Resistance to all major groups of antibiotics has arisen hand in hand with their extensive use in medicine and animal husbandry, and macrolide antibiotics are no exception. The therapeutic utility of macrolides has been severely compromised by the emergence of drug resistance in many pathogenic bacteria. The molecular mechanisms by which bacteria become resistant are manifold, but in general these can be collectively characterized as involving either drug efflux, drug inactivation, or alterations in the drug target site. The target site for macrolides is the large (50S) subunit of the bacterial ribosome. Many cases of macrolide resistance in clinical strains can be linked to alteration of specific nucleotides in 23S rRNA within the large ribosomal subunit.
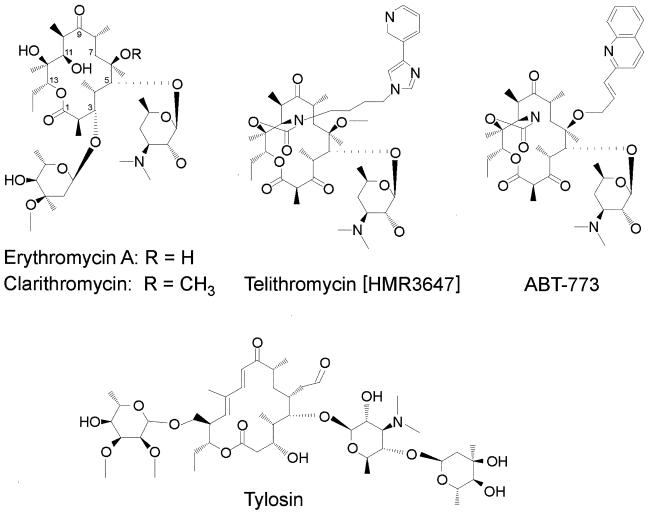
Macrolides are natural polyketide products of secondary metabolism in many actinomycete species (51, 140). Clinically useful macrolides consist of a 14-, 15-, or 16-member lactone ring (Table 1) that is generally substituted with two or more neutral and/or amino sugars (16). The structures of the 14- and 16-member-ring macrolides erythromycin and tylosin and of some semisynthetic erythromycin derivatives are shown in Fig. 1. The inhibitory action of erythromycin, and probably that of the other 14-member-ring macrolides, is effected at the early stages of protein synthesis when the drug blocks the growth of the nascent peptide chain (7, 140), presumably causing premature dissociation of the peptidyl-tRNA from the ribosome (85). The antimicrobial action of these drugs is compounded by their inhibition of the assembly of new large ribosomal subunits, which leads to gradual depletion of functional ribosomes in the cell (23). The mode of action of the 16-member-ring macrolides is less well characterized, although it is clear that they bind to the same region of the large subunit as the 14-member-ring macrolides and inhibit peptide bond formation in a more direct manner (reviewed in reference 140).
TABLE 1.
Macrolide antibiotics and their derivatives discussed in this review
| Antibiotic(s) | Phenotype designation | Mol wt | Lactone ring size | Description |
|---|---|---|---|---|
| ABT-773 | 765 | 14 | Ketolide | |
| Azithromycin | Azm | 749 | 15 | Azalide |
| Carbomycin | Cbm | 842 | 16 | Macrolide |
| Clarithromycin | Clr | 748 | 14 | Macrolide |
| Erythromycin A | Ery | 734 | 14 | Macrolide |
| Josamycin | 828 | 16 | Macrolide | |
| Spiramycin I, II, III | Spi | 843, 885, 899 | 16 | Macrolide |
| Telithromycin | Tel | 812 | 14 | Ketolide |
| Tylosin | Tyl | 916 | 16 | Macrolide |
| Macrolides | M14 | 14 | 14-member ring only | |
| M16 | 16 | 16-member ring only | ||
| Mac | All macrolides |
FIG. 1.
Selected clinically important macrolide antibiotics and their derivatives. Two naturally occurring macrolides are shown: erythromycin A, which was the first therapeutic macrolide and possesses a 14-member ring, and tylosin, a 16-member-ring macrolide which has been used extensively in the farming industry both therapeutically and as a growth promoter. Clarithromycin is the 6-methoxy derivative of erythromycin and is presently the drug of choice in H. pylori eradication. The ketolides telithromycin and ABT-773 represent the most recent generation of drugs and are characterized by the 3-ketone group that substitutes the 3-cladinose sugar residue in erythromycin and clarithromycin. Both ketolides have a C-11–C-12 carbamate, which is extended by an alkyl-aryl group in the case of telithromycin. This extension enables telithromycin to make an alternative interaction with domain II of 23S rRNA (see text). Both ketolides are presently undergoing clinical trials, with ABT-773 in the early stage and telithromycin in the final stage of the process.
Shortly after the introduction of erythromycin in therapy in the 1950s, resistance to the drug was observed in bacterial pathogens (reviewed in reference 76). More disquieting was the observation that erythromycin-resistant strains were cross-resistant not only to all other macrolides but also to the chemically unrelated lincosamide and streptogramin B drugs. This phenomenon was first observed in Staphylococcus aureus and came to be termed the macrolide-lincosamide-streptogramin B (MLSB) antibiotic resistance phenotype. In these S. aureus strains, MLSB resistance can be induced by exposure to low concentrations of erythromycin (151), which leads to expression of a methyltransferase enzyme (ErmC). ErmC specifically methylates 23S rRNA (74) at the N-6 position of adenosine 2058 (A2058) (Escherichia coli numbering) (121), which is a pivotal nucleotide for the binding of MLSB antibiotics (see below). Subsequently, several dozen erm methyltransferase genes have been identified. Many of these are constitutively expressed, and their products all presumably methylate A2058. A new nomenclature system has recently been proposed for the different erm genes, which clarifies their phylogenetic relatedness (105). For a comprehensive account of the action of Erm methyltransferases, see the review by Weisblum (149).
Since the discovery of erm genes, another means of resistance involving alteration of rRNA structure has been identified. Under laboratory conditions, single base substitutions introduced into rRNA were shown to confer macrolide resistance. This form of resistance was first observed in the single rRNA (rrn) operon of yeast mitochondria, which was mutated at position A2058 in the large-subunit rRNA (123). Shortly afterwards, similar phenotypes were obtained in E. coli by expression of mutant rrn alleles from multiple-copy plasmids (see, e.g., references 120 and 143). About 6 years ago, reports of rRNA mutations conferring macrolide resistance in clinical pathogens began to appear in the literature. While it is conceptually gratifying to establish that the mutations appearing in pathogens are identical to those previously isolated in laboratory strains, the clinical implications of this are quite disturbing. The 23S rRNA mutations reported so far to cause macrolide resistance are shown in Table 2. Generally, pathogenic species that develop macrolide resistance through mutations at A2058 (or neighboring nucleotides) possess only one or two rrn operons, such as in the case of Helicobacter pylori and Mycobacterium species. Resistance in bacteria with multiple rrn operons, such as Enterococcus, Streptococcus, and Staphylococcus species, is generally conferred by Erm methylation of A2058 (Table 3) or by efflux (see e.g., references 70 and 110). However, there are cases of macrolide resistance by drug inactivation (reviewed in reference 150), and there are recent reports of macrolide resistance in Streptococcus pneumoniae strains conferred by mutations in ribosomal proteins L4 and L22 and in rRNA (129; P. Appelbaum, personal communication). Macrolide and ketolide resistance is additionally conferred in E. coli by the expression of small, specific peptides (134), although the level of resistance is probably too low to be a problem in the treatment of clinical strains.
TABLE 2.
23S rRNA mutations reported to confer macrolide resistance
| E. coli 23S rRNA positiona | Organismb | Nucleotide(s)
|
Phenotypec | Reference(s) | |
|---|---|---|---|---|---|
| Wild type | Mutant | ||||
| 754 | Escherichia coli | U | A | Erylr Tellr | 156 |
| 2057 | Chlamydomonas reinhardtii chloroplast | G | A | Eryr Lins | 63 |
| Escherichia coli | G | A | Eryr M16s Lins SBs | 47 | |
| Propionibacteria | G | A | Erylr M16s | 111 | |
| 2057+ | Escherichia coli | G+G | A+A | Eryr Linr | 39 |
| 2032 | Helicobacter pylori | A+G | G+A | Clrr Azmr Eryr | 64 |
| 2058 | Brachyspira hyodysenteriae | A | G, U | Eryr Tylr Linr | 69 |
| Chlamydomonas reinhardtii chloroplast | A | G | Eryr Linr | 63 | |
| Escherichia coli | A | G | Eryr Linr | 39, 143 | |
| A | U | MLSBr | 120 | ||
| Helicobacter pylori | A | C | Clrr | 125 | |
| Macr Linr | 94 | ||||
| MLSBr | 148 | ||||
| Clar | 34 | ||||
| A | G | Clar | 142 | ||
| Macr Linp | 94 | ||||
| MLSBp | 148 | ||||
| Clar | 34 | ||||
| A | U | MLSBr | 148 | ||
| Clar | 34 | ||||
| Mycobacterium abscessus | A | G | Clrr | 146 | |
| Mycobacterium avium | A | C, G, U | Clrr | 90 | |
| Mycobacterium chelonae | A | C, G | Clrr | 146 | |
| Mycobacterium intracellulare | A | C, G, U | Clrr | 84 | |
| Mycobacterium kansasii | A | U | Clrr | 18 | |
| Mycobacterium smegmatis | A | G | Clrr | 113 | |
| Mycoplasma pneumoniae | A | G | Eryhr Spimr Tyls Linhr | 79 | |
| Propionibacteria | A | G | MLSBr | 111 | |
| Streptococcus pneumoniae | A | G | MLSBr | 129 | |
| Streptomyces ambofaciens | A | G | MLSBr | 98 | |
| Saccharomyces cerevisiae mitochondrion | A | G | Eryr | 123 | |
| Treponema pallidum | A | G | Eryr | L. V. Stamm and H. L. Bergen, Letter, Antimicrob. Agents Chemother. 44:806–807, 2000 | |
| 2059 | Helicobacter pylori | A | C | Macr Linr SBs | 148 |
| Clrr | 34 | ||||
| A | G | Clrr | 142 | ||
| Macr Linr | 94 | ||||
| Macr Linr SBs | 148 | ||||
| Clar | 34 | ||||
| Mycobacterium abscessus | A | C,G | Clrr | 146 | |
| Mycobacterium chelonae | A | G | Clrr | 146 | |
| Mycobacterium intracellulare | A | C | Clrr Azmr | 84 | |
| Mycobacterium avium | A | C | Clrr Azmr | 84 | |
| Mycobacterium smegmatis | A | G | Clrr | 113 | |
| Mycoplasma pneumoniae | A | G | Erymr Spihr Tyllr Linmr | 79 | |
| Streptococcus pneumoniae | A | G | Macr | 129 | |
| Propionibacteria | A | G | Machr Linlr | 111 | |
| 2452 | Sulfolobus acidocaldarius | C | U | Cbmr Linr | 1 |
| 2611 | Chlamydomonas moewusii chloroplast | C | G | Eryr Spilr | 54 |
| Chlamydomonas reinhardtii chloroplast | C | G, U | Eryr Linmr | 63 | |
| Escherichia coli | C | U | Eryr Spis Tyls Lins | 139 | |
| Streptococcus pneumoniae | C | A, G | Macr SBs | 129 | |
| Saccharomyces cerevisiae mitochrondrion | C | G | Eryr Spir | 122 | |
| Saccharomyces cerevisiae mitochondrion | C | U | Erys Spir | 122 | |
Nucleotide positions are numbered according to the corresponding positions in E. coli 23S rRNA. Consistent use of the E. coli system facilitates comparison between the different organisms and avoids discrepancies in some of the other notation systems, such as that for H. pylori (132).
Pathogenic organisms are in boldface (the E. coli strains are nonvirulent laboratory strains).
The phenotypes conferred to the different types of macrolide antibiotics are given when these were specified in the original articles (the lack of a notation does not imply sensitivity but merely indicates that no specific phenotype was reported). Similarly, in some reports the levels of resistance are arbitrarily categorized, and when this is the case these are recounted here (r, resistant; s, sensitive; h, high; m, medium; l, low). Phenotype designations: Lin, lincosamides; SB, streptogramin B group; MLSB, macrolides, lincosamides, and streptogramin B. Other designations are given in Table 1.
TABLE 3.
Macrolide resistance mechanisms found in some pathogens and their numbers of rRNA operons
| Organism | Mechanism (reference[s]a) | No. of rRNA operons | Reference(s) for rRNA operons |
|---|---|---|---|
| Brachyspira hyodysenteriae | 23S RNA mutation | 1 | 157 |
| Mycoplasma pneumoniae | 23S RNA mutation | 1 | 55 |
| Mycobacterium chelonae | 23S RNA mutation | 1 | 146 |
| Mycobacterium abscessus | 23S RNA mutation | 1 | 146 |
| Mycobacterium avium | 23S RNA mutation | 1 | 84, 90 |
| Mycobacterium intracellulare | 23S RNA mutation | 1 | 13, 84, 90 |
| Propionibacterium avidum | 23S RNA mutation | 1 | 111 |
| Helicobacter pylori | 23S RNA mutation | 2 | 17, 68, 133 |
| Propionibacterium granulosum | 23S RNA mutation | 2 | 111 |
| Treponema pallidum | 23S RNA mutation | 2 | 22 |
| Propionibacterium acnes | 23S RNA mutation | 3 | 111 |
| Streptococcus pneumoniae | 23S RNA mutation | 4 | 129; Tait-Kamradt et al., Abstr. ICMASKO V Meet. |
| Corynebacterium diphtheriae | erm (149) | NAb | |
| Neisseria gonorrhoeae | erm and efflux (61, 104) | 4 | 14 |
| Enterococcus | erm (67) | 4–6 | 116, 117 |
| Lactobacillus reuteri | erm (149) | NA | |
| Bacillus anthracis | erm (149) | NA | |
| Bacteroides fragilis | erm (149) | NA | |
| Staphylococcus | erm and efflux (43) | NA | |
| Staphylococcus aureus | erm (67) | 6 | 145 |
| Streptococcus pneumoniae | Ribosomal protein L4 (129; Tait-Kamradt et al., Abstr. ICMASKO V Meet.) | 4 | 129; Tait-Kamradt et al., Abstr. ICMASKO V Meet. |
| Streptococcus pneumoniae | erm and efflux (73, 128) | 4 or 6 | 10, 53, 129; Tait-Kamradt et al., Abstr. ICMASKO V Meet. |
| Streptococcus agalactiae | erm and efflux (28, 149) | 6 | 37 |
| Streptococcus pyogenes | erm and efflux (70, 128) | 6 | 128 |
| Clostridium perfringens | erm (149) | 9 | 19 |
In the following sections of this review, we first look at the current state of knowledge of the bacterial ribosome target site for macrolide antibiotics. A detailed model of a drug target site is a prerequisite for understanding the molecular mechanisms of drug binding and drug resistance and for rational design of new drugs. Our present state of knowledge, although far from being complete, supports the view that the macrolide target site is highly conserved within the ribosomes of all bacteria. We then direct attention to the pathogens, and in particular to H. pylori, that have been shown to attain resistance by rRNA mutation, and we consider the possibility of this form of resistance emerging in other pathogens. Finally, some suggestions are made regarding how future macrolide derivatives might be best equipped to combat bacteria with resistant rRNAs.
THE RIBOSOME TARGET FOR MACROLIDES
The drug binding site.
Our knowledge of the tertiary structure of the ribosome has increased enormously within the last year. Models at resolutions approaching 5 Å have been obtained by X-ray crystallographic analysis of the small (30S) (29) and large (50S) subunits (11), as well as of the functional 70S ribosome complex of these two subunits (21). In addition to this, the structure of the ribosome at specific steps of protein synthesis has been deduced by cryoelectron microscopy (see, e.g., references 4 and 124), albeit at lower resolution. The macrolide binding site is presumably situated at the base of the deep cleft that provides access to the peptide exit channel of the large subunit (11, 21). This is at, or very close to, the location where the aminoacyl and peptidyl ends of tRNAs become aligned within the large subunit to catalyze the formation of peptide bonds. The X-ray crystallographers promise data at even better resolution in the near future, which will eventually reveal the molecular details of the antibiotic binding sites (see Addendum in Proof). For the moment, however, we must rely heavily on biochemical and molecular genetic data for our understanding of macrolide binding.
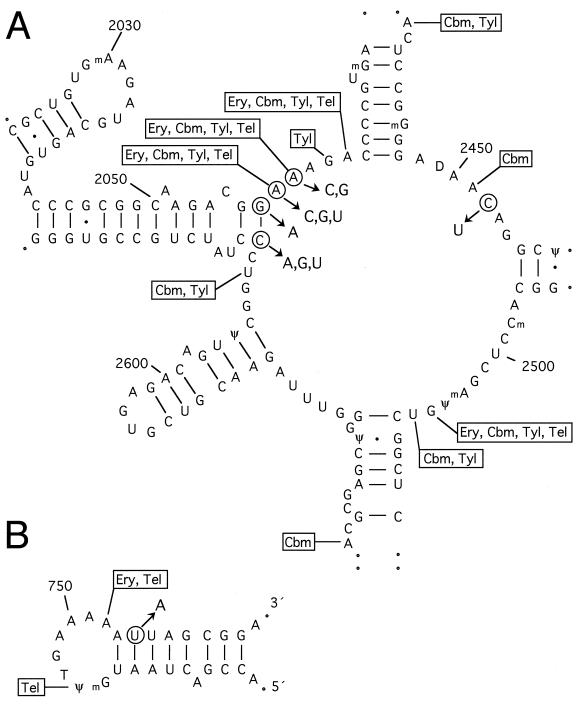
The site of peptide bond formation on the large ribosomal subunit (the peptidyl transferase center) is associated with the central loop in domain V of 23S rRNA (Fig. 2) (32, 93). The interactions of macrolides, and other MLSB drugs, have been mapped here by chemical footprinting (39, 40, 62, 87, 99, 107, 138, 156). The 16-member-ring macrolides seem to make more extensive interactions in this rRNA region than the 14-member-ring macrolides (Fig. 2), which is undoubtedly related to the respective manner in which the drugs interfere with protein synthesis.
FIG. 2.
Secondary-structure models of the peptidyl transferase center in domain V of 23S rRNA (A) and hairpin 35 in domain II (B) (60). Nucleotides at which macrolide drugs interact (as defined by chemical footprinting experiments) are indicated (62, 87, 107, 156). The circled nucleotides indicate the positions of mutations that confer macrolide drug resistance in bacterial pathogens and laboratory strains (details and references are given in Table 2). These data are depicted here on the secondary structure of the E. coli rRNA; the rRNA secondary structures of all other organisms are believed to be the same (60, 93). The single-stranded nucleotides involved in macrolide interaction and resistance are conserved in all of the wild-type bacterial rRNAs discussed in this review. However, the identities of the base-paired nucleotides (at positions 754, 2057, and 2611) can vary between different bacteria (see text). Drug abbreviations and classifications are giving in Table 1. Erythromycin and clarithromycin interaction sites on the rRNA are identical.
The interaction sites of erythromycin and ketolide derivatives have additionally been mapped to hairpin 35 in domain II of the rRNA (Fig. 2) (62, 156). A single molecule of erythromycin binds per large ribosomal subunit (reviewed in reference 140), and this holds true for the ketolide derivatives (62), indicating that the same drug molecule simultaneously contacts domains II and V of 23S rRNA. As these drugs are small relative to the ribosome, such interactions would be possible only if the rRNA is folded so that hairpin 35 and the peptidyl transferase loop are adjacent. Evidence from other approaches, including phylogenetic comparisons of rRNA sequences (60) and RNA cross-linking (88), strongly supports the idea of contact between these two rRNA regions. In addition, mutations in ribosomal proteins L4 and L22 that confer erythromycin resistance in E. coli laboratory strains (27, 96, 152) presumably do so by perturbing the 23S rRNA structure. In the resistant L22 mutant, the configuration of the hairpin 35 loop is clearly affected (59). Most recent evidence indicates that the 16-member-ring macrolide tylosin also interacts with the peptidyl transferase and hairpin 35 loops. Two resistance determinants, tlrA and tlrD in the tylosin-producing actinomycete Streptomyces fradiae, encode Erm homologs that methylate A2058 (149), whereas a third resistance determinant, tlrB, encodes another type of methyltransferase that methylates G748 in the hairpin 35 loop (reference 80 and see Addendum in Proof).
The structure of the MLSB drug binding pocket within the large ribosomal subunit is defined by the tertiary configuration of 23S rRNA. Hairpin 35 and the peptidyl transferase loop seem to be the main, although not the sole, components of this binding pocket. Nucleotide 2032 within the loop of 23S rRNA hairpin 72 is also implicated. Mutations at this nucleotide confer resistance to lincosamides (31, 39) but increase sensitivity to erythromycin (39) and perturb the peptidyl transferase loop structure (41). Also, several nucleotides within helices radiating from the peptidyl transferase loop interact with the aminoacyl end of tRNA (92), which places these regions near the site of peptide bond formation. The elucidation of an exact model of the tertiary folding and spatial orientation of these 23S rRNA components is beyond the scope of biochemical and molecular genetic approaches and is now in the hands of the X-ray crystallographers. However, the data that are presently available do enable us to go quite some way towards understanding the mechanisms of macrolide binding and resistance and make it possible to predict what new resistant strains might emerge and how these could best be combated therapeutically.
rRNA mutations confer resistance.
The rRNA mutations reported for laboratory and clinical strains that have relevance for macrolide binding and resistance are listed in Table 2. Pertinent information on cross-resistance to other MLSB drugs is included. Mutations at A2058, or at A2059 for certain macrolides, confer the highest levels of resistance. All of the mutations in Table 2 presumably, to greater or lesser degrees, perturb the structure of the drug binding pocket and thereby reduce the ability of the drug to interact with and inhibit ribosomes (41, 94). Methylation of the rRNA at A2058 by Erm methyltransferases is thought to confer resistance by a similar mechanism (56). Lower-level drug resistance is provided by mutations at positions 2057, 2452, and 2611 (Fig. 2), which are close by in the secondary structure although slightly outside the focal point of macrolide interaction. Low-level macrolide resistance is conferred in an E. coli laboratory strain by a mutation at position 754 in hairpin 35 (156), which provides additional support for the proximity of this hairpin and the peptidyl transferase loop in the rRNA tertiary structure.
It can be seen from the data in Table 2 that while all of the mutations discovered in clinical strains have also been observed in laboratory strains, the converse is not the case. The distinction is that rRNA resistance mutations in a clinical pathogen often first become apparent after a drug therapy program has failed to eradicate the pathogen. Drug therapies are generally as aggressive as is expedient, and thus strains containing mutations that confer the highest resistance will be selected. In contrast, rRNA mutations created under laboratory conditions have been done so intentionally to increase our understanding of drug interaction mechanisms. Under the controlled conditions of the laboratory, a range of less effective resistance phenotypes can be nurtured. Such rRNA mutations are useful in helping us to delineate the macrolide interaction site on the ribosome, but, unless they segregate with another resistance mechanism, it is not expected that they will be observed in clinical isolates. Clinical pathogens in which rRNA mutations have been shown to confer macrolide resistance are considered below.
(i) Resistance in H. pylori.
H. pylori colonizes the stomach in over 30% of the adult population. Although the majority of infections are asymptomatic, H. pylori is nevertheless the main etiological agent in most duodenal and many gastric ulcers; H. pylori has also been linked with the development of some types of gastric cancer (30). The preferred treatment for aggressive infections is a drug combination including the erythromycin derivative clarithromycin (Fig. 1), which has improved acid stability and uptake properties compared to erythromycin (57). H. pylori is susceptible to many antibiotics in vitro, although treatment in vivo is less trivial as the stomach is a difficult environment in which to carrying out successful antimicrobial therapy (58). Clinical treatment often entails multiple drug therapy, consisting of two antimicrobial agents in addition to a proton pump inhibitor, with bismuth as an extra option (reference 97 and references therein).
Recently, clarithromycin resistance was shown to arise during drug therapy and was traced to mutations at positions A2058 or A2059 in the 23S rRNA (142). A number of similar reports have subsequently been made (Table 2). No erm genes or macrolide efflux systems have yet been found in H. pylori despite searches for them (35, 64), and resistance mechanisms thus seem to be confined to rRNA mutations. The presence of a gastric H. pylori infection can be rapidly ascertained by any of several methods (see, e.g., references 5, 26 and 136), although more-involved procedures are required to establish whether the infecting strain has 23S rRNA mutations that confer macrolide resistance. H. pylori is slow to culture in vitro, and thus microbiological approaches to determine a resistance profile are often inappropriate in the case of an acute infection. A solution to this problem is offered by techniques based on PCR that facilitate rapid analysis of a relatively small number of H. pylori cells in a gastric juice or gastric biopsy sample. The H. pylori 23S RNA gene region around nucleotide A2058 has been amplified and analyzed for altered restriction enzyme patterns (82, 94, 119) and by hybridization to oligonucleotide probes (83, 137). Such methods are potentially valuable tools for optimizing drug therapy and avoiding relapse, and it is envisaged that they will also be used to identify resistance in other slow-growing bacteria with few RNA operons.
(ii) Resistance in other pathogens.
Erythromycin-resistant isolates of Mycoplasma pneumoniae with A2058G and A2059G mutations display phenotypes similar to those of H. pylori mutants (79). The same mutations were found in resistant clinical isolates of propionibacteria, although in some isolates resistance was conferred by a G2057-to-A mutation (111). Pathogenic species of mycobacteria also develop resistance during clarithromycin treatment (references 90 and 113 and references therein). In Mycobacterium intracellulare and Mycobacterium avium all three possible base substitutions have been observed at position 2058 (84), whereas substitution at position 2059 is more restrictive (Table 2). Brachyspira hyodysenteriae, the etiological agent of swine dysentery, possesses a single rrn operon. Isolates of B. hyodysenteriae that are resistant to tylosin (which is commonly used both as a therapeutic agent and as a growth promoter in swine production) exhibited G or U substitutions at position 2058 (69). The resistance phenotypes conferred by the various base substitutions are considered in greater detail below.
PHENOTYPIC CONSEQUENCES OF TARGET SITE MUTATIONS
Phylogenetic conservation of rRNA.
Change in the structure of rRNA has been subject to severe limitations during the course of evolution. The overall shape of rRNA, determined by secondary and tertiary structural folding, is remarkably similar in all organisms (60, 93). The base sequences within the paired stems of the rRNA can vary a great deal between species, because the size and shape of stems can be maintained by a variety of different Watson-Crick and other base-pairing interactions. However, within certain single-stranded loop regions of the rRNA, such as those depicted in Fig. 2, sequences tend to be highly conserved. Nucleotide 2058 is conserved as an adenosine in all (wild-type) bacteria, whereas this position is a guanosine in most archaeal ribosomes and in all eukaryal cytoplasmic ribosomes (which are refractory to macrolides). Nucleotide 2059 is conserved as an adenosine in all organisms. The identities of the bases at positions 2057 and 2611, which form the base pair closing the neighboring stem structure (Fig. 2), are not conserved, although a Watson-Crick pair is generally found here in all organisms. A priori it might be expected that the higher the level of phylogenetic conservation of a base the more drastic would be the phenotypic consequence of changing it. Surprisingly, this is not always the case.
Genetic stability of rRNA mutations.
Depending on a nucleotide's position and functional importance in the rRNA, its substitution either can be phenotypically silent, can be deleterious, or can confer an advantage such as drug resistance. It might then be asked why a substitution such as A2058G, which obviously confers a clear advantage to the cell, has not been consolidated as the “wild-type” sequence in all bacteria. This probably reflects the fact that the phenotypic effect of a mutation may vary according to the environmental conditions. Competitive growth experiments with low levels of clarithromycin show that H. pylori with an A2058G or an A2059G mutation has a clear advantage compared to the wild-type strain or to strains with any of the other bases at these positions (147). However, in stationary-phase cultures of E. coli that are maintained in the absence of drug, A2058G mutant ribosomes are distinctly less stable than wild-type ribosomes (2). The advantage conferred by A2058G in the presence of macrolides must be weighed against any disadvantage of having this substitution in the absence of drug and whether the disadvantage can be ameliorated by other factors. The biological cost of maintaining such mutations will determine how stable they are in pathogen rRNA, which in turn is important for determining subsequent drug therapy.
H. pylori, when grown in vitro in the absence of antibiotic selective pressure, stably maintained the A2058G and A2059G mutations through 21 (34) and 50 (64) passages, whereas A2058U and A2059C mutations were less stable (34). It should be noted, however, that another study showed a considerable loss of resistance over only five generations (155), although here the genetic basis for the resistance was not known. In a clinical setting, H. pylori with resistant rRNA mutations persisted in patients 3 months after completion of an unsuccessfully therapy with clarithromycin (64). In other drug resistance systems it has been shown that the biological cost of maintaining a resistance mutation can be alleviated by a second mutation at another site (6). Possibly a second-site mutation in the rRNA or in another ribosomal component allows the mutations at positions 2058 and 2059 to be maintained at no extra cost to the bacterium. Whether such second-site mutations exist and whether they compensate for the initial mutation under all growth conditions are not presently known.
Clinically important rRNA mutations.
Given the conservation in structure and function of ribosomes, it is tempting to predict that identical mutations will give the same phenotype in different bacterial species. This seems to be generally the case, although a few disparities exist. The sites of rRNA mutations conferring macrolide resistance in clinical pathogens are considered in detail below.
(i) Position 2057.
The occurrence of mutations at position 2057 in clinical isolates is presently limited to a group of erythromycin-resistant propionibacteria (111) and to a clarithromycin-resistant, double mutant strain of H. pylori (Table 2). The latter strain contained a mutation at position 2032 in addition to the 2057 substitution (64), although the 2057 substitution most likely determines the macrolide-resistant phenotype (39). The 2057 substitutions disrupt the 2057-2611 base pair at the end of the stem adjacent to the drug interaction site (Fig. 2). This confers low-level resistance to 14-member-ring macrolides and no resistance to 16-member-ring macrolides (47, 111). Substitution of position 2611 results in a similar disruption in the rRNA structure and confers a similar phenotype (139, 144). Resistant 2611 mutant isolates of S. pneumoniae have been noted after extensive in vitro selection with the macrolide derivative azithromycin (Table 1) (129).
(ii) Position A2058.
Many independent lines of evidence indicate that adenosine 2058 is the key nucleotide involved in macrolide interaction on the ribosome. A2058 to G was the first rRNA mutation shown to confer erythromycin resistance and is presently the most frequent clinically isolated substitution (38, 94, 141). Relative to other rRNA mutations, A2058G gives the highest level of resistance to 14-member-ring macrolides (34, 126, 148). The A2058G mutation does not seem to influence growth rate adversely in the absence of drug, although as mentioned above, A2058G mutant rRNA is preferentially degraded in E. coli (2).
C and U mutations at nucleotide 2058 also confer resistance (Table 2), but the phenotype apparently varies according to the organism. A2058 to C seems to be lethal in E. coli (S. Gregory, personal communication), whereas in H. pylori, A2058C confers a resistance level similar to that conferred by the G substitution (34, 94, 148). Another species discrepancy is seen with the A2058-to-U mutation, which in E. coli confers resistance to MLSB antibiotics (120), and this mutant rrn allele can be stably maintained on a plasmid without affecting growth rates in the absence of drug (our unpublished observations); however, in H. pylori, A2058 to U gives lower resistance, strongly decreases growth, and is easily lost in the absence of drug selection (34, 148). No A2058-to-U mutation has yet been identified in clinical H. pylori isolates. B. hyodysenteriae isolates selected for tylosin resistance were shown to possess either G or U at position 2058 (69). All three possible base substitutions at position 2058 have been found in two different species of Mycobacterium, where they all seem to be functional and to confer resistance (84, 90). Considering the high phylogenetic conservation of this rRNA region, it appears to be counterintuitive that a particular substitution can confer such varied phenotypes in different bacterial groups. This variation may yet be shown to be caused either by differences in the sequences of rRNA regions that interact with A2058 or by peculiarities in other ribosomal components in the individual species.
Mutations at position 2058 are the only substitutions to confer “true” MLSB resistance, defined as high resistance to all the drugs in this group. This should be viewed with the caveat that the term MLSB resistance has been assigned in a number of different ways, often without due reference to a comprehensive set of 14- and 16-member-ring macrolide, lincosamide, and streptogramin B antibiotics. Mutations that have been conclusively demonstrated to exhibit the MLSB phenotype are A2058U in E. coli (120), A2058C/G/U in H. pylori (148), A2058G in Propionibacterium spp. (111), and A2058G in Streptomyces ambofaciens (98). However, the present indications make it judicious to assume that the 2058G mutation would confer true MLSB resistance in any bacterium with a low rrn copy number. In addition, an S. pneumoniae strain with A2058G in two of its four rrn alleles exhibits the MLSB-resistant phenotype (P. Appelbaum, personal communication).
(iii) Position A2059.
As shown in Table 2, A2059-to-C or -G mutations have been found in vivo in mycobacteria, propionibacteria, H. pylori, and, most recently, S. pneumoniae. Mutations at position 2059 have also arisen under in vitro selection in M. pneumoniae, and S. pneumoniae. H. pylori 2059 mutants have lower levels of clarithromycin resistance than 2058 mutants in growth experiments in vitro (34, 148). A2059 to C in H. pylori is not very stable, and the U substitution cannot be maintained (34). The H. pylori A2059-to-G and -C mutations give moderate resistance to clarithromycin and clindamycin (a lincosamide) but no resistance to quinupristin (a streptogramin B) (148). During treatment for H. pylori infection, there seems to be variation in the relative frequency with which the 2058 and 2059 mutations occur (38, 82, 94, 141). This is probably dependent on a number of factors, including the therapeutic regimes employed (which are not always stipulated). A clinical macrolide-resistant isolate of S. pneumoniae was recently reported to contain A2059G substitutions in three of its four rrn operons (A. Tait-Kamradt, T. Davies, L. Brennan, F. Depardieu, P. Courvalin, J. Duignan, J. Petitpas, A. Walker, L. Wondrack, M. Jacobs, P. Appelbaum, and J. Sutcliffe, Abstr. 5th Int. Conf. Macrolides, Azalides, Streptogramins, Ketolides, Oxazolidinones, abstr. 2.22, 2000). It is presently unclear whether this is an exceptional case or whether this form of resistance is prevalent in pneumococci and has previously escaped detection.
In propionibacteria, A2059G confers resistance to both 14- and 16-member-ring macrolides but gives significantly higher resistance to the 16-member-ring macrolide josamycin than that seen for A2058G (111). This is consistent with the same mutation in M. pneumoniae, which confers higher resistance than the A2058G mutation to 16-member-ring macrolides such as tylosin and spiramycin (79). This could reflect subtly different modes of interaction of 14- and 16-member-ring macrolides with 23S rRNA. Both types of macrolides protect positions 2058 and 2059 from modification by dimethyl sulfate (Fig. 2), but the focus of the interaction of the bulkier 16-member-ring macrolides is possibly shifted towards position 2059.
Phenotypic variability.
Recently, reports of different H. pylori phenotypes arising from the same rRNA mutation have been made: strains with A-to-G mutations at position 2059 exhibited high resistance to erythromycin but variable levels of resistance to clarithromycin (52, 83). An explanation for these observations is not immediately clear, although to avoid conflict with a basic premise of microbial genetics (that isogenic strains will display the same phenotype under the same growth conditions), one must assume that these strains were not isogenic. Unexpectedly high diversity in the genetic footprints of H. pylori strains has been established (30) and is possibly one of the causal factors in the aberrant phenotypes. In addition, H. pylori has two rrn operons (17, 68, 133), and although both operons often contain the same mutation (34, 148), heterozygous strains, which exhibit intermediate or high levels of drug resistance have been found (64, 126, 142). Paradoxes about resistance phenotypes are best resolved using strains engineered by in vitro site-directed mutagenesis (34, 148), where the specific effect of a single substitution can be ascertained unambiguously.
FUTURE PERSPECTIVES
Predicted resistance in other pathogens.
After exposure to macrolide antibiotics, the types of rRNA mutations described above can rapidly dominate bacterial populations in which the individual cells possess only one or two rrn operons. Table 3 summarizes the relationship between the number of rrn operons in a pathogen and the mechanism by which resistance occurs. A general pattern emerges indicating that the fewer rrn operons a bacterium possesses, the greater the likelihood that macrolide resistance, if and when it arises, will be conferred by rRNA mutations. These spontaneous mutations are constantly arising at a low frequency in any bacterial population, and the drugs merely exert a selective pressure towards their proliferation. In this context, the potential influence of adaptive mutation mechanisms, which can come into play in residual populations of nondividing or slowly dividing cells (103), should also be noted.
In bacteria with multiple rrn operons, the effect of a beneficial mutation in one operon is likely to be diluted out so that it offers no significant phenotypic advantage. However, amplification of a mutant allele, so that it occupies the majority of the bacterium's rrn operons, could confer a resistant phenotype, as has been observed in S. pneumoniae (Table 2). In general, however, in bacteria with multiple rrn copies resistance is mediated by an erm-encoded methyltransferase, which can potentially modify all ribosomes, or by an efflux system such as that encoded by msrA in Staphylococcus (110). While implementation of both of these latter systems requires the acquisition of exogenous genetic material, moderate levels of macrolide resistance have been observed in Neisseria gonorrhoeae upon overexpression of an endogenous membrane transport system (61). Probably many pathogens have inherent efflux mechanisms that provide some tolerance to macrolides and other antimicrobial agents, e.g., the mmr gene in Mycobacterium tuberculosis (36) and the acrAB homolog in Haemophilus influenzae (112).
The occurrence of macrolide resistance in many bacterial pathogens remains largely undocumented. Examples of pathogens where this is the case are listed together with their rrn copy numbers in Table 4. It is predicted that there is a high potential for macrolide resistance to occur by mutations in the 23S rRNAs of the bacteria in the upper portion of the table. The probability of resistance developing would of course depend on the types and quantities of drug to which these organisms are exposed. Development of macrolide resistance in any of the remaining bacteria in the lower portion of Table 4 would be most likely linked to rRNA methylation or drug efflux. The potential risks of resistance developing by modification of endogenous efflux systems such as mtrRCDE of N. gonorrhoeae (61) or by drug inactivation remain to be assessed. So far there have only been a few reports of resistance conferred by macrolide inactivation, which include strains of enterobacteria (9, 95), an isolate of S. aureus (154), and a drug-producing actinomycete (66).
TABLE 4.
Copy numbers of rRNA operons in pathogens for which macrolide resistance mechanisms have not been reporteda
| Organism | No. of rRNA operons | Reference(s) |
|---|---|---|
| Chlamydia pneumoniae | 1 | 50 |
| Coxiella burnetii | 1 | 3 |
| Mycobacterium leprae | 1 | 118 |
| Mycobacterium tuberculosis | 1 | 13 |
| Mycoplasma genitalium | 1 | 48 |
| Mycoplasma hyopneumoniae | 1 | 130 |
| Rickettsia prowazekii | 1 | 8 |
| Borrelia burgdorferi | 2b | 33, 115 |
| Chlamydia trachomatis | 2 | 46 |
| Leptospira interrogans | 2 | 49 |
| Mycobacterium celatum | 2 | 102 |
| Mycoplasma gallisepticum | 2c | 25 |
| Bordetella pertussis | 3 | 89 |
| Campylobacter jejuni-C. coli | 3 | 71, 131 |
| Moraxella catarrhalis | 4 | 91 |
| Neisseria meningitidis | 4 | 153 |
| Pseudomonas aeruginosa | 4 | 108 |
| Bacillus cereus group | 6–10 | 101 |
| Haemophilus influenzae | 6 | 77 |
| Listeria monocytogenes | 6 | 86 |
| Salmonella spp. | 7 | 78 |
| Vibrio cholerae | 9 | 75 |
Most of these bacteria are sensitive to macrolide antibiotics, at least in vitro (see, e.g., references 12, 15, 24, 65, 72, 81, 100, 114, 127, and 135). For M. tuberculosis, some controversy exist about the effect of macrolides in vivo (81, 135). It is expected that macrolide resistance conferred by rRNA mutations is more likely to arise in the bacteria in the upper portion of the table (see text).
One 16S RNA gene and two 23S RNA genes.
One operon of rRNA genes plus a separate set of 16S and 23S RNA genes.
Drug development to overcome resistance.
Naturally occurring macrolides have been derivatized in most conceivable ways to improve their acid stability, uptake, resilience to modification and efflux, and improve ribosome binding, not least to MLSB-resistant ribosomes. The latest generation of macrolides, the ketolides, include telithromycin (HMR 3647) and ABT-773 (20), which possess a 3-keto group instead of cladinose and a carbamate at C-11–C-12 (Fig. 1). Telithromycin is presently nearing the end of clinical trials and is showing considerable promise against bacterial pathogens (see, e.g., references 44, 45, 106, and 109). Telithromycin binds to ribosomes with up to 10-fold-higher affinity than erythromycin (62), and this appears to be a direct consequence of improved contact between an alkyl-aryl extension from the C-11–C-12 carbamate of the drug and the loop of hairpin 35 in domain II of the rRNA (62, 156).
Telithromycin binding is appreciably reduced by the A2058G mutation in E. coli ribosomes, although its binding remains over 20-fold higher than that of erythromycin and clarithromycin (42). It appears that the improved domain II interaction enables the ketolide to maintain a precarious, but possibly crucial, foothold on resistant ribosomes. The drug-domain II interaction is only just beginning to be understood and is undoubtedly capable of further improvement. As discussed above, structural models of the ribosome will soon become available at a resolution that is high enough to disclose additional sites for potential drug contact. This will not only enable further macrolide and ketolide development but should reveal new ribosome targets against which novel drugs can be designed.
Future therapies against infections caused by pathogens with a low rrn copy number have the potential to be improved in several ways. A rapid pretreatment analysis of the infecting strain to ascertain the rrn genotype would facilitate an optimal choice of drugs. Prescription of a tailor-made drug cocktail, leading to quick and complete eradication of an infection, would minimize the occurrence of resistance mutations in rRNA. Previous experience has shown, however, that the best that can be hoped for is a delay in the development of bacterial resistance, which can be expected to continue to evolve and spread in step with drug development and use. It is therefore important to base therapeutic strategies upon an accurate and detailed understanding of antibiotic action and resistance mechanisms and hopefully in this way to stay one step ahead of intractable bacterial infections.
ACKNOWLEDGMENTS
We thank Alexander Mankin, Peter Appelbaum, Todd Davies, Joyce Sutcliffe, Steven Gregory, Diane Taylor, André Bryskier, and Erik Böttger for scientific discussions and for their comments on the manuscript.
This work was supported by grants from the Danish Natural Sciences Biotechnology Program, the Carlsberg Foundation, and the Danish Medical Research Council.
ADDENDUM IN PROOF
The most recent high-resolution crystallographic structure of the 50S subunit (N. Ban, P. Nissen, J. Hansen, P. B. Moore, and T. A. Steitz, Science 289:905–920, 2000) clearly reveals the positions of all the 23S rRNA nucleotides and shows how position G748 nin domain II lies close to (within 10 Å of) A2058 in domain V. The site of peptide bond formation is close by and is catalyzed by domain V of the rRNA (P. Nissen, J. Hansen, N. Ban, P. B. Moore, and T. A. Steitz, Science 289:920–930, 2000). Model of comparable resolution are also available for the 30S subunit (F. Schluenzen, A. Tocilj, R. Zarivach, J. Harms, M. Gluehmann, D. Janell, A. Bashan, H. Bartels, I. Agmon, F. Franceschi, and A. Yonath, Cell 102:615–623, 2000; B. T. Wimberly, D. E. Brodersen, W. M. Clemons, R. J. Morgan-Warren, A. P. Carter, C. Vonrhein, T. Hartsch, and V. Ramakrishnan, Nature 407:327–339, 2000). The rRNA mutations described, as well as a comprehensive list of other rRNA mutations, can be found in the rRNA database (http://ribosome.fandm.edu) that is maintained by Kathleen Triman, Franklin and Marshall College, Lancaster, Pa.
REFERENCES
- 1.Aagaard C, Phan H, Trevisanato S, Garrett R A. A spontaneous point mutation in the single 23S rRNA gene of the thermophilic arachaeon Sulfolobus acidocaldarius confers multiple drug resistance. J Bacteriol. 1994;176:7744–7747. doi: 10.1128/jb.176.24.7744-7747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard C, Rosendahl G, Dam M, Powers T, Douthwaite S. Specific structural probing of plasmid-coded ribosomal RNAs from Escherichia coli. Biochimie. 1991;73:1439–1444. doi: 10.1016/0300-9084(91)90176-2. [DOI] [PubMed] [Google Scholar]
- 3.Afseth G, Mallavia L P. Copy number of the 16S rRNA gene in Coxiella burnetii. Eur J Epidemiol. 1997;13:729–731. doi: 10.1023/a:1007384717771. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal R K, Penczek P, Grassucci R A, Li Y, Leith A, Nierhaus K H, Frank J. Direct visualization of A-, P-, and E-site transfer RNAs in the Escherichia coli ribosome. Science. 1996;271:1000–1002. doi: 10.1126/science.271.5251.1000. [DOI] [PubMed] [Google Scholar]
- 5.Andersen L P, Kiilerick S, Pedersen G, Thoreson A C, Jorgensen F, Rath J, Larsen N E, Børup O, Krogfelt K, Scheibel J, Rune S. An analysis of seven different methods to diagnose Helicobacter pylori infections. Scand J Gastroenterol. 1998;33:24–30. doi: 10.1080/00365529850166167. [DOI] [PubMed] [Google Scholar]
- 6.Andersson D I, Levin B R. The biological cost of antibiotic resistance. Curr Opin Microbiol. 1999;2:489–493. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 7.Andersson S, Kurland C G. Elongating ribosomes in vivo are refractory to erythromycin. Biochimie. 1987;69:901–904. doi: 10.1016/0300-9084(87)90218-5. [DOI] [PubMed] [Google Scholar]
- 8.Andersson S G, Zomorodipour A, Winkler H H, Kurland C G. Unusual organization of the rRNA genes in Rickettsia prowazekii. J Bacteriol. 1995;177:4171–4175. doi: 10.1128/jb.177.14.4171-4175.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthur M, Andremont A, Courvalin P. Distribution of erythromycin esterase and rRNA methylase genes in members of the family Enterobacteriaceae highly resistant to erythromycin. Antimicrob Agents Chemother. 1987;31:404–409. doi: 10.1128/aac.31.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacot C M, Reeves R H. Novel tRNA gene organization in the 16S–23S intergenic spacer of the Streptococcus pneumoniae rRNA gene cluster. J Bacteriol. 1991;173:4234–4236. doi: 10.1128/jb.173.13.4234-4236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ban N, Nissen P, Hansen J, Capel M, Moore P B, Steitz T A. Placement of protein and RNA structures into a 5 Å-resolution map of the 50S ribosomal subunit. Nature. 1999;400:841–847. doi: 10.1038/23641. [DOI] [PubMed] [Google Scholar]
- 12.Bauernfeind A. In-vitro activity of dirithromycin in comparison with other new and established macrolides. J Antimicrob Chemother. 1993;31(Suppl. C):39–49. doi: 10.1093/jac/31.suppl_c.39. [DOI] [PubMed] [Google Scholar]
- 13.Bercouvier H, Kafri O, Sela S. Mycobacteria possess a surprisingly small number of ribosomal RNA genes in relation to the size of their genome. Biochem Biophys Res Commun. 1986;136:1136–1141. doi: 10.1016/0006-291x(86)90452-3. [DOI] [PubMed] [Google Scholar]
- 14.Bihlmaier A, Römling U, Meyer T F, Tümmler B, Gibbs C P. Physical and genetic map of the Neisseria gonorrhoeae strain MS11–N198 chromosome. Mol Microbiol. 1991;5:2529–2539. doi: 10.1111/j.1365-2958.1991.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 15.Boswell F J, Andrews J M, Ashby J P, Fogarty C, Brenwald N P, Wise R. The in-vitro activity of HMR 3647, a new ketolide antimicrobial agent. J Antimicrob Chemother. 1998;42:703–709. doi: 10.1093/jac/42.6.703. [DOI] [PubMed] [Google Scholar]
- 16.Bryskier A J, Butzler J P, Neu H C, Tulkens P M. Macrolides: chemistry, pharmacology and clinical uses. Paris, France: Arnette Blackwell; 1993. [Google Scholar]
- 17.Bukanov N O, Berg D E. Ordered cosmid library and high-resolution physical-genetic map of Helicobacter pylori strain NCTC11638. Mol Microbiol. 1994;11:509–523. doi: 10.1111/j.1365-2958.1994.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 18.Burman W J, Stone B L, Brown B A, Wallace R J, Jr, Böttger E C. AIDS-related Mycobacterium kansasii infection with initial resistance to clarithromycin. Diagn Microbiol Infect Dis. 1998;31:369–371. doi: 10.1016/s0732-8893(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 19.Canard B, Cole S T. Genome organization of the anaerobic pathogen Clostridium perfringens. Proc Natl Acad Sci USA. 1989;86:6676–6680. doi: 10.1073/pnas.86.17.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capobianco J O, Cao Z, Shortridge V D, Ma Z, Flamm R K, Zhong P. Studies of the novel ketolide ABT-773: transport, binding to ribosomes, and inhibition of protein synthesis in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44:1562–1567. doi: 10.1128/aac.44.6.1562-1567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cate J H, Yusupov M M, Yusupova G Z, Earnest T N, Noller H F. X-ray crystal structures of 70S ribosome functional complexes. Science. 1999;285:2095–2104. doi: 10.1126/science.285.5436.2095. [DOI] [PubMed] [Google Scholar]
- 22.Centurion-Lara A, Castro C, van Voorhis W C, Lukehart S A. Two 16S–23S ribosomal DNA intergenic regions in different Treponema pallidum subspecies contain tRNA genes. FEMS Microbiol Lett. 1996;143:235–240. doi: 10.1111/j.1574-6968.1996.tb08486.x. [DOI] [PubMed] [Google Scholar]
- 23.Champney W S, Tober C L. Superiority of 11,12 carbonate macrolide antibiotics as inhibitors of translation and 50S ribosomal subunit formation in Staphylococcus aureus. Cells Curr Microbiol. 1999;38:342–348. doi: 10.1007/pl00006814. [DOI] [PubMed] [Google Scholar]
- 24.Charles L, Segreti J. Choosing the right macrolide antibiotic. A guide to selection. Drugs. 1997;53:349–357. doi: 10.2165/00003495-199753030-00002. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Finch L R. Novel arrangement of rRNA genes in Mycoplasma gallisepticum: separation of the 16S gene of one set from the 23S and 5S genes. J Bacteriol. 1989;171:2876–2878. doi: 10.1128/jb.171.5.2876-2878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chey W D, Murthy U, Toskes P, Carpenter S, Laine L. The 13C-urea blood test accurately detects active Helicobacter pylori infection: a United States, multicenter trial. Am J Gastroenterol. 1999;94:1522–1524. doi: 10.1111/j.1572-0241.1999.1137_r.x. [DOI] [PubMed] [Google Scholar]
- 27.Chittum H S, Champney W S. Ribosomal protein gene sequence changes in erythromycin-resistant mutants of Escherichia coli. J Bacteriol. 1994;176:6192–6198. doi: 10.1128/jb.176.20.6192-6198.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clancy J, Dib-Hajj F, Petitpas J W, Yuan W. Cloning and characterization of a novel macrolide efflux gene, mreA, from Streptococcus agalactiae. Antimicrob Agents Chemother. 1997;41:2719–2723. doi: 10.1128/aac.41.12.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemons W M, Jr, May J L, Wimberly B T, McCutcheon J P, Capel M S, Ramakrishnan V. Structure of a bacterial 30S ribosomal subunit at 5.5 A resolution. Nature. 1999;400:833–840. doi: 10.1038/23631. [DOI] [PubMed] [Google Scholar]
- 30.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 31.Cseplö A, Etzold T, Schell J, Schreier P H. Point mutations in the 23 S rRNA genes of four lincomycin resistant Nicotiana plumbaginifolia mutants could provide new selectable markers for chloroplast transformation. Mol Gen Genet. 1988;214:295–299. doi: 10.1007/BF00337724. [DOI] [PubMed] [Google Scholar]
- 32.Cundliffe E. Recognition sites for antibiotics in rRNA. In: Hill W, Dahlberg A, Garrett R A, Moore P B, Schlessinger D, Warner J, editors. The structure, function, and evolution of ribosomes. Washington, D.C.: American Society for Microbiology; 1990. pp. 479–490. [Google Scholar]
- 33.Davidson B E, MacDougall J, Saint Girons I. Physical map of the linear chromosome of the bacterium Borrelia burgdorferi 212, a causative agent of Lyme disease, and localization of rRNA genes. J Bacteriol. 1992;174:3766–3774. doi: 10.1128/jb.174.11.3766-3774.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debets-Ossenkopp Y J, Brinkman A B, Kuipers E J, Vandenbroucke-Grauls C M, Kusters J G. Explaining the bias in the 23S rRNA gene mutations associated with clarithromycin resistance in clinical isolates of Helicobacter pylori. Antimicrob Agents Chemother. 1998;42:2749–2751. doi: 10.1128/aac.42.10.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debets-Ossenkopp Y J, Sparrius M, Kusters J G, Kolkman J J, Vandenbroucke-Grauls C M J E. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol Lett. 1996;142:37–42. doi: 10.1111/j.1574-6968.1996.tb08404.x. [DOI] [PubMed] [Google Scholar]
- 36.De Rossi E, Branzoni M, Cantoni R, Milano A, Riccardi G, Ciferri O. mmr, a Mycobacterium tuberculosis gene conferring resistance to small cationic dyes and inhibitors. J Bacteriol. 1998;180:6068–6071. doi: 10.1128/jb.180.22.6068-6071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dmitriev A, Suvorov A, Totolian A. Physical and genetic chromosomal maps of Streptococcus agalactiae, serotypes II and III; rRNA operon organization. FEMS Microbiol Lett. 1998;167:33–39. doi: 10.1111/j.1574-6968.1998.tb13204.x. [DOI] [PubMed] [Google Scholar]
- 38.Domingo D, Alarcon T, Sanz J C, Sánchez I, López-Brea M. High frequency of mutations at position 2144 of the 23S rRNA gene in clarithromycin-resistant Helicobacter pylori strains isolated in Spain. J Antimicrob Chemother. 1998;41:573–574. doi: 10.1093/jac/41.5.573. [DOI] [PubMed] [Google Scholar]
- 39.Douthwaite S. Functional interactions within 23S rRNA involving the peptidyltransferase center. J Bacteriol. 1992;174:1333–1338. doi: 10.1128/jb.174.4.1333-1338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douthwaite S. Interaction of the antibiotics clindamycin and lincomycin with Escherichia coli 23S ribosomal RNA. Nucleic Acids Res. 1992;20:4717–4720. doi: 10.1093/nar/20.18.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douthwaite S, Aagaard C. Erythromycin binding is reduced in ribosomes with conformational alterations in the 23S rRNA peptidyl transferase loop. J Mol Biol. 1993;232:725–731. doi: 10.1006/jmbi.1993.1426. [DOI] [PubMed] [Google Scholar]
- 42.Douthwaite S, Hansen L H, Mauvais P. The macrolide-ketolide inhibition of MLS-resistant ribosomes is improved by alternative drug interaction with domains II of 23S rRNA. Mol Microbiol. 2000;36:183–192. doi: 10.1046/j.1365-2958.2000.01841.x. [DOI] [PubMed] [Google Scholar]
- 43.Eady E A, Ross J I, Tipper J L, Walters C E, Cove J H, Noble W C. Distribution of genes encoding erythromycin ribosomal methylases and an erythromycin efflux pump in epidemiologically distinct groups of staphylococci. J Antimicrob Chemother. 1993;31:211–217. doi: 10.1093/jac/31.2.211. [DOI] [PubMed] [Google Scholar]
- 44.Ednie L M, Spangler S K, Jacobs M R, Appelbaum P C. Antianaerobic activity of the ketolide RU 64004 compared to activities of four macrolides, five beta-lactams, clindamycin, and metronidazole. Antimicrob Agents Chemother. 1997;41:1037–1041. doi: 10.1128/aac.41.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ednie L M, Spangler S K, Jacobs M R, Appelbaum P C. Susceptibilities of 228 penicillin- and erythromycin-susceptible and -resistant pneumonococci to RU 64004, a new ketolide, compared with susceptibilities to 16 other agents. Antimicrob Agents Chemother. 1997;41:1033–1036. doi: 10.1128/aac.41.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engel J N, Ganem D. Chlamydial rRNA operons: gene organization and identification of putative tandem promoters. J Bacteriol. 1987;169:5678–5685. doi: 10.1128/jb.169.12.5678-5685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ettayebi M, Prasad S M, Morgan E A. Chloramphenicol-erythromycin resistance mutations in a 23S rRNA gene of Escherichia coli. J Bacteriol. 1985;162:551–557. doi: 10.1128/jb.162.2.551-557.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraser C M, Gocayne J D, White O, Adams M D, Clayton R A, Fleischmann R D, Bult C J, Kerlavage A R, Sutton G, Kelly J M, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 49.Fukunaga M, Mifuchi I. Unique organization of Leptospira interrogans rRNA genes. J Bacteriol. 1989;171:5763–5767. doi: 10.1128/jb.171.11.5763-5767.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukushi H, Hirai K. Restriction fragment length polymorphisms of rRNA as genetic markers to differentiate Chlamydia spp. Int J Syst Bacteriol. 1993;43:613–617. doi: 10.1099/00207713-43-3-613. [DOI] [PubMed] [Google Scholar]
- 51.Gale E F, Cundliffe E, Reynolds P E, Richmond M H, Waring M J. The molecular basis for antibiotic action. London, United Kingdom: John Wiley and Sons; 1981. [Google Scholar]
- 52.Garcia-Arata M I, Baquero F, de Rafael L, Martin de Argila C, Gisbert J P, Bermejo F, Boixeda D, Canton R. Mutations in 23S rRNA in Helicobacter pylori conferring resistance to erythromycin do not always confer resistance to clarithromycin. Antimicrob Agents Chemother. 1999;43:374–376. doi: 10.1128/aac.43.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gase A-M, Kauc L, Barraille P, Sicard M, Goodgal S. Gene localization, size, and physical map of the chromosome of Streptococcus pneumoniae. J Bacteriol. 1991;173:7361–7367. doi: 10.1128/jb.173.22.7361-7367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gauthier A, Turmel M, Lemieux C. Mapping of chloroplast mutations conferring resistance to antibiotics in Chlamydomonas: evidence for a novel site of streptomycin resistance in the small subunit rRNA. Mol Gen Genet. 1988;214:192–197. doi: 10.1007/BF00337710. [DOI] [PubMed] [Google Scholar]
- 55.Göbel U, Butler G H, Stanbridge E J. Comparative analysis of mycoplasma ribosomal RNA operons. Isr J Med Sci. 1984;20:762–764. [PubMed] [Google Scholar]
- 56.Goldman R C, Kadam S K. Binding of novel macrolide structures to macrolide-lincosamide-streptogramin B-resistant ribosomes inhibits protein synthesis and bacterial growth. Antimicrob Agents Chemother. 1989;33:1058–1066. doi: 10.1128/aac.33.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldman R C, Zakula D, Flamm R, Beyer J, Capobianco J. Tight binding of clarithromycin, its 14-(R)-hydroxy metabolite, and erythromycin to Helicobacter pylori ribosomes. Antimicrob Agents Chemother. 1994;38:1496–1500. doi: 10.1128/aac.38.7.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham D Y. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 59.Gregory S T, Dahlberg A E. Erythromycin resistance mutations in ribosomal proteins L22 and L4 perturb the higher order structure of 23 S ribosomal RNA. J Mol Biol. 1999;289:827–834. doi: 10.1006/jmbi.1999.2839. [DOI] [PubMed] [Google Scholar]
- 60.Gutell R R, Larsen N, Woese C R. Lessons from an evolving rRNA: 16S and 23S rRNA structures from a comparative perspective. Microbiol Rev. 1994;58:10–26. doi: 10.1128/mr.58.1.10-26.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hagman K E, Pan W, Spratt B G, Balthazar J T, Judd R C, Shafer W M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 62.Hansen L H, Mauvais P, Douthwaite S. The macrolide-ketolide antibiotic binding site is formed by structures in domains II and V of 23S ribosomal RNA. Mol Microbiol. 1999;31:623–631. doi: 10.1046/j.1365-2958.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 63.Harris E H, Burkhart B D, Gillham N W, Boynton J E. Antibiotic resistance mutations in the chloroplast 16S and 23S rRNA genes of Chlamydomonas reinhardtii: correlation of genetic and physical maps of the chloroplast genome. Genetics. 1989;123:281–292. doi: 10.1093/genetics/123.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Húlten K, Gibreel A, Sköld O, Engstrand L. Macrolide resistance in Helicobacter pylori: mechanism and stability in strains from clarithromycin-treated patients. Antimicrob Agents Chemother. 1997;41:2550–2553. doi: 10.1128/aac.41.11.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ives T J, Manzewitsch P, Regnery R L, Butts J D, Kebede M. In vitro susceptibilities of Bartonella henselae, B. quintana, B. elizabethae, Rickettsia rickettsii, R. conorii, R. akari, and R. prowazekii to macrolide antibiotics as determined by immunofluorescent-antibody analysis of infected Vero cell monolayers. Antimicrob Agents Chemother. 1997;41:578–582. doi: 10.1128/aac.41.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jenkins G, Cundliffe E. Cloning and characterization of two genes from Streptomyces lividans that confer inducible resistance to lincomycin and macrolide antibiotics. Gene. 1991;108:55–62. doi: 10.1016/0378-1119(91)90487-v. [DOI] [PubMed] [Google Scholar]
- 67.Jensen L B, Frimodt-Moller N, Aarestrup F M. Presence of erm gene classes in gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol Lett. 1999;170:151–158. doi: 10.1111/j.1574-6968.1999.tb13368.x. [DOI] [PubMed] [Google Scholar]
- 68.Jiang Q, Hiratsuka K, Taylor D E. Variability of gene order in different Helicobacter pylori strains contributes to genome diversity. Mol Microbiol. 1996;20:833–842. doi: 10.1111/j.1365-2958.1996.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 69.Karlsson M, Fellstrom C, Heldtander M U, Johansson K E, Franklin A. Genetic basis of macrolide and lincosamide resistance in Brachyspira (Serpulina) hyodysenteriae. FEMS Microbiol Lett. 1999;172:255–260. doi: 10.1111/j.1574-6968.1999.tb13476.x. [DOI] [PubMed] [Google Scholar]
- 70.Kataja J, Huovinen P, Skurnik M, Seppäla H. Erythromycin resistance genes in group A streptococci in Finland. Antimicrob Agents Chemother. 1999;43:48–52. doi: 10.1128/aac.43.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim N W, Lombardi R, Bingham H, Hani E, Louie H, Ng D, Chan V L. Fine mapping of the three rRNA operons on the updated genomic map of Campylobacter jejuni TGH9011 (ATCC 43431) J Bacteriol. 1993;175:7468–7470. doi: 10.1128/jb.175.22.7468-7470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein J O. History of macrolide use in pediatrics. Pediatr Infect Dis J. 1997;16:427–31. doi: 10.1097/00006454-199704000-00025. [DOI] [PubMed] [Google Scholar]
- 73.Klugman K P, Capper T, Widdowson C A, Koornhof H J, Moser W. Increased activity of 16-membered lactone ring macrolides against erythromycin-resistant Streptococcus pyogenes and Streptococcus pneumoniae: characterization of South African isolates. J Antimicrob Chemother. 1998;42:729–734. doi: 10.1093/jac/42.6.729. [DOI] [PubMed] [Google Scholar]
- 74.Lai C J, Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci USA. 1971;68:856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lan R, Reeves P R. Recombination between rRNA operons created most of the ribotype variation observed in the seventh pandemic clone of Vibrio cholerae. Microbiology. 1998;144:1213–1221. doi: 10.1099/00221287-144-5-1213. [DOI] [PubMed] [Google Scholar]
- 76.Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee J J, Smith H O, Redfield R J. Organization of the Haemophilus influenzae Rd genome. J Bacteriol. 1989;171:3016–3024. doi: 10.1128/jb.171.6.3016-3024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu S L, Sanderson K E. Homologous recombination between rrn operons rearranges the chromosome in host-specialized species of Salmonella. FEMS Microbiol Lett. 1998;164:275–281. doi: 10.1111/j.1574-6968.1998.tb13098.x. [DOI] [PubMed] [Google Scholar]
- 79.Lucier T S, Heitzman K, Liu S K, Hu P C. Transition mutations in the 23S rRNA of erythromycin-resistant isolates of Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1995;39:2770–2773. doi: 10.1128/aac.39.12.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lui M, Kirpekar F, van Wezel G P, Douthwaite S. The tylosin resistance gene tlrB of Streptomyces fradiae encodes a methyltransferase that targets G748 in 23S rRNA. Mol Microbiol. 2000;37:811–820. doi: 10.1046/j.1365-2958.2000.02046.x. [DOI] [PubMed] [Google Scholar]
- 81.Luna-Herrera J, Reddy V M, Daneluzzi D, Gangadharam P R. Antituberculosis activity of clarithromycin. Antimicrob Agents Chemother. 1995;39:2692–2695. doi: 10.1128/aac.39.12.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maeda S, Yoshida H, Ogura K, Kanai F, Shiratori Y, Omata M. Helicobacter pylori specific nested PCR assay for the detection of 23S rRNA mutation associated with clarithromycin resistance. Gut. 1998;43:317–321. doi: 10.1136/gut.43.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marais A, Monteiro L, Occhialini A, Pina M, Lamouliatte H, Mégraud F. Direct detection of Helicobacter pylori resistance to macrolides by a polymerase chain reaction/DNA enzyme immunoassay in gastric biopsy specimens. Gut. 1999;44:463–467. doi: 10.1136/gut.44.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meier A, Kirschner P, Springer B, Steingrube V A, Brown B A, Wallace R J, Jr, Böttger E C. Identification of mutations in 23S rRNA gene of clarithromycin-resistant Mycobacterium intracellulare. Antimicrob Agents Chemother. 1994;38:381–384. doi: 10.1128/aac.38.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Menninger J R. Mechanism of inhibition of protein synthesis by macrolide and lincosamide antibiotics. J Basic Clin Physiol Pharmacol. 1995;6:229–250. doi: 10.1515/jbcpp.1995.6.3-4.229. [DOI] [PubMed] [Google Scholar]
- 86.Michel E, Cossart P. Physical map of the Listeria monocytogenes chromosome. J Bacteriol. 1992;174:7098–7103. doi: 10.1128/jb.174.22.7098-7103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moazed D, Noller H F. Chloramphenicol, erythromycin, carbomycin and vernamycin B protect overlapping sites in the peptidyl transferase region of 23S ribosomal RNA. Biochimie. 1987;69:879–884. doi: 10.1016/0300-9084(87)90215-x. [DOI] [PubMed] [Google Scholar]
- 88.Mueller F, Sommer I, Baranov P, Matadeen R, Stoldt M, Wohnert J, Gorlach M, van Heel M, Brimacombe R. The 3D arrangement of the 23 S and 5 S rRNA in the Escherichia coli 50 S ribosomal subunit based on a cryo-electron microscopic reconstruction at 7.5 Å resolution. J Mol Biol. 2000;298:35–59. doi: 10.1006/jmbi.2000.3635. [DOI] [PubMed] [Google Scholar]
- 89.Müller M, Hildebrandt A. Nucleotide sequences of the 23S rRNA genes from Bordetella pertussis, B. parapertussis, B. bronchiseptica and B. avium, and their implications for phylogenetic analysis. Nucleic Acids Res. 1993;21:3320. doi: 10.1093/nar/21.14.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nash K A, Inderlied C B. Genetic basis of macrolide resistance in Mycobacterium avium isolated from patients with disseminated disease. Antimicrob Agents Chemother. 1995;39:2625–2630. doi: 10.1128/aac.39.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen K T, Hansen E J, Farinha M A. Construction of a genomic map of Moraxella (Branhamella) catarrhalis ATCC 25238 and physical mapping of virulence-associated genes. Can J Microbiol. 1999;45:299–303. [PubMed] [Google Scholar]
- 92.Noller H F. Ribosomal RNA and translation. Annu Rev Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- 93.Noller H F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- 94.Occhialini A, Urdaci M, Doucet-Populaire F, Bébéar C M, Lamouliatte H, Mégraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother. 1997;41:2724–2728. doi: 10.1128/aac.41.12.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O'Hara K, Kanda T, Ohmiya K, Ebisu T, Kono M. Purification and characterization of macrolide 2′-phosphotransferase from a strain of Escherichia coli that is highly resistant to erythromycin. Antimicrob Agents Chemother. 1989;33:1354–1357. doi: 10.1128/aac.33.8.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pardo D, Rosset R. Properties of ribosomes from erythromycin resistant mutants of Escherichia coli. Mol Gen Genet. 1977;156:267–271. doi: 10.1007/BF00267181. [DOI] [PubMed] [Google Scholar]
- 97.Peitz U, Hackelsberger A, Malfertheiner P. A practical approach to patients with refractory Helicobacter pylori infection, or who are re-infected after standard therapy. Drugs. 1999;57:905–920. doi: 10.2165/00003495-199957060-00006. [DOI] [PubMed] [Google Scholar]
- 98.Pernodet J L, Boccard F, Alegre M T, Blondelet-Rouault M H, Guérineau M. Resistance to macrolides, lincosamides and streptogramin type B antibiotics due to a mutation in an rRNA operon of Streptomyces ambofaciens. EMBO J. 1988;7:277–282. doi: 10.1002/j.1460-2075.1988.tb02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Porse B T, Garrett R A. Sites of interaction of streptogramin A and B antibiotics in the peptidyl transferase loop of 23 S rRNA and the synergism of their inhibitory mechanisms. J Mol Biol. 1999;286:375–387. doi: 10.1006/jmbi.1998.2509. [DOI] [PubMed] [Google Scholar]
- 100.Prescott J F, Nicholson V M. Antimicrobial drug susceptibility of Leptospira interrogans serovar hardjo isolated from cattle. Can J Vet Res. 1988;52:286–287. [PMC free article] [PubMed] [Google Scholar]
- 101.Prüss B M, Francis K P, von Stetten F, Scherer S. Correlation of 16S ribosomal DNA signature sequences with temperature-dependent growth rates of mesophilic and psychrotolerant strains of the Bacillus cereus group. J Bacteriol. 1999;181:2624–2630. doi: 10.1128/jb.181.8.2624-2630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reischl U, Feldmann K, Naumann L, Gaugler B J, Ninet B, Hirschel B, Emler S. 16S rRNA sequence diversity in Mycobacterium celatum strains caused by presence of two different copies of 16S rRNA gene. J Clin Microbiol. 1998;36:1761–1764. doi: 10.1128/jcm.36.6.1761-1764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riesenfeld C, Everett M, Piddock L J, Hall B G. Adaptive mutations produce resistance to ciprofloxacin. Antimicrob Agents Chemother. 1997;41:2059–2060. doi: 10.1128/aac.41.9.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roberts M C, Chung W O, Roe D, Xia M, Marquez C, Borthagaray G, Whittington W L, Holmes K K. Erythromycin-resistant Neisseria gonorrhoeae and oral commensal Neisseria spp. carry known rRNA methylase genes. Antimicrob Agents Chemother. 1999;43:1367–1372. doi: 10.1128/aac.43.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roberts M C, Sutcliffe J, Courvalin P, Jensen L B, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roblin P M, Hammerschlag M R. In vitro activity of a new ketolide antibiotic, HMR 3647, against Chlamydia pneumoniae. Antimicrob Agents Chemother. 1998;42:1515–1516. doi: 10.1128/aac.42.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodriguez-Fonseca C, Amils R, Garrett R A. Fine structure of the peptidyl transferase centre on 23 S-like rRNAs deduced from chemical probing of antibiotic-ribosome complexes. J Mol Biol. 1995;247:224–235. doi: 10.1006/jmbi.1994.0135. [DOI] [PubMed] [Google Scholar]
- 108.Römling U, Duchene M, Essar D W, Galloway D, Guidi-Rontani C, Hill D, Lazdunski A, Miller R V, Schleifer K H, Smith D W, et al. Localization of alg, opr, phn, pho, 4.5S RNA, 6S RNA, tox, trp, and xcp genes, rrn operons, and the chromosomal origin on the physical genome map of Pseudomonas aeruginosa PAO. J Bacteriol. 1992;174:327–330. doi: 10.1128/jb.174.1.327-330.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosato A, Vicarini H, Bonnefoy A, Chantot J F, Leclercq R. A new ketolide, HMR 3004, active against streptococci inducibly resistant to erythromycin. Antimicrob Agents Chemother. 1998;42:1392–1396. doi: 10.1128/aac.42.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ross J I, Eady E A, Cove J H, Cunliffe W J, Baumberg S, Wootton J C. Inducible erythromycin resistance in staphylococci is encoded by a member of the ATP-binding transport super-gene family. Mol Microbiol. 1990;4:1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 111.Ross J I, Eady E A, Cove J H, Jones C E, Ratyal A H, Miller Y W, Vyakrnam S, Cunliffe W J. Clinical resistance to erythromycin and clindamycin in cutaneous propionibacteria isolated from acne patients is associated with mutations in 23S rRNA. Antimicrob Agents Chemother. 1997;41:1162–1165. doi: 10.1128/aac.41.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez L, Pan W, Vinas M, Nikaido H. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J Bacteriol. 1997;179:6855–6857. doi: 10.1128/jb.179.21.6855-6857.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sander P, Prammananan T, Meier A, Frischkorn K, Böttger E C. The role of ribosomal RNAs in macrolide resistance. Mol Microbiol. 1997;26:469–480. doi: 10.1046/j.1365-2958.1997.5811946.x. [DOI] [PubMed] [Google Scholar]
- 114.Schönwald S, Kuzman I, Oreskovic K, Burek V, Skerk V, Car V, Bozinovic D, Culig J, Radosevic S. Azithromycin: single 1.5 g dose in the treatment of patients with atypical pneumonia syndrome—a randomized study. Infection. 1999;27:198–202. doi: 10.1007/BF02561528. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz J J, Gazumyan A, Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1992;174:3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sechi L A, Zanetti S, Dupre I, Cappiello M G, Delogu G, Mortensen J E, Daneo-Moore L, Fadda G. Molecular epidemiology by ribotyping and PCR-ribotyping of Enterococcus faecium strains isolated from intercontinental areas. New Microbiol. 1998;21:113–122. [PubMed] [Google Scholar]
- 117.Sechi L A, Zuccon F M, Mortensen J E, Daneo-Moore L. Ribosomal RNA gene (rrn) organization in enterococci. FEMS Microbiol Lett. 1994;120:307–313. doi: 10.1111/j.1574-6968.1994.tb07051.x. [DOI] [PubMed] [Google Scholar]
- 118.Sela S, Clark-Curtiss J E, Bercovier H. Characterization and taxonomic implications of the rRNA genes of Mycobacterium leprae. J Bacteriol. 1989;171:70–73. doi: 10.1128/jb.171.1.70-73.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sevin E, Lamarque D, Delchier J C, Soussy C J, Tankovic J. Co-detection of Helicobacter pylori and of its resistance to clarithromycin by PCR. FEMS Microbiol Lett. 1998;165:369–372. doi: 10.1111/j.1574-6968.1998.tb13172.x. [DOI] [PubMed] [Google Scholar]
- 120.Sigmund C D, Ettayebi M, Morgan E A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984;12:4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Skinner R, Cundliffe E, Schmidt F J. Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics. J Biol Chem. 1983;258:12702–12706. [PubMed] [Google Scholar]
- 122.Sor F, Fukuhara H. Erythromycin and spiramycin resistance mutations of yeast mitochondria: nature of the rib2 locus in the large ribosomal RNA gene. Nucleic Acids Res. 1984;12:8313–8318. doi: 10.1093/nar/12.22.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sor F, Fukuhara H. Identification of two erythromycin resistance mutations in the mitochondrial gene coding for the large ribosomal RNA in yeast. Nucleic Acids Res. 1982;10:6571–6577. doi: 10.1093/nar/10.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stark H, Rodnina M V, Rinke-Appel J, Brimacombe R, Wintermeyer W, van Heel M. Visualization of elongation factor Tu on the Escherichia coli ribosome. Nature. 1997;389:403–406. doi: 10.1038/38770. [DOI] [PubMed] [Google Scholar]
- 125.Stone G G, Shortridge D, Flamm R K, Versalovic J, Beyer J, Idler K, Zulawinski L, Tanaka S K. Identification of a 23S rRNA gene mutation in clarithromycin-resistant Helicobacter pylori. Helicobacter. 1996;1:227–228. doi: 10.1111/j.1523-5378.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 126.Stone G G, Shortridge D, Versalovic J, Beyer J, Flamm R K, Graham D Y, Ghoneim A T, Tanaka S K. A PCR-oligonucleotide ligation assay to determine the prevalence of 23S rRNA gene mutations in clarithromycin-resistant Helicobacter pylori. Antimicrob Agents Chemother. 1997;41:712–714. doi: 10.1128/aac.41.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sturgill M G, Rapp R P. Clarithromycin: review of a new macrolide antibiotic with improved microbiologic spectrum and favorable pharmacokinetic and adverse effect profiles. Ann Pharmacother. 1992;26:1099–1108. doi: 10.1177/106002809202600912. [DOI] [PubMed] [Google Scholar]
- 128.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tait-Kamradt A, Davies T, Cronan M, Jacobs M R, Appelbaum P C, Sutcliffe J. Mutations in 23S rRNA and L4 ribosomal protein account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob Agents Chemother. 2000;44:2118–2125. doi: 10.1128/aac.44.8.2118-2125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taschke C, Klinkert M Q, Wolters J, Herrmann R. Organization of the ribosomal RNA genes in Mycoplasma hyopneumoniae: the 5S rRNA gene is separated from the 16S and 23S rRNA genes. Mol Gen Genet. 1986;205:428–433. doi: 10.1007/BF00338078. [DOI] [PubMed] [Google Scholar]
- 131.Taylor D E, Eaton M, Yan W, Chang N. Genome maps of Campylobacter jejuni and Campylobacter coli. J Bacteriol. 1992;174:2332–2337. doi: 10.1128/jb.174.7.2332-2337.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taylor D E, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41:2621–2628. doi: 10.1128/aac.41.12.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Venter J C, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 134.Tripathi S, Kloss P S, Mankin A S. Ketolide resistance conferred by short peptides. J Biol Chem. 1998;273:20073–20077. doi: 10.1074/jbc.273.32.20073. [DOI] [PubMed] [Google Scholar]
- 135.Truffot-Pernot C, Lounis N, Grosset J H, Ji B. Clarithromycin is inactive against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:2827–2828. doi: 10.1128/aac.39.12.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vaira D, Holton J, Menegatti M, Ricci C, Landi F, Ali A, Gatta L, Acciardi C, Farinelli S, Crosatti M, Berardi S, Miglioli M. New immunological assays for the diagnosis of Helicobacter pylori infection. Gut. 1999;45(Suppl. 1):123–27. doi: 10.1136/gut.45.2008.i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Doorn L J, Debets-Ossenkopp Y J, Marais A, Sanna R, Mégraud F, Kusters J G, Quint W G. Rapid detection, by PCR and reverse hybridization, of mutations in the Helicobacter pylori 23S rRNA gene associated with macrolide resistance. Antimicrob Agents Chemother. 1999;43:1779–1782. doi: 10.1128/aac.43.7.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vannuffel P, Di Giambattista M, Cocito C. The role of rRNA bases in the interaction of peptidyltransferase inhibitors with bacterial ribosomes. J Biol Chem. 1992;267:16114–16120. [PubMed] [Google Scholar]
- 139.Vannuffel P, Di Giambattista M, Morgan E A, Cocito C. Identification of a single base change in ribosomal RNA leading to erythromycin resistance. J Biol Chem. 1992;267:8377–8382. [PubMed] [Google Scholar]
- 140.Vazquez D. Inhibitors of protein synthesis. Berlin, Germany: Springer-Verlag; 1979. [Google Scholar]
- 141.Versalovic J, Osato M S, Spakovsky K, Dore M P, Reddy R, Stone G G, Shortridge D, Flamm R K, Tanaka S K, Graham D Y. Point mutations in the 23S rRNA gene of Helicobacter pylori associated with different levels of clarithromycin resistance. J Antimicrob Chemother. 1997;40:283–286. doi: 10.1093/jac/40.2.283. [DOI] [PubMed] [Google Scholar]
- 142.Versalovic J, Shortridge D, Kibler K, Griffy M V, Beyer J, Flamm R K, Tanaka S K, Graham D Y, Go M F. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996;40:477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vester B, Garrett R A. A plasmid-coded and site-directed mutation in Escherichia coli 23S RNA that confers resistance to erythromycin: implications for the mechanism of action of erythromycin. Biochimie. 1987;69:891–900. doi: 10.1016/0300-9084(87)90217-3. [DOI] [PubMed] [Google Scholar]
- 144.Vester B, Hansen L H, Douthwaite S. The conformation of 23S rRNA nucleotide A2058 determines its recognition by the ErmE methyltransferase. RNA. 1995;1:501–509. [PMC free article] [PubMed] [Google Scholar]
- 145.Wada A, Ohta H, Kulthanan K, Hiramatsu K. Molecular cloning and mapping of 16S–23S rRNA gene complexes of Staphylococcus aureus. J Bacteriol. 1993;175:7483–7487. doi: 10.1128/jb.175.22.7483-7487.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wallace R J, Jr, Meier A, Brown B A, Zhang Y, Sander P, Onyi G O, Böttger E C. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob Agents Chemother. 1996;40:1676–1681. doi: 10.1128/aac.40.7.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang G, Rahman M S, Humayun M Z, Taylor D E. Multiplex sequence analysis demonstrates the competitive growth advantage of the A-to-G mutants of clarithromycin-resistant Helicobacter pylori. Antimicrob Agents Chemother. 1999;43:683–685. doi: 10.1128/aac.43.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang G, Taylor D E. Site-specific mutations in the 23S rRNA gene of Helicobacter pylori confer two types of resistance to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob Agents Chemother. 1998;42:1952–1958. doi: 10.1128/aac.42.8.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weisblum B. Macrolide resistance. Drug Resistance Updates. 1998;1:29–41. doi: 10.1016/s1368-7646(98)80212-4. [DOI] [PubMed] [Google Scholar]
- 151.Weisblum B, Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: survey of antibiotic classes involved. J Bacteriol. 1969;98:447–452. doi: 10.1128/jb.98.2.447-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wittmann H G, Stöffler G, Apirion D, Rosen L, Tanaka K, Tamaki M, Takata R, Dekio S, Otaka E. Biochemical and genetic studies on two different types of erythromycin resistant mutants of Escherichia coli with altered ribosomal proteins. Mol Gen Genet. 1973;127:175–189. doi: 10.1007/BF00333665. [DOI] [PubMed] [Google Scholar]
- 153.Wolff K, Sperka S, Stern A. Phylogeny and nucleotide sequence of a 23S rRNA gene from Neisseria gonorrhoeae and Neisseria meningitidis. Nucleic Acids Res. 1992;20:4657. doi: 10.1093/nar/20.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wondrack L, Massa M, Yang B V, Sutcliffe J. Clinical strain of Staphylococcus aureus inactivates and causes efflux of macrolides. Antimicrob Agents Chemother. 1996;40:992–998. doi: 10.1128/aac.40.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xia H X, Buckley M, Keane C T, O'Morain C A. Clarithromycin resistance in Helicobacter pylori: prevalence in untreated dyspeptic patients and stability in vitro. J Antimicrob Chemother. 1996;37:473–481. doi: 10.1093/jac/37.3.473. [DOI] [PubMed] [Google Scholar]
- 156.Xiong L, Shah S, Mauvais P, Mankin A S. A ketolide resistance mutation in domain II of 23S rRNA reveals the proximity of hairpin 35 to the peptidyl transferase centre. Mol Microbiol. 1999;31:633–639. doi: 10.1046/j.1365-2958.1999.01203.x. [DOI] [PubMed] [Google Scholar]
- 157.Zuerner R L, Stanton T B. Physical and genetic map of the Serpulina hyodysenteriae B78T chromosome. J Bacteriol. 1994;176:1087–1092. doi: 10.1128/jb.176.4.1087-1092.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]