Abstract
Background:
There have been multiple waves in the COVID-19 pandemic in many countries. We sought to compare mortality and respiratory, cardiovascular and renal dysfunction between waves in 3 Canadian provinces.
Methods:
We conducted a substudy of the ARBs CORONA I study, a multicentre Canadian pragmatic observational cohort study that examined the association of pre-existing use of angiotensin receptor blockers with outcomes in adults admitted to hospital with acute COVID-19 up to April 2021 from 9 community and teaching hospitals in 3 Canadian provinces (British Columbia, Ontario and Quebec). We excluded emergency department admissions without hospital admission, readmissions and admissions for another reason. We used logistic and 0–1-inflated β regression models to compare 28-day and in-hospital mortality, and the use of invasive mechanical ventilation, vasopressors and renal replacement therapy (RRT) between the first 3 waves of the COVID-19 pandemic in these provinces.
Results:
A total of 520, 572 and 245 patients in waves 1, 2 and 3, respectively, were included. Patients in wave 3 were on average younger and had fewer comorbidities than those in waves 1 and 2. The unadjusted 28-day mortality rate was significantly lower in wave 3 (7.8%) than in wave 1 (18.3%) (odds ratio [OR] 0.43, 95% confidence interval [CI] 0.24–0.78) and wave 2 (16.3%) (OR 0.46, 95% CI 0.27–0.79). After adjustment for differences in baseline characteristics, the difference in 28-day mortality remained significant (adjusted OR wave 3 v. wave 1: 0.46, 95% CI 0.26–0.81; wave 3 v. wave 2: 0.52, 95% CI 0.29–0.91). In-hospital mortality findings were similar. Use of invasive mechanical ventilation or vasopressors was less common in waves 2 and 3 than in wave 1, and use of RRT was less common in wave 3 than in wave 1.
Interpretation:
Severity of illness decreased (lower mortality and less use of organ support) across waves among patients admitted to hospital with acute COVID-19, possibly owing to changes in patient demographic characteristics and management, such as increased use of dexamethasone. Continued application of proven therapies may further improve outcomes.
Study registration:
There have been 5 waves of the COVID-19 pandemic in Canada, including the omicron-driven wave (as of Dec. 17, 20211). Mortality from COVID-19 varied as the waves moved across the world, and changes in patient mix, the emergence of successful treatment and improvements in quality of care affected acute COVID-19 mortality. Some studies showed decreased mortality after wave 1.2,3 In a 43-country study,3 there was lower mortality in wave 2 than in wave 1. Domingo and colleagues4 found higher mortality among patients in Spain in wave 1 than in wave 2 that was explained by differences in intensive care unit (ICU) admission and ventilation use. In another study, with data from 14 countries that each had at least 4000 deaths from COVID-19 as of Jan. 14, 2021, the age distribution of those who died was similar between waves 1 and 2, but there were fewer deaths among nursing home residents in wave 2.5 More use of anticoagulation and corticosteroids in wave 2 may explain the lower mortality.6 In Spain, patients from wave 2 were more often treated with noninvasive ventilation and corticosteroids, and less often with invasive ventilation, usual oxygen therapy and anticoagulants.7 Other studies showed increased mortality in later waves in Africa8 and South Korea.9
Although prior studies showed differences in case numbers across waves,2–9 we are not aware of any reports of differences in organ dysfunction and use of organ-supporting care (ventilation, vasopressors and renal replacement therapy [RRT]) across waves. Differences in outcomes between waves may be due to differences in patient characteristics (age, comorbidities2–9), viral characteristics (viral load10,11 and variants of concern12), natural immunity,13 genetics,14 postvaccination immunity,15 resources (hospital16 and ICU17,18 bed availability) and treatments (dexamethasone19,20). Examination of differences in patient baseline characteristics between waves would require adjusted analyses, but not all studies did such analyses.
In this cohort study, we sought to compare mortality and use of respiratory, cardiovascular and renal support among adults admitted to hospital with acute COVID-19 in 3 Canadian provinces between pandemic waves 1, 2 and 3. Our hypothesis was that there were differences in patient characteristics, mortality and use of ventilation, vasopressors and RRT across these waves.
Methods
Study design
This was a substudy of the ARBs CORONA I study,21 a multicentre Canadian pragmatic observational cohort study to examine the association of pre-existing use of angiotensin receptor blockers with outcomes in patients admitted to hospital with COVID-19. We used data from the ARBs CORONA I study to compare mortality and respiratory, cardiovascular and renal dysfunction between the first 3 waves in 3 Canadian provinces (British Columbia, Ontario and Quebec). The present study is reported in accordance with the STROBE checklist.22
Setting
Sites for the ARBs CORONA I study (Appendix 1, Table S1, available at www.cmajopen.ca/content/10/2/E379/suppl/DC1) were 9 community and teaching hospitals in British Columbia, Ontario and Quebec that saw large numbers of patients admitted with acute COVID-19.
Two authors (T.L. and J.A.R.) independently derived definitions of waves 1, 2 and 3 in BC, Ontario and Quebec from the Canadian national COVID-19 daily epidemiology update website23 through visual identification of the start and end of cycles in daily case count for each province. Differences were resolved through discussion.
Participants
Those eligible for the study were adults (age > 18 yr) who had SARS-CoV-2 infection confirmed by clinically approved laboratory SARS-CoV-2 testing at local hospital or provincial laboratories and were admitted to hospital for acute COVID-19. Patients were defined as having acute COVID-19 based on best evidence at the time24–28 and when the site investigator judged that the admitting illness (to a ward or the ICU) was consistent with a clinical presentation of acute COVID-19.
We excluded readmissions for acute COVID-19, emergency department admissions without hospital admission, and hospital admissions of patients with a positive SARS-CoV-2 test result but whose acute illness was not due to acute COVID-19. Sites that enrolled only patients admitted to the ICU were excluded, as crude comparisons between waves would have been confounded by the proportions of patients from these sites in each wave (Appendix 1).
Data sources
Patients were identified prospectively at the sites, and data were collected by ARBs CORONA I research coordinators at each site using specifically designed electronic case report forms (Appendix 1). Baseline data were the first data available within 24 hours of admission. Quebec sites were unable to recruit patients in wave 3 owing to research coordinator shortages. Random samples of 15% of the records were reviewed for accuracy by the data monitoring team. There were no concerns regarding the quality of data, and any missing data were requested and included in the database.
Outcomes
For 28-day mortality, patients discharged alive before day 28 and lost to follow-up were assumed to be survivors at day 28.19,20,29
We scored organ dysfunction as the use of invasive mechanical ventilation, vasopressors or RRT, and as days alive and free (DAF) of invasive mechanical ventilation, vasopressors and RRT within the first 14 days (Appendix 1).
Medications looked at are listed in Appendix 1, Relevant variables captured on ARBs CORONA I case report forms.
Sample size
No formal sample size calculation was performed for this analysis as it was a substudy of the ARBs CORONA I study. The initial planned sample size of the ARBs CORONA I study was 497;21 this was later increased to 1600 because several prior studies of association of exposure to angiotensin receptor blockers with outcomes were published, so we reasoned that a larger sample would be more clinically relevant.
Statistical analysis
We compared patient baseline characteristics using the χ2 test, Fisher exact test, analysis of variance or Kruskal– Wallis test, as appropriate. To compare outcomes across waves, we performed unadjusted and adjusted regression analyses, adjusting for predefined factors in ARBs CORONA I, including age, sex, comorbidities (chronic heart disease, hypertension, chronic kidney disease and diabetes, the comorbidities most commonly associated with death in patients with COVID-1924,27,30) and baseline systolic blood pressure, plus organ dysfunction confounders (baseline heart rate, oxygen saturation level and creatinine level), which were different across waves.
We used logistic regression to compare 28-day and in-hospital mortality, and any use of invasive mechanical ventilation, vasopressors or RRT during the hospital stay or the first 14 days. Results were expressed as odds ratio (OR) and 95% confidence interval (CI). We compared DAF between waves using 0–1-inflated β regression (Appendix 1), as the observed data had a U-shaped distribution; results were expressed as mean difference in DAF. Adjusted analysis for DAF of RRT was not feasible because too few patients received RRT during the first 14 days.
Within each regression model, we compared outcomes between pairs of waves. Because the regional distribution of patients was different across waves owing to varying levels of site participation over time, we accounted for site effect in all regression analyses (unadjusted and adjusted) by including a site effect term in the model. Site effect was considered as random in logistic regression but as fixed in 0–1-inflated β regression owing to numeric issues and computational limitations. In a sensitivity analysis, we restricted our analyses to BC, which contributed data throughout the 3 waves.
As there were minimal missing data, we excluded patients with missing data from the corresponding analysis. Around 5% of patients were excluded from the adjusted regression analyses as they did not have complete data on all the required variables.
Analyses were conducted in SAS 9.4 (SAS Institute) and R 4.0.4 (R Foundation for Statistical Computing). A p value < 0.05 was considered statistically significant.
Ethics approval
The study was approved by Providence Health Care and the University of British Columbia Human Research Committee and by each of the contributing clinical sites. Anonymized clinical data were deemed low risk, and informed consent was deemed not necessary for this research.
Results
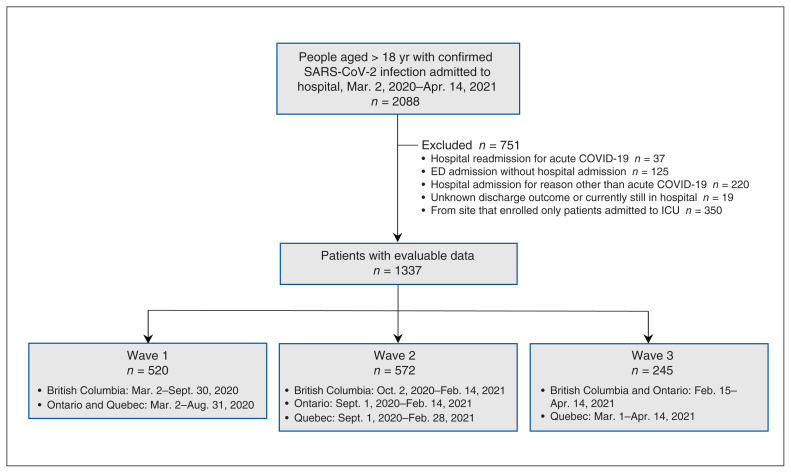
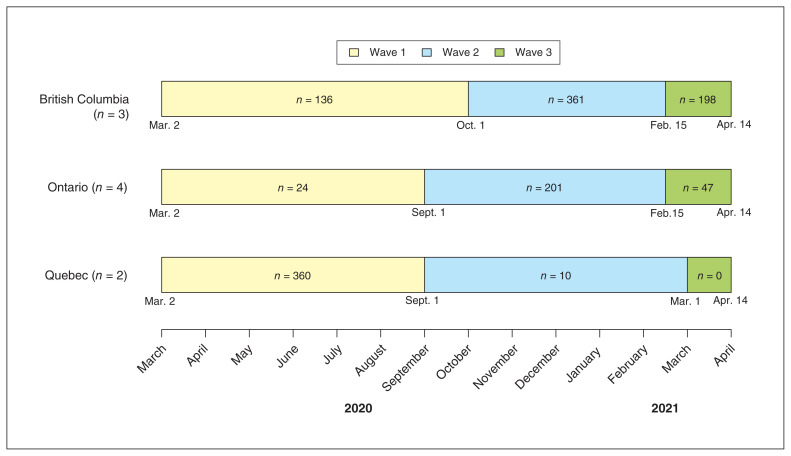
Of 1337 patients with evaluable data admitted from Mar. 2, 2020, to Apr. 14, 2021, 520 (38.9%), 572 (42.8%) and 245 (18.3%) were in waves 1, 2 and 3, respectively (Figure 1). The regional distribution of patients varied across waves owing to varying levels of site participation (Figure 2; Appendix 1, Table S1).
Figure 1:
Flow diagram showing patient selection. Note: ED = emergency department, ICU = intensive care unit.
Figure 2:
Number of patients with acute COVID-19 enrolled in each wave, by province.
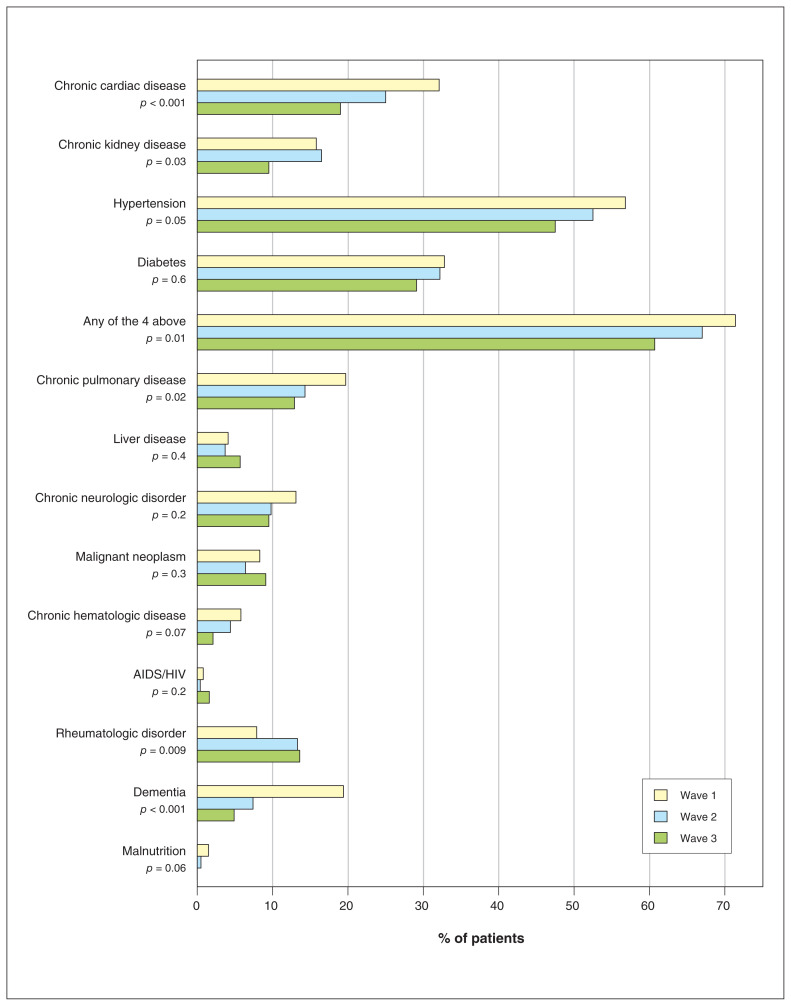
Compared to patients in waves 1 and 2, those in wave 3 were significantly younger (mean age 63.3 yr, 65.8 yr and 70.2 yr in waves 3, 2 and 1, respectively, p < 0.001) and less likely to have chronic cardiac disease, hypertension, chronic kidney disease or diabetes (148/244 [60.7%], 381/569 [67.0%] and 370/518 [71.4%] in waves 3, 2 and 1, respectively, p = 0.01) (Figure 3, Table 1; Appendix 1, Table S2).
Figure 3:
Comorbidities of patients in waves 1, 2 and 3. p value based on χ2 test or Fisher exact test, as appropriate.
Table 1:
Baseline characteristics of patients admitted to hospital with acute COVID-19, overall and in pandemic waves 1, 2 and 3
| Characteristic | No. (%) of patients* | p value† | |||
|---|---|---|---|---|---|
| Overall n = 1337 |
Wave 1 n = 520 |
Wave 2 n = 572 |
Wave 3 n = 245 |
||
| Admission date | – | ||||
| March–May 2020 | 429 (32.1) | 429 (82.5) | – | – | |
| June–August 2020 | 34 (2.5) | 34 (6.5) | – | – | |
| September–November 2020 | 276 (20.6) | 57 (11.0) | 219 (38.3) | – | |
| December 2020–February 2021 | 386 (28.9) | – | 353 (61.7) | 33 (13.5) | |
| March–April 2021 | 212 (15.9) | – | – | 212 (86.5) | |
| SARS-CoV-2 confirmed status | < 0.001 | ||||
| Positive on screening test | 82 (6.1) | 67 (12.9) | 13 (2.3) | 2 (0.8) | |
| Positive on definitive test | 1255 (93.9) | 453 (87.1) | 559 (97.7) | 243 (99.2) | |
| Positive for other pathogen | 11 (0.8) | 9 (1.7) | 2 (0.3) | 0 (0.0) | 0.02 |
| Sex | 0.3 | ||||
| Male | 791 (59.2) | 293 (56.5) | 348 (60.8) | 150 (61.2) | |
| Female | 545 (40.8) | 226 (43.5) | 224 (39.2) | 95 (38.8) | |
| Unknown | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0 (0.0) | |
| Age, mean ± SD, yr (range) | 67.0 ± 17.1 (20–103) | 70.2 ± 16.3 (23–103) | 65.8 ± 17.3 (20–100) | 63.3 ± 17.1 (23–101) | < 0.001 |
| Received COVID-19 vaccine before admission | 24 (1.8) | 0 (0.0 ) | 3 (0.5) | 21 (8.6) | < 0.001 |
| Admitted to intensive care unit on hospital admission day | 218 (16.3) | 104 (20.0) | 83 (14.5) | 31 (12.8) | 0.01 |
| Organ support on day of admission | |||||
| Invasive mechanical ventilation | 102 (7.6) | 55 (10.6) | 34 (5.9) | 13 (5.3) | 0.005 |
| Renal replacement therapy or dialysis | 18/1320 (1.4) | 8/511 (1.6) | 7/567 (1.2) | 3/242 (1.2) | 0.9 |
| Vasopressor | 81 (6.1) | 35 (6.7) | 33 (5.8) | 13 (5.3) | 0.7 |
| Temperature, mean ± SD, °C | 37.5 ± 0.9 n = 1306 |
37.5 ± 0.9 n = 501 |
37.4 ± 0.9 n = 563 |
37.4 ± 0.8 n = 242 |
0.1 |
| Heart rate, mean ± SD, beats/min | 94.2 ± 20.3 n = 1325 |
91.4 ± 20.6 n = 514 |
95.5 ± 20.3 n = 569 |
97.4 ± 19.1 n = 242 |
< 0.001 |
| Respiratory rate, mean ± SD, breaths/min | 24.0 ± 7.3 n = 1313 |
22.9 ± 6.4 n = 505 |
24.9 ± 7.9 n = 567 |
24.2 ± 7.5 n = 241 |
< 0.001 |
| Systolic blood pressure, mean ± SD, mm Hg | 129.4 ± 22.7 n = 1328 |
128.8 ± 22.9 n = 518 |
130.6 ± 23.4 n = 567 |
128.0 ± 20.5 n = 243 |
0.2 |
| Diastolic blood pressure, mean ± SD, mm Hg | 73.8 ± 12.6 n = 1312 |
73.7 ± 11.9 n = 517 |
74.4 ± 13.2 n = 558 |
72.5 ± 12.5 n = 237 |
0.2 |
| Oxygen saturation level, mean ± SD, % | 91.9 ± 7.2 n = 1320 |
93.5 ± 4.2 n = 507 |
90.8 ± 8.6 n = 570 |
91.1 ± 7.9 n = 243 |
< 0.001 |
| Required oxygen therapy | 436/1290 (33.8) | 186/516 (36.0) | 174/541 (32.2) | 76/233 (32.6) | 0.4 |
| Leucocyte count, median (IQR), × 103/μL | 6.6 (4.9–9.0) n = 1310 |
6.5 (4.9–8.6) n = 503 |
7.0 (5.1–9.2) n = 566 |
6.4 (4.7–9.1) n = 241 |
0.09 |
| Hemoglobin level, median (IQR), g/L | 132.0 (117.0–145.0) n = 1311 |
130.0 (118.0–145.0) n = 505 |
132.0 (117.0–145.0) n = 565 |
134.0 (119.0–145.0) n = 241 |
0.5 |
| Creatinine level, median (IQR), μmol/L | 85.0 (69.0–115.0) n = 1309 |
84.0 (68.0–114.0) n = 510 |
87.0 (70.0–121.0) n = 561 |
82.0 (66.0–107.0) n = 238 |
0.04 |
Note = IQR = interquartile range, SD = standard deviation.
Except where noted otherwise.
For the comparison between the 3 waves.
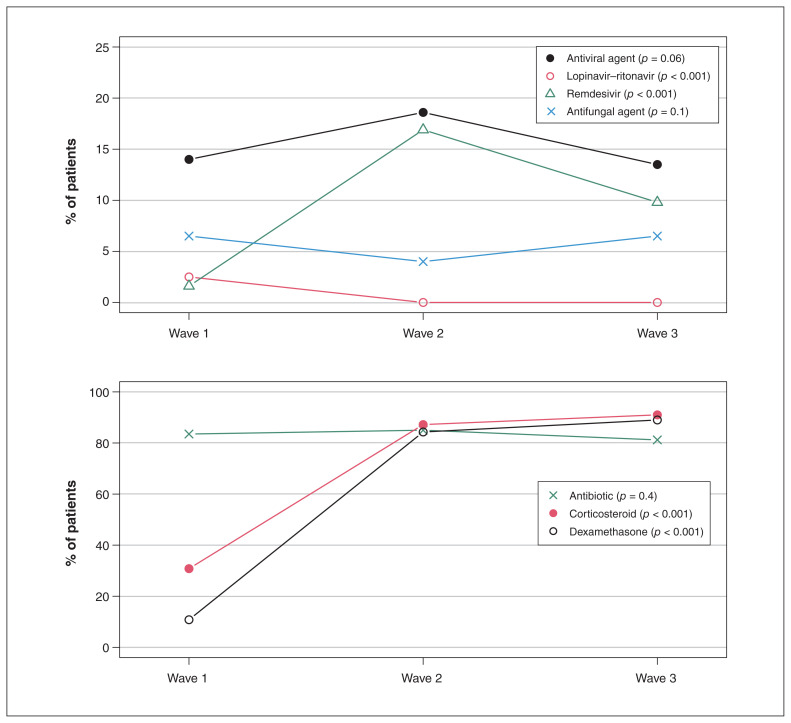
Treatments differed across waves. Lopinavir–ritonavir was administered to 13/516 patients (2.5%) in wave 1 versus no patients in waves 2 and 3 (p < 0.001) (Figure 4; Appendix 1, Table S3). Remdesivir use increased between waves 1 and 2 (8/516 [1.6%] to 96/569 [16.9%]) and then decreased in wave 3 (24/245 [9.8%]) (p < 0.001). Corticosteroid use
Figure 4:
COVID-19 therapies administered during the hospital stay. p value based on χ2 test or Fisher exact test, as appropriate.
increased from wave 1 (160 [30.8%]) to wave 3 (223 [91.0%]), as did dexamethasone use (56 [10.8%] and 218 [89.0%], respectively) (both p < 0.001). Among patients who ever received dexamethasone, treatment was initiated by day 1 in 39/54 patients (72.2%), 409/459 patients (89.1%) and 195/209 (93.3%) patients in waves 1, 2 and 3, respectively (p < 0.001).
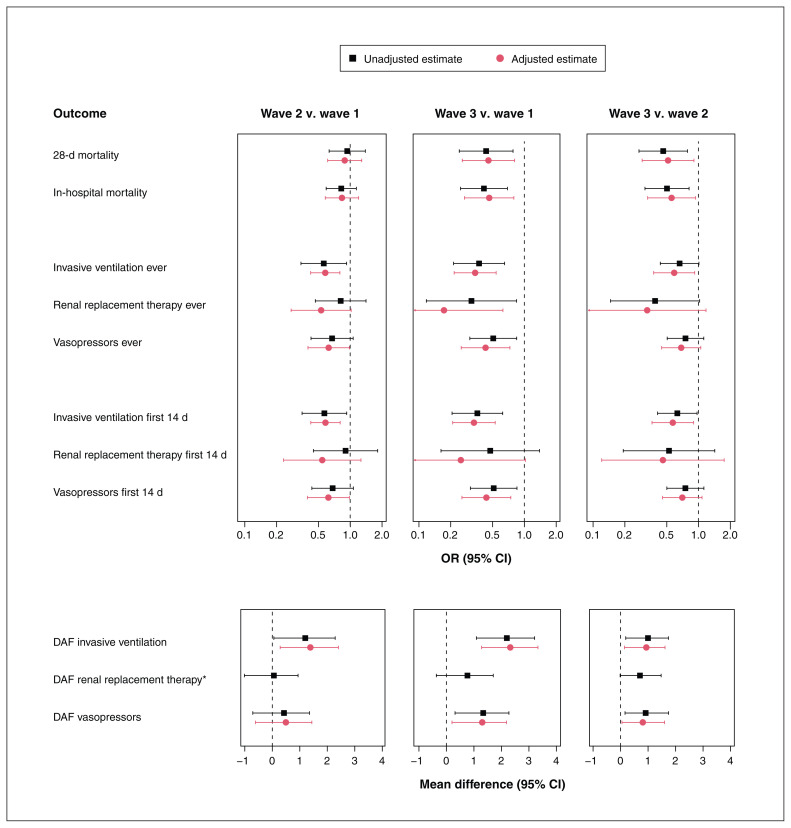
The 28-day mortality rate was 18.3%, 16.3% and 7.8% among patients in waves 1, 2 and 3, respectively. It was significantly lower in wave 3 than in wave 1 (adjusted OR 0.46, 95% CI 0.26–0.81) and wave 2 (adjusted OR 0.52, 95% CI 0.29–0.91) (Figure 5; Appendix 1, Table S4). In-hospital mortality findings were similar.
Figure 5:
Comparison of outcomes between waves by regression analysis. The following factors were accounted for in the adjusted analysis: age, sex, chronic heart disease, hypertension, chronic kidney disease, diabetes, baseline systolic blood pressure, baseline heart rate, baseline oxygen saturation level, baseline creatinine level and site. *Adjusted regression analysis was not feasible numerically as too few patients received renal replacement therapy during the first 14 days. Note: CI = confidence interval, DAF = days alive and free, OR = odds ratio.
There was significantly less use of invasive mechanical ventilation in the later waves (wave 3 v. wave 1: adjusted OR 0.34, 95% CI 0.22–0.54; wave 3 v. wave 2: adjusted OR 0.59, 95% CI 0.38–0.92; and wave 2 v. wave 1: adjusted OR 0.58, 95% CI 0.42–0.80) (Figure 5, Table 2; Appendix 1, Tables S5 and S6). Vasopressor use declined in waves 2 and 3 versus wave 1. Use of RRT declined in wave 3 versus wave 1.
Table 2:
Outcomes, overall and in waves 1, 2 and 3
| Variable | No. (%) of patients* | |||
|---|---|---|---|---|
| Overall n = 1337 |
Wave 1 n = 520 |
Wave 2 n = 572 |
Wave 3 n = 245 |
|
| 28-day mortality† | 207 (15.5) | 95 (18.3) | 93 (16.3) | 19 (7.8) |
| In-hospital death | 238 (17.8) | 111 (21.3) | 103 (18.0) | 24 (9.8) |
| Admitted to intensive care unit | 495/1336 (37.1) | 201 (38.7) | 215 (37.6) | 79/244 (32.4) |
| Organ support during hospital stay | ||||
| All patients | ||||
| Invasive mechanical ventilation | 281 (21.0) | 130 (25.0) | 116 (20.3) | 35 (14.3) |
| Renal replacement therapy or dialysis | 68/1320 (5.2) | 33/511 (6.5) | 30/567 (5.3) | 5/242 (2.1) |
| Vasopressor | 285 (21.3) | 120 (23.1) | 123 (21.5) | 42 (17.1) |
| Patients admitted to intensive care unit | ||||
| Invasive mechanical ventilation | 276/495 (55.8) | 130/201 (64.7) | 111/215 (51.6) | 35/79 (44.3) |
| Renal replacement therapy or dialysis | 49/484 (10.1) | 25/193 (13.0) | 20/213 (9.4) | 4/78 (5.1) |
| Vasopressor | 276/495 (55.8) | 118/201 (58.7) | 117/215 (54.4) | 41/79 (51.9) |
| Organ support during first 14 d | ||||
| Invasive mechanical ventilation | 275 (20.6) | 127 (24.4) | 115 (20.1) | 33 (13.5) |
| Renal replacement therapy or dialysis | 55/1315 (4.2) | 26/508 (5.1) | 24/565 (4.2) | 5/242 (2.1) |
| Vasopressor | 278/1335 (20.8) | 116 (22.3) | 121/571 (21.2) | 41/244 (16.8) |
| DAF of invasive mechanical ventilation during first 14 d, mean ± SD | 10.8 ± 5.4 n = 1330 |
10.0 ± 5.9 n = 518 |
11.0 ± 5.3 n = 570 |
12.2 ± 4.3 n = 242 |
| DAF of renal replacement therapy during first 14 d, mean ± SD | 12.2 ± 4.6 n = 1319 |
11.8 ± 5.0 n = 509 |
12.3 ± 4.5 n = 567 |
13.1 ± 3.4 n = 243 |
| DAF of vasopressor first 14 d, mean ± SD | 11.3 ± 5.1 n = 1331 |
10.7 ± 5.5 n = 518 |
11.3 ± 5.0 n = 570 |
12.4 ± 3.9 n = 243 |
| Hospital length of stay, d | ||||
| Survivors | ||||
| Median (IQR) | 9.0 (5.0–19.0) | 13.0 (7.0–25.0) | 8.0 (5.0–15.0) | 9.0 (5.0–15.0) |
| Range | 2.0–135.0 | 2.0–135.0 | 2.0–77.0 | 2.0–66.0 |
| Decedents | ||||
| Median (IQR) | 11.0 (6.0–20.0) | 11.0 (6.0–21.0) | 11.0 (7.0–20.0) | 12.0 (9.5–24.0) |
| Range | 0.0–63.0 | 0.0–63.0 | 1.0–44.0 | 2.0–62.0 |
| Intensive care unit length of stay, d | ||||
| Survivors | ||||
| Median (IQR) | 8.0 (4.0–14.0) | 10.0 (4.0–17.0) | 7.0 (4.0–11.0) | 7.0 (2.0–13.5) |
| Range | 0.0–86.0 | 0.0–86.0 | 1.0–39.0 | 1.0–54.0 |
| Decedents | ||||
| Median (IQR) | 14.0 (6.0–24.0) | 15.0 (5.0–26.0) | 12.0 (7.0–21.0) | 16.0 (10.0–49.0) |
| Range | 0.0–61.0 | 0.0–61.0 | 0.0–38.0 | 3.0–57.0 |
Note: DAF = days alive and free, IQR = interquartile range, SD = standard deviation.
Except where noted otherwise.
Patients who were discharged alive before day 28 and were lost to follow-up were assumed to be survivors at day 28 (n = 185, 177 and 81 for waves 1, 2 and 3, respectively).
Days alive and free of ventilation was greater in the later waves (adjusted mean difference 1.4 [95% CI 0.3–2.4] for wave 2 v. wave 1; 2.3 [95% CI 1.3–3.3] for wave 3 v. wave 1; and 0.9 [95% CI 0.1–1.6] for wave 3 v. wave 2), as was DAF of vasopressors (adjusted mean difference 1.3 [95% CI 0.2–2.2] for wave 3 v. wave 1, and 0.8 [95% CI 0.05–1.6] for wave 3 v. wave 2) (Figure 5; Appendix 1, Table S7). Days alive and free of RRT was not significantly different across waves.
Findings were similar in the sensitivity analysis of BC data (Appendix 1, Table S8).
Interpretation
There were large differences in outcomes across waves 1–3 of the COVID-19 pandemic in patients admitted to hospital with acute COVID-19. Mortality rates and use of invasive mechanical ventilation, vasopressors and RRT were significantly lower in wave 3 than in wave 1 in analyses adjusted for confounders. Use of dexamethasone was increased in the later waves.
In several aspects, our findings are consistent with those of other global reports. Our results align with those of other studies showing that mortality was lower in wave 2 than in wave 1.2–9 It adds evidence in showing even further decreases in mortality and organ dysfunction (as reflected by the use of respiratory, cardiovascular and renal support therapies) in wave 3. Our methods for risk factor adjustment were appropriate because we adjusted for the major factors associated with increased mortality of COVID-19: age, comorbidities,24,27,30 baseline systolic blood pressure and additional potential confounders of organ dysfunction that were different across waves. These adjustments allowed us to tease out the differences in mortality and organ dysfunction across waves while adjusting for risk variables that may have differed or did differ across waves.
Our study differed from prior studies in that it was multicentre, it was based on Canadian data, we had detailed data regarding use of invasive mechanical ventilation, vasopressors and RRT during the hospital stay, and we had data on therapies such as dexamethasone, antibiotics, and antiviral and antifungal agents used in hospital.
Use of dexamethasone was shown to decrease mortality19 and use of ventilation20 in COVID-19 trials. In our study, it correlated with less use of ventilation and vasopressors, and with increased use of remdesivir in waves 2 and 3. Dexamethasone use may have decreased the need for ventilation and perhaps vasopressors in our study: use increased from 10.8% in wave 1 to 84.3% and 89.0% in waves 2 and 3, respectively, and coincided with less use of invasive mechanical ventilation, vasopressors and RRT. We suspect that the positive results of the pivotal trials of dexamethasone19 and clinical awareness of its use in the COVID-19 pandemic likely drove the rapid change in practice we observed.
Studies later in the pandemic supported early weaning off mechanical ventilation,31,32 early physiotherapy,33,34 reductions in use of sedation and muscle relaxants35 and other ICU liberation strategies35 that decrease mortality and length of stay. Early in the pandemic (before COVID-19 vaccines were available), these strategies may not have been implemented in order to decrease transmission to health care workers, likely to the detriment of patients.36 The return to more usual ICU care of critically ill patients with COVID-19 that increased direct contact by bedside providers — as the latter became more vaccinated and accustomed to personal protective equipment — may plausibly be associated with better outcomes. In addition, improved consistency and organization of COVID-19-specific ward care may have developed with successive waves, which would have contributed to reliable care and interventions, and improved patient outcomes.37 Perhaps some of these factors affected outcomes of patients with acute COVID-19 that we observed.
Limitations
In this association study, we could not determine causation. However, the study adds evidence regarding differences in patient characteristics, treatments and outcomes between waves 1, 2 and 3. The regional distribution of enrolled patients changed across waves, partly owing to research coordinator shortage in Quebec, which may have confounded our crude results. Another potential limitation is inadequate sample size, particularly in wave 3, which limited statistical power. Site selectivity was a limitation because our study was based on a combination of community and teaching hospitals. These centres were located in downtown cores and suburbs of large cities in 3 provinces with the largest COVID-19 caseloads, which limits the representativeness of our results somewhat. Our research coordinators used SARS-CoV-2 tests with a positive result in the hospital laboratory to find patients, but some patients may have been missed. Lower mortality in wave 3 may mean that patients had less severe disease or that treatments were better, or both. We would have needed to see measures of disease severity to conclude that patients had less severe illness, but there was no such measure for COVID-19 at that time.
We do not know whether variants of concern played a role in the decreased use of invasive mechanical ventilation and vasopressors in wave 3 compared to waves 1 and 2 because we did not measure SARS-CoV-2 genotype for variants of concern. Variants of concern increased in frequency in later waves in Italy (yet mortality was lower compared to the first wave38), Japan39 and Hong Kong.40 Because we did not assess immunity in our patients, we could not determine whether immunity played a role in the decreased use of ventilation and vasopressors in wave 3. Barallat and colleagues41 found that, in wave 1, the seroprevalence of anti-SARS-CoV-2 IgG in health care workers in Barcelona was higher than that in the general population in the same geographic area. Utrero-Rico and colleagues42 showed that interleukin 6 levels predicted mortality in both the first and second waves in Europe.
We focused on organ support of the respiratory, cardiovascular and renal systems by measuring use of invasive mechanical ventilation, vasopressors and RRT. We did not assess neurologic dysfunction as there was no comparable neurologic support system. Furthermore, assessment of neurologic dysfunction in critically ill people is difficult because of confounding by sedation. The lack of adjudication as to whether patients had acute COVID-19 was mitigated by having centres with extensive experience with acute COVID-19.
Conclusion
Outcomes of patients admitted to hospital with acute COVID-19 in 3 Canadian provinces improved in wave 3 of the pandemic, possibly related to patient demographic characteristics, improved COVID-specific therapies and return to better baseline ICU care. Patients in wave 3 were younger, had fewer comorbidities and lower mortality, and needed less organ-supporting care than patients in the earlier waves, even after we accounted for these differences. Changes in at-risk groups and management strategies (such as corticosteroid treatment) may explain these improved outcomes.
Supplementary Material
Acknowledgements
The authors thank the patients and families who participated in the ARBs CORONA I study. They also thank the many dedicated clinicians (doctors, nurses, therapists and others) who cared for these patients and comforted their families, as well as the ARBs CORONA I study investigators (Appendix 1) for their ongoing support and tireless collaboration.
Footnotes
Competing interests: James Russell reports an investigator-initiated grant from Grifols that was provided to and administered by the University of British Columbia (UBC). He reports patents owned by UBC that are related to the use of PCSK9 inhibitor(s) in sepsis and the use of vasopressin in septic shock, and a patent owned by Ferring Pharmaceuticals for use of selepressin in septic shock. He is an inventor on these patents. He was a founder, director and shareholder in Cyon Therapeutics and is a shareholder in Molecular You. He reports consulting fees from SIB Therapeutics (developing a sepsis drug) and Ferring Pharmaceuticals (manufactures vasopressin and developing selepressin). He is no longer actively consulting for any industry. He was a nonfunded science advisor and member of the Government of Canada COVID-19 Therapeutics Task Force (June 2020–December 2021) and a nonfunded member of the data and safety monitoring board of a trial of plasma in COVID-19 (PassITON) (2020–2021) sponsored by the National Institutes of Health. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Terry Lee, Joel Singer, Karen Tran, Puneet Mann, Kathryn Donohoe, Greg Haljan and James Russell conceived and designed the study. Terry Lee analyzed the data. Terry Lee and James Russell drafted the manuscript. All of the authors interpreted the data, revised the manuscript critically for important intellectual content, approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by grants from the Canadian Institutes of Health Research (CIHR #439993) and St. Paul’s Hospital Foundation to James Russell. John Boyd is a recipient of a Providence Health Care Research Scholarship. Keith Walley is supported by CIHR Foundation Grant FDN 154311. Donald Vinh is supported by the Fonds de recherche du Québec – Santé Clinician-Scientist Scholar Program. Srinivas Murthy is supported by the Health Research Foundation of Innovative Medicines Canada Chair in Pandemic Preparedness Research.
Data sharing: The data are not available for use by other researchers.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/2/E379/suppl/DC1.
References
- 1.Smart K. Tough choices needed to slow new wave of COVID-19 [news release] Ottawa: Canadian Medical Association; 2021. Dec 17, [accessed 2021 Dec. 21]. Available: https://www.cma.ca/news-releases-and-statements/tough-choices-needed-slow-new-wave-covid-19. [Google Scholar]
- 2.Soriano V, de Mendoza C, Gómez-Gallego F, et al. Third wave of COVID-19 in Madrid, Spain. Int J Infect Dis. 2021;107:212–4. doi: 10.1016/j.ijid.2021.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan G, Yang Z, Lin Q, et al. Decreased case fatality rate of COVID-19 in the second wave: a study in 53 countries or regions. Transbound Emerg Dis. 2021;68:213–5. doi: 10.1111/tbed.13819. [DOI] [PubMed] [Google Scholar]
- 4.Domingo P, Pomar V, Mur I, et al. Not all COVID-19 pandemic waves are alike. Clin Microbiol Infect. 2021;27:1040.e7–10. doi: 10.1016/j.cmi.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannidis JPA, Axfors C, Contopoulos-Ioannidis DG. Second versus first wave of COVID-19 deaths: shifts in age distribution and in nursing home fatalities. Environ Res. 2021;195:110856. doi: 10.1016/j.envres.2021.110856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radovanovic D, Santus P, Coppola S, et al. Characteristics, outcomes and global trends of respiratory support in patients hospitalized with COVID-19 pneumonia: a scoping review. Minerva Anestesiol. 2021;87:915–26. doi: 10.23736/S0375-9393.21.15486-0. [DOI] [PubMed] [Google Scholar]
- 7.Iftimie S, Lopez-Azcona AF, Vallverdu I, et al. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus, Spain. PLoS One. 2021;16:e0248029. doi: 10.1371/journal.pone.0248029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salyer SJ, Maeda J, Sembuche S, et al. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. Lancet. 2021;397:1265–75. doi: 10.1016/S0140-6736(21)00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seong H, Hyun HJ, Yun JG, et al. Comparison of the second and third waves of the COVID-19 pandemic in South Korea: importance of early public health intervention. Int J Infect Dis. 2021;104:742–5. doi: 10.1016/j.ijid.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawasuji H, Takegoshi Y, Kaneda M, et al. Transmissibility of COVID-19 depends on the viral load around onset in adult and symptomatic patients. PLoS One. 2020;15:e0243597. doi: 10.1371/journal.pone.0243597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avadhanula V, Nicholson EG, Ferlic-Stark L, et al. Viral load of severe acute respiratory syndrome coronavirus 2 in adults during the first and second wave of coronavirus disease 2019 pandemic in Houston, Texas: the potential of the superspreader. J Infect Dis. 2021;223:1528–37. doi: 10.1093/infdis/jiab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somerville M, Curran JA, Dol J, et al. Public health implications of SARS-CoV-2 variants of concern: a rapid scoping review. BMJ Open. 2021;11:e055781. doi: 10.1136/bmjopen-2021-055781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–88e11. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pairo-Castineira E, Clohisey S, Klaric L, et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–8. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra S, Wang L, Ma H, et al. Estimated surge in hospital and intensive care admission because of the coronavirus disease 2019 pandemic in the Greater Toronto Area, Canada: a mathematical modelling study. CMAJ Open. 2020;8:E593–604. doi: 10.9778/cmajo.20200093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palamim CVC, Marson FAL. COVID-19: the availability of ICU beds in Brazil during the onset of pandemic. Ann Glob Health. 2020;86:100. doi: 10.5334/aogh.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoukat A, Wells CR, Langley JM, et al. Projecting demand for critical care beds during COVID-19 outbreaks in Canada. CMAJ. 2020;192:E489–96. doi: 10.1503/cmaj.200457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomazini BM, Maia IS, Cavalcanti AB, et al. COALITION COVID-19 Brazil III Investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–16. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell JA, Marshall JC, Slutsky A, et al. Study protocol for a multicentre, prospective cohort study of the association of angiotensin II type 1 receptor blockers on outcomes of coronavirus infection. BMJ Open. 2020;10:e040768. doi: 10.1136/bmjopen-2020-040768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 23.COVID-19 daily epidemiology update. Ottawa: Government of Canada; [accessed 2021 June 1]. Available: https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html. [Google Scholar]
- 24.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy S, Archambault PM, Atique A, et al. SPRINT-SARI Canada Investigators and the Canadian Critical Care Trials Group. Characteristics and outcomes of patients with COVID-19 admitted to hospital and intensive care in the first phase of the pandemic in Canada: a national cohort study. CMAJ Open. 2021;9:E181–8. doi: 10.9778/cmajo.20200250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piroth L, Cottenet J, Mariet AS, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251–9. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RECOVERY Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:143–51. doi: 10.1016/S0140-6736(21)01825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ovadya D, Bachar K, Peled M, et al. Weaning of severe COVID-19 mechanically ventilated patients: experience within a dedicated unit in Israel. Isr Med Assoc J. 2020;22:733–5. [PubMed] [Google Scholar]
- 32.Bordon J, Akca O, Furmanek S, et al. Acute respiratory distress syndrome and time to weaning off the invasive mechanical ventilator among patients with COVID-19 pneumonia. J Clin Med. 2021;10:2935. doi: 10.3390/jcm10132935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curci C, Negrini F, Ferrillo M, et al. Functional outcome after inpatient rehabilitation in postintensive care unit COVID-19 patients: findings and clinical implications from a real-practice retrospective study. Eur J Phys Rehabil Med. 2021;57:443–50. doi: 10.23736/S1973-9087.20.06660-5. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JK, Lapin B, Green K, et al. Frequency of physical therapist intervention is associated with mobility status and disposition at hospital discharge for patients with COVID-19. Phys Ther. 2021;101:pzaa181. doi: 10.1093/ptj/pzaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasa P, Azoulay E, Khanna AK, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care. 2021;25:106. doi: 10.1186/s13054-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen LH, Drew DA, Graham MS, et al. COronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–83. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frost DW, Shah R, Melvin L, et al. Principles for clinical care of patients with COVID-19 on medical units. CMAJ. 2020;192:E720–6. doi: 10.1503/cmaj.200855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coccia M. The impact of first and second wave of the COVID-19 pandemic in society: comparative analysis to support control measures to cope with negative effects of future infectious diseases. Environ Res. 2021;197:111099. doi: 10.1016/j.envres.2021.111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko K, Nagashima SEB, Ouoba S, et al. Molecular characterization and the mutation pattern of SARS-CoV-2 during first and second wave outbreaks in Hiroshima, Japan. PLoS One. 2021;16:e0246383. doi: 10.1371/journal.pone.0246383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan WM, Ip JD, Chu AWH, et al. Phylogenomic analysis of COVID-19 summer and winter outbreaks in Hong Kong: an observational study. Lancet Reg Health West Pac. 2021;10:100130. doi: 10.1016/j.lanwpc.2021.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barallat J, Fernández-Rivas G, Quirant-Sánchez B, et al. Seroprevalence of SARS-CoV-2 IgG specific antibodies among healthcare workers in the Northern Metropolitan Area of Barcelona, Spain, after the first pandemic wave. PLoS One. 2020;15:e0244348. doi: 10.1371/journal.pone.0244348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Utrero-Rico A, Ruiz-Hornillos J, González-Cuadrado C, et al. IL-6-based mortality prediction model for COVID-19: validation and update in multicenter and second wave cohorts. J Allergy Clin Immunol. 2021;147:1652–61e1. doi: 10.1016/j.jaci.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.