Abstract
After oral administration of 500 mg of levofloxacin to 12 volunteers, we investigated the pharmacokinetics and serum bactericidal activities (SBAs) against five strains of members of the family Enterobacteriaceae. Pharmacokinetic data were as follows: maximum concentration in serum, 6.36 ± 0.57 mg/liter; area under the concentration-time curve, 43.6 ± 6.23 mg · h/liter; elimination half-life 4.23 ± 0.87 h. SBAs were present for 24 h against Escherichia coli and Citrobacter freundii. The SBAs at 1, 12, and 24 h after administration against E. coli were 1:108, 1:29, and 1:7, respectively, and those against Citrobacter freundii were 1:74, 1:25, and 1:7, respectively. The SBAs were present for 12 h against the other three organisms tested. The SBAs against Serratia marcescens were 1:28 and 1:9 at 1 and 12 h, respectively; the SBAs against Klebsiella pneumoniae were 1:25 and 1:7 at 1 and 12 h, respectively; and the SBAs against Enterobacter cloacae were 1:24 and 1:10 at 1 and 12 h, respectively.
Levofloxacin is the active l-isomer of the racemate ofloxacin, a fluoroquinolone with a broad spectrum of activity (5, 14). We assessed the pharmacokinetics of levofloxacin and the serum bactericidal activities (SBAs) against five different species of members of the family Enterobacteriaceae. Twelve healthy female volunteers (mean age, 33.8 ± 6.1 years; mean body weight, 64.1 ± 10.2 kg) were included after they provided written informed consent. Exclusion criteria were hypersensitivity to quinolones, pregnancy, drug or alcohol abuse, or the use of a concomitant medication except for contraceptives. The study was approved by the ethical committee of the Physicians' Association of Hessen, Frankfurt, Germany.
Levofloxacin (lot HR355/1014, batch no. 20; Hoechst, Frankfurt, Germany) was administered orally as an open single dose of 500 mg (in tablet form) on an empty stomach after 10 h of fasting. Blood samples were collected just before medication and at 0.5, 1, 2, 4, 6, 8, 12, and 24 h after medication. Serum levofloxacin concentrations were determined enantioselectively by high-performance liquid chromatography by a previously described method (12). The assay was linear over the concentration range studied, and the lower limit of quantitation was 20 ng/ml. The inter- and intraday coefficients of variation were both <3%.
We analyzed 10 clinically relevant strains of five different species of the family Enterobacteriaceae isolated by the Department of Clinical Microbiology of the City Hospital Zehlendorf/Heckeshorn and of the Departments of Microbiology of the University Hospitals Benjamin-Franklin and Charité-Virchow, Berlin, Germany. The species of all isolates were determined with the API 20E system (API BioMérieux, Nürtingen, Germany). The minimal bactericidal concentrations (MBCs) of levofloxacin were determined in triplicate by the standardized microdilution method described in the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) (16). All samples were serially diluted in a 96-well plate (Falcon; Becton Dickinson, Lincoln Park, N.J.) from a titer of 1:4 to a titer of 1:512 with an automatic microdilutor (Titertek; Denley, Billinghurst, United Kingdom). The diluent was Mueller-Hinton broth without Mg2+ and Ca2+ (Difco Laboratories, Detroit, Mich.). Every well was inoculated with the final inoculum of bacteria (5 × 105 CFU/ml). The trays were incubated at 35°C for 24 h, and the wells were assessed for visible turbidity. One microliter from each well was spotted with a multipoint inoculator (Microtiter; Denley) on Mueller-Hinton agar plates, which were then incubated for 24 h at 35°C. The concentration of the dilution titer at which no bacterial growth occurred was considered the MBC. SBAs were determined in triplicate by the standardized microdilution method described in the NCCLS guidelines (15), with the dilution procedure for the sera being similar to that used for the MBC method. As the diluent, a mixture of equal parts of Mueller-Hinton broth without Mg2+ and Ca2+ and heat-inactivated (56°C, 30 min) pooled human serum was used. Pseudomonas aeruginosa ATCC 27853 (MIC, 1 to 4 mg/liter) and Escherichia coli ATCC 25922 (MIC, 0.016 to 0.06 mg/liter) were used as quality control strains.
Pharmacokinetics were analyzed compartmentally and noncompartmentally. All pharmacokinetic parameters were normalized to a body weight of 70 kg before averaging; the clearance values were normalized to a body surface of 1.73 m2. The maximum concentration (Cmax), the time to Cmax (Tmax), and the elimination half-life (t1/2) were calculated by assuming an open one-compartment model for extravascular application. The decision was based on the Schwarz criterion (22). An iterative least-squares method was used to fit the regression curve to the experimentally obtained values. Nonlinear regression analysis was performed to minimize the following objective function:
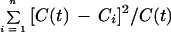 |
where Ci is the concentration measured at time ti (i = 1, … n), and C(t) is the concentration measured at time t.
The area under the serum concentration-time curve (AUC) was calculated noncompartmentally by the log trapezoidal summation method (24). The apparent terminal half-life was calculated from the adjustment of a single exponential function (Ce−zt) to the terminal phase of the concentration-time profiles, where C is a constant and z is the apparent terminal rate constant. The adjustments were done by using the method of least squares. The half-lives were then calculated as t1/2;z = ln(2)/z, where t1/2;z is the terminal half-life. The relative total volume of distribution at steady state (Vss/ƒ) and the clearance (CL/ƒ) were calculated noncompartmentally, where ƒ is the bioavailability. SBA data are indicated as geometric mean titers and as the mean ± standard deviation (SD) of the log2 of all reciprocal serum bactericidal titers. Spearman's rank order correlation coefficient (rs,t) corrected for ties (18) was used to analyze the correlation between C(t)/MIC and the corresponding SBA.
Pharmacokinetic data are presented in Table 1. There were only mild adverse effects in four volunteers (mild peripheral edema and mild headache); no abnormal physical or laboratory findings were observed. The MBC of levofloxacin was ≤0.008 mg/liter for all isolates of E. coli. For Klebsiella pneumoniae, the MBCs were ≤0.016 mg/liter (5 strains), ≤0.25 mg/liter (four strains), and 1 mg/liter (one strain). For Enterobacter cloacae, the MBCs were ≤0.008 mg/liter (five strains), ≤0.5 mg/liter (three strains), 1 mg/liter (one strain), and 4 mg/liter (one strain). For Serratia marcescens, the MBCs were ≤0.063 mg/liter for all strains. For Citrobacter freundii, the MBCs were ≤0.008 mg/liter (eight strains), 0.016 mg/liter (one strain), and 0.063 mg/liter (one strain). The MBCs for reference strains P. aeruginosa ATCC 27853(MBC, 2 mg/liter) and E. coli ATCC 25922 (MBC, 0.016 mg/liter) were within the acceptable quality control ranges of NCCLS (16). The SBA titers are indicated in detail in Table 2.
TABLE 1.
Pharmacokinetic data after oral administration of a single dose of 500 mg of levofloxacin to 12 healthy female volunteersa
| Unit | Cmax (mg/liter) | Tmax (h) | Tlag (h) | AUC (μg · h/ml) | t1/2;z (h) | MRT (h) | Vss/f (liters/70 kg) | CL/f (ml/min/1.73 m2) |
|---|---|---|---|---|---|---|---|---|
| Mean | 6.36 | 0.81 | 0.26 | 43.6 | 4.23 | 6.52 | 69.7 | 176 |
| SD | 0.57 | 0.37 | 0.11 | 6.23 | 0.87 | 1.1 | 3.95 | 21.6 |
| Lower range | 5.42 | 0.52 | 0.17 | 36.8 | 3.13 | 5.32 | 63.3 | 135 |
| Upper range | 7.03 | 1.65 | 0.46 | 57.9 | 6.31 | 9.29 | 75.6 | 203 |
Tlag, lag time; MRT, mean residence time; the other abbreviations are defined in the text.
TABLE 2.
SBAs after administration of a single oral dose of 500 mg of levofloxacin to 12 healthy volunteers against five species of clinically relevant members of the family Enterobacteriaceae
| Pathogena | Time (h) | Mean titerb | Highest titer | Lowest titer | Rec. log 2 ± SDc |
|---|---|---|---|---|---|
| E. coli | 1 | 1 /108 | 1 /256 | 1 /32 | 6.76 ± 0.40 |
| 2 | 1 /105 | 1 /256 | 1 /32 | 6.72 ± 0.31 | |
| 12 | 1 /29 | 1 /64 | 1 /8 | 4.85 ± 0.44 | |
| 24 | 1 /7 | 1 /32 | 1 /4 | 2.82 ± 0.34 | |
| C. freundii | 1 | 1 /74 | 1 /256 | 1 /8 | 6.20 ± 0.97 |
| 2 | 1 /58 | 1 /256 | 1 /8 | 5.87 ± 0.73 | |
| 12 | 1 /25 | 1 /64 | 1 /4 | 4.63 ± 0.87 | |
| 24 | 1 /7 | 1 /32 | <1 /4 | 2.87 ± 1.02 | |
| S. marcescens | 1 | 1 /28 | 1 /128 | 1 /8 | 4.79 ± 0.56 |
| 2 | 1 /31 | 1 /128 | 1 /16 | 4.96 ± 0.43 | |
| 12 | 1 /9 | 1 /32 | 1 /4 | 3.18 ± 0.33 | |
| 24 | <1 /4 | 1 /8 | <1 /4 | 1.22 ± 0.26 | |
| K. pneumoniae | 1 | 1 /25 | 1 /128 | 1 /4 | 4.66 ± 1.22 |
| 2 | 1 /23 | 1 /128 | 1 /4 | 4.51 ± 1.35 | |
| 12 | 1 /7 | 1 /32 | <1 /4 | 2.77 ± 1.32 | |
| 24 | <1 /4 | 1 /8 | <1 /4 | 1.64 ± 0.55 | |
| E. cloacae | 1 | 1 /24 | 1 /256 | <1 /4 | 4.56 ± 2.05 |
| 2 | 1 /24 | 1 /256 | <1 /4 | 4.60 ± 2.20 | |
| 12 | 1 /10 | 1 /64 | <1 /4 | 3.28 ± 1.65 | |
| 24 | 1 /4 | 1 /32 | <1 /4 | 1.87 ± 0.92 |
Ten strains of each species were tested.
Mean titer, = geometric mean of SBA.
Rec. log 2 ± SD, arithmetic mean of the reciprocal log2 ± SD.
There was a strong positive correlation between the concentration in serum/MIC ratio and the corresponding SBA at l, 2, 12, and 24 h after drug administration (rs,t = 0.846 for 2300 datum pairs; P < 0.0001). Similarly, the Cmax/MIC ratio was strongly correlated with the SBA at 1 h after drug administration (rs, t = 0.808 for 600 datum pairs; P < 0.001). The AUC from time zero to 24 h (AUC0–24)/MIC ratio (indicated as inverse serum inhibitory titer [SIT] integrated over time) had a strong correlation with the area under the reciprocal of the SBA curve (rs,t = 0.882 for 540 datum pairs; P < 0.0001).
Our pharmacokinetic data correspond to published data that indicate a Cmax of 5.2 to 5.9 mg/liter within 1 to 2 h after administration of 500 mg of levofloxacin and a t1/2;z of 6 to 8 h (2, 3, 5, 7, 11, 14). The bactericidal activities of levofloxacin are within the range of those of other quinolones, with MICs at which 90% of isolates are inhibited of ≤1 mg/liter for most members of the family Enterobacteriaceae (1, 8, 9, 17).
The SBA test has been used for many years to monitor antimicrobial activity in vivo (20, 25). SBA is supposed to occur at concentrations of 4× the MIC or greater, although patients with reduced immunity may require higher SBA titers of 8× to 16× the MIC (10, 17, 23). Our data confirm that SBA against members of the family Enterobacteriaceae occurs at a concentration in serum/MIC ratio of ≥4.
The antimicrobial effects of fluoroquinolones are concentration dependent (4, 13). An AUC0–24/MIC ratio of >125 SIT−1 · h is accepted to be the significant breakpoint for probabilities of clinical and microbiological cures (13). However, there is evidence that the Cmax/MIC ratio may be better correlated with the antimicrobial effect than the AUC0–24/MIC ratio, with the breakpoint being a Cmax/MIC ratio of 13:1 to 20:1 (6, 19, 21). Our data show that both the Cmax/MIC ratio and the AUC0–24/MIC ratio are significantly correlated with the SBA of levofloxacin.
Acknowledgments
This work was supported by an unrestricted grant from Hoechst Marion Roussel AG, Frankfurt am Main, Germany.
REFERENCES
- 1.Bauernfeind A. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 2.Chien S C, Chow A T, Natarajan J, Williams R R, Wong F A, Rogge M C, Nayak R K. Absence of age and gender effects on the pharmacokinetics of a single 500-milligram oral dose of levofloxacin in healthy subjects. Antimicrob Agents Chemother. 1997;41:1562–1565. doi: 10.1128/aac.41.7.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien S C, Rogge M C, Gisclon L G, Curtin C, Wong F, Natarajan J, Williams R R, Fowler C L, Cheung W K, Chow AT. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother. 1997;41:2256–2260. doi: 10.1128/aac.41.10.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing in mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 5.Davis R, Bryson H M. Levofloxacin—a review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 1994;47:677–700. doi: 10.2165/00003495-199447040-00008. [DOI] [PubMed] [Google Scholar]
- 6.Drusano G L, Johnson D, Rosen M, Standiford M C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993;37:483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fish D N, Chow A T. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32:101–109. doi: 10.2165/00003088-199732020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Fu K P, Lafredo S C, Foleno B, Isaacson D M, Barrett J F, Tobia A J, Rosenthale M E. In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob Agents Chemother. 1992;36:860–866. doi: 10.1128/aac.36.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto T, Mitsuhashi S. In vitro antibacterial activity of DR-3355, the S-(−)-isomer of ofloxacin. Chemotherapy (Tokyo) 1990;36:268–276. doi: 10.1159/000238777. [DOI] [PubMed] [Google Scholar]
- 10.Klastersky J, Didier D, Swing G, Werts D. Antibacterial activity in serum and urine as a therapeutic guide in bacterial infections. J Infect Dis. 1974;129:187–193. doi: 10.1093/infdis/129.2.187. [DOI] [PubMed] [Google Scholar]
- 11.Lee L J, Hafkin B, Lee I D, Dix R. Effects of food and sucralfate on a single oral dose of 500 milligrams of levofloxacin in healthy subjects. Antimicrob Agents Chemother. 1997;41:2196–2200. doi: 10.1128/aac.41.10.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehr K H, Damm P. Quantification of the enantiomers of ofloxacin in biological fluids by HPLC. J Chromatogr. 1988;425:153. doi: 10.1016/0378-4347(88)80015-x. [DOI] [PubMed] [Google Scholar]
- 13.Lode H, Borner K, Koeppe P. Pharmacodynamics of fluoroquinolones. Clin Infect Dis. 1998;27:33–39. doi: 10.1086/514623. [DOI] [PubMed] [Google Scholar]
- 14.Martin S J, Meyer J M, Chuck S K, Jung R, Messick C R, Pendland S L. Levofloxacin and sparfloxacin: new quinolone antibiotics. Ann Pharmacother. 1998;32:320–326. doi: 10.1345/aph.17178. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methodology for the serum bactericidal test; tentative guideline. NCCLS document M21-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. Approved standard M7–A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 17.Odenholt I, Löwdin E, Cars O. Bactericidal effects of levofloxacin in comparison with those of ciprofloxacin and sparfloxacin. Clin Microbiol Infect. 1998;4:264–270. doi: 10.1111/j.1469-0691.1998.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 18.Ott L. An introduction to statistical methods and data analysis. 3rd ed. Boston, Mass: PWS-Kent Publishing Company; 1988. pp. 323–324. [Google Scholar]
- 19.Palmer S M, Rybak M J. Pharmacodynamics of once- or twice-daily levofloxacin versus vancomycin, with or without rifampin, against Staphylococcus aureus in an in vitro model with infected platelet-fibrin clots. Antimicrob Agents Chemother. 1996;40:701–705. doi: 10.1128/aac.40.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pien F D, Vosti K L. Variation in performance of the serum bactericidal test. Antimicrob Agents Chemother. 1974;6:330–333. doi: 10.1128/aac.6.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston S L, Drusano G L, Berman A L, Fowler C L, Chow A T, Dornseif B, Reichl V, Natarajan J, Corrado M. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 23.Sculier J P, Klastersky J. Significance of serum bactericidal activity in gram-negative bacillary bacteremia in patients with and without granulocytopenia. Am J Med. 1984;76:429–435. doi: 10.1016/0002-9343(84)90662-4. [DOI] [PubMed] [Google Scholar]
- 24.Shargel L, Yu A B C. Applied biopharmaceutics and pharmacokinetics. 3rd ed. Norwalk, Conn: Appleton and Lange; 1993. [Google Scholar]
- 25.Wolfson J S, Swartz M N. Serum bactericidal activity as a monitor of antibiotic therapy. N Engl J Med. 1985;312:968–975. doi: 10.1056/NEJM198504113121507. [DOI] [PubMed] [Google Scholar]


