103(2):388–396. doi: 10.1210/jc.2017-01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chambers JC, Abbott J, Zhang W, Turro E, Scott WR, Tan ST, et al. The South Asian genome. PLoS ONE. 2014;9(8):e102645. doi: 10.1371/journal.pone.0102645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leduc MS, Shimmin LC, Klos KL, Hanis C, Boerwinkle E, Hixson JE. Comprehensive evaluation of apolipoprotein H gene (APOH) variation identifies novel associations with measures of lipid metabolism in GENOA. J Lipid Res. 2008;49(12):2648–2656. doi: 10.1194/jlr.M800155-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harper AR, Bowman M, Hayesmoore JBG, Sage H, Salatino S, Blair E, et al. Reevaluation of the South Asian MYBPC3(Delta25bp) intronic deletion in hypertrophic cardiomyopathy. Circ Genom Precis Med. 2020;13(3):e002783. doi: 10.1161/CIRCGEN.119.002783. [DOI] [PMC free article] [PubMed] [Google Scholar]