Abstract
The development of nanoparticles (NPs) with potential therapeutic uses represents an area of vast interest in the scientific community during the last years. Recently, the pandemic caused by COVID-19 motivated a race for vaccines creation to overcome the crisis generated. This is a good demonstration that nanotechnology will most likely be the basis of future immunotherapy. Moreover, the number of publications based on nanosystems has significantly increased in recent years and it is expected that most of these developments can go on to experimentation in clinical stages soon. The therapeutic use of NPs to combat different diseases such as cancer, allergies or autoimmune diseases will depend on their characteristics, their targets, and the transported molecules. This review presents an in-depth analysis of recent advances that have been developed in order to obtain novel nanoparticulate based tools for the treatment of allergies, autoimmune diseases and for their use in vaccines. Moreover, it is highlighted that by providing targeted delivery an increase in the potential of vaccines to induce an immune response is expected in the future. Definitively, the here gathered analysis is a good demonstration that nanotechnology will be the basis of future immunotherapy.
Keywords: Immunomodulation therapy, Nanoparticles, Allergy, Autoimmune disease, Immune stimulation, Immunosuppressants
1. Introduction
Immunomodulating agents are substances that have the ability to increase or decrease the immune response. From a therapeutic point of view, this modulation capacity has broad potential as adjuvant therapy in neoplastic, allergic and immunodeficient diseases. The immune system is formed by a set of cells, molecules and tissues organized to defend the body from foreign agents and maintain homeostasis. This is a complex control system used to fight pathogens as well as to maintain a dynamic equilibrium among proimmune/inflammatory processes, regulatory/suppressive functions, and levels of homeostatic activity. The immune response involves the coordination of both innate and adaptive immunity [1], [2].
The first line of defense is the innate immunity, often followed by the adaptive immune response. Innate immunity involves physical, chemical and cellular responses against pathogens. Innate immune cells activation (macrophages, neutrophils, mast cells, eosinophils, basophiles, dendritic cells, natural killer cells, among others) is responsible for the secretion of specific cytokines at the reaction site, producing local inflammation. The adaptive immune response frequently follows this initial inflammation. It involves the activation of T and B lymphocytes, which are antigen-specific cells that target pathogens and instigators of the immune system. Both immune responses are interconnected and highly dependent on which pathway of the immune system is activated [3].
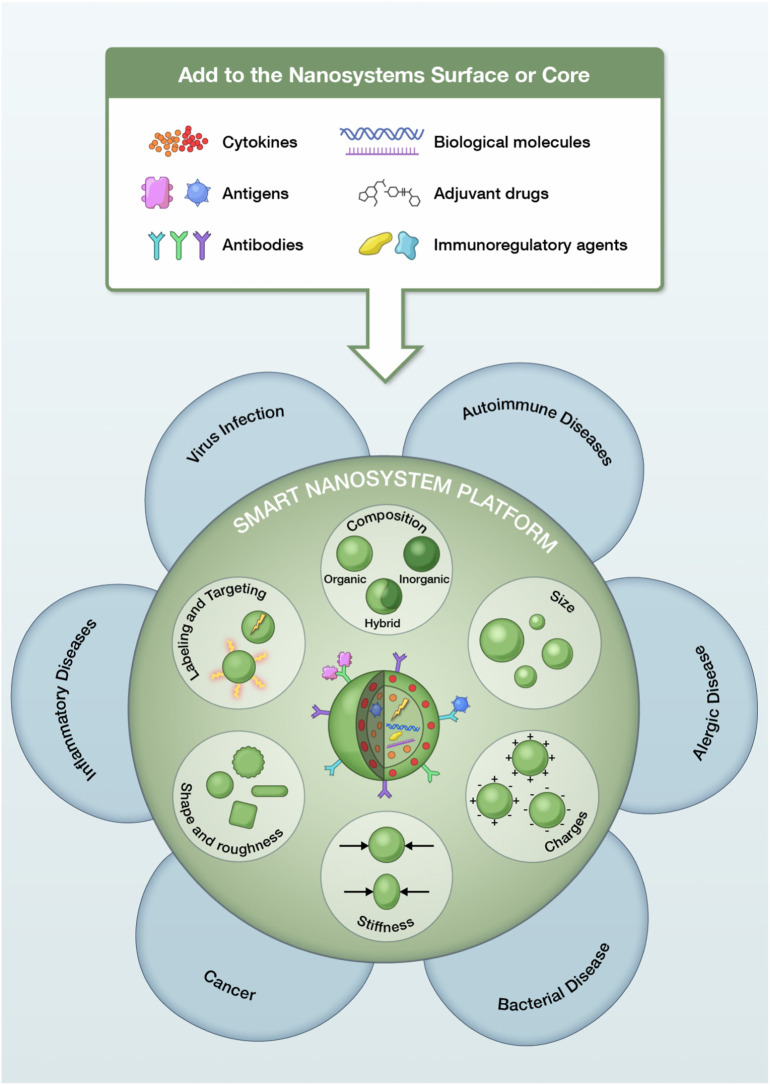
At the forefront of recent developments in the treatment of systemic diseases, such as inflammatory disorders, is the innovation in agents which can modulate the immune system. In this sense, nanomedicine has emerged as a successful strategy to produce engineered nanomaterials that can target specific organs or tissues [4], [5], [6], [7] and deliver therapeutic agents [8], [9] while avoiding undesirable immunosuppressive or immunostimulatory effects [10], [11], [12], [13]. Indeed, various nanomaterials are under different stages of clinical trials or have been approved by the United States Food and Drug Administration (FDA) [14]. Fig. 1 shows a schematic representation of different smart nanosystems. The impact of this nanoparticles (NPs) on the immune response for use with therapeutic purposes (i.e.: inflammatory, autoimmune, allergic, cancer diseases) will depend on their nature (i.e.: composition, size, charges, stiffness, shape, roughness, etc.) as well as their labeling and targeting (i.e.: antibodies) and the cargo it carries (i.e.: molecules, antigens, cytokines, drugs, immunoregulatory agents, etc.) (Fig. 1). For example, cell uptake and intracellular distribution or circulation time and elimination rate are mediated by particle shape [15]. Moreover, the analysis is more complex considering diverse species, models and associated methodologies employed to assess the immune responses to NPs [16].
Fig. 1.
Schematic representation of different smart nanosystems and several biological molecules that can be used in the treatment of diverse pathologies.
Definitively, the characteristics of NPs can be exploited to modulate the immune system [17]. For example, the purpose of modulation will be to increase the intensity of the immune response that is diminished by some causes (i.e.: immunosuppression, stress, chronic infections), while in other cases, it will be reduced when it is out of regulation or control (i.e.: autoimmune diseases, allergies, transplants, hypersensitivity). NPs can be applied from two different approaches:
-
(i)
Using NPs as adjuvants to modify the specific response of immune cells subsets. In particular, the adjuvant activity of NPs depends on their physiochemical properties and their capacity to be internalized in different cells, which can modify their responses. Moreover, the preferential access to specific immune cell populations would be achieve with the development of engineered nanosized carriers grafted with targeting moieties.
-
(ii)
Designing NPs in order to reach a subset of immune cells. This would be the basis for the development of immunotherapy that involves the stimulation of the immune response through the administration of immunomodulatory molecules. For these purposes, the characteristics of the nanocarriers (i.e.: particle size, surface charge or shape) can be modified to facilitate their interaction with immune cells; however, cell-specific activation and selective recognition can only be achieved through the use of active targeting ligands.
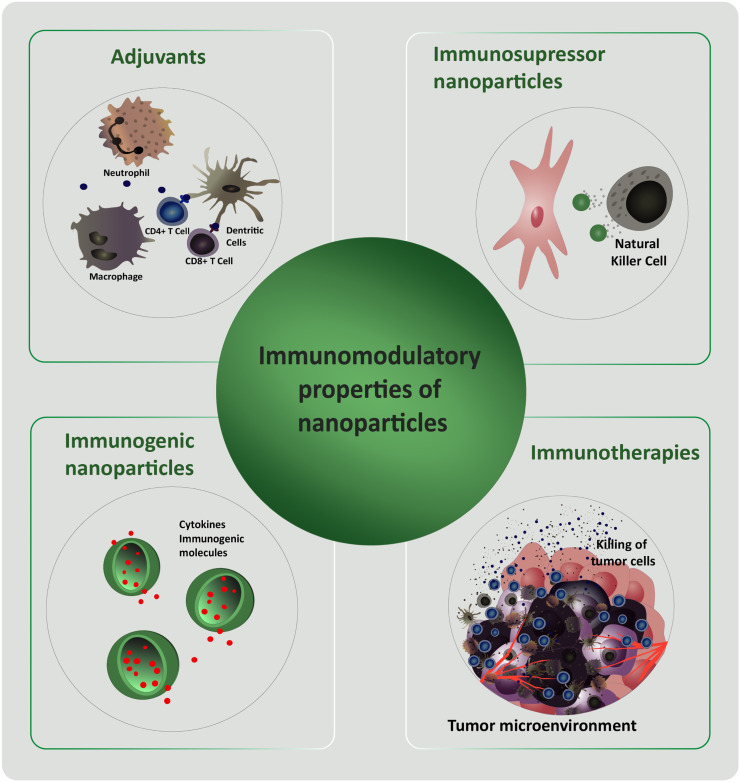
Based on the characteristics of the immune system and the NPs of different materials, immunomodulatory therapies using nanomaterials have been investigated and designed. For example, Scheiblhofer et al. reviewed researches in which by covalently linking allergens and polysaccharides to NPs, a versatile hypoallergenic tool that specifically target antigen-presenting cells can be obtain [18]. On the other hand, publications such as Jurj et al. and Jia et al. summarize the researches and developments of nanoscale immunotherapy and drug delivery for cancer by activating the immune system for clinical purpose [19], [20]. Different NPs were developed to induce immune response against cancer cells, override cancer-mediated immunosuppression, or generate long-term disease control with memory immune cells [21]. Other investigations describe the use of NPs and microparticles as delivery systems for immunomodulatory agents or the immunomodulatory effects of NPs themselves [22], [23], [24], [25], [26]. In this sense, Fig. 2 presents a scheme of the NPs characteristics that can be exploited to modulate the immune system response. In short, NPs could be used as immunomodulatory tools with immunosuppressive or immunogenic properties. In the first case, immunosuppressive NPs could be used to stop an undesirable immune response (autoimmune or allergic diseases), while immunogenic NPs could transport cytokines and molecules that enhance the immune response (useful for immunosuppressed patients' treatment). In addition, immunotherapeutic NPs for cancer and adjuvant NPs to improve vaccines are continuously being developed [27], [28], [29], [30], [31], [32]. For example, the innate immune response through macrophages, natural killer cells (NKs), neutrophiles and dendritic cells (DCs) has the ability to kill tumor cells. In this sense, tumor-associated antigens stimulate the maturation of DCs which in turns migrate to lymph nodes. Once in lymph nodes, promotes the activation of T cells (adaptive immune system). Subsequently, activated T cells migrate and recognize tumor cells antigens. Finally, tumor cells are killed by cytotoxic T cells (Fig. 3 ). Nanosystems would contribute to radiotherapy, chemotherapy and/or immunotherapy developments for cancer treatments [33], [34], [35], [36], [37], [38], [39] (Fig. 3).
Fig. 2.
Scheme of the NPs characteristics that can be exploited to modulate the immune system response.
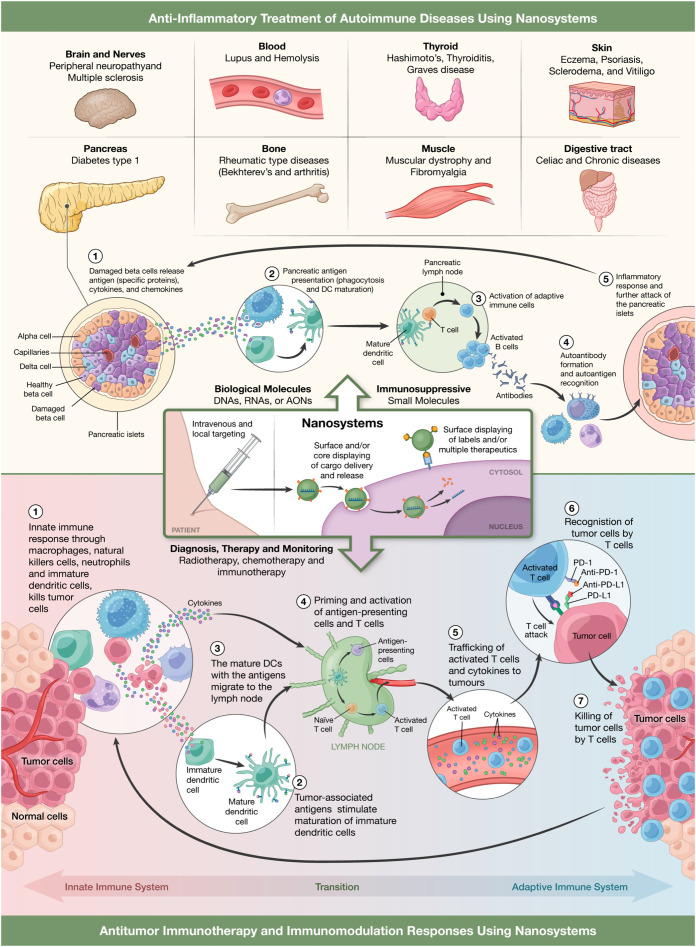
Fig. 3.
Representation of anti-inflammatory nanosystems for the treatment of different autoimmune diseases with special focus, as pathophysiological model, in type 1 diabetes progression (upper part of the figure) or as immunotherapeutics against cancer where it can be seen the different stages of innate and adaptive immunity where nanosystems can intervene (lower part of the figure).
2. Therapeutic strategies with nano and microparticles for autoimmune diseases and allergies treatment
2.1. The fundamental role of the immune system
The immune response plays a fundamental role in the maintenance of homeostasis, since it is an important system for the protection of the body against foreign substances and signs of danger, but an abnormal immune response, which could include both, a state of immunosuppression and immune stimulation, will inevitably lead to the disease. Recent studies reveal that both, the inflammatory processes (immune stimulation) as well as the presence of immunological tolerance (absence of reaction against own antigens or some foreign antigens that are innocuous) are influenced by the way in which antigens are presented [40], [41]. A process of immune stimulation can give rise to a strong adverse response, as in the case of autoimmune diseases. These are a group of pathologies that occur when the tissues of the body are attacked by their own immune system. On the other hand, allergic diseases are a series of conditions caused by a hypersensitivity reaction of the immune system to substances in the environment that are typically harmless. Immunosuppressants are among the most used drugs for autoimmune diseases and allergies treatment. These drugs reduce or inhibit the self-reaction of the immune response but have a number of adverse effects.
2.2. Engineered nanoparticles as therapeutic tools for autoimmune and allergies diseases treatment
It has been described that NPs, once inside the body, depending on the route by which they do it, will find phagocytic cells, such as macrophages and DCs or molecules, such as components of complement. When administered in living systems, NPs act as foreign materials and can suppress and/or stimulate the immune system [42]. These effects are determined by their chemistry nature [43] and in many cases, may be undesirable. However, NPs could decrease or increase the immune response, thus their immunomodulation properties could be useful for several diseases prevention or treatment [44], [45], [46]. In this way, the understanding of NPs and immune system interaction became fundamental for NPs application in clinical treatment in a safe way.
It would be possible to think that NPs that causes immunosuppressive effects could be used as anti-inflammatory therapeutic agents or to counteract the effects of autoimmune or allergic diseases. Unlike, NPs that stimulate the immune system could be employed as vaccine adjuvants or in cancer therapy [47] (Fig. 3). In the particular case of the treatment of autoimmune diseases it would be ideal that NPs generate phenotypes of anti-inflammatory M2 macrophages. The generation of this profile depends not only on the size of the NPs (their small size allows them to cross cell membranes and interact with biological molecules), but also on the material with which NPs have been generated, since some have been described as suppressors of the immune response [48]. Thus, it is possible to denominate as tolerogenic NPs the tool that researchers are trying to obtain for autoimmune disease treatment or allergies suppression.
Tolerance can be defined as the balance between molecules and cells of the immune system that avoid the response against self-antigens or harmless foreign antigens. When this process became unbalanced autoimmune pathologies and allergies appear. NPs are interesting candidates as potential immunotherapeutics. As mentioned before, NPs can subvert, enhance or suppress the immune system [42]. This effect can be achieved by particles to deliver genes, proteins or drugs and recent results have yielded models of NPs based therapies for controlling severe inflammation in autoimmune disease without impairing immunity against infections and tumors [49], [50], [51], [52], [53]. As can be seen in Fig. 3, the cargo carried by the NPs together with the targeting that can be given to them by attaching antibodies and/or ligands to their surface could transform them into useful tools for the treatment of autoimmune diseases such as multiple sclerosis, lupus, psoriasis, diabetes, etc. Regulatory T cells (Tregs) are responsible for controlling or suppressing other cells of the immune system and the response against both self and foreign antigens, helping to prevent autoimmune diseases. For example, damaged beta cells in the pancreas release specific proteins that would be recognized as antigens. This would lead to phagocytosis and maturation of DCs. In the pancreatic lymph node mature DCs interacts with T cell causing the activation of the adaptive immune response. Furthermore, activated B cell produces autoantibodies which recognize autoantigens. In conclusion, an inflammatory response is generated which further attacks pancreatic islets (Fig. 3). In this sense, immunosuppressive NPs with therapeutic molecules would greatly contribute to suppress this type of autoimmune disease (Fig. 3). Indeed, autoimmune diseases are associated with a decreased function of Tregs, so one of the objectives of therapy will be focused in restore Tregs activity [54], [55], [56].
2.3. Immunosuppressive nanoparticles
Several works described NPs of different materials with immunosuppression activities by itself [57], [58]. The common limitations of current immunosuppressive and biological therapies can be overcome with nanotechnology based strategies [58]. In this section, key examples of the effect of NPs with different composition are analyzed. Moreover, Table 1 presents a summary of NPs assayed for autoimmune and allergies diseases treatment.
Table 1.
Summary of NPs assayed for autoimmune and allergies diseases treatment.
| Nanoparticle | Cells implicated in immune response/tissue affected | Molecules regulated | References |
|---|---|---|---|
| CNTs. Oral administration. | Reduce T cell (fundamentally Th17) and NK cells activation, modulate DCs functions. | Increase IL-10, IL-27 | [59], [62], [63] |
| CNTs. Intravenous administration. | Induce Th2 cells and neutrophils influx. | Increase IL-5, IL-4 and promote a critical role of IL-33 | [59], [60] |
| Multi-walled carbon nanotubes | Fibrosis and functional damage in lung. | [61] | |
| Fullerene (carbon) | Inhibit degranulation of neutrophils and mast cells. Affect fibroblast, lymphocytes and macrophages activation |
Inhibition of oxidative burst, the release of NETs, ROS and histamine. Suppressed TNF-α. |
[44], [64], [65] |
| Graphene oxide | Reduction of inflammation in an autoimmune encephalomyelitis model and sepsis model. | [66] | |
| Functionalized graphene oxide | Activate both cellular and humoral immunity | [69] | |
| Gold NPs | Intra-articular administration reduced the development of polyarthritis. | Inhibit the cellular responses induced by IL-1β. Reduction of the production of TNF-α and IL-6 and in the maintenance of IL-1Ra levels |
[81], [82], [83] |
| Nanoceria | Anti-inflammatory effect on macrophages and APCs. Antipsoriatic effect. |
Free radical scavengers and affect iNOS expression. Induced the secretion of IL-10 and Th2 profile. Downregulation of inflammatory proteins (NF-κB, COX-2 and GSK3) and inhibiting Th-cell mediated IL-17/IL-23 |
[84], [85], [86], [87] |
| QDs | Colonic epithelial cells and macrophages. Affect proliferation of LT CD4 and macrophages. Phagocytosis of large aggregates is lower than smaller QDs on fish and bivalves. Intraperitoneally injected, diminish intestinal inflammation. |
Induce ROS. Diminish TNF-α, IL-8 and nitric oxide. |
[88], [89], [90], [91], [92] |
| Polymeric NPs | Inhibit inflammatory processes in the lungs (polystyrene NPs). Inhibit lung DCs proliferation and lymph node drainage. No significant alterations in oxidative stress neither pathological changes in liver, lung or cortex tissues. |
[70], [73] | |
| TiO2 nanotubes | Diminish splenocytes proliferation. Probably block Th-1 cell. | Diminish IL-2 and INF-γ. Slightly elevated levels of IL-4. | [94], [96] |
| DNPs | Stimulated splenic DCs (activating Tregs and suppressing effectors T cells). | Reduced pro-inflammatory cytokines | [74], [75], [76], [77], [78], [80] |
| SiNPs | ≥200 nm diminishes monocyte-macrophages proliferation. Large NPs induce monocyte-macrophages activation (increase CD86, CD80, CD40 and CD14expression) but affect membrane integrity and viability of cells. | Large NPs Increased the production of nitrites, IL-8 and IL-12. | [97], [106], [107] |
| Iron oxide NPs. | Do not induce inflammatory responses of monocyte-macrophages and aortic endothelia cells but may induce oxidative Attenuate Th17 cell responses in vitro and in vivo. Inhibit or induce Th2 lymphocytes? Inflammatory or Anti-inflammatory effects? |
[108], [109], [110], [111], [112], [115] |
2.3.1. Carbon based particles
2.3.1.1. Carbon nanotubes (CNTs)
CNTs can generate immunosuppressive effects depending on how they are administered. The inhalation of CNTs induced systemic immunosuppression in mice with increase gene expression of IL-10 in spleen (an anti-inflammatory cytokine), reduced T cell proliferation and decreased NK cell function in C57BL/6 adult mice [59]. On the contrary, the effect of CNTs in the same adult mice but with intravenously administration resulted in a Th2 immune responses (T helper type 2 -Th2- cells are CD4+ effectors T cells, required for humoral immunity and with an important role in coordinating the immune response to large extracellular pathogens and in allergies) that could promote adverse allergic reactions with neutrophil influx, increase IL-5, IL-4 and a critical role of IL-33 [59], [60].While other authors described that certain forms of CNTs induce inflammatory processes and fibrosis in the lungs [61].However, other studies confirmed that aspiration of CNTs in a mice model have direct effects on DCs, modulating systemic immunity and suppressing the responsiveness of T cells [62]. According to this, Moraes et al. described that the stimulation of antigen presenting cells (APCs) with CNTs increase IL-27 levels and inhibit the development of the Th17 cells (a type of effectors T lymphocytes differentiated from helper T lymphocytes with an important role in immune response against extracellular bacteria and fungi) resulting in less severe experimental autoimmune encephalomyelitis (EAE) a model of human multiple sclerosis [63].
2.3.1.2. Fullerenes and graphene
Fullerene, an allotrope of carbon, was described to have anti-inflammatory effects and interfere with the innate immune system in a fish model causing inhibition of oxidative burst and suppression of NETs release (DNA mesh that encloses histones and antimicrobial proteins, released by neutrophils into the extracellular space) as well as degranulation of neutrophils [64]. In the same way, type I hypersensitivity was suppressed by C60 fullerene. This carbon NPs decrease the release of histamine by human mast cells and the level of reactive oxygen species (ROS), that could be a potential way to control asthma, inflammatory arthritis and multiple sclerosis [44]. Other authors observed that fullerene suppressed induction of the proinflammatory cytokine TNF-α in human cells (fibroblast, lymphocytes and macrophages), and reduced synovitis in a rat arthritis model [65]. These observations were based primarily in the free radical scavenger capacity of fullerenes since free radicals are important as messengers in the pathogenesis of arthritis.
Alternatively, graphene oxides have important therapeutic applications such as their potential use to reduce inflammation as described in an autoimmune encephalomyelitis and sepsis models [66], [67]. However, in some cases, nanotoxicity hinders its application. Indeed, acute graphene oxide exposure induces innate immune gene expression but the innate immune response is considerably less pronounced after acute amino-functionalized graphene oxide exposure [68]. On the other hand graphene oxide NPs with surface modifications were described as an useful potential tool to improve its biocompatibility with the aim to employ them as both carriers and adjuvants. Functionalized graphene oxide was shown to activate both cellular and humoral immunity, that is why it was postulated as a true vaccine component carrier and adjuvant [69].
2.3.1.3. Polymeric nanoparticles
Polymeric NPs are polymers within the size range of 1 to 1000 nm of different nature. These can be loaded with active compounds trapped inside or adsorbed on their surface. Polymeric NPs have shown great potential for targeted drug delivery for the treatment of various diseases.
2.3.1.3.1. Polystyrene nanoparticles
Some polymeric NPs have been shown to inhibit inflammatory processes in vivo, as observed in the lungs of mice previously exposed to an allergen and after intratracheal administration of polystyrene NPs. These NPs inhibited the proliferation of lung DCs and lymph node drainage [70]. Alternatively, polyelectrolyte nanocapsules were efficiently applied to transfer different RNAs to various cell lines including primary T cells [71]. Moreover, by using carboxyl- and amino-functionalized polystyrene NPs, Fuchs et al., demonstrated that the surface modification is a valuable tool for reprogramming the M1/M2 polarization macrophage subsets [72]. While polystyrene 50-nm NPs coated with glycine, a neutral amino acid, are not inflammatory and are taken up preferentially by DCs in the periphery [70]. Although, it has been found that there are no significant alterations in oxidative stress neither pathological change in liver, lung or cortex tissues when polystyrene NPs were administrated orally for 30 days in mice [73].
2.3.1.3.2. DNA nanoparticles
Other polymeric NPs under development that represent a promising approach to treat a wide variety of clinical diseases are DNA nanoparticles (DNPs). These NPs are widely used as vehicles for gene knock down, gene transduction or gene transfer. On the other hand, it was described that immunity and autoimmunity can be suppressed after systemic administration of DNPs in mice models. DNPs stimulated the activity of a subset of splenic DCs specialized to activate Foxp3-lineage regulatory CD4 T cells (Tregs) and suppress effectors T cell responses. One way to avoid the release of the stimulating IFNγ in response to DNPs is the removal of CpG motifs from cargo DNA. Different DNPs delivery routes are frequently employed to provoke immunogenic responses however systemic administration was the most important to incite regulatory responses. DNP treatments attenuated antigen-induced arthritis and EAE in mice indicating that regulatory responses to DNPs were critically dependent on DCs activity. DNPs reduced pro-inflammatory cytokine production and antigen-specific T-cell responses in spleen and attenuated T cell infiltration into central nervous system (CNS) tissues [74], [75], [76], [77], [78]. On the contrary, DNA microcapsules made of cytosine–phosphate–guanosine oligodeoxynucleotides arranged into 3D nanostructures were developed to improve the serum stability and immunostimulatory effect. Interestingly, these DNA capsules can serve as both adjuvants to stimulate an immune reaction and vehicles to encapsulate vaccine peptides/genes to achieve synergistic immune effects [79]. Definitively, in the last years, there are increase evidence demonstrating that nucleic acid NPs can be used to modulate the immune response [80].
2.3.2. Non-carbon-based nanoparticles
2.3.2.1. Gold nanoparticles
It was reported that other NPs with anti-inflammatory properties are citrate coated Gold NPs that inhibited the cellular responses induced by IL-1β in a size dependent manner, both in vitro and in vivo [81]. IL-1β is an important cytokine involved in inflammatory disorders such as rheumatoid arthritis and psoriasis. Beside this, Leonavičienė et al. demonstrated that intra-articular administration of gold NPs reduced inflammation, joint swelling, and the development of polyarthritis [82]. It is important to note that gold compounds have been used from decades to treat rheumatic diseases [82]. A negative consequence of this behavior was described by Swartzwelter et al. (2020). They observed a decrease of BCG-stimulated monocyte response in presence of gold NPs. Indeed, a reduction of the production of TNF-α and IL-6 and in the maintenance of IL-1Ra levels was observed. However, such effect could be important in a context of excessive immune reactions against this or other antigens [83].
2.3.2.2. Cerium oxide nanoparticles
Cerium oxide NPs (nanoceria) have the ability to act as free radical scavengers. Nitric Oxide is an important inflammation mediator. Some studies indicated that nanoceria affect inducible nitric oxide synthase (iNOS) expression and provoke an important anti-inflammatory effect in murine macrophages. For this reason, nanoceria were postulated as potential NPs in chronic inflammation diseases therapy. These NPs also induced the secretion of IL-10 by APCs and Th-2 profile [84], [85]. Furthermore, Eitan et al. demonstrated that combination of lenalidomide and nanoceria reduce demyelination and neurological symptoms in EAE mice model by suppressing oxidative stress and inflammation [86]. In the same way, it was demonstrated that nanoceria presents an antipsoriatic effect by down regulation of inflammatory proteins (NF-κB, COX-2 and GSK3) and inhibiting Th-cell mediated IL-17/IL-23 [87].
2.3.2.3. Quantum dots (QDs)
It was reported that QDs can produce damage in nuclei, mitochondria, and plasma membrane. Moreover, QDs induce the generation of ROS leading to cell death. In addition, it was observed that sub toxic levels of QDs diminish the levels of TNF-α, IL-8 and nitric oxide in colonic epithelial cells and macrophages [88], [89]. In this sense, it was demonstrated that graphene QDs, intraperitoneally injected, diminish intestinal inflammation by inhibiting Th1/Th17 polarization in a chronic and acute colitis model as well as switch macrophages polarization to M2 type and increase the presence of Tregs in intestinal tissue [90]. QDs did not generate immune responses in vitro and in vivo although the proliferation of CD4 lymphocytes (T helper cells) and macrophages was affected [91]. Interestingly, the size of the QDs aggregates is of paramount importance in their resulting effect. Indeed, the phagocytes of large aggregates of CdS/CdTe QDs (25–100 nm) is lower than smaller QDs (<25 nm) on fish and bivalves [92]. However, the study of adverse effects of QDs is still under study, such as their effect over liver, kidney, lung, spleen and brain [93].
2.3.2.4. Titanium oxide (TiO2) nanoparticles
TiO2 nanotubes, NPs or fine particles have been described to diminish splenocytes proliferation (~20–35%) in concentrations of 50 μg/ml for NPs and nanotubes or 100 μg/ml for fine particles. These authors also found lower levels of IL-2 and INF-γ in a mix lymphocyte reaction assay, probably by blocking Th-1 cell (T cells that lead to an increased cell-mediated response, typically directed against intracellular bacteria and protozoa) response in vitro [94]. The importance of this finding resides in the fact that low levels of IL-2 maintain Tregs cells activity, preventing autoimmune diseases. Likewise, the inhibition of INF-γ, which is involved in inflammatory response, prevents it from promoting the cytotoxic CD8 T cells involved in the immune cellular response of several autoimmune diseases. On the other hand, in this work slightly elevated levels of IL-4 were found. This molecule, a Th-2 cytokine, induces the production of IgE and mast cell activation, both implicated in allergies diseases. Other work of the same authors, described that TiO2 NPs ameliorate EAE and collagen induced arthritis [94]. On the other hand, it was described that oral consumption of TiO2 NPs increase cytokines levels and the presence of pro-inflammatory immune cells in the colonic mucosa, indicating an inflammatory state [95], [96].
2.3.2.5. Silica nanoparticles (SiNPs)
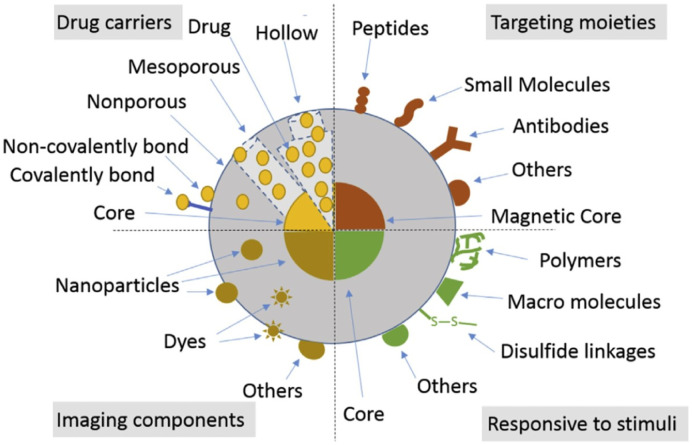
The physicochemical properties and biological effects of SiNPs are remarkable [97], [98]. Indeed, the development of SiNPs for various purposes has been successfully reported [99], [100], [101]. Moreover, a variety of biomedical applications of SiNPs have entered clinical trials [102]. Among them, it is highlighted their use for the administration of biologically active agents and drugs, the targeting ability due to molecules on its surface, stimuli responsive behavior (pH, magnetic field, light, temperature, etc.) and their applications for obtaining bioimages (Fig. 4 ). It was observed that the effects of these NPs depend on their concentration, size and charge [103], [104], [105]. Indeed, the existence of a size-dependent effect of silica particles on cell proliferation was observed. When THP-1 cultures cells were exposed to SiOH particles, only those of ≥200 nm diminish cell proliferation [106]. In addition, most of the silica particles evaluated, increased the production of nitrites, IL-8 and IL-12. Moreover, the levels of cytokine secretion increase during the times evaluated. However, 10 nm SiOH NPs did not induce the production of nitrites and IL-12 during all times assayed. Similarly, SiOH particles induce cell activation, evidenced by an increased expression of CD86, CD80, CD40 and CD14 molecules on the THP-1 cell membrane but all SiOH particles except 10 nm ones, which decreases CD11 expression. Finally, large sized SiOH particles affect membrane integrity and viability of cells. All these evidence, suggest a proinflammatory role of silica particles since these molecules, employed in the proper concentrations, and could be used as an adjuvant for immune response against microbial antigens. On the contrary, results with NPs (10 nm) suggest that these NPs could be more biocompatible and less proinflammatory with the potential to be used as delivery of molecules for several purposes unlike immune stimulation or with the correct modifications be used in autoimmune or allergies diseases treatment [106]. In conclusion, SiNPs inhibited proliferation and induced monocyte/macrophages activation but amine grafted silica NPs did not alter these parameters so activation properties of SiNPs could be hindered after grafting with amine moieties [107].
Fig. 4.
Schematic representation of SiNPs. Types of SiNPs for delivering biologically active agents and drugs. Targeting moieties on the surface of SiNPs or magnetic composites. SiNPs responding to stimuli (e.g. pH, glutathione, magnetic field, light and temperature). SiNPs for optical, magnetic resonance and other bioimaging applications. “Reprinted from Mebert et al. [97] Copyright (2017), with permission from Elsevier”.
2.3.2.6. Iron nanoparticles
Recent in vitro studies demonstrated the absence of toxicity of iron oxide NPs. Moreover, they did not induce inflammatory responses on human monocyte-macrophages and human aortic endothelia cells but may induce oxidative stress [108], [109]. However, in vivo studies showed complex results. For example, Ban et al., (2012) described anti-inflammatory effects after iron oxide NPs instillations and that intratracheally administration inhibited Th2 lymphocytes (related to allergic process) [110]. Lower doses of 147 nm NPs had no significant effect, while 35 nm NPs induced specific Th2 response to ovalbumin [111]. On the other way, both 35 and 147 nm NPs affect lung function, increasing inflammation but decreasing immune responses against sheep erythrocytes. Shen et al. (2012) demonstrated that iron oxide NPs modified Th balance toward Th2 cells and suppressed hypersensitivity after intravenously administration in mice. But most applications of iron oxide are related to in vivo imaging yet [112]. These characteristics could be related to the fact that their accumulation in macrophages induces transient phenotypic and functional changes [113]. In conclusion, there are evidences for both immunostimulatory and immunosuppressive effects of iron NPs [114]. Iron oxide NPs can affect Th balance, but also the immune responses of Th17 cells, a subset of T cells related with some inflammatory pathologies. According to Hsiao et al. (2017), iron oxide NPs decrease infiltration of CCR6+, IL-6+, IL17+ and ROR-γ+ cells in inflamed footpads of ovalbumin (OVA)-sensitized mice and they cause a direct suppressive effect on the expression of IL-6, IL-17 and ROR-γt by OVA-primed splenocytes in culture. All this data indicates that iron oxide NPs attenuate Th17 cell responses in vitro and in vivo [115].
2.3.3. Engineered nanoparticles
With the aim to obtain tools for immunotherapy, researchers work in the construction of complex NPs with different molecules [116], [117], [118], [119]. Table 2 presents a summary of complex NPs assayed for autoimmune and allergies diseases treatment. Indeed, different NPs carrying molecules have been tested in diverse biological models. For example, nanomaterials were developed from peptide amphiphiles that respond to the increased levels of matrix metalloproteinases or reactive oxygen species for immunotherapeutic delivery [120]. Engineered nanomaterials have progressed from being a proof of concept to improve traditional applications, including cargo delivery, immune modulation, therapy and imaging [121], [122], [123].
Table 2.
Summary of complex NPs assayed for autoimmune and allergies diseases treatment.
| Nanoparticle | Cells implicated in immune response/tissue affected | Molecules regulated | References |
|---|---|---|---|
| Antisense oligonucleotides included in PEG/PVP | Convert DCs into a suppressive phenotype | [124] | |
| Small interfering ribonucleic acids (siRNA) transported by small lipid NPs | Decrease the recruitment of monocytes | Silencing the expression of CCR2. | [125] |
| Iron oxide core coated with dextran and conjugated to siRNA | Antigen presenting cells | Down regulate the expression of MHC class I molecules. | [126] |
| Oligonucleotides complexed with pegylated cationic lipid NPs | Reduction of obstructive airway remodeling and CD68 immunoreactivity preventing airway inflammation in a model of asthma | Reduction of whole-lung IL-4 levels. | [127] |
| Polymer NPs with rapamycin | Decreased lymphocytic infiltration in a Sjogren's syndrome model. | [129] | |
| Iron NPs coated with MHC class I or II presenting specific peptides | Induce the expansion of antigen specific regulatory cells | [132], [133], [134] | |
| T cell epitope transported by gold particles-polyethylene glycol (PEG) complex NPs | Phagocyted by DCs. Expanded Foxp3+ Tregs. | [135], [136] | |
| Poly(lactide-co-glycolide) NPs with autoantigens and rapamycin(PLG). | Inhibit CD8+ and CD4+T cells activation and increase regulatory cells. | [137], [138] | |
| Liposomal NPs with inhibitory ligands and antigens | Induce B cell tolerance | Induce inhibitory antibodies | [139] |
| PLG NPs coating with red blood cell membranes | Use as target for pathological antibodies | [140] | |
| PLG NPs with antigens | Inhibit of Th2 response. | [141], [143] | |
| Myelin antigen coupled to PLG NPs | Reduced the presence of Th1 and Th17 lymphocytes and macrophages in the central nervous system | [142] | |
| Self-antigen in QDs | Immunological tolerance | [40] | |
| Antigen-decorated PLA NPs particles | Inactivate pathogenic T cells and activated Tregs cells. Uptake by macrophages | [144] | |
| PLGA NPs delivering OVA and decorated with ligands for scavenger and mannose receptors. | Suppress airway eosinophilia and induce the presence of Foxp3+ Tregs in the lung. | Induction of TGF-β, IL-4, and IL-10 production in vitro. | [51] |
| Fucan-coated Silver NPs | Macrophages | Increase of IL-10, IL-6, TNF-α and nitric oxide. | [180] |
2.3.3.1. Oligonucleotide-bearing nanoparticles
Antisense oligonucleotides included in PEG/PVP particles have been used to convert DCs into a suppressive phenotype in a mice model [124]. In the same way, Leuschner et al. succeeded in silencing the expression of CCR2 (chemokine receptor) in order to decrease the recruitment of monocytes and therefore the activation of the innate immune response, using small interfering ribonucleic acids (siRNA) transported by small lipid NPs [125]. Other authors reported NPs of an iron oxide core coated with dextran and conjugated to siRNA to downregulate the expression of major histocompatibility complex (MHC) class I molecules [126]. Ramelli et al. described oligonucleotides complexed with pegylated cationic lipid NPs that reduced obstructive airway remodeling and reduced CD68 immunoreactivity preventing airway inflammation in a model of asthma [127]. In this sense, different NPs that transport anti-inflammatory oligonucleotides are under study [128].
2.3.3.2. Immunosuppressant drugs bearing nanoparticles
Polymer NPs with rapamycin, an immunosuppressant, was developed with the aim to limit its exposure to the kidneys, when was administered via the tail vein into NOD mice. This complex decreased lymphocytic infiltration and represented a low release system that reduces its toxicity in a Sjogren's syndrome model. These preparations worked better than the drug alone [129]. Liposomes loaded with nintedanib and colchicine provided an alternative strategy to module M1/M2 macrophages into a balanced status and consequently module the innate immune response [130]. These type of NPs have interesting features and been also postulated for their application in transplantation medicine [131].
2.3.3.3. Nanoparticles as antigens carriers for immune response control
Other studies use NPs specific to autoantigens. Iron NPs coated with MHC class I or II presenting specific peptides implicated in autoimmune disease induce the expansion of antigen specific regulatory cells, which suppressed autoantigen presentation and restore normoglycemia in different mice models including a humanized one without compromising systemic immunity [132]. This construction showed efficacy in EAE model, collagen-induced arthritis (CIA) and Type 1 Diabetes (T1D) [132], [133], [134]. T cell epitope transported by gold particles-polyethylene glycol (PEG) complex NPs are taken by DCs and expanded Foxp3+ Tregs in vitro reducing the severity in EAE and T1D in NOD mice [135], [136]. Another study, reported that in order to induce tolerance, authors employed poly(lactide-co-glycolide) NPs with autoantigens and rapamycin (PLG) [137]. These NPs inhibit CD8+ and CD4+T cells activation and increase regulatory cells in EAE and hemophilia A mice models [137], [138]. Liposomal NPs with inhibitory ligands and antigens induce B cell tolerance and in this way the formation of inhibitory antibodies responsible for Hemophilia A is repressed [139]. Coating PLG NPs with red blood cell membranes were assayed as an alternative target for pathological antibodies in an anemia in vitro and in vivo model [140].
Other reports employed antigen associated polystyrene or PLG NPs or microparticles in the effective inhibition of allergic airway inflammation in animals with Th2 sensitization [141]. Myelin antigen coupled to PLG NPs reduced the presence of Th1 and Th17 lymphocytes and macrophages in the central nervous system, inducing tolerance in EAE model [142]. Other authors described tolerogenic NPs composted of PLG NPs with antigens that eliminate completely the disease in an EAE mice model [143]. In other work that use a mouse model of multiple sclerosis (MS), Hess et al. showed that by controlling the density of self-antigen in QDs, immunological tolerance can be generated [40]. In addition, intravenous administration of antigen-decorated 500 nm polystyrene or PLA particles inactivated pathogenic T cells and, on the contrary, activated Tregs cells after being uptake by macrophages of the marginal zone. This effect caused T-cell tolerance and ameliorated EAE [144]. More recently, PLGA NPs that deliver OVA and decorated with ligands for scavenger and mannose receptors was developed. These NPs induced TGF-β, IL-4, and IL-10 production in vitro. In vivo experiments demonstrated that NPs suppress anti-OVA IgE responses, Th2 cytokine production, airway eosinophilia and induce the presence of Foxp3+ Tregs in the lung [51].
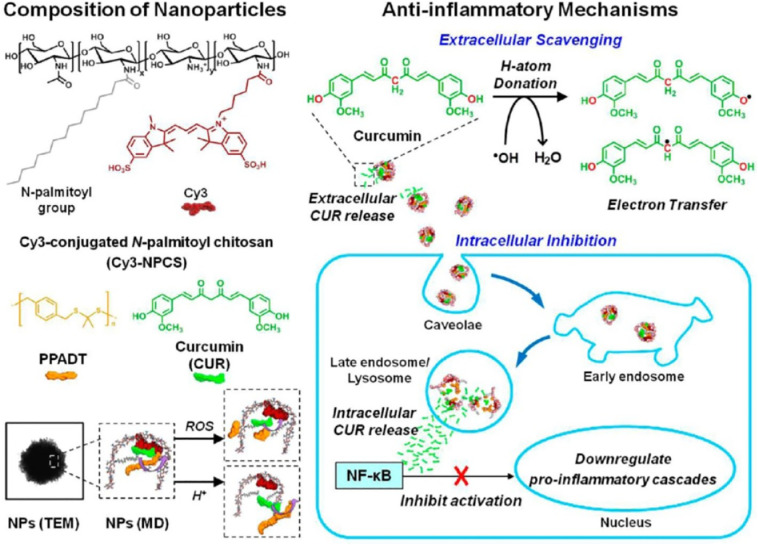
2.3.3.4. Nanoparticles for pH and oxidative stress control
Many inflammatory diseases involve oxidative stress and reduced pH. Pu et al. (2014) described a NP system that is reactive to both reduction of pH and oxidative stress, commonly present in an inflammatory environment. These NPs effectively release curcumin, which is a potent antioxidant and anti-inflammatory agent, and reduce the excess oxidants produced by the lipopolysaccharide (LPS)-stimulated macrophages. This system allows monitoring the intracellular release behavior because of the presence of Förster resonance energy transfer between the carrier and curcumin. Curcumin-loaded NPs applications were also validated in a mouse model with ankle inflammation induced by LPS. This system is a promising tool for treating oxidative stress-related diseases [145]. Moreover, the dual-responsive NPs possess interesting extracellular/intracellular anti-inflammatory mechanisms (Fig. 5 ).
Fig. 5.
Schematic illustrations showing the composition/structure of the dual-responsive NPs developed in this study and their extracellular/intracellular anti-inflammatory mechanisms. “Reprinted from Pu et al. [145] Copyright (2014), with permission from American Chemical Society”.
3. Applications of nanoparticles as adjuvants for vaccine formulation
3.1. Vaccines adjuvants
Many of the current modern vaccines contain purified or recombinant antigens in suspension with excipients, possible components from the production process (egg proteins, formaldehyde, antibiotics, etc.) and adjuvants. Adjuvants are used to enhance the immune response against vaccine antigens.
For many years, only aluminum salts were approved as adjuvants for human use for the FDA of the United States. These aluminum salts-based adjuvants directly activate DCs and can induce strong antibody responses. The mechanism of action involves the adsorption of the desired antigens on the surface of aluminum compounds. This phenomenon can contribute to retain the antigens at a higher concentration in the injection site, therefore providing a sufficient uptake time for DCs [146], [147]. Even though, these adjuvants cannot be applied with all antigens, because in some cases can induce local reactions in the injection site and sometimes fail to generateCD8+ T-cell immunity [148]. Currently, there are other adjuvants approved by FDA like MF59, an oil-in-water emulsion of squalene oil used in Fluad, a vaccine for the prevention of seasonal influenza in adults older than 65 years. Another example is CpG 1018, an adjuvant based on synthetic DNA sequences used for Heplisav-B, a vaccine for the prevention of hepatitis B virus infections in 18 years old adults and older.
Actually, it is well-known that APCs, such as DCs, plays a fundamental role in determining the direction of the adaptive immune response. They are the link between the innate and adaptive responses. Therefore, it was suggested that the ideal vaccine, would initiate an innate immune response competent to direct the adaptive immune response toward a specific pathogen, followed by the development of immune memory. If the vaccines fail in inducing APCs maturation, protective immunity is limited [149].
The employment of adjuvants during vaccine formulation ensures the generation of a soft local inflammatory reaction which can efficiently stimulate the recruitment of immune cells and induce the adaptive immunity rapidly. This effect is of particular interest for the induction of protective specific adaptive immunity to vaccine antigens. Most of the particulate adjuvants apparently contribute to the activation of the inflammasome, which is an intracytoplasmic protein complex with the role of detecting stress signals and activating enzymes involved in the production of inflammatory cytokines. Especially, the activation of the inflammasome induces the production of IL-1β. This is an inflammatory cytokine with an important immunostimulatory role [150]. Understanding the mechanisms of interactions of nanomaterials with the inflammasome will provide strategies for safer nanomaterial design and therapy [151].
3.2. Nanoparticles in vaccine formulations
Until now, a great progress has been achieved in the development of conventional vaccines. However, in some cases they require further improvements to completely afford concerns about: i) intrinsic instability in vivo, ii) toxicity, iii) weak immunogenicity, and iv) requirement of multiple administrations. Nanotechnology has emerged as a true candidate to overcome these problems and improve vaccine development. Indeed, nanoparticulate delivery systems provide the possibility to improve both, the humoral and cellular immune responses. The nanoscale size possesses various interesting advantages for vaccine developments among which the facile uptake by phagocytic cells, the mucosa-associated lymphoid tissue and the gut-associated lymphoid tissue, would lead to improvements in antigen recognition and presentation. Moreover, surface modifications of the nanocarriers with different targeting moieties allow the stimulation of selective and specific immune responses through the delivery of antigens to specific receptors present in cell surfaces. In addition, some nanocarriers have been designed to co-deliver both an antigen and an adjuvant. Indeed, nanocarriers can facilitate the targeting and/or sustained release of antigens or adjuvants to APCs [152]. Nowadays, it is clear that nanovaccines represent a key milestone in the prevention of classical and emerging diseases [153].
The employment of NPs with biomedical purposes has brought new concerns that must be addressed. One of these points involves the analysis of the time that they persist in the organism and produce their effects before being recognized and eliminated by the defensive systems. Another important point requires the analysis of the NPs modulation of the immune responses in order to obtain optimal effects. These analyses are highly important for the safety use of NPs in vaccine formulations since NPs can accomplish a dual function. In one hand, NPs can work as delivery system of antigens to enhance antigen processing. On the other hand, NPs can also work as an immunostimulatory adjuvants inducing and amplifying protective immunity. In addition, the degradation rate of the NPs within the cells is another factor that must be considered. NPs must persist enough time to allow efficient antigen uptake, processing, and presentation, although NPs can induce chronic inflammasome activation and pathological unresolved inflammation if they persist too much time. However, NPs can be very good adjuvants because of their physicochemical properties (e.g., shape, size, surface charge), biocompatibility and the possibility to be tailored to attain different immunological effects [150], [154].
An important topic to consider before developing NPs vaccines is that after parenteral administration, host proteins are immediately adsorbed on NPs surface, thus immune cells encountering vaccine adjuvant particles do not interact with a clean surface. Instead, such particles incorporate a complex mixture of surface-adsorbed protein immediately after immunization. However, the importance of ‘protein corona’ consequence on vaccine formulations is still underappreciated. While great effort are being performed to clarify the relevance of such interactions, it is clear that if composition and reproducibly of production are not well controlled, it could not be possible to reliably characterize the interactions with the biological environment and the resulting immune responses [155], [156].
3.3. Nanoparticles being studied for the development of vaccines
There are different types of NPs such as hard material NPs (i.e.: silica, iron oxide, gold, silver) and organic-based NPs (i.e.: polymeric, lipidic), among others. Depending on the material of the NPs, structure and chemical modifications on the surface, the effects over immune system could change (Table 3 ). Indeed, various material properties are being exploited to develop effective vaccines [157].
Table 3.
Summary of NPs being studied for the development of vaccines.
| Nanoparticle | Cells implicated in immune response/tissue affected | Molecules regulated | References |
|---|---|---|---|
| Liposomes composed by DDA. Subcutaneal injection. | Induce the cell-mediated immune response. | Production of IFN-γ and IL-17 in some variants with mycobacterial lipid monomycoloyl glycerol | [162] |
| Chitosan NPs coated with the Salmonella surface F-protein. | Induction of lymphocyte proliferation. | Increased expression of TLR-4, TLR-2, TGF-β, IL-4, and IFN-γ mRNAs. | [165] |
| Gold nanorods. Intranasal administration. |
Monocytes, macrophages, T cells, NK cells and DCs. | Decrease of TNF-α, GM-CSF, IL-17 and IL-12p70 and increase of IL-9 in comparison with respiratory syncytial virus infected animals | [166] |
| E2 protein conjugated Gold NPs | Activate CD4+ and CD8+ T cells and balance Th1 and Th2 cellular responses | Increased production of IFN-γ and IL-10. | [167] |
| Hydrophobic Zwittwerionic Functionalized Gold NPs in presence of LPS |
Macrophages. Pro inflammatory profile. | Increase TNF-α. | [168] |
| Hydrophilic Zwittwerionic Functionalized Gold NPs in presence of LPS |
Macrophages. Interactions between macrophages and LPS blocked. | Inhibition of oxidative burst, the release of NETs, ROS and histamine. Suppressed expression of TNF-α. |
[168] |
| Carbon Dots and Ricin toxin binding subunit B. Oral vaccine adjuvant. | Promotion of macrophages proliferation | Increase of TNF-α, IL-6 and NO. | [159] |
| Silica-based NPs coated with nevirapine (NVP) | Peripheral blood mononuclear cells. NVP coated NPs showed less cytotoxicity than free NVP. | [173], [174] | |
| Pulullan-coated iron oxide NPs conjugated with an antigen from Plasmodium yoelii. | CD4+ T cells reactive to this antigen in a rodent malaria challenge model. | Induce secretion of specific antibodies and IFN-γ | [179] |
| Polymeric NPs releasing curcumin | Macrophages | Decrease of oxidants (ROS/RNS) produced by the LPS-stimulated Macrophages | [145] |
3.3.1. Carbon based particles
3.3.1.1. Carbon nanoparticles
Carbon NPs are also studied for vaccine and drug delivery. They present good biocompatibility and can be synthesized into a variety of mesoporous spheres and nanotubes. Mesoporous carbon NPs have been studied as an oral vaccine adjuvant [158]. Sometimes, a molecule has adjuvant activity but is unstable in physiological conditions, and the binding to a nanoplatform can offer an increase of stability and efficiency. Li et al. (2018) prepared stable NPs by supramolecular assembling of carbon dots (CDs) and Ricin toxin binding subunit B (RTB). However, RTB can modulate cell-mediated immunity and promote the activation of macrophages; its biomedical applications are significantly limited because of the intrinsic properties of proteins, like low efficacy of cellular uptake and poor stability. The formed CDs-RTB possessed robust stability and protected RTB against enzymatic hydrolysis. More importantly, CDs-RTB promoted macrophages proliferation, enhanced the generation of TNF-α, IL-6 and NO in RAW 264.7 cells and increased the expression of mRNA, suggesting the improved immunomodulatory activity of CDs-RTB [159]. Similarly, a nanocomplex formed by multiwalled carbon nanotubes (MWCNTs) and a noncovalent attached synthetic peptide was able to generate a stronger immune response compared to the antigen vaccine without the MWCNTs [160].
3.3.1.2. Liposomes and emulsions
One of the nanosystems of great interest for the scientific community is the liposomes that are biocompatible NPs composed of phospholipid bilayers [161]. They are capable of deliver both hydrophilic and hydrophobic molecules, properties that facilitate the co-delivery of different molecules, such as antigens and adjuvants. The physicochemical properties of liposomes, including their lipid composition, structure and size can be tuned according to the properties of the vaccine antigen to maximize immunogenicity. These properties are also significant for the induction of the immune response. Liposomes composed of dimethyldioctadecyl ammonium (DDA) can affect only the cell-mediated immune response, but not the humoral ones, in a size depending way [162].
In parallel, adjuvant formulations with emulsions have been long studied as vaccine delivery systems. Emulsions are dispersions of two or more immiscible liquids composed of oil, emulsifiers, and excipients. Emulsions can be classified in two main classes: oil-in-water emulsions and water-in-oil emulsions. The first emulsion type is usually used in adjuvant formulations [152]. Emulsions can carry antigens inside their core for efficient vaccine delivery or can also be simply mixed with the antigen. One frequently employed emulsion is MF59, which has been licensed as a potent and safe vaccine adjuvant in more than 20 countries. Indeed, it has been extensively studied for therapeutic application in influenza vaccines. A frequently used adjuvant delivery system in DNA vaccine studies involves a combination of 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) modified cationic liposome and a cationic polymer (usually protamine) condensed DNA, which are called liposome-polycation-DNA NPs (LPD) [158]. Liposome-polymer hybrid NPs were successfully employed to deliver a multi-epitope self-replication DNA vaccine. This platform induced a strong humoral and cellular immune responses representing a promising alternative for the development of DNA vaccines for various infectious diseases [163].
3.3.1.3. Polysaccharide polymeric nanoparticles
Nanosized adjuvants were also prepared employing natural polymers like polysaccharides (i.e.: chitosan, alginate, inulin, pullulan). Among this group, chitosan-based NPs have been extensively studied due to their biodegradability, biocompatibility, and possibility to be processed into desired sizes and shapes. Indeed, chitosan NPs have been employed in the formulation of different vaccines including Newcastle disease vaccines, HBV vaccines and DNA vaccines. Another potent adjuvant and well-known activator of complement via the alternative pathway is Inulin. Inulin derived NPs were employed as adjuvants. In this sense, AdvaxTM, generated a higher immune response in vaccines against different viruses including respiratory syncytial virus [164]. Other work studied chitosan NPs coated with the Salmonella surface F-protein in chickens. These NPs induced higher levels of specific mucosal IgA production and lymphocyte proliferation as well as increased the expression of TLR-4, TLR-2, TGF-β, IL-4, and IFN-γ mRNAs. Chitosan NPs induced antigen-specific T and B cell responses against Salmonella, a common poultry pathogen [165]. A minimal association between antigen and NPs is needed for the formulation of immune potentiator adjuvant. Thus, in most cases the simple mix of NPs and adjuvant shortly prior to injection should be enough to prepare the NPs with a target antigen. This hypothesis was investigated with hard-material NPs adjuvants. It was observed that even when not conjugated to the antigen, NPs can work as a size-dependent immune potentiator adjuvant [158]. Different works further confirms the induction of inflammatory immune responses after injection of hard material NPs without antigen [158].
3.3.2. Non-carbon-based nanoparticles
3.3.2.1. Gold nanoparticles
Gold NPs are platforms extensively studied and with many applications in biomedicine thanks to their binding capacity to multiple molecules as proteins, antibodies and oligonucleotides. The binding between these molecules and gold NPs can modify their physicochemical properties as surface plasmon resonance, conductivity, and redox behavior, giving a possibility to detect signals. Gold nanorods with antigens conjugated on their surfaces were employed in the development of carrier for an antigen derived from respiratory syncytial virus [166]. Different types of gold NPs were used as carriers for various virus derived antigens such as classical swine fever, foot-and-mouth disease and influenza, or as a DNA vaccine adjuvant for human immunodeficiency virus (HIV) [158], [167]. Moyano et al. (2016) reported the use of 2-nm-core gold NPs functionalized with zwitterionic and non-ionic tetraethylene glycol ligands. These particles were engineered based on the NPs surface chemistry and biological activities relationship analysis. In this way, it was shown that NPs bearing hydrophobic zwitterionic improve inflammatory response more than hydrophilic zwitterionic ones. However, tetra (ethylene glycol) head groups produce an important anti-inflammatory response (in vitro and in vivo) indicating that the surface ligands alter immunomodulatory NPs properties [168].
3.3.2.2. Silica-based nanoparticles
Silica emerged as a candidate to develop delivery systems and the interest for its application in nanovaccinology design is growing fast. SiNPs are generally recognized as safe and possess important physicochemical properties as nanocarriers for different applications, such as real-time multimodal imaging, selective tumor targeting and vaccine delivery. Indeed, these NPs conjugated in a vaccine with inactivated transmissible gastroenteritis virus enhanced early cellular immune response and long-term humoral response. The vaccine with the NPs (70 nm) increased the production of IFN-γ, TNF-α and IL-6, generated high levels of antibodies, and induced a high CD4+/CD8+ T lymphocyte ratio with stimulation of CD3+ T cells proliferation [169].
Moreover, different templating methods are available to prepare porous particles like mesoporous SiNPs (MSNs) and hollow SiNPs. Porous particles can be employed as a multifunctional platform to simultaneously deliver various cargo molecules with different molecular weights and the ability to functionalize their surface highlights silica-based NPs as a promising platform for various applications [107], [170], [171], [172]. Indeed, silica NPs provide the possibility to improve both, the cellular and humoral immune responses [148]. MSNs with sizes in the range of 50–200 nm have been studied as both nanocarriers and adjuvants for delivery of effective antigens, such as those derived from porcine circovirus and HIV [173], [174]. Biocompatibility and internalization of MSNs in macrophage like THP-1 cells is dependent of the particle size, despite their cargo, as it has been shown by Huang et al. (2020). Their results indicated that adjuvant potential of SiNPs can vary depending of their size [175].
The potential of mesoporous silica particles as a vaccine adjuvant was early identify by Mercuri et al., employing SBA-15 as a transport and adjuvant for bacterial recombinant protein Int1b [176]. The authors reported an increased immune response in mice. Moreover, SBA-15 presented better adjuvant properties than Al(OH)3. Although, there was no evidence of in vitro release of the protein from carriers. Mice treated with SBA-15 containing Int1b presented higher antibody titles compared to the mice immunized with protein plus Al(OH)3 adjuvant [148]. SBA-16 was also reported as adjuvant system. Recently, through the incorporation of a specific antigen of pathogenic fungus Paracoccidioides brasiliensis at silanized SBA-16 (APTES-SBA-16), it has been created a nanosystem with a sustained release profile without significant cytotoxicity on normal human lineage cells [177].
3.3.2.3. Iron oxide nanoparticles
Selective drug transport and its controlled release at the target site can be achieved with aminosilane-coated iron oxide magnetic NPs. DNA vaccines developed with Fe3O4-Glu-polyethyleneimine NPs induced a strong immune response and generate a statistically significant increase in the protective efficacy against infections [178]. Pulullan-coated iron oxide NPs are non-toxic. They were conjugated with an antigen from Plasmodium yoelii to induce both, specific antibodies and IFN-γ CD4+ T cells reactive to this antigen in a rodent malaria challenge model [179].
3.3.2.4. Silver nanoparticles coated with biomolecules
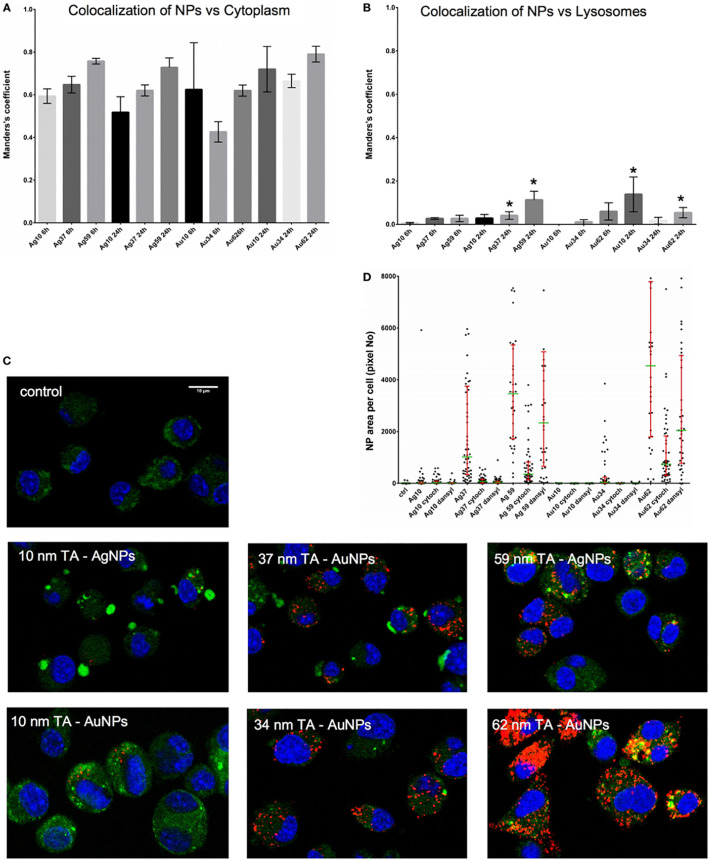
Fernandes-Negreiros et al. (2017), synthesized silver NPs containing fucans from Dictyotamertensii (Martius) Kützing using an environmentally friendly method. Fucan-coated silver NPs (FN) were size-stable for 16 months. They inhibited melanoma tumor cell line B16F10 proliferation. Additionally, they increased the release of cytokines (IL-10; IL-6 and TNF-α) and nitric oxide up to 7000 times. In addition, the FN showed Gram-positive and -negative bacteria inhibitory effects [180]. Sharma et al. synthesized curcumin-stabilized silver 45 nm NPs (Cur-AgNP). They observed a significantly reduced HIV 1 replication in cells treated with Cur-AgNP by inhibition of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) and translocation of nuclear NF-κB [181]. Although, the interaction of silver NPs with immune system is not really understood. Orlowski et al. (2018), studied the ability of tannic acid-modified silver NPs to induce DCs maturation and activation. Both types of NPs were efficiently internalized by DCs and induced maturation and TLR9 expression. Moreover, the uptake of NPs was blocked by an inhibitor of clathrin-mediated endocytosis of NPs and there was a low co-localization of NPs with lysosomes (Fig. 6 ) [182].
Fig. 6.
Intracellular localization of TA-Ag/AuNPs. The Manders' coefficients for co-localization of TA-Ag/AuNPs and cytoplasm (A) or lysosomes (B) in JAWS II cell culture exposed to 10 nm, 37 nm, 59 nm TA-AgNPs and 10 nm, 34 nm, 62 nm TA-AuNPs for 24 h at 2.5 μg/ml. *Significant differences with p ≤ 0.05. (C) Representative images for lysosomes (green), NPs (red) and nuclei (blue) in cells exposed to NPs, as described above. (D) NPs content in cells subjected to pretreatment with 10 μg/ml monodansylcadeverine and 5 μg/ml cytochalasin D, and then to incubation with TA-Ag/AuNPs at 2.5 μg/ml for 6 h. Reprinted from [182] with permission from Frontiers under the terms of the Creative Commons Attribution License (CC BY).
4. Nanoparticles targeting
Disease treatments have significantly changed with the development of NPs with the ability to accurately target desired cells or organs (Table 4 ). For example, TLR receptors can be efficiently targeted employing NPs grafted with surface TLR ligands which can bind and activate them. The adjuvant AS04 is a precursor of this concept made of TLR4 ligands (MPL) on alum particles. Moreover, NPs can be engineered or modified in order to improve the rupture of the phagolysosome after being incorporated by APCs. Examples of these engineered NPs include the crystalline particles (e.g., alum) and membrane-active particles (e.g., the protonic sponge particles). As a consequence of the phagolysosomal rupture, antigens and inflammasome-activating molecules escape to the cytoplasm and this process enhance cross-presentation. For that, to act as great adjuvants NPs must provide to the cells all the stimuli required, including the ability to upregulate IL-1β gene and activate inflammasome [183], [184], [185]. However, IL-1β and inflammasome are also involved in a great variety of diseases, including degenerative diseases, acute and chronic inflammatory diseases, cancer, autoimmune diseases, among others. So, the balance between local beneficial (protective) inflammation and pathological inflammation is diffuse, and is principally based on the duration and persistence of the stimulus [150].
Table 4.
Summary of NPs targeting.
| Nanoparticle | Cells implicated in immune response/tissue affected | Molecules regulated | References |
|---|---|---|---|
| Lipid NPs with immunomodulatory oligonucleotide (IMO-2125) | Induce stronger Th1-type response. Antigen specific cell-mediated immune responses enhanced. | Production of antigen-specific IFN-γ, TNF-α and IL-2. | [186] |
| Tumor-targeted lipid-dendrimer‑calcium-phosphate (TT-LDCP) NPs with thymine-functionalized dendrimers | Tumoral infiltration and activation of CD8+ T cells | [187] | |
| Encapsulated MSNs into polyion complex vesicles | Cytotoxicity against cultured tumor cells and suppression of lung tumor in vivo. | [193] | |
| PRINT hydrogels of biocompatible hydroxy-poly(ethylene glycol) (PEG) | APCs, B cells and CD4+ T Helper cells | [108] | |
| Cationic stearylamine lipid-polymer hybrid NPs delivering Amphotericin B | Macrophages and splenocytes | Increase of IFN-γ, TNF-α and IL-12. Decrease of IL-10, IL-4 and TGF-β. | [188] |
| Hyaluronic acid decorated pH sensitive LPNPs delivering erlotinib and bevacizumab | Suppression of non-small cell lung cancer. | [189] | |
| E2 protein NPs with CpG oligonucleotides | APCs | [190] | |
| pSiNPs with anti-DC-SIGN antibodies and loaded with rapamycin | DCs | [191] | |
| Functionalized pSi NPs conjugated with an antibody against polysialylated neural cell adhesion molecule and loaded with SC-79. | Endogenous neuroblasts. | [192] |
4.1. Carbon based particles
4.1.1. Lipid nanoparticles
Swaminathan et al. (2015) analyzed a novel Merck-proprietary lipid NP (LNP) in order to improve immune stimulation against viral antigens. BALB/c and C57BL/6 mice models immunized with LNPs alone or in combination with TLR9 agonist, immunomodulatory oligonucleotides, IMO-2125 (IMO), presented significantly enhanced immune responses to hepatitis B virus surface antigen (HBsAg) and ovalbumin (OVA). B-cell responses to both antigens tested were enhanced with LNPs, to levels comparable to known adjuvants including aluminum-based adjuvant, a TLR4 agonist, 3-O-deactytaled monophosphoryl lipid A (MPL), and IMO alone. LNP/IMO agonist combination elicited a stronger Th1-type response. Moreover, antigen specific cell-mediated immune responses were significantly enhanced by the LNP adjuvant. Furthermore, LNPs elicited potent antigen-specific CD8+ and CD4+ T-cell responses. In the same way, LNP and LNP/IMO formulated antigens led to higher frequency of antigen-specific CD8+T-cell responses, than antigens alone or vaccine with only IMO. These results show that lipid NPs can be useful as future vaccine adjuvant to in vivo improve both T-cell and B-cell responses [186]. On the other hand, targeted NPs could be useful for cancer treatment. In this sense, it was reported an engineered tumor-targeted lipid-dendrimer-calcium-phosphate (TT-LDCP) NPs with thymine-functionalized dendrimers, that enhanced gene delivery capacity with adjuvant properties. TT-LDCP NPs delivered siRNA against ligand PD-L1 and IL-2 achieving tumoral infiltration and activation of CD8+ T cells [187].
4.1.2. Lipid-polymer hybrid NPs (LPNPs)
Asthana et al. (2015) developed macrophage targeted cationic stearylamine lipid–polymer hybrid NPs (LPNPs) with liposomes and polymeric NPs. LPNPs were adapted for the delivery of Amphotericin B (AmpB) in order to enhance therapeutic efficacy and diminishing toxic effect. LPNPs core-shell structure has the ability to encapsulate amphiphilic AmpB in higher amount in a stabilized formulation and sustain drug release. LPNPs demonstrated safe applicability for parenteral administration, high macrophage incorporation and great anti-leishmanial efficacy in vitro and in vivo due to Th-1 biased immune-alteration mediated by drug-free LPNPs which increased macrophages microbicidal mediators. AmpB-LPNPs could be a promising alternative to commercial AmpB-formulations for the eradication of intra-macrophage diseases [188]. Other authors developed a hyaluronic acid (HA) decorated, pH sensitive LPNPs to deliver two compounds: erlotinib and bevacizumab for targeting and suppressing non-small cell lung cancer. NPs reduced the tumor volume respect control group, so it was postulated as a promising system for the therapy of this type of cancer [189].
4.1.3. Oligonucleotides to target APCs
Efficient delivery of antigens is of great concern in immunotherapies. Molino et al., (2017) conjugated E2 protein NPs (CpG-PEG-E2) with CpG oligonucleotides to target APCs. CpG-PEG-E2 was uptake by APC in vitro and in vivo, and showed enhanced lymph node retention up to at least 48 h. Both parameters are helpful for vaccine success. This suggests that enhanced APC uptake of NPs mediated by oligonucleotide display may help vaccine development overcoming delivery barriers [190].
4.2. Non-carbon-based nanoparticles
4.2.1. Porous silica NPs
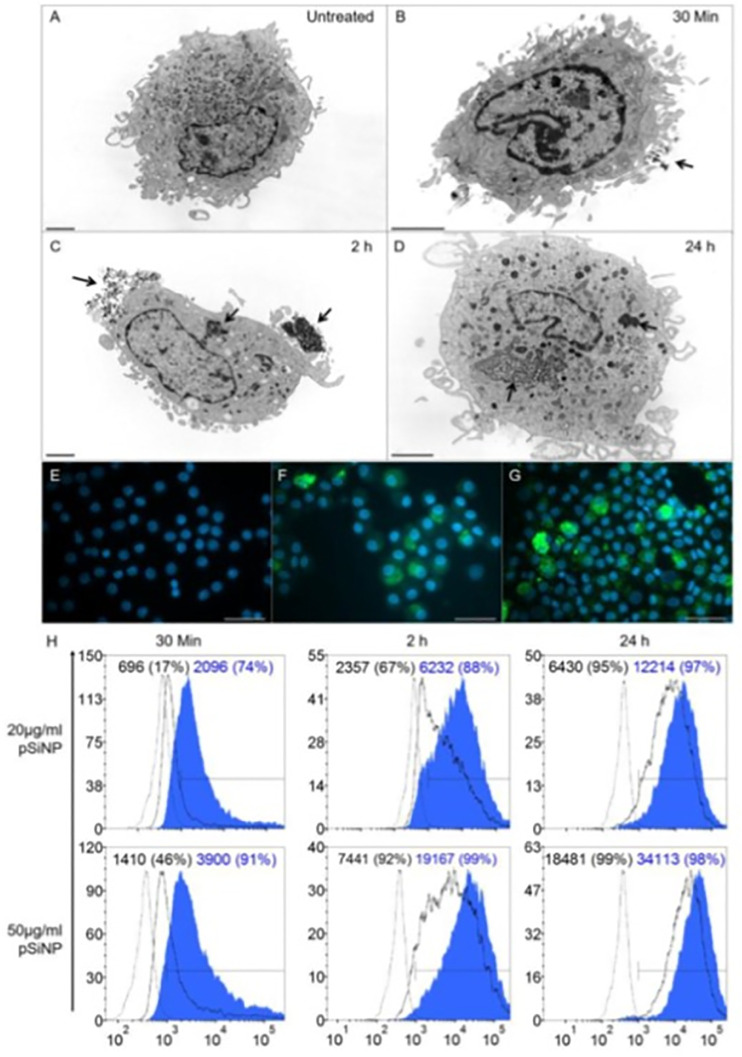
DCs are the most potent APCs and are fundamental for transplant tolerance. A possible target for DCs therapy is the DCs specific intracellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN; CD209) receptor. In this way, biodegradable porous silicon (pSi) NPs with high-surface area displaying antibodies anti-DC-SIGN and loaded with the immunosuppressant rapamycin (Sirolimus) was developed to target DCs. These NPs not only target but were phagocytosed by monocyte-derived and myeloid DCs in a time- and dose-dependent manner (Fig. 7 ). Rapamycin-loaded NPs, resulted in a maturation resistant phenotype of DCs and significantly suppressed proliferation of allogeneic T-cell [191]. Other work described that functionalized pSi NPs conjugated with a specific antibody against polysialylated neural cell adhesion molecule and loaded as a novel tool for regenerating functional neurocircuitry after stoking [192].
Fig. 7.
(A–D) TEM micrographs of mature DC (mDC). (A) Untreated mDC. (B, C, D) mDC treated with 100 μg/ml of DC-SIGN pSiNP and cultured for 30 min, 2 h and 24 h. Arrows indicate surface binding and internalization of pSiNP. Scale barre presents 2 μm. (E–G) Fluorescence microscopy of mDC. (E) Untreated mDC. mDC cultured with 100 μg/ml of FITC-labelled isotype pSiNP (F) or DCSIGNpSiNP (G) taken at 24 h. Scale bar represents 40 μm at 40× magnification. (H) Flow cytometry histograms representing NPs uptake was dependent on DC-SIGN display. Monocyte-derived DCs treated with 20 μg/ml or 50 μg/ml of isotype pSiNP (black line) or DC-SIGN pSiNP (blue shaded)at 30 min, 2 h and 24 h. (n = 9, data is representative of one blood donor). Dashed line represents untreated DC control. Histograms show mean fluorescence intensity (MFI) and % positivity in parentheses. Reproduced from [191] with permission from Elsevier.
4.3. Engineered nanoconstructions
Some nanoconstructions have a complex structure. Polyion complex vesicles (PICsomes) are polymeric hollow capsules. They are versatile platform for drug-loaded nano-formulation composed by a semipermeable membrane. Goto et al. (2017), successfully encapsulated MSNs into the PICsomes (MSN@PICsome). MSN@PICsome was stably under physiological condition and mice blood circulation. Furthermore, the surface of MSN in MSN@PICsome can be modified, obtaining amino-functionalized and sulfonate-functionalized MSN@PICsomes (A-MSN@PICsome and S-MSN@PICsome, respectively). Both surface-modified MSN@PICsomes were successfully loaded with charged water-soluble low-molecular-weight compounds (LMWCs). Particularly, S-MSN@PICsome with gemcitabine (GEM) released it in a continued manner. GEM-loaded S-MSN@PICsome demonstrated marked cytotoxicity against cultured tumor cells, and suppressed the growth of lung tumor in in vivo model [193]. Furthermore, Battistella et al., (2019), reported that covalently packaging low molecular weight immunotherapeutics in enzyme-responsive NPs maintains drug efficacy while decreasing immunotoxicity. Indeed, after parent administration of the immunotherapeutic small molecule (1V209), mice experience significantly increased plasma levels of proinflammatory cytokines IP-10, IL-6, and MCP-1, while no increase was observed with the nanomaterial, thus, providing an interesting platform for cancer immunotherapeutic delivery [194].
Mueller et al. (2015) developed a vaccine delivery platform based on PRINT hydrogels of biocompatible hydroxy-poly(ethylene glycol) (PEG). This platform was able to activate the alternative pathway of complement system. These lymph node-targeting NPs showed comparable adjuvant effect to Alum and promoted the immunogenicity of ovalbumin, demonstrating that an antigen-specific humoral response is correlated with antigen delivery to the draining lymph nodes. The easy chemistry used in antigen conjugation to PRINT NPs confers versatility, allowing for potential application to many infectious diseases [108].
5. Some considerations to keep in mind
Until now, it has been described in many reported studies that NPs can produce an immunomodulatory phenomenon. Therefore, analyze which factors are needed to produce immunostimulant or immunosuppression effects represents a key field of research. NPs nature such as composition, size, shape, surface chemistry and protein-binding capability establishes NPs interactions, but it must be taken into account the individual difference and exposure route [195], [196]. Systemic administration of NPs favors accumulation in the organs and tissues but it should be note that the size of particles influences which will be the accumulation site [197]. The immune response is dependent on the route of NPs entrance because different cells and molecules are present in different organs such as epithelium (i.e.: DCs, macrophages) or blood, (i.e.: neutrophil, eosinophil, basophile, lymphocyte, monocyte) with different results in immune stimulation. The reticule endothelial system and the mononuclear phagocyte system clearance can be avoided by NPs < 100 nm, but those <5.5 nm can be excreted in the urine [198]. NPs-cells interaction is also determined by NPs size. In this way, Shao et al. described that DC preferentially internalize small sized particles, while larger ones are taken up by macrophages [199]. Immunity was induced by series of polystyrene nanobeads with different size. It was observed that IFN-γ induction from CD8+ T-cells was limited to 40 and 49 nm beads. Meanwhile 93–123 nm beads induced CD4+ T-cell activation and increased the levels of IL-4. These results highlight the effect of the NPs size on the cytokine balance. Thus, the size is a key factor to be considered in the development of vaccines against common human pathogens. Same NPs demonstrate that inflammatory activity increase with the size (gold NPs) but others such as iron oxide NPs and CdS/CdTe QD have the inverse behavior [200].
As discussed previously, the composition of NPs has an important participation in the type of interactions established with the immune system. Moreover, in the case of NPs with similar composition, surface properties can change the interaction with the immune system. Indeed, various NPs (i.e.: silica, GNPs, CNTs, fullerenes) can be grafted with different moieties on their surface, that modifies the immune response induced [201]. Furthermore, other important factor is the charge of NPs; this can be modified with surface coatings and, in this way, tune their interaction with the immune system. Positive particles would primarily elicit a Th1 cells response and would be more readily captured by DCs [202], [203], [204]. On the other way, negative particles are associated with MARCO, a scavenger receptor of macrophages [205]. NPs shape and structure are also determinant for the uptake by cells and immune system interaction [206], [207], [208]. Finally, biomolecules NPs interaction are important for corona formation and further effects on cellular uptake and biodistribution. Protein corona formation also depends on NPs size and charge [209], [210]. Plasma proteins are able to bind NPs surfaces, contributing to the interaction with receptors and the consequent activation/deactivation of cells [211], [212]. Definitively, the interactions of cells with NPs and consequently the biological responses generated are affected by the amount and composition of proteins adsorbed on the NPs surface [213], [214]. The induced immune response by NPs could be of great utility in nanomedicine applications such as bone [215] or periodontal tissue regeneration [216]. Increasing the knowledge about immune response and their interaction with NPs is crucial for NPs immunomodulation therapies development.
6. Conclusion
The development of NPs with potential therapeutic uses is an area of vast interest in the scientific community during the last years. Recently, the pandemic caused by COVID-19 motivated a race for the development of vaccines to overcome the crisis generated. In this sense, some of the most advanced developments under study are based on NPs [217], [218], [219]. This is a good demonstration that nanotechnology will most likely be the basis of future immunotherapy. Indeed, NPs have been used for a variety of therapeutic applications. A great number of delivery systems based on NPs design have been explored to improve the safety and effectiveness of drugs. The fundamental advantages of NPs are: i) targeted delivery to the immune system, ii) improved delivery of water-insoluble drugs, iii) co-delivery of two or more drugs for combined therapy, iv) gene silencing by gene therapy, and v) theragnostic uses. In the last years, the number of publications based on these systems has increased and it is expected that there will be many developments soon that go on to experimentation in clinical stages.
In this review we have described many of the advances that have been developed in order to obtain novel tools for the treatment of allergies, autoimmune diseases and for their use in vaccines. Today many classes of NPs are under study to develop, in the near future, new therapeutic strategies that employ our own immune system in order to direct it to a specific objective, while minimizing the adverse effects present in many of the current treatments.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
E. G. is grateful for his doctoral fellowship granted by CONICET. The authors would like to acknowledge the support of grants from the Universidad de Buenos Aires UBACYT 20020150100056BA and PIDAE 2019 (to M.F.D.), and from Universidad Nacional de Luján CDD-CB:075-16 and 119-18 (to M.C.D.M). Gorka Orive wish to thank the Spanish Ministry of Economy, Industry, and Competitiveness (PID2019-106094RB-I00/AEI/10.13039/501100011033) and technical assistance from the ICTS NANBIOSIS (Drug Formulation Unit, U10) at the University of the Basque Country. We also appreciate the support from the Basque Country Government (Grupos Consolidados, No ref.: IT907-16). ADP would like to acknowledge the Danish Council for Independent Research (Technology and Production Sciences, 8105–00003B) and the VIDI research programme with project number R0004387, which is (partly) financed by the Netherlands Organization for Scientific Research (NWO).
References
- 1.Medzhitov R., Janeway C.A. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. doi: 10.1016/S0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson L.B. The immune system. Essays Biochem. 2016;60:275–301. doi: 10.1042/EBC20160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flajnik M.F., Du Pasquier L. Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol. 2004;25:640–644. doi: 10.1016/j.it.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Ren H., He Y., Liang J., Cheng Z., Zhang M., Zhu Y., Hong C., Qin J., Xu X., Wang J. Role of liposome size, surface charge, and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl. Mater. Interfaces. 2019;11:20304–20315. doi: 10.1021/acsami.8b22693. [DOI] [PubMed] [Google Scholar]
- 5.Cheng T., Zhang Y., Liu J., Ding Y., Ou H., Huang F., An Y., Liu Y., Liu J., Shi L. Ligand-switchable micellar nanocarriers for prolonging circulation time and enhancing targeting efficiency. ACS Appl. Mater. Interfaces. 2018;10:5296–5304. doi: 10.1021/acsami.7b18137. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Dai R., Wei Q., Li W., Zhu G., Chi H., Guo Z., Wang L., Cui C., Xu J., Ma K. Dual-functionalized janus mesoporous silica nanoparticles with active targeting and charge reversal for synergistic tumor-targeting therapy. ACS Appl. Mater. Interfaces. 2019;11:44582–44592. doi: 10.1021/acsami.9b15434. [DOI] [PubMed] [Google Scholar]
- 7.Hajiali H., Ouyang L., Llopis-Hernandez V., Dobre O., Rose F.R.A.J. Review of emerging nanotechnology in bone regeneration: progress, challenges, and perspectives. Nanoscale. 2021 doi: 10.1039/D1NR01371H. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y., Zhang M., Zeng F., Jin H., Xu Q., Huang Y. Dual-targeting magnetic PLGA nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy. ACS Appl. Mater. Interfaces. 2016;8:32159–32169. doi: 10.1021/acsami.6b10175. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Jayakumar M.K.G., Shikha S., Zhang Y., Zheng X., Zhang Y. Modularly assembled upconversion nanoparticles for orthogonally controlled cell imaging and drug delivery. ACS Appl. Mater. Interfaces. 2020;12:12549–12556. doi: 10.1021/acsami.0c00672. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020 doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner A.M., Knipe J.M., Orive G., Peppas N.A. Quantum dots in biomedical applications. Acta Biomater. 2019;94:44–63. doi: 10.1016/j.actbio.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oroojalian F., Beygi M., Baradaran B., Mokhtarzadeh A., Shahbazi M.-A. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small. 2021;17 doi: 10.1002/smll.202006484. [DOI] [PubMed] [Google Scholar]
- 13.Lenders V., Koutsoumpou X., Sargsian A., Manshian B.B. Biomedical nanomaterials for immunological applications: ongoing research and clinical trials. Nanoscale Adv. 2020;2:5046–5089. doi: 10.1039/D0NA00478B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan S., Prakash G., Bal Ozturk A., Saghazadeh S., Farhan Sohail M., Seo J., Remzi Dokmeci M., Zhang Y.S., Khademhosseini A. Evolution and clinical translation of drug delivery nanomaterials. Nano Today. 2017;15:91–106. doi: 10.1016/j.nantod.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadji H., Bouchemal K. Effect of micro- and nanoparticle shape on biological processes. J. Control. Release. 2021 doi: 10.1016/j.jconrel.2021.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Swartzwelter B.J., Mayall C., Alijagic A., Barbero F., Ferrari E., Hernadi S., Michelini S., Navarro Pacheco N.I., Prinelli A., Swart E., Auguste M. Cross-species comparisons of nanoparticle interactions with innate immune systems: a methodological review. Nanomaterials. 2021;11 doi: 10.3390/nano11061528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyano D.F., Liu Y., Peer D., Rotello V.M. Modulation of immune response using engineered nanoparticle surfaces. Small. 2016;12:76–82. doi: 10.1002/smll.201502273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheiblhofer S., Machado Y., Feinle A., Thalhamer J., Hüsing N., Weiss R. Potential of nanoparticles for allergen-specific immunotherapy–use of silica nanoparticles as vaccination platform. Expert Opin. Drug Deliv. 2016;13:1777–1788. doi: 10.1080/17425247.2016.1203898. [DOI] [PubMed] [Google Scholar]
- 19.Jurj A., Braicu C., Pop L.A., Tomuleasa C., Gherman C.D., Berindan-Neagoe I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des. Devel. Ther. 2017;11:2871–2890. doi: 10.2147/DDDT.S142337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia Y., Omri A., Krishnan L., McCluskie M.J. Potential applications of nanoparticles in cancer immunotherapy. Hum. Vaccin. Immunother. 2017;13:63–74. doi: 10.1080/21645515.2016.1245251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong Y., Wang Y., Tiruthani K. Tumor immune microenvironment and nano-immunotherapeutics in colorectal cancer. Nanomed. Nanotechnol. Biol. Med. 2019;21 doi: 10.1016/j.nano.2019.102034. [DOI] [PubMed] [Google Scholar]
- 22.Lappas C.M. The immunomodulatory effects of titanium dioxide and silver nanoparticles. Food Chem. Toxicol. 2015;85:78–83. doi: 10.1016/j.fct.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Klinman D.M., Sato T., Shimosato T. Use of nanoparticles to deliver immunomodulatory oligonucleotides. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8:631–637. doi: 10.1002/wnan.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jatana S., Palmer B.C., Phelan S.J., Delouise L.A. Immunomodulatory effects of nanoparticles on skin allergy. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-03729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boraschi D., Alijagic A., Auguste M., Barbero F., Ferrari E., Hernadi S., Mayall C., Michelini S., Navarro Pacheco N.I., Prinelli A., Swart E., Swartzwelter B.J., Bastús N.G., Canesi L., Drobne D., Duschl A., Ewart M.-A., Horejs-Hoeck J., Italiani P., Kemmerling B., Kille P., Prochazkova P., Puntes V.F., Spurgeon D.J., Svendsen C., Wilde C.J., Pinsino A. Addressing nanomaterial immunosafety by evaluating innate immunity across living species. Small. 2020;16 doi: 10.1002/smll.202000598. [DOI] [PubMed] [Google Scholar]
- 26.Wu H., Wang M.-D., Liang L., Xing H., Zhang C.-W., Shen F., Huang D.-S., Yang T. Nanotechnology for hepatocellular carcinoma: from surveillance, diagnosis to management. Small. 2021;17 doi: 10.1002/smll.202005236. [DOI] [PubMed] [Google Scholar]
- 27.Moodley T., Singh M. Current stimuli-responsive mesoporous silica nanoparticles for cancer therapy. Pharmaceutics. 2021;13:71. doi: 10.3390/pharmaceutics13010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batty C.J., Heise M.T., Bachelder E.M., Ainslie K.M. Vaccine formulations in clinical development for the prevention of severe acute respiratory syndrome coronavirus 2 infection. Adv. Drug Deliv. Rev. 2021;169:168–189. doi: 10.1016/j.addr.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billingsley M.M., Singh N., Ravikumar P., Zhang R., June C.H., Mitchell M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guimarães P.P.G., Gaglione S., Sewastianik T., Carrasco R.D., Langer R., Mitchell M.J. Nanoparticles for immune cytokine TRAIL-based cancer therapy. ACS Nano. 2018;12:912–931. doi: 10.1021/acsnano.7b05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., Anderson D.G., Langer R., Blankschtein D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun B., Zhao X., Wu Y., Cao P., Movahedi F., Liu J., Wang J., Xu Z.P., Gu W. Mannose-functionalized biodegradable nanoparticles efficiently deliver DNA vaccine and promote anti-tumor immunity. ACS Appl. Mater. Interfaces. 2021;13:14015–14027. doi: 10.1021/acsami.1c01401. [DOI] [PubMed] [Google Scholar]
- 33.Bai S., Yang L.-L., Wang Y., Zhang T., Fu L., Yang S., Wan S., Wang S., Jia D., Li B., Xue P., Kang Y., Sun Z.-J., Xu Z. Prodrug-based versatile nanomedicine for enhancing cancer immunotherapy by increasing immunogenic cell death. Small. 2020;16 doi: 10.1002/smll.202000214. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R., Zhu Z., Lv H., Li F., Sun S., Li J., Lee C.-S. Immune checkpoint blockade mediated by a small-molecule nanoinhibitor targeting the PD-1/PD-L1 pathway synergizes with photodynamic therapy to elicit antitumor immunity and antimetastatic effects on breast cancer. Small. 2019;15 doi: 10.1002/smll.201903881. [DOI] [PubMed] [Google Scholar]
- 35.Gasparri A.M., Sacchi A., Basso V., Cortesi F., Freschi M., Rrapaj E., Bellone M., Casorati G., Dellabona P., Mondino A., Corti A., Curnis F. Boosting Interleukin-12 antitumor activity and synergism with immunotherapy by targeted delivery with isoDGR-tagged nanogold. Small. 2019;15 doi: 10.1002/smll.201903462. [DOI] [PubMed] [Google Scholar]
- 36.Saleh B., Dhaliwal H.K., Portillo-Lara R., Shirzaei Sani E., Abdi R., Amiji M.M., Annabi N. Local immunomodulation using an adhesive hydrogel loaded with miRNA-laden nanoparticles promotes wound healing. Small. 2019;15 doi: 10.1002/smll.201902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Zhang R., Xu Z.P. Nanoparticle-based nanomedicines to promote cancer immunotherapy: recent advances and future directions. Small. 2019;15 doi: 10.1002/smll.201900262. [DOI] [PubMed] [Google Scholar]
- 38.Lin J., Ding B., Zheng P., Li D., Wang M., Jiang F., Wang Z., Ma P. Tumor microenvironment-triggered in-situ cancer vaccines with dual immunogenic cell death inductions for elevated antitumor and antimetastatic therapy. Nanoscale. 2021 doi: 10.1039/D1NR02018H. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Guo X., Ling J., Tang Z., Huang G., He L., Chen T. Nanomedicine-based cancer immunotherapies developed by reprogramming tumor-associated macrophages. Nanoscale. 2021;13:4705–4727. doi: 10.1039/D0NR08050K. [DOI] [PubMed] [Google Scholar]
- 40.Hess K.L., Oh E., Tostanoski L.H., Andorko J.I., Susumu K., Deschamps J.R., Medintz I.L., Jewell C.M. Engineering immunological tolerance using quantum dots to tune the density of self-antigen display. Adv. Funct. Mater. 2017;27:1–11. doi: 10.1002/adfm.201700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Froimchuk E., Carey S.T., Edwards C., Jewell C.M. Self-assembly as a molecular strategy to improve immunotherapy. Acc. Chem. Res. 2020 doi: 10.1021/acs.accounts.0c00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y., Hardie J., Zhang X., Rotello V.M. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol. 2017;34:25–32. doi: 10.1016/j.smim.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobrovolskaia M.A., McNeil S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 44.Ryan J.J., Bateman H.R., Stover A., Gomez G., Norton S.K., Zhao W., Schwartz L.B., Lenk R., Kepley C.L. Fullerene nanomaterials inhibit the allergic response. J. Immunol. 2007;179:665–672. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- 45.Parween S., Gupta P.K., Chauhan V.S. Induction of humoral immune response against PfMSP-119 and PvMSP-119using gold nanoparticles along with alum. Vaccine. 2011;29:2451–2460. doi: 10.1016/j.vaccine.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Michelini S., Barbero F., Prinelli A., Steiner P., Weiss R., Verwanger T., Andosch A., Lütz-Meindl U., Puntes V.F., Drobne D., Duschl A., Horejs-Hoeck J. Gold nanoparticles (AuNPs) impair LPS-driven immune responses by promoting a tolerogenic-like dendritic cell phenotype with altered endosomal structures. Nanoscale. 2021;13:7648–7666. doi: 10.1039/D0NR09153G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiao Q., Li L., Mu Q., Zhang Q. Immunomodulation of nanoparticles in nanomedicine applications. Biomed. Res. Int. 2014;2014:1–19. doi: 10.1166/jbn.2014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsieh J.Y., Smith T.D., Meli V.S., Tran T.N., Botvinick E.L., Liu W.F. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 2017;47:14–24. doi: 10.1016/j.actbio.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.dos Santos Rodrigues B., Lakkadwala S., Kanekiyo T., Singh J. Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties. Int. J. Nanomedicine. 2019;14:6497–6517. doi: 10.2147/IJN.S215941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo M., Lewik G., Ratcliffe J.C., Choi C.H.J., Mäkilä E., Tong W.Y., Voelcker N.H. Systematic evaluation of transferrin-modified porous silicon nanoparticles for targeted delivery of doxorubicin to glioblastoma. ACS Appl. Mater. Interfaces. 2019;11:33637–33649. doi: 10.1021/acsami.9b10787. [DOI] [PubMed] [Google Scholar]
- 51.Liu Q., Wang X., Liu X., Kumar S., Gochman G., Ji Y., Liao Y.-P., Chang C.H., Situ W., Lu J., Jiang J., Mei K.-C., Meng H., Xia T., Nel A.E. Use of polymeric nanoparticle platform targeting the liver to induce treg-mediated antigen-specific immune tolerance in a pulmonary allergen sensitization model. ACS Nano. 2019;13:4778–4794. doi: 10.1021/acsnano.9b01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naskalska A., Dabrowska A., Nowak P., Szczepanski A., Jasik K., Milewska A., Ochman M., Zeglen S., Rajfur Z., Pyrc K. Novel coronavirus-like particles targeting cells lining the respiratory tract. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwiatkowski A.J., Stewart J.M., Cho J.J., Avram D., Keselowsky B.G. Nano and Microparticle emerging strategies for treatment of autoimmune diseases: multiple sclerosis and type 1 diabetes. Adv. Healthc. Mater. 2020;9 doi: 10.1002/adhm.202000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mccarthy D.P., Hunter Z.N., Chackerian B., Shea L.D., Miller S.D. Targeted immunomodulation using antigen-conjugated nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014;6:298–315. doi: 10.1002/wnan.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Getts D.R., Shea L.D., Miller S.D., King N.J.C. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015;36:419–427. doi: 10.1016/j.it.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serra P., Santamaria P. Nanoparticle-based autoimmune disease therapy. Clin. Immunol. 2015;160:3–13. doi: 10.1016/j.clim.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Ngobili T.A., Daniele M.A. Nanoparticles and direct immunosuppression. Exp. Biol. Med. 2016;241:1064–1073. doi: 10.1177/1535370216650053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gharagozloo M., Majewski S., Foldvari M. Therapeutic applications of nanomedicine in autoimmune diseases: from immunosuppression to tolerance induction, nanomedicine nanotechnology. Biol. Med. 2015;11:1003–1018. doi: 10.1016/j.nano.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell L.A., Gao J., Vander Wal R., Gigliotti A., Burchiel S.W., McDonald J.D. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol. Sci. 2007;100:203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- 60.Wang X., Podila R., Shannahan J.H., Rao A.M., Brown J.M. Intravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. Int. J. Nanomedicine. 2013;8:1733–1748. doi: 10.2147/IJN.S44211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong J. Signaling pathways implicated in carbon nanotube-induced lung inflammation. Front. Immunol. 2020;11:3250. doi: 10.3389/fimmu.2020.552613. https://www.frontiersin.org/article/10.3389/fimmu.2020.552613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tkach A.V., Shurin G.V., Shurin M.R., Kisin E.R., Ashley R., Young S., Star A., Fadeel B., Kagan V.E., Shvedova A.A. Direct effects of carbon nanotubes on dendritic cells. Am. Chem. Soc. 2011;5:5755–5762. doi: 10.1021/nn2014479.Direct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moraes A.S., Paula R.F.O., Pradella F., Santos M.P.A., Oliveira E.C., von Glehn F., Camilo D.S., Ceragioli H., Peterlevitz A., Baranauskas V., Volpini W., Farias A.S., Santos L.M.B. The suppressive effect of il-27 on encephalitogenic th17 cells induced by multiwalled carbon nanotubes reduces the severity of experimental autoimmune encephalomyelitis. CNS Neurosci. Ther. 2013;19:682–687. doi: 10.1111/cns.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jovanović B., Anastasova L., Rowe E.W., Palić D. Hydroxylated fullerenes inhibit neutrophil function in fathead minnow (Pimephales promelas rafinesque. Aquat. Toxicol. 1820;101(2011):474–482. doi: 10.1016/j.aquatox.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Yudoh K., Karasawa R., Masuko K., Kato T. Water-soluble fullerene (c60) inhibits the development of arthritis in the rat model of arthritis. Int. J. Nanomedicine. 2009;4:217–225. doi: 10.2147/ijn.s7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tosic J., Stanojevic Z., Vidicevic S., Isakovic A., Ciric D., Martinovic T., Kravic-Stevovic T., Bumbasirevic V., Paunovic V., Jovanovic S., Todorovic-Markovic B., Markovic Z., Danko M., Micusik M., Spitalsky Z., Trajkovic V. Graphene quantum dots inhibit T cell-mediated neuroinflammation in rats. Neuropharmacology. 2019;146:95–108. doi: 10.1016/j.neuropharm.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 67.Lee S.W., Park H.J., Van Kaer L., Hong S., Hong S. Graphene oxide polarizes iNKT cells for production of TGFβ and attenuates inflammation in an iNKT cell-mediated sepsis model. Sci. Rep. 2018;8:10081. doi: 10.1038/s41598-018-28396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rive C., Reina G., Wagle P., Treossi E., Palermo V., Bianco A., Delogu L.G., Rieckher M., Schumacher B. Improved biocompatibility of amino-functionalized graphene oxide in Caenorhabditis elegans. Small. 2019;15 doi: 10.1002/smll.201902699. [DOI] [PubMed] [Google Scholar]
- 69.Cao W., He L., Cao W., Huang X., Jia K., Dai J. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant. Acta Biomater. 2020;112:14–28. doi: 10.1016/j.actbio.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 70.Hardy C.L., LeMasurier J.S., Belz G.T., Scalzo-Inguanti K., Yao J., Xiang S.D., Kanellakis P., Bobik A., Strickland D.H., Rolland J.M., O’Hehir R.E., Plebanski M. Inert 50-nm polystyrene nanoparticles that modify pulmonary dendritic cell function and inhibit allergic airway inflammation. J. Immunol. 2012;188:1431–1441. doi: 10.4049/jimmunol.1100156. [DOI] [PubMed] [Google Scholar]
- 71.Tarakanchikova Y., Alzubi J., Pennucci V., Follo M., Kochergin B., Muslimov A., Skovorodkin I., Vainio S., Antipina M.N., Atkin V., Popov A., Meglinski I., Cathomen T., Cornu T.I., Gorin D.A., Sukhorukov G.B., Nazarenko I. Biodegradable nanocarriers resembling extracellular vesicles deliver genetic material with the highest efficiency to various cell types. Small. 2020;16 doi: 10.1002/smll.201904880. [DOI] [PubMed] [Google Scholar]
- 72.Fuchs A.K., Syrovets T., Haas K.A., Loos C., Musyanovych A., Mailänder V., Landfester K., Simmet T. Carboxyl- and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials. 2016;85:78–87. doi: 10.1016/j.biomaterials.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 73.Xiao J., Jiang X., Zhou Y., Sumayyah G., Zhou L., Tu B., Qin Q., Qiu J., Qin X., Zou Z., Chen C. Results of a 30-day safety assessment in young mice orally exposed to polystyrene nanoparticles. Environ. Pollut. 2022;292 doi: 10.1016/j.envpol.2021.118184. [DOI] [PubMed] [Google Scholar]
- 74.Mellor A.L., Munn D.H. Physiologic control of the functional status of Foxp3+ regulatory T cells. J. Immunol. 2011;186:4535–4540. doi: 10.4049/jimmunol.1002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang L., Lemos H.P., Li L., Li M., Chandler P.R., Baban B., McGaha T.L., Ravishankar B., Lee J.R., Munn D.H., Mellor A.L. Engineering DNA nanoparticles as immunomodulatory reagents that activate regulatory T cells. J. Immunol. 2012;188:4913–4920. doi: 10.4049/jimmunol.1103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang L., Li L., Lemos H., Chandler P.R., Pacholczyk G., Baban B., Barber G.N., Hayakawa Y., McGaha T.L., Ravishankar B., Munn D.H., Mellor A.L. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J. Immunol. 2013;191:3509–3513. doi: 10.4049/jimmunol.1301419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemos H., Huang L., Chandler P.R., Mohamed E., Souza G.R., Li L., Pacholczyk G., Barber G.N., Hayakawa Y., Munn D.H., Mellor A.L. Activation of the STING adaptor attenuates experimental autoimmune encephalitis. J. Immunol. 2014;192:5571–5578. doi: 10.4049/jimmunol.1303258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lemos H., Huang L., McGaha T., Mellor A.L. STING, nanoparticles, autoimmune disease and cancer: a novel paradigm for immunotherapy? Expert. Rev. Clin. Immunol. 2014;11:155–165. doi: 10.1586/1744666X.2015.995097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qu Y., Ju Y., Cortez-Jugo C., Lin Z., Li S., Zhou J., Ma Y., Glab A., Kent S.J., Cavalieri F., Caruso F. Template-mediated assembly of DNA into microcapsules for immunological modulation. Small. 2020;16:2002750. doi: 10.1002/smll.202002750. [DOI] [PubMed] [Google Scholar]
- 80.Durbin J.K., Miller D.K., Niekamp J., Khisamutdinov E.F. Modulating immune response with nucleic acid nanoparticles. Molecules. 2019;24 doi: 10.3390/molecules24203740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sumbayev V.V., Yasinska I.M., Garcia C.P., Gilliland D., Lall G.S., Gibbs B.F., Bonsall D.R., Varani L., Rossi F., Calzolai L. Gold nanoparticles downregulate interleukin-1β-induced pro-inflammatory responses. Small. 2013;9:472–477. doi: 10.1002/smll.201201528. [DOI] [PubMed] [Google Scholar]
- 82.Leonavičienė L., Kirdaite G., Bradunaite R., Vaitkiene D., Vasiliauskas A., Zabulyte D., Ramanavičienė A., Ramanavičius A., Ašmenavičius T., Mackiewicz Z. Effect of gold nanoparticles in the treatment of established collagen arthritis in rats. Medicine. 2012;48:91–101. doi: 10.4324/9780203841273. [DOI] [PubMed] [Google Scholar]
- 83.Swartzwelter B.J., Barbero F., Verde A., Mangini M., Pirozzi M., De Luca A.C., Puntes V.F., Leite L.C.C., Italiani P., Boraschi D. Gold nanoparticles modulate BCG-induced innate immune memory in human monocytes by shifting the memory response towards tolerance. Cells. 2020;9 doi: 10.3390/cells9020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirst S.M., Karakoti A.S., Tyler R.D., Sriranganathan N., Seal S., Reilly C.M. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5:2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 85.Schanen B.C., Das S., Reilly C.M., Warren W.L., Self W.T., Seal S., Drake D.R. Immunomodulation and T helper TH1/TH2Response polarization by CeO2and TiO2Nanoparticles. PLoS One. 2013;8:16–26. doi: 10.1371/journal.pone.0062816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eitan E., Hutchison E.R., Greig N.H., Tweedie D., Celik H., Ghosh S., Fishbein K.W., Spencer R.G., Sasaki C.Y., Ghosh P., Das S., Chigurapati S., Raymick J., Sarkar S., Chigurupati S., Seal S., Mattson M.P. Combination therapy with lenalidomide and nanoceria ameliorates CNS autoimmunity. Exp. Neurol. 2015;273:151–160. doi: 10.1016/j.expneurol.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Domala A., Bale S., Godugu C. Protective effects of nanoceria in imiquimod induced psoriasis by inhibiting the inflammatory responses. Nanomedicine. 2019;15:5–22. doi: 10.2217/nnm-2018-0515. [DOI] [PubMed] [Google Scholar]
- 88.Lovrić J., Cho S.J., Winnik F.M., Maysinger D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem. Biol. 2005;12:1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Nguyen K.C., Seligy V.L., Tayabali A.F. Cadmium telluride quantum dot nanoparticle cytotoxicity and effects on model immune responses to Pseudomonas aeruginosa. Nanotoxicology. 2013;7:202–211. doi: 10.3109/17435390.2011.648667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Byung-Chul L., Young L.J., Juhee K., Min Y.J., Insung K., Jae-Jun K., Nari S., Jin K.D., Won C.S., Donghoon K., Hee H.B., Kyung-Sun K. Graphene quantum dots as anti-inflammatory therapy for colitis. Sci. Adv. 2021;6 doi: 10.1126/sciadv.aaz2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoshino A., Hanada S., Manabe N., Nakayama T., Yamamoto K. Nanocrystal quantum dots in vitro and in vivo, 52. IEEE Trans. Nanobiosci. 2009;8:51–57. doi: 10.1109/TNB.2009.2016550. [DOI] [PubMed] [Google Scholar]
- 92.Bruneau A., Fortier M., Gagne F., Gagnon C., Turcotte P., Tayabali A., Davis T.L., Auffret M., Fournier M. Size distribution effects of cadmium tellurium quantum dots (CdS/CdTe) immunotoxicity on aquatic organisms. Environ Sci Process Impacts. 2013;15:596–607. doi: 10.1039/c2em30896g. [DOI] [PubMed] [Google Scholar]
- 93.Liang Y., Zhang T., Tang M. Toxicity of quantum dots on target organs and immune system. J. Appl. Toxicol. 2022;42:17–40. doi: 10.1002/jat.4180. [DOI] [PubMed] [Google Scholar]
- 94.Latha S., Lomada D., Dharani P.K., Muthukonda S.V., Reddy M.C. Ti-O based nanomaterials ameliorate experimental autoimmune 2 encephalomyelitis and collagen-induced arthritis. RSC Adv. 2016;6:8870–8880. doi: 10.1039/C5RA18974H. [DOI] [Google Scholar]
- 95.Cao X., Han Y., Gu M., Du H., Song M., Zhu X., Ma G., Pan C., Wang W., Zhao E., Goulette T., Yuan B., Zhang G., Xiao H. Foodborne titanium dioxide nanoparticles induce stronger adverse effects in obese mice than non-obese mice: gut microbiota dysbiosis, colonic inflammation, and proteome alterations. Small. 2020;16 doi: 10.1002/smll.202001858. [DOI] [PubMed] [Google Scholar]
- 96.Lamas B., Martins Breyner N., Houdeau E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: potential consequences for host health. Part. Fibre Toxicol. 2020;17:19. doi: 10.1186/s12989-020-00349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mebert A.M., Baglole C.J., Desimone M.F., Maysinger D. Nanoengineered silica: properties, applications and toxicity. Food Chem. Toxicol. 2017;109:753–770. doi: 10.1016/j.fct.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 98.Foglia M.L., Alvarez G.S., Catalano P.N., Mebert A.M., Diaz L.E., Coradin T., Desimone M.F. Recent patents on the synthesis and application of silica nanoparticles for drug delivery. Recent Pat. Biotechnol. 2011:54–61. doi: 10.2174/187220811795655887. [DOI] [PubMed] [Google Scholar]
- 99.Alvarez Echazú M., Renou S., Alvarez G., Desimone M., Olmedo D. A collagen-silica-based biocomposite for potential application in bone tissue engineering. J. Biomed. Mater. Res. Part A. 2022;110:331–340. doi: 10.1002/jbm.a.37291. [DOI] [PubMed] [Google Scholar]
- 100.Alvarez Echazú M.I., Olivetti C.E., Peralta I., Alonso M.R., Anesini C., Perez C.J., Alvarez G.S., Desimone M.F. Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata cav. Extract with antioxidant activity. Colloids Surf. B: Biointerfaces. 2018;169:82–91. doi: 10.1016/j.colsurfb.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 101.Belen S.M., Sofía N.T., Romina M., Belén A.M., Santiago C., María Julieta F.L., Pablo R., Cristina V., Martín D., Mauricio D.M., Emilio M., Marisa F. Optimized surface plasmon resonance immunoassay for staphylococcal enterotoxin G detection using silica nanoparticles. Biochem. Biophys. Res. Commun. 2021;558:168–174. doi: 10.1016/j.bbrc.2021.04.077. [DOI] [PubMed] [Google Scholar]
- 102.Janjua T.I., Cao Y., Yu C., Popat A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021;6:1072–1074. doi: 10.1038/s41578-021-00385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mebert A.M., Camporotondi D.E., Foglia M.L., Alvarez G.S., Orihuela P.L.S., Diaz L.E., Desimone M.F. Controlling the interaction between cells and silica nanoparticles. J. Biomater. Tissue Eng. 2013;3:108–121. doi: 10.1166/jbt.2013.1069. [DOI] [Google Scholar]
- 104.Alvarez Echazú M.I., Tuttolomondo M.V., Foglia M.L., Mebert A.M., Alvarez G.S., Desimone M.F. Advances in collagen, chitosan and silica biomaterials for oral tissue regeneration: from basics to clinical trials. J. Mater. Chem. B. 2016;4:6913–6929. doi: 10.1039/c6tb02108e. [DOI] [PubMed] [Google Scholar]
- 105.Santo-Orihuela P.L., Foglia M.L., Targovnik A.M., Miranda M.V., Desimone M.F. Nanotoxicological effects of SiO2 nanoparticles on Spodoptera frugiperda Sf9 cells. Curr. Pharm. Biotechnol. 2016;17:465–470. doi: 10.2174/138920101705160303165604. [DOI] [PubMed] [Google Scholar]
- 106.De Marzi M.C., Saraceno M., Mitarotonda R., Todone M., Fernandez M., Malchiodi E.L., Desimone M.F. Evidence of size-dependent effect of silica micro-and nano-particles on basal and specialized monocyte functions. Ther. Deliv. 2017;8:1035–1049. doi: 10.4155/tde-2017-0053. [DOI] [PubMed] [Google Scholar]
- 107.Mitarotonda R., Saraceno M., Todone M., Giorgi E., Malchiodi E.L., Desimone M.F., De Marzi M.C. Surface chemistry modification of silica nanoparticles alters the activation of monocytes. Ther. Deliv. 2021;12:443–459. doi: 10.4155/tde-2021-0006. [DOI] [PubMed] [Google Scholar]
- 108.Mueller S.N., Tian S., Desimone J.M. Rapid and persistent delivery of antigen by lymph node targeting PRINT nanoparticle vaccine carrier to promote humoral immunity. Mol. Pharm. 2015;12:1356–1365. doi: 10.1021/mp500589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gojova A., Guo B., Kota R.S., Rutledge J.C., Kennedy I.M., Barakat A.I. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ. Health Perspect. 2007;115:403–409. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ban M., Langonné I., Huguet N., Goutet M. Effect of submicron and nano-iron oxide particles on pulmonary immunity in mice. Toxicol. Lett. 2012;210:267–275. doi: 10.1016/j.toxlet.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 111.Ban M., Langonné I., Huguet N., Guichard Y., Goutet M. Iron oxide particles modulate the ovalbumin-induced Th2 immune response in mice. Toxicol. Lett. 2013;216:31–39. doi: 10.1016/j.toxlet.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 112.Shen C.C., Liang H.J., Wang C.C., Liao M.H., Jan T.R. Iron oxide nanoparticles suppressed T helper 1 cell-mediated immunity in a murine model of delayed-type hypersensitivity. Int. J. Nanomedicine. 2012;7:2729–2737. doi: 10.2147/IJN.S31054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pedro L., Harmer Q., Mayes E., Shields J.D. Impact of locally administered carboxydextran-coated super-paramagnetic iron nanoparticles on cellular immune function. Small. 2019;15 doi: 10.1002/smll.201900224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shah A., Dobrovolskaia M.A. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: therapeutic benefits, toxicity, mechanistic insights, and translational considerations, nanomedicine nanotechnology. Biol. Med. 2018;14:977–990. doi: 10.1016/j.nano.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hsiao Y.-P., Shen C.-C., Huang C.-H., Lin Y.-C., Jan T.-R. Iron oxide nanoparticles attenuate T helper 17 cell responses in vitro and in vivo. Int. Immunopharmacol. 2018;58:32–39. doi: 10.1016/j.intimp.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 116.Surendran S.P., Moon M.J., Park R., Jeong Y.Y. Bioactive nanoparticles for cancer immunotherapy. Int. J. Mol. Sci. 2018;19:3877. doi: 10.3390/ijms19123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Y., Crawford B.M., Vo-Dinh T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy. 2018;10:1175–1188. doi: 10.2217/imt-2018-0029. [DOI] [PubMed] [Google Scholar]
- 119.Zang X., Zhao X., Hu H., Qiao M., Deng Y., Chen D. Nanoparticles for tumor immunotherapy. Eur. J. Pharm. Biopharm. 2017;115:243–256. doi: 10.1016/j.ejpb.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 120.Peters E.B., Tsihlis N.D., Karver M.R., Chin S.M., Musetti B., Ledford B.T., Bahnson E.M., Stupp S.I., Kibbe M.R. Atheroma niche-responsive nanocarriers for immunotherapeutic delivery. Adv. Healthc. Mater. 2019;8 doi: 10.1002/adhm.201801545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu L., Wu J., Gao J., Lu X. Bacteria-derived nanoparticles: multifunctional Containers for Diagnostic and Therapeutic Applications. Adv. Healthc. Mater. 2020;9:2000893. doi: 10.1002/adhm.202000893. [DOI] [PubMed] [Google Scholar]
- 122.Wang S., Sun Z., Hou Y. Engineering nanoparticles toward the modulation of emerging cancer immunotherapy. Adv. Healthc. Mater. 2021;10 doi: 10.1002/adhm.202000845. [DOI] [PubMed] [Google Scholar]
- 123.Cheng H.-W., Tsao H.-Y., Chiang C.-S., Chen S.-Y. Advances in magnetic nanoparticle-mediated cancer immune-theranostics. Adv. Healthc. Mater. 2021;10 doi: 10.1002/adhm.202001451. [DOI] [PubMed] [Google Scholar]
- 124.Phillips B., Nylander K., Harnaha J., Machen J., Lakomy R., Styche A., Gillis K., Brown L., Lafreniere D., Gallo M., Knox J., Hogeland K., Trucco M., Giannoukakis N. Vol. 57. 2008. Phillips_micro vaccine against diabetes_2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leuschner F., Dutta P., Gorbatov R., Novobrantseva T.I., Donahoe J.S., Courties G., Lee K.M., Kim J.I., Markmann J.F., Marinelli B., Panizzi P., Lee W.W., Iwamoto Y., Milstein S., Epstein-Barash H., Cantley W., Wong J., Cortez-Retamozo V., Newton A., Love K., Libby P., Pittet M.J., Swirski F.K., Koteliansky V., Langer R., Weissleder R., Anderson D.G., Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang P., Yigit M.V., Ran C., Ross A., Wei L., Dai G., Medarova Z., Moore A. A theranostic small interfering RNA nanoprobe protects pancreatic islet grafts from adoptively transferred immune rejection. Diabetes. 2012;61:3247–3254. doi: 10.2337/db12-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ramelli S.C., Comer B.S., McLendon J.M., Sandy L.L., Ferretti A.P., Barrington R., Sparks J., Matar M., Fewell J., Gerthoffer W.T. Nanoparticle delivery of anti-inflammatory LNA oligonucleotides prevents airway inflammation in a HDM model of asthma. Mol. Ther. - Nucleic Acids. 2020;19:1000–1014. doi: 10.1016/j.omtn.2019.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mehta M., Deeksha D., Tewari G., Gupta R., Awasthi H., Singh P., Pandey D.K., Chellappan R., Wadhwa T., Collet P.M., Hansbro S.R., Kumar L., Thangavelu P., Negi K., Dua S. Satija, oligonucleotide therapy: an emerging focus area for drug delivery in chronic inflammatory respiratory diseases. Chem. Biol. Interact. 2019;308:206–215. doi: 10.1016/j.cbi.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shah M., Edman M.C., Janga S.R., Shi P., Dhandhukia J., Liu S., Louie S.G., Rodgers K., MacKay J.A., Hamm-Alvarez S.F. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjögren’s syndrome. J. Control. Release. 2013;171:269–279. doi: 10.1016/j.jconrel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang X., Xing L., Wang Y., Zhou T.-J., Shen L.-J., Jiang H.-L. Nanoengineered immunosuppressive therapeutics modulating M1/M2 macrophages into the balanced status for enhanced idiopathic pulmonary fibrosis therapy. Nanoscale. 2020;12:8664–8678. doi: 10.1039/D0NR00750A. [DOI] [PubMed] [Google Scholar]
- 131.Yao C.G., Martins P.N. Nanotechnology applications in transplantation medicine. Transplantation. 2020:104. doi: 10.1097/TP.0000000000003032. https://journals.lww.com/transplantjournal/Fulltext/2020/04000/Nanotechnology_Applications_in_Transplantation.9.aspx [DOI] [PubMed] [Google Scholar]
- 132.Tsai S., Shameli A., Yamanouchi J., Clemente-Casares X., Wang J., Serra P., Yang Y., Medarova Z., Moore A., Santamaria P. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 133.Clemente-Casares X., Blanco J., Ambalavanan P., Yamanouchi J., Singha S., Fandos C., Tsai S., Wang J., Garabatos N., Izquierdo C., Agrawal S., Keough M.B., Yong V.W., James E., Moore A., Yang Y., Stratmann T., Serra P., Santamaria P. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 134.Singha S., Shao K., Yang Y., Clemente-Casares X., Solé P., Clemente A., Blanco J., Dai Q., Song F., Liu S.W., Yamanouchi J., Umeshappa C.S., Nanjundappa R.H., Detampel P., Amrein M., Fandos C., Tanguay R., Newbigging S., Serra P., Khadra A., Chan W.C.W., Santamaria P. Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat. Nanotechnol. 2017;12:701–710. doi: 10.1038/nnano.2017.56. [DOI] [PubMed] [Google Scholar]
- 135.Yeste A., Nadeau M., Burns E.J., Weiner H.L., Quintana F.J. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. 2012;109:11270–11275. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yeste A., Takenaka M.C., Mascanfroni I.D., Nadeau M., Kenison J.E., Patel B., Tukpah A.M., Babon J.A.B., Denicola M., Kent S.C., Pozo D., Quintana F.J. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci. Signal. 2016;9 doi: 10.1126/scisignal.aad0612. [DOI] [PubMed] [Google Scholar]
- 137.Maldonado R.A., LaMothe R.A., Ferrari J.D., Zhang A.-H., Rossi R.J., Kolte P.N., Griset A.P., O’Neil C., Altreuter D.H., Browning E., Johnston L., Farokhzad O.C., Langer R., Scott D.W., von Andrian U.H., Kishimoto T.K. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl. Acad. Sci. 2015;112:E156–E165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang A.H., Rossi R.J., Yoon J., Wang H., Scott D.W. Tolerogenic nanoparticles to induce immunologic tolerance: prevention and reversal of FVIII inhibitor formation. Cell. Immunol. 2016;301:74–81. doi: 10.1016/j.cellimm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 139.Macauley M.S., Pfrengle F., Rademacher C., Nycholat C.M., Gale A.J., Von Drygalski A., Paulson J.C. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J. Clin. Invest. 2013;123:3074–3083. doi: 10.1172/JCI69187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Copp J.A., Fang R.H., Luk B.T., Hu C.-M.J., Gao W., Zhang K., Zhang L. Clearance of pathological antibodies using biomimetic nanoparticles. Proc. Natl. Acad. Sci. 2014;111:13481–13486. doi: 10.1073/pnas.1412420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smarr C.B., Yap W.T., Neef T.P., Pearson R.M., Hunter Z.N., Ifergan I., Getts D.R., Bryce P.J., Shea L.D., Miller S.D. Biodegradable antigen-associated PLG nanoparticles tolerize Th2-mediated allergic airway inflammation pre- and postsensitization. Proc. Natl. Acad. Sci. 2016;113:5059–5064. doi: 10.1073/pnas.1505782113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hunter Z., McCarthy D.P., Yap W.T., Harp C.T., Getts D.R., Shea L.D., Miller S.D. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8:2148–2160. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McCarthy D.P., Yap J.W.T., Harp C.T., Song W.K., Chen J., Pearson R.M., Miller S.D., Shea L.D. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy, nanomedicine nanotechnology. Biol. Med. 2017;13:191–200. doi: 10.1016/j.nano.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Getts D.R., Martin A.J., Mccarthy D.P., Terry R.L., Hunter Z.N., Yap W.T., Getts M.T., Pleiss M., Luo X., King N.J.C., Shea L.D., Miller S.D. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pu H.L., Chiang W.L., Maiti B., Liao Z.X., Ho Y.C., Shim M.S., Chuang E.Y., Xia Y., Sung H.W. Nanoparticles with dual responses to oxidative stress and reduced pH for drug release and anti-inflammatory applications. ACS Nano. 2014;8:1213–1221. doi: 10.1021/nn4058787. [DOI] [PubMed] [Google Scholar]
- 146.Shi S., Zhu H., Xia X., Liang Z., Ma X., Sun B. Vaccine adjuvants: understanding the structure and mechanism of adjuvanticity. Vaccine. 2019;37:3167–3178. doi: 10.1016/j.vaccine.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 147.Del Giudice G., Rappuoli R., Didierlaurent A.M. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin. Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 148.Mody K.T., Popat A., Mahony D., Cavallaro A.S., Yu C., Mitter N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale. 2013;5:5167–5179. doi: 10.1039/c3nr00357d. [DOI] [PubMed] [Google Scholar]
- 149.Pasquale A., Preiss S., Silva F., Garçon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines. 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boraschi D., Italiani P. From antigen delivery system to adjuvanticy: the board application of nanoparticles in vaccinology. Vaccines. 2015;3:930–939. doi: 10.3390/vaccines3040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun B., Wang X., Ji Z., Li R., Xia T. NLRP3 inflammasome activation induced by engineered nanomaterials. Small. 2013;9:1595–1607. doi: 10.1002/smll.201201962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim M.G., Park J.Y., Shon Y., Kim G., Shim G., Oh Y.K. Nanotechnology and vaccine development. Asian J. Pharm. Sci. 2014;9:227–235. doi: 10.1016/j.ajps.2014.06.002. [DOI] [Google Scholar]
- 153.Durán-Lobato M., López-Estévez A.M., Cordeiro A.S., Dacoba T.G., Crecente-Campo J., Torres D., Alonso M.J. Nanotechnologies for the delivery of biologicals: historical perspective and current landscape. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113899. [DOI] [PubMed] [Google Scholar]
- 154.Almeida J.P.M., Figueroa E.R., Drezek R.A. Gold nanoparticle mediated cancer immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2014;10:503–514. doi: 10.1016/j.nano.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.O’Hagan D.T., Fox C.B. New generation adjuvants - from empiricism to rational design. Vaccine. 2015;33:B14–B20. doi: 10.1016/j.vaccine.2015.01.088. [DOI] [PubMed] [Google Scholar]
- 156.Vu M.N., Kelly H.G., Wheatley A.K., Peng S., Pilkington E.H., Veldhuis N.A., Davis T.P., Kent S.J., Truong N.P. Cellular interactions of liposomes and PISA nanoparticles during human blood flow in a microvascular network. Small. 2020;16 doi: 10.1002/smll.202002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Leleux J., Roy K. Micro and nanoparticle-based delivery Systems for Vaccine Immunotherapy: an immunological and materials perspective. Adv. Healthc. Mater. 2013;2:72–94. doi: 10.1002/adhm.201200268. [DOI] [PubMed] [Google Scholar]
- 158.Zhao L., Seth A., Wibowo N., Zhao C.X., Mitter N., Yu C., Middelberg A.P.J. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 159.Li Y., Liu W., Sun C., Zheng M., Zhang J., Liu B., Wang Y., Xie Z., Xu N. Hybrids of carbon dots with subunit B of ricin toxin for enhanced immunomodulatory activity. J. Colloid Interface Sci. 2018;523:226–233. doi: 10.1016/j.jcis.2018.03.108. [DOI] [PubMed] [Google Scholar]
- 160.Pimentel L.S., Turini C.A., Santos P.S., de Morais M.A., Souza A.G., Barbosa M.B., Coutinho L.B., Furtado C.A., Ladeira L.O., Martins J.R., Goulart L.R., de Faria P.C.B., Martins E.M.do N. Balanced Th1/Th2 immune response induced by MSP1a functional motif coupled to multiwalled carbon nanotubes as anti-anaplasmosis vaccine in murine model. Nanomed. Nanotechnol. Biol. Med. 2020;24 doi: 10.1016/j.nano.2019.102137. [DOI] [PubMed] [Google Scholar]
- 161.Ahmed K.S., Hussein S.A., Ali A.H., Korma S.A., Lipeng Q., Jinghua C. Liposome: composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019;27:742–761. doi: 10.1080/1061186X.2018.1527337. [DOI] [PubMed] [Google Scholar]
- 162.Nordly P., Korsholm K.S., Pedersen E.A., Khilji T.S., Franzyk H., Jorgensen L., Nielsen H.M., Agger E.M., Foged C. Incorporation of a synthetic mycobacterial monomycoloyl glycerol analogue stabilizes dimethyldioctadecylammonium liposomes and potentiates their adjuvant effect in vivo. Eur. J. Pharm. Biopharm. 2011;77:89–98. doi: 10.1016/j.ejpb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 163.Zhao Z., Ma X., Zhang R., Hu F., Zhang T., Liu Y., Han M.H., You F., Yang Y., Zheng W. A novel liposome-polymer hybrid nanoparticles delivering a multi-epitope self-replication DNA vaccine and its preliminary immune evaluation in experimental animals. Nanomed. Nanotechnol. Biol. Med. 2021;35 doi: 10.1016/j.nano.2020.102338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wong T.M., Petrovsky N., Bissel S.J., Wiley C.A., Ross T.M. Delta inulin-derived adjuvants that elicit Th1 phenotype following vaccination reduces respiratory syncytial virus lung titers without a reduction in lung immunopathology. Hum. Vaccin. Immunother. 2016;12:2096–2105. doi: 10.1080/21645515.2016.1162931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.R. S, AD M., YS L., RK S., GJ R. Oral deliverable mucoadhesive chitosan-salmonella subunit nanovaccine for layer chickens. Int. J. Nanomedicine. 2020;15:761–777. doi: 10.2147/IJN.S238445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bawage S.S., Tiwari P.M., Singh A., Dixit S., Pillai S.R., Dennis V.A., Singh S.R. Gold nanorods inhibit respiratory syncytial virus by stimulating the innate immune response. Nanomed. Nanotechnol. Biol. Med. 2016;12:2299–2310. doi: 10.1016/j.nano.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li Y., Jin Q., Ding P., Zhou W., Chai Y., Li X., Wang Y., Zhang G. Gold nanoparticles enhance immune responses in mice against recombinant classical swine fever virus E2 protein. Biotechnol. Lett. 2020;42:1169–1180. doi: 10.1007/s10529-020-02853-w. [DOI] [PubMed] [Google Scholar]
- 168.Moyano D.F., Liu Y., Ayaz F., Hou S., Puangploy P., Duncan B., Osborne B.A., Rotello V.M. Immunomodulatory effects of coated gold nanoparticles in LPS-stimulated in vitro and in vivo murine model systems. Chemistry. 2016;1:320–327. doi: 10.1016/j.chempr.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jin X.-H., Zheng L.-L., Song M.-R., Xu W.-S., Kou Y.-N., Zhou Y., Zhang L.-W., Zhu Y.-N., Wan B., Wei Z.-Y., Zhang G.-P. A nano silicon adjuvant enhances inactivated transmissible gastroenteritis vaccine through activation the Toll-like receptors and promotes humoral and cellular immune responses. Nanomed. Nanotechnol. Biol. Med. 2018;14:1201–1212. doi: 10.1016/j.nano.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 170.Vivero-Escoto J.L., Slowing I.I., Trewyn B.G., Lin V.S.-Y. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6:1952–1967. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- 171.Frontera E., Desimone M.F., De Marzi M.C., Guerra L.N. N-acetylcysteine delivery with silica nanoparticles into 3T3-L1 adipocytes. Ther. Deliv. 2021;12:287–296. doi: 10.4155/tde-2020-0093. [DOI] [PubMed] [Google Scholar]
- 172.Baudou F.G., Fusco L., Giorgi E., Diaz E., Municoy S., Desimone M.F., Leiva L., De Marzi M.C. Physicochemical and biological characterization of nanovenoms, a new tool formed by silica nanoparticles and Crotalus durissus terrificus venom. Colloids Surf. B: Biointerfaces. 2020;193 doi: 10.1016/j.colsurfb.2020.111128. [DOI] [PubMed] [Google Scholar]
- 173.Guo H.-C., Feng X.-M., Sun S.-Q., Wei Y.-Q., Sun D.-H., Liu X.-T., Liu Z.-X., Luo J.-X., Yin H. Immunization of mice by hollow mesoporous silica nanoparticles as carriers of porcine circovirus type 2 ORF2 protein. Virol. J. 2012;9:108. doi: 10.1186/1743-422X-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fotooh Abadi L., Kumar P., Gajbhiye V., Paknikar K.M., Kulkarni S. Non-nuke HIV-1 inhibitor shuttled by mesoporous silica nanoparticles effectively slows down HIV-1 replication in infected human cells. Colloids Surf. B: Biointerfaces. 2020 doi: 10.1016/j.colsurfb.2020.111227. [DOI] [PubMed] [Google Scholar]
- 175.Huang X., Cavalcante D.P., Townley H.E. Macrophage-like THP-1 cells show effective uptake of silica nanoparticles carrying inactivated diphtheria toxoid for vaccination. J. Nanopart. Res. 2020;22:23. doi: 10.1007/s11051-019-4720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mercuri L.P., Carvalho L.V., Lima F.A., Quayle C., Fantini M.C.A., Tanaka G.S., Cabrera W.H., Furtado M.F.D., Tambourgi D.V., Matos J.D.R., Jaroniec M., Sant’Anna O.A. Ordered mesoporous silica SBA-15: a new effective adjuvant to induce antibody response. Small. 2006;2:254–256. doi: 10.1002/smll.200500274. [DOI] [PubMed] [Google Scholar]
- 177.Soares D.C.Ferreira, Soares L.M., de Goes A.Miranda, Melo E.M., de Barros A.L.Branco, Bicalho T.C.Alves Santos, Leao N.M., Tebaldi M.L. Mesoporous SBA-16 silica nanoparticles as a potential vaccine adjuvant against Paracoccidioides brasiliensis. Microporous Mesoporous Mater. 2020;291 doi: 10.1016/j.micromeso.2019.109676. [DOI] [Google Scholar]
- 178.Yu F., Wang J., Dou J., Yang H., He X., Xu W., Zhang Y., Hu K., Gu N. Nanoparticle-based adjuvant for enhanced protective efficacy of DNA vaccine Ag85A-ESAT-6-IL-21 against Mycobacterium tuberculosis infection. Nanomed. Nanotechnol. Biol. Med. 2012;8:1337–1344. doi: 10.1016/j.nano.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 179.Powles L., Wilson K.L., Xiang S.D., Coppel R.L., Ma C., Selomulya C., Plebanski M. Pullulan-coated iron oxide nanoparticles for blood-stage malaria vaccine delivery. Vaccines. 2020;8 doi: 10.3390/vaccines8040651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fernandes-Negreiros M., Araújo Machado R., Bezerra F., Nunes Melo M., Alves M., Alves Filgueira L., Morgano M., Trindade E., Costa L., Rocha H. Antibacterial, antiproliferative, and immunomodulatory activity of silver nanoparticles synthesized with fucans from the alga Dictyota mertensii. Nanomaterials. 2017;8:6. doi: 10.3390/nano8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sharma R.K., Cwiklinski K., Aalinkeel R., Reynolds J.L., Sykes D.E., Quaye E., Oh J., Mahajan S.D., Schwartz S.A. Immunomodulatory activities of curcumin-stabilized silver nanoparticles: efficacy as an antiretroviral therapeutic. Immunol. Investig. 2017;46:833–846. doi: 10.1080/08820139.2017.1371908. [DOI] [PubMed] [Google Scholar]
- 182.Orlowski P., Tomaszewska E., Ranoszek-Soliwoda K., Gniadek M., Labedz O., Malewski T., Nowakowska J., Chodaczek G., Celichowski G., Grobelny J., Krzyzowska M. Tannic acid-modified silver and gold nanoparticles as novel stimulators of dendritic cells activation. Front. Immunol. 2018;9:1115. doi: 10.3389/fimmu.2018.01115. https://www.frontiersin.org/article/10.3389/fimmu.2018.01115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sharma B., McLeland C.B., Potter T.M., Stern S.T., Adiseshaiah P.P. In: Assessing NLRP3 Inflammasome Activation by Nanoparticles BT - Characterization of Nanoparticles Intended for Drug Delivery. McNeil S.E., editor. Springer; New York, New York, NY: 2018. pp. 135–147. [DOI] [PubMed] [Google Scholar]
- 184.Ruwona T.B., Xu H., Li X., Taylor A.N., Shi Y., Cui Z. Toward understanding the mechanism underlying the strong adjuvant activity of aluminum salt nanoparticles. Vaccine. 2016;34:3059–3067. doi: 10.1016/j.vaccine.2016.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cibulski S.P., Rivera-Patron M., Mourglia-Ettlin G., Casaravilla C., Yendo A.C.A., Fett-Neto A.G., Chabalgoity J.A., Moreno M., Roehe P.M., Silveira F. Quillaja brasiliensis saponin-based nanoparticulate adjuvants are capable of triggering early immune responses. Sci. Rep. 2018;8:13582. doi: 10.1038/s41598-018-31995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Swaminathan G., Thoryk E.A., Cox K.S., Meschino S., Dubey S.A., Vora K.A., Celano R., Gindy M., Casimiro D.R., Bett A.J. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine. 2016;34:110–119. doi: 10.1016/j.vaccine.2015.10.132. [DOI] [PubMed] [Google Scholar]
- 187.Kuan-Wei H., Fu-Fei H., Timothy Q.J., Guann-Jen C., Yi-An L., Chih-Chun C., Yu-Ting H., Yun-Chieh S., Cheng-Chin C., Rui-Lin H., Chu-Chi L., Kieu D.T., Hsi-Chien H., Yu-Chuan S., Donia A., Chun-Yen L., Yung-Chang L., Po-Chiao C., Shu-Yi L., Yunching C. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2021;6 doi: 10.1126/sciadv.aax5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Asthana S., Jaiswal A.K., Gupta P.K., Dube A., Chourasia M.K. European journal of pharmaceutics and biopharmaceutics th-1 biased immunomodulation and synergistic antileishmanial activity of stable cationic lipid – polymer hybrid nanoparticle : biodistribution and toxicity assessment of encapsulated amphotericin B. Eur. J. Pharm. Biopharm. 2015;89:62–73. doi: 10.1016/j.ejpb.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 189.Pang J., Xing H., Sun Y., Feng S., Wang S. Non-small cell lung cancer combination therapy: hyaluronic acid modified, epidermal growth factor receptor targeted, pH sensitive lipid-polymer hybrid nanoparticles for the delivery of erlotinib plus bevacizumab. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109861. [DOI] [PubMed] [Google Scholar]
- 190.Molino N.M., Neek M., Tucker J.A., Nelson E.L., Wang S.W. Display of DNA on nanoparticles for targeting antigen presenting cells. ACS Biomater. Sci. Eng. 2017;3:496–501. doi: 10.1021/acsbiomaterials.7b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stead S.O., McInnes S.J.P., Kireta S., Rose P.D., Jesudason S., Rojas-Canales D., Warther D., Cunin F., Durand J.O., Drogemuller C.J., Carroll R.P., Coates P.T., Voelcker N.H. Manipulating human dendritic cell phenotype and function with targeted porous silicon nanoparticles. Biomaterials. 2018;155:92–102. doi: 10.1016/j.biomaterials.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 192.Balasubramanian V., Domanskyi A., Renko J.-M., Sarparanta M., Wang C.-F., Correia A., Mäkilä E., Alanen O.S., Salonen J., Airaksinen A.J., Tuominen R., Hirvonen J., Airavaara M., Santos H.A. Engineered antibody-functionalized porous silicon nanoparticles for therapeutic targeting of pro-survival pathway in endogenous neuroblasts after stroke. Biomaterials. 2020;227 doi: 10.1016/j.biomaterials.2019.119556. [DOI] [PubMed] [Google Scholar]
- 193.Goto A., Yen H.C., Anraku Y., Fukushima S., Lai P.S., Kato M., Kishimura A., Kataoka K. Facile preparation of delivery platform of water-soluble low-molecular-weight drugs based on polyion complex vesicle (PICsome) encapsulating mesoporous silica nanoparticle. ACS Biomater. Sci. Eng. 2017;3:807–815. doi: 10.1021/acsbiomaterials.6b00562. [DOI] [PubMed] [Google Scholar]
- 194.Battistella C., Callmann C.E., Thompson M.P., Yao S., Yeldandi A.V., Hayashi T., Carson D.A., Gianneschi N.C. Delivery of immunotherapeutic nanoparticles to tumors via enzyme-directed assembly. Adv. Healthc. Mater. 2019;8 doi: 10.1002/adhm.201901105. [DOI] [PubMed] [Google Scholar]
- 195.Neef T., Miller S.D. Tolerogenic nanoparticles to treat islet autoimmunity. Curr. Diab. Rep. 2017;17 doi: 10.1007/s11892-017-0914-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Youshia J., Ali M.E., Stein V., Lamprecht A. Nanoparticles’ properties modify cell type-dependent distribution in immune cells. Nanomed. Nanotechnol. Biol. Med. 2020;29 doi: 10.1016/j.nano.2020.102244. [DOI] [PubMed] [Google Scholar]
- 197.Garciafigueroa Y., Trucco M., Giannoukakis N. A brief glimpse over the horizon for type 1 diabetes nanotherapeutics. Clin. Immunol. 2015;160:36–45. doi: 10.1016/j.clim.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 198.Choi M.R., Stanton-Maxey K.J., Stanley J.K., Levin C.S., Bardhan R., Akin D., Badve S., Sturgis J., Robinson J.P., Bashir R., Halas N.J., Clare S.E. A cellular trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 199.Shao K., Singha S., Clemente-Casares X., Tsai S., Yang Y., Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 200.Fallarini S., Paoletti T., Battaglini C.O., Ronchi P., Lay L., Bonomi R., Jha S., Mancin F., Scrimin P., Lombardi G. Factors affecting T cell responses induced by fully synthetic glyco gold-nanoparticles. Nanoscale. 2013;5:390–400. doi: 10.1039/c2nr32338a. [DOI] [PubMed] [Google Scholar]
- 201.Gallud A., Delaval M., Kinaret P., Marwah V.S., Fortino V., Ytterberg J., Zubarev R., Skoog T., Kere J., Correia M., Loeschner K., Al-Ahmady Z., Kostarelos K., Ruiz J., Astruc D., Monopoli M., Handy R., Moya S., Savolainen K., Alenius H., Greco D., Fadeel B. Multiparametric profiling of engineered nanomaterials: unmasking the surface coating effect. Adv. Sci. 2020;7 doi: 10.1002/advs.202002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fromen C.A., Robbins G.R., Shen T.W., Kai M.P., Ting J.P.Y., DeSimone J.M. Controlled analysis of nanoparticle charge on mucosal and systemic antibody responses following pulmonary immunization. Proc. Natl. Acad. Sci. 2015;112:488–493. doi: 10.1073/pnas.1422923112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bal S.M., Hortensius S., Ding Z., Jiskoot W., Bouwstra J.A. Co-encapsulation of antigen and toll-like receptor ligand in cationic liposomes affects the quality of the immune response in mice after intradermal vaccination. Vaccine. 2011;29:1045–1052. doi: 10.1016/j.vaccine.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 204.Koppolu B., Zaharoff D.A. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials. 2013;34:2359–2369. doi: 10.1016/j.biomaterials.2012.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chao Y., Makale M., Karmali P.P., Sharikov Y., Tsigelny I., Merkulov S., Kesari S., Wrasidlo W., Ruoslahti E., Simberg D. Recognition of dextran-superparamagnetic iron oxide nanoparticle conjugates (feridex) via macrophage scavenger receptor charged domains. Bioconjug. Chem. 2012;23:1003–1009. doi: 10.1021/bc200685a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Stone J.W., Thornburg N.J., Blum D.L., Kuhn S.J., Wright D.W., Crowe J.E. Gold nanorod vaccine for respiratory syncytial virus. Nanotechnology. 2013;24 doi: 10.1088/0957-4484/24/29/295102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Karch C.P., Li J., Kulangara C., Paulillo S.M., Raman S.K., Emadi S., Tan A., Helal Z.H., Fan Q., Khan M.I., Burkhard P. Vaccination with self-adjuvanted protein nanoparticles provides protection against lethal influenza challenge. Nanomed. Nanotechnol. Biol. Med. 2017;13:241–251. doi: 10.1016/j.nano.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 208.Doll T.A.P.F., Neef T., Duong N., Lanar D.E., Ringler P., Müller S.A., Burkhard P. Optimizing the design of protein nanoparticles as carriers for vaccine applications. Nanomed. Nanotechnol. Biol. Med. 2015;11:1705–1713. doi: 10.1016/j.nano.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Monopoli M.P., Åberg C., Salvati A., Dawson K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 210.Lundqvist M., Stigler J., Elia G., Lynch I., Cedervall T., Dawson K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. 2008;105:14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Walczyk D., Bombelli F.B., Monopoli M.P., Lynch I., Dawson K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010;132:5761–5768. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- 212.Marano F., Hussain S., Rodrigues-Lima F., Baeza-Squiban A., Boland S. Nanoparticles: molecular targets and cell signalling. Arch. Toxicol. 2011;85:733–741. doi: 10.1007/s00204-010-0546-4. [DOI] [PubMed] [Google Scholar]
- 213.Cedervall T., Lynch I., Lindman S., Berggard T., Thulin E., Nilsson H., Dawson K.A., Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Deng Z.J., Liang M., Toth I., Monteiro M., Minchin R.F. Plasma protein binding of positively and negatively charged polymer-coated gold nanoparticles elicits different biological responses. Nanotoxicology. 2013;7:314–322. doi: 10.3109/17435390.2012.655342. [DOI] [PubMed] [Google Scholar]
- 215.Xu C., Xiao L., Cao Y., He Y., Lei C., Xiao Y., Sun W., Ahadian S., Zhou X., Khademhosseini A., Ye Q. Mesoporous silica rods with cone shaped pores modulate inflammation and deliver BMP-2 for bone regeneration. Nano Res. 2020;13:2323–2331. doi: 10.1007/s12274-020-2783-z. [DOI] [Google Scholar]
- 216.Xu C., Cao Y., Lei C., Li Z., Kumeria T., Meka A.K., Xu J., Liu J., Yan C., Luo L., Khademhosseini A., Popat A., He Y., Ye Q. Polymer-mesoporous silica nanoparticle Core-Shell nanofibers as a dual-drug-delivery system for guided tissue regeneration. ACS Appl. Nano Mater. 2020;3:1457–1467. doi: 10.1021/acsanm.9b02298. [DOI] [Google Scholar]
- 217.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O’Dell S., Schmidt S.D., Swanson P.A., Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA vaccine against SARS-CoV-2 — preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O’Dell S., Schmidt S.D., Corbett K.S., Swanson P.A., Padilla M., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.V., Floyd K., Suthar M.S., Martinez D.R., Baric R., Buchanan W., Luke C.J., Phadke V.K., Rostad C.A., Ledgerwood J.E., Graham B.S., Beigel J.H. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Farfán-Castro S., García-Soto M.J., Comas-García M., Arévalo-Villalobos J.I., Palestino G., González-Ortega O., Rosales-Mendoza S. Synthesis and immunogenicity assessment of a gold nanoparticle conjugate for the delivery of a peptide from SARS-CoV-2, nanomedicine nanotechnology. Biol. Med. 2021;34 doi: 10.1016/j.nano.2021.102372. [DOI] [PMC free article] [PubMed] [Google Scholar]