Abstract
Background:
Few studies have modeled smoking histories by combining smoking intensity and duration to show what profile of smoking behavior is associated with highest risk of bladder cancer. This study aims to provide insight into the association between smoking exposure history and bladder cancer risk by modeling both smoking intensity and duration in a pooled analysis.
Methods:
We used data from 15 case–control studies included in the bladder cancer epidemiology and nutritional determinants study, including a total of 6,874 cases and 17,727 controls. To jointly interpret the effects of intensity and duration of smoking, we modeled excess odds ratios per pack–year by intensity continuously to estimate the risk difference between smokers with long duration/low intensity and short duration/high intensity.
Results:
The pattern observed from the pooled excess odds ratios model indicated that for a fixed number of pack–years, smoking for a longer duration at lower intensity was more deleterious for bladder cancer risk than smoking more cigarettes/day for a shorter duration. We observed similar patterns within individual study samples.
Conclusions:
This pooled analysis shows that long duration/low intensity smoking is associated with a greater increase in bladder cancer risk than short duration/high intensity smoking within equal pack–year categories, thus confirming studies in other smoking-related cancers and demonstrating that reducing exposure history to a single metric such as pack–years was too restrictive.
Keywords: Bladder cancer, Cancer risk, Pooled analysis, Smoking history, statistical modeling
Smoking is an important modifiable risk factor for urothelial bladder cancer (UBC) and studies demonstrate a differential dose–response pattern for intensity and duration.1 Many studies have investigated smoking behavior in relation to UBC, showing separate risk estimates for intensity, duration, and pack–year, but to our knowledge only a few studies have modeled complex smoking histories including all aspects of exposure such as duration, intensity, and time since smoking cessation.2,3
Most studies establishing the association between smoking history and various diseases use cumulative exposure (i.e., pack–years) in an attempt to go beyond smoking status only.4 However, more recently, consensus has been reached that modeling pack–years alone is not sufficient to identify possible mechanisms underlying such associations.5 Several researchers have discussed whether pack–years should be used to measure effects of smoking or whether pack–years can be useful in making biologically credible models that provide unbiased information on complex smoking exposure histories5,6 and circumventing multicollinearity issues.7 Although simultaneous and interpretable modeling of the effects of smoking behavior has been a research topic for several decades for other diseases, this have been infrequently investigated in UBC research.8,9
Two case–control studies in UBC both suggested that among equal pack–year categories, individuals who had smoked relatively fewer cigarettes per day for longer duration were at a higher risk of bladder cancer compared with those who smoked more cigarettes per day over a shorter duration.2,3 In these studies, estimates of the excess odds ratio (EOR) per pack–year were compared across categories of smoking intensity. Recently, similar models have been further developed and tested to also include time since smoking cessation10 or stratification by age category to consider timing of exposure.11 Using an alternative approach, two other case–control studies data also showed that duration is the over-riding factor in determining the risk of bladder cancer.12,13
The aim of this study was to investigate the association between cumulative smoking exposure and UBC risk, and to model and interpret the various smoking effects, in a uniquely large pooled sample of case–control studies.
METHODS
Study Data
The bladder cancer epidemiology and nutritional determinants (BLEND) consortium currently consists of 19 case–control studies and 14 cohort studies investigating the association between lifestyle behaviors and UBC risk. For this analysis, we included 15 case–control studies providing complete data on smoking behavior, including smoking status, intensity, and duration, These included 6,824 cases and 17,727 controls originating from Italy,13–15 Germany,16,17 Belgium,18 Sweden,19 Canada,20 the USA,21–26 and China.27 All smoking data were either collected through interview-administered questionnaires (n = 6) or self-administered questionnaires (n = 9). Further details on the methodology of this consortium have been described.28
Statistical Analysis and Delivery Rate of Exposure
We used a statistical approach described by Vlaanderen et al.10 The pooled smoking data were divided into quintile categories of pack–years, years of smoking, cigarettes per day, and time since smoking cessation. We obtained odds ratios (ORs) for these categories using a multilevel random effect logistic regression model adjusting for study, age, and sex as covariates. Subsequently, total pack–years were cross-classified by cigarettes smoked per day and years of smoking to estimate the ORs in combined exposure categories with never smokers as the reference group. Finally, we fitted an exponential model to estimate the EOR per pack–year by smoking intensity to investigate the independent effect of cigarette smoking duration and intensity of cigarette smoking on bladder cancer risk. In other words, with these models, long duration/low intensity smokers are compared with short duration/high intensity smokers with equal pack–years.
We used the model:
where the model was fitted using continuous pack–years (d), continuous intensity (n), and g1 as a three-knot restricted cubic spline function of continuous smoking intensity (knots located at 20th, 50th, and 80th percentile of the distribution of intensity of all smokers). This model was applied to each of the 15 studies.
The results from such models describe delivery rate patterns of exposure to tobacco smoking in relation to UBC risk. The delivery rate is described through estimating how increasing intensity or duration within a fixed number of pack–years influences bladder cancer risk. For example, an inverse exposure rate effect for intensity would mean that the EOR/pack–year (the strength of association) decreases with more cigarettes smoked per day (and thus decrease duration) or alternatively the EOR/pack–year increases with fewer cigarettes per day (and increased duration). Consequently, for two individuals with equal total pack–years, greater risk accrues to the individual smoking for longer duration at lower intensity.
A sensitivity analysis was performed with data from five studies that provided detailed data on time since smoking cessation by adding an extra three-knot restricted cubic spline (knots at the 20th, 50th, and 80th percentiles of the distribution of time since cessation of all former smokers) to the model, as incorporating time since cessation into these models might provide a better fit with the data.10 Additionally, different knot locations (at the 10th, 50th, and 90th and 5th, 50th, and 95th percentiles) were applied to assess the robustness of the associations. The fit of the models with different knot locations were tested using the Akaike information criterion (AIC). Ninety-five percent confidence intervals (CIs) for the EOR models were estimated through bootstrapping via 1,000 replications of the original data. The 2.5th and the 97.5th percentile of the subsequent distribution are shown in the fitted model. To assess the level of heterogeneity underlying this EOR model, we also repeated it in individual BLEND study populations.
RESULTS
Smoking Characteristics in Included Studies
Table 1 shows baseline characteristics for all included case–control studies. In most studies, at least 80% of current smokers at baseline smoked more than 10 cigarettes a day. The only study in which this proportion was much lower than the mean proportion for both current smokers (14%) and former smokers (4%) was the Swedish study19 (Table 1). Nine of the 15 studies demonstrated that 90% of current smokers had smoked for at least 20 years. This percentage was lower among former smokers (between 70% and 80%; Table 1). One study from the USA22 provided details on smoking behavior among current smokers only.
TABLE 1.
Smoking Behavior and Number of Cases and Controls in all Included BLEND Studies
| All Cases–Controls (n = 24,551) |
Current Smokers (n = 9,147) |
Former Smokers (n = 6,928) |
||||||
|---|---|---|---|---|---|---|---|---|
| Study Location | Total (Cases/Controls) | Current | Former | Never | >10 Cigarettes/Day | >20 Years of Cigarette Smoking | >10 Cigarettes/Day | >20 Years of Cigarette Smoking |
|
| ||||||||
| Total | 24,551 (6,824/17,727) | 9,147 | 6,928 | 8,476 | 4,523 | 6,179 | 4,751 | 5,044 |
| Europe | ||||||||
| Belgium13 | 582 (200/382) | 105 | 286 | 191 | 88 (84%) | 103 (98%) | 237 (83%) | 204 (71%) |
| Germany (1)14 | 561 (278/283) | 143 | 264 | 154 | 111 (78%) | 136 (95%) | 168 (64%) | 181 (69%) |
| Germany (2)15 | 421 (191/230) | 89 | 198 | 135 | 79 (90%) | 84 (95%) | 164 (83%) | 138 (70%) |
| Italy (1)16 | 1,734 (702/1,032) | 691 | 529 | 514 | 578 (84%) | 637 (92%) | 437 (83%) | 433 (82%) |
| Italy (2)17 | 413 (200/213) | 162 | 181 | 70 | 130 (80%) | 117 (72%) | 147 (81%) | 116 (64%) |
| Italy (3)18 | 1,324 (669/655) | 418 | 573 | 333 | 377 (90%) | 404 (97%) | 494 (86%) | 455 (79%) |
| Sweden19 | 748 (240/508) | 241 | 242 | 265 | 31 (14%) | 225 (93%) | 8 (4%) | 196 (81%) |
| North America | ||||||||
| Canada20 | 5,689 (898/4,800) | 1,205 | 2,390 | 2,103 | 1,078 (89%) | 1,023 (85%) | 1,872 (78%) | 1,462 (61%) |
| USA (1)21 | 3,179 (1,641/1,538) | 1,240 | 1,133 | 806 | 1,085 (88%) | 698 (56%) | 1,068 (94%) | 1,065 (94%) |
| USAa (2)22 | 533 (122/411) | 163 | – | 370 | 157 (96%) | 155 (95%) | – | – |
| USA (3)23 | 6,834 (399/6,435) | 3,363 | 125 | 3,346 | 2,585 (82%) | 2,203 (66%) | 97 (82%) | 91 (73%) |
| USA (4)24 | 664 (374/290) | 175 | 301 | 188 | 164 (95%) | 160 (91%) | 262 (87%) | 200 (66%) |
| USA (5)25 | 451 (184/267) | 63 | 229 | 159 | 55 (87%) | 47 (75%) | 213 (93%) | 162 (71%) |
| USA (6)26 | 457 (243/214) | 89 | 249 | 119 | 86 (97%) | 84 (94%) | 231 (93%) | 175 (70%) |
| Asia | ||||||||
| China27 | 952 (483/469) | 330 | 228 | 394 | 277 (84%) | 313 (95%) | 184 (81%) | 187 (82%) |
This study provided duration and intensity data for current smokers only.
Risk Estimates for Smoking Behavior
Based on the pooled results, current smokers had a higher UBC risk than never smokers (OR = 2.23, 95% CI = 2.05–2.42; Table 2). Tests for linear trend showed increasing risks across quintile categories of intensity, duration, and pack–years (P-values < 0.001). Furthermore, smoking cessation was related to a lower bladder cancer risk compared with current smokers (Table 2), with an OR of 0.40 (95% CI = 0.32–0.51) for those who had quit smoking more than 30 years before diagnosis. Bladder cancer risk for those who had quit smoking 30 years before diagnosis was very similar to those who had never smoked (OR = 1.04, 95% CI = 0.81–1.32).
TABLE 2.
Odds Ratios (ORs) and 95% Confidence Intervals (95% CI) for Bladder Cancer According to Smoking Status, Quintile Categories for Pack–Years, Duration and Intensity, and Time Since Smoking Cessation Overall and by Sex
| All Subjects |
Men |
Women |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 6,824) | Controls (n = 17,727) | ORa | 95% CI | Cases (n = 5,305) | Controls (n = 9,320) | ORa | 95% CI | Cases (n = 1,519) | Controls (n = 8,407) | ORa | 95% CI | |
|
| ||||||||||||
| Smoking status | ||||||||||||
| Never smoker | 1,493 | 7,654 | 1.00 | Ref | 834 | 2,662 | 1.00 | Ref | 659 | 4,992 | 1.00 | Ref |
| Former smoker | 2,765 | 4,163 | 1.94 | 1.78–2.11 | 2,337 | 3,092 | 2.05 | 1.85–2.27 | 428 | 1,071 | 1.85 | 1.57–2.19 |
| Current smoker | 2,566 | 5,910 | 2.23 | 2.05–2.42 | 2,134 | 3,566 | 2.29 | 2.06–2.53 | 432 | 2,344 | 2.24 | 1.91–2.62 |
| Pack–years | ||||||||||||
| Quintile 1 (<9) | 642 | 2,794 | 1.18 | 1.05–1.33 | 473 | 1,289 | 1.21 | 1.05–1.40 | 169 | 1,505 | 1.18 | 0.97–1.45 |
| Quintile 2 (9–17) | 684 | 2,191 | 1.60 | 1.43–1.80 | 525 | 1,315 | 1.50 | 1.31–1.72 | 159 | 876 | 1.91 | 1.54–2.38 |
| Quintile 3 (18–30) | 1,221 | 2,107 | 2.31 | 2.09–2.56 | 1,020 | 1,617 | 2.19 | 1.94–2.46 | 201 | 490 | 2.72 | 2.19–3.37 |
| Quintile 4 (31–46) | 1,248 | 1,446 | 2.76 | 2.48–3.06 | 1,054 | 1,099 | 2.70 | 2.39–3.05 | 194 | 347 | 3.13 | 2.49–3.94 |
| Quintile 5 (≥47) | 1,536 | 11,535 | 2.96 | 2.67–3.28 | 1,399 | 1,338 | 3.01 | 2.68–3.38 | 137 | 197 | 2.77 | 2.11–3.65 |
| P for linear trend | <0.001 | <0.001 | <0.001 | |||||||||
| Duration (in years) | ||||||||||||
| Quintile 1 (<16) | 603 | 2,492 | 1.14 | 1.01–1.28 | 475 | 1,262 | 1.20 | 1.04–1.38 | 128 | 1,230 | 1.12 | 0.89–1.41 |
| Quintile 2 (16–25) | 827 | 2,297 | 1.59 | 1.43–1.78 | 686 | 1,344 | 1.68 | 1.48–1.92 | 141 | 953 | 1.48 | 1.18–1.85 |
| Quintile 3 (26–35) | 1,277 | 2,191 | 2.24 | 2.03–2.47 | 1,071 | 1,484 | 2.31 | 2.05–2.60 | 206 | 707 | 2.14 | 1.74–2.62 |
| Quintile 4 (36–43) | 1,193 | 1,476 | 2.64 | 2.38–2.94 | 968 | 1,165 | 2.55 | 2.26–2.89 | 225 | 311 | 3.32 | 2.67–4.14 |
| Quintile 5 ((≥44) | 1,431 | 1,617 | 3.10 | 2.79–3.44 | 1,271 | 1,403 | 3.18 | 2.81–3.59 | 160 | 214 | 3.27 | 2.54–4.21 |
| P for linear trend | <0.001 | <0.001 | <0.001 | |||||||||
| Intensity (in cigarettes/day) | ||||||||||||
| Quintile 1 (<7) | 551 | 1,901 | 1.39 | 1.22–1.57 | 394 | 933 | 1.37 | 1.17–1.60 | 157 | 968 | 1.44 | 1.16–1.79 |
| Quintile 2 (7–10) | 775 | 2,932 | 1.80 | 1.62–2.01 | 597 | 1,649 | 1.74 | 1.52–1.99 | 178 | 1,283 | 1.82 | 1.48–2.24 |
| Quintile 3 (11–19) | 777 | 1,082 | 2.19 | 1.95–2.47 | 648 | 805 | 2.15 | 1.87–2.47 | 129 | 277 | 2.66 | 2.05–3.44 |
| Quintile 4 (20–29) | 1,862 | 2,362 | 2.37 | 2.16–2.60 | 1,590 | 1,865 | 2.34 | 2.09–2.61 | 272 | 497 | 2.85 | 2.33–3.49 |
| Quintile 5 (≥30) | 1,366 | 1,794 | 2.62 | 2.36–2.91 | 1,242 | 1,404 | 2.70 | 2.40–3.04 | 124 | 390 | 2.09 | 1.58–2.74 |
| P for linear trend | <0.001 | <0.001 | <0.001 | |||||||||
| Time since smoking cessationb | ||||||||||||
| Current smoker | 907 | 1,566 | 1.00 | Ref | 748 | 1,057 | 1.00 | ref | 159 | 509 | 1.00 | Ref |
| 1–5 years | 220 | 372 | 0.93 | 0.76–1.13 | 194 | 275 | 0.96 | 0.77–1.19 | 26 | 97 | 0.88 | 0.54–1.44 |
| 6–10 years | 193 | 428 | 0.76 | 0.62–0.93 | 164 | 294 | 0.80 | 0.64–1.00 | 29 | 134 | 0.66 | 0.41–1.04 |
| 11–15 years | 171 | 400 | 0.72 | 0.58–0.89 | 153 | 272 | 0.83 | 0.66–1.05 | 18 | 128 | 0.38 | 0.22–0.66 |
| 16–20 years | 138 | 401 | 0.59 | 0.47–0.74 | 115 | 279 | 0.60 | 0.47–0.77 | 23 | 122 | 0.61 | 0.37–1.01 |
| 21–30 years | 198 | 600 | 0.58 | 0.48–0.71 | 180 | 410 | 0.70 | 0.56–0.87 | 18 | 190 | 0.27 | 0.16–0.46 |
| >30 years | 123 | 445 | 0.40 | 0.32–0.51 | 113 | 327 | 0.48 | 0.37–0.62 | 10 | 118 | 0.17 | 0.08–0.33 |
| P for linear trend | <0.001 | <0.001 | <0.001 | |||||||||
Adjusted for age. Overall estimates also adjusted for sex.
Data only present in 5/15 studies.
Delivery Rate Patterns of Exposure to Smoking in Relation to Urothelial Bladder Cancer Risk
We calculated 15 ORs, with never smokers as reference category, in the analysis stratified by intensity quintile (Figure 1), while 20 ORs were estimated in the analysis stratified by duration quintile (eFigure 1; http://links.lww.com/EDE/B457) because data were sparse in the intensity categories. None of the associations showed any departures from linearity (P > 0.05 for all categories), which means that the EOR model as it is presented is valid in meeting the assumption about linearity of association between exposure and disease.
FIGURE 1.
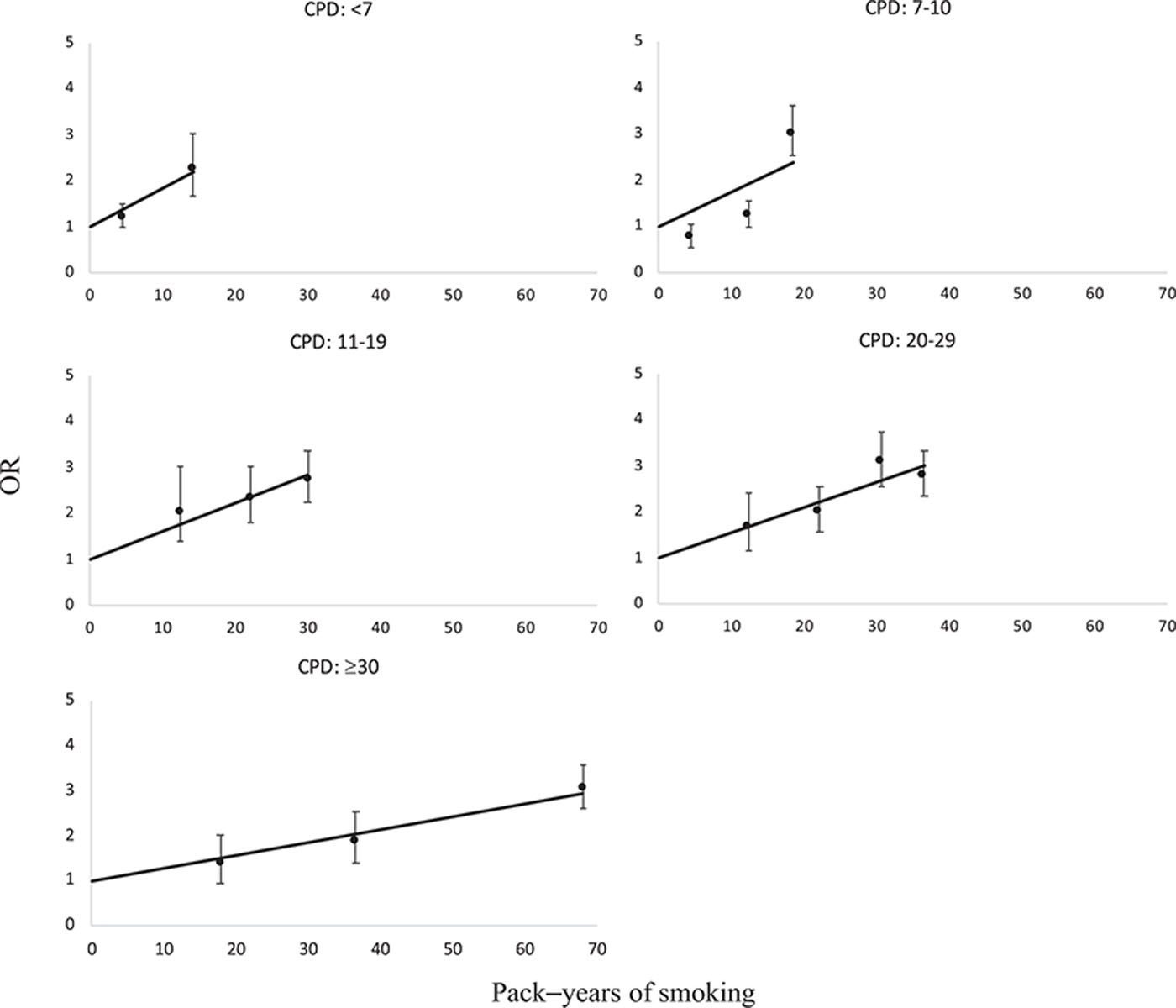
Odds ratios (OR) for bladder cancer by cross-classified categories of pack–years and quintile categories of number of cigarettes smoked per day (CPD). Lines indicate fitted linear odds ratio models in pack–years, bars indicate 95% confidence intervals. Pooled data were limited to never and current smokers.
The EOR per pack–year and 95% CI by continuous smoking intensity (cigarettes/day) resulting from the cubic spline model are plotted in Figure 2. Additionally, the slope resulting from the model including splines for time since smoking cessation (TSC) is also shown. The model excluding TSC had a slightly better fit to the data (AIC = 23,14) compared with the model including TSC (AIC = 24,22), probably because the effect of TSC was heterogeneous between the few included studies. Both curves show an inverse delivery rate pattern, whereby with increasing cigarettes smoked per day (and decreasing duration) the EOR per pack–year decreases. This indicates that for equal pack–years, smoking for a longer duration (at few cigarettes/day) is more strongly associated with UBC risk than smoking more cigarettes per day (for a shorter duration). As can be observed from the bootstrapped 95% CI, the plotted curve had the highest number of participants for individuals smoking between 10 and 40 cigarettes per day, which included 79% of all smokers in this consortium, and therefore the shape of the curve is most reliable on this interval.
FIGURE 2.
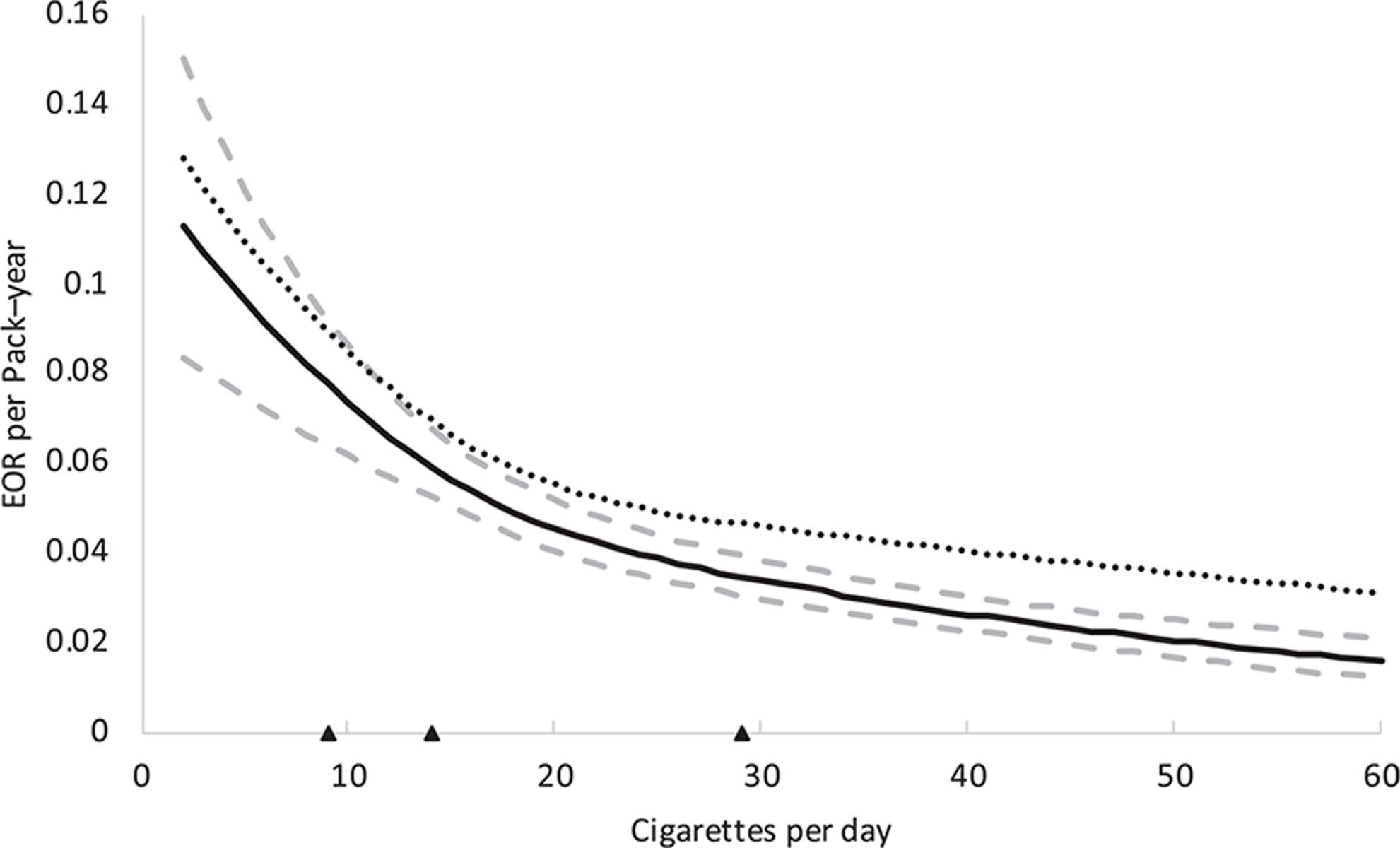
Estimated excess odds ratio (EOR) per pack–year for bladder cancer by cigarettes per day with bootstrapped 95% confidence intervals. The dotted line indicates a model including an extra spline for time since smoking cessation. Triangles depict locations of the knots of the restricted cubic splines (20th, 50th, and 80th percentile).
Heterogeneity was small among the 10 individual studies in which EOR models could be fit with the original spline settings (eFigure 2A; http://links.lww.com/EDE/B457). For 3 studies14,23,25 the model did not fit because of their data distribution (e.g., 19 cigarettes per day represented the 44th percentile and 20 cigarettes per day represented the 82th percentile of the data), and there was limited power within two studies16,27 (too many levels of intensity with no cases). When moving the splines to positions fitting the data distribution in the three studies with a different data distribution, the three added curves show a similar shape to the EOR curves from the 10 studies that were estimated with the original spline settings (eFigure 3; http://links.lww.com/EDE/B457). Additionally, the EOR models within the three studies that included sufficient data on TSC15,18,20 were also similar (eFigure 2B; http://links.lww.com/EDE/B457).
DISCUSSION
We have provided insight into the complex exposure patterns of lifetime smoking behavior and the impact on UBC risk. We have shown an inverse delivery rate pattern indicating that, for equal pack–years of smoking, fewer cigarettes per day over a longer duration is more deleterious for UBC risk than smoking more cigarettes per day over a shorter duration. The results of this pooled analysis of 15 case–control studies are in line with data from two other previous case–control studies on bladder cancer applying a similar approach.2,3
Robustness of Results
We applied the model as described by Vlaanderen et al. but a similar approach was first described in a lung cancer study,29 known as the L-C (Lubin-Caparaso) model, which has also been applied in a pooled analysis of case–control studies on head and neck cancer30 and in two individual UBC case–control studies.2,3 Alongside these models, Brennan et al. described a different approach in 2000 that was based on stratification of both duration and intensity and estimating ORs in all strata. They also observed that duration was more important in predicting bladder cancer risk than intensity.12 Nevertheless, the approach Brennan et al. took does not allow for an unambiguous interpretation of the separate RRs; the question remains whether the increase in risk derives from duration or from pack–years, which increases concurrently. The modeling approach applied in our study does answer this question since the results show the risk difference between long duration/low intensity and short duration/high intensity smokers with equal pack–years.
Similar ORs were observed for both women and men, although more men smoked at least 10 cigarettes per day (86%) compared with women (73%), possibly explaining differences in precision of risk estimates, in addition to the smaller sample of women in the included studies. Furthermore, observed ORs for smokers might be underestimated since the pooled OR for current smokers was markedly lower than observed in a large meta-analysis1 (OR = 2.23 in the current sample vs. OR = 3.14 in the meta-analysis). This might be explained by some misclassification of smoking information collected through self-administered questionnaires in the 15 included studies or differences in data collection in the meta-analysis.
Notwithstanding, the selection of 15 studies that agreed to participate in this consortium might also not be representative of all bladder cancer case–control studies present, since most participating studies are from either Europe and the USA.
Little heterogeneity in the range of predicted EORs per pack–year by cigarettes per day between the included studies was observed (eFigure 2; http://links.lww.com/EDE/B457). However, some heterogeneity in magnitude of estimated EORs per pack–year remains between the studies, which may be explained by several factors such as geographic location1 and calendar year in which cases and controls were recruited.31 As only five studies provided sufficient data on TSC, this pooled analysis might not have had sufficient power to include TSC as an extra spline.
Strengths and Limitations of the Excess Odds Ratio Model and Interpretation of Results
Although the EOR model can provide a more detailed insight into the association between smoking behavior and disease risk, there are some other factors not in this model that also need to be considered. Since a more vigorous inhalation pattern has been shown to be associated with a higher UBC risk,32,33 the observed inverse delivery rate pattern might reflect differences in inhalation patterns among cigarette smokers. It is generally believed that light smokers inhale more vigorously compared with heavier smokers to achieve the same amount of nicotine consumption,34,35 therefore possibly confounding the risk estimates comparing heavy to light smokers. However, inhalation was not found to be a confounder of pack–years-adjusted cigarettes per day patterns in a lung cancer study.29 Data on inhalation patterns were not available for the study participants within BLEND.
Moreover, since no data were available on time periods during which study participants might have smoked less (or more) than their average estimated intensity, we could not account for this.
Owing to the retrospective nature of data collection in case–control studies including such detailed data on smoking behavior would not have been possible in this pooled analysis; however, in prospective studies such periodical changes in smoking intensity could be accounted for when applying the EOR models by adding TSC or time since moderation splines if data are gathered. Nevertheless, the five case–control studies that did gather data on TSC showed a similar shape of the EOR curve (Figure 2). This EOR model provides one of the most detailed UBC risk predictions following different durations and intensities of smoking. Nevertheless, there have been other methods to model smoking history in relation to cancer such as the comprehensive smoking index, which also incorporates intensity, duration, and time since cessation.36
Smoking Behavior and Molecular Pathways to Bladder Carcinogenesis
Tobacco smoke contains many carcinogens that can contribute to carcinogenesis in the bladder. These carcinogens can form DNA adducts and, when multiple types of DNA adducts are combined, they contribute greatly to human cancer risk.37 Several studies have shown that nicotine-derived nitrosamine ketones (NNK), methyl and other DNA adducts are more frequently present in UBC patients who have smoked compared with those who have never smoked.38,39 Nevertheless, it is not clear how NT2 status is involved in an inverse smoking intensity effect in at population level. It has not been measured whether rapid acetylators are more likely to be high intensity/short duration smokers. Moreover, there is heterogeneity in the efficiency of DNA repair pathways between individuals; for example, those who have a slow N-acetyltransferase phenotype have a higher risk of UBC when they smoke,40 and DNA repair processes can also be negatively influenced by longer smoking duration or higher cumulative exposure (in pack–years).41 This indicates that the DNA adduct pathway of UBC pathogenesis is important in smoking-related UBC. Although not directly implied from our data as we did not measure DNA adducts, the risk difference between long duration/low intensity smokers and short duration/high intensity smokers could be explained by the longer exposure period for accumulation of smoking-related DNA adducts in long duration/low intensity smokers. The results from our study, as well as of other studies in UBC,2,3 lung cancer,10,29 and head and neck cancer,30 are consistent in showing that smoking fewer cigarettes over a longer duration increases disease risk more than smoking at a higher intensity for a shorter duration when pack–years are equal. Therefore, future studies should investigate differences in DNA repair pathways between long duration/low intensity versus short duration/high intensity smokers as the studies discussed in this paragraph focus only on intensity or duration separately in relation to DNA adducts. Nevertheless, these results have major implications for prevention at public health level and can impact the public’s perception on smoking and health risks.
CONCLUSION
We have demonstrated that long duration/low intensity smoking behavior is most strongly associated with UBC risk within equal pack–year categories in this pooled analysis, thereby confirming studies in two case–control studies on UBC as well as other smoking-related cancers. Furthermore, with this model we found that reducing complex exposure history to a single metric such as pack–years was too restrictive, and future research should focus on interpretable ways to model complex cumulative exposures such as lifetime smoking behavior.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge all principal investigators of the studies included in the BLEND consortium for their willingness to participate in this collaborative project. This study was partly funded by the World Cancer Research Fund.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
Data availability:
Computing code is available by request to the corresponding author. Data from this consortium is not available offsite and cannot be shared online.
REFERENCES
- 1.van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP. Quantified relations between exposure to tobacco smoking and bladder cancer risk: a meta-analysis of 89 observational studies. Int J Epidemiol 2016;45:857–870. [DOI] [PubMed] [Google Scholar]
- 2.Lubin JH, Kogevinas M, Silverman D, et al. Evidence for an intensity-dependent interaction of NAT2 acetylation genotype and cigarette smoking in the Spanish Bladder Cancer Study. Int J Epidemiol. 2007;36:236–241. [DOI] [PubMed] [Google Scholar]
- 3.Baris D, Karagas MR, Verrill C, et al. A case-control study of smoking and bladder cancer risk: emergent patterns over time. J Natl Cancer Inst. 2009;101:1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burch JD, Rohan TE, Howe GR, et al. Risk of bladder cancer by source and type of tobacco exposure: a case-control study. Int J Cancer. 1989;44:622–628. [DOI] [PubMed] [Google Scholar]
- 5.de Vocht F, Burstyn I, Sanguanchaiyakrit N. Rethinking cumulative exposure in epidemiology, again. J Expo Sci Environ Epidemiol. 2015;25:467–473. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DC. Invited commentary: is it time to retire the “pack-years” variable? Maybe not! Am J Epidemiol. 2014;179:299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leffondré K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. Am J Epidemiol. 2002;156:813–823. [DOI] [PubMed] [Google Scholar]
- 8.Moolgavkar SH, Dewanji A, Luebeck G. Cigarette smoking and lung cancer: reanalysis of the British doctors’ data. J Natl Cancer Inst. 1989;81:415–420. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DC. Statistical Methods in Environmental Epidemiology. Oxford: Oxford University Press; 2009. [Google Scholar]
- 10.Vlaanderen J, Portengen L, Schüz J, et al. Effect modification of the association of cumulative exposure and cancer risk by intensity of exposure and time since exposure cessation: a flexible method applied to cigarette smoking and lung cancer in the SYNERGY Study. Am J Epidemiol. 2014;179:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63:6556–6562. [PubMed] [Google Scholar]
- 12.Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000;86:289–294. [DOI] [PubMed] [Google Scholar]
- 13.Polesel J, Bosetti C, di Maso M, et al. Duration and intensity of tobacco smoking and the risk of papillary and non-papillary transitional cell carcinoma of the bladder. Cancer Causes Control. 2014;25:1151–1158. [DOI] [PubMed] [Google Scholar]
- 14.Hung RJ, Boffetta P, Brennan P, et al. GST, NAT, SULT1A1, CYP1B1 genetic polymorphisms, interactions with environmental exposures and bladder cancer risk in a high-risk population. Int J Cancer. 2004;110:598–604. [DOI] [PubMed] [Google Scholar]
- 15.Randi G, Pelucchi C, Negri E, et al. Family history of urogenital cancers in patients with bladder, renal cell and prostate cancers. Int J Cancer. 2007;121:2748–2752. [DOI] [PubMed] [Google Scholar]
- 16.Pohlabeln H, Jöckel KH, Bolm-Audorff U. Non-occupational risk factors for cancer of the lower urinary tract in Germany. Eur J Epidemiol. 1999;15:411–419. [DOI] [PubMed] [Google Scholar]
- 17.Golka K, Heitmann P, Gieseler F, et al. Elevated bladder cancer risk due to colorants–a statewide case-control study in North Rhine-Westphalia, Germany. J Toxicol Environ Health A. 2008;71:851–855. [DOI] [PubMed] [Google Scholar]
- 18.Kellen E, Zeegers M, Paulussen A, Van Dongen M, Buntinx F. Fruit consumption reduces the effect of smoking on bladder cancer risk. The Belgian case control study on bladder cancer. Int J Cancer. 2006;118:2572–2578. [DOI] [PubMed] [Google Scholar]
- 19.Augustsson K, Skog K, Jägerstad M, Dickman PW, Steineck G. Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: a population-based study. Lancet. 1999;353:703–707. [DOI] [PubMed] [Google Scholar]
- 20.Gaertner RR, Trpeski L, Johnson KC; Canadian Cancer Registries Epidemiology Research Group. A case-control study of occupational risk factors for bladder cancer in Canada. Cancer Causes Control. 2004;15:1007–1019. [DOI] [PubMed] [Google Scholar]
- 21.Jiang X, Castelao JE, Yuan JM, et al. Cigarette smoking and subtypes of bladder cancer. Int J Cancer. 2012;130:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang L, Zirpoli GR, Guru K, et al. Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:938–944. [DOI] [PubMed] [Google Scholar]
- 23.Quirk JT, Li Q, Natarajan N, Mettlin CJ, Cummings KM. Cigarette smoking and the risk of bladder cancer in men and women. Tob Induc Dis. 2004;2:141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karagas MR, Tosteson TD, Blum J, Morris JS, Baron JA, Klaue B. Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a U.S. population. Environ Health Perspect. 1998;106(Suppl 4):1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao W, Cai L, Rao JY, et al. Tobacco smoking, GSTP1 polymorphism, and bladder carcinoma. Cancer. 2005;104:2400–2408. [DOI] [PubMed] [Google Scholar]
- 26.Taylor JA, Umbach DM, Stephens E, et al. The role of N-acetylation polymorphisms in smoking-associated bladder cancer: evidence of a gene-gene-exposure three-way interaction. Cancer Res. 1998;58:3603–3610. [PubMed] [Google Scholar]
- 27.Hemelt M, Hu Z, Zhong Z, et al. Fluid intake and the risk of bladder cancer: results from the South and East China case-control study on bladder cancer. Int J Cancer. 2010;127:638–645. [DOI] [PubMed] [Google Scholar]
- 28.Goossens ME, Isa F, Brinkman M, et al. International pooled study on diet and bladder cancer: the bladder cancer, epidemiology and nutritional determinants (BLEND) study: design and baseline characteristics. Arch Public Health. 2016;74:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15:517–523. [DOI] [PubMed] [Google Scholar]
- 30.Lubin JH, Purdue M, Kelsey K, et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2009;170:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryan RT, Zeegers MP, van Roekel EH, et al. A comparison of patient and tumour characteristics in two UK bladder cancer cohorts separated by 20 years. BJU Int 2013;112:169–175. [DOI] [PubMed] [Google Scholar]
- 32.Zeegers MP, Goldbohm RA, van den Brandt PA. A prospective study on active and environmental tobacco smoking and bladder cancer risk (The Netherlands). Cancer Causes Control 2002;13:83–90. [DOI] [PubMed] [Google Scholar]
- 33.López-Abente G, González CA, Errezola M, et al. Tobacco smoke inhalation pattern, tobacco type, and bladder cancer in Spain. Am J Epidemiol. 1991;134:830–839. [DOI] [PubMed] [Google Scholar]
- 34.Fidler JA, Stapleton JA, West R. Variation in saliva cotinine as a function of self-reported attempts to reduce cigarette consumption. Psychopharmacology (Berl). 2011;217:587–593. [DOI] [PubMed] [Google Scholar]
- 35.Patterson F, Benowitz N, Shields P, et al. Individual differences in nicotine intake per cigarette. Cancer Epidemiol Biomarkers Prev. 2003;12: 468–471. [PubMed] [Google Scholar]
- 36.Leffondré K, Abrahamowicz M, Xiao Y, Siemiatycki J. Modelling smoking history using a comprehensive smoking index: application to lung cancer. Stat Med. 2006;25:4132–4146. [DOI] [PubMed] [Google Scholar]
- 37.Poirier MC. Chemical-induced DNA damage and human cancer risk. Discov Med. 2012;14:283–288. [PMC free article] [PubMed] [Google Scholar]
- 38.Jin F, Thaiparambil J, Donepudi SR, et al. Tobacco-Specific Carcinogens Induce Hypermethylation, DNA Adducts, and DNA Damage in Bladder Cancer. Cancer Prev Res (Phila). 2017;10:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Talaska G, al-Juburi AZ, Kadlubar FF. Smoking related carcinogen-DNA adducts in biopsy samples of human urinary bladder: identification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl as a major adduct. Proc Natl Acad Sci U S A. 1991;88:5350–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vineis P, Marinelli D, Autrup H, et al. Current smoking, occupation, N-acetyltransferase-2 and bladder cancer: a pooled analysis of genotype-based studies. Cancer Epidemiol Biomarkers Prev. 2001;10:1249–1252. [PubMed] [Google Scholar]
- 41.Corral R, Lewinger JP, Van Den Berg D, et al. Comprehensive analyses of DNA repair pathways, smoking and bladder cancer risk in Los Angeles and Shanghai. Int J Cancer. 2014;135:335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Computing code is available by request to the corresponding author. Data from this consortium is not available offsite and cannot be shared online.


