Abstract
Plastic pollution in a growing problem globally. In addition to the continuous flow of plastic particles to the environment from direct sources, and through the natural wear and tear of items, the plastics that are already there have the potential to breakdown further and therefore provide an immense source of plastic particles. With the continued rise in levels of plastic production, and consequently increasing levels entering our marine environments it is imperative that we understand its impacts. There is evidence microplastic and nanoplastic (MNP) pose a serious threat to all the world's marine ecosystems and biota, across all taxa and trophic levels, having individual- to ecosystem-level impacts, although these impacts are not fully understood. Microplastics (MPs; 0.1–5 mm) have been consistently found associated with the biota, water and sediments of all coral reefs studied, but due to limitations in the current techniques, a knowledge gap exists for the level of nanoplastic (NP; <1 µm). This is of particular concern as it is this size fraction that is thought to pose the greatest risk due to their ability to translocate into different organs and across cell membranes. Furthermore, few studies have examined the interactions of MNP exposure and other anthropogenic stressors such as ocean acidification and rising temperature. To support the decision-making required to protect these ecosystems, an advancement in standardised methods for the assessment of both MP and NPs is essential. This knowledge, and that of predicted levels can then be used to determine potential impacts more accurately.
Keywords: anthropogenic stressors, coral reefs, microplastics, nanoplastics
Introduction
Since plastics came into mainstream use its production has increased exponentially, increasing from 2 million tonnes per year in 1950 to 368 million tonnes in 2019 [1]. Plastics are now used in every aspects of our daily lives. With this increase in plastic production has come an increase in plastic pollution. This has resulted in a global problem, affected aquatic and terrestrial environments, from the Arctic to Antarctic [2–4], the highest mountain tops [5], to remote islands [6] and the deep ocean [7].
The weathering of large plastic items results in them gradually being broken into progressively smaller fragments. The processes involved in weathering include mechanical abrasion resulting from natural wear and tear (e.g. car tyres and clothing), hydrolysis, UV photodegradation, biodegradation and biological ingestion [8–13]. These plastic particles <5 mm are referred to as ‘secondary’ microplastics (MPs) (Figure 1) [14], whilst nanoplastics (NPs) are generally defined as particles of either <100 nm or <1 µm [15,16]. Another class of these plastic particles are referred to as ‘primary’ microplastic and nanoplastic (MNP), and are manufactured within this size range and include, but are not limited to, such things as microbeads and fragments used in consumer products and industrial abrasives, glitter and preproduction pellets (nurdles) (Figure 1) [17–20]. These may enter the environment directly through accidental spills or in wastewater effluent [21–24].
Figure 1. Beached plastic pollution.
(A) Plastic drink bottles are an example of macroplastics, which through weathering will fragment into meso- micro- and nanoplastics. (B,C) Microplastics gathered from the last high tide mark. Preproduction pellets (nurdles) and fragments of polyethylene and polypropylene are often predominant due to their low density.
Coastal environments are generally considered to be the areas amongst those most highly impacted by plastic pollution [25]. Modelling has suggested that in 2016 around 19–23 million tonnes (11% of plastic waste generated globally that year) entered aquatic ecosystems [26]. Due to the scarcity of data the levels of small plastic fragments are uncertain but estimates suggest that there are 15–51 trillion particles present in the oceans, weighing between 93 000 and 236 000 tonnes [27].
The fate of MNPs on entering the marine environment is dependent on its density. Whilst there has been focus on buoyant plastics aggregating in the five major oceanic gyres it has been estimated that 77% of buoyant marine plastic debris originating from land is either beached or floating in coastal waters [28] and it is not necessarily associated with a local source, which is evident by the high levels of plastic debris found in remote and uninhabited islands [28,29]. Although many studies have looked at the levels and types of MPs associated with the coral reef ecosystems relatively little is known, and nothing is known of the levels of NPs due to the limitations of current methods. Differences in sampling protocols and isolation and identification methods directly influences the results, consequently, in combination with natural variability (spatial and temporal) it is not possible to accurately compare studies. There is, therefore, a significant need for standardised methods.
The ingestion of plastics by animals is most commonly associated with macroplastics and larger vertebrates such as seabirds, cetaceans, sirenians and sea turtles [30–32], and MPs by filter-feeding bivalves. Organisms at different trophic levels have been found to ingest MNPs directly and indirectly through trophic transfer [33,34]. Zooplankton and phytoplankton have been shown in laboratory studies that they are capable of MNP uptake [35–38] and therefore MNPs are able to enter the food chain at the very bottom.
The potential impacts of MNPs are not fully understood but range from physical damage and nutritional impairment to physiological, and have been found to affect all taxa so far examined, as well as across trophic levels, feeding strategies and niches. The type and degree of the MNPs have found to be influenced by their physical and chemical characteristics, including: size, morphology (bead, fibre, fragment, film), base polymer (e.g. polypropylene) and associated chemicals (organic and inorganic), as well as the species that they interact with. They also present an indirect threat at the organism and ecosystem level as they provide a substrate for microorganisms, therefore, potentially altering biogeochemical processes, and for the settlement of biota and therefore pose a risk by acting as a vector for invasive species and potential pathogens.
Here, we assess the current knowledge of the presence and impacts of MNPs in coral reef ecosystems and identify gaps and future research needs.
Occurrence of microplastics within the coral reef ecosystem
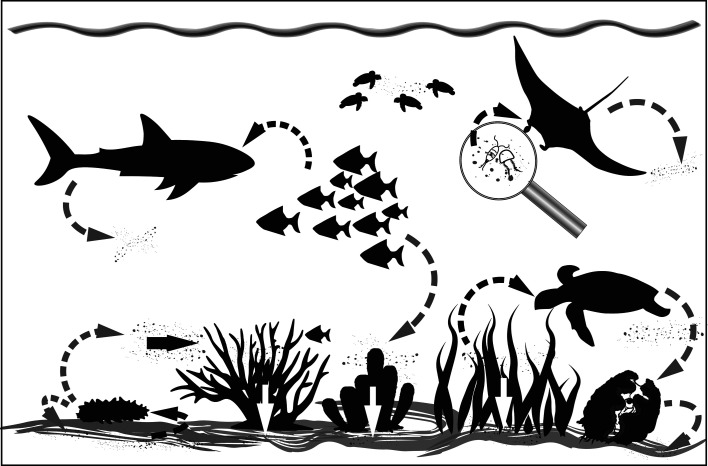
Although many studies have looked at the levels and types of MPs associated with coral reef ecosystems [39,40] the lack of standardised methods, including the units used for reporting, in combination with spatial and temporal variation, makes it difficult to make accurate comparisons between sites and organism. Due to the highly resistant nature of plastics, they are continuously cycled within the coral reef ecosystem (Figure 2), with few points at which to be removed permanently from circulation. This, therefore, means that the continued input will, over time, result in a gradual increase in concentration.
Figure 2. Cycling of micro- and nanoplastics (MNPs) within the coral reef system.
MNPs may be ingested (black dashed lines) either directly or indirectly via trophic transfer. Ingestion may also result when particles area adhered to the surface of food items, for example, those adhered to seagrass will be ingested by grazing turtles. Due to the resistant nature of plastic, it remains undigested and passes through the digestive tract and cycles back into the system (grey dashed arrows). The complex 3D structure of species such as corals and seagrass cause the transfer of MNPs from the water column to the sediments (solid black and white arrows).
Surface water
MPs in the surface waters (Table 1) of remote coral reef areas are lower than those of coastal reefs close to high human populations [51,62]. For example, Connors [44] found concentrations in the waters off Mo'orea of 0.74 MP m−2, and Tan [29] of ∼6 × 10−2 MP m−2 of surface waters off the remote uninhabited coral reefs of Nansha Islands in the South China Sea. In contrast, levels of 4.3 MP m−2 have been reported in the surface waters of near-shore reefs on the Great Barrier Reef, Australia [63]. An exception to this is the surface waters of the Red Sea [53] which are significantly lower (3.546 × 10−3 ± 8.154 × 10−3 MP m−2) which may be a result of the low inputs of land-based MP pollution, due to low coastal populations, coupled with low rainfall and therefore fluvial and stormwater inputs [64]. It should, however, be acknowledged that although potential MNPs inputs are low, concentrations in both the water column and sediments may increase disproportionately compared with other areas due to the low rate of water exchange and high evaporation rate of this nearly enclosed marginal sea [65]. Although levels may vary between locations, fibres and fragments are consistently found to be the most common morphotype, and polyethylene, polypropylene, polyethylene terephthalate and nylon are the most common polymer types [42,45,57,66,67].
Table 1. Examples of microplastic concentrations in coral reef water, sediments and biota.
| Location | Size | Abundance | Reference | |
|---|---|---|---|---|
| Reef water | ||||
| Great Barrier Reef, Australia | 100–500 µm | 2 MP per 11 m3 | [41] | |
| Great Barrier Reef, Australia | 0.355–5 mm | 0.04–0.48 MP m−3 | [42] | |
| Australian waters | 0.4–82.6 mm | 4.26 × 10−2 ± 7.4 × 10−3 MP km−2 (0–4.89 × 10−2 MP m−2) | [43] | |
| Mo'orea, French Polynesia | 0.05–5 mm | 0.74 MP m−2 | [44] | |
| Faafu Atoll, Maldives | 330 µm–5 mm | 0.26 particles m−3(0.02–0.48 MP m−3) | [45] | |
| Faafu Atoll, Maldives | 200 µm–5 mm | 0.12 ± 0.09 particles m−3 (0.03–0.65 MP m−3) | [46] | |
| Xisha Islands, South China Sea | 7–4856 µm | 200–45 200 MP m−3 | [47] | |
| Nansha Islands, South China Sea | 1.6–5000 µm | 0.469 ± 0.219 MP m−3 (0.148–0.842 MP m−3) | [48] | |
| 50 µm–5 mm | 4933 ± 1369 MP m−3 (1400–8100 MP m−3) | [49] | ||
| 48 µm–5 mm | 1733 MP m−3 (1250–3200 MP m−3) | [50] | ||
| 20 µm–5 mm | 0.0556 ± 0.0355 MP m−3 (0.0112–0.149 MP m−3) | [29] | ||
| Gulf of Mannar, India | 0.8–5 mm | 60 000–126 000 MP m−3 | [51] | |
| Albuquerque Atoll, Caribbean Sea | 1–5 mm | 0.059 MP m−3 | [52] | |
| Red Sea | 0.26–30 mm | 3.546 × 10−3 ± 8.154 × 10−3 MP km−2 | [53] | |
| Sediment | ||||
| Vavvaru Island, Maldives | >1 mm | 35.8 ± 42.5 MP m−2; 1029 ± 1134 MP m−2 in accumulation zones | [54] | |
| Four Coral Islands, Hong Kong | 0.3–5 mm | 194.5 ± 49.9 MP kg−1 (171.7 ± 57.6 to 223 ± 51.4 MP kg−1) | [55] | |
| Xisha Is. Nansha is, Weizhou Is. and Sanya Lu Hui Tou, South China Sea | 0.3–5 mm | 60 ± 3 to 610 ± 11 MP kg−1; 40 ± 4 to 100 ± 2 MP kg−1; 60 ± 2 to 90 ± 5 MP kg−1, and; 50 ± 3 to 350 ± 7 MP kg−1, respectively | [56] | |
| Gulf of Mannar, India | 0.8–5 mm | 50–103.8 MP kg−1 | [51] | |
| 2–5 mm | 55 ± 2a1 to 259 ± 88 MP kg−1 | [57] | ||
| Lhaviyani Atoll, Maldives | 63 µm–4 mm | 277.90 ± 24.98 MP kg−1 (55–1127.5 MP kg−1) | [58] | |
| Kepulauan Seribu, Indonesia | 125 µm–5 mm | 59.47 ± 78.49 MP kg−1 | [59] | |
| Biota | ||||
| Taxa | Size | Abundance | ||
| Western Australia | Turtle | <1 mm | 0–343 MP per individual (10.83 ± 36.72 MP per individual) | [60] |
| Rhode Island, U.S.A. | Coral | 0.4–5 mm | 112 MP per polyp | [61] |
| Xisha Island, South China Sea | Coral | 24–4729 µm | 1–44 MP per individual | [47] |
| Nansha Islands, South China Sea | Fish | <500 µm | 1–14 MP per individual | [50] |
Standard methods are better established for the assessment of surface water MNPs than sediments and biota. This is likely due to MNPs first being examined in plankton trawls and therefore using an already accepted method. However, as more studies are carried out, and with the introduction of new methods, there is a risk that the ability to compare between studies will be compromised. Therefore, official internationally agreed standard methods are crucial to understanding this global problem.
Sediments
Plastic particles that enter the oceans can become deposited in sediments, either directly due to their density or as a result of biofouling, and ingestion/egestion by biota (Figure 2). A wide range of concentrations have been reported for coral sediments [47,49,51,55–59] (Table 1); however, due to the lack of standardised methods and units for reporting it is difficult to make direct comparisons. The predominant morphotype and polymer type vary between studies. Fibres and fragments are the most common types, and a wide range of polymers have been identified, with polyethylene, polypropylene, polyethylene terephthalate and nylon the most common [68].
Reef sediments act as sinks for MPs and their associated chemical contaminants [69,70]. Sediments >3.5 cm depth act as permanent sinks as they are unlikely to be resuspended under modal sea conditions. Under extreme conditions, the sediments act as a source of MPs back to the local area [59]. The distribution of MPs within the coral reef system is influenced by the presence of habitat-forming species. The complex three-dimensional structure of these habitat-forming species results in the physical deposition of MNPs from the water column [69,71–73], and translocation into the sediments (Figure 2). Habitat-forming species may also influence the translocation of suspended MNPs into the food chain. Particles that have settled on the surface of seagrasses and macroalgae can be ingested by herbivores [74], and then either transferred back into the sediments via faeces, or transferred up the food chain (Figure 2).
Association and impact on coral reef biota
The ingestion of MNPs have been identified in a broad range of coral reef biota, across taxonomic groups and trophic levels, and may be ingested directly or indirectly through prey (Figure 2) [33,34,75,76]. Here, we will discuss some key reef taxa and the known or potential effects of MNP exposure.
Scleractinian corals have been shown to interact directly with MNPs, and respond to chemosensory cues [77]. The level of interaction exhibits species-specificity and may be influenced by polyp and prey size, as well as feeding strategy [41,71,78–81]. Plastic particles of varying size, morphotype and polymer type have been observed to attach to tentacles and mesenterial filaments, and become trapped in the mucus layer, and may be ingested [33,41,61,71,77,79,82–85]. Laboratory experiments have demonstrated that MNP feeding rates vary between individual colonies with some showing a rate similar to the ingestion of plankton [41,86]. Whilst others discriminate between prey and plastic particles with increased handling times [81]. In all cases, the majority of plastic particles were egested within 24–48 h [33,77,82]. Where the MNPs were preconditioned with a biofilm the rate of uptake and retention time were affected [71]. This is thought due to corals often relying on chemoreception to capture prey, and therefore a natural biofilm will trigger a feeding response but also reduce the coral's ability to discriminate against inert particles, resulting in longer retention times [71]. Overall, the lack of avoidance and increased handling time of plastic particles relative to prey items results in the impairment of feeding efficiency, and ultimately reduce fitness [87].
Plastic particles have been found embedded within the skeletal matrix of both experimental and colonies collected from the wild [79,88–92]. Two mechanisms by which the particles become trapped within the skeleton have been suggested. The first results from the growth of polyps over the particles. This has been observed in acroporid and poritid species, and occurred predominantly in areas where surface cleaning mechanisms were ineffective and where passive removal of surface sediments were restricted due to tissues or skeletal morphology, colony orientation and water movement [79]. The second mechanism is thought to occur when non-egested MNP particles pass through the endoderm and calicoblastic layers and become incorporated within the skeletal matrix as aragonite is laid down.
Responses seen in corals include increased mucus production, reduced feeding rates and increased particle handling [79]. Overall, these responses are energetically costly to the corals, and may lead to reduced energy budget and have subsequent effects on health and fecundity.
The level of knowledge around the effect of MNPs on corals remains unclear, and further research is required. Many negative impacts have been identified and include host–symbiont relationship, photosynthetic efficiency, tissue necrosis, calcification rates, energy demand, reproductive success and overall fitness [41,79,80,82,83,86,87,89,92–99]. Perturbation of photophysiology, bleaching and tissue necrosis have been observed in laboratory-based studies in response to exposure to MNPs [79,85,89,96] and may demonstrate coral species-specificity. The exact mechanisms involved remain unknown but in addition to causing the release of zooxanthellae the dietary exposure of MNPs has been observed to supress the uptake of new cells, and were associated with the translocation of MNPs into the endodermal cells of the mesenterial filaments [33,88].
Larger plastic debris has been implicated in disease transmission and physical trauma resulting in susceptibility [100,101]. It is plausible that MPs also pose a threat, either through pathogen transmission, tissue damage or alteration of the coral microbiome [102,103], resulting in reduced fitness and susceptibility to disease. This includes the skeletal microbiome which is thought to play a critical role in the health of the colony [102,104]. The biofilms that form on MPs, referred to as the ‘plastisphere’, differ significantly from those in the water column and associated with natural particles, and may cause dysbiosis of the coral microbiome resulting in susceptibility to disease, or may harbour potential pathogens which have been seen to preferentially colonise plastics [102,105–113]. Contaminants associated with the plastics [51,114–117] have also been found to enter the tissues [51,118] after ingestion or overgrowth with potential direct toxicological effects [119] including affecting the microbiome. As the levels have been found to correlate with the environmental MP levels [46] and exposure is predicted to increase significantly over the coming decades [26] it is imperative that the knowledge gap is filled.
It remains unclear what key characteristics of MNPs are responsible for eliciting an impact on corals, but size, morphology, base polymer, associated chemicals (inherent and acquired) and microbes are all thought to play a role, and may also have a co-effect. There is some evidence that other anthropogenic stressors have a synergistic effect with MNPs in Anthozoa. For example, thermally bleached anemones and corals ingested more MNPs relative to prey, and experience greater internal exposure to MNPs due to longer retention times [120,121]. This is of particular concern given that coral reef ecosystems are facing increases in frequency and intensity of bleaching events due to ocean warming in conjunction with the predicted increase in MNP exposure.
Studies examining the ingestion and effects of MNPs on other anthozoa had similar results to those seen in scleractinia [44,103,122] but there needs to be more research done before we fully understand the impacts, including using environmentally relevant MNP types and concentrations and exposure periods. Being able to achieve this is reliant upon the development of internationally agreed standard methods which are able to accurately determine the levels and characteristics (e.g. size and morphology) of MNPs in the environment.
The ingestion of MNPs by higher trophic level taxa may be both direct and indirect. The mechanisms involved and impacts through the food chain are becoming increasingly studied. Teleosts have been a group that have received a lot of attention due to their importance as food. The majority of these studies have looked at ingestion by adult fish, and the presence of plastics within the gastrointestinal tract with relation to human health risk [123]. Coral reef fish are not exempt from the ingestion of MNPs [42,50,124]. The impacts on the fish themselves is not fully understood, and appears to be influenced by a range of factors [125], including the MNPs themselves, fish species and the life stage at exposure [126]. Impacts range from no observable effect [127,128], to affecting growth and body condition due to reducing nutritional intake [129], and even resulting in behavioural change such as feeding activity and boldness in the presence of others [130,131]. Both of which can significantly affect survival.
Echinoderms play an important role in the coral reef ecosystem, from the control of macroalgae to the maintenance of sediment health by those species that burrow into or feed directly on them and egestion of undigested material back into the water column. They are exposed to MNPs both through their diet and respiration. The majority of work to date has been on sea cucumbers. Individuals collected from the wild and experimental conspecifics were shown to ingest MNPs as they fed on sediments [132–134], with some studies showing translocation from the gut to the coelomic fluid. Ingestion rates were also found to correlate to water temperature [133] and therefore rising sea surface temperatures may result in increased dietary exposure. There is also some evidence of internalisation of MNPs directly from the water column [134]. The concentration of MNPs within pseudofaeces are found to be higher than those in the surrounding sediments, suggesting that preferential selection occurs [75,135,136]. To date, few studies have examined the impacts of MNPs on sea cucumbers, and results are variable. Laboratory exposure experiments of juvenile and adult sea cucumbers to environmentally relevant levels of MNPs were found to impact gastric function and physiological change including to the immune system and metabolism, as well as physical damage to the respiratory trees. In addition to the uptake of MNPs, plastic-associated chemicals (e.g. phthalates) and heavy metals [137–139] adsorbed onto MNPs from the environment are found to transfer into the tissues of the sea cucumbers. The impacts of this, and their role in the effects of MNPs have not yet been elucidated. Sea cucumbers are also suggested to play a key role in maintaining the bioavailabiliy of MPs [136] within the system through the resuspension of MNPs from the sediments in pseudofaeces, preventing them from entering deeper sediments that act as a sink for MNPs [69]. Sea urchins have also recently become a focus for the impacts of MNPs. Experimental evidence has demonstrated MNP uptake directly from water through the madreporite [140], and indirectly via their diet [12]. In adult urchins, MNPs are seen to translocate between tissues and organs, and elicit an immune response [140], with growth and development impaired in early life stages [141–143]. Further work is required to fill the knowledge gap of uptake and impacts of MNPs on all coral reef Echinodermata classes.
Molluscs fill multiple niches within the coral reef ecosystem, from grazers of algae and seagrasses, to providing nursery and refuge for juvenile and adult fish. Until relatively recently it has been considered that it has only been the filter-feeding species that are subject to MNP exposure due to their ability to filter large volumes of water. It may, therefore, be considered that reef species will be similarly affected as non-reef species. However, a growing number of studies have identified that species with different feeding strategies are also exposed to MNPs through their diet. Examples of species from other ecosystems have identified ingestion by both bivalves and gastropods [144], and particles may translocate from the guts to other organs [145]. The coral reef sea hare, Dolabella auricularia, has been found to be exposed to MNPs via their diet due to the particles becoming entrained and adhered to the surface of the seagrass they graze on [74]. Taxa such as Tridacna which play various ecological roles in the coral reef ecosystem [146] may be subject direct impacts of MNPs and also via their impact on the symbiont–host relationship [79,80,85,89,93,96]. The impacts so far identified range from physiological to behavioural [147–149], therefore, having a potential impact at both the individual and ecosystem level. Further work is required to understand the impacts on coral reef molluscan species.
Porifera are widely distributed through the coral reef ecosystem and filter large volumes of water, removing a wide size range of particles. It remains unclear whether selective uptake occurs due to variability and accuracy of the methods used to determine MNPs in different species of coral reef sponge, representing different functional growth forms [150–152]. Due to the important role sponges play in the reef system, from nutrient recycling to providing habitats, more work is required to fill this knowledge gap, and to understand whether sponge health and system function is impacted.
Megafauna are more commonly associated the ingestion of meso- and macroplastics (5–25 mm and >25 mm, respectively [153,154]. They are, however, exposed to MNPs either through direct ingestion or through trophic transfer. Turtles are one of the most studied taxa, with MNPs having been found in all seven species [31,60,155–157], occupying different trophic levels and reinforcing the assertion that ingestion occurs via multiple pathways, including contaminated water, sediments and prey/forage items. It remains unclear the impact MNPs have on turtles. In adults they are not thought to cause blockage of the gastrointestinal tract, gross physical damage or false satiety, resulting in nutrient deficiencies [31,158,159], although this may occur in juveniles [60]. These effects on juveniles may, therefore, have significant impacts at the population-scale.
Other reef megafauna such as sharks and manta rays are also vulnerable to the impacts of MNPs, with exposure via trophic transfer or directly in their diet (Figure 2). For example, egested material from manta rays has been found to contain high levels of MNPs, and predictions of ingest rates from surface water trawls in the same area as these animals was between ∼25 and 63 pieces of plastic h−1. Predictions of the ingestion rates by whale sharks in the same area, were more than twice this rate [160]. Other visitors of the coral reef ecosystems such as dolphins and whales have also been found to ingest MPs [161–164]. The impacts are similarly unknown and requires further study.
A major and critical components of the coral reef ecosystem that is frequently overlooked is that of the microbes. Consequently, the impacts of plastics on the ecology of coral reef microbes are not well understood [102]. Plastic particles provide a new substrate on which diverse microbial and metazoan communities, sometimes referred to as the ‘plastisphere’, rapidly colonise marine plastic [113]. Where plastisphere communities differ from the those on natural surfaces, there is a potential that the presence of plastics may disrupt the microbially driven processes that occur. These may including, for example, disruption of biogeochemical processes [165–167], the dysbiosis of an organism's microbiome [102,103] or altered pathogen exposure [105,107,168,169]. The plastisphere may also play host to the early life stages of non-indigenous species (NIS) [170]. The resilience of the plastic, therefore, supports the range expansion and introduction of NIS into new regions, which could pose a significant threat to coral reefs.
Conclusion
Our knowledge of the distribution and impacts of MPs is still not fully understood, with even less known about NPs. While this area of research is still in its relative infancy, results to date have shown their ubiquity in different ecosystem compartments and suggest that MNPs pose a significant risk to a wide range of coral reef organisms, across all taxa, trophic levels and feeding strategies. The factors determining the adverse effects include MNP size, polymer, morphology, associated chemical and microbial contaminants, and may be species-specific. There is, therefore, significant research required to fully elucidate their presence in these highly diverse ecosystems. Before we can accurately [171] determine the impacts MNPs are having we need to first determine their exposure. To be able to do this technical limitations need to be overcome to allow the more accurate assessment of MNPs in environmental samples, and internationally agreed standard methods are required to enable accurate comparisons between studies. By improving our knowledge of the levels, types of MNPs, and their associated contaminants it will allow the more accurate assessment of impacts, through the use of ecologically relevant concentrations, morphotypes, polymers and associated contaminants (microbial and chemical) in controlled experiments. Considering the importance of coral reefs to marine ecosystems, it is important that the impacts of MNPs is done in conjunction with the other anthropogenic stressors they are under, which are also predicted to increase in severity over the coming decades [26,172], to determine potential synergies these stressors may have. This information is important to predict species and areas under greatest threat, and help us better allocate resources for the management and protection of coral reefs.
Summary
MPs are found in all coral reef compartments; water, sediment and biota.
MPs and NPs have been found to have an effect at the organism- to ecosystem-scale.
A knowledge gap exists around the impacts of plastics particles, including under future climate conditions.
Coral reef communities facilitate both the bioavailability of plastic particles and sequestration into the sediments.
Standardised methods are needed to enable the improved assessment of MNP pollution.
Abbreviations
- NP
nanoplastic
- MP
microplastic
- MNP
micro- and nanoplastic
Competing Interests
The author declares that there are no competing interests associated with this manuscript.
References
- 1.Europe P. Plastics - The Facts. 2021 Available from: https://www.plasticseurope.org/en/resources/publications/4312-plastics-facts-2020.
- 2.Waller, C.L., Griffiths, H.J., Waluda, C.M., Thorpe, S.E., Loaiza, I., Moreno, B.et al. (2017) Microplastics in the Antarctic marine system: an emerging area of research. Sci. Total Environ. 598, 220–227 10.1016/j.scitotenv.2017.03.283 [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, M., Mützel, S., Primpke, S., Tekman, M.B., Trachsel, J. and Gerdts, G. (2019) White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 5, eaax1157 10.1126/sciadv.aax1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Guen, C., Suaria, G., Sherley, R.B., Ryan, P.G., Aliani, S., Boehme, L.et al. (2020) Microplastic study reveals the presence of natural and synthetic fibres in the diet of King Penguins (Aptenodytes patagonicus) foraging from South Georgia. Environ. Int. 134, 105303 10.1016/j.envint.2019.105303 [DOI] [PubMed] [Google Scholar]
- 5.Napper, I.E. Davies, B.F.R., Clifford, H., Elvin, S., Koldewey, H.J., Mayewski, P.A.et al. (2020) Reaching new heights in plastic pollution: preliminary findings of microplastics on Mount Everest. One Earth 3, 621–630 10.1016/j.oneear.2020.10.020 [DOI] [Google Scholar]
- 6.Lavers, J.L. and Bond, A.L. (2017) Exceptional and rapid accumulation of anthropogenic debris on one of the world's most remote and pristine islands. Proc. Natl Acad. Sci. U.S.A. 114, 6052 10.1073/pnas.1619818114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amon, D.J., Kennedy, B.R.C., Cantwel, K., Suhre, K., Glickson, D., Shank, T.M.et al. (2020) Deep-sea debris in the central and western Pacific Ocean. Front. Mar. Sci. 7 10.3389/fmars.2020.00369 [DOI] [Google Scholar]
- 8.Dawson, A.L., Kawaguchi, S., King, C.K., Townsend, K.A., King, R., Huston, W.M.et al. (2018) Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 9, 1001 10.1038/s41467-018-03465-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez, L.M., Xu, E.G.. Larsson, H.C.E., Tahara, R., Maisuria, V.B. and Tufenkji, N. (2019) Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 53, 12300–12310 10.1021/acs.est.9b02540 [DOI] [PubMed] [Google Scholar]
- 10.Liu, P., Zhan, X., Wu, X., Li, J., Wang, H. and Gao, S. (2020) Effect of weathering on environmental behavior of microplastics: properties, sorption and potential risks. Chemosphere 242, 125193 10.1016/j.chemosphere.2019.125193 [DOI] [PubMed] [Google Scholar]
- 11.Po, B.H.K., Lo, H.S., Cheung, S.G. and Lai, K.P. (2020) Characterisation of an unexplored group of microplastics from the South China Sea: can they be caused by macrofaunal fragmentation? Mar. Pollut. Bull. 155, 111151 10.1016/j.marpolbul.2020.111151 [DOI] [PubMed] [Google Scholar]
- 12.Porter, A., Smith, K.E. and Lewis, C. (2019) The sea urchin Paracentrotus lividus as a bioeroder of plastic. Sci. Total Environ. 693, 133621 10.1016/j.scitotenv.2019.133621 [DOI] [PubMed] [Google Scholar]
- 13.Weinstein, J.E., Crocker, B.K. and Gray, A.D. (2016) From macroplastic to microplastic: degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ. Toxicol. Chem. 35, 1632–1640 10.1002/etc.3432 [DOI] [PubMed] [Google Scholar]
- 14.Thompson, R.C. (2015) Microplastics in the marine environment: sources, consequences and solutions. In Marine Anthropogenic Litter (Bergmann, M., Gutow, L. and Klages, M., eds), pp. 185–200, Springer International Publishing, Cham [Google Scholar]
- 15.Hartmann, N.B., Huffer, T., Thompson, R.C., Hassellov, M., Verschoor, A., Daugaard, A.E.et al. (2019) Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 53, 1039–1047 10.1021/acs.est.8b05297 [DOI] [PubMed] [Google Scholar]
- 16.Koelmans, A.A., Besseling, E. and Shim, W.J. (2015) Nanoplastics in the aquatic environment. Critincal review. In Marine Anthropogenic Litter (Bergmann, M., Gutow, L. and Klages, M., eds), pp. 325–340, SpringerOpen, Switzerland [Google Scholar]
- 17.Gouin, T., Roche, N., Lohmann, R. and Hodges, G. (2011) A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ. Sci. Technol. 45, 1466–1472 10.1021/es1032025 [DOI] [PubMed] [Google Scholar]
- 18.Green, D.S., Jefferson, M., Boots, B. and Stone, L. (2021) All that glitters is litter? Ecological impacts of conventional versus biodegradable glitter in a freshwater habitat. J. Hazard. Mater. 402, 124070 10.1016/j.jhazmat.2020.124070 [DOI] [PubMed] [Google Scholar]
- 19.Perosa, M., Guerranti, C., Renzi, M. and Bevilacqua, S. (2021) Taking the sparkle off the sparkling time. Mar. Pollut. Bull. 170, 112660 10.1016/j.marpolbul.2021.112660 [DOI] [PubMed] [Google Scholar]
- 20.Rochman, C.M., Kross, S.M., Armstrong, J.B., Bogan, M.T., Darling, E.S., Green, S.J.et al. (2015) Scientific evidence supports a ban on microbeads. Environ. Sci. Technol. 49, 10759–10761 10.1021/acs.est.5b03909 [DOI] [PubMed] [Google Scholar]
- 21.Browne, M.A., Crump, P., Niven, S.J., Teuten, E., Tonkin, A., Galloway, T.et al. (2011) Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ. Sci. Technol. 45, 9175–9179 10.1021/es201811s [DOI] [PubMed] [Google Scholar]
- 22.Cao, Y., Wang, Q., Ruan, Y., Wu, R., Chen, L., Zhang, K.et al. (2020) Intra-day microplastic variations in wastewater: a case study of a sewage treatment plant in Hong Kong. Mar. Pollut. Bull. 160, 111535 10.1016/j.marpolbul.2020.111535 [DOI] [PubMed] [Google Scholar]
- 23.Hernández-Arenas, R., Beltrán-Sanahuja, A., Navarro-Quirant, P. and Sanz-Lazaro, C. (2021) The effect of sewage sludge containing microplastics on growth and fruit development of tomato plants. Environ. Pollut. 268, 115779 10.1016/j.envpol.2020.115779 [DOI] [PubMed] [Google Scholar]
- 24.Ziajahromi, S., Neale, P.A., Rintoul, L. and Leusch, F.D. (2017) Wastewater treatment plants as a pathway for microplastics: development of a new approach to sample wastewater-based microplastics. Water Res. 112, 93–99 10.1016/j.watres.2017.01.042 [DOI] [PubMed] [Google Scholar]
- 25.Browne, M.A., Galloway, T.S. and Thompson, R.C. (2010) Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 44, 3404–3409 10.1021/es903784e [DOI] [PubMed] [Google Scholar]
- 26.Borrelle, S.B., Ringma, J., Law, K.L., Monnahan, C.C., Lebreton, L., McGivern, A.et al. (2020) Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369, 1515 10.1126/science.aba3656 [DOI] [PubMed] [Google Scholar]
- 27.van Sebille, E., Wilcox, C., Lebreton, L., Maximenko, N., Hardesty, B.D., van Franeker, J.A.et al. (2015) A global inventory of small floating plastic debris. Environ. Res. Lett. 10, 124006 10.1088/1748-9326/10/12/124006 [DOI] [Google Scholar]
- 28.Onink, V., Jongedijk, C.E., Hoffman, M.J., van Sebille, E. and Laufkotter, C. (2021) Global simulations of marine plastic transport show plastic trapping in coastal zones. Environ. Res. Lett. 16, 064053 10.1088/1748-9326/abecbd [DOI] [Google Scholar]
- 29.Tan, F., Yang, H.Q., Xu, X.R., Fang, Z., Xu, H.L., Shi, Q.et al. (2020) Microplastic pollution around remote uninhabited coral reefs of Nansha Islands, South China Sea. Sci. Total Environ. 725, 138383 10.1016/j.scitotenv.2020.138383 [DOI] [PubMed] [Google Scholar]
- 30.Provencher, J.F., Bond, A.L., Avery-Gomm, S., Borrelle, S.B., Bravo Rebolledo, E.L., Hammer, S.et al. (2017) Quantifying ingested debris in marine megafauna: a review and recommendations for standardization. Anal. Methods 9, 1454–1469 10.1039/C6AY02419J [DOI] [Google Scholar]
- 31.Santos, R.G., Andrades, R., Demetrio, G.R., Kuwai, G.M., Sobral, M.F., JdS, V.et al. (2020) Exploring plastic-induced satiety in foraging Green turtles. Environ. Pollut. 265, 114918 10.1016/j.envpol.2020.114918 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka, K., Takada, H., Yamashita, R., Mizukawa, K., Fukuwaka, M.A. and Watanuki, Y. (2013) Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 69, 219–222 10.1016/j.marpolbul.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 33.Okubo, N., Takahashi, S. and Nakano, Y. (2018) Microplastics disturb the anthozoan-algae symbiotic relationship. Mar. Pollut. Bull. 135, 83–89 10.1016/j.marpolbul.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 34.Setälä, O., Fleming-Lehtinen, V. and Lehtiniemi, M. (2014) Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 185, 77–83 10.1016/j.envpol.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 35.Besseling, E., Wang, B., Lurling, M. and Koelmans, A.A. (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 48, 12336–12343 10.1021/es503001d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole, M., Lindeque, P., Fileman, E., Halsband, C. and Galloway, T.S. (2015) The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 49, 1130–1137 10.1021/es504525u [DOI] [PubMed] [Google Scholar]
- 37.Cole, M., Lindeque, P., Fileman, E., Halsband, C., Goodhead, R., Moger, J.et al. (2013) Microplastic ingestion by zooplankton. Environ. Sci. Technol. 47, 6646–6655 10.1021/es400663f [DOI] [PubMed] [Google Scholar]
- 38.Geng, X.H., Wang, J., Zhang, Y. and Jiang, Y. (2021) How do microplastics affect the marine microbial loop? Predation of microplastics by microzooplankton. Sci. Total Environ. 758, 144030 10.1016/j.scitotenv.2020.144030 [DOI] [PubMed] [Google Scholar]
- 39.Huang, W., Chen, M., Song, B., Deng, J.Q., Shen, M.C., Chen, Q.et al. (2021) Microplastics in the coral reefs and their potential impacts on corals: a mini-review. Sci. Total Environ. 762, 143112 10.1016/j.scitotenv.2020.143112 [DOI] [PubMed] [Google Scholar]
- 40.John, J., Nandhini, A.R., Velayudhaperumal Chellam, P. and Sillanpää, M. (2021) Microplastics in mangroves and coral reef ecosystems: a review. Environ. Chem. Lett. 1–20 10.1007/s10311-021-01326-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall, N.M., Berry, K.L.E., Rintoul, L. and Hoogenboom, M.O. (2015) Microplastic ingestion by scleractinian corals. Mar. Biol. 162, 725–732 10.1007/s00227-015-2619-7 [DOI] [Google Scholar]
- 42.Jensen, L.H., Motti, C.A., Garm, A.L., Tonin, H. and Kroon, F.J. (2019) Sources, distribution and fate of microfibres on the Great Barrier Reef, Australia. Sci. Rep. 9, 9021 10.1038/s41598-019-45340-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reisser, J., Shaw, J., Wilcox, C., Hardesty, B.D., Proietti, M., Thums, M.et al. (2013) Marine plastic pollution in waters around Australia: characteristics, concentrations, and pathways. PLoS One 8, e80466 10.1371/journal.pone.0080466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connors, E.J. (2017) Distribution and biological implications of plastic pollution on the fringing reef of Mo'orea, French Polynesia. PeerJ 5, e3733 10.7717/peerj.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saliu, F., Montano, S., Garavaglia, M.G., Lasagni, M., Seveso, D. and Galli, P. (2018) Microplastic and charred microplastic in the Faafu Atoll, Maldives. Mar. Pollut. Bull. 136, 464–471 10.1016/j.marpolbul.2018.09.023 [DOI] [PubMed] [Google Scholar]
- 46.Saliu, F., Montano, S., Leoni, B., Lasagni, M. and Galli, P. (2019) Microplastics as a threat to coral reef environments: detection of phthalate esters in neuston and scleractinian corals from the Faafu Atoll, Maldives. Mar. Pollut. Bull. 142, 234–241 10.1016/j.marpolbul.2019.03.043 [DOI] [PubMed] [Google Scholar]
- 47.Ding, J.F., Jiang, F.H., Li, J.X., Wang, Z.X., Sun, C.J., Wang, Z.Y.et al. (2019) Microplastics in the coral reef systems from Xisha Islands of South China Sea. Environ. Sci. Technol. 53, 8036–8046 10.1021/acs.est.9b01452 [DOI] [PubMed] [Google Scholar]
- 48.Wang, T., Zou, X., Li, B., Yao, Y., Zang, Z., Li, Y.et al. (2019) Preliminary study of the source apportionment and diversity of microplastics: taking floating microplastics in the South China Sea as an example. Environ. Pollut. 245, 965–974 10.1016/j.envpol.2018.10.110 [DOI] [PubMed] [Google Scholar]
- 49.Huang, Y.J., Yan, M.T., Xu, K.H., Nie, H.Y., Gong, H. and Wang, J. (2019) Distribution characteristics of microplastics in Zhubi Reef from South China Sea. Environ. Pollut. 255, 113133 10.1016/j.envpol.2019.113133 [DOI] [PubMed] [Google Scholar]
- 50.Nie, H., Wang, J., Xu, K., Huang, Y. and Yan, M. (2019) Microplastic pollution in water and fish samples around Nanxun Reef in Nansha Islands, South China Sea. Sci. Total Environ. 696, 134022 10.1016/j.scitotenv.2019.134022 [DOI] [PubMed] [Google Scholar]
- 51.Patterson, J., Jeyasanta, K.I., Sathish, N., Edward, J.K.P. and Booth, A.M. (2020) Microplastic and heavy metal distributions in an Indian coral reef ecosystem. Sci. Total Environ. 744, 140706 10.1016/j.scitotenv.2020.140706 [DOI] [PubMed] [Google Scholar]
- 52.Portz, L., Manzolli, R.P., Herrera, G.V., Garcia, L.L. and Villate, D.A. and Ivar do Sul J.A. (2020)Marine litter arrived: distribution and potential sources on an unpopulated atoll in the Seaflower Biosphere Reserve, Caribbean Sea. Mar. Pollut. Bull. 157, 111323 10.1016/j.marpolbul.2020.111323 [DOI] [PubMed] [Google Scholar]
- 53.Marti, E., Martin, C., Cozar, A. and Duarte, C.M. (2017) Low abundance of plastic fragments in the surface waters of the Red Sea. Front. Mar. Sci. 4 10.3389/fmars.2017.00333 [DOI] [Google Scholar]
- 54.Imhof, H.K., Sigl, R., Brauer, E., Feyl, S., Giesemann, P., Klink, S.et al. (2017) Spatial and temporal variation of macro-, meso- and microplastic abundance on a remote coral island of the Maldives, Indian ocean. Mar. Pollut. Bull. 116, 340–347 10.1016/j.marpolbul.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 55.Cheang, C.C., Ma, Y. and Fok, L. (2018) Occurrence and composition of microplastics in the seabed sediments of the coral communities in proximity of a metropolitan area. Int. J. Environ. Res. Public Health 15, 2270 10.3390/ijerph15102270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, L.L., Zhang, S.P., Wang, Y.H., Yu, K.F. and Li, R.L. (2019) The spatial distribution of microplastic in the sands of a coral reef island in the South China Sea: comparisons of the fringing reef and atoll. Sci. Total Environ. 688, 780–786 10.1016/j.scitotenv.2019.06.178 [DOI] [PubMed] [Google Scholar]
- 57.Jeyasanta, K.I., Patterson, J., Grimsditch, G. and Edward, J.K.P. (2020) Occurrence and characteristics of microplastics in the coral reef, sea grass and near shore habitats of Rameswaram Island, India. Mar. Pollut. Bull. 160, 111674 10.1016/j.marpolbul.2020.111674 [DOI] [PubMed] [Google Scholar]
- 58.Patti, T.B. and Fobert, E.K.,. Reeves, S.E. and da Silva, K.B. (2020) Spatial distribution of microplastics around an inhabited coral island in the Maldives, Indian ocean. Sci. Total Environ. 748 10.1016/j.scitotenv.2020.141263 [DOI] [PubMed] [Google Scholar]
- 59.Utami, D.A., Reuning, L., Konechnaya, O. and Schwarzbauer, J. (2021) Microplastics as a sedimentary component in reef systems: a case study from the Java Sea. Sedimentology 68, 2270–2292 10.1111/sed.12879 [DOI] [Google Scholar]
- 60.Duncan, E.M., Broderick, A.C., Critchell, K., Galloway, T.S., Hamann, M., Limpus, C.J.et al. (2021) Plastic pollution and small juvenile marine turtles: a potential evolutionary trap. Front. Mar. Sci. 8 10.3389/fmars.2021.699521 [DOI] [Google Scholar]
- 61.Rotjan, R.D., Sharp, K.H., Gauthier, A.E., Yelton, R., Lopez, E.M.B., Carilli, J.et al. (2019) Patterns, dynamics and consequences of microplastic ingestion by the temperate coral, Astrangia poculata. Proc. R. Soc. B-Biol. Sci. 286 10.1098/rspb.2019.0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bauer-Civiello, A., Critchell, K., Hoogenboom, M. and Hamann, M. (2019) Input of plastic debris in an urban tropical river system. Mar. Pollut. Bull. 144, 235–242 10.1016/j.marpolbul.2019.04.070 [DOI] [PubMed] [Google Scholar]
- 63.Reisser, J., Shaw, J., Hallegraeff, G., Proietti, M., Barnes, D.K.A., Thums, M.et al. (2014) Millimeter-sized marine plastics: a new pelagic habitat for microorganisms and invertebrates. PLoS One 9, e100289 10.1371/journal.pone.0100289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kleinhaus, K., Al-Sawalmih, A., Barshis, D.J., Genin, A., Grace, L.N., Hoegh-Guldberg, O.et al. (2020) Science, diplomacy, and the Red Sea's unique coral reef: it's time for action. Front. Mar. Sci. 7 10.3389/fmars.2020.00090 [DOI] [Google Scholar]
- 65.Xie, J., Krokos, G., Sofianos, S. and Hoteit, I. (2019) Interannual variability of the exchange flow through the strait of Bab-al-Mandeb. J. Geophys. Res.: Oceans 124, 1988–2009 10.1029/2018JC014478 [DOI] [Google Scholar]
- 66.Cordova, M.R., Purwiyanto, A.I.S. and Suteja, Y. (2019) Abundance and characteristics of microplastics in the northern coastal waters of Surabaya, Indonesia. Mar. Pollut. Bull. 142, 183–188 10.1016/j.marpolbul.2019.03.040 [DOI] [PubMed] [Google Scholar]
- 67.Dharmadasa, W., Andrady, A.L., Kumara, P., Maes, T. and Gangabadage, C.S. (2021) Microplastic pollution in marine protected areas of Southern Sri Lanka. Mar. Pollut. Bull. 168, 112462 10.1016/j.marpolbul.2021.112462 [DOI] [PubMed] [Google Scholar]
- 68.Harris, P.T. (2020) The fate of microplastic in marine sedimentary environments: a review and synthesis. Mar. Pollut. Bull. 158 10.1016/j.marpolbul.2020.111398 [DOI] [PubMed] [Google Scholar]
- 69.de Smit, J.C., Anton, A., Martin, C., Rossbach, S., Bouma, T.J. and Duarte, C.M. (2021) Habitat-forming species trap microplastics into coastal sediment sinks. Sci. Total Environ. 772 10.1016/j.scitotenv.2021.145520 [DOI] [PubMed] [Google Scholar]
- 70.Sandre, F., Dromard, C.R., Le Menach, K., Bouchon-Navaro, Y., Cordonnier, S., Tapie, N.et al. (2019) Microplastic distribution and detection of chlordecone on microplastics in marine sediments in Guadeloupe: a preliminary study. Gulf Caribb. Res. 30, GCFI8–GCFI14 10.18785/gcr.3001.14 [DOI] [Google Scholar]
- 71.Corona, E., Martin, C., Marasco, R. and Duarte, C.M. (2020) Passive and active removal of marine microplastics by a mushroom coral (Danafungia scruposa). Front. Mar. Sci. 7 10.3389/fmars.2020.00128 [DOI] [Google Scholar]
- 72.de los Santos, C.B., Krang, A.S. and Infantes, E. (2021) Microplastic retention by marine vegetated canopies: simulations with seagrass meadows in a hydraulic flume. Environ. Pollut. 269 10.1016/j.envpol.2020.116050 [DOI] [PubMed] [Google Scholar]
- 73.Martin, C., Agusti, S. and Duarte, C.M. (2019) Seasonality of marine plastic abundance in central Red Sea pelagic waters. Sci. Total Environ 688, 536–541 10.1016/j.scitotenv.2019.06.240 [DOI] [PubMed] [Google Scholar]
- 74.Priscilla, V., Sedayu, A. and Patria, M.P. (2019) Microplastic abundance in the water, seagrass, and sea hare Dolabella auricularia in Pramuka Island, Seribu Islands, Jakarta Bay, Indonesia. J. Phys.: Conf. Ser. 1402, 033073 10.1088/1742-6596/1402/3/033073 [DOI] [Google Scholar]
- 75.Renzi, M., Blaskovic, A., Bernardi, G. and Russo, G.F. (2018) Plastic litter transfer from sediments towards marine trophic webs: a case study on holothurians. Mar. Pollut. Bull. 135, 376–385 10.1016/j.marpolbul.2018.07.038 [DOI] [PubMed] [Google Scholar]
- 76.Nelms, S.E., Galloway, T.S., Godley, B.J., Jarvis, D.S. and Lindeque, P.K. (2018) Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 238, 999–1007 10.1016/j.envpol.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 77.Allen, A.S., Seymour, A.C. and Rittschof, D. (2017) Chemoreception drives plastic consumption in a hard coral. Mar. Pollut. Bull. 124, 198–205 10.1016/j.marpolbul.2017.07.030 [DOI] [PubMed] [Google Scholar]
- 78.Oldenburg, K.S., Urban-Rich, J., Castillo, K.D. and Baumann, J.H. (2021) Microfiber abundance associated with coral tissue varies geographically on the Belize mesoamerican Barrier Reef System. Mar. Pollut. Bull. 163, 111938 10.1016/j.marpolbul.2020.111938 [DOI] [PubMed] [Google Scholar]
- 79.Reichert, J., Schellenberg, J., Schubert, P. and Wilke, T. (2018) Responses of reef building corals to microplastic exposure. Environ. Pollut. 237, 955–960 10.1016/j.envpol.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 80.Ripken, C., Khalturin, K. and Shoguchi, E. (2020) Response of coral reef dinoflagellates to nanoplastics under experimental conditions suggests downregulation of cellular metabolism. Microorganisms 8 10.3390/microorganisms8111759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Savinelli, B., Fernandez, T.V., Galasso, N.M., D'Anna, G., Pipitone, C., Prada, F.et al. (2020) Microplastics impair the feeding performance of a Mediterranean habitat-forming coral. Mar. Environ. Res. 155 10.1016/j.marenvres.2020.104887 [DOI] [PubMed] [Google Scholar]
- 82.Hankins, C., Duffy, A. and Drisco, K. (2018) Scleractinian coral microplastic ingestion: Potential calcification effects, size limits, and retention. Mar. Pollut. Bull. 135, 587–593 10.1016/j.marpolbul.2018.07.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hankins, C., Moso, E. and Lasseigne, D. (2021) Microplastics impair growth in two atlantic scleractinian coral species, Pseudodiploria clivosa and Acropora cervicornis. Environ. Pollut. 275 10.1016/j.envpol.2021.116649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin, C., Corona, E., Mahadik, G.A. and Duarte, C.M. (2019) Adhesion to coral surface as a potential sink for marine microplastics. Environ. Pollut. 255, 113281 10.1016/j.envpol.2019.113281 [DOI] [PubMed] [Google Scholar]
- 85.Syakti, A.D., Jaya, J.V., Rahman, A., Hidayati, N.V., Raza'i, T.S., Idris, F.et al. (2019) Bleaching and necrosis of staghorn coral (Acropora formosa) in laboratory assays: immediate impact of LDPE microplastics. Chemosphere 228, 528–535 10.1016/j.chemosphere.2019.04.156 [DOI] [PubMed] [Google Scholar]
- 86.Chapron, L., Peru, E., Engler, A., Ghiglione, J.F., Meistertzheim, A.L., Pruski, A.M.et al. (2018) Macro- and microplastics affect cold-water corals growth, feeding and behaviour. Sci. Rep. 8 10.1038/s41598-018-33683-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reichert, J., Arnold, A.L., Hoogenboom, M.O., Schubert, P. and Wilke, T. (2019) Impacts of microplastics on growth and health of hermatypic corals are species-specific. Environ. Pollut. 254 10.1016/j.envpol.2019.113074 [DOI] [PubMed] [Google Scholar]
- 88.Okubo, N., Tamura-Nakano, M. and Watanabe, T. (2020) Experimental observation of microplastics invading the endoderm of anthozoan polyps. Mar. Envion. Res. 162 10.1016/j.marenvres.2020.105125 [DOI] [PubMed] [Google Scholar]
- 89.Mendrik, F.M., Henry, T.B., Burdett, H., Hackney, C.R., Waller, C., Parsons, D.R.et al. (2021) Species-specific impact of microplastics on coral physiology. Environ. Pollut. 269 10.1016/j.envpol.2020.116238 [DOI] [PubMed] [Google Scholar]
- 90.Krishnakumar, S., Anbalagan, S., Hussain, S.M., Bharani, R., Godson, P.S. and Srinivasalu, S. (2021) Coral annual growth band impregnated microplastics (Porites sp.): a first investigation report. Wetl. Ecol. Manag. 29, 677–687 10.1007/s11273-021-09786-9 [DOI] [Google Scholar]
- 91.Hierl, F., Wu, H.C. and Westphal, H. (2021) Scleractinian corals incorporate microplastic particles: identification from a laboratory study. Environ. Sci. Pollut. Res. 28, 37882–37893 10.1007/s11356-021-13240-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao, B.H., Li, D.D., Liao, B.L., Zheng, H.N., Yang, X.D., Xie, Y.Q.et al. (2021) Effects of microplastics exposure on the Acropora sp. antioxidant, immunization and energy metabolism enzyme activities. Front. Microbiol. 12 10.3389/fmicb.2021.666100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lanctot, C.M., Bednarz, V.N., Melvin, S., Jacob, H., Oberhaensli, F., Swarzenski, P.W.et al. (2020) Physiological stress response of the scleractinian coral Stylophora pistillata exposed to polyethylene microplastics. Environ. Pollut. 263 10.1016/j.envpol.2020.114559 [DOI] [PubMed] [Google Scholar]
- 94.Liao, B.L., Wang, J.J., Xiao, B.H., Yang, X.D., Xie, Z.Q., Li, D.D.et al. (2021) Effects of acute microplastic exposure on physiological parameters in Tubastrea aurea corals. Mar. Pollut. Bull. 165 10.1016/j.marpolbul.2021.112173 [DOI] [PubMed] [Google Scholar]
- 95.Jiang, S.Q., Zhang, Y.Y., Feng, L.M., He, L., Zhou, C.X., Hong, P.Z.et al. (2021) Comparison of short- and long-term toxicity of microplastics with different chemical constituents on button polyps (Protopalythoa sp). ACS Earth Space Chem. 5, 12–22 10.1021/acsearthspacechem.0c00213 [DOI] [Google Scholar]
- 96.Tang, J., Wu, Z.J., Wan, L., Cai, W.Q., Chen, S.Q., Wang, X.J.et al. (2021) Differential enrichment and physiological impacts of ingested microplastics in scleractinian corals in situ. J. Hazard. Mater. 404 10.1016/j.jhazmat.2020.124205 [DOI] [PubMed] [Google Scholar]
- 97.Tang, J., Ni, X.Z., Zhou, Z., Wang, L.G. and Lin, S.J. (2018) Acute microplastic exposure raises stress response and suppresses detoxification and immune capacities in the scleractinian coral Pocillopora damicornis. Environ. Pollut. 243, 66–74 10.1016/j.envpol.2018.08.045 [DOI] [PubMed] [Google Scholar]
- 98.Berry, K.L.E., Epstein, H.E., Lewis, P.J., Hall, N.M. and Negri, A.P. (2019) Microplastic contamination has limited effects on coral fertilisation and larvae. Diversity 11 [Google Scholar]
- 99.Su, Y.L., Zhang, K.D., Zhou, Z., Wang, J.R., Yang, X.H., Tang, J.et al. (2020) Microplastic exposure represses the growth of endosymbiotic dinoflagellate Cladocopium goreaui in culture through affecting its apoptosis and metabolism. Chemosphere 244 10.1016/j.chemosphere.2019.125485 [DOI] [PubMed] [Google Scholar]
- 100.Bidegain, G. and Paul-Pont, I. (2018) Commentary: plastic waste associated with disease on coral reefs. Front. Mar. Sci. 5 10.3389/fmars.2018.00237 [DOI] [PubMed] [Google Scholar]
- 101.Lamb, J.B., Willis, B.L., Fiorenza, E.A., Couch, C.S., Howard, R., Rader, D.N.et al. (2018) Plastic waste associated with disease on coral reefs. Science 359, 460 10.1126/science.aar3320 [DOI] [PubMed] [Google Scholar]
- 102.Lear, G., Kingsbury, J.M., Franchini, S., Gambarini, V., Maday, S.D.M., Wallbank, J.A.et al. (2021) Plastics and the microbiome: impacts and solutions. Environ. Microbiome 16 10.1186/s40793-020-00371-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corinaldesi, C., Canensi, S., Dell'Anno, A., Tangherlini, M., Di Capua, I., Varrella, S.et al. (2021) Multiple impacts of microplastics can threaten marine habitat-forming species. Commun. Biol. 4 10.1038/s42003-021-01961-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ricci, F., Marcelino, V.R., Blackall, L.L., Kuhl, M., Medina, M. and Verbruggen, H. (2019) Beneath the surface: community assembly and functions of the coral skeleton microbiome. Microbiome 7 10.1186/s40168-019-0762-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bowley, J., Baker-Austin, C., Porter, A., Hartnell, R. and Lewis, C. (2021) Oceanic hitchhikers - assessing pathogen risks from marine microplastic. Trends Microbiol. 29, 107–116 10.1016/j.tim.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 106.Bryant, J.A., Clemente, T.M., Viviani, D.A., Fong, A.A., Thomas, K.A., Kemp, P.et al. (2016) Diversity and activity of communities inhabiting plastic debris in the North Pacific Gyre. mSystems 1, e00024–16 10.1128/mSystems.00024-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feng, L.M., He, L., Jiang, S.Q., Chen, J.J., Zhou, C.X., Qian, Z.J.et al. (2020) Investigating the composition and distribution of microplastics surface biofilms in coral areas. Chemosphere 252 10.1016/j.chemosphere.2020.126565 [DOI] [PubMed] [Google Scholar]
- 108.Kirstein, I.V., Kirmizi, S., Wichels, A., Garin-Fernandez, A., Erler, R., Loder, M.et al. (2016) Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 120, 1–8 10.1016/j.marenvres.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 109.La Beur, L., Henry, L.A., Kazanidis, G., Hennige, S., McDonald, A., Shaver, M.P.et al. (2019) Baseline assessment of marine litter and microplastic ingestion by cold-water coral reef benthos at the east mingulay marine protected area (Sea of the Hebrides. Western Scotland). Front. Mar. Sci. 6 10.3389/fmars.2019.00080 [DOI] [Google Scholar]
- 110.Li, W.J., Zhang, Y., Wu, N., Zhao, Z., Xu, W.A., Ma, Y.Z.et al. (2019) Colonization characteristics of bacterial communities on plastic debris influenced by environmental factors and polymer types in the Haihe Estuary of Bohai Bay, China. Environ. Sci. Technol. 53, 10763–10773 10.1021/acs.est.9b03659 [DOI] [PubMed] [Google Scholar]
- 111.Oberbeckmann, S., Loeder, M.G., Gerdts, G. and Osborn, A.M. (2014) Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol. Ecol. 90, 478–492 10.1111/1574-6941.12409 [DOI] [PubMed] [Google Scholar]
- 112.Rohwer, F., Seguritan, V., Azam, F. and Knowlton, N. (2002) Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 243, 1–10 10.3354/meps243001 [DOI] [Google Scholar]
- 113.Zettler, E.R., Mincer, T.J. and Amaral-Zettler, L.A. (2013) Life in the “Plastisphere": microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 7137–7146 10.1021/es401288x [DOI] [PubMed] [Google Scholar]
- 114.Browne, M.A., Galloway, T. and Thompson, R. (2007) Microplastic: an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 3, 559–561 10.1002/ieam.5630030412 [DOI] [PubMed] [Google Scholar]
- 115.Engler, R.E. (2012) The complex interaction between marine debris and toxic chemicals in the ocean. Environ. Sci. Technol. 46, 12302–12315 10.1021/es3027105 [DOI] [PubMed] [Google Scholar]
- 116.Bakir, A., Rowland, S.J. and Thompson, R.C. (2012) Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 64, 2782–2789 10.1016/j.marpolbul.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 117.Hermabessiere, L., Dehaut, A., Paul-Pont, I., Lacroix, C., Jezequel, R., Soudant, P.et al. (2017) Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere 182, 781–793 10.1016/j.chemosphere.2017.05.096 [DOI] [PubMed] [Google Scholar]
- 118.Jafarabadi, A.R., Mashjoor, S., Bakhtiari, A.R. and Cappello, T. (2021) Ecotoxico linking of phthalates and flame-retardant combustion byproducts with coral solar bleaching. Environ. Sci. Technol. 55, 5970–5983 10.1021/acs.est.0c08730 [DOI] [PubMed] [Google Scholar]
- 119.Maity, S., Biswas, C., Banerjee, S., Guchhait, R., Adhikari, M., Chatterjee, A.et al. (2021) Interaction of plastic particles with heavy metals and the resulting toxicological impacts: a review. Environ. Sci. Pollut. Res. 28, 60291–60307 10.1007/s11356-021-16448-z [DOI] [PubMed] [Google Scholar]
- 120.Axworthy, J.B. and Padilla-Gamino, J.L. (2019) Microplastics ingestion and heterotrophy in thermally stressed corals. Sci. Rep. 9 10.1038/s41598-019-54698-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Orte, M.R., Clowez, S. and Caldeira, K. (2019) Response of bleached and symbiotic sea anemones to plastic microfiber exposure. Environ. Pollut. 249, 512–517 10.1016/j.envpol.2019.02.100 [DOI] [PubMed] [Google Scholar]
- 122.Rocha, R.J.M., Rodrigues, A.C.M., Campos, D., Cicero, L.H., Costa, A.P.L., Silva, D.A.M.et al. (2020) Do microplastics affect the zoanthid Zoanthus sociatus? Sci. Total Environ. 713 10.1016/j.scitotenv.2020.136659 [DOI] [PubMed] [Google Scholar]
- 123.Jiang, B., Kauffman, A.E., Li, L., McFee, W., Cai, B., Weinstein, J.et al. (2020) Health impacts of environmental contamination of micro- and nanoplastics: a review. Environ. Health Prev. Med. 25, 29 10.1186/s12199-020-00870-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kroon, F.J., Motti, C.E., Jensen, L.H. and Berry, K.L.E. (2018) Classification of marine microdebris: a review and case study on fish from the Great Barrier Reef, Australia. Sci. Rep. 8 10.1038/s41598-018-34590-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thomas, P.J., Perono, G., Tommasi, F., Pagano, G., Oral, R., Buric, P.et al. (2021) Resolving the effects of environmental micro- and nanoplastics exposure in biota: a knowledge gap analysis. Sci. Total Environ. 780 10.1016/j.scitotenv.2021.146534 [DOI] [PubMed] [Google Scholar]
- 126.Guerrera, M.C., Aragona, M., Porcino, C., Fazio, F., Laura, R., Levanti, M.et al. (2021) Micro and nano plastics distribution in fish as model organisms: histopathology, blood response and bioaccumulation in different organs. Appl. Sci. Basel. 11 10.3390/app11135768 [DOI] [Google Scholar]
- 127.Jacob, H., Gilson, A., Lanctot, C., Besson, M., Metian, M. and Lecchini, D. (2019) No effect of polystyrene microplastics on foraging activity and survival in a post-larvae coral-reef fish, Acanthurus triostegus. Bull. Environ. Contam. Toxicol. 102, 457–461 10.1007/s00128-019-02587-0 [DOI] [PubMed] [Google Scholar]
- 128.Nanninga, G.B., Scott, A. and Manica, A. (2020) Microplastic ingestion rates are phenotype-dependent in juvenile anemonefish. Environ. Pollut. 259 10.1016/j.envpol.2019.113855 [DOI] [PubMed] [Google Scholar]
- 129.Critchell, K. and Hoogenboom, M.O. (2018) Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Acanthochromis polyacanthus). PLoS One 13 10.1371/journal.pone.0193308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rios-Fuster, B., Arechavala-Lopez, P., García-Marcos, K., Alomar, C., Compa, M., Álvarez, E.et al. et al. (2021) Experimental evidence of physiological and behavioral effects of microplastic ingestion in Sparus aurata. Aquat. Toxicol. 231 10.1016/j.aquatox.2020.105737 [DOI] [PubMed] [Google Scholar]
- 131.McCormick, M.I., Chivers, D.P., Ferrari, M.C.O., Blandford, M.I., Nanninga, G.B., Richardson, C.et al. (2020) Microplastic exposure interacts with habitat degradation to affect behaviour and survival of juvenile fish in the field. Proc. R. Soc. B-Biol. Sci. 287, 20201947 10.1098/rspb.2020.1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mohsen, M., Wang, Q., Zhang, L.B., Sun, L., Lin, C.G. and Yang, H.S. (2019) Microplastic ingestion by the farmed sea cucumber Apostichopus japonicus in China. Environ. Pollut. 245, 1071–1078 10.1016/j.envpol.2018.11.083 [DOI] [PubMed] [Google Scholar]
- 133.Iwalaye, O.A., Moodley, G.K. and Robertson-Andersson, D.V. (2020) The possible routes of microplastics uptake in sea cucumber Holothuria cinerascens (Brandt, 1835). Environ. Pollut. 264 10.1016/j.envpol.2020.114644 [DOI] [PubMed] [Google Scholar]
- 134.Mohsen, M., Zhang, L., Sun, L., Lin, C., Wang, Q. and Yang, H. (2020) Microplastic fibers transfer from the water to the internal fluid of the sea cucumber Apostichopus japonicus. Environ. Pollut. 257, 113606 10.1016/j.envpol.2019.113606 [DOI] [PubMed] [Google Scholar]
- 135.Renzi, M. and Blaskovic, A. (2020) Chemical fingerprint of plastic litter in sediments and holothurians from Croatia: assessment & relation to different environmental factors. Mar. Pollut. Bull. 153 10.1016/j.marpolbul.2020.110994 [DOI] [PubMed] [Google Scholar]
- 136.Bulleri, F., Ravaglioli, C., Anselmi, S. and Renzi, M. (2021) The sea cucumber Holothuria tubulosa does not reduce the size of microplastics but enhances their resuspension in the water column. Sci. Total Environ. 781, 146650 10.1016/j.scitotenv.2021.146650 [DOI] [PubMed] [Google Scholar]
- 137.Jiménez-Skrzypek, G., Hernández-Sánchez, C., Ortega-Zamora, C., González-Sálamo, J., González-Curbelo, M.Á and Hernández-Borges, J. (2021) Microplastic-adsorbed organic contaminants: analytical methods and occurrence. Trends Anal. Chem. 136, 116186 10.1016/j.trac.2021.116186 [DOI] [Google Scholar]
- 138.Sharifi, M., Yegdaneh, A., Sajjadi, S.E. and Shushizadeh, M. (2017) Identification and quantification of phthalate pollution in Holothuria atra: a sea cucumber from the Persian Gulf (Iran). Jundishapur J. Nat. Pharm. Prod. 12 10.5812/jjnpp.65055 [DOI] [Google Scholar]
- 139.Mohsen, M., Wang, Q., Zhang, L.B., Sun, L.N., Lin, C.G. and Yang, H.S. (2019) Heavy metals in sediment, microplastic and sea cucumber Apostichopus japonicus from farms in China. Mar. Pollut. Bull. 143, 42–49 10.1016/j.marpolbul.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 140.Murano, C., Agnisola, C., Caramiello, D., Castellano, I., Casotti, R., Corsi, I.et al. (2020) How sea urchins face microplastics: uptake, tissue distribution and immune system response. Environ. Pollut. 264 10.1016/j.envpol.2020.114685 [DOI] [PubMed] [Google Scholar]
- 141.Della Torre, C., Bergami, E., Salvati, A., Faleri, C., Cirino, P., Dawson, K.A.et al. (2014) Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ. Sci. Technol. 48, 12302–12311 10.1021/es502569w [DOI] [PubMed] [Google Scholar]
- 142.Nobre, C.R., Santana, M.F.M., Maluf, A., Cortez, F.S., Cesar, A., Pereira, C.D.S.et al. (2015) Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 92, 99–104 10.1016/j.marpolbul.2014.12.050 [DOI] [PubMed] [Google Scholar]
- 143.Kaposi, K.L., Mos, B., Kelaher, B.P. and Dworjanyn, S.A. (2014) Ingestion of microplastic has limited impact on a Marine Larva. Environ. Sci. Technol. 48, 1638–1645 10.1021/es404295e [DOI] [PubMed] [Google Scholar]
- 144.De-la-Torre, G.E., Apaza-Vargas, D.M. and Santillan, L. (2020) Microplastic ingestion and feeding ecology in three intertidal mollusk species from Lima, Peru. Rev. Biol. Mar. Oceanogr. 55, 167–171 10.22370/rbmo.2020.55.2.2502 [DOI] [Google Scholar]
- 145.Li, R., Zhang, S., Zhang, L., Yu, K.-F., Wang, S. and Wang, Y. (2020) Field study of the microplastic pollution in sea snails (Ellobium chinense) from mangrove forest and their relationships with microplastics in water/sediment located on the north of Beibu Gulf. Environ. Pollut. 263, 114368 10.1016/j.envpol.2020.114368 [DOI] [PubMed] [Google Scholar]
- 146.Neo, M.L., Eckman, W., Vicentuan, K., Teo, S.L.M. and Todd, P.A. (2015) The ecological significance of giant clams in coral reef ecosystems. Biol. Conserv. 181, 111–123 10.1016/j.biocon.2014.11.004 [DOI] [Google Scholar]
- 147.Chae, Y. and An, Y.J. (2020) Nanoplastic ingestion induces behavioral disorders in terrestrial snails: trophic transfer effects via vascular plants. Environ. Sci.-Nano 7, 975–983 10.1039/C9EN01335K [DOI] [Google Scholar]
- 148.Seuront, L. (2018) Microplastic leachates impair behavioural vigilance and predator avoidance in a temperate intertidal gastropod. Biol. Lett. 14 10.1098/rsbl.2018.0453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sadri, S.S. and Thompson, R.C. (2014) On the quantity and composition of floating plastic debris entering and leaving the Tamar Estuary, Southwest England. Mar. Pollut. Bull. 81, 55–60 10.1016/j.marpolbul.2014.02.020 [DOI] [PubMed] [Google Scholar]
- 150.Girard, E.B., Fuchs, A., Kaliwoda, M., Lasut, M., Ploetz, E., Schmahl, W.W.et al. (2021) Sponges as bioindicators for microparticulate pollutants? Environ. Pollut. 268 10.1016/j.envpol.2020.115851 [DOI] [PubMed] [Google Scholar]
- 151.Fallon, B.R. and Freeman, C.J. (2021) Plastics in Porifera: the occurrence of potential microplastics in marine sponges and seawater from Bocas del Toro, Panamá. PeerJ 9, e11638-e 10.7717/peerj.11638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Celis-Hernandez, O., Avila, E., Ward, R.D., Rodriguez-Santiago, M.A. and Aguirre-Tellez, J.A. (2021) Microplastic distribution in urban vs pristine mangroves: Using marine sponges as bioindicators of environmental pollution. Environ. Pollut. 284 10.1016/j.envpol.2021.117391 [DOI] [PubMed] [Google Scholar]
- 153.Andrady, A.L. (2011) Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605 10.1016/j.marpolbul.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 154.Cole, M., Lindeque, P., Halsband, C. and Galloway, T.S. (2011) Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62, 2588–2597 10.1016/j.marpolbul.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 155.Nelms, S.E., Duncan, E.M., Broderick, A.C., Galloway, T.S., Godfrey, M.H., Hamann, M.et al. (2016) Plastic and marine turtles: a review and call for research. ICES J. Mar. Sci. 73, 165–181 10.1093/icesjms/fsv165 [DOI] [Google Scholar]
- 156.Duncan, E.M., Broderick, A.C., Fuller, W.J., Galloway, T.S., Godfrey, M.H., Hamann, M.et al. (2018) Microplastic ingestion ubiquitous in marine turtles. Global Change Biol. 25, 744–752 10.1111/gcb.14519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Caron, A.G.M., Thomas, C.R., Berry, K.L.E., Motti, C.A., Ariel, E. and Brodie, J.E. (2018) Ingestion of microplastic debris by Green sea turtles (Chelonia mydas) in the Great Barrier Reef: validation of a sequential extraction protocol. Mar. Poll. Bull. 127, 743–751 10.1016/j.marpolbul.2017.12.062 [DOI] [PubMed] [Google Scholar]
- 158.Machovsky-Capuska, G.E., Amiot, C., Denuncio, P., Grainger, R. and Raubenheimer, D. (2019) A nutritional perspective on plastic ingestion in wildlife. Sci. Total Environ. 656, 789–796 10.1016/j.scitotenv.2018.11.418 [DOI] [PubMed] [Google Scholar]
- 159.Machovsky-Capuska, G.E., Andrades, R. and Santos, R.G. (2020) Debris ingestion and nutritional niches in estuarine and reef Green turtles. Mar. Pollut. Bull. 153 10.1016/j.marpolbul.2020.110943 [DOI] [PubMed] [Google Scholar]
- 160.Germanov, E., Marshall, A.D., Hendrawan, I.G., Admiraal, R., Rohner, C.A., Argeswara, J.et al. (2019) Microplastics on the menu: plastics pollute Indonesian manta ray and whale shark feeding grounds. Front. Mar. Sci. 6 10.3389/fmars.2019.00679 [DOI] [Google Scholar]
- 161.Besseling, E., Foekema, E.M., Van Franeker, J.A., Leopold, M.F., Kuhn, S., Rebolledo, E.L.B.et al. (2015) Microplastic in a macro filter feeder: humpback whale Megaptera novaeangliae. Mar. Pollut. Bull. 95, 248–252 10.1016/j.marpolbul.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 162.Battaglia, F.M., Beckingham, B.A. and McFee, W.E. (2020) First report from North America of microplastics in the gastrointestinal tract of stranded bottlenose dolphins (Tursiops truncatus). Mar. Pollut. Bull. 160 10.1016/j.marpolbul.2020.111677 [DOI] [PubMed] [Google Scholar]
- 163.Lusher, A.L., Hernandez-Milian, G., Berrow, S., Rogan, E. and O'Connor, I. (2018) Incidence of marine debris in cetaceans stranded and bycaught in Ireland: Recent findings and a review of historical knowledge. Environ. Pollut. 232, 467–476 10.1016/j.envpol.2017.09.070 [DOI] [PubMed] [Google Scholar]
- 164.Nelms, S.E., Barnett, J., Brownlow, A., Davison, N.J., Deaville, R., Galloway, T.S.et al. (2019) Microplastics in marine mammals stranded around the British coast: ubiquitous but transitory? Sci. Rep. 9, 1075 10.1038/s41598-018-37428-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tetu, S.G., Sarker, I., Schrameyer, V., Pickford, R., Elbourne, L.D.H., Moore, L.R.et al. (2019) Plastic leachates impair growth and oxygen production in Prochlorococcus, the ocean's most abundant photosynthetic bacteria. Commun. Biol. 2, 184 10.1038/s42003-019-0410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Huang, Y., Li, W., Gao, J., Wang, F., Yang, W., Han, L.et al. (2021) Effect of microplastics on ecosystem functioning: Microbial nitrogen removal mediated by benthic invertebrates. Sci. Total Environ. 754 [DOI] [PubMed] [Google Scholar]
- 167.Hope, J.A., Coco, G. and Thrush, S.F. (2020) Effects of polyester microfibers on microphytobenthos and sediment-dwelling infauna. Environ. Sci. Technol. 54, 7970–7982 10.1021/acs.est.0c00514 [DOI] [PubMed] [Google Scholar]
- 168.Wu, N., Zhang, Y., Zhao, Z., He, J.H., Li, W.J., Li, J.F.et al. (2020) Colonization characteristics of bacterial communities on microplastics compared with ambient environments (water and sediment) in Haihe Estuary. Sci. Total Environ. 708 10.1016/j.scitotenv.2019.134876 [DOI] [PubMed] [Google Scholar]
- 169.Gkoutselis, G., Rohrbach, S., Harjes, J., Obst, M., Brachmann, A., Horn, M.A.et al. (2021) Microplastics accumulate fungal pathogens in terrestrial ecosystems. Sci. Rep. 11 10.1038/s41598-021-92405-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Audrézet, F., Zaiko, A., Lear, G., Wood, S.A., Tremblay, L.A. and Pochon, X. (2021) Biosecurity implications of drifting marine plastic debris: current knowledge and future research. Mar. Pollut. Bull. 162, 111835 10.1016/j.marpolbul.2020.111835 [DOI] [PubMed] [Google Scholar]
- 171.Weis, J.S. and Palmquist, K.H. (2021) Reality check: experimental studies on microplastics lack realism. Appl. Sci. 11, 8529 10.3390/app11188529 [DOI] [Google Scholar]
- 172.IPCC (2021). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the sixth assessment report of the Intergovernmental Panel on Climate Change In press