To the Editor:
The coronavirus disease 2019 (COVID-19) pandemic has heightened awareness of the primary immunodeficiency/inborn errors of immunity community, and its impact on those with immunodeficiency diseases has been reported recently.1 Management of these patients often involves administration of therapeutic immunoglobulin (IgG); however, the formation of antibodies to novel pathogens, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) lags behind plasma donor convalescence or vaccination. Confirming the presence of neutralizing antibodies has value for future immunoglobulin-based modalities and is of clear benefit to this patient population.
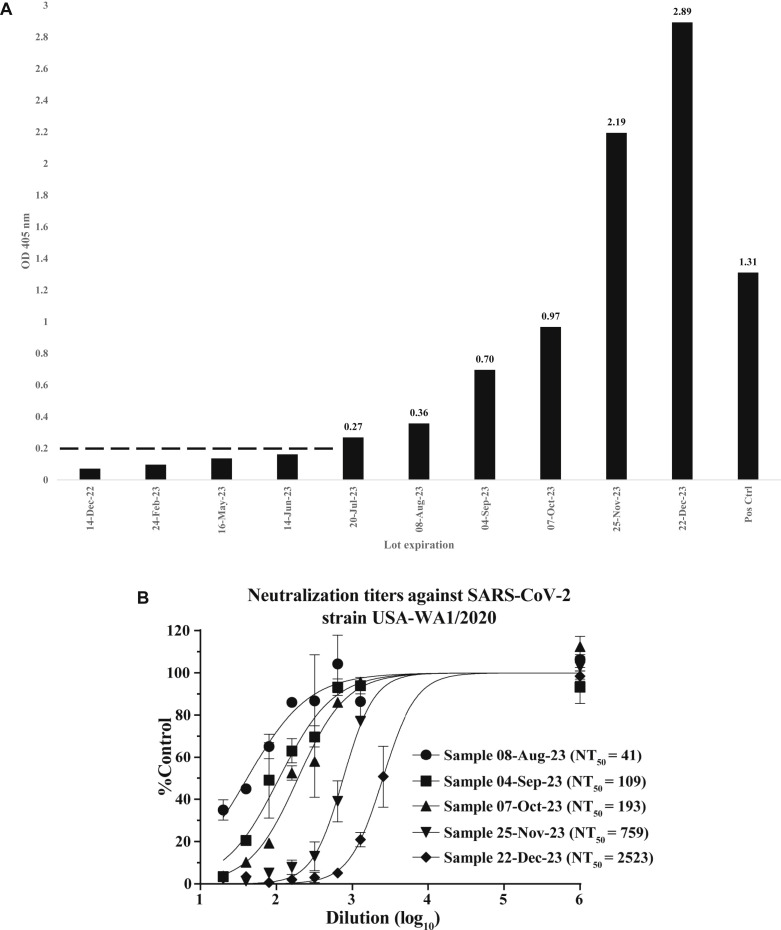
To evaluate the presence of SARS-CoV-2 antibodies in currently available preparations of Hizentra (20% liquid for subcutaneous infusion), 10 recent lots representing expiration dates encompassing 1 year were analyzed by using a SARS-CoV-2 ELISA. Our customized ELISA provided broad SARS-CoV-2 antibody detection using whole cell antigen generated from VeroE6 cells infected with the SARS-CoV-2 strain USA-WA1/2020, thus allowing for the greatest breadth of epitope coverage recognizing both natural and vaccine-elicited antibodies.2 For analysis, Hizentra samples were serially diluted to empiric ranges for detection. Positive detection was based on an OD405 nm value of 0.2 or higher. The first sequential sample to cross this threshold was lot 5 (expiration date July 20, 2023 [Fig 1 , A]). To better quantify titers, we compared OD results for the 1:1600 dilution, which showed a steady increase in titers culminating in a 10.7-fold elevation (sample 10 vs sample 5) by sample lot 10 (expiration date December 22, 2023). Interestingly, the titer for lot 10 was 2.2-fold higher than that for SARS-CoV-2 convalescent serum, which served as a positive control (Fig 1, A).
Fig 1.
SARS-CoV-2 antibody assessment of Hizentra lots. A, Ten temporal lots of Hizentra were diluted for detection within the linear range of a SARS-CoV-2–specific ELISA. Lot 1 (expiration date December 14, 2022) is the leftmost sample, and lot 10 (expiration date December 22, 2023) is the rightmost sample, followed by the positive control (de-identified SARS-CoV-2 convalescent patient serum). The results for the 1:1600 dilution are depicted, indicating relative antibody levels (OD405 nm) at lot expiration dates. The threshold for ELISA positivity is shown as a dashed line (OD405 nm ≥ 0.2) established by a 1:400 dilution (not shown). B, All 10 sample lots (lots 6 through 10 are shown) were measured for neutralizing activities against SARS-CoV-2 engineered with an mNeonGreen (mNG) fluorescent reporter virus on VeroE6 cells by fluorescent focus reduction NT (FFRNT) as described previously.3,4 Error bars indicate SDs from duplicates. The nonlinear regression curves of the relative infectivity versus the Hizentra dilutions (log10 values) were created with Prism 9 (GraphPad Software, San Diego, Calif) and used to determine the fold dilution that neutralized 50% of mNG SARS-CoV-2 infectivity (defined as FFRNT50). The calculated NT50 values are shown for sample lots 6 to 10 (expiration dates Aug 8 through December 22, 2023).
Neutralization studies were carried out by using our mNeonGreen SARS-CoV-2 fluorescent reporter system, which is equivalent to well-established plaque reduction assays.3 Hizentra lots 1 through 5 were devoid of detectable neutralization (defined as neutralization negative at a dilution of 1:20). However, by sample lot 6 (expiration date August 8, 2023), neutralization activity was detected, and it escalated in each subsequent lot from titers of 41 to 2523, culminating in a 61.5-fold increase by lot 10 (a 50% neutralization titer [NT50] of 2523 for sample 10 [expiration date December 22, 2023] vs an NT50 of 41 for sample 6 [expiration date August 8, 2023] [Fig 1, B]).
Collectively, these data confirmed the chronologic availability of SARS-CoV-2–neutralizing antibodies in Hizentra. Detection was correlated with neutralization and increased temporally, ultimately reaching impressive titer increases of 10.7-fold and 61.5-fold, respectively (Fig 1, A and B). The NTs in sample lot 10 (NT50 = 2523) were 4.2-fold higher than the reported average titer observed in 64 patient sera collected 1 month after natural infection (NT50 = 601).4
Although our study is limited in terms of both product brand and scale, we believe that it is important to expedite findings that may directly affect patients undergoing IgG replacement therapy. "Does my immunoglobulin contain SARS-CoV-2 antibodies?" is a question of intense interest that is frequently asked by both patients with primary immunodeficiency and the health care provider community. According to a recent publication devoted to current manufacturer-directed inquiries, 65% of patients and 45% of health care providers have inquired about the presence of SARS-CoV-2 antibodies in their immunoglobulin products.5 This level of inquiry warrants experimental investigation and timely reporting to answer the question. From the results of our study, the initial detection of SARS-CoV-2–neutralizing antibodies has been observed in current patient-accessible lots of the IgG therapeutic Hizentra.
Acknowledgments
We thank L.M. for supplying essential assay reagents.
Footnotes
Supported by the National Institutes of Health (grants HHSN272201600013C, AI134907, AI145617, and UL1TR001439 [to P.-Y.S.]) and awards from the Sealy & Smith Foundation, the Kleberg Foundation, the John S. Dunn Foundation, the Amon G. Carter Foundation, the Gillson-Longenbaugh Foundation, and the Summerfield Roberts Foundation (to P.-Y.S.).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest. Hizentra samples were obtained and evaluated with no input or affiliation from the manufacturer, CSL Behring.
References
- 1.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 3.Muruato A.E., Fontes-Garfias C.R., Ren P., Garcia-Blanco M.A., Menachery V.D., Xie X., et al. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun. 2020;11:4059. doi: 10.1038/s41467-020-17892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou J., Xia H., Xie X., Kurhade C., Machado R.R.G., Weaver S.C., et al. Neutralization against Omicron SARS-CoV-2 from previous non-Omicron infection. Nat Commun. 2022;13:852. doi: 10.1038/s41467-022-28544-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stinca S., Barnes T.W., Vogel P., Meyers W., Schulte-Pelkum J., Filchtinski D., et al. Modelling the concentration of anti-SARS-CoV-2 immunoglobulin G in intravenous immunoglobulin product batches. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259731. [DOI] [PMC free article] [PubMed] [Google Scholar]