Abstract
It is established that vitamin D deficiency is correlated with the disease severity in COVID-19 patients. However, the reliable and sensitive quantitation of vitamin D3 (D3) and its metabolites remains a difficult challenge. Herein, a novel ultrasensitive and reliable UHPLC-ESI-MS/MS method was developed and validated for the quantitation of D3 and its major metabolites in COVID-19 patients. The mass spectral sensitivity was augmented via controlled microwave-assisted derivatization reaction (CMDR) with 2-nitrosopyridine (Pyr-NO) at 65 °C for 2 min. CMDR hyphenation with UHPLC-MS/MS improves detection sensitivity while shortening separation and derivatization reaction times. The precursor to product ion transitions for D3, 25-hydroxy D3 (25(OH)D3), 1,25-dihydroxy D3 (1,25-(OH)2D3) and calcipotriol (CPT) as an internal standard were m/z 493.4 → 231.3, m/z 509.4 → 231.3, m/z 525.4 → 247.3, and m/z 521.4 → 247.3; respectively. The separation of the formed derivatives was conducted using a gradient elution mode with mobile phase A: formic acid (0.1%) in water and mobile phase B: formic acid (0.1%) in acetonitrile. The elution started with 40% (v/v) of B for 0.3 min then increased linearly to 90% (v/v) at 2 min on an Agilent EclipsePlus C18 (50 × 2.1 mm, 1.8 μm) column at a flow rate of 0.3 mL min−1. The method was validated using FDA standards for bioanalytical method validation over a concentration range of 0.02–50 ng mL−1 with correlation coefficient ≥0.9987 and the lower limit of quantitation (LLOQ) were 0.02–0.05 ng mL−1 in human plasma. The developed method has demonstrated excellent comparability to a well-established chemiluminescent immunoassay (CLIA) method for the analysis of D3 metabolites in human samples. The developed UHPLC-ESI-MS/MS method was implemented for routine and reliable quantitation of D3 and its major metabolites in COVID-19 patients.
Keywords: Vitamin D3; 25-hydroxyvitamin D3; 1,25-dihydroxyvitamin D3; 2-nitrosopyridine; UHPLC-ESI-MS/MS; CMDR; COVID-19
Graphical abstract
1. Introduction
The roles of vitamin D in calcium homeostasis, bone metabolism, as well as the immunological, cardiovascular, and reproductive system's functions are well-recognized [[1], [2], [3]]. Recent studies have emphasized the role of vitamin D deficiency in the pathogenesis of a variety of diseases including autoimmune diseases, cardiovascular disorders, infectious diseases, and several malignancies [[4], [5], [6]]. Vitamin D occurs in 2 major forms: the first is vitamin D3 (D3), which is formed by ultraviolet B radiations from its precursor 7-dehydrocholesterol in the skin induced transformation. The second is vitamin D2 (D2) which is derived from dietetic supplies together with some small percentage of D3 [7]. D3 is converted to 25-hydroxy D3 (25(OH)D3) in the liver and then released into the bloodstream, where it is predominantly bound to vitamin D binding protein (VDBP). The most often used biomarker for vitamin D status is the plasma level of 25(OH)D3, which functions as a reservoir for further hydroxylation to 1,25-dihydroxy D3 (1,25-(OH)2D3) in the kidney and extrarenal tissues [8].
Coronavirus disease 2019 (COVID-19) has sparked a worldwide public health emergency and resulted in millions of deaths all over the world. As a result, preventative health measures to lower the infection risk, disease progress, and severity are urgently needed [9]. Recent investigations have aimed at the prospective impacts of D3 in lowering the threat of COVID-19 and its relationship with disease severity in COVID-19 patients [[10], [11], [12]]. Hence, the necessity for assessing D3 and its major metabolites for clinical diagnosis and fostering our understanding of its function in disease management has expanded dramatically in recent years.
The quantitation of plasma levels of D3 and its major metabolites is challenging as they are strongly bound to VDBP due to their lipophilicity and they exist in extremely low levels in plasma, ranging from nanomolar to picomolar for 25(OH)D3 and 1,25-(OH)2D3 [13]. There are considerable variations in D3 and/or its major metabolite assessment results between laboratories and methods, and currently, there is no gold standard method targeting the quantitation of D3 and its major metabolites [14]. Although various laboratories use automated immunoassays [15,16] for D3 metabolites assessment, the accuracy and precision of these methods were unsatisfactory and interlaboratory variations in analysis results were claimed [17]. The poor selectivity, the ineffective release of D3 metabolites from plasma protein, and matrix interferences are major obstacles in immunoassay techniques. High-performance liquid chromatography (HPLC) with a UV detector was used for quantifying 25(OH)D3 [18]. However, the unsatisfactory limit of detection (LOD) and large sample size (∼10 mL) hindered its application in clinical assays. Quantification of the D3 metabolite has also been reported using gas chromatography with mass spectrometry (GC-MS) [19]. Even though, the metabolite degradation at the high GC temperatures restricted its use [19]. Liquid chromatography-tandem mass spectrometry (LC-MS/MS), deemed as the unique standard method for assessing D3 and its metabolites, provides a dependable platform with improved sensitivity and selectivity. The capability of LC-MS/MS technologies to make simultaneous detection of numerous analytes has initiated trials to measure several analytes in a single run [13,20]. Therefore, several LC-MS/MS were found in the literature using different ionization techniques such as fast atom bombardment (FAB) [21], thermospray (TSP) [22], electrospray ionization (ESI) [14,[23], [24], [25], [26]], and atmospheric pressure chemical ionization (APCI) [27] and atmospheric pressure photoionization (APPI) [28]. ESI is the best ionization technique because it provides superior sensitivity and a lower background signal for the determination of D3 and its metabolites. Additionally, it works better at low flow rates adopted for ultraperformance liquid chromatography (UHPLC) which, provides fast determinations [29]. However, the absence of easily ionizable groups in the structures of D3 and its metabolites can reduce LC-ESI-MS/MS method sensitivity [23]. Hence, derivatization procedures have been used to increase ion intensities and enhance the method sensitivity in ESI.
Several derivatization protocols were employed for D3 and/or its metabolites with Cookson reagents including 1,2,4-triazoline-3,5-dione derivatives as 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) [30], 4-(4′-dimethylaminophenyl)-1,2,4-triazoline-3,5-dione (DAPTAD) [23] and 4-[2-(6,7-dimethoxy-4-methyl-3,4-dihydro-quinoxalinyl)ethyl]-1,2,4-triazoline-3,5-dione (DMEQ-TAD) [31] and Amplifex [24]. However, these derivatization techniques lacked the sensitivity needed to quantify low plasma levels of D3 metabolites in COVID-19 patients. Besides, long derivatization times and sharing the same m/z for the product ions cause cross-interference for the poor separating metabolites with similar m/z for the precursor ions. The newly introduced click reagent, 2-Nitrosopyridine (Pyr-NO), is a superior dienophile for Diels-Alder derivatization that presents an improved sensitivity while preserving the good chromatographic separation [32]. However, the derivatization reaction time oscillates between 30 and 60 min which is not suitable for high throughput analysis in clinical studies. To speed up the reaction kinetics and improve derivatization repeatability, microwave reactors with sealed reaction vessels and an online monitoring platform have recently been developed [33,34]. The microwave-assisted derivatization reactions (CMDR) protocol was adopted to improve derivatization reaction speed and efficiency [35].
For the reproducible assessment of D3 and its metabolites, it is essential to optimize the sample extraction method to decrease ion suppression in the MS detector caused by biological matrices' interferents. Salting-out assisted liquid-liquid extraction (SALLE) appeared to be the best candidate to segregate the analytes from the binding proteins, improve the extraction recovery, and diminish the MS detector's ion suppression.
In the current approach, we developed and validated a new ultrasensitive UHPLC-ESI-MS/MS method for the simultaneous determination of D3 and its metabolites in COVID-19 patients. The method dedicated CMDR with Pyr-NO reagent to the derivatization reaction and triggered the mass spectral sensitivity. The clinical performance of the developed methodology was verified by comparison with routine chemiluminescent immunoassay (CLIA) methods for the analysis of 25(OH)D3and 1,25(OH)2D3. Finally, the developed approach was used to analyze D3 and its metabolites in positive COVID-19 cases.
2. Experimental
2.1. Chemicals and reagents
Analytical standards of D3, 25-hydroxy D3, 1,25-dihydroxy D3, and calcipotriol (I.S.) were purchased from Sigma Aldrich (Seelze, Germany). 2-nitrosopyridine (Pyr-NO) reagent was from Toronto Research Chemicals, Inc. (Toronto, Ontario, Canada). Sigma Aldrich (Germany) provided all additional reagents and LC-MS grade solvents. A Millipore water filtration system (Bedford, MA, USA) was utilized to get ultrapure water. VD-DC mass spect gold® serum, as a vitamin D-free human serum, was bought from Sigma Aldrich (Germany). Liaison®25OH vitamin D total assay kit and Liaison®XL 1,25(OH)2-vitamin D kits were from Diasorin (MN, USA).
2.2. Preparation of stock standard solutions, calibrators, and quality control samples
Stock standard solutions of D3, 25(OH)D3, 1,25-(OH)2D3, and I.S. were prepared via dissolving accurately weighed amounts in methanol in amber glass vials at a concentration of 1 mg mL−1. The calibrator solutions were made by adding suitable quantities of the standard solutions to VD-DC mass spect gold® serum to generate 6 calibrators at nominal concentrations of 0.02, 0.05, 0.5, 5, 20, and 50 ngmL−1. Similarly, Quality control (QC) samples were done in VD-DC mass spect gold® serum at three concentration levels: high QC level (HQC; 50 ng mL−1), medium QC level (MQC; 5 ng mL−1), low QC level (LQC; 0.05 ng mL−1). The spiked calibrators and QC samples in sealed, light-resistant vials were frozen at - 30 °C then permitted to thaw for 15 min at room temperature instantly before use.
2.3. Sample preparation
An aliquot of 100 μL of serum was transferred to a 1.5 mL Eppendorf tube and combined with 10 μL of the IS solution (100 ngmL-1) then 500 μL of methanol was added to dissociate the analytes from VD binding proteins and spun down the proteins. After vortex mixing for 1 min, 100 μL of 5 M ammonium sulfate was put in for salting out induction afterwards the mixture was centrifuged at 25 °C for 10 min at 15,000 rpm. The supernatant was pipetted into a new Eppendorf tube and was dried under a gentle nitrogen gas stream. The dried residue was then subjected to the CMDR derivatization procedures.
2.4. CMDR procedures
100 μL aliquot of 1 mM Pyr-NO, prepared in methanol, was added to the extraction residue. After vortex mixing for 10 s, the reaction mixture was placed in the vessel of the microwave reactor. The microwave method setup was set at irradiation for 2 min after ramping for 1 min, microwave temperature at 65 °C, microwave power at 250 W, and maximum pressure at 250 psi while maintaining air cooling and stirring on throughout the reaction. The derivatization mixture was transferred to the UHPLC-ESI-MS/MS instrument for quantitation.
2.5. UHPLC-ESI-MS/MS conditions
UHPLC-ESI-MS/MS platform composed an Agilent UHPLC system (Agilent, CA, United States) hyphenated to an Ultivo triple quadrupole mass spectrometer was used. The UHPLC system was composed of a 1260 Infinity II quaternary pump with a degassing unit, a 1260 Infinity II auto-sampler, and a thermostatic column oven (Agilent Technologies). A nitrogen generator from LNI Swissgas, Switzerland (NG CASTORE XS iQ) was merged with the system for nitrogen gas supply. The UHPLC-MS/MS system was controlled using Mass Hunter® software. The chromatographic run was carried out using an EclipsePlus C18 RRHD (50 × 2.1 mm, 1.8 μm) column from Agilent working in a programmed gradient mode at a flow rate of 0.3 mL min−1 using a mobile phase A: formic acid (0.1%) in water and mobile phase B: formic acid (0.1%) in acetonitrile. The elution started with 40% (v/v) of B for 0.3 min then increased linearly to 90% (v/v) at 2 min the column oven was thermostatically adjusted at 40 °C for the complete run. Positive electrospray ionization (ESI) in the multiple reaction monitoring (MRM) mode was used to detect the generated derivatives of D3 and its metabolites. The MRM transitions were selected based on the extremely intense product ion. The precursor to product ion transitions for D3, 25-hydroxy D3 (25(OH)D3), 1,25-dihydroxy D3 (1,25-(OH)2D3) and calcipotriol (CPT) as an internal standard were m/z 493.4 → 231.3, m/z 509.4 → 231.3, m/z 525.4 → 247.3, and m/z 521.4 → 247.3; respectively. MRM transitions were given at 25 ms dwell time. The ESI Jet Stream source parameters were optimized to provide the strongest MRM signals. The optimized MS settings were set at: 300 °C for gas temperature, 10 L/min for gas flow, 15 psi for nebulizer gas, 4000 V for capillary voltage, the fragmentor voltage 120 V for D3 and CPT while 100 V for 25(OH)D3 and 1,25-(OH)2D3. The collision energy (CE) was set at 45 V for D3 and CPT and 35 V for 25(OH)D3 and 1,25-(OH)2D3.
2.6. Validation of the developed UHPLC-ESI-MS/MS method
In accordance with the FDA's bioanalytical method validation requirements, the performance of the developed UHPLC-ESI-MS/MS method was verified [36].
The calibration curves for D3, 25(OH)D3, and 1,25-(OH)2D3 were established using linear regression of analyte/IS peak area ratios vs the relevant calibrator's concentration. The calibration curves (n = 5) were prepared using six different nominal concentrations (0.02, 0.05, 0.5, 5, 20 and 50 ngmL−1). The linearity was evaluated by calculating the correlation coefficient (r) from linear regression analysis. Calibration curves were judged linear if the six calibrators' recalculated concentrations were within 15% of the nominal concentration (20% at LLOQ). The method's sensitivity was also measured by calculating the lower limit of quantitation (LLOQ). LLOQ was assessed as the lowest calibrator concentration with 20% accuracy, expressed as % mean relative error (RE%), calculated for each analyte using five replicates in three consecutive runs.
Method selectivity has been evaluated by examining chromatograms of blank human serum samples for any interference at D3, 25(OH)D3, 1,25-(OH)2D3, and I.S retention times. If the chromatographic peaks’ areas of the co-eluted components are <20% of the analytes' peak areas at the LLOQ level, the interference is nonsignificant, thus the method is considered selective.
For evaluation of the within-batch and between batches accuracy and precision, five replicates of QC samples of D3, 25(OH)D3, and 1,25(OH)2D3 were analyzed using the developed method at three different days using three QC levels: 50 ng mL−1(HQC), 5 ng mL−1(MQC) and 0.05 ng mL−1(LQC). The precision was stated as the relative standard deviation percentage (RSD%), while the accuracy was calculated as the relative error (RE%) between the estimated and nominal concentrations. The method is considered accurate and precise if the RE% and RSD% for the assessed samples are within 15% (20% at LLOQ).
The extraction recovery for D3 and its metabolites from human serum using the SALLE method was evaluated at three QC levels: 50 ng mL−1(HQC), 5 ng mL−1(MQC), and 0.05 ng mL−1(LQC). The extraction recovery percentage was estimated by comparing the peak areas of five replicates of pre-spiked VD-DC mass spect gold® serum extract to peak area unextracted QC samples with similar nominal concentration levels.
To determine the potential matrix effect in human serum, the peak areas of spiked samples extracts were compared to the peak area of pure standards with equivalent concentrations at the three QC levels. The absolute ion suppression produced by the matrix was expressed as normalized I.S. matrix factors (MF).
The matrix effect was assessed similarly at the three QC levels by comparison of the peak area of post-extraction spiked samples to that of the pure standard of equivalent concentration and was expressed as IS normalized matrix factors (MF).
To extend the upper concentration limit with appropriate precision and accuracy, the dilution integrity was performed. It was determined via dilution of the ultrahigh quality control sample (UHQC; 200 ng mL−1) with VD-DC mass spect gold® serum (n = 5) by 10 and 100 times to 20 ng mL−1 and 2 ng mL−1; respectively. The diluted samples were extracted and analyzed using the developed UHPLC-ESI-MS/MS method and the obtained concentrations were compared to the nominal concentrations.
Short-term, long-term, autosampler and freeze-thaw, and stabilities were assessed via analysis of 5 replicates at 3 QC levels HQC (50 ng mL−1), MQC (5 ng mL−1), and LQC (0.05 ng mL−1). For evaluation of short-term stability, unprocessed QC samples were allowed to defrost on the bench for 6 h at 25 °C afterwards extraction, derivatization, and analysis procedures were carried out. Similarly, the long-term stability was assessed by freezing the unprocessed QC samples for 3 weeks at −30 °C then thawed, processed, and analyzed. The autosampler stability was assessed by analysis of the processed QC samples following storage for 24 h at 4 °C in the autosampler. Freeze-thaw stability was carried out by three cycles of freezing (24 h at −30 °C) and defrosting (at −25 °C) before processing and analysis. Acceptable stabilities were for results within 15% accuracy, expressed as RE%, compared to the nominal concentrations.
2.7. Method's clinical performance and application to quantitation in COVID-19 patients
The novel UHPLC-MS/MS method was compared, in terms of performance, to DiaSorin® Liaison chemiluminescent immunoassay (CLIA) kits for assessments of 25(OH)D3 and 1,25-(OH)2D3 by blind measurements of 20 samples in triplicate. The CLIA was conducted as stated by the manufacturer's instructions. The results were compared via Bland-Altman plot analysis using MedCalc software. The developed method was used for assessment of serum concentration levels of D3, 25(OH)D3, and 1,25(OH)2D3 in COVID-19 blood samples collected from 20 patients (40.45 ± 12.3 years) admitted to Ohud Hospital, Al Madinah AlMunawarah, Saudi Arabia by trained professionals from the infection control unit, taking all necessary precautions. The study does not involve any drug intervention and samples were collected and analyzed for the endogenous content in a double-blind study. Samples were also analyzed for 25(OH)D3 and 1,25(OH)2D3 using the CLIA method and results were compared using Student's t-test. COVID-19 test status was determined through positive real-time reverse transcriptase-polymerase chain reaction (RT‐PCR) test results. The patients were followed up at Ohud Hospital, Saudi Arabia, and given the COVID-19 treatment protocol approved by the Ministry of Health, Saudi Arabia. Ethical approval (No. H‐03‐M‐085) was given from the review board of the health affairs general directorate in Al Madinah AlMunawarah.
3. Results and discussion
Currently, LC-MS/MS is regarded as the best practice platform for routine assessment of D3 and its major metabolites in clinical applications attributed to its high throughput, sensitivity, and selectivity.
However, the lack of ionizable polar groups in their structures leads to poor ionization efficiency and low sensitivity. Therefore, several derivatization protocols have been developed to enhance its detection response [23,24,30,31]. Diels-Alder derivatization with Pyr-NO was a superior protocol as it offered an improved sensitivity with the good chromatographic separation for D3 metabolites [32]. However, long derivatization times and lack of reproducibility were major drawbacks that hampered the high throughput analysis of D3 and its metabolites in clinical applications. Since the introduction of CMDR in the derivatization reaction, it has been employed to decrease the reaction times, increase the reaction yield, and improve method reproducibility [33,34]. Consequently. CMDR was employed in this study in combination with UHPLC-ESI-MS/MS detection. CPT was the chosen internal standard in the current work due to structural and physicochemical characteristics similarity.
3.1. Optimization of CMDR conditions
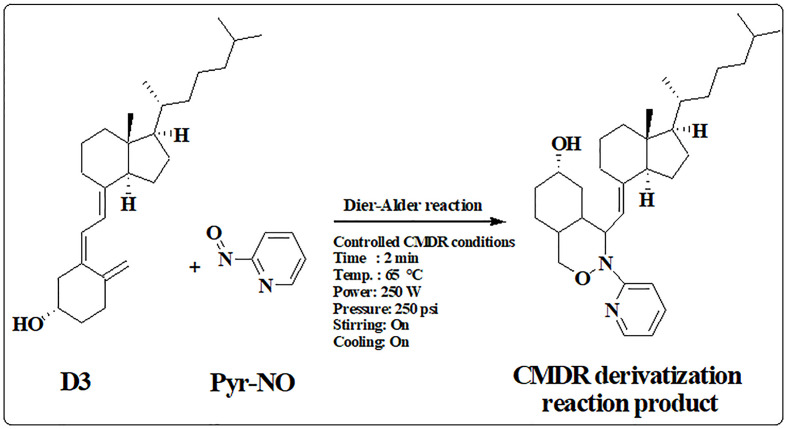
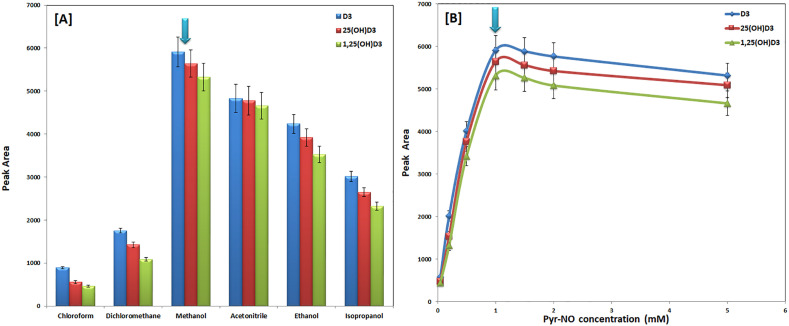
The newly developed microwave reactor with online controlled reaction conditions was devoted to accelerating the derivatization reaction of D3 and its metabolites with Pyr-NO as well as to providing superior reaction yield and reproducibility. Diels-Alder's derivatization reaction of D3 with Pyr-NO as a model for CMDR derivatization reaction was presented in Fig. 1 . The experimental factors influencing the CMDR derivatization reaction with Pyr-NO were tuned in this study. The incubation solvent in the CMDR derivatization reaction was screened for acetonitrile, methanol, ethanol, isopropanol, chloroform, and dichloromethane. The results were shown in Fig. 2 [A]. It was found that non-polar solvents gave a low reaction yield while the best results were obtained for methanol compared to other polar solvents. This may be attributed to the increase in reaction rate with increasing solvent polarity [37]. Hence, methanol was used as a solvent for the Pyr-NO reagent which is the main solvent in this CMDR reaction. Furthermore, the impacts of reagent Pyr-NO concentration on the effectiveness of the CMDR derivatization process were investigated. Results were shown in Fig. 2 [B]. It was found that the best reaction yield was obtained using 1 mM of Pyr-NO in methanol. Hence, 1 mM of Pyr-NO was used for subsequent works.
Fig. 1.
Scheme for the CMDR derivatization reaction of D3 with Pyr-NO.
Fig. 2.
Effects of Pyr-NO reagent solvent [A] and concentration [B] on peak areas of D3, 25(OH)D3, and 1,25(OH)2D3 using the developed UHPLC-ESI-MS/MS method.
In the current study, derivatization was carried out in a microwave reactor with sealed reaction containers and an online monitoring platform that displayed reaction time, temperature, and pressure. This approach combined microwave-assisted dielectric heating with autoclave-style sealed-vessel technology [38]. Firstly, the CMDR was examined in both open and closed vessel systems in preliminary studies. Though, the closed vessel system gave a 2 times yield compared to the open vessel. The pressure inside the reaction vessel was also checked for its effect on the reaction yield. The better results were obtained when using 250 psi pressure in the closed vessel with no increase in the yield with pressure increase. As a result, a closed vessel system with a maximum pressure of 250 psi was proposed in this CMDR method.
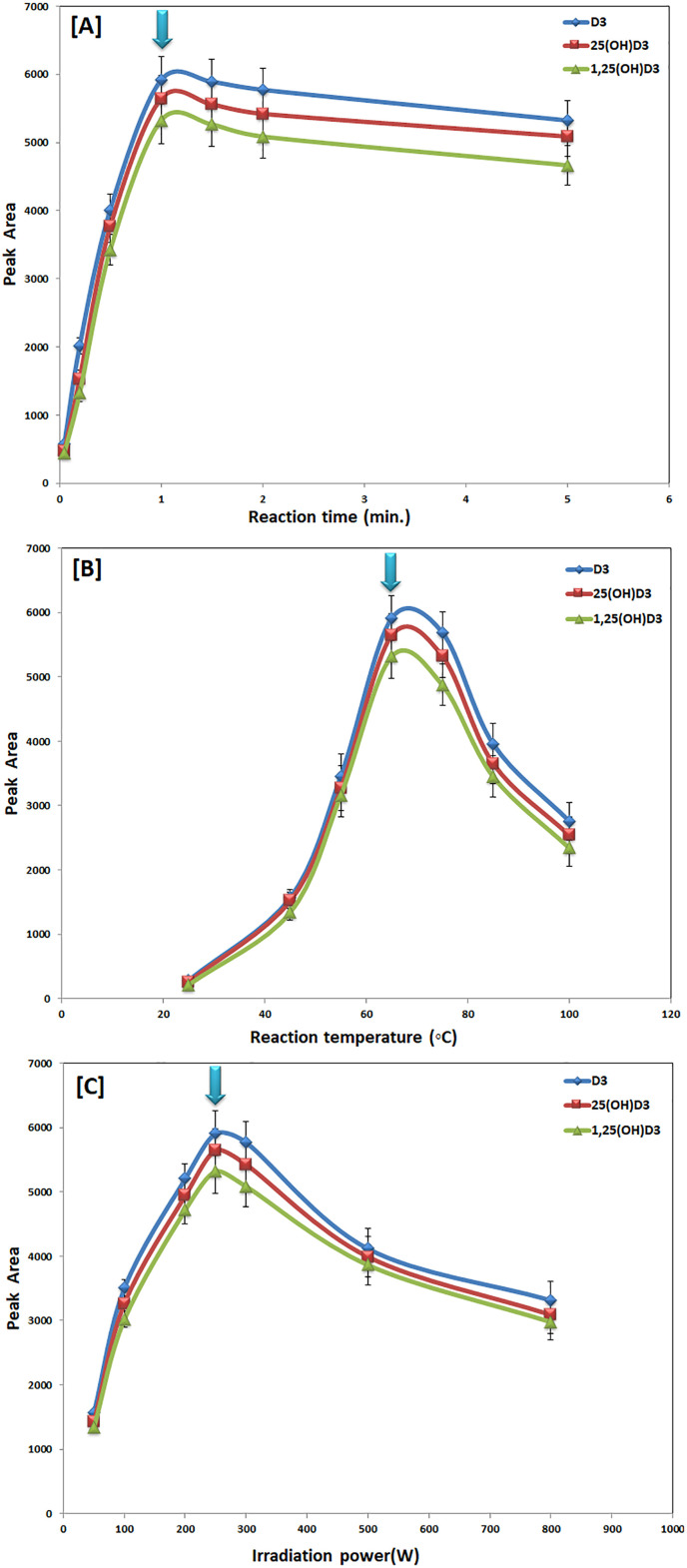
The irradiation conditions were also tuned in this CMDR method for the irradiation times, temperature, and microwave power. Various derivatization reaction times were examined in ranges of 0.05–5 min at 60 °C and 250 W microwave irradiation power. As shown in Fig. 3 [A], the reaction yield was best when the derivatization reaction time was 2 min. A closed-vessel with an in-situ cooling system was used to investigate the influence of microwave reaction temperature within the range of 25–100 °C. Lower reaction temperatures were shown to be insufficient for completing the derivatization process, whereas increasing the irradiation temperature over 65 °C reduced the reaction yield (Fig. 3 [B]). This might be due to the produced derivatization product being hydrolyzed at high temperatures. As a result, a 65 °C irradiation temperature was recommended. In this investigation, the in-situ air cooling system was turned on to prevent the reaction mixture from overheating by continuously removing the reaction latent heat, as detailed in a prior study [39]. Over the range of 50–800 W, the effect of microwave irradiation power was also investigated as shown in Fig. 3 [C]. Derivatization reaction products were low when using low microwave irradiation power at reduced reaction times (2 min). The greatest reaction yields were obtained by raising the microwave irradiation power to 250 W for 2 min. Higher microwave irradiation power, on the other hand, reduced reaction yields. At this high microwave strength, the derivatization products breakdown may be claimed. Hence, the CMDR was performed in a closed vessel system using microwave irradiation (250 W) at 65 °C for a 2 min reaction time while the air-cooling system was turned on.
Fig. 3.
Effects of microwave reaction time [A], reaction temperature [B] and microwave irradiation power [C] on peak areas of D3, 25(OH)D3, and 1,25(OH)2D3 using the developed UHPLC-ESI-MS/MS method.
3.2. Optimization of UHPLC-ESI-MS/MS method
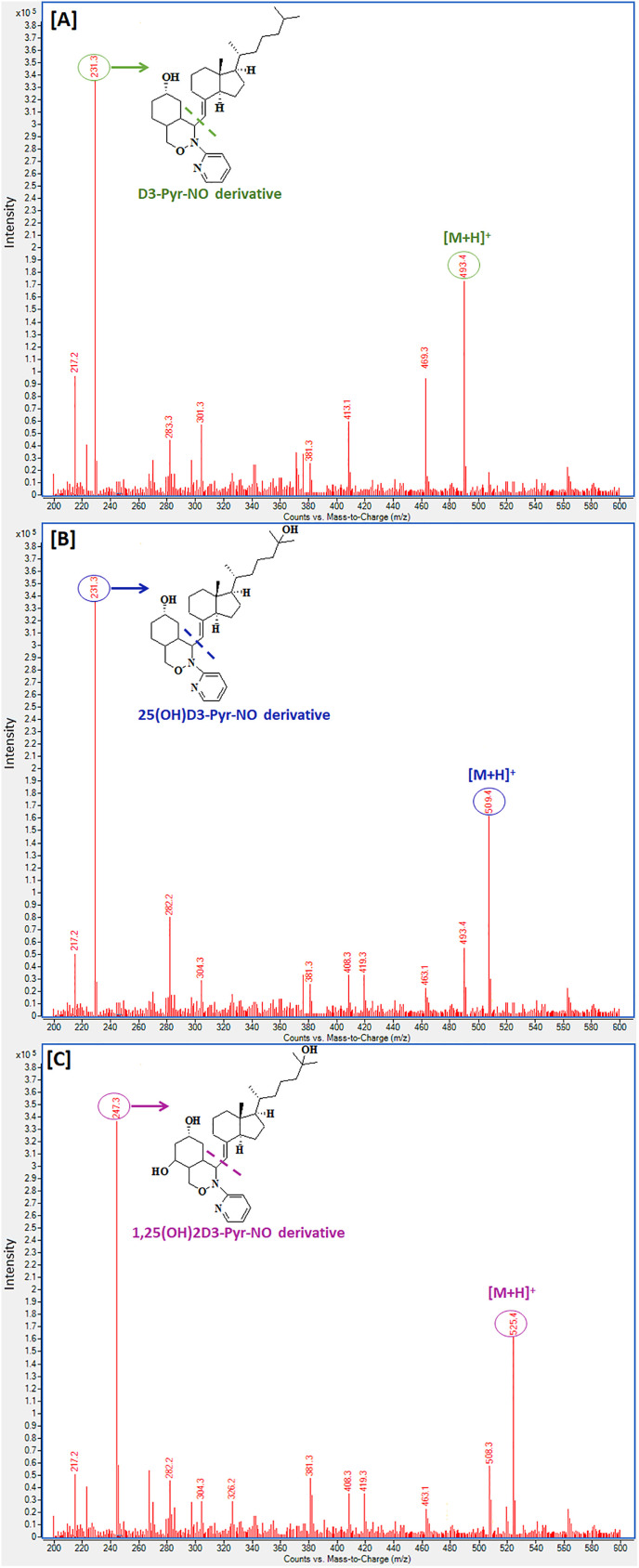
UHPLC-ESI-MS/MS settings were tuned for the greatest resolution, peak shape, and analytical response in a short retention time to accommodate the high-throughput capability in clinical research. At first, the ESI-MS/MS conditions were optimized in multiple MRM using an automated tool provided by Agilent. The MassHunter Optimizer application offers a versatile tool for automating the optimization of MRM parameters such as the selection of precursor and product ions, as well as the precise tuning of collision energies and fragmentor voltages. It was discovered that, under ESI circumstances, the target CMDR products show a higher response in positive ion detection mode rather than in negative ion detection mode, which may be attributed to the presence of a basic amine functional group in the derivatization product structures. A low-pH mobile phase was utilized to improve the ionization in the positive ion mode. Several acids were tried; however, the best response was attained when 0.1% formic acid was used in the mobile phase. The most abundant precursor ions were tuned for CMDR derivatization products for positive ions, different adducts, and charge states. Ions with m/z 200 and low abundance precursor ions were omitted from consideration. Based on the entered formula, chemical structure, mobile phase content, and ionization mode, the observed m/z values were monitored. The collision-induced dissociation mass spectra of the derivatization products of D3, 25(OH)D3, and 1,25(OH)2D3 with Pyr-NO are seen in Fig. 4 . The spectra showed significant protonated molecular ions [M + H]+ at m/z 493.4, 509.4 and 525.4 for the derivatization products of D3, 25(OH)D3, and 1,25(OH)2D3; respectively (Fig. 4 [A], [B] and [C]). On the other hand, stable and intense product ions were observed at m/z 231.3 for D3 and 25(OH)D3 corresponding to the loss of 7-methyl-4-methylene-1-(6-methylheptan-2-yl) indene moiety (Fig. 4 [A] and [B]). The product ion for 1,25(OH)2D3 was seen at m/z 247.3 corresponding to loss of 3-methyl-7-methylene-1-inden-3-yl-2-methylheptan-2-ol moiety (Fig. 4 [C]). The precursor ion and product ions of the derivatization product of CPT (IS) were seen at m/z 521.4 and m/z 247.3, respectively.
Fig. 4.
Collision-induced dissociation mass spectrum of the CMDR reaction product of [A] D3, [B] 25(OH)D3 and [C] 1,25(OH)2D3 with 1 mM Pyr-NO reagent in positive ion electrospray ionization mode.
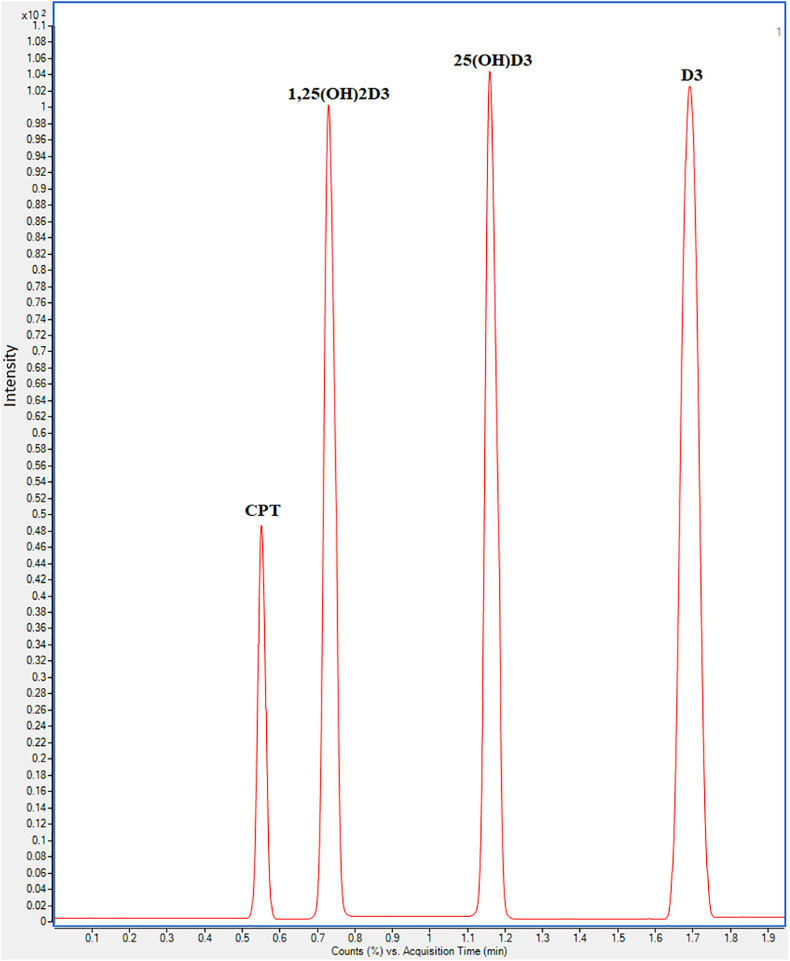
Furthermore, the fragmentor voltage and collision energies (CE) were correctly set for the CMDR derivatization products to match the target selectivity and sensitivity of the proposed UHPLC-ESI-MS/MS method. The system initially adjusts the coarse fragmentor voltage to achieve the highest ion intensity of the selected precursor ions and then refines the fragmentor voltage by stepping in smaller increments to achieve the optimal response. The voltage range of 100–200 V was tested for the fragmentors. D3 and CPT had the highest ion intensity at 120 V, whereas 25(OH)D3 and 1,25-(OH)2D3 had the highest at 100 V. While keeping the capillary voltage at 4000 V, the CE was tuned by acquiring the precursor ion to product ion transition within the range of 0–100 V. The highest abundance was gained at 45 V for D3 and CPT and 35 V for 25(OH)D3 and 1,25-(OH)2D3. As a result, these values were picked as the best MRM settings. Unfortunately, only one transition for each analyte gave the highest abundance while the second transition was not sufficient for the quantitative purposes in the current study especially for 1,25-(OH)2D3. Additionally, sharing the same m/z for the product ions causes cross-interferences especially for metabolites with similar precursor ions. Therefore, only one transition for each analyte was used for the quantitation of D3, 25(OH)D3, and 1,25-(OH)2D3 in COVID-19 patients while giving considerable attention for testing the method selectivity in the validation phase. The dwell time was also tuned for the greatest detector response, and a dwell period of 25 ms is recommended for the stated MRM transitions. Afterwards, UHPLC conditions were optimized for the chromatographic separation of Pyr-NO derivatives of D3, 25(OH)D3, 1,25(OH)2D3 and IS for the best resolution and peak shape in a short analysis time. The effect of the organic modifier on the separation of the target analytes was tested. Although various organic solvents including acetonitrile, methanol, and ethanol were examined, acetonitrile showed the best resolution in a short analysis time (<2.0 min). The effect of formic acid in the range of 0.01–1% was also examined and 0.1% formic acid was the most excellent for peak shape and resolution. The separation was attained using gradient elution using water with formic acid (0.1%) and acetonitrile with formic acid (0.1%). The elution program started with 40% (v/v) of acetonitrile for 0.3 min then increased linearly to 90% (v/v) at 2 min at 0.3 mL min−1 flow rate. The column oven was thermostatically controlled to hold the column at 40 °C during the run. MRM chromatogram of blank serum spiked with 20 ng mL−1 of D3, 25(OH)D3, 1,25(OH)2D3, and 5 ng mL−1 I.S. using the developed UHPLC -ESI-MS/MS method was shown in Fig. 5 . It is noticeable that high peak resolution for the target analytes was achieved in less than 2 min.
Fig. 5.
Typical MRM chromatogram of VD-DC mass spect gold® serum spiked with 20 ng mL−1 of D3, 25(OH)D3, 1,25(OH)2D3 and 5 ng mL−1 I.S. using the developed UHPLC-ESI-MS/MS method (transitions were m/z 493.4 → 231.3 for D3, m/z 509.4 → 231.3 for 25(OH)D3, m/z 525.4 → 247.3 for 1,25(OH)2D3, and m/z 521.4 → 247.3 for I.S). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Optimization of SALLE method
SALLE with water-miscible organic solvent has demonstrated a good approach in vitamin D3 extraction for its ability to separate D3 from binding proteins, enhance extraction recovery, and reduce ion suppression in the MS detector [23]. The extraction solvent type is a crucial aspect of the SALLE technique. The optimum extraction solvent should be water-miscible, polar, and capable of inducing phase separation upon salt addition. In this work, the effects of acetonitrile, methanol, ethanol, and isopropanol on the extraction of target analytes were investigated. Methanol was the chosen SALLE due to its ability to segregate the analytes from the plasma protein besides the high extraction recovery. Moreover, the choice of salting-out mediator is another effective parameter in the sample preparation phase. Ammonium acetate was reported as a salting-out mediator for vitamin D3 metabolites [23]. Although, we noticed low extraction efficiency in the current methodology. In our way to seek the most suitable salting-out mediator, several salts were used including magnesium chloride (MgCl2), calcium chloride (CaCl2), sodium chloride (NaCl), and ammonium sulfate ((NH4)2SO4). Results are shown in Table 1 . The best extraction recoveries were obtained with (NH4)2SO4 with % recoveries ranging from 98.4 to 100.7. The amount of used (NH4)2SO4 was adjusted for the best extraction recovery. It was noticed that 100 μL of 5 M (NH4)2SO4 gave the highest extraction efficiency therefore, it was chosen for further tests.
Table 1.
Effects of several types of salting-out mediators on the extraction efficiency of D3 and its major metabolites from human serum and assay using the developed UHPLC-ESI-MS/MS method.
| Analytea | % Recoveryb ± S.D. |
|||
|---|---|---|---|---|
| MgCl2 | CaCl2 | NaCl | (NH4)2SO4 | |
| D3 | 81.3 ± 2.8 | 71.5 ± 2.0 | 62.3 ± 3.5 | 99.4 ± 3.1 |
| 25(OH)D3 | 85.5 ± 2.6 | 73.3 ± 2.7 | 70.5 ± 2.9 | 100.7 ± 2.7 |
| 1,25(OH)2D3 | 87.4 ± 3.0 | 70.8 ± 2.4 | 68.8 ± 3.3 | 98.4 ± 2.9 |
Concentration used from D3, 25(OH)D3 and 1,25(OH)2D3 was 5 ng mL−1.
Data presented as mean ± S.D. of three experiments.
3.4. Validation of the developed UHPLC-ESI-MS/MS method
Calibration curves for D3, 25(OH)D3, and 1,25-(OH)2D3 were created by graphing the peak area ratios of the reference standard and CPT (I.S.) MRM signals against the serum concentration level. The linearity ranges and regression analysis data for D3, 25(OH)D3, and 1,25-(OH)2D3 using five data points were summarized in Table 2 . Over the assessed calibration range, the linear regression study demonstrated excellent correlation coefficients (r ≥ 0.9987). The re-calculated concentrations of the measured calibrators were within ±10% indicating good linearity (data was not shown). The sensitivity was assessed by calculating the LLOQ of D3, 25(OH)D3, and 1,25-(OH)2D3 in human serum. The calculated LLOQ was 0.02 ng mL−1 for 25(OH)D3, and 1,25-(OH)2D3 and 0.05 ng mL−1 for D3 indicating good sensitivity of the developed method. The slopes of the calibration curves were 1.18 for D3, 1.54 for 25(OH)D3, and 1.69 for 1,25-(OH)2D3. The sensitivity of this UHPLC-ESI-MS/MS was about 100 times than HPLC-UV method [18], 50 times than GC-MS method [19], and 2–40 times than LC-MS/MS methods [13,14,[20], [21], [22], [23], [24], [25], [26], [27], [28]].
Table 2.
The linear regression analyses for the calibration curves data and sensitivity for D3 and its major metabolites in human serum using the developed UHPLC-ESI-MS/MS method.
| Analyte | Calibration curvea (n = 5) |
LLOQb (ng mL−1) | |||
|---|---|---|---|---|---|
| Range (ng mL−1) | Slopeb (±SD) | Interceptb (±SD) | r | ||
| D3 | 0.05–50 | 1.18 (±0.05) | 0.056(±0.002) | 0.9991 | 0.05 |
| 25(OH)D3 | 0.02–50 | 1.54 (±0.06) | 0.024 (±0.002) | 0.9990 | 0.02 |
| 1,25(OH)2D3 | 0.02–50 | 1.69 (±0.07) | 0.019 (±0.001) | 0.9987 | 0.02 |
Peak area ratio of D3, 25(OH)D3 or 1,25(OH)2D3 and I.S. versus serum concentration (ng mL−1).
Data presented as mean (n = 5) ± SD.
Selectivity of the developed UHPLC-ESI-MS/MS method was tested by looking for any interfering peaks in the chromatograms of human serum at the retention times of D3, 25(OH)D3, 1,25(OH)2D3, and I.S at LLOQ. It observed that the peak areas of co-eluted substances did not go beyond 7% of the peak areas of the target analytes. Hence, the interference was nonsignificant, thus the method was believed to be selective.
Within-batch and between batches accuracy and precision were assessed using QCs of D3, 25(OH)D3, and 1,25(OH)2D3 at three QC levels. Results were shown in Table 3 . Within-batch precision, expressed as RSD%, was ranged from 1.5 to 3.5% while between batches precision was varied from 1.8 to 4.7%. Similarly, within-batch accuracy, as RE% between the calculated and nominal concentration was within −3.1-1.2% and between batches accuracy was between −5.7-1.4%. As a result, the new UHPLC -ESI-MS/MS method demonstrated remarkable repeatability in clinical investigations.
Table 3.
Accuracy and precision for assay of D3 and its major metabolites from human serum using the developed UHPLC-ESI-MS/MS method.
| Analyte | Concentration (ng mL−1) | Within-batch (n = 5) |
Between-batches (n = 3) |
||
|---|---|---|---|---|---|
| Accuracy (RE%) | Precision (RSD%) | Accuracy (RE%) | Precision (RSD%) | ||
| D3 | 0.05 | −3.1 | 3.5 | −5.4 | 4.7 |
| 5 | 0.8 | 3.4 | −1.1 | 3.6 | |
| 50 | −1.5 | 2.1 | 1.4 | 2.7 | |
| 25(OH)D3 | 0.05 | −2.9 | 3.8 | −3.7 | 4.0 |
| 5 | −1.8 | 2.8 | −2.6 | 3.7 | |
| 50 | −0.9 | 1.9 | 1.2 | 2.2 | |
| 1,25(OH)2D3 | 0.05 | −3.0 | 3.3 | −4.9 | 4.1 |
| 5 | 1.2 | 2.9 | 1.7 | 2.9 | |
| 50 | 0.6 | 1.5 | −1.3 | 1.8 | |
The extraction recoveries of D3, 25(OH)D3, and 1,25(OH)2D3 from human serum were evaluated at three quality control levels as seen in Table 4 . The recoveries of the target analytes utilizing the SALLE procedure were found to be within 95.9–101.0%, suggesting satisfactory extraction recovery. Likewise, the potential matrix effect in human serum was assessed as normalized I.S. matrix factors (MF). The IS-normalized matrix factors were varied from 0.955 to 1.007. These results show that the extraction approach provides adequate extraction effectiveness without significant interference from coeluted serum matrix components with the analyte peaks.
Table 4.
Extraction recovery and matrix effect of D3 and its major metabolites from human serum after SALLE and assay using the developed UHPLC-ESI-MS/MS method.
| Analyte | Concentration (ng mL−1) | Extraction recoverya (%) ± S.D. | Matrix factora (IS-normalized) |
|---|---|---|---|
| D3 | 0.05 | 95.9 ± 4.6 | 0.955 |
| 5 | 98.7 ± 3.0 | 0.984 | |
| 50 | 99.3 ± 1.9 | 1.004 | |
| 25(OH)D3 | 0.05 | 96.1 ± 4.1 | 0.975 |
| 5 | 97.7 ± 2.7 | 0.988 | |
| 50 | 100.4 ± 1.9 | 0.999 | |
| 1,25(OH)2D3 | 0.05 | 96.9 ± 4.8 | 0.979 |
| 5 | 99.1 ± 2.9 | 0.990 | |
| 50 | 101.0 ± 1.6 | 1.007 |
Average of five determinations.
To extend the UHQC 200 ng mL−1 with satisfactory precision and accuracy, the dilution integrity was tested by dilution with blank serum 10- and 100 times. Results are shown in Table 5 . The dilution integrity RE% for of 10- and 100-times dilution was found to range from −2.5 to 2.0 while the RSD% results were varied from 1.96% to 3.08%. Hence, the method calibration limit could be extended to 200 ng mL−1 with appropriate accuracy and precision.
Table 5.
Dilution integrity of D3 and its major metabolites in human serum after dilution and assay using the developed UHPLC-ESI-MS/MS method.
| Analyte | Dilution timesa | Nominal conc. (ng mL −1) | Measured concb ± SD (ng mL −1) | Accuracy (RE%) | Precision (RSD%) |
|---|---|---|---|---|---|
| D3 | 10 | 20 | 19.60 ± 0.53 | - 2.0 | 2.70 |
| 100 | 2 | 1.95 ± 0.06 | −2.5 | 3.08 | |
| 25(OH)D3 | 10 | 20 | 19.75 ± 0.49 | - 1.25 | 2.48 |
| 100 | 2 | 2.04 ± 0.04 | 2.0 | 1.96 | |
| 1,25(OH)2D3 | 10 | 20 | 20.15 ± 0.61 | 0.75 | 3.03 |
| 100 | 2 | 1.94 ± 0.05 | −3.0 | 2.58 |
Using ultrahigh quality control sample (UHQC; 200 ng mL−1) from each analyte.
Average of five determinations.
Short-term, long-term, autosampler and freeze-thaw stabilities were evaluated under a variety of storing and processing conditions. Stability results at the mentioned settings are presented in Table 6 . The short-term stability findings of D3, 25(OH)D3, and 1,25(OH)2D3 were varied from −3.6 to 2.1%, while the stability results of long-term storage were from −4.9-2.9%. After three cycles of freeze-thaw, the stability was found to be −4.8-2.9%, while the autosampler stability was found to be −2.8-1.2%. The performed stability experiments demonstrate no obvious deterioration results given that the RE% for all samples were within 15% and proved to be stable in human serum under the storage and processing conditions.
Table 6.
Stability data for of D3 and its major metabolites in human serum under different storage conditions using the developed UHPLC-ESI-MS/MS method.
| Nominal conc. (ng mL −1) | Accuracya (RE%) |
|||
|---|---|---|---|---|
| Stability tests | D3 | 25(OH)D3 | 1,25(OH)2D3 | |
| Short-term stability | 0.05 | −3.2 | −3.2 | −3.6 |
| 5 | 2.1 | 1.9 | 1.3 | |
| 50 | −1.4 | - 2.1 | −1.0 | |
| Long term stability | 0.05 | - 4.1 | −4.2 | −4.9 |
| 5 | −3.2 | −2.6 | −3.4 | |
| 50 | 2.2 | 2.7 | 2.9 | |
| Autosampler stability | 0.05 | −2.8 | −2.4 | −2.1 |
| 5 | −2.2 | −2.0 | −1.8 | |
| 50 | 0.4 | 0.8 | 1.2 | |
| Freeze–thaw stability | 0.05 | −3.8 | −4.3 | −4.8 |
| 5 | 2.9 | 2.5 | 2.7 | |
| 50 | −1.7 | −1.8 | −2.1 | |
Average of five determinations.
3.5. Method's clinical performance and application to quantitation in COVID-19 patients
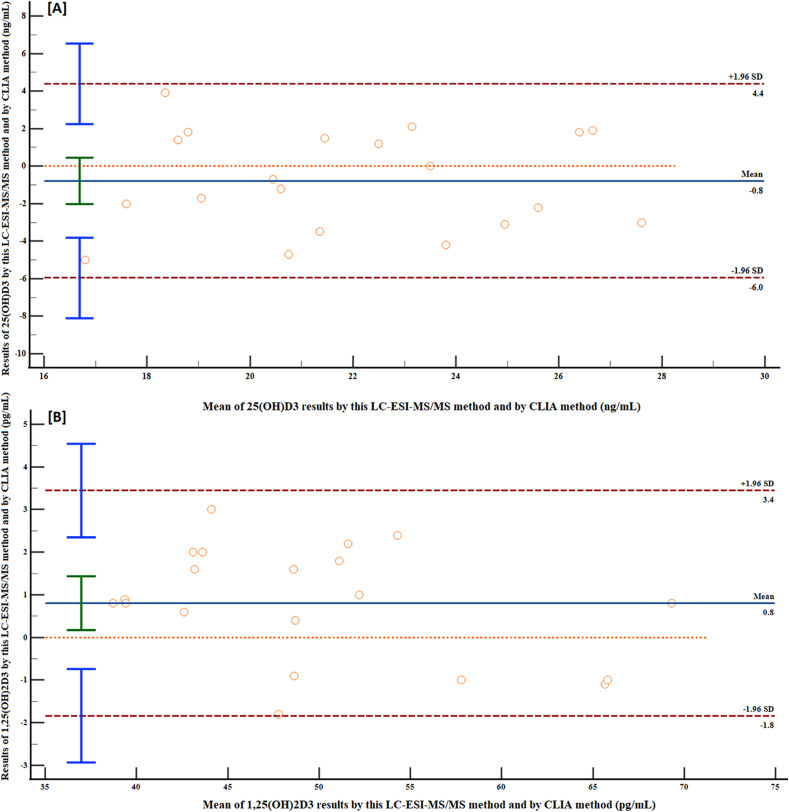
To assess the clinical performance of our newly designed UHPLC-ESI-MS/MS approach, a statistical comparison of its performance versus the already established CLIA method was performed. It is thought that assessing performance differences rather than just comparing methods is a superior way to determine comparability [40]. As a result, we used a Bland-Altman plot to examine the differences between the CLIA method and the developed UHPLC-ESI-MS/MS method. For this purpose, 20 serum samples from healthy subjects were analyzed blindly for 25(OH)D3 and 1,25(OH)2D3 using the developed UHPLC-ESI-MS/MS and the commercial DiaSorin® Liaison immunoassay kits (CLIA method). The differences between the two methods were evaluated using a Bland-Altman plot for bias assessment (Fig. 6 A and B). When comparing our LC-MS/MS approach to the CLIA method, the Bland-Altman plot analysis revealed that our LC-MS/MS method had a minimal mean bias of - 0.8 and 0.8 for 25(OH)D3 and 1,25(OH)2D3, respectively. The low bias level indicates similar limits of agreements between both methods. The obtained results for our method compared to the CLIA method at the existed serum concentrations of 25(OH)D3 and 1,25(OH)2D3 may be described by the fact that other molecules that cross-react with the immunoassay are minimal.
Fig. 6.
Results of Bland-Altman plot for 25(OH)D3 [A] and 1,25(OH)2D3 [B] analysis bias assessment between the developed UHPLC-ESI-MS/MS method and CLIA method.
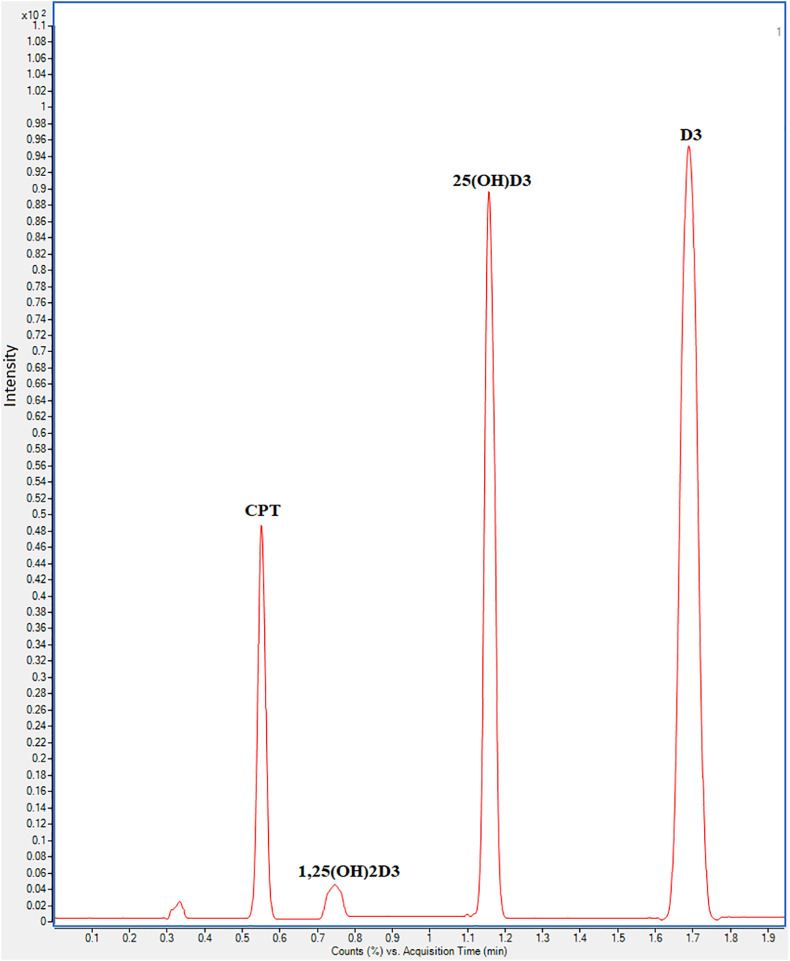
To assess the clinical importance of our method and to maximize its utility, the developed UHPLC-ESI-MS/MS approach was utilized for simultaneous determination of serum levels of D3, 25(OH)D3, and 1,25(OH)2D3 in twenty positive COVID-19 cases. MRM chromatogram of COVID-19 patients serum sample extracted, spiked with IS (5 ng mL−1), and analyzed by the developed UHPLC-ESI-MS/MS method is shown in Fig. 7 . The tested samples were also analyzed for 25(OH)D3 and 1,25(OH)2D3 using the CLIA method and results were compared using Student's t-test. D3 was analyzed using UHPLC-ESI-MS/MS method only due to the deficiency of a suitable immunoassay kit for D3. Results are presented in Table 7 . A good agreement between the developed method and the routine CLIA method with P-values > 0.05 was given that proved the clinical significance of the developed method. However, as far as we see, this is the first study on the simultaneous quantification of D3, 25(OH)D3, and 1,25(OH)2D3 in human sera that could be a very important diagnostic marker in many diseases related to a vitamin D3 deficiency.
Fig. 7.
MRM chromatogram of COVID-19 patients serum sample extracted, spiked with IS (5 ng mL−1) and analyzed by the developed UHPLC-ESI-MS/MS method (transitions were m/z 493.4 → 231.3 for D3, m/z 509.4 → 231.3 for 25(OH)D3, m/z 525.4 → 247.3 for 1,25(OH)2D3, and m/z 521.4 → 247.3 for I.S).
Table 7.
Mean serum levels of D3 and its major metabolites in COVID-19 patients analyzed by the developed UHPLC-ESI-MS/MS method and CLIA method.
| Analyte | Patient age (years) | Serum level using this UHPLC-ESI-MS/MS method (Mean ± SD) | Serum level using CLIA method (Mean ± SD) | P-Valuea |
|---|---|---|---|---|
| D3 | 40.45 ± 12.30 | 18.78 ± 6.88 ng mL−1 | – | – |
| 25(OH)D3 | 16.07 ± 3.79 ng mL−1 | 17.29 ± 3.47 ng mL−1 | P > 0.05 | |
| 1,25(OH)2D3 | 40.02 ± 9.96 pg mL−1 | 39.38 ± 9.23 pg mL−1 | P > 0.05 |
Calculated using Student's t-test.
4. Conclusion
This report presents a novel ultrasensitive and reliable UHPLC-ESI-MS/MS approach for simultaneous quantification of D3 and its major metabolites in COVID-19 patients. The method was based on the enhancement of the detection sensitivity and method reproducibility via a derivatization reaction with Pyr-NO in a modern microwave reactor with controlled reaction conditions. The hyphenation of CMDR with UHPLC-MS/MS permitted precise and comprehensive derivatization outcomes in short reaction times. The new approach demonstrated fast separation and derivatization reaction times, superior derivatization reaction yields, and enhanced product purities by reducing the undesirable side reactions. The new approach provided enhanced mass spectral sensitivity about 2–100 times compared to the reported methods with a short run time (<2.0 min). As far as we know, this report is the first approach for the hyphenation of CMDR with LC-MS/MS techniques guiding toward reproducible and fast bioanalytical approaches. A simple and efficient SALLE procedure was implemented for the clean-up of D3 and its major metabolites from serum samples. The developed method was applied successfully for simultaneous analysis of D3, 25(OH)D3, and 1,25(OH)2D3 in COVID-19 patients. The methodology is regarded as an effective diagnostic tool for the assessment of the correlation of vitamin D status and severity of COVID-19.
Author statement
Sameh Ahmed: Conceptualization, Methodology, Funding acquisition, Formal analysis, Resources Writing – original draft, Writing – review & editing and Visualization. Hani M. Khojah: Conceptualization, Project administration, Writing – review & editing, and Supervision. Sultan S. Al-Thagfan: Conceptualization, Project administration, Writing – review & editing, and Supervision. Yasser M. Alahmadi: Conceptualization, Methodology, Investigation, Validation, Data curation, and Writing – original draft. Yasser A. Mohammed: Conceptualization, Methodology, Investigation, Validation, Data curation, and Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors express their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work (project no. 112/442) Also, the authors would like to extend their thanks to Taibah University for its supervision support.
References
- 1.Sharma D.K., Sawyer R.K., Robertson T.S., Stamenkov R., Solomon L.B., Atkins G.J., Clifton P.M., Morris H.A., Anderson P.H. Elevated serum 25-hydroxyvitamin D levels are associated with improved bone formation and micro-structural measures in elderly hip fracture patients. J. Clin. Med. 2019;8(11):1988–1994. doi: 10.3390/jcm8111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell T.D., Demay M.B., Burnett-Bowie S.A. The biology and pathology of vitamin D control in bone. J. Cell. Biochem. 2010;111(1):7–13. doi: 10.1002/jcb.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norman A.W. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008;88(2):491s–499s. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 4.Holick M.F. Vitamin D status: measurement, interpretation, and clinical application. Ann. Epidemiol. 2009;19(2):73–88. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R., Naughton D.P. Vitamin D in health and disease: current perspectives. Nutr. J. 2010;9:65–77. doi: 10.1186/1475-2891-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Thagfan S.S., Ahmed S., Emara M.M., Awadallah M.F. Impacts of deficiency in vitamin D derivatives on disease severity in adult bronchial asthma patients. Pulm. Pharmacol. Therapeut. 2021:102073–102077. doi: 10.1016/j.pupt.2021.102073. [DOI] [PubMed] [Google Scholar]
- 7.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barragry J.M., France M.W., Boucher B.J., Cohen R.D. Metabolism of intravenously administered cholecalciferol in man. Clin. Endocrinol. 1979;11(5):491–495. doi: 10.1111/j.1365-2265.1979.tb03101.x. [DOI] [PubMed] [Google Scholar]
- 9.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health. 2020;13(10):1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mardani R., Alamdary A., Mousavi Nasab S.D., Gholami R., Ahmadi N., Gholami A. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020;289:198148–198152. doi: 10.1016/j.virusres.2020.198148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campi I., Gennari L., Merlotti D., Mingiano C., Frosali A., Giovanelli L., Torlasco C., Pengo M.F., Heilbron F., Soranna D., Zambon A., Di Stefano M., Aresta C., Bonomi M., Cangiano B., Favero V., Fatti L., Perego G.B., Chiodini I., Parati G., Persani L. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect. Dis. 2021;21(1):566–572. doi: 10.1186/s12879-021-06281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crafa A., Cannarella R., Condorelli R.A., Mongioì L.M., Barbagallo F., Aversa A., La Vignera S., Calogero A.E. Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: a systematic review and meta-analysis. EClinicalMedicine. 2021;37:100967–100980. doi: 10.1016/j.eclinm.2021.100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser W.D., Tang J.C.Y., Dutton J.J., Schoenmakers I. Vitamin D measurement, the debates continue, new analytes have emerged, developments have variable outcomes. Calcif. Tissue Int. 2020;106(1):3–13. doi: 10.1007/s00223-019-00620-2. [DOI] [PubMed] [Google Scholar]
- 14.Ding S., Schoenmakers I., Jones K., Koulman A., Prentice A., Volmer D.A. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Anal. Bioanal. Chem. 2010;398(2):779–789. doi: 10.1007/s00216-010-3993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geno K.A., Tolan N.V., Singh R.J., Nerenz R.D. Improved recognition of 25-hydroxyvitamin D2 by 2 automated immunoassays. J. Appl. Lab Med. 2020;5(6):1287–1295. doi: 10.1093/jalm/jfaa070. [DOI] [PubMed] [Google Scholar]
- 16.Li L., Zeng Q., Yuan J., Xie Z. Performance evaluation of two immunoassays for 25-hydroxyvitamin D. J. Clin. Biochem. Nutr. 2016;58(3):186–192. doi: 10.3164/jcbn.15-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah I., James R., Barker J., Petroczi A., Naughton D.P. Misleading measures in Vitamin D analysis: a novel LC-MS/MS assay to account for epimers and isobars. Nutr. J. 2011;10:46–54. doi: 10.1186/1475-2891-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimada K., Mitamura K., Kitama N. Quantitative determination of 25-hydroxyvitamin D3 3-sulphate in human plasma using high performance liquid chromatography. Biomed. Chromatogr. 1995;9(5):229–232. doi: 10.1002/bmc.1130090508. [DOI] [PubMed] [Google Scholar]
- 19.Coldwell R.D., Trafford D.J.H., Makin H.L.J. Mass fragmentographic assay for 25-hydroxyvitamin D in plasma without derivatization: enhanced sensitivity for metabolites of vitamins D2 and D3 after pre-column dehydration. J. Mass Spectrom. 1995;30(2):348–356. [Google Scholar]
- 20.Zelzer S., Goessler W., Herrmann M. Measurement of vitamin D metabolites by mass spectrometry, an analytical challenge. J. Lab. Precis Med. 2018;3:99–112. [Google Scholar]
- 21.Yeung B., Vouros P., Reddy G.S. Characterization of vitamin D3 metabolites using continuous-flow fast atom bombardment tandem mass spectrometry and high-performance liquid chromatography. J. Chromatogr. 1993;645(1):115–123. doi: 10.1016/0021-9673(93)80625-i. [DOI] [PubMed] [Google Scholar]
- 22.Watson D., Setchell K.D.R., Ross R. Analysis of vitamin D and its metabolites using thermospray liquid chromatography/mass spectrometry. Biomed. Chromatogr. 1991;5(4):153–160. doi: 10.1002/bmc.1130050404. [DOI] [PubMed] [Google Scholar]
- 23.Alshabrawy A.K., Bergamin A., Sharma D.K., Hickey S.M., Brooks D.A., O'Loughlin P., Wiese M.D., Anderson P.H. LC-MS/MS analysis of vitamin D3 metabolites in human serum using a salting-out based liquid-liquid extraction and DAPTAD derivatization. J. Chromatogr. B. 2021;1173:122654–122660. doi: 10.1016/j.jchromb.2021.122654. [DOI] [PubMed] [Google Scholar]
- 24.Hedman C.J., Wiebe D.A., Dey S., Plath J., Kemnitz J.W., Ziegler T.E. Development of a sensitive LC/MS/MS method for vitamin D metabolites: 1,25 Dihydroxyvitamin D2&3 measurement using a novel derivatization agent. J. Chromatogr. B. 2014;953–954:62–77. doi: 10.1016/j.jchromb.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Senn T., Kalhorn T., Zheng X.E., Zheng S., Davis C.L., Hebert M.F., Lin Y.S., Thummel K.E. Simultaneous measurement of plasma vitamin D(3) metabolites, including 4β,25-dihydroxyvitamin D(3), using liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2011;418(1):126–133. doi: 10.1016/j.ab.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouweland J.M.W.v.d. Analysis of vitamin D metabolites by liquid chromatography-tandem mass spectrometry. Trends Anal. Chem. 2016;84:117–130. [Google Scholar]
- 27.Tai S.S.C., Nelson M.A. Candidate reference measurement procedure for the determination of (24r),25-dihydroxyvitamin D3 in human serum using isotope-dilution liquid chromatography–tandem mass spectrometry. Anal. Chem. 2015;87(15):7964–7970. doi: 10.1021/acs.analchem.5b01861. [DOI] [PubMed] [Google Scholar]
- 28.Adamec J., Jannasch A., Huang J., Hohman E., Fleet J.C., Peacock M., Ferruzzi M.G., Martin B., Weaver C.M. Development and optimization of an LC-MS/MS-based method for simultaneous quantification of vitamin D2 , vitamin D3 , 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3. J. Separ. Sci. 2011;34(1):11–20. doi: 10.1002/jssc.201000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Ac A., Segura P.A., Viglino L., Gagnon C., Sauvé S. Comparison of APPI, APCI and ESI for the LC-MS/MS analysis of bezafibrate, cyclophosphamide, enalapril, methotrexate and orlistat in municipal wastewater. J. Mass Spectrom. 2011;46(4):383–390. doi: 10.1002/jms.1904. [DOI] [PubMed] [Google Scholar]
- 30.Aronov P.A., Hall L.M., Dettmer K., Stephensen C.B., Hammock B.D. Metabolic profiling of major vitamin D metabolites using Diels–Alder derivatization and ultra-performance liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2008;391(5):1917–1922. doi: 10.1007/s00216-008-2095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann M., Gallagher J.C., Peacock M., Schlingmann K.-P., Konrad M., DeLuca H.F., Sigueiro R., Lopez B., Mourino A., Maestro M., St-Arnaud R., Finkelstein J.S., Cooper D.P., Jones G. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J. Clin. Endocrinol. Metab. 2014;99(7):2567–2574. doi: 10.1210/jc.2013-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan D., Yang J., Barnych B., Hwang S.H., Lee K.S.S., Cui Y., Niu J., Watsky M.A., Hammock B.D. A new sensitive LC/MS/MS analysis of vitamin D metabolites using a click derivatization reagent, 2-nitrosopyridine. J. Lipid Res. 2017;58(4):798–808. doi: 10.1194/jlr.D073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damm M., Rechberger G., Kollroser M., Kappe C.O. An evaluation of microwave-assisted derivatization procedures using hyphenated mass spectrometric techniques. J. Chromatogr. A. 2009;1216(31):5875–5881. doi: 10.1016/j.chroma.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Aziz H.A., Ghabbour H.A., Bhat M.A., Fun H.-K. Microwave-assisted synthesis and characterization of certain oximes, hydrazones, and olefins derived from β-Keto sulfones. J. Chem. 2014;2014:532467–532473. [Google Scholar]
- 35.Ahmed S., Atia N.N. Controlled microwave derivatization reaction for reproducible trace analysis of budesonide in human plasma. Anal. Chim. Acta. 2019;1048:132–142. doi: 10.1016/j.aca.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Department of Health and Human Services, Food and Drug Administration . Regist.; Rockville, MD: 2001. Guidance for Industry on Bioanalytical Method Validation, Fed. [Google Scholar]
- 37.Sauer J., Sustmann R. Mechanistic aspects of diels-alder reactions: a critical survey. Angew Chem. Int. Ed. Engl. 1980;19(10):779–807. [Google Scholar]
- 38.Kappe C.O. Controlled microwave heating in modern organic synthesis. Angew Chem. Int. Ed. Engl. 2004;43(46):6250–6284. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]
- 39.La Regina G., Gatti V., Piscitelli F., Silvestri R. Open vessel and cooling while heating microwave-assisted synthesis of Pyridinyl N-aryl hydrazones. ACS Comb. Sci. 2011;13(1):2–6. doi: 10.1021/co100015b. [DOI] [PubMed] [Google Scholar]
- 40.Giavarina D. Understanding Bland altman analysis. Biochem. Med. 2015;25(2):141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]