Abstract
Objective
This study aimed to conduct a retrospective observational study to understand the status of characteristics of pain and identify potential variables influencing the clinical presentation of breakthrough cancer pain (BTP) in advanced cancer patients.
Methods
Advanced cancer patients over 18 years of age; diagnosed with cancer of any type and stage III or IV in the palliative care ward with available data were enrolled between 2018 and 2020. Demographic data and pain-related information were collected by using structured electronic extraction form from Hospital Information System (HIS). Patients who had well-controlled background pain with an intensity ≤4 on a 0–10 numerical scale for >12 hours/day, the presence of transient exacerbations of pain with moderate-severe intensity (≧5), and clearly distinguish from background pain were regarded to have suffered BTP. Spearman correlation was conducted to explore the relationship between pain score and demographics characteristics. Factors significant in univariate analysis were included in the multiple regression model to explore independent predictive factors associated with the BTP.
Results
Of 798 advanced cancer patients, the mean age was 56.7 (SD = 11.84) years. Lung cancer (29.95%) was the most common cancer, and pain (93%) was the most common symptom. More than half (n = 428, 53.6%) of the patients experienced BTP. The median number of BTP episodes was 4 (IQR = 2, 7, range: 1–42). The median intensity of BTP was 6 (IQR = 6, 7, range 5–10). Patients with severe background pain or BTP had longer hospital stay and more symptoms. Besides, more severe background pain was related to higher activity of daily living. Intramuscular injection of hydromorphone hydrochloride was the main medication for BTP onset. Younger age, background pain, anorexia, and constipation were independently associated with the presentation of BTP. BTP pain intensity was independently associated with bloating. Symptom numbers were an independent factor and positively associated with BTP episodes.
Conclusions
BTP resulted in poor prognosis, which has a variable presentation depending on interdependent relationships among different characteristics. Good controlling of background pain and assessment of pain-related symptoms are essential for BTP management. BTP should be managed individually, especially the invisible pain among aged patients. Furthermore, BTP-related education and training were still needed.
1. Introduction
Cancer pain is one of the most frequent and disturbing of all cancer-related symptoms [1]. Unrelieved pain denies patients' comfort and greatly affects their activities, motivation, interactions with family and friends, and overall quality of life (QOL) [2]. Breakthrough pain (BTP) is a serious problem in cancer patients, with prolonged pain episodes and severe intensity despite analgesic [3], which was proved to be the independent risk factor of poor pain control [4]. BTP has not yet been clearly defined. Association for Palliative Medicine (APM) of Great Britain and Ireland defined BTP as a “transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger despite relatively stable and adequately controlled background pain” [5]. The prevalence of BTP is commonly high in cancer population. Meanwhile, the highest BTP prevalence was found in patients from palliative care or advanced cancer patients admitted to a hospice [6]. A prospective longitudinal study revealed that BTP episodes in terminally ill cancer patients reached an average of 7.2 episodes per patient over 7 days [7]. BTP is associated with a variety of physical, psychological, social complications, more disability, and decreased QOL [8–10]. However, managing BTP is challenging, for various factors like demographic data, diagnosis, psychological distress, sleep disturbances, cognitive function, addictive behavior, and even the performance status could lead to different BTP characteristics and response to treatment [6, 11]. Besides, BTP can have different causes, comorbidities, and pathophysiology, which make it complex to diagnose, assess, and manage [12]. For these heterogeneous natures, guidelines endorse the recommendation that management of BTP should be set on the individual patient's condition [8]. BTP can occur spontaneously or in relation to specific and predictable or unpredictable triggers [9]. About 30.5% of BTP were still predictable [13]. The predictability of BTP, no matter in occurrence or its characteristics, offers the chance of early identification and advanced intervention before an onset [14]. Meanwhile, it avoids undermanagement or overtreatment with opioids [15]. So far, some researches have been conducted to anticipate the BTP. Age, diagnosis, PS score, and background pain intensity were demonstrated to be associated with the BTP episodes and intensity in cancer patients [13]. The IQ-BTP is an 11-item questionnaire with satisfactory psychometric and validity properties, which enable potential BTP to be identified and differentiated into three likelihood classes (no BTP or high, intermediate, or low likelihood for BTP) [16]. Also, risk-prediction models for BTP were developed, and the accuracy between machine learning and regression techniques was compared, though in labour epidural analgesia [17]. Although BTP has been better characterized in recent years, failures to detect BTP remain common especially in advanced cancer patients. Effective management of BTP requires early prediction and reliable identification. Thus, this study aims to assess the status of BTP and identify potential variables influencing the presentation characteristics of BTP in advanced cancer patients, optimize the management of BTP, and provide enlightenment of BTP prediction model construction in further future.
2. Materials and Methods
2.1. Study Design and Participants
We performed a retrospective cross-sectional analysis of data obtained from the Hospital Information System (HIS) of a specialized tertiary cancer hospital located in Hunan, China. Convenience sampling was used, and patients who received palliative care from January 2018 to December 2020 were included.
Patients selected for this study met the following inclusion criteria: over 18 years of age and diagnosed with cancer of any type and stage III or IV according to the National Cancer Institute codes. Patients with ostensible cognitive deficits or serious psychiatric dysfunctions were excluded. Any case with missing data was excluded. Among those included populations, patients who had well-controlled and stable background pain with an intensity ≤4 on a 0–10 numerical scale for >12 hours/day, the presence of transient exacerbations of pain with moderate-severe intensity (≧5), and clearly distinguish from background pain were regarded to have suffered BTP.
The sample size was calculated based on the rate of BTP among cancer patients. The occurrence rate varies greatly in different studies, from 25.7% to 80% [18–21]. A multicenter, observational, cross-sectional study of 3,765 cancer patients showed that 48% suffered moderate BTP [6]. Considering the average value, the rate of 40% was used in the present study, and the sample size was calculated using the formula N = Z2PQ/(0.1P)2 where Q = 1 − P, Z = 1.96 (≈ 2.00) represents the chi-squared value [22]. The sample size calculated was N = 600. Given the possible 20% loss of data, the sample size of this study should be at least 750. This study was approved by the ethics committee of our hospital and conducted following the principles of the Helsinki Declaration.
2.2. Data Collection
Relevant data were obtained for analysis, which can be divided into two categories:
General patient demographics, including age, gender, spouse (have or no), present residence (rural, city), education level (primary, middle, graduate, and above), body mass index [BMI, BMI < 18.4 (lower weight), 18.4 ≦ BMI ≦ 23.9 (normal weight), 24 ≦ BMI ≦ 27.9 (overweight), BMI ≧ 28 (obesity)], ECOG performance status score (PS), Barthel index, distress thermometer score (DT), the average length of stay, primary tumor, metastasis (site, number), and presence of symptoms (category, number). PS is a reliable indicator for a patient's general condition (0–5) [23], and we divided it into good PS group (0–2) and poor PS group (3–5). DT had been proved to be efficacious in screening for psychological distress in advanced cancer patients with pain (0–10) [24] with 4 as the cutoff value. Patients who scored greater than 4 were regarded to have psychological distress. Barthel index [25] is used to measure activities of daily living (ADL), ranging from 0–100, which can be divided into severely dependent (0–40), moderately dependent (41–60), slightly dependent (61–99), and independent (100). All the data were collected before the first BTP onset.
For background pain, we collected mean pain score before the first BTP outset as basic background pain and pain score at discharge as an evaluation indicator of pain controlling during hospitalization. For BTP, median episodes, and intensity, major BTP interventions were recorded. The pain score was measured by the Numerical Rating Scale (NRS) ranging from 0–10, which has to be one of the most tools to measure pain intensity [26] and can be identified into three categories levels: mild (NRS 1–4), moderate (5–6), and severe (7–10) [27].
To improve the efficiency of data collection and avoid the bias, we designed a structured electronic extraction form, which was specifically designed for the study. All the information we need were listed, and collectors only need to import numbers we valued in advance, for example, binary variable “0” and “1” represented “no” and “yes”. All collectors had completed research courses (including medical statistics and nursing informatics) and received training on how to extract medical data. Details of extraction form and raw data of the participants were presented in Supplementary Material.
2.3. Statistical Analysis
Data were exported from Excel 2019 to SPSS Version 22.0 for analysis. Enumeration data were described as N (%) and median (interquartile range, IQR), while measurement data were described as mean ± standard deviation (SD). In order to find differences among demographic and clinical characteristics, chi-square was used for enumeration data, two-sample independent tests and variance analysis were used to evaluate measurement data of normal distribution, while Kruskal–Wallis Test and Mann–Whitney were used for measurement data of nonnormal distribution. Spearman correlation was conducted to explore the relationship between pain score and demographics characteristics. Factors significant in univariate analysis were included in multiple logistic regression model to explore independent predictive factors associated with the presence of BTP. Significant variables in univariate analysis then were selected in multiple linear regression for BTP intensity and episodes. The normal distribution of residuals was also tested to confirm the validation of the model. Variance inflation factor (VIF), tolerance, and factor analysis were used to judge multicollinearity. All statistical tests were two-sided, P values <0.05 were considered to be significant.
3. Results
3.1. Patient Characteristics
The initial search retrieved 830 records. After meticulous inspection, 798 inpatients from 2018 to 2020 were enrolled in this study (Figure 1). Table 1 summarizes the patients' characteristics. The age ranged from 16 to 85 years with the mean age being 56.7 (SD = 11.84) years. Most of them graduated from high school (53.3%). Majority of the samples (95.6%) have a spouse, and 55% lived in rural. Nearly half of the proportion (51%) had a normal BMI. Most samples had PS of 0–2 (69.8%), and 55.8% experienced distant metastasis with the most metastatic site in bone (31.1%). The most common cancer was lung cancer (29.95%), colorectal cancer (11.15%), and cervical cancer (7.14%). The most common symptom was pain (93%), followed by anorexia (36.5%) and sleep disorders (34.2%). More than half of the participants (60.7%) had symptoms no more than 4. The median distress score and Barthel index were 2 (2, 2) and 65 (50, 80), respectively.
Figure 1.
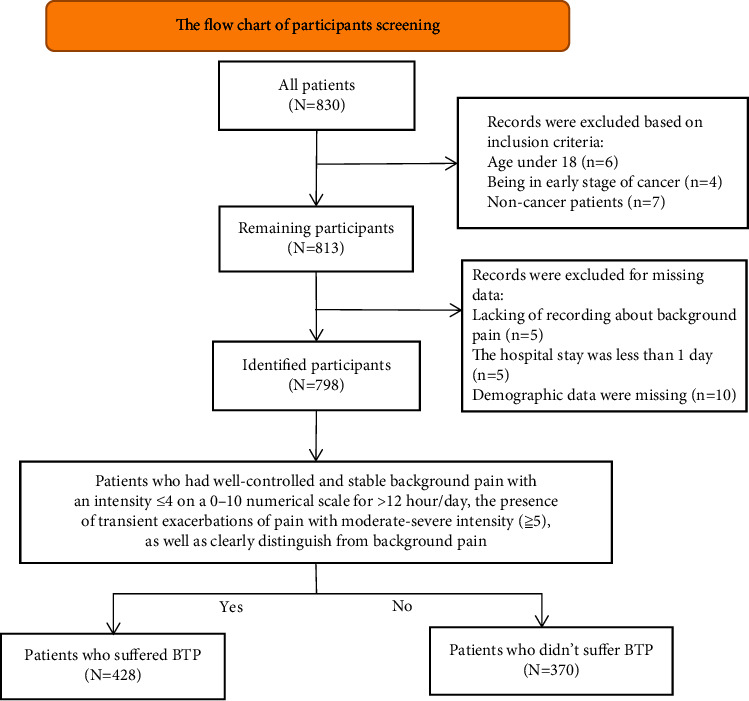
The flowchart of participants' screening.
Table 1.
Demographic, clinical, and pain data characteristics of patients (n = 798).
| Characteristics | Patients (N%) | |
|---|---|---|
| Age (years) | 56.7 ± 11.84 | |
| 18–64 | 573 (71.8) | |
| ≧65 | 225 (28.2) | |
|
| ||
| Gender | Male | 452 (56.6) |
| Female | 346 (43.4) | |
|
| ||
| Education level | Primary | 268 (33.6) |
| Middle school | 425 (53.3) | |
| Graduate and above | 105 (13.2) | |
|
| ||
| Having spouse | Yes | 763 (95.6) |
| No | 35 (4.4) | |
|
| ||
| Present residence | City | 359 (45) |
| Rural | 439 (55) | |
|
| ||
| BMI | 21.1 ± 3.86 | |
| Low weight | 211 (26.4) | |
| Normal | 407 (51.0) | |
| Overweight | 130 (16.3) | |
| Obesity | 50 (6.3) | |
|
| ||
| PS score | 2 (1, 3) | |
| Good (0–2) | 557 (69.8) | |
| Poor (3–5) | 241 (30.2) | |
|
| ||
| Metastasis | Yes | 445 (55.8) |
| No | 353 (44.2) | |
|
| ||
| Metastasis site | Bone | 248 (31.1) |
| Lymph | 147 (18.42) | |
| Liver | 122 (15.29) | |
|
| ||
| Metastasis number | 0 | 353 (44.20) |
| 1 | 201 (25.2) | |
| 2 | 150 (18.80) | |
| 3 | 94 (11.8) | |
|
| ||
| Symptom numbers | 4 (2, 6) | |
| ≦4 | 484 (60.7) | |
| 5–9 | 284 (35.6) | |
| ≧10 | 30 (3.8) | |
|
| ||
| Background pain score | 4 (3, 5) | |
| Mild (1–4) | 553 (69.3) | |
| Moderate (5–6) | 209 (26.2) | |
| Severe (7–10) | 36 (4.5) | |
| Pain score at discharge | 2 (2, 2) | |
|
| ||
| Breakthrough pain | Yes | 428 (53.6) |
| No | 370 (46.4) | |
| BTP episodes | 4 (2, 7) | |
| BTP intensity | 6 (6, 7) | |
| Average length of stay | 9 (6.75, 15) | |
|
| ||
| Distress score | 2 (2, 2) | |
| Severe distress (≦4) | 783 (98.1) | |
| Mild stress (>4) | 15 (1.9) | |
|
| ||
| Barthel index | 65 (50, 80) | |
| Severely dependent (0–40) | 128 (16) | |
| Moderately dependent (41–60) | 263 (33) | |
| Slightly dependent (61–99) | 345 (43.2) | |
| Independent (100) | 62 (7.8) | |
3.2. Characteristics of Background Pain
The median background pain intensity on the day of assessment before the first BTP was 4 (3, 5). We divided patients into three levels based on background pain scores, and there were 69.3%, 26.2%, and 4.5% of patients in mild, moderate, and severe pain groups, respectively (Table 1). Sever background pain was related to longer hospital stay, higher Barthel index, and symptoms number (Table 2).
Table 2.
Comparison and correlation analysis in patients with different background pain level.
| Background pain level | Median (IQR) | Coefficient | P | |
|---|---|---|---|---|
| BTP intensity | Mild | 6 (6, 7) | 5.954∗ | 0.051 |
| Moderate | 6 (6, 7) | |||
| Severe | 7 (6, 7) | |||
| 0.086a | 0.074 | |||
|
| ||||
| BTP episodes | Mild | 3 (2, 6) | 3.633∗ | 0.163 |
| Moderate | 4 (2, 7) | |||
| Severe | 5 (2, 7) | |||
| 0.107a | 0.027 | |||
|
| ||||
| Length of stay | Mild | 9 (6, 15) | 3.287∗ | 0.038 |
| Moderate | 10 (7, 15.5) | |||
| Severe | 11 (8.25, 18.75) | |||
| 0.05a | 0.154 | |||
|
| ||||
| PS | Mild | 2 (1, 3) | 1.689∗ | 0.430 |
| Moderate | 2 (1, 3) | |||
| Severe | 2 (1, 2.75) | |||
| 0.116a | 0.001 | |||
|
| ||||
| Distress score | Mild | 2 (2, 2) | 2.631∗ | 0.268 |
| Moderate | 2 (2, 2.5) | |||
| Severe | 2 (1, 2) | |||
| 0.039a | 0.272 | |||
|
| ||||
| Barthel index | Mild | 65 (50, 80) | 6.061∗ | 0.048 |
| Moderate | 60 (50, 75) | |||
| Severe | 70 (55, 80) | |||
| −0.008a | 0.822 | |||
|
| ||||
| Symptom numbers | Mild | 4 (2, 6) | 11.347∗ | 0.003 |
| Moderate | 4 (2.5, 6) | |||
| Severe | 4 (2, 6.75) | |||
| 0.135a | <0.001 | |||
∗ Kruskal–Wallis test; IQR, interquartile range; aSpearman correlation.
3.3. Characteristics of BTP
More than half (n = 428, 53.6%) of the patients experienced breakthrough. The median number of BTP episodes was 4 (IQR = 2, 7, range: 1–42). The median intensity of BTP was 6 (IQR = 6, 7, range 5–10). The majority of patients (n = 360, 84.1%) had an intensity of ≥7. Spearman correlation analysis showed BTP episodes and intensity were both negatively related to age (r = −0.081, −0.124, P < 0.05). Patients with BTP had longer hospital stay (Z = −7.134, P < 0.001), pain score at discharge (Z = −2.986, P=0.003), and more symptoms number (Z = −6.852, P < 0.001). Medications for BTP onset were intramuscular injection of hydromorphone hydrochloride (n = 116, 27.1%), subcutaneous morphine (n = 112, 26.16%), intramuscular injection of ketorolac tromethamine (n = 96, 22.43%), oral morphine (n = 36, 8.4%), intravenous analgesia (n = 24, 5.6%), PCIA (n = 20, 4.7%), and other analgesic ways (n = 24, 5.6%).
4. Factors Influencing BTP Clinical Presentation
4.1. Factors Influencing the Occurrence of Breakthrough Pain
Univariate analysis revealed age-group, education level, background pain level, symptoms number, metastasis number, bone metastasis, liver metastasis, the occurrence of anorexia, sleep disorder, constipation, and fatigue (Table 3) were significantly associated with higher BTP prevalence. There was no significant difference in BTP incidence among various cancer types (χ2 = 0.004–2.443, P=0.085–0.666). Table 4 showed the results of multivariate analysis for BTP occurrence. Finally, older age, background pain level, the occurrence of anorexia, and constipation were confirmed. Factor analysis showed there existed no multicollinearity among independent variables.
Table 3.
Univariate analysis of BTP incidence among demographic data.
| BTP (%) | χ 2 | P | BTP (%) | χ 2 | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age | 18–64 | 57.2 | 10.641 | <0.001 | Liver and cholecyst cancer | Yes | 62.7 | 1.810 | 0.177 |
| ≧65 | 44.4 | No | 53 | ||||||
|
| |||||||||
| Gender | Male | 56.5 | 3.781 | 0.052 | Lung cancer | Yes | 50.8 | 1.064 | 0.302 |
| Female | 49.7 | No | 54.8 | ||||||
|
| |||||||||
| Education level | Primary | 47.2 | 7.4 | 0.025 | Breast cancer | Yes | 47.7 | 0.653 | 0.419 |
| Middle | 57.8 | ||||||||
| Advanced | 53.3 | No | 54 | ||||||
|
| |||||||||
| Spouse | Yes | 54 | 0.923 | 0.337 | Colorectal cancer | Yes | 57.3 | 0.542 | 0.461 |
| No | 45.7 | No | 53.2 | ||||||
|
| |||||||||
| Living place | City | 52.1 | 0.626 | 0.429 | Cervical cancer | Yes | 42.1 | 3.281 | 0.07 |
| Country | 54.9 | No | 54.5 | ||||||
|
| |||||||||
| BMI | 1 | 55 | 0.686 | 0.877 | Head and neck cancer | Yes | 55.8 | 0.167 | 0.682 |
| 2 | 52.8 | No | 53.4 | ||||||
| 3 | 55.4 | Fatigue | Yes | 60.9 | 7.428 | 0.006 | |||
| 4 | 50 | No | 50.5 | ||||||
|
| |||||||||
| Metastasis number | 0 | 47.9 | 9.45 | 0.024 | Pain | Yes | 56.6 | 37.497 | <0.001 |
| 1 | 56.7 | No | 14.3 | ||||||
| 2 | 57.3 | Constipation | Yes | 65.2 | 15.432 | <0.001 | |||
| ≧3 | 62.8 | No | 49.5 | ||||||
|
| |||||||||
| Bone metastasis | Yes | 59.3 | 4.6 | 0.032 | Anorexia | Yes | 66.3 | 29.665 | <0.001 |
| No | 51.1 | No | 46.4 | ||||||
|
| |||||||||
| Lymph metastasis | Yes | 57.8 | 1.27 | 0.259 | Sleep disorder | Yes | 65.6 | 23.764 | <0.001 |
| No | 52.7 | No | 47.4 | ||||||
|
| |||||||||
| Liver metastasis | Yes | 62.3 | 4.344 | 0.037 | Bloating | Yes | 57.5 | 0.753 | 0.386 |
| No | 52.1 | No | 53 | ||||||
|
| |||||||||
| PS | Good | 52.4 | 1.087 | 0.297 | Cough | Yes | 50.6 | 0.853 | 0.356 |
| Poor | 56.4 | No | 54.5 | ||||||
|
| |||||||||
| Distress score | Severe | 60 | 0.249 | 0.618 | Nausea and vomiting | Yes | 58.3 | 2.505 | 0.114 |
| Mild | 53.5 | No | 52 | ||||||
|
| |||||||||
| Barthel index | Severe | 51.6 | 1.963 | 0.58 | Dyspnea | Yes | 58.8 | 1.938 | 0.164 |
| Moderate | 57 | ||||||||
| Slight | 52.5 | ||||||||
| Independent | 50 | No | 52.5 | ||||||
|
| |||||||||
| Background pain level | 1 | 44.7 | 52.282 | <0.001 | Symptom number | ≦4 | 45.5 | 33.764 | <0.001 |
| 2 | 73.7 | 5–9 | 65.5 | ||||||
| 3 | 75 | ≧10 | 73.3 | ||||||
Table 4.
Multivariate analysis for presence of BTP.
| B | SE | Wald | P | AOR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Age (≧65) | −0.470 | 0.177 | 7.037 | 0.008 | 0.625 | 0.441 | 0.884 |
| Primary school (ref) | 4.410 | 0.110 | |||||
| Middle school | 0.329 | 0.176 | 3.505 | 0.061 | 1.390 | 0.985 | 1.962 |
| Graduate and above | −0.007 | 0.257 | 0.001 | 0.979 | 0.993 | 0.601 | 1.643 |
| Bone metastasis | 0.090 | 0.225 | 0.160 | 0.689 | 1.094 | 0.705 | 1.699 |
| Liver metastasis | 0.280 | 0.265 | 1.121 | 0.290 | 1.324 | 0.788 | 2.225 |
| No metastasis (ref) | 0.896 | 0.826 | |||||
| Metastasis number (1) | 0.207 | 0.223 | 0.861 | 0.354 | 1.230 | 0.794 | 1.907 |
| Metastasis number (2) | 0.094 | 0.270 | 0.121 | 0.728 | 1.098 | 0.647 | 1.862 |
| Metastasis number (≧3) | 0.119 | 0.362 | 0.109 | 0.742 | 1.127 | 0.555 | 2.289 |
| Symptom numbers (≦4) (Ref) | 3.280 | 0.194 | |||||
| Symptom numbers (5–9) | 0.394 | 0.220 | 3.228 | 0.072 | 1.484 | 0.965 | 2.281 |
| Symptom numbers (≧10) | 0.426 | 0.491 | 0.752 | 0.386 | 1.531 | 0.585 | 4.006 |
| Mild background pain (ref) | 48.998 | <0.001 | |||||
| Moderate background pain | 1.251 | 0.189 | 43.935 | <0.001 | 3.495 | 2.414 | 5.061 |
| Severe background pain | 1.222 | 0.410 | 8.902 | 0.003 | 3.395 | 1.521 | 7.579 |
| Anorexia | 0.639 | 0.201 | 10.168 | 0.001 | 1.895 | 1.279 | 2.808 |
| Sleep disorder | 0.221 | 0.198 | 1.248 | 0.264 | 1.247 | 0.846 | 1.838 |
| Constipation | 0.391 | 0.187 | 4.372 | 0.037 | 1.479 | 1.025 | 2.133 |
| Fatigue | −0.307 | 0.209 | 2.154 | 0.142 | 0.736 | 0.489 | 1.108 |
Ref, reference; B, regression coefficient; SE, standard deviation; Wald, Wald coefficient; AOR, adjusted odds ratio; CI, confidence interval; Hosmer–Lemeshow test, P=0.881; adjusted Nagelkerke, R2 = 19.5%; overall predictive ability, 66.4%; −2 log likelihood = 976.037.
4.2. Intensity of BTP
Mann–Whitney test and Kruskal–Wallis test showed that variables associated with a higher BTP intensity were BMI (H = 8.874, P=0.031) and bloating (Z = −1.973, P=0.049). There was no linear relation between BTP intensity and BMI, so dummy variables were set. Table 5 shows the results of multivariate analysis and bloating was an independent influencing factor of BTP intensity. The residual was in normal distribution, and there existed no multiple collinearity.
Table 5.
Multiple linear regression of BTP intensity.
| B | SE | Standard beta | t | P | 95% CI | Tolerance | VIF | |
|---|---|---|---|---|---|---|---|---|
| Normal (ref) | ||||||||
| Low weight | 0.127 | 0.096 | 0.067 | 1.323 | 0.186 | −0.062, 0.316 | 0.891 | 1.122 |
| Overweight | −0.188 | 0.113 | −0.083 | −1.655 | 0.099 | −0.411, 0.035 | 0.900 | 1.111 |
| Obesity | 0.323 | 0.176 | 0.090 | 1.835 | 0.067 | −0.023, 0.670 | 0.950 | 1.053 |
| Bloating | 0.238 | 0.115 | 0.099 | 2.060 | 0.040 | 0.011, 0.464 | 0.998 | 1.002 |
F = 3.649, P=0.006; Ref, reference; B, standardized coefficients; SE, standard deviation; Durbin–Watson, 1.745.
4.3. Number of BTP Episodes during Hospitalization
Mann–Whitney test and Kruskal–Wallis test showed that variables associated with higher BTP episodes were colorectal cancer (Z = −2.058, P=0.04), distress score (Z = −2.222, P=0.026), bloating (Z = −3.01, P=0.03), anorexia (Z = −2.714, P=0.007), sleep disorder (Z = −2.599, P=0.009), fatigue (Z = −3.165, P=0.002), and symptoms number (H = 18.011, P < 0.001). Symptoms number was positively related with BTP episodes (r = 0.279, P < 0.001). Considered the multicollinearity among symptoms, we choose stepwise selection in multiple linear regression. More symptom numbers were independently associated with a higher number of BTP episodes (Table 6). The residual was in normal distribution.
Table 6.
Multiple linear regression of BTP episodes during hospitalization.
| B | SE | Standard beta | t | P | 95% CI | Tolerance | VIF | |
|---|---|---|---|---|---|---|---|---|
| Symptoms number | 0.302 | 0.093 | 0.156 | 3.255 | 0.001 | 0.120, 0.484 | 1.000 | 1.000 |
F = 10.593, P=0.001; B, standardized coefficients; SE, standard deviation; Durbin–Watson, 1.505.
5. Discussion
5.1. Characteristics of Background Pain and BTP
Cancer-related pain was the most popular symptom [28, 29]. A systematic review concluded in advanced cancer that 35% to 96% of cancer patients experienced pain [30], while more than 50% of cancer patients experienced moderate to severe pain [31]. Though, totally, patients had mild background pain score in our study, more than 50% then experienced BTP with high episodes and intensity, which was consistent with current studies that the BTP occurrence rate varies from 25.7% to 80% [18–21]. It seemed that there was an interaction between background pain and BTP. On the one hand, high background pain intensity was proved to be associated with frequent BTP [21]. We also revealed the slight positive correlation between BTP episodes and background level. Unfortunately, though patients with severe background pain score had stronger BTP intensity, significant statistical difference was not found. However, the importance of optimizing background pain management still needs to be emphasized, for optimization of background analgesia is vitally important to decrease BTP episodes, peak intensity, and duration [32, 33]. On the other hand, patients with BTP had higher pain score at discharge, indicating the controlling of BTP is helpful to improve treatment outcome.
Existing suffering of background pain or BTP aggravated the physical and symptoms burden. We found that the patients with BTP and those with more severe background pain score had longer hospital stays and more symptoms numbers, as the previous study showed [34]. Our study showed the occurrence of pain or BTP was related to poor prognosis, which was consistent with the finding that patients with BTP had more pain-related interference in function, worse physical health and mental health, and more disability [18]. Pain was considered to have strong influence on ADL difficulties [35]. Interestingly, we found the severe background pain was associated with better activity of daily life, which was contradictory to some conclusions that pain intensity was significantly negatively correlated with ADL [36, 37]. The possible interpretation was patients with better ADL more easily triggered the pain due to more activity. For example, patients may experience incident pain each time they get up from a chair or perform another specific activity [14]. Thus, NCCN guideline is recommended to optimize activities of daily living [2]. Activity movement was also proved to be the principal triggers for predictable BTP [13, 38], though there was no relationship between ADL and BTP in our study. No statistical difference was found between pain, BTP, and cancer types. We hypothesized that the participants were all in advanced stage. Thus, the cancer type played a less important role compared with disease condition.
Increased odds of persistent pain seemed to be related to younger age. Our study showed the elder (age ≧ 65) had a lower prevalence of BTP compared to younger groups. Meanwhile, age was negatively related to BTP intensity and episodes, and age was one of the independent influencing factors of BTP presentation. Sebastiano et al. found younger patients had higher background pain, a fast onset, and predictable BTP [13]. Pain is inherently subjective, and patient self-report is the current standard for assessment. However, less pain manifestation of elderly does not mean the less pain-related suffering, but some complications made self-reporting of pain more difficult. One of the biggest challenges is that most older adults and health-care practitioners perceive that pain is a normal part of aging, which impede history taking and pain expression [14]. Communication may be another challenge for memory failure, impaired cognition, sensory impairment (visual, hearing, and circulation problems), and stoicism with aging that can impact both assessment and management of pain older adults [39]. For elderly, prioritizing pain assessment so as to decrease the chance of inadequate analgesia is important. In addition to self-assessment pain, observation of pain-related behaviors and discomfort (like facial expression, body movements, changes in interpersonal interactions, and routine activity) are alternative strategies for assessing the presence of pain [40].
As for the intervention of BTP, opioids are the drug rescue of choice for BTP and guideline recommends offering oral immediate-release morphine for the first-line rescue medication of BTP [41, 42]. Recent data indicate that there were large disparities in the use of opioid analgesics to control BTP worldwide [43], due to cultural differences and overall awareness. We found intramuscular injection of hydromorphone hydrochloride and subcutaneous morphine were the most common analgesic form for BTP onset, while oral morphine accounted for only 8.4%. The reason for not adopting oral morphine as first-line treatment for BTP was as follows. Firstly, patients usually are reluctant to choose oral analgesics for they harbor a variety of fears and misconceptions such as opioid addiction, tolerance, and series of side effects [44]. Second is the unconsciousness or low compliance of medical members to guideline. Recent study showed despite oncologist's clinical practice on BTP treatment was increasingly guided by clinical guideline, it suffers from limited compliance [45]. Our study indicated improved dissemination and education were needed to enhance the awareness and guideline implementation in Chinese context.
5.2. Factors Influencing BTP Clinical Presentation
BTP was proved to be related to the presence of more than one pain, a vertebral pain syndrome, pain due to plexopathy, and English-speaking country [46]. This study indicates that BTP may have different characteristics and influence by many factors especially various concomitant symptoms. Patients aged <65 accompanied by moderate and severe background pain and the occurrence of symptoms including anorexia and constipation easily suffer BTP. There were some studies that proved compared to adults, the old-age group experienced less BTP [47]. Older patients might have multiple complex and serious complications which obstructed the presentation of BTP. A cross-sectional study reported subjects with older age had lower odds of reporting cancer alarm symptoms [48]. Our study showed patients with anorexia, fatigue, and constipation more easily suffer BTP. Symptoms like fatigue, sleep disorders, and anorexia were the common symptoms for cancer patients, which easily promoted the occurrence of the pain [42]. Anorexia and fatigue were often related to reduced energy intake, which lead to malnutrition and muscle strength. There were some researches that have proved the greater muscle strength was associated with less pain [37, 49, 50]. A randomized controlled trial revealed muscle strengthening and balancing exercises were effective in reducing chemotherapy-induced peripheral neuropathic pain and improving QOL among cancer patients [51]. Also, nutrition interventions had a significant effect on pain reduction [52]. The finding emphasized the importance to strengthen nutrition and recovery of myodynamia to reduce the possibility of BTP for patients with potential nutrition-related risk factors. Constipation is one of the most common possible side effects of analgesia (such as opioids) for moderate-to-severe pain (BTP) [53], which is not only a common factor contributing to pain but also a reversible cause of BTP. Thus, using laxatives is recommended during constipation to avoid BTP. Moderate and severe background pain was proved to be an influencing factor of BTP presentation. That's because higher background pain intensity may favor the development of BTP episodes, and severe background pain intensity was a powerful predictor of BTP scores [13]. Bloating was the only independent influencing factor of BTP intensity, which can be caused by constipation.
Symptoms number was proved to be the independent influencing factor of BTP episodes. The more the symptoms, the more the episodes. We hypothesize that less symptoms present a related good situation and thus less likely to suffer BTP onset. Symptoms and BTP onset interacted with each other. On one hand, symptoms promote the occurrence of BTP, and on the other hand, BTP results in significantly worse outcomes on functional and symptom [54].
5.3. Strengths and Limitations of the Study
There was limited literature to explore the characteristics of BTP and its influencing factors among advanced cancer patients. Firstly, our study confirmed the high prevalence of BTP among advanced cancer patients in the palliative care ward and the status characteristics of BTP episodes and intensity. Again, we emphasized the importance of the background pain controlling for better BTP management as well as optimized BTP scheme for better treatment outcome. Severe background pain and BTP were both related to poor prognosis, like longer hospital stay and more symptoms. Besides, more severe background pain was related to higher activity of daily living. Further research about the reason why oral opioid is not used as first-line therapy for BTP and related solutions should be considered. Several influencing factors of BTP presentation, such as older age, background pain level, the occurrence of anorexia, and constipation, were identified. Meanwhile, the bloating and symptoms number were independent factors of BTP intensity and episodes, respectively. For these heterogeneous clinical presentations, our finding indicated the problem should be solved individually. We should pay attention to the potential masked pain among elderly. This study has certain limitations. One of them is a retrospective study in which data were collected from the medical record and the fact that the data collection was conducted in a single hospital. The findings may not apply to all patients with advanced cancer. Though some influencing factors of BTP were confirmed, our retrospective design does not allow to establish a causal relationship for this association. As for the possibility of time rhythm of BTP, we failed to collect its onset characteristics including onset time, time to maximum pain intensity, mean duration of untreated episodes, and time to meaningful pain relief after intervention. Further research is needed in this regard for better BTP management.
Acknowledgments
This study was funded by Key Research and Development Program of Science and Technology Department of Hunan Province (2020SK2121) and Natural Science Foundation of Hunan Province (2020JJ8021).
Data Availability
The data are provided in the Supplementary Information files.
Ethical Approval
This is an observational study, and this article does not contain any clinical studies with human participants or animals performed by any of the authors. The Hunan Cancer Hospital Research Ethics Committee has approved the study (Ethics Approval Number is 12034209).
Consent
Written information was used to explain the study to all participants and their family members, including that the study would use anonymized medical information. Informed consent was obtained with an opt-out policy before the study could begin. The participant has consented to the submission of the original study including their data to the journal.
Conflicts of Interest
All the authors have declared that no conflicts of interest exist.
Authors' Contributions
All authors contributed to the study conception and design. The study was designed by Xuying Li, Yongyi Chen, and Rongrong Fan. Material preparation and data collection were performed by Rongrong Fan and Siyu Yang. Data analysis was performed by Xiaofan Bu and Siyu Yang. Cui Ling, Boyong Shen, and Wang Ying interpreted the results. The first draft of the manuscript was written by Rongrong Fan. All authors commented on previous versions of the manuscript, and all authors read and approved the final manuscript.
Supplementary Materials
Details of extraction form and raw data of the participants were presented in Supplementary Material.
References
- 1.Chung M., Kim H. K., Abdi S. Update on cannabis and cannabinoids for cancer pain. Current Opinion in Anaesthesiology . 2020;33(6):825–831. doi: 10.1097/aco.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 2.Swarm R. A., Paice J. A., Anghelescu D. L., et al. Adult cancer pain, version 3.2019, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network . 2019;17(8):977–1007. doi: 10.6004/jnccn.2019.0038. [DOI] [PubMed] [Google Scholar]
- 3.Fink R. M., Brant J. M. Complex cancer pain assessment. Hematology-Oncology Clinics of North America . 2018;32(3):353–369. doi: 10.1016/j.hoc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Wang N., Dong Y., Zhao L., Zhao H., Li W., Cui J. Factors associated with optimal pain management in advanced cancer patients. Current Problems in Cancer . 2019;43(1):77–85. doi: 10.1016/j.currproblcancer.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Bernardes S. F., Matos M., Mourão S., Vauclair C.-M. Cultural adaptation and psychometric validation of the Portuguese breakthrough pain assessment tool with cancer patients. Scandinavian Journal of Pain . 2021;21(4):688–695. doi: 10.1515/sjpain-2021-0002. [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Hernández C., Blasco A., Gándara Á., et al. Prevalence and characterization of breakthrough pain in patients with cancer in Spain: the CARPE-DIO study. Scientific Reports . 2019;9(1):p. 17701. doi: 10.1038/s41598-019-54195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campagna S., Sperlinga R., Milo A., et al. The circadian rhythm of breakthrough pain episodes in terminally-ill cancer patients. Cancers . 2018;11(1):p. 18. doi: 10.3390/cancers11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies A. N., Elsner F., Filbet M. J., et al. Breakthrough cancer pain (BTcP) management: a review of international and national guidelines. BMJ Supportive & Palliative Care . 2018;8(3):241–249. doi: 10.1136/bmjspcare-2017-001467. [DOI] [PubMed] [Google Scholar]
- 9.Gonella S., Sperlinga R., Sciannameo V., Dimonte V., Campagna S. Characteristics of breakthrough pain and its impact on quality of life in terminally ill cancer patients. Integrative Cancer Therapies . 2019;18 doi: 10.1177/1534735419859095.153473541985909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brant J., Rodgers B., Gallagher E., Sundaramurthi T. Breakthrough cancer pain: a systematic review of pharmacologic management. Clinical Journal of Oncology Nursing . 2017;21(3):71–80. doi: 10.1188/17.Cjon.S3.71-80. [DOI] [PubMed] [Google Scholar]
- 11.Scarborough B. M., Smith C. B. Optimal pain management for patients with cancer in the modern era. CA: A Cancer Journal for Clinicians . 2018;68(3):182–196. doi: 10.3322/caac.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liossi C., Greenfield K., Schoth D. E., et al. A systematic review of measures of breakthrough pain and their psychometric properties. Journal of Pain and Symptom Management . 2021;62(5):1041–1064. doi: 10.1016/j.jpainsymman.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Mercadante S., Marchetti P., Cuomo A., et al. Factors influencing the clinical presentation of breakthrough pain in cancer patients. Cancers . 2018;10(6):p. 175. doi: 10.3390/cancers10060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fink R. M., Gallagher E. Cancer pain assessment and measurement. Seminars in Oncology Nursing . 2019;35(3):229–234. doi: 10.1016/j.soncn.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Currow D. C., Clark K. Opioids for breakthrough cancer pain. The Oncologist . 2020;25(7):p. e1133. doi: 10.1634/theoncologist.2020-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samolsky Dekel B. G., Gori A., Gunnellini M., et al. The Italian questionnaire for cancer breakthrough pain diagnosis, a multicenter validation study. Pain and Therapy . 2021;10(2):1171–1188. doi: 10.1007/s40122-021-00274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan H. S., Liu N., Sultana R., et al. Prediction of breakthrough pain during labour neuraxial analgesia: comparison of machine learning and multivariable regression approaches. International Journal of Obstetric Anesthesia . 2021;45:99–110. doi: 10.1016/j.ijoa.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Narayana A., Katz N., Shillington A. C., et al. National breakthrough pain study. Pain . 2015;156(2):252–259. doi: 10.1097/01.j.pain.0000460305.41078.7d. [DOI] [PubMed] [Google Scholar]
- 19.Ferrero V. T., Oset M. M., Masferrer J. P., Pardo E. H., Sorolla E. J., Largo S. C. Prevalence and characterization of breakthrough pain in cancer patients with proctalgia treated with 3D pelvic radiotherapy. Clinical and Translational Oncology . 2019;21(12):1707–1711. doi: 10.1007/s12094-019-02102-1. [DOI] [PubMed] [Google Scholar]
- 20.Bhatnagar S., Upadhyay S., Mishra S. Prevalence and characteristics of breakthrough pain in patients with head and neck cancer: a cross-sectional study. Journal of Palliative Medicine . 2010;13(3):291–295. doi: 10.1089/jpm.2009.0266. [DOI] [PubMed] [Google Scholar]
- 21.Wang N., Liu Y., Shi L., He H., Wang C., Li H. Characteristics and prognostic factors for pain management in 152 patients with lung cancer. Patient Preference and Adherence . 2016;10:571–577. doi: 10.2147/ppa.S103276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jing L.-W., Wang F.-L., Zhang X.-L., Yao T., Xing F.-M. Occurrence of and factors influencing elderly homebound in Chinese urban community. Medicine . 2017;96(26) doi: 10.1097/md.0000000000007207.e7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sok M., Zavrl M., Greif B., Srpčič M. Objective assessment of WHO/ECOG performance status. Supportive Care in Cancer . 2019;27(10):3793–3798. doi: 10.1007/s00520-018-4597-z. [DOI] [PubMed] [Google Scholar]
- 24.Guan B., Wang K., Shao Y., et al. The use of distress thermometer in advanced cancer inpatients with pain. Psycho-Oncology . 2019;28(5):1004–1010. doi: 10.1002/pon.5032. [DOI] [PubMed] [Google Scholar]
- 25.Collin C., Wade D. T., Davies S., Horne V. The Barthel ADL Index: a reliability study. International Disability Studies . 1988;10(2):61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 26.Caraceni A., Shkodra M. Cancer pain assessment and classification. Cancers . 2019;11(4):p. 510. doi: 10.3390/cancers11040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ripamonti C. I., Santini D., Maranzano E., Berti M., Roila F. Management of cancer pain: ESMO clinical practice guidelines. Annals of Oncology . 2012;23(7):vii139–vii154. doi: 10.1093/annonc/mds233. [DOI] [PubMed] [Google Scholar]
- 28.Rojas-Concha L., Hansen M. B., Petersen M. A., Groenvold M. Which symptoms and problems do advanced cancer patients admitted to specialized palliative care report in addition to those included in the EORTC QLQ-C15-PAL? A register-based national study. Supportive Care in Cancer . 2020;28(4):1725–1735. doi: 10.1007/s00520-019-04976-x. [DOI] [PubMed] [Google Scholar]
- 29.Kirkova J., Rybicki L., Walsh D., Aktas A. Symptom prevalence in advanced cancer. American Journal of Hospice and Palliative Medicine . 2012;29(2):139–145. doi: 10.1177/1049909111410965. [DOI] [PubMed] [Google Scholar]
- 30.Henson L. A., Maddocks M., Evans C., Davidson M., Hicks S., Higginson I. J. Palliative care and the management of common distressing symptoms in advanced cancer: pain, breathlessness, nausea and vomiting, and fatigue. Journal of Clinical Oncology . 2020;38(9):905–914. doi: 10.1200/jco.19.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett M., Paice J. A., Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. American Society of Clinical Oncology Educational Book . 2017;37(37):705–713. doi: 10.1200/edbk_180469. [DOI] [PubMed] [Google Scholar]
- 32.Mercadante S., Valle A., Porzio G., et al. Relationship between background cancer pain, breakthrough pain, and analgesic treatment: a preliminary study for a better interpretation of epidemiological and clinical studies. Current Medical Research and Opinion . 2013;29(6):667–671. doi: 10.1185/03007995.2013.792247. [DOI] [PubMed] [Google Scholar]
- 33.Mercadante S., Caraceni A., Cuomo A., et al. A longitudinal study of breakthrough cancer pain: an extension of IOPS-MS study. Journal of Clinical Medicine . 2021;10(11):p. 2273. doi: 10.3390/jcm10112273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valeberg B. T., Miaskowski C., Hanestad B. R., Bjordal K., Paul S., Rustøen T. Demographic, clinical, and pain characteristics are associated with average pain severity groups in a sample of oncology outpatients. The Journal of Pain . 2008;9(10):873–882. doi: 10.1016/j.jpain.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Connolly D., Garvey J., McKee G. Factors associated with ADL/IADL disability in community dwelling older adults in the Irish longitudinal study on ageing (TILDA) Disability and Rehabilitation . 2017;39(8):809–816. doi: 10.3109/09638288.2016.1161848. [DOI] [PubMed] [Google Scholar]
- 36.Solbakken G., Løseth S., Froholdt A., et al. Pain in adult myotonic dystrophy type 1: relation to function and gender. BMC Neurology . 2021;21(1):p. 101. doi: 10.1186/s12883-021-02124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luc-Harkey B. A., Safran-Norton C. E., Mandl L. A., Katz J. N., Losina E. Associations among knee muscle strength, structural damage, and pain and mobility in individuals with osteoarthritis and symptomatic meniscal tear. BMC Musculoskeletal Disorders . 2018;19(1):p. 258. doi: 10.1186/s12891-018-2182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercadante S., Masedu F., Valenti M., Aielli F. Breakthrough pain in patients with lung cancer. A secondary analysis of IOPS MS study. Journal of Clinical Medicine . 2020;9(5):p. 1337. doi: 10.3390/jcm9051337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brant J. M. Assessment and management of cancer pain in older adults: strategies for success. Asia-Pacific Journal of Oncology Nursing . 2018;5(3):248–253. doi: 10.4103/apjon.apjon_11_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallon M., Giusti R., Aielli F., et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Annals of Oncology . 2018;29(4):iv166–iv191. doi: 10.1093/annonc/mdy152. [DOI] [PubMed] [Google Scholar]
- 41.National Institute for Health and Care Excellence. Palliative Care for Adults: Strong Opioids for Pain Relief, London, UK: National Institute for Health and Care Excellence (UK); 2018. Clinical Guidelines (2016) [PubMed] [Google Scholar]
- 42.Jara C., Del Barco S., Grávalos C., et al. SEOM clinical guideline for treatment of cancer pain (2017) Clinical and Translational Oncology . 2018;20(1):97–107. doi: 10.1007/s12094-017-1791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olarte J. M. N. Breakthrough cancer pain and rational drug use. Supportive Care in Cancer . 2017;25(S1):11–17. doi: 10.1007/s00520-017-3636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhiner M. I., von Gunten C. F. Cancer breakthrough pain in the presence of cancer-related chronic pain: fact versus perceptions of health-care providers and patients. Journal of Supportive Oncology . 2010;8(6):232–238. doi: 10.1016/j.suponc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 45.López López R., Camps Herrero C., Khosravi-Shahi P., et al. Oncologist’s knowledge and implementation of guidelines for breakthrough cancer pain in Spain: CONOCE study. Clinical and Translational Oncology . 2018;20(5):613–618. doi: 10.1007/s12094-017-1756-5. [DOI] [PubMed] [Google Scholar]
- 46.Caraceni A., Martini C., Zecca E., et al. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliative Medicine . 2004;18(3):177–183. doi: 10.1191/0269216304pm890oa. [DOI] [PubMed] [Google Scholar]
- 47.Ahuja D., Choudhary N., Kumar V., Gupta N., Bharati S. J. Managing breakthrough pain for advanced malignancy in elderly patients: a real challenge. Journal of Opioid Management . 2020;16(3):219–222. doi: 10.5055/jom.2020.0571. [DOI] [PubMed] [Google Scholar]
- 48.Svendsen R. P., Paulsen M. S., Larsen P. V., et al. Associations between reporting of cancer alarm symptoms and socioeconomic and demographic determinants: a population-based, cross-sectional study. BMC Public Health . 2012;12(1):p. 686. doi: 10.1186/1471-2458-12-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos T. R. T., Oliveira B. A., Ocarino J. M., Holt K. G., Fonseca S. T. Effectiveness of hip muscle strengthening in patellofemoral pain syndrome patients: a systematic review. Brazilian Journal of Physical Therapy . 2015;19(3):167–176. doi: 10.1590/bjpt-rbf.2014.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S., Shaharudin S., Abdul Kadir M. R. Effects of blood flow restriction training on muscle strength and pain in patients with knee injuries. American Journal of Physical Medicine & Rehabilitation . 2021;100(4):337–344. doi: 10.1097/phm.0000000000001567. [DOI] [PubMed] [Google Scholar]
- 51.Dhawan S., Andrews R., Kumar L., Wadhwa S., Shukla G. A randomized controlled trial to assess the effectiveness of muscle strengthening and balancing exercises on chemotherapy-induced peripheral neuropathic pain and quality of life among cancer patients. Cancer Nursing . 2020;43(4):269–280. doi: 10.1097/ncc.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 52.Brain K., Burrows T. L., Rollo M. E., et al. A systematic review and meta-analysis of nutrition interventions for chronic noncancer pain. Journal of Human Nutrition and Dietetics . 2019;32(2):198–225. doi: 10.1111/jhn.12601. [DOI] [PubMed] [Google Scholar]
- 53.National Institute for Health and Care Excellence. End of Life Care for Infants, Children and Young People with Life-Limiting Conditions: Planning and Management . London, UK: National Institute for Health and Care Excellence (UK); 2020. Clinical Guidelines (2019) [PubMed] [Google Scholar]
- 54.Hjermstad M. J., Kaasa S., Caraceni A., et al. Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Supportive & Palliative Care . 2016;6(3):344–352. doi: 10.1136/bmjspcare-2015-000887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of extraction form and raw data of the participants were presented in Supplementary Material.
Data Availability Statement
The data are provided in the Supplementary Information files.


