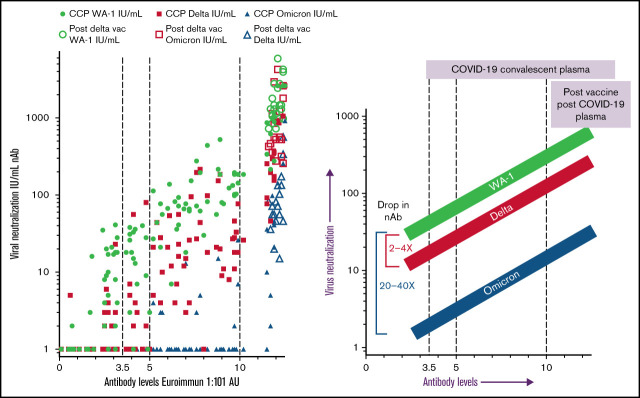
Key Points
All of the post-δ COVID-19/postvaccination convalescent plasma effectively neutralizes the ο and δ variants.
High-titer CCP and hyperimmunoglobulin neutralizes SARS-CoV-2 variants despite no previous donor exposure to the variants.
Visual Abstract
Abstract
The ongoing evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants severely limits available effective monoclonal antibody therapies. Effective drugs are also supply limited. COVID-19 convalescent plasma (CCP) qualified for high antibody levels effectively reduces immunocompetent outpatient hospitalization. The Food and Drug Administration currently allows outpatient CCP for the immunosuppressed. Viral-specific antibody levels in CCP can range 10- to 100-fold between donors, unlike the uniform viral-specific monoclonal antibody dosing. Limited data are available on the efficacy of polyclonal CCP to neutralize variants. We examined 108 pre-δ/pre-ο donor units obtained before March 2021, 20 post-δ COVID-19/postvaccination units, and 1 pre-δ/pre-ο hyperimmunoglobulin preparation for variant-specific virus (vaccine-related isolate [WA-1], δ, and ο) neutralization correlated to Euroimmun S1 immunoglobulin G antibody levels. We observed a two- to fourfold and 20- to 40-fold drop in virus neutralization from SARS-CoV-2 WA-1 to δ or ο, respectively. CCP antibody levels in the upper 10% of the 108 donations as well as 100% of the post-δ COVID-19/postvaccination units and the hyperimmunoglobulin effectively neutralized all 3 variants. High-titer CCP neutralizes SARS-CoV-2 variants despite no previous donor exposure to the variants.
Introduction
Commercial serologic assays predictive of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and variant neutralization are important for effective clinical use of COVID-19 convalescent plasma (CCP) because substantial heterogeneity exists in CCP donor responses with higher antibody levels associated with virus neutralization.1-3 The SARS-CoV-2 ο variant BA.1 rendered many monoclonals ineffective in laboratory virus neutralization tests, necessitating their removal as outpatient monoclonal therapies for acute COVID-19.4,5 Although Sotrovimab retained activity against ο BA.1, activity was lost against the BA.1.1 and BA.2 ο variants. Tixagevimab/cilgavimab (Evusheld), approved only for preexposure prophylaxis, was ineffective at neutralizing ο BA.2 and showed reduced activity toward Omicrons BA.1 and BA.1.1.6 Bebtelovimab neutralizes ο BA.1 but will have limited availability.7
A recent large clinical trial over the period from June 2020 to October 2021 demonstrated that early outpatient CCP reduced the risk of hospitalizations by more than half.8 Ninety percent of the trial’s >300 unique CCP donor units were collected prior to January 2021, representing nonvaccinated pre-α/δ/ο variant plasma. In December 2021, the Food and Drug Administration extended CCP from hospital use to immunosuppressed outpatients while simultaneously increasing commercial serologic benchmarks by 1.5-fold for CCP qualification.9
Recent ο variant studies measured virus neutralization without concomitant commercial serologic testing for general antibody levels.5 Wang and colleagues measured a 10-fold virus neutralization reduction from SARS-CoV-2 wild-type to ο in 16 individual plasma samples obtained from January to March 2020 in China.10 Röllser et al examined only 10 CCP units from donors infected with variants and showed a lack of CCP neutralization of ο but substantial neutralization with post–COVID-19 postvaccination plasma.11 These studies have only tested a few samples and do not correlate the results to a commercial assay necessary to qualify therapeutic CCP units. Considering that CCP donors vary significantly in terms of virus neutralization capacity, the use of commercial assays that predict neutralization of SARS-CoV-2 and its variants becomes important to select the optimal therapeutic CCP units.
This study tested a total of 129 samples: 108 CCP donor units, 20 post-δ COVID-19/postvaccination donor units, and 1 pre-δ/pre-ο hyperimmunoglobulin preparation for ability to neutralize 3 SARS-CoV-2 isolates/variants (WA-1, δ, and ο), correlating results to the Euroimmun spike-S1 antibody plasma level.
Methods
Participants
After the outpatient clinical research trial transfusions from >300 unique donors were complete, 108 remnant-qualified (positive antibody presence after 1:320 dilution using a validated spike protein Clinical Laboratory Improvement Amendment Enzyme-Linked Immunosorbent Assay) donor plasma units were available for WA-1, δ, and ο virus neutralizations.8 Seventy-one out of 108 (66%) pre-δ/pre-ο units were donated before January 2021. Approvals were obtained from the institutional review boards at Johns Hopkins University School of Medicine as single Institutional Review Board for all participating sites and the Department of Defense Human Research Protection Office. All participants provided written informed consent. The 20 post-δ COVID-19/postvaccination (1 J&J Ad26.COV2.S, 5 Moderna mRNA-1273, and 14 Pfizer BNT162b2) plasma aliquots were obtained from Innovative Transfusion Medicine after research informed consent for CCP collection. The collections occurred late August through October 2021, representing δ variant infection. The viral strain associated to convalescent plasma was associated by timing of donation to existing variants, not sequence confirmed.
The hyperimmunoglobulin sample was prepared from 101 CCP units, collected between 16 June 2020 and 13 January 2021 (representing pre-δ/pre-ο plasma) from recovered patients by an apheresis plasma collection technique following Mayo Clinic Blood Donor Center’s standard operating procedures. Pooled plasma was loaded on the protein A resin to capture immunoglobulin G at a neutral pH, washed to remove low affinity proteins, and eluted at low pH. The final product was formulated as a 5% protein solution in glycine/acetic acid.
Microneutralization studies
Plasma neutralizing antibodies were determined as described for SARS-CoV-2.1,12 WA-1 (SARS-CoV-2/USA-WA1/2020 EPI_ISL_404895) was obtained from BEI Resources, and the δ (hCoV19/USA/MD-HP05660/2021 EPI_ISL_2331507) and ο (hCoV19/USA/MD-HP20874/2021 EPI_ISL_7160424) variants were isolated from COVID-19 patients at Johns Hopkins Hospital as previously described.13 The neutralizing antibodies titer was calculated as the highest serum dilution that eliminated the cytopathic effect in 50% of the wells (NT50), and the area under the curve was calculated using Graphpad Prism. The total plasma levels of antibodies against spike region S1 were measured using the ELISA Euroimmun assay as described.14
Results and discussion
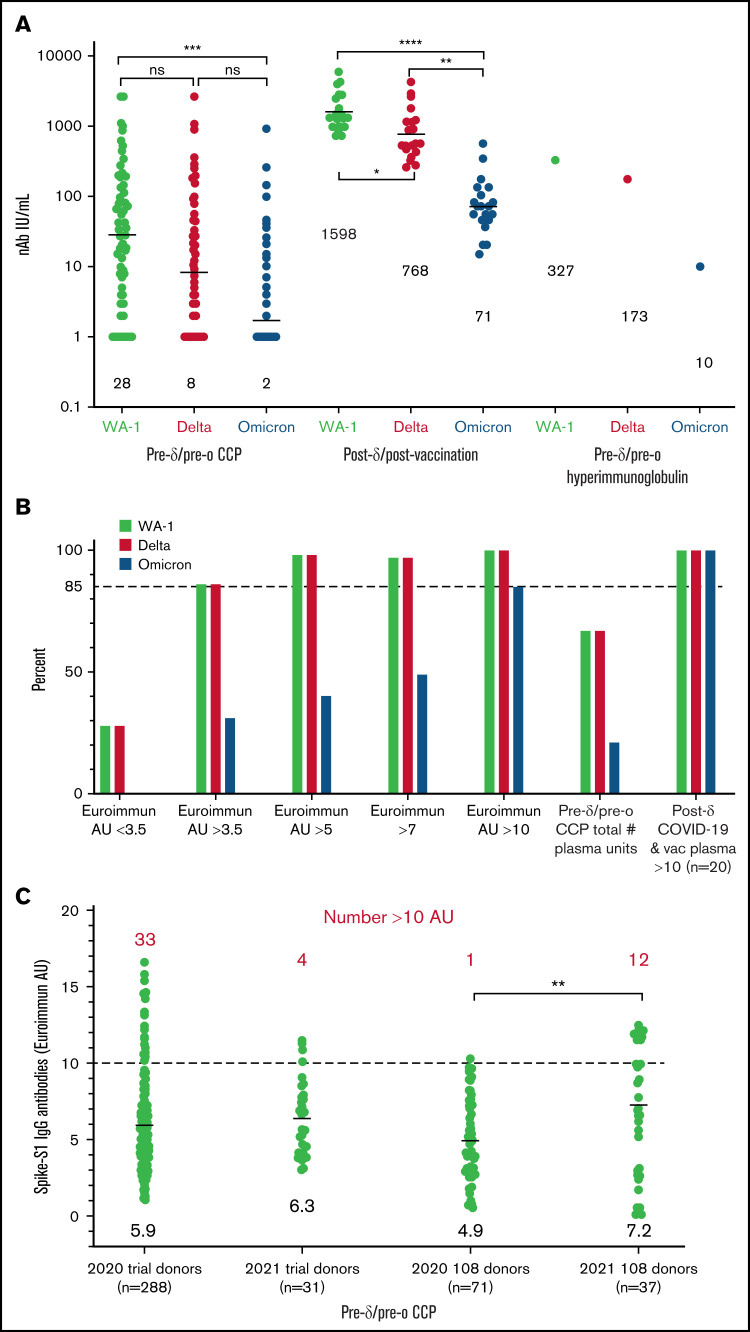
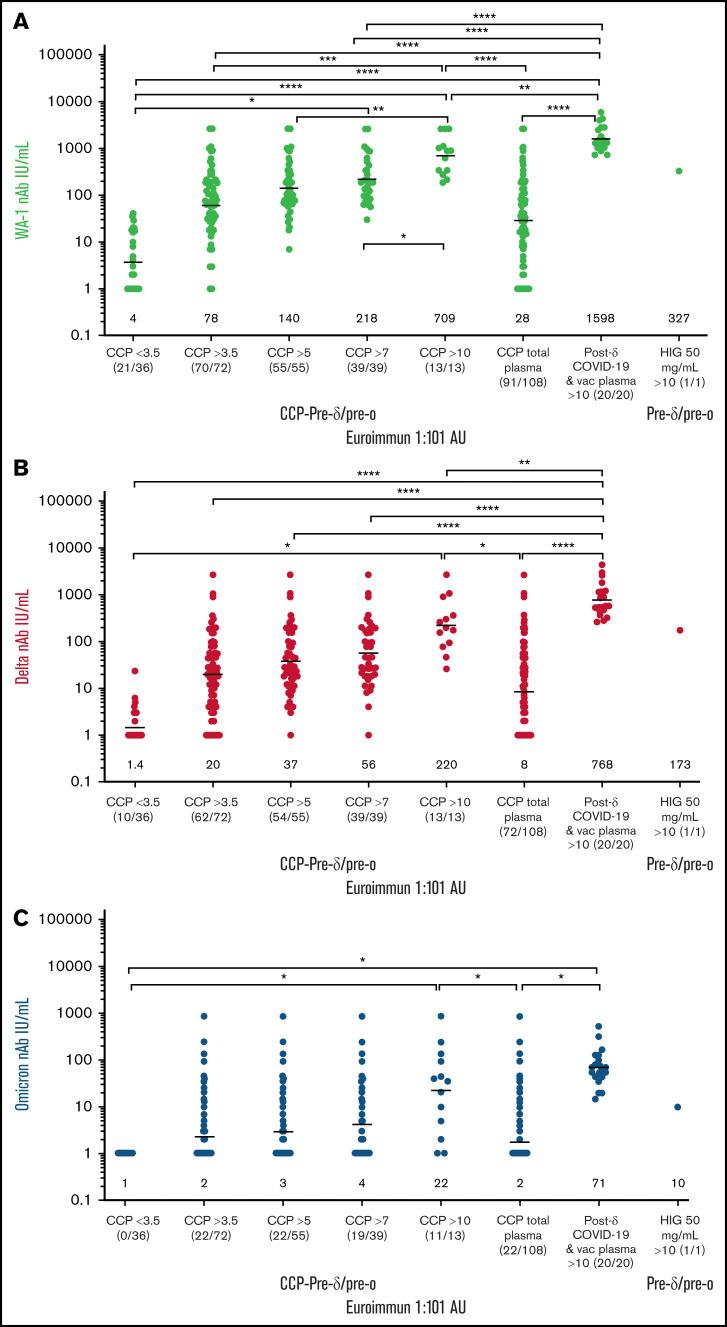
Compared with the WA-1 isolate, we observed a two- to fourfold decrease in virus neutralization of δ and 20- to 40-fold neutralization decrease of ο in all plasma samples (108 pre-δ/pre-ο CCP units, the 20 post-δ COVID-19/postvaccination units, and pre-δ/pre-ο hyperimmunoglobulin) (Figure 1). The 108 research trial remnant units with Euroimmun AU over 3.5 have an 85% rate of positive virus neutralization for WA-1 and δ. However, Euroimmun over 10 AU was necessary to retain similar neutralization for ο (Figure 1). All of the post-δ COVID-19/postvaccination donor plasma as well as the hyperimmunoglobulin, with Euroimmun AU over 10, effectively neutralized the WA-1 isolate, δ and ο variants. As a benchmark for clinical effectiveness, the early treatment CCP trial, successful at preventing hospitalizations principally with unvaccinated ancestral virus, had >10% of pre-δ/pre-ο 2020 prevaccination donor units or 2021 donor units with Euroimmun AU over 10 (Figure 1). For all these clinical trial transfused units, the WA-1 neutralizing antibody geometric mean (geomean) was 26 IU/mL,8 whereas remnant 108 CCP plasma units had a geomean of 28 IU/mL. However, sorting to increasing Euroimmun AUs from >3.5 to 7 and to 10 AUs increased twofold and 10-fold, respectively, WA-1, δ, and ο neutralization (Figure 2). All of the post-δ COVID-19/postvaccination samples measured Euroimmun AU over 10, with WA-1 neutralization at geomean 1598 with comparable reduction in δ activity of twofold and 20-fold with ο (Figure 2). The pre-δ/pre-ο hyperimmune globulin immunoglobulin G showed a similar fold decrease in neutralization across the 3 isolates (Figure 2). The wider span of both high and low levels of neutralization antibodies in CCP may account for the larger decrease in δ activity compared with the narrower high range of virus neutralizations with the post-δ COVID-19/postvaccination units. This data suggests that Euroimmun AU over 5 (∼1.5 × 3.5) would be effective for δ and over 10 (∼3 × 3.5) for ο CCP therapy.
Figure 1.
Reduction in virus neutralization sorted by plasma type as well as an increase by Euroimmun antibody levels. (A) WA-1, δ, and ο virus microneutralization sorted by the 108 CCP units, post-δ COVID-19/post vaccination, and hyperimmunoglobin. IU/mL geomeans are shown above x-axis. (B) Antibody levels over 3.5 Euroimmun AU show 85% microneutralization with WA-1 and δ, whereas ο neutralization requires Euroimmun AU over 10 for 85% of the 108 CCP donors. Post-δ COVID-19/postvaccination retains 100% virus neutralization for the 3 variants. Virus neutralization is any positive IU/mL over 1. (C) Range of Euroimmun antibody levels sorted by prevaccination 2020 donor collections and early January to March 2021 for the large number of clinical trial donors and the 108 remnant donors. Geomeans near x-axis and number above 10 AU in red above values. More than 10% of units have Euroimmun AU over 10 except for the 2020 remnant units. Three of the 12 2021 108 donors were vaccinated along with documented COVID-19. The vaccine status of the other 9 was not recorded at time of donation. The P values were Tukey’s multiple comparisons of 1-way ANOVA. ****P < .0001; ***P < .001; **P < .01. AU, arbitrary units.
Figure 2.
Sorting higher Euroimmun categories indicates virus neutralization to WA-1, δ, and ο. WA-1 (A), δ (B), and ο (C) virus microneutralization measured in CCP, post-δ COVID-19/postvaccination, and hyperimmune globulin (HIG) sorted for viral-specific antibody levels by Euroimmun AU at 1:101 dilution. IU/mL geometric means are shown above x-axis. The ratio of donor plasma neutralizations to total tested is shown below x-axis (neutralization number/total number).The P values were Tukey’s multiple comparisons of 1-way ANOVA. ****P < .0001; ***P < .001; **P < .01; *P < .05.
Commercial serologic assays were adjusted ∼1.5-fold by the Food and Drug Administration to be in the effective range for ο.9 The Euroimmun assay is one of many that can be used for donor plasma qualification. Many published studies on variant virus neutralization by CCP characterize 10 to 30 individual donors, deeming them inactive against variants with small sample sizes. In contrast, here we compared over 100 CCP donor units to show that high viral-specific antibodies defined by commercial serologic tests indicates ο neutralization. The post-δ COVID-19/postvaccination donor units and hyperimmune globulin retain broad activity against variants through ο, indicating the improved potential efficacy of a polyclonal antibody treatment compared with monoclonal antibody treatments as a way to keep up as a therapeutic tool against the evolving virus. This study did not characterize ο convalescent plasma neutralization of older variants. Another limitation is the use of only in vitro data, for which the true relevance to clinical use is not known. We posit that high-titer polyclonal CCP donors characterized with commercial serologic assays with a mismatch to existing variants still neutralize SARS-CoV-2 variants as well as lower titer donor plasma units with a direct match of plasma-to-virus variants. These polyclonal high-titer units and hyperimmune globulin retain efficacy as antibody therapy in the face of variants.
Acknowledgments
The authors gratefully acknowledge the generous contributions of the study participants and plasma donors who gave of their time and specimens.
This study was funded principally by the US Department of Defense’s Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense (JPEO-CBRND), in collaboration with the Defense Health Agency (DHA) (contract number: W911QY2090012); with additional support from Bloomberg Philanthropies; State of Maryland; the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) 3R01AI152078-01S1; NIH NIAID contract N7593021C00045 to the Johns Hopkins Center of Excellence in Influenza Research and Response (JH CEIRR); NIH National Center for Advancing Translational Sciences U24TR001609 and UL1TR003098; Division of Intramural Research NIAID NIH; Mental Wellness Foundation; Moriah Fund; Octapharma; HealthNetwork Foundation; and the Shear Family Foundation.
Appendix: COVID-19 Serologic Studies Consortium members
The members of the COVID-10 Serologic Studies Consortium (CSSC) are: Anne Arundel Medical Center (Barry R. Meisenberg); Ascada Research (Matthew Abinante, Kevin Oei); Baylor College of Medicine (Yuriko Fukuta); MedStar Georgetown University Hospital (Seble Kessaye); Johns Hopkins Center for American Indian Health (Laura L. Hammitt, Catherine G. Sutcliffe) Johns Hopkins Bloomberg School of Public Health (David J. Sullivan, Bryan Lau, Atika Singh, David M. Shade, Stephan Ehrhardt, Sheriza N. Baksh, Andrew Pekosz, Sabra L. Klein, Arturo Casadevall); Johns Hopkins University (Kelly A. Gebo, Shmuel Shoham, Evan M. Bloch, Christi E. Marshall, Anusha Yarava, Karen Lane, Nichol A. McBee, Amy L. Gawad, Nicky Karlen, Daniel E. Ford, Douglas A. Jabs, Lawrence J. Appel, Oliver Laeyendecker, Aaron A.R. Tobian, Daniel F. Hanley); Lifespan/Brown University Rhode Island Hospital (Adam C. Levine); Mayo Clinic, Phoenix (Janis E. Blair); MedStar Washington Hospital Center (Aarthi Shenoy); NorthShore University HealthSystem (Giselle S. Mosnaim, Thomas J. Gniadek); The Bliss Group (Michael Roth); The Next Practice Group (Colin Foster); University of California Los Angeles (Judith S. Currier); University of Alabama at Birmingham (Sonya L. Health); University of California, Irvine Health (Donald N. Forthal); University of California, San Diego (Edward R. Cachay, Elizabeth S. Allen); University of Cincinnati Medical Center (Moises A. Huaman); University of Massachusetts Worcester (Jonathan M. Gerber); University of Miami (Shweta Anjan); University of New Mexico (Jay S. Raval); University of Rochester (Martin S. Zand); University of Texas Health Science Center at Houston (Bela Patel); University of Utah Health (Emily S. Spivak, J. Robinson Singleton); Vassar Brothers Medical Center (Valerie C. Cluzet, Daniel Cruser); Wayne State University (James H. Paxton); Western Connecticut Health Network, Danbury Hospital (Joann R. Petrini, Patrick B. Broderick, William Rausch, MarieElena Cordisco); Western Connecticut Health Network, Norwalk Hospital (Jean Hammel, Benjamin Greenblatt).
Authorship
Contribution: D.J.S., A.C., A.P., T.J.G., and A.A.R.T. designed experiments; M.L., C.W., C.B., A.P., E.J.B., and Y.E. performed experiments; M.J.J.., T.D.W., and A.B.D. purified the hyperimmunoglobulin; G.R.C., W.B., and R.O.M. provided post-δ COVID-19/postvaccination plasma; A.Y., and K.L. managed trial research blood bank; A.J.S., E.M.B., A.T., and T.J.G. were contributing blood bankers; E.R.C., B.R.M., M.A.H., Y.F., B.P., S.L.H., O.L., A.C.L., J.H.P., S.A., and J.M.G. contributed to the conduct of the related clinical trial; D.J.S., K.A.G., D.F.H., E.M.B., P.C., A.A.R.T., and S.S. organized clinical trial studies as source of CCP; D.J.S., M.L., and A.P. analyzed data and wrote the manuscript with input from all authors; and all authors approved the final version of the manuscript. The full list of COVID-19 Serologic Studies Consortium (CSSC) authors appears in “Appendix.”
Conflict-of-interest disclosure: T.J.G. reports paid consultancy for Fresenius Kabi; G.R.C. and R.O.M. are on the Board of Innovative Transfusion Medicine; W.B. reports Board of Blood Centers of America membership; A.C. reports Scientific Advisory Board of Sabtherapeutics (cow-derived human immunoglobulins COVID-19 treatment and other infectious diseases) and Ortho Diagnostics Speakers Bureau membership; and E.B. reports FDA Blood Products Advisory Committee membership. The remaining authors declare no competing financial interests.
Correspondence: David J. Sullivan, Room W4606, 615 N. Wolfe St, Baltimore, MD 21205; e-mail: dsulliv7@jhmi.edu.
References
- 1.Klein SL, Pekosz A, Park HS, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141-6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59(2):e02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang MS, Case JB, Franks CE, et al. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin Chem. 2020;66(12):1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671-675. [DOI] [PubMed] [Google Scholar]
- 5.Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602(7898):664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou H, Tada T, Dcosta B, Landau N. Neutralization of SARS-CoV-2 Omicron BA.2 by therapeutic monoclonal antibodies. bioRxiv. 2022;480166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westendorf K, Wang L, Zentelis S, et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. bioRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan DJ, Gebo KA, Shoham S, et al. Randomized controlled trial of early outpatient COVID-19 treatment with high-titer convalescent plasma. medRxiv. 2021. [Google Scholar]
- 9.O'Shaughnessy JA. US Food and Drug Administration. Convalescent Plasma EUA Letter of Authorization 12282021. Available at: https://www.fda.gov/media/141477/download. Accessed 30 December 2021.
- 10.Wang Y, Ma Y, Xu Y, et al. Resistance of SARS-CoV-2 Omicron variant to convalescent and CoronaVac vaccine plasma. Emerg Microbes Infect. 2022;11(1):424-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386(7):698-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaecher SR, Stabenow J, Oberle C, et al. An immunosuppressed Syrian golden hamster model for SARS-CoV infection. Virology. 2008;380(2):312-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fall A, Eldesouki RE, Sachithanandham J, et al. A quick displacement of the SARS-CoV-2 variant Delta with Omicron: unprecedented spike in COVID-19 cases associated with fewer admissions and comparable upper respiratory viral loads. medRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caturegli G, Materi J, Howard BM, Caturegli P. Clinical validity of serum antibodies to SARS-CoV-2: A case-control study. Ann Intern Med. 2020;173(8):614-622. [DOI] [PMC free article] [PubMed] [Google Scholar]