Abstract
Objective
We sought to determine if corticosteroid administration is associated with a SARS-CoV-2 nucleic acid test-positive result and to describe therapies administered to SARS-CoV-2 infected children.
Methods
We collected cross-sectional data from participants recruited in 41 pediatric emergency departments (ED) in 10 countries between March 2020 and June 2021. Participants were <18 years old, had signs or symptoms of, or risk factors for acute SARS-CoV-2 infection, and had nucleic acid testing performed. To determine if SARS-CoV-2 test status was independently associated with corticosteroid administration, we used a multivariable conditional logistic regression model matched by study site to compare treatments administered based on SARS-CoV-2 test and disposition status. This analysis was repeated for the subgroup of study participants who were hospitalized.
Results
30.3% (3,121/10,315) of participants were SARS-CoV-2-positive. Although remdesivir was more commonly administered to SARS-CoV-2-positive children, use was infrequent (25/3120 [0.8%] vs 1/7188 [0.01%]; P = .001). Corticosteroid use was less common among SARS-CoV-2-positive children (219/3120 [7.0%] vs 759/7190 [10.6%]; P < .001). Among hospitalized children, there were no differences in provision of inotropes, respiratory support, chest drainage or extracorporeal membrane oxygenation between groups. Corticosteroid administration was associated with age, history of asthma, wheezing, study month, hospitalization and intensive care unit admission; it was not associated with a positive SARS-CoV-2 test result overall (aOR: 0.91; 95%CI: 0.74, 1.12) or among the subgroup of those hospitalized (aOR: 1.04; 95%CI: 0.75, 1.44).
Conclusions
Few disease-specific treatments are provided to SARS-CoV-2-positive children; clinical trials evaluating therapies in children are urgently needed.
Keywords: adrenal cortex hormones; child, hospitalized; COVID-19; emergency service, hospital; SARS-CoV-2
What's New.
Few SARS-CoV-2 infected children receive SARS-CoV-2 specific therapies. There was no independent association between corticosteroid use and SARS-CoV-2 test status, even among those hospitalized. There is a need for clinical trials that provide pediatric-specific data to guide therapy in children.
Alt-text: Unlabelled box
Over the course of the SARS-CoV-2 pandemic, the pediatric burden of disease has increased. As of December 2021, children represented 15.7% of all cases reported in the United States,1 and the hospitalization rate among children during the fourth wave exceeds those seen during earlier waves2 with more than 30% of hospitalized children having severe COVID-19.3 However, we have limited knowledge of treatments provided to children with COVID-19 in emergency departments (ED) and during hospitalization and such data can identify interventions in need of pediatric-specific evidence to inform use.
Reports from early 2020 that included 115 hospitalized children described the use of hydroxychloroquine (30%–37%), remdesivir (6%–8%), corticosteroids (4%–11%), and tocilizumab (2%–5%).4 , 5 In one study, additional treatments included anticoagulation (28%), azithromycin (20%), and anakinra (11%).4 A recent multinational database study reported significant heterogeneity across countries in the use of treatments among hospitalized children.6 Notably, corticosteroids were administered to between 25% and 35% of children in the US but just 7% of children in Korea. Moreover, a study from the United Kingdom reported that 69% of children hospitalized with COVID-19 received antibiotics.7
Although several drugs have recently been approved for use in children with COVID-19 (eg, monoclonal antibodies), the extension of their use to include neonates and young children was based primarily on adolescent and adult safety and efficacy data.8 Moreover, the mainstay of therapy among critically ill adults, dexamethasone,9 remains essentially an unevaluated option in children and although pediatric data are lacking, a clinicaltrials.gov search on December 17, 2021 revealed no active COVID-19 corticosteroid pediatric studies. Thus, there are few pediatric SARS-CoV-2 specific treatment options available based on pediatric clinical trial data.10
To shed light on this issue, we sought to describe and compare treatments provided to children tested for SARS-CoV-2 infection based on test result and disposition status. Treatments provided were grouped based on timing of administration: 1) prior to the ED visit (ie, prehospital), and 2) during the hospital visit (ie, in the ED or the subsequent hospitalization (if required)). We additionally determined if, among hospitalized children, SARS-CoV-2 test-result status was associated with corticosteroid administration. As most children with COVID-19 discharged home from the ED are unlikely to experience complications, we hypothesized that among these children, treatments would not differ by SARS-CoV-2 test status. Among those hospitalized, given the benefits seen in adults associated with corticosteroid administration, we hypothesized that a higher proportion of SARS-CoV-2-positive children would receive corticosteroids.
Methods
Study Design & Setting
The Pediatric Emergency Research Network's (PERN)-COVID-19 prospective study recruited children with suspected acute SARS-CoV-2 infections presenting for care in one of 41 participating pediatric EDs in 10 countries between March 18, 2020 and June 15, 2021.11 We report cross-sectional data (database export performed July 26, 2021) related to the index ED visit and subsequent hospitalization, if required.
Participants & Recruitment
Eligible participants were <18 years old, tested for SARS-CoV-2 because of suspected acute infections, and included SARS-CoV-2-positive and negative children. Children diagnosed or presumed to have multisystem inflammatory syndrome in children (MIS-C) were excluded from this analysis. Testing indications varied by institution and were modified as the pandemic evolved. Only those who underwent nucleic acid testing from the nasopharynx, nares, or throat were included in this analysis (ie, those who only underwent antibody testing were excluded given the low sensitivity of these tests during the acute illness)12. Those whose caregivers declined consent and children who declined assent, when appropriate, were excluded. Research ethics board approval was obtained at all participating institutions, with varying requirements for documentation of consent and assent (eg, verbal, electronic, and written).
At most study sites, research assistants received a list of all potentially eligible children daily along with their test results. At study initiation, nucleic acid testing capacity was limited, thus we attempted to recruit all children tested for SARS-CoV-2 infections. Over time, the number of children tested for SARS-CoV-2 increased. Therefore, the protocol was revised to have sites consecutively recruit all SARS-CoV-2-positive children and to sequentially contact test-negative children (starting with the first children tested each day) to a maximum of 5 children per day. As the positivity rate varied between sites, to minimize sampling bias and promote similar proportions of test-positive participants across study sites, on September 8, 2020, the recruitment model was revised to have sites recruit all positive children along with two SARS-CoV-2 test-negative children from the same day for each positive child recruited (ie, 1:2 ratio).
Data Collection
Participant demographic, epidemiologic, chronic conditions/comorbidities, and clinical data were collected from caregivers shortly after the ED visit. Information was extracted from the medical record regarding the index ED visit and subsequent hospitalization, if required. Case report forms were modified as therapeutic considerations and our understanding of COVID-19 in children evolved. This led us to add data fields during the course of the study (eg, anosmia, ageusia); eTable 1. Study procedures were standardized through the use of a manual of operations. Data quality rules were programmed within REDCap, with data quality checks performed by the study statistician. Queries were issued for key variables that were missing, out of range or discrepant using REDCap's data resolution workflow.
SARS-CoV-2 Testing & Treatment
This pragmatic observational study did not specify the approach to detection of SARS-CoV-2 infection. Local practices and illness severity dictated isolation, investigations, treatment, and hospitalization decisions.
Sample Size
The PERN-COVID-19 prospective study intended to recruit up to 12,500 participants to acquire the 50+ COVID-positive children who experienced the study's primary objective.11 When the target number of children experiencing the primary outcome had been recruited, no additional participants were enrolled. During planning of the parent study, we anticipated that the proposed target number of participants would provide sufficient precision for the current study, as reflected by the 95% confidence intervals.
Statistical Analysis
Continuous and categorical variables were summarized with medians and interquartile ranges and frequencies and percentages, respectively. For between-group comparisons, Cochran–Mantel–Haenszel and van Elteren tests stratified by study site were used for categorical and continuous variables, respectively. Predictors of corticosteroid administration were evaluated in all study participants, and in an a priori subgroup of those who were hospitalized, using multivariable conditional logistic regression, with the study site variable used to form the matched sets of observations.13 The dependent variable (corticosteroids – oral, or intravenous) was limited to administration in the ED, hospital, or prescribed at ED discharge (ie, administration prior to the ED visit was excluded). The following independent variables were included in the model – SARS-CoV-2 nucleic acid result, age, time-period (ie, each month identified chronologically), history of asthma, other chronic conditions/comorbidities, and wheezing on ED examination. In the model with all participants, we included hospitalization as a covariate; in the hospitalized participant analysis, we included intensive care unit (ICU) admission as a covariate. Children admitted to an observation unit were classified as ‘hospitalized.’ In both models, we included interaction terms between SARS-CoV-2 test results and study time period. As corticosteroids are routinely administered to children with asthma, we performed a sensitivity analysis excluding those with a history of asthma and those who were diagnosed as having an asthma exacerbation, wheeze, or reactive airways disease to allow for a focused assessment of corticosteroid use in COVID-19 disease. No correction for multiple pairwise comparisons was performed because of the exploratory nature of the study. Because only 1% of participants had missing data related to key variables, missing data were not imputed (eTable 2 in the Supplement). Statistical significance was designated as P < .05 (2-sided). Analyses were performed using SPSS 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) and Stata 15.0 (College Station, TX: StataCorp LLC).
Results
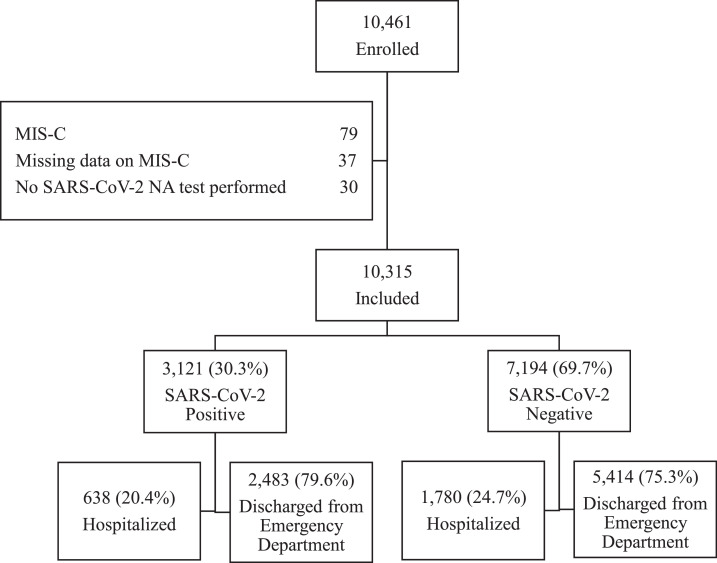
We enrolled 10,315 children who met all study eligibility criteria were enrolled; Figure . SARS-CoV-2 nucleic acid testing was positive for 3121 (30.3%) study participants, with the proportion positive varying by geographic region and race and/or ethnicity; Table 1 , eTables 3, and 4. Compared to test-negative children, SARS-CoV-2-positive children were more likely to have been febrile and have respiratory symptoms; eTable 5. While they more often reported ageusia and anosmia (P < .001), these symptoms were uncommon, reported by 6.8% (166/2444) and 6.5% (160/2444) of SARS-CoV-2 positive children, respectively. SARS-CoV-2-positive children were less likely to have an underlying medical condition and gastrointestinal symptoms; Table 1, eTables 5, and 6. Prior to the index ED visit, SARS-CoV-2-positive children were less likely to have received a corticosteroid, antibiotics, and ibuprofen (all P < .001; Table 2 ). Although SARS-CoV-2-positive children were less frequently hospitalized at the index ED visit (638/3121 [20.4%] vs 1780/7194 [24.7%]; P < .001), ICU admission did not differ between test-positive and negative children (90/3121 [2.9%] vs. 190/7194 [2.6%]; P = .53).
Figure.
Study participants. NA, Nucleic Acid; MIS-C, Multisystem inflammatory syndrome in children.
Table 1.
Demographic and Clinical Characteristics of Study Participants Based on Hospitalization and SARS-CoV-2 Test Result Status
| All Study Participants |
Hospitalized Study Participants |
|||||
|---|---|---|---|---|---|---|
| SARS-CoV-2 PositiveN = 3121 | SARS-CoV-2 NegativeN = 7194 | P Value | SARS-CoV-2 PositiveN = 638 | SARS-CoV-2 NegativeN = 1780 | P Value | |
| Demographic Characteristics | ||||||
| Age† | ||||||
| <60 Days, n (%) | 147/3121 (4.7) | 206/7194 (2.9) | <.001 | 72/638 (11.3) | 120/1780 (6.7) | <.001 |
| 60 Days– < 2 Years, n (%) | 1066/3121 (34.2) | 2553/7194 (35.5) | .20 | 157/638 (24.6) | 452/1780 (25.4) | .02 |
| 2 Years– < 5 Years, n (%) | 529/3121 (16.9) | 1619/7194 (22.5) | <.001 | 102/638 (16.0) | 308/1780 (17.3) | .16 |
| 5 Years– <12 Years, n (%) | 754/3121 (24.2) | 1787/7194 (24.8) | .48 | 146/638 (22.9) | 492/1780 (27.6) | .06 |
| 12 Years– <18 Years, n (%) | 625/3121 (20.0) | 1029/7194 (14.3) | <.001 | 161/638 (25.2) | 408/1780 (22.9) | .002 |
| Male sex, n (%) | 1630/3121 (52.2) | 3865/7194 (53.7) | .15 | 348/638 (54.5) | 982/1780 (55.2) | .39 |
| Underlying Medical Condition, n (%) | 471/3118 (15.1) | 1509/7178 (21.0) | <.001 | 177/637 (27.8) | 586/1779 (32.9) | .77 |
| Race, Self-Reported, US Only, n (%) | ||||||
| American Indian or Alaska Native | 9/1974 (0.5) | 12/3490 (0.3) | .65 | 2/372 (0.5) | 1/1201 (0.1) | .18 |
| Asian | 51/1974 (2.6) | 108/3490 (3.1) | .32 | 8/372 (2.2) | 39/1201 (3.9) | .12 |
| Black or African American | 695/1974 (35.2) | 911/3490 (26.1) | <.001 | 103/372 (27.7) | 289/1201 (24.1) | .73 |
| Hispanic or Latino | 694/1974 (35.2) | 680/3490 (19.5) | <.001 | 106/372 (28.5) | 180/1201 (15.0) | <.001 |
| Native Hawaiian or Other Pacific Islander | 12/1974 (0.6) | 7/3490 (0.2) | .02 | 2/372 (0.5) | 3/1201 (0.2) | .41 |
| White | 319/1974 (16.2) | 1357/3490 (38.9) | <.001 | 104/372 (28.0) | 568/1201 (47.3) | <.001 |
| Other or mixed | 194/1974 (9.8) | 415/3490 (11.9) | .02 | 47/372 (12.6) | 121/1201 (10.1) | .44 |
| Enrolment Period | ||||||
| March–May 2020, n (%) | 218/3121 (7.0) | 1996/7194 (27.7) | <.001 | 63/638 (9.9) | 574/1780 (32.2) | <.001 |
| June–August 2020, n (%) | 842/3121 (27.0) | 2807/7194 (39.0) | <.001 | 189/638 (29.6) | 781/1780 (43.9) | .001 |
| September–November, n (%) | 751/3121 (24.1) | 918/7194 (12.8) | <.001 | 148/638 (23.2) | 195/1780 (11.0) | <.001 |
| December 2020–February 2021, n (%) | 753/3121 (24.1) | 768/7194 (10.7) | <.001 | 142/638 (22.3) | 137/1780 (7.7) | <.001 |
| March 2021–June 2021, n (%) | 557/3121 (17.8) | 705/7194 (9.8) | <.001 | 96/638 (15.0) | 93/1780 (5.2) | .004 |
| Emergency Department Clinical Examination | ||||||
| Triage Temperature, °C, Median (IQR) | 37.1 (36.7, 37.8) N = 3069 |
37.1 (36.7, 37.9) N = 7105 |
.97 | 37.1 (36.7, 38.0) N = 626 |
37.0 (36.7, 37.6) N = 1757 |
.15 |
| Triage Heart Rate, beats/minute, Median (IQR) | 122 (102, 145) N = 3024 |
126 (104, 148) N=7051 |
.002 | 126 (106, 150) N = 621 | 125 (102, 149) N = 1742 |
.22 |
| Triage Respiratory Rate, Breaths/Minute, Median (IQR) | 26 (20, 33) N = 2882 | 26 (22, 34) N = 6825 | <.001 | 26 (22, 36) N = 591 | 26 (20, 36) N = 1670 | .09 |
| Triage Oxygen Saturation, Room Air, Median (IQR) | 98 (97, 100) N = 2792 | 98 (97, 100) N = 6341 | <.001 | 98 (97, 100) N = 569 | 98 (97, 100) N = 1562 | .002 |
| Altered mental state (including obtunded, not easily arousable, confused, inappropriately agitated, unconscious), n (%) | 37/3103 (1.2) | 112/7173 (1.6) | .39 | 29/631 (4.6) | 81/1776 (4.6) | .82 |
| Crackles on examination, n (%) | 46/3103 (1.5) | 164/7180 (2.3) | .03 | 24/631 (3.8) | 87/1775 (4.9) | .12 |
| Wheezing on examination, n (%) | 80/3103 (2.6) | 493/7181 (6.9) | <.001 | 37/631 (5.9) | 211/1776 (11.9) | <.001 |
| Radiographic Imaging | ||||||
| Chest radiographs performed while in ED, n (%) | 553/3118 (17.7) | 1201/7192 (16.7) | .12 | 279/638 (43.7) | 539/1780 (30.3) | .15 |
| Radiographic Pneumonia while in ED, n (%) | 149/550 (27.1) | 319/1196 (26.7) | .35 | 103/279 (36.9) | 196/536 (36.6) | .11 |
Column percentages are reported.
ED indicates Emergency Department.
The P-values were obtained from Cochran–Mantel–Haenszel tests stratified by study sites for categorical variables, and van Elteren tests stratified by study sites for continuous variables as appropriate.
Radiographic Pneumonia definition: interstitial infiltrates/perihilar opacity, cavitation/abscess/pneumatocele, probable or definite pneumonia/consolidation, linear/streak opacity, pleural effusion.
Age was collected in whole numbers for those >1 year of age.
Table 2.
Prehospital Visit Therapies Administered, Including Children (and Treatments Provided) Transferred to the Study Institution. All Cells Represent n (%)
| SARS-CoV-2Positive N = 3121 | SARS-CoV-2Negative N = 7194 | P Value | AdmittedN = 2418 | DischargedN = 7897 | P Value | |
|---|---|---|---|---|---|---|
| Any Antiviral† | 2/3113 (0.1) | 6/7169 (0.1) | .51 | 6/2416 (0.2) | 2/7866 (0.03) | .02 |
| Any Corticosteroid | 65/3096 (2.1) | 293/7135 (4.1) | <.001 | 131/2400 (5.5) | 227/7831 (2.9) | <.001 |
| Oral | 31/3096 (1.0) | 104/7135 (1.5) | .46 | 72/2400 (3.0) | 63/7831 (0.8) | <.001 |
| Intravenous | 2/3096 (0.1) | 14/7135 (0.2) | .03 | 13/2400 (0.5) | 3/7831 (0.04) | <.001 |
| Inhaled | 32/3096 (1.0) | 175/7135 (2.5) | <.001 | 46/2400 (1.9) | 161/7831 (2.1) | .04 |
| Intramuscular | 0/3096 (0) | 3/7135 (0.04) | n/a | 3/2400 (0.1) | 0/7831 (0) | n/a |
| Any Antibiotic | 91/3113 (2.9) | 429/7171 (6.0) | <.001 | 225/2416 (9.3) | 295/7868 (3.7) | <.001 |
| Amoxicillin | 29/3113 (0.9) | 151/7171 (2.1) | <.001 | 46/2416 (1.9) | 134/7868 (1.7) | .08 |
| Antipyretics | 1511/3113 (48.5) | 3863/7173 (53.9) | .32 | 1034/2415 (42.8) | 4340/7871 (55.1) | <.001 |
| Acetaminophen | 1184/3111 (38.1) | 3116/7168 (43.5) | .30 | 801/2411 (33.2) | 3499/7868 (44.5) | .003 |
| Ibuprofen | 592/3110 (19.0) | 1833/7169 (25.6) | <.001 | 500/2412 (20.7) | 1925/7867 (24.5) | .66 |
| Salicylate | 20/3112 (0.6) | 35/7170 (0.5) | .56 | 24/2415 (1.0) | 31/7867 (0.4) | .02 |
The P-values were obtained from Cochran–Mantel–Haenszel tests stratified by study sites.
n/a not estimable by Cochran–Mantel–Haenszel test.
Includes acyclovir, oseltamivir, valganciclovir.
Treatments by SARS-CoV-2 Status
Few SARS-CoV-2-positive children received specific therapies including corticosteroids (219/3120; 7.0%), antivirals (43/3120; 1.4%), intravenous immunoglobulin (IVIG; 14/2518; 0.6%), and convalescent plasma (4/2518; 0.2%); Table 3 . Although corticosteroid use was less common among SARS-CoV-2-positive, compared with test-negative children (219/3120 [7.0%] vs 759/7190 [10.6%]; P < .001), usage was more frequent among SARS-CoV-2 positive children who experienced severe outcomes (38/89 [42.7%] vs 180/3017 [6.0%]; P < .001). Similar proportions of SARS-CoV-2-positive and negative children received antiviral medications except for remdesivir which was more frequently administered to SARS-CoV-2-positive children (25/3120 [0.8%] vs 1/7188 [0.01%]; P = .001), particularly those who experienced severe outcomes (18/89 [20.2%]). Antibiotics were less commonly administered to SARS-CoV-2 test-positive children (396/3120 [12.7%] vs 1138/7192 [15.8%]; P < .001); Table 3, eTable 7.
Table 3.
Emergency Department, Observation Unit or In-Patient Therapies Administered. All Cells Represent n (%)
| All Participants |
SARS-CoV-2 Positive Participants Only |
|||||
|---|---|---|---|---|---|---|
| SARS-CoV-2 PositiveN = 3121 | SARS-CoV-2 NegativeN = 7194 | P Value | Severe OutcomeN = 89 | No Severe OutcomeN = 3018 | P Value | |
| Albuterol | 142/3091 (4.6) | 652/7145 (9.1) | <.001 | 31/87 (35.6) | 110/2991 (3.7) | <.001 |
| Any Antibiotic† | 396/3120 (12.7) | 1138/7192 (15.8) | <.001 | 60/89 (67.4) | 334/3017 (11.1) | <.001 |
| Antifungal agent | 19/3119 (0.6) | 54/7188 (0.8) | .72 | 5/88 (5.7) | 14/3017 (0.5) | <.001 |
| Antipyretics | 1098/3120 (35.2) | 2940/7190 (40.9) | .06 | 63/89 (70.8) | 1031/3017 (34.2) | <.001 |
| Acetaminophen | 712/3120 (22.8) | 2083/7190 (29.0) | .01 | 58/89 (65.2) | 654/3017 (21.7) | <.001 |
| Ibuprofen | 560/3120 (17.9) | 1588/7190 (22.1) | .05 | 23/89 (25.8) | 533/3017 (17.7) | .02 |
| Salicylate | 16/3120 (0.5) | 44/7190 (0.6) | .45 | 4/89 (4.5) | 12/3017 (0.4) | <.001 |
| Any Antiviral | 43/3120 (1.4) | 47/7188 (0.7) | <.001 | 22/89 (24.7) | 21/3017 (0.7) | <.001 |
| Remdesivir | 25/3120 (0.8) | 1/7188 (0.01) | .001 | 18/89 (20.2) | 7/3017 (0.2) | <.001 |
| Acyclovir | 15/3120 (0.5) | 40/7188 (0.6) | .72 | 4/89 (4.5) | 11/3017 (0.4) | .001 |
| Ganciclovir | 1/3120 (0.03) | 3/7188 (0.04) | .91 | 0/89 (0) | 1/3017 (0.03) | n/a |
| Lopinavir/Ritonavir | 1/3120 (0.03) | 0/7188 (0) | n/a | 0/89 (0) | 1/3017 (0.03) | n/a |
| Oseltamivir | 1/3120 (0.03) | 2/7188 (0.03) | .80 | 0/89 (0) | 1/3017 (0.03) | n/a |
| Palivizumab | 0/3120 (0) | 1/7188 (0.01) | n/a | 0/89 (0) | 0/3017 (0) | n/a |
| Anakinra | 1/3115 (0.03) | 3/7181 (0.4) | .93 | 0/89 (0) | 1/3012 (0.03) | n/a |
| Infliximab | 0/3115 (0) | 1/7181 (0.01) | n/a | 0/89 (0) | 0/3012 (0) | n/a |
| Any Corticosteroid | 219/3120 (7.0) | 759/7190 (10.6) | <.001 | 38/89 (42.7) | 180/3017 (6.0) | <.001 |
| Inhaled | 18/3120 (0.6) | 106/7190 (1.5) | .001 | 3/89 (3.4) | 15/3017 (0.5) | .005 |
| Intravenous | 61/3120 (2.0) | 174/7190 (2.4) | .03 | 27/89 (30.3) | 34/3017 (1.1) | <.001 |
| Oral | 147/3120 (4.7) | 505/7190 (7.0) | .02 | 13/89 (14.6) | 133/3017 (4.4) | <.001 |
| Convalescent Plasma* | 4/2518 (0.2) | 2/4643 (0.04) | .27 | 2/71 (2.8) | 2/2435 (0.1) | .02 |
| ECMO | 0/3083 (0) | 2/7142 (0.03) | n/a | 0/85 (0) | 0/2985 (0) | n/a |
| Hydration | ||||||
| Intravenous fluids | 569/3121 (18.2) | 1524/7192 (21.2) | <.001 | 66/89 (74.2) | 503/3018 (16.7) | <.001 |
| Nasogastric fluids | 17/3121 (0.5) | 53/7191 (0.7) | .09 | 7/89 (7.9) | 10/3018 (0.3) | <.001 |
| Hydroxychloroquine | 8/3120 (0.3) | 4/7190 (0.1) | .03 | 2/89 (2.2) | 6/3017 (0.2) | .07 |
| Hypertonic Saline | 12/3085 (0.4) | 49/7142 (0.7) | .17 | 4/86 (4.7) | 8/29986 (0.3) | <.001 |
| Intravenous Immunoglobulin* | 14/2518 (0.6) | 17/4644 (0.4)‡ | .67 | 10/71 (14.1) | 4/2435 (0.2) | <.001 |
| Inotropic support | 19/3085 (0.6) | 28/7141 (0.4) | .47 | 19/87 (21.8) | 0/2985 (0) | n/a |
| Oxygen and Respiratory Support, Any | 151/3090 (4.9) | 402/7146 (5.6) | .002 | 64/89 (71.9) | 86/2988 (2.9) | <.001 |
| CPAP | 6/3090 (0.2) | 13/7146 (0.2) | .71 | 6/89 (6.7) | 0/2988 (0) | n/a |
| BiPAP | 12/3090 (0.4) | 21/7146 (0.3) | .65 | 12/89 (13.5) | 0/2988 (0) | n/a |
| Endotracheal Intubation/LMA | 22/3090 (0.7) | 43/7146 (0.6) | .98 | 22/89 (24.7) | 0/2988 (0) | n/a |
| Renal Replacement Therapy | 1/3085 (0.03) | 11/7142 (0.2) | .16 | 1/86 (1.2) | 0/2986 (0) | n/a |
CPAP indicates Continuous Positive Airway Pressure; BiPAP, Bilevel Positive Airway Pressure; and ECMO, Extracorporeal Membrane Oxygenation.
The P-values were obtained from Cochran–Mantel–Haenszel tests stratified by study sites.
n/a not estimable by Cochran–Mantel–Haenszel test.
Data field added to survey after study launch.
For more detailed antibiotic usage – see Supplementary Table 5.
Of the 17 SARS-CoV-2 test-negative administered intravenous immunoglobulin, 13 with Kawasaki Disease, one with Idiopathic Thrombocytopenia Purpura, on with encephalitis, one with scarlet fever, and one child had acute inflammatory demyelinating polyneuropathy. None of these children had SARS-CoV-2 antibody testing performed.
Treatments by SARS-CoV-2 Status in Hospitalized Participants
Among hospitalized children, medications administered included corticosteroids (507/2416; 21.0%), antivirals (88/2416; 3.6%), and IVIG (31/1515; 2.0%); Table 4 . Corticosteroid use was less frequent in SARS-CoV-2 test-positive compared with test-negative children (110/637 [17.3%] vs 397/1779 [22.3%]; P = .04). When comparing only children without histories of asthma or wheezing on examination, there was no difference in corticosteroid use between those who were SARS-CoV-2 test-positive (49/487 [10.1%]) and test-negative (131/1273 [10.3%]); P = .90, eTable 8, and eFigure 1 in the Supplement. Remdesivir was administered almost exclusively to SARS-CoV-2-positive children ([25/637 [3.9%] vs 1/1779 [0.1%]; P = .001); Table 4. While albuterol use was less common among SARS-CoV-2-positive hospitalized children ([88/609 {14.4%} vs 350/1734 [20.2%]; P = .001), the use of respiratory ([148/608 {24.3%} vs 376/1735 [21.7%]; P = .53] and inotropic support ([20/603 {3.3%} vs 27/1730 [1.6%]; P = .09) did not differ between groups.
Table 4.
Unadjusted Odds of Emergency Department, Observation Unit or Inpatient Therapies Administered to Hospitalized Children With a Positive SARS-CoV-2 Test Result
| SARS-CoV-2Positive; N = 638 | SARS-CoV-2Negative; N = 1780 | Unadjusted OR (95%CI) | P Value | |
|---|---|---|---|---|
| Albuterol | 88/609 (14.4) | 350/1734 (20.2) | 0.60 (0.45, 0.81) | .001 |
| Antibiotics† | 317/637 (49.8) | 857/1780 (48.1) | 1.04 (0.84, 1.29) | .70 |
| Any Antiviral† | 42/637 (6.6) | 46/1779 (2.6) | 3.27 (1.93, 5.54) | <.001 |
| Acyclovir | 15/637 (2.4) | 39/1779 (2.2) | 1.60 (0.81, 3.14) | .17 |
| Ganciclovir | 1/637 (0.2) | 3/1779 (0.2) | 1.83 (0.15, 22.87) | .64 |
| Lopinavir/Ritonavir | 1/637 (0.2) | 0/1779 (0) | n/a | n/a |
| Oseltamivir | 0/637 (0) | 2/1779 (0.1) | n/a | n/a |
| Palivizumab | 0/637 (0) | 1/1779 (0.1) | n/a | n/a |
| Remdesivir | 25/637 (3.9) | 1/1779 (0.1) | 21.11 (3.63, 122.59) | .001 |
| Anakinra | 1/635 (0.2) | 3/1776 (0.2) | 1.40 (0.13, 15.58) | .79 |
| Infliximab | 0/635 (0) | 1/1776 (0) | n/a | n/a |
| Antifungal Agents | 15/636 (2.4) | 50/1779 (2.8) | 1.03 (0.55, 1.94) | .93 |
| Any Corticosteroid | 110/637 (17.3) | 397/1779 (22.3) | 0.76 (0.58, 0.99) | .04 |
| Inhaled | 15/637 (2.4) | 89/1779 (5.0) | 0.54 (0.30, 0.97) | .04 |
| Intravenous | 56/637 (8.8) | 157/1779 (8.8) | 0.91 (0.63, 1.33) | .63 |
| Oral | 47/637 (7.4) | 177/1779 (9.9) | 0.81 (0.56, 1.18) | .28 |
| Chest Drainage Procedure | 5/602 (0.8) | 7/1734 (0.4) | 1.86 (0.49, 7.02) | .36 |
| Convalescent Plasma* | 4/492 (0.8) | 2/1023 (0.2) | 6.60 (0.62, 69.94) | .12 |
| Extracorporeal Membrane Oxygenation | 0/601 (0) | 2/1731 (0.1) | n/a | n/a |
| Hydroxychloroquine | 7/637 (1.1) | 4/1779 (0.2) | 6.30 (1.43, 27.70) | .02 |
| Hypertonic Saline | 8/603 (1.3) | 40/1731 (2.3) | 0.82 (0.36, 1.88) | .63 |
| Inotropic Support | 20/603 (3.3) | 27/1730 (1.6) | 1.85 (0.91, 3.77) | .09 |
| Intravenous Immunoglobulin* | 14/492 (2.8) | 17/1023 (1.7) | 1.12 (0.46, 2.70) | .81 |
| Oxygen and Respiratory Support | 148/608 (24.3) | 376/1735 (21.7) | 0.92 (0.71, 1.20) | .53 |
| Continuous Positive Airway Pressure | 6/608 (1.0) | 13/1735 (0.7) | 0.83 (0.26, 2.63) | .75 |
| Bilevel Positive Airway Pressure | 13/608 (2.1) | 21/1735 (1.2) | 1.39 (0.63, 3.04) | .41 |
| Invasive ventilation by ETT/LMA | 22/608 (3.6) | 48/1735 (2.8) | 0.97 (0.54, 1.75) | 0.92 |
| Renal Replacement Therapy | 2/603 (0.3) | 11/1731 (0.6) | 0.65 (0.12, 3.45) | .61 |
ETT indicates Endotracheal Tube; LMA, Laryngeal Mask Airway.
The P-values and OR's were obtained from Cochran–Mantel–Haenszel tests stratified by study sites.
n/a not estimable by Cochran–Mantel–Haenszel test.
Data field added to survey after study launch.
Only those administered to >1.0% of any subgroup are specifically listed.
Corticosteroid Administration
SARS-CoV-2 detection was not associated with corticosteroid administration (aOR: 0.58 [0.27, 1.26]); Table 5 . In the full participant analysis, corticosteroid administration was associated with age, history of asthma and other chronic conditions/comorbidities, wheezing on examination, and hospitalization. In the subgroup of hospitalized children, the same variables, except for chronic conditions/comorbidities, remained associated with corticosteroid use while SARS-CoV-2 detection was not associated with corticosteroid use (aOR: 0.65 [0.23, 1.84]). Sensitivity analysis excluding participants with histories of asthma and those diagnosed as having an asthma exacerbations, wheeze, or reactive airways disease did not alter the findings; eTable 9 in the Supplement.
Table 5.
Multivariable Conditional Logistic Regression Model Matching by Study Site Evaluating Likelihood of Receiving an Oral or Intravenous Corticosteroid at the Index Emergency Department Visit, at Discharge, or During Hospitalization
| All Study Participants (N = 10237, Received = 906)* |
Hospitalized at Index Visit (N = 2336, Received = 431)† |
|||
|---|---|---|---|---|
| Odds Ratio (95%CI) | P-Value | Odds Ratio (95%CI) | P-Value | |
| SARS-CoV-2 Nucleic Acid Test, Positive | 0.58 (0.27, 1.26) | .17 | 0.65 (0.23, 1.84) | .41 |
| Age Group | ||||
| <60 Days | 0.17 (0.08, 0.39) | <.001 | 0.18 (0.08, 0.40) | <.001 |
| 60 Days - <2.0 Years | 0.52 (0.40, 0.68) | <.001 | 0.34 (0.23, 0.51) | <.001 |
| 2.0 Years - <5.0 Years | 1.32 (1.03, 1.70) | .03 | 1.23 (0.85, 1.78) | .28 |
| 5.0 Years - <12.0 Years | 0.98 (0.77, 1.25) | .86 | 0.94 (0.68, 1.32) | .74 |
| 12.0 Years - <18.0 Years | reference | reference | ||
| Month of Enrolment | ||||
| March–May 2020 June–August 2020 September–November 2020 December 2020–February 2021 March–June 2021 |
Reference 1.07 (0.84, 1.37) 1.27 (0.91, 1.79) 1.22 (0.85, 1.75) 1.33 (0.93, 1.90) |
.60 .17 .28 .13 |
Reference 1.01 (0.71, 1.43) 0.88 (0.52, 1.50) 0.74 (0.40, 1.36) 0.52 (0.23, 1.17) |
.95 .64 .34 .11 |
| History of Asthma, Yes | 1.96 (1.61, 2.38) | <.001 | 1.55 (1.14, 2.11) | .005 |
| Chronic Condition/Comorbidity (Excluding Asthma), Yes | 1.27 (1.05, 1.53) | .01 | 1.01 (0.77, 1.31) | .97 |
| Wheezing on History or Examination, Yes | 12.2 (10.20, 14.59) | <0.001 | 8.91 (6.49, 12.23) | <.001 |
| Hospitalized at the Index Visit, Yes | 4.6 (3.79, 5.61) | <.001 | N/A | |
| Intensive Care Unit Admission, Yes | N/A | 2.46 (1.75, 3.45) | <.001 | |
| Interaction Terms | ||||
| Overall | .35 | .12 | ||
| SARS-CoV-2 positive × June to August 2020 | 1.31 (0.55, 3.10) | .54 | 0.95 (0.29, 3.11) | .93 |
| SARS-CoV-2 positive × September to November 2020 | 1.60 (0.67, 3.81) | .29 | 2.28 (0.67, 7.73) | .19 |
| SARS-CoV-2 positive × December 2020 to February 2021 | 1.53 (0.64, 3.70) | .34 | 1.93 (0.55, 6.86) | .31 |
| SARS-CoV-2 positive × March to June 2021 | 2.18 (0.91, 5.24) | .08 | 3.17 (0.78, 12.93) | .11 |
N/A, the variable was not included in the regression model.
2 sites (22 observations) dropped because of no steroid prescribed in these sites.
5 sites (55 observations) dropped because of no steroid prescribed in these sites; 2 sites (15 observations) dropped because of all prescribed steroid in this site.
Discussion
Our multinational study revealed that although SARS-CoV-2 test-positive children receive a variety of treatments, no therapies are consistently administered, either to inpatients or outpatients. Corticosteroid administration, the most evidence-based treatment in SARS-CoV-2 infected adults, was not independently associated with SARS-CoV-2 test status, even among hospitalized children and after excluding children with asthma (history or active disease). Although infrequently provided, several treatments were administered more commonly to SARS-CoV-2 test-positive children including remdesivir, hydroxychloroquine, IVIG, and inotropic drugs.
Based primarily on the results of the RECOVERY trial which reported reduced mortality at 28 days associated with dexamethasone treatment of SARS-CoV-2 infected adults who were hospitalized and on supplemental oxygen or receiving mechanical ventilation,9 corticosteroids are recommended for use in adults with severe COVID-19.14 , 15 Consequently, dexamethasone use among hospitalized adults with COVID-19 has become routine.16 , 17 Although the RECOVERY trial was modified (Version 5) to include children with respiratory COVID-19 infections,18 few children have been enrolled and no conclusive evidence exists regarding corticosteroid use in this population. In our study, we found no independent association between corticosteroid use and SARS-CoV-2 status among hospitalized children including those admitted to the ICU. Corticosteroid use was associated with hospitalization, ICU admission (usage increasing in those more unwell), history of asthma, and the presence of wheeze. Thus, corticosteroid administration was likely performed to treat asthma and not SARS-CoV-2 infection. Moreover, as we did not find evidence of interaction between SARS-CoV-2 test status and time, it does not appear that evidence from adult trials is leading to adoption in children.
Although remdesivir trials, which have included more than 13,500 adults, report that use improves clinical outcomes among hospitalized patients,19 access and regulatory approval varies by country.20 As was seen with dexamethasone, remdesivir usage among hospitalized adults has increased as reflected in data from the University of California medical centers where administration increased from 4.9% to 62.5% between June 1 and December 31, 2020.16 Although pediatric pharmacokinetic data analyzing the association between drug dose, plasma exposure, and intracellular drug exposure remain unavailable, some groups have endorsed remdesivir for use in children with severe COVID-1921 and case-series describing its use have emerged.22 A systematic review estimates that remdesivir is administered to 6.6% of children with severe COVID-19.23 In our study, only 22 of the 151 SARS-CoV-2-positive children requiring oxygen therapy (ie, severe COVID-19) received remdesivir (Table 2). The limited use of this treatment may reflect recruitment prior to the maturation of evidence, lack of pediatric data, limited access, and/or conflicting guidelines.
Monoclonal antibody treatments have recently received emergency use authorizations (EUA) with approved indications including children and newborns with COVID-19 who are at high risk for progressing to severe COVID-19 and/or hospitalization.8 However, given the limited safety and efficacy data in children, the American Academy of Pediatrics recommends infectious disease consultation prior to use in children <12 years of age, and a North American expert panel recommended against the routine administration of monoclonal antibodies to children, including those identified in the EUA.24
The ramifications of excluding children from COVID-19 clinical trials has increased as the pandemic has evolved. As adult vaccination rates rise, children are increasingly bearing the burden of disease and are treated based on limited evidence often with off-label therapies approved for adults. While early in the pandemic young children were infrequently infected by SARS-CoV-2,25 in our study nearly 80% of cases occurred in children <12 years of age. The latter finding is in keeping with an international network cohort study that used real-world data from European primary care records, South Korean and US insurance claims, and hospital databases to conclude that most COVID-19 diagnoses occur among children aged <4 years of age.6 This finding highlights the importance of closing the gap which continues to expand, with evidence regarding the care of adults, particularly those who are critically ill continuously increasing, while progress in pediatric treatments lags and remains limited. A bibliographic analysis identified no pediatric interventional studies and few articles regarding therapeutics.26 Of the initial 275 COVID-19 interventional clinical trials registered on ClinicalTrials.gov, only 30 (11%) were open to patients <18 years old.27 In January 2021, the situation remained unchanged, with <10% of registered interventional COVID-19 trials including children.18
The lack of regulatory authorization and high-quality data to guide therapeutic management of children is not a new problem. In the United States, the proportion of medications prescribed to children that are off-label ranges from 62% to 85%.28, 29, 30 In 2003, the Pediatric Research Equity Act was signed into law in the United States, with the goal of ensuring earlier access to information on drug approvals for children. Although only 34% of pediatric postmarketing studies mandated by the Pediatric Research Equity Act had been completed after 7 years of follow-up,31 the COVID pandemic and has highlighted the failure of this law to achieve its aims.
Novel approaches are needed to generate the data to inform the care of children. Bayesian designs can permit the use of historical adult data to generate a probability distribution to inform the design of pediatric trials which can integrate the adult data into the analytic plan, thereby reducing the required sample size.32 Bayesian hierarchical modeling can also be used to combine data from simultaneously conducted adult and pediatric trials.33 Such an approach can provide improved efficacy estimates in each population based on the similarity of effect. In the best case scenario, the effects are similar; if the effects are discordant, the adult estimate would have limited effect on that of the pediatric one.
Propensity matched designs are an alternative approach that can be used to examine and infer treatment effects when an adequately powered trial is not possible. However, since investigators have no control over treatment allocation in observational studies, confounding by indication may lead to misleading treatment effect estimates. To reduce the impact of confounding, propensity scores can be used to estimate the probability, based on pre-treatment characteristics, that a patient would receive the experimental treatment. The propensity score can be used to perform matching or stratification34 and such analyses tend to produce treatment effects that are similar to randomized clinical trials.35
Our study has several limitations. We do not know why some children received certain treatments and others did not and we are unable to clearly evaluate if an intervention improved outcomes. We could not correlate intervention timing to clinician knowledge of SARS-CoV-2 test results as the latter data was not recorded. Delays in test turnaround time may have led to the empiric administration of COVID-specific treatments to children who were test-negative. This is particularly relevant to those enrolled in the early days of the pandemic, as there were often delays in the reporting of SARS-CoV-2 test results. We did not factor into our analysis the timing of availability of evidence supporting various interventions, the age range for which certain treatments are approved, and participant severity of illness which varied between participating centers based on testing policies. While study participants were matched based on day of testing and study site, no additional matching was performed to guide recruitment. Consequently, our SARS-CoV-2-positive and negative children differ in some characteristics. While several of these were adjusted for in our model, other confounders likely remain. The hospitalization rate in our study is higher than population estimates for COVID-19-infected children; this likely reflects the fact that children seeking ED care are more unwell than those seeking treatment at their primary care provider's office. Moreover, participating sites were predominantly tertiary care centers and some participants were transferred to these sites. Additionally, testing regimes varied between and within sites and over time. Early in the pandemic, SARS-CoV-2 testing was limited to the most unwell children. As the test positivity rate varied during the recruitment period, and between countries, our recruitment approach was modified to minimize its impact on our analytical inferences due to uneven recruitment between sites and countries. Further, to overcome confidentiality and privacy barriers associated with data sharing in an international study, we did not record data from potentially eligible children who declined to participate. Lastly, while we did record race for participants in Canada and the United States, due to the challenges of recording race separately for each of our European countries, those data were not collected.
In conclusion, we found that few SARS-CoV-2 infected children received any specific SARS-CoV-2 therapies. We found no independent association between corticosteroid use and SARS-CoV-2 test status in children, even among those hospitalized. Thus, there is an urgent need for research to include children to provide pediatric-specific data that can be used to guide the treatment of children who are now bearing a significant burden of COVID-19 disease.
Data Access, Responsibility and Analysis
Drs. Stephen Freedman, Jianling Xie, and Daniel Tancredi had full access to all the data in the study and they take responsibility for the integrity of the data and the accuracy of the data analysis.
CIHR Open Access Policy
All research papers generated from CIHR funded projects must be freely accessible through the Publisher's website or an online repository within 12 months of publication.
Data Sharing
Any reasonable requests to share data will be considered by the PERN-COVID-19 study group steering committee subject to institutional agreements and ethics approvals. Data requests should be sent to the corresponding author.
Contributors’ Statement
Stephen B. Freedman designed the study, contributed to data acquisition, analysis and interpretation, drafted the manuscript, obtained funding, provided administrative, technical and material support and provided supervision.
Nathan Kuppermann, and Todd A. Florin designed the study, contributed to data acquisition, analysis and interpretation, critically reviewed the manuscript, obtained funding, provided administrative, technical and material support and provided supervision.
Anna L. Funk designed the study, contributed to data acquisition, analysis and interpretation, critically reviewed the manuscript, obtained funding, and provided administrative, technical and material support.
Kelly Kim and Lilliam Ambroggio contributed to conception and design, data acquisition, analysis and interpretation and critically reviewed the manuscript.
Jianling Xie contributed to data acquisition, analysis and interpretation, critically reviewed the manuscript, and conducted the statistical analyses.
Daniel Tancredi contributed to conception and design, data acquisition, analysis and interpretation, critically reviewed the manuscript, provided statistical analysis guidance and obtained funding.
Stuart R Dalziel, Mark I. Neuman and Santiago Mintegi contributed to conception and design, data acquisition, analysis and interpretation, critically reviewed the manuscript, obtained funding and provided supervision.
Amy C. Plint, Terry P Klassen and Marina Salvadori contributed to conception and design, data acquisition, analysis and interpretation, critically reviewed the manuscript, and obtained funding.
Richard Malley and Daniel C. Payne contributed to conception and design, interpretation of data, critically reviewed the manuscript, and obtained funding.
Jessica Gómez-Vargas, Yaron Finkelstein, Kristen A. Breslin, Pradip P Chaudhari, Kelly R. Bergmann, Jasmine R. Nebhrajani, Fahd A. Ahmad, Nipam P. Shah, Vikram J. Sabhaney, Meredith L. Borland, Kerry Caperell, Usha Avva, Michael A. Gardiner, Nidhya Navanandan, Maren M. Lunoe, Iker Gangoiti, Laura F. Sartori, Carmen Campos, April J. Kam, Jonathan C. Cherry, Bruce Wright, Alexander J. Rogers, Claudia R. Morris, Sarah M. Becker, Usha Sethuraman, Ana F. Dragovetzky, Viviana Pavlicich, Isabel Beneyto, Andrea K. Morrison, Shu-Ling Chong, Naveen Poonai, Maria Y. Kwok, Laura Palumbo, Michelle Eckerle, and Muhammad Wassem contributed to acquisition of data, interpretation of data and critically reviewed the manuscript.
All authors approved the final version to be published.
Funding
This work was supported by grants from the Canadian Institutes of Health Research (Operating Grant: COVID-19 - Clinical management), Alberta Innovates, the Alberta Health Service – University of Calgary - Clinical Research Fund, the Alberta Children's Hospital Research Institute, and the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis. Dr. Stephen Freedman is supported by the Alberta Children's Hospital Foundation Professorship in Child Health and Wellness. Dr. Stuart Dalziel is supported by Cure Kids New Zealand. Anna Funk, PhD, is supported by the University of Calgary Eyes-High Post-Doctoral Research Fund. None of the funders played any role in the design or conduct of the study, collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript and decision to submit the manuscript for publication.
Acknowledgments
Pediatric Emergency Research Network-COVID-19 Study Team
Kristen A. Breslin MD,1 Pradip P Chaudhari MD,2 Kelly R. Bergmann DO, MS3 Jasmine R. Nebhrajani MD,4 Fahd A. Ahmad MD, MSCI5 Nipam P. Shah MD, MBBS, MPH6 Vikram J. Sabhaney MD,7 Meredith L. Borland MD,8 Kerry Caperell MD, MS, MBA,9 Usha Avva MD,10 Michael A. Gardiner MD,11 Nidhya Navanandan MD,12 Maren M. Lunoe MD,13 Iker Gangoiti MD,14 Laura F. Sartori MD, MPH15 Carmen Campos MD,16 April J. Kam MD,17 Jonathan C. Cherry MD,18 Bruce Wright MD,19 Alexander J. Rogers MD,20 Claudia R. Morris MD,21 Sarah M. Becker MD,22 Usha Sethuraman MD,23 Ana F. Dragovetzky MD,24 Viviana Pavlicich MD,25 Isabel Beneyto MD,26 Andrea K. Morrison MD, MS27 Shu-Ling Chong MPH,28 Naveen Poonai MD,29 Maria Y. Kwok MD, MPH30 Laura Palumbo MD,31 Michelle Eckerle MD,32 Muhammad Wassem MD33
1 Children's National Hospital, Washington, DC, United States
2 Division of Emergency and Transport Medicine, Children's Hospital Los Angeles and Keck School of Medicine of the University of Southern California, Los Angeles, United States
3 Department of Emergency Medicine, Children's Minnesota, Minneapolis, United States
4 St. Mary's Medical Center, West Palm Beach, United States
5 Department of Pediatrics, Washington University School of Medicine, St. Louis, United States
6 Division of Pediatric Emergency Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham, United States
7 Department of Paediatrics, University of British Columbia, Vancouver, Canada
8 Perth Children's Hospital; Divisions of Emergency Medicine and Paediatrics, School of Medicine, University of Western Australia, Perth, Australia
9 University of Louisville, Norton Children's Hospital, Louisville, United States
10 Division of Pediatric Emergency Medicine, Joseph M Sanzari Children's Hospital, Hackensack UMC, Hackensack Meridian Health, Hackensack, United States (Current Affiliation: Department of Emergency Medicine, Chilton Medical Center, Atlantic Health, Pompton Plains, United States)
11 Department of Pediatrics, University of California San Diego, Rady Children's Hospital, San Diego, United States
12 Department of Pediatrics, University of Colorado School of Medicine, Aurora, United States
13 UPMC Children's Hospital of Pittsburgh, Pittsburgh, United States
14 Pediatric Emergency Department, Biocruces Bizkaia Health Research Institute, Hospital Universitario Cruces, University of the Basque Country, UPV/EHU, Bilbao, Spain
15 Children's Hospital of Philadelphia, Division of Pediatric Emergency Medicine, Department of Pediatrics, Philadelphia, United States
16 Hospital Universitario Miguel Servet, Pediatric Emergency Department, Zaragoza, Spain
17 Department of Pediatrics, Division of Emergency Medicine, McMaster Children's Hospital, Hamilton, Canada
18 Department of Pediatric Emergency Medicine, IWK Health Centre, Dalhousie University, Halifax, Canada
19 Department of Pediatrics, University of Alberta, Women's and Children's Research Institute, Edmonton, Canada
20 Departments of Emergency Medicine and Pediatrics, University of Michigan School of Medicine, Ann Arbor, United States
21 Department of Pediatrics, Division of Emergency Medicine, Emory University School of Medicine, Children's Healthcare of Atlanta, Atlanta, United States
22 Primary Children's Hospital, Intermountain Healthcare, Salt Lake City, United States
23 Division of Emergency Medicine and Department of Pediatrics, Children's Hospital of Michigan, Central Michigan University, Detroit, United States
24 Hospital de Pediatría "Prof. Dr. Juan P. Garrahan", RIDEPLA, Buenos Aires, Argentina
25 Pediatric Emergency Department, Hospital General Pediátrico Niños de Acosta Ñu, Facultad de Medicina, Universidad Privada del Pacífico, San Lorenzo, Paraguay
26 Pediatrics, Hospital Francesc de Borja, Gandia, Spain
27 Division of Emergency Medicine, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, United States
28 Department of Emergency Medicine, KK Women's and Children's Hospital, Duke-NUS Medical School, SingHealth Duke-NUS Global Health Institute, Singapore
29 Departments of Pediatrics, Internal Medicine, Epidemiology and Biostatistics, Schulich School of Medicine & Dentistry, Child Health Research Institute, Division of Paediatric Emergency Medicine, London, Canada
30 Department of Emergency Medicine, New York Presbyterian Morgan Stanley Children's Hospital, Columbia University Irving Medical Center, New York City, United States
31 ASST Spedali Civili di Brescia - Pronto soccorso pediatrico, Brescia, Italy
32 Department of Pediatrics, University of Cincinnati College of Medicine; Cincinnati Children's Hospital, Division of Pediatric Emergency Medicine, Cincinnati, United States
33 Lincoln Medical Center, Bronx New York, New York City, United States.
Footnotes
The authors have no conflicts of interest to disclose. The study sponsors played no role in study design, data collection, analysis or interpretation, report writing, or in the decision to submit the manuscript for publication. The first draft of the manuscript was written by Stephen Freedman. No honorarium, grant or other form of payment was provided to produce the manuscript.
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.acap.2022.04.006.
Appendix. Supplementary Data
References
- 1.Centers for Disease Control and Prevention. Cases of coronavirus disease (COVID-19) in the U.S. Available at: https://covid.cdc.gov/covid-data-tracker/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-updates%2Fcases-in-us.html#demographics. Accessed December 17, 2021.
- 2.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Available at:https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html. Accessed August 6, 2021.
- 3.Preston LE, Chevinsky JR, Kompaniyets L, et al. Characteristics and disease severity of US children and adolescents diagnosed with COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.5298. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kainth MK, Goenka PK, Williamson KA, et al. Northwell Health COVID-19 Research Consortium Early experience of COVID-19 in a US Children’s Hospital. Pediatrics. 2020;146 doi: 10.1542/peds.2020-003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020;174 doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duarte-Salles T, Vizcaya D, Pistillo A, et al. Thirty-day outcomes of children and adolescents with COVID-19: an international experience. Pediatrics. 2021;148 doi: 10.1542/peds.2020-042929. [DOI] [PubMed] [Google Scholar]
- 7.Swann OV, Holden KA, Turtle L, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food & Drug Administration. FDA expands authorization of two monoclonal antibodies for treatment and post-exposure prevention of COVID-19 to younger pediatric patients, including newborns. Available at: https://www.fda.gov/news-events/press-announcements/fda-expands-authorization-two-monoclonal-antibodies-treatment-and-post-exposure-prevention-covid-19. Accessed December 17, 2021.
- 9.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan K, Beck C, Chauvin-Kimoff L, et al. The acute management of paediatric coronavirus disease 2019 (COVID-19). Available at: https://www.cps.ca/en/documents/position/the-acute-management-of-paediatric-coronavirus-disease-2019covid-19. Accessed February 11, 2021.
- 11.Funk AL, Florin TA, Dalziel SR, et al. Prospective cohort study of children with suspected SARS-CoV-2 infection presenting to paediatric emergency departments: a Paediatric Emergency Research Networks (PERN) study protocol. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisboa Bastos M, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breslow N. Covariance adjustment of relative-risk estimates in matched studies. Biometrics. 1982;38:661–672. [PubMed] [Google Scholar]
- 14.Nasa P, Azoulay E, Khanna AK, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care. 2021;25:106. doi: 10.1186/s13054-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed December 17, 2021. [PubMed]
- 16.Watanabe JH, Kwon J, Nan B, et al. Medication use patterns in hospitalized patients with COVID-19 in California during the pandemic. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.10775. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crothers K, DeFaccio R, Tate J, et al. Dexamethasone in hospitalised coronavirus-19 patients not on intensive respiratory support. Eur Respir J. 2021 doi: 10.11183/13993003.02532-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming PF, Gale C, Molloy EJ, et al. Paediatric research in the times of COVID-19. Pediatr Res. 2021;90:267–271. doi: 10.1038/s41390-021-01479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai CC, Chen CH, Wang CY, et al. Clinical efficacy and safety of remdesivir in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2021;76:1962–1968. doi: 10.1093/jac/dkab093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hordijk L, Patnaik P. Covid-19: EU countries spent over €220m stockpiling remdesivir despite lack of effectiveness, finds investigation. BMJ. 2020;371:m4749. doi: 10.1136/bmj.m4749. [DOI] [PubMed] [Google Scholar]
- 21.Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter Interim Guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc. 2021;10:34–48. doi: 10.1093/jpids/piaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman DL, Aldrich ML, Hagmann SHF, et al. Compassionate use of remdesivir in children with severe COVID-19. Pediatrics. 2021;147 doi: 10.1542/peds.2020-047803. [DOI] [PubMed] [Google Scholar]
- 23.Panda PK, Sharawat IK, Natarajan V, et al. COVID-19 treatment in children: a systematic review and meta-analysis. J Family Med Prim Care. 2021;10:3292–3302. doi: 10.4103/jfmpc.jfmpc_2583_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf J, Abzug MJ, Wattier RL, et al. Initial guidance on use of monoclonal antibody therapy for treatment of coronavirus disease 2019 in children and adolescents. J Pediatric Infect Dis Soc. 2021;10:629–634. doi: 10.1093/jpids/piaa175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 26.Stilwell PA, Munro APS, Basatemur E, et al. Bibliography of published COVID-19 in children literature. Arch Dis Child. 2021;107:168–172. doi: 10.1136/archdischild-2021-321751. [DOI] [PubMed] [Google Scholar]
- 27.Hwang TJ, Randolph AG, Bourgeois FT. Inclusion of children in clinical trials of treatments for coronavirus disease 2019 (COVID-19) JAMA Pediatrics. 2020;174:825–826. doi: 10.1001/jamapediatrics.2020.1888. [DOI] [PubMed] [Google Scholar]
- 28.Bazzano AT, Mangione-Smith R, Schonlau M, et al. Off-label prescribing to children in the United States outpatient setting. Acad Pediatr. 2009;9:81–88. doi: 10.1016/j.acap.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Czaja AS, Reiter PD, Schultz ML, et al. Patterns of off-label prescribing in the pediatric intensive care unit and prioritizing future research. J Pediatr Pharmacol Ther. 2015;20:186–196. doi: 10.5863/1551-6776-20.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanovska V, Rademaker CMA, van Dijk L, et al. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134:361–372. doi: 10.1542/peds.2013-3225. [DOI] [PubMed] [Google Scholar]
- 31.Hwang TJ, Orenstein L, Kesselheim AS, et al. Completion rate and reporting of mandatory pediatric postmarketing studies under the US Pediatric Research Equity Act. JAMA Pediatrics. 2019;173:68–74. doi: 10.1001/jamapediatrics.2018.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huff RA, Maca JD, Puri M, et al. Enhancing pediatric clinical trial feasibility through the use of Bayesian statistics. Pediatr Res. 2017;82:814–821. doi: 10.1038/pr.2017.163. [DOI] [PubMed] [Google Scholar]
- 33.McGlothlin AE, Viele K. Bayesian hierarchical models. JAMA. 2018;320:2365–2366. doi: 10.1001/jama.2018.17977. [DOI] [PubMed] [Google Scholar]
- 34.Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32:1704–1708. doi: 10.1093/eurheartj/ehr031. [DOI] [PubMed] [Google Scholar]
- 35.Kuss O, Legler T, Borgermann J. Treatments effects from randomized trials and propensity score analyses were similar in similar populations in an example from cardiac surgery. J Clin Epidemiol. 2011;64:1076–1084. doi: 10.1016/j.jclinepi.2011.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.