Abstract
Introduction
Vulvodynia (chronic vulvar pain) is a sexually debilitating disorder with a prevalence of ∼10%.
Aim
To investigate the effectiveness of therapy with local anesthetics (TLA) in women with severe vulvodynia, we conducted a prospective, non-controlled observational study.
Methods
45 patients with severe chronic vulvodynia (primary and secondary vulvodynia, 0–10 numeric analogue scale (NAS) ≥6, median 7.9, duration ≥6 months, median 65.2 months) in an outpatient practice in Germany were treated with TLA in 3–12 sessions using procaine 1% as local anesthetic. Effectiveness was analyzed with Wilcoxon signed rank tests and Wilcoxon rank sum tests.
Outcomes
Therapeutic success as a reduction of pain to ≤4 NAS lasting for ≥6 months after end of therapy.
Results
TLA successfully reduced vulvodynia in 36 of 45 patients (80 %, responders). The NAS reduction was from 7.9 to 2.4 (P < .001). Even patients denominated as non-responders experienced a significant reduction in NAS (P = .03). In responders, long-term success was observed for 6.8–125 months (median 24.1 months). No adverse events occurred.
Clinical Translation
A promising new treatment for a hard-to-treat chronic female pain disorder.
Strengths and Limitations
Limitation: Monocentric, non-controlled observational design; Strength: the high number of patients treated.
Conclusion
The high success rate of TLA in this investigation offers new perspectives on the etiology of vulvodynia as a complex pain syndrome affecting several nerves of the pelvic floor, and also provides early insight into the effectiveness of TLA in women with vulvodynia.
Weinschenk S, Benrath J, Kessler E, et al. Therapy With Local Anesthetics to Treat Vulvodynia. A Pilot Study. Sex Med 2022;10:100482.
Key Words: Neurogenic Inflammation, Pudendal Neuralgia, Vestibulitis, Vulvar Pain, Complex Pain Syndrome, Neural Therapy
INTRODUCTION
Vulvodynia is defined as “chronic vulvar pain of at least three months duration, without clear identifiable cause, which may have potential associated factors.”1 Two major types are frequently found: Localized provoked vulvodynia (LPV), formerly vulvar vestibulitis, and generalized vulvodynia (GVD), formerly chronic vulvar pain.1 The definition of vulvodynia by the International Society for the Study of Vulvar Diseases (ISSVD)1 distinguishes primary (idiopathic), and secondary vulvodynia, which occur after an identifiable event such as trauma, vaginal delivery, or chronic dermal diseases, such as lichen sclerosis and genital herpes.
Vulvodynia has a lifetime prevalence of 7–16%, with most patients suffering on average 3 years before diagnosis.2 Therapy regimes include3: Steroids, hormonal therapy, anticonvulsants, antidepressants, psychotherapy, physical therapy,4 acupuncture,5 and even vulvectomy.6,7 In a multimodal setting, 63% of vulvodynia patients saw no improvement after six years of treatment.8
Pathophysiology theories include genetic,9 psychological,10 and musculofascial factors.4 The rate of pelvic floor dysfunction11, 12, 13 was significantly higher in patients with vulvodynia.12 However, it is unclear, if this is a primary cause, or a sequela of vulvodynia. There is increasing evidence that vulvodynia may be a neuropathic disorder with central and/or peripheral sensitization. Histopathological specimens after vestibulectomy reveal an elevated nerve density14 and a higher concentration of free nerve endings,15 identified as nociceptors16 with transient receptor potential (TRP) channels.17 Together with an increased sensitization to thermal stimuli,18 these findings suggest that vulvodynia can result from pudendal neuralgia.19
Consequently, vulvodynia has been treated with local anesthetics in several studies. Topical self-application of lidocaine decreased dyspareunia.20 Injections of procaine 1%, a short-acting local anesthetic (LA) to the pudendal nerve via trans-gluteal21 or dorsal access were performed.22 Combined injections of LAs to the pudendal nerve, affected vulvar area, and impar ganglion improved both forms of vulvodynia, LPV and GVD.23,24 However, cost-intensive radiologic surveillance is necessary, and it only has an efficacy of 50% local anesthesia.21,25,26
We tested the effectiveness of Therapeutic Local Anesthesia (TLA, in Central Europe also known as Neural Therapy)27 on vulvodynia. TLA is an effective method to treat chronic pain.28,29 A first case report with successful TLA in vulvodynia was published in 2013.30 Application routes were: Local infiltrations,24,31 intracutaneous injections, or regional blockades such as transperineal pudendal nerve blockade.32 This technique consists of repeatedly injecting LA into the respective nerve, is highly effective (90% anesthesia of the perineum), and makes therapy more comfortable for patients than trans-gluteal21 or intravaginal techniques.23 A local approach was chosen by Rey-Novea et al31 who injected LAs in tender vulvar areas with good success rates for six months or more.
The aim of this study was to evaluate the effectiveness of TLA on vulvodynia in a large cohort of affected patients. We aimed to test the hypothesis: “Can TLA result in long-term relief of vulvodynia symptoms in patients in which all treatment methods thus far have been unsuccessful?”
MATERIALS AND METHODS
Patients
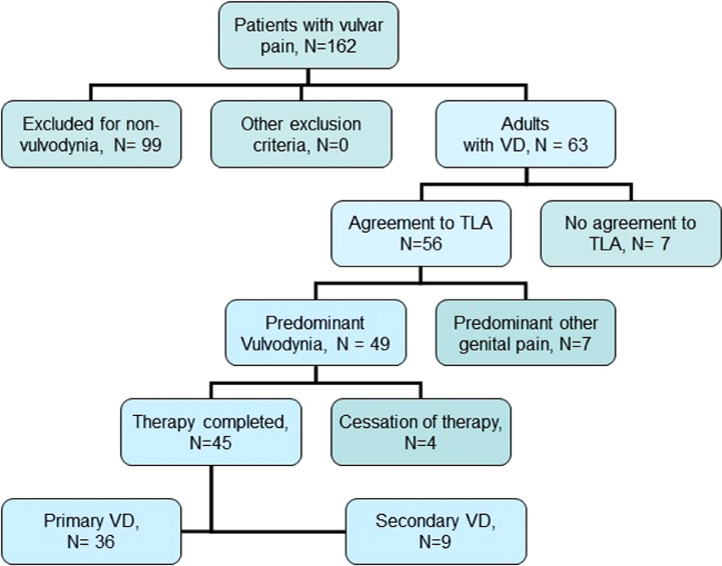
This observational prospective study was approved by the ethical board of the University Hospital Heidelberg (approval S487-2011 of December 06, 2011). Written informed consent was obtained from all participants. 162 consecutive patients presented with vulvar pain in an outpatient practice specialized in chronic gynecological diseases in Karlsruhe, Germany, between April 2008 and December 2018, and were screened for vulvodynia. Most of them (n = 99) suffered from lighter forms of vulvodynia (NAS 5 or less) or had temporary complaints (<6 months). 63 of them suffered from a severe form of chronic vulvodynia (NAS ≥6 and ≥6 months of duration) and met the ISSVD criteria of vulvodynia.1 All of them have received multimodal pain therapy and had seen 5–15 physicians and pain clinics before. Therapy with Local Anesthetics (TLA) of the genital region was offered to all 63 patients and consisted of a regimen of at least three therapy sessions. 56 of them (88.9%) gave their informed consent for TLA and for pseudonymized data evaluation. Inclusion criteria were: Primary and secondary chronic vulvodynia, according to the above-mentioned definition; valid informed consent to TLA and to pseudonymized data evaluation, and age ≥18 years. Patients were excluded for the following reasons (see Figure 1): Mixed pain with major complaints not primarily located in the vulvar region; discontinuation of TLA after the first 1–2 therapy sessions, and missing informed consent.
Figure 1.
Flow chart of patient selection according to the STARD criteria.33
Among the 56 patients treated with TLA, seven patients suffered not only from vulvodynia, but from other predominant genital pain disorders as well: two with painful bladder syndrome, two with chronic vaginal pain, and three with chronic pelvic pain. Another complex and extended TLA regimen was applied in these patients; thus, their data were excluded from evaluation. In the end, 49 patients with isolated vulvodynia could be included in the data evaluation. All patients received TLA in 1–12 treatment sessions. Four patients cancelled treatment after the first one or two sessions. Among the 45 patients completing the therapy, we identified 36 with primary, and 9 with secondary vulvodynia. For patient selection, see Figure 1.
Physical Examination
A full gynecological examination was performed to exclude pregnancy, infections, morphological and microbiological causes, dermatosis (psoriasis, neurodermatitis), and oncological diseases. A Q-Tip test was performed to classify the type of vulvodynia and the extent of complaints. Pelvic floor assessment before therapy performed by palpation revealed normal findings in all patients. According to ISSVD criteria,1 each patient was allocated to the respective subgroup of vulvodynia. All examinations and treatments were performed by one of the authors, who has over two decades experience in TLA and the treatment of chronic gynecological pain.
TLA Treatment Regimen
Patients included received 3–12 treatment sessions, depending on the individual's improvement to their complaints. Therapy was ended as soon a therapeutic success was observed (see definition in section 2.4) or study observation was finished after 12 sessions, respectively. To exclude unknown LA allergies in patients, an allergenicity test with intracutaneous injections to exclude a type-I-allergy to LA was performed before the first session. In all sessions 3–20 ml procaine 1% without additional pharmaceuticals were used. Procaine requires only short surveillance time due to its ultra-short anesthesia time, has a low allergenicity,34 and has the highest anti-inflammatory potential of all frequently used LA.35 It has been used in previous vulvodynia studies as well.31
The first three sessions consisted of administering a bilateral perineal pudendal nerve blockade. This simple technique32 consists of sagittal injection of 5mL each, lateral to the greater labia with a 0.4 × 40 needles. If no success was achieved (no NAS reduction to ≤4) by the 3rd session, therapy was extended to other nerve blockades, such as the genitofemoral nerve via the inguinal channel,36 or the hypogastric plexus, which is performed by injecting 2 mL of LA transvaginally, with a 0.6 × 80 needle lateral to the cervix at 4 hour and 8 hour.37 Further injections were given according to co-morbidities: If a history of recurrent cystitis was described, also the bladder triangle (trigonum vesicae) was infiltrated.38 If present, infiltrations of genital scars (eg, from hysterectomy or laparoscopy) were performed. The respective injection techniques were applied as described in TLA textbooks.28
In order to standardize the otherwise individual therapy regimes, the first 5 sessions were planned with intervals of 1–3 weeks. The following sessions, if necessary, were appointed in individual intervals depending on the pain (NAS) and the duration of the pain-free interval since the previous session. Therapy could be discontinued once the therapy goal (≥50% improvement) has been achieved. A final examination was performed ≥6 months after the last treatment.
Pain Measurement and Treatment Evaluation
Patients rated their pain daily on a 0–10 nominal analogue scale (NAS) chart.3 Successful treatment was defined when persistent reduction to NAS ≤4 was achieved by treatment session 12. Patients with a successful treatment were defined as responders, all others as non-responders for TLA therapy. Further treatment beyond the 12th session and study protocol was offered to patients on demand in non-response cases.
Subsequent controls after 12 treatment sessions were done in regular preventive gynecological examinations together with a Q-tip test and the annual Pap smear. If the patient had been referred from other institutions, we performed telephone interviews with the patient for long-term assessment of the treatment results.
Statistical Analysis
Demographic variables of the patients were described as frequencies and percentages for categorical variables, and as means and standard deviations or median and range for continuous variables. Effectiveness of therapy as well as differences in NAS between groups was analyzed using Wilcoxon signed rank tests and Wilcoxon rank sum tests. Description of the progress of the NAS over time was illustrated by boxplots. Due to the exploratory character of the study, no missing data was imputed, P-values have a descriptive meaning, and if they were smaller than 0.05, they were defined as significant. All analyses were done using the statistical software R version ≥3.5.0 (R-Foundation for Statist. Computing, Vienna, Austria).
RESULTS
Patients
Patients’ characteristics are described in Table. 2. Patients (mean age 44.5 ± 14.9 years) had suffered from vulvodynia on average 5.43 years (SD = 5.6, range 1–22 years) before study inclusion. Patients had seen ∼7 physicians on average before coming to us (Table. 2).
Table 2.
Description of patients with vulvodynia included into data evaluation
| Variable | Measure | All patients | Primary | Secondary | P |
|---|---|---|---|---|---|
| Sample size | N(%) | 45 | 36 (80%) | 9 (20%) | |
| Biographic data | |||||
| Age | mean ± SD | 44.5 ± 14.9 | 44.3 ± 14.5 | 50.9 ± 15.7 | .153 |
| BMI | mean ± SD | 22.6 ± 4.1 | 22.6 ± 4.3 | 22.6 ± 3.2 | .773 |
| History of Deliveries | .239 | ||||
| 0 | N (%) | 22 (48.9) | 19 (52.8) | 3 (33.3) | |
| 1 | N (%) | 10 (22.2) | 8 (22.2) | 2 (22.2) | |
| 2 | N (%) | 10 (22.2) | 7 (19.4) | 3 (33.3) | |
| 3 | N (%) | 3 (6.7) | 2 (5.6) | 1 (11.1) | |
| VULVODYNIA Type | .514 | ||||
| GS (general - spontaneous) | N (%) | 30 (66.7) | 24 (66.7) | 6 (66.7) | |
| GP (general - provoked) | N (%) | 4 (8.9) | 4 (11.1) | 0 (0) | |
| LS (localized - spontaneous) | N (%) | 6 (13.3) | 5 (13.9) | 1 (11.1) | |
| LP (localized - provoked) | N (%) | 5 (11.1) | 3 (8.3) | 2 (22.2) | |
| Comorbidity | n. a. | ||||
| Vulvar lichen sclerosus | N (%) | 3 (6.7) | 0 | 3 (33.3) | |
| Genital herpes | N (%) | 1 (2.2) | 0 | 1 (11.1) | |
| Genital scars | N (%) | 3 (6.7) | 0 | 3 (33.3) | |
| Vulvar precancerosis | N (%) | 2 (4.4) | 0 | 2 (22.2) | |
| Onset cause | .150 | ||||
| - Inflammation | N (%) | 13 (28.9) | 10 (27.8) | 3 (33.3) | |
| - Trauma | N (%) | 21 (46.7) | 15 (41.7) | 6 (66.7) | |
| - Other | N (%) | 11 (24.4) | 11 (30.5) | 0 (0) | |
| Number of HCP seen before | mean ± SD | 7.4 ± 5.2 | 6.7 ± 5.2 | 9.8 ± 4.7 | n.s. |
| Number of therapy visits | Median (range) | 10 (5–14) | 11 (5–14) | 8 (6–14) | .224 |
| Admission waiting time (days) | Median (range) | 29 (0–216) | 29.5 (0–216) | 14 (1–149) | .754 |
BMI = body mass index; HCP = health care professionals; n. a. = not applicable; VD = vulvodynia.
The term “trauma” consists of different entities: Traumatic gynecological surgery, delivery, pain experience, or traumatic psychosocial experience. “Inflammation”: Complaints began after recurrent vaginitis or vulvitis.
Patients had undergone multiple previous therapies. This included therapy with fungicides, vaginal ointments, corticoids (topical and injected), topical and/or systemic hormones, systemic non-steroidal analgesics, antidepressants, general physical therapy, and treatment by a psychiatrist or psychotherapist. All therapeutic treatments that were still running at the start of our study were continued in all patients. Two patients complained of pain associated with lichen sclerosus, and two suffered from painful recurrent genital herpes. According to the ISSVD definition, these four patients suffered from secondary forms of vulvodynia.
Effectiveness of TLA
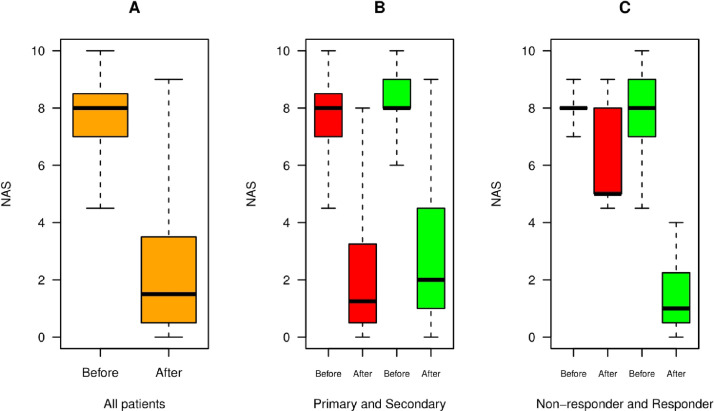
We performed TLA in addition to existing ongoing therapy. Sessions varied from 3-12 TLA treatments with an average of 7.8 ± 3.13 treatments. The timespan of therapy ranged two to 47.6 weeks with a median of 6.3 ± 10 weeks. The effectiveness of TLA is seen in Figure 2. Patients rated their vulvodynia pain with a mean NAS 7.9 before treatment and 2.4 after treatment (P < .001, see Figure 2A). The difference before and after therapy significantly decreased in both primary and secondary vulvodynia groups (P = .014, and P < .001, respectively), see Figure 2B.
Figure 2.
Therapy effect of TLA in Vulvodynia. (A) Pain score on a 0–10 nominal analogue scale (NAS) for vulvar pain before and after therapy in all patients (orange). The NAS decreases from 8.0 ± 1.1 on admission to 1.6 ± 1.4 on average. (B) Comparison of treatment effect in primary (red) and secondary vulvodynia (green bars). There is no significant difference between these two groups after treatment (NAS 1.2 vs 1.95 in secondary forms). (C) Therapy effect in the responder vs the non-responder subgroup. Improvement in responders is from 8.0 ± 1.1 on admission to 0.97 ± 0.7 (green bars), whereas non-responders (red) react slower, need more sessions, and experience improvement down to only 4.9 ± 1.8 (red bars).
The success rate increased from 68.3 % at visit 3 to 80% after including injections to other structures from the 4th therapy session on. Nine patients were found to be non-responders according to the definition from section 2.4. Among those nine patients, two had lichen sclerosus, one a combination of vulvodynia with pain in the periosteum of the ischium, and one a history of extended resection of a phlegmon of the left thigh, leaving a 9 × 8 cm scar in the region of the genitofemoral nerve. Five patients discontinued therapy after the 5th or 6th session with a final NAS of more than 4 (mean NAS on admission 8.2, last NAS 5.8). Significant pain reduction on NAS was also observed in non-responders (P = .036) (Figure 2C), but it did not meet our strict definition of therapy success.
Therapy was discontinued for one of the following reasons: Achievement of sufficient therapeutic success, no success after ≥12 therapy sessions, or non-compliance (discontinuation after the 1st or 2nd treatment session). In the responder group, discontinuation was more frequent due to successful therapy, with only 50% of patients treated seven or more sessions (Table. 1). In the non-responder group, ∼50% of patients were treated ≥11 times, with limited success. These patients were advised to end therapy after the 12th session (Table. 1).
Table 1.
Duration of therapy. 50% of the patients from the responder group discontinued the therapy until the 7th session, 50% of the non-responder group discontinued until the 11th session (numbers in bold)
| Patients in therapy | Adm | V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 | V10 | V11 | V12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 45 | 43 | 43 | 42 | 40 | 34 | 29 | 24 | 22 | 21 | 17 | 12 | 9 |
| Responder | 36 | 34 | 34 | 34 | 32 | 27 | 23 | 18 | 16 | 15 | 11 | 7 | 6 |
| NR | 9 | 9 | 9 | 8 | 8 | 7 | 6 | 6 | 6 | 6 | 6 | 5 | 3 |
Adm = admission date; V1–12 = treatment session 1–12; NR = non-responder.
Adverse Effects
During and immediately after therapy we observed no adverse effects (AE) such as circulatory depression, hypotension-induced dizziness, type I-allergy, anaphylaxis, bleeding from the injection site of >2 ml, or hematoma >1 cm2. All patients left the clinic in good health within 20–60 minutes. Long-term AE were structurally assessed in the subsequent therapy appointments. No other AE were reported, especially no neurological sequelae, no late allergy, no major bleeding, and no major hematoma after injection.
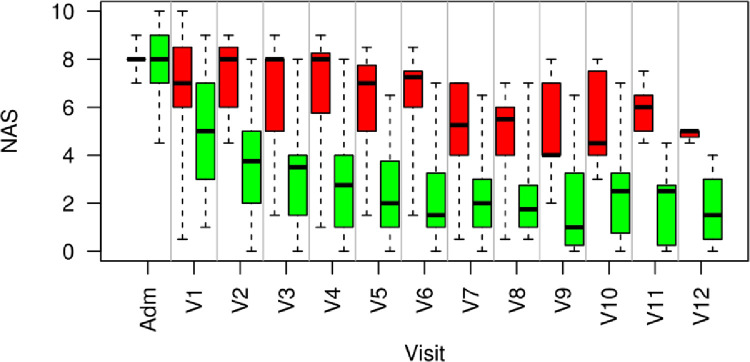
Timeline of Therapeutic Effects
Figure 3 shows box plots for the NAS time course of the two groups, responders and non-responders. The number of patients receiving treatment differed from N = 45 at admission to N = 9 patients (6 non-responders vs 3 responder) by visit 12, due to early success of therapy.
Figure 3.
Timeline of NAS-Score illustrated by boxplots for each visit for therapy responders (green) and non-responders (red). Adm = Admission; V1–V12 = number of treatment session. NAS decreases quickly in responders (green bars) within the first three sessions from 8.0 ± 1.2 on admission to 3.8 ± 1.1 after the first three treatments, whereas non-responders (red bars) react slower, but also experience an improvement to NAS = 5.1 ± 1.5 after the 7th session.
Most responders experienced their first improvement after the first few sessions. The time course of responders and non-responders can be clearly distinguished after the 2nd treatment session (P < .001).
Long Term Success Rate
Out of 45 patients included into data evaluation, 38 could be followed more than three months after the last therapy session, 32 (88.9%) of the responders, and 6 (67%) of the non-responders. Follow-up period of the 38 patients was 3.8–121.3 months, with a median of 21.1 months. From the nine non-responders, three were treated further in the open label phase of the study after the 12th therapy session, as continuation of the TLA was required by these patients. Among the 38 follow-up patients, all 32 responders remained within the response status. Furthermore, two out of the six non-responders became responders after the defined limit of 12 therapy sessions.
DISCUSSION
Main Findings
We investigated pain reduction with TLA in a non-placebo controlled prospective study with a homogeneous group of patients having clearly diagnosed vulvodynia according to ISSVD definition. 80% of the patients experienced therapeutic success, strictly defined by a permanent decrease in NAS to ≤4. Furthermore, NAS decreased on average from NAS 7.9 to 2.4 (P < .001) for all patients after 3–12 TLA therapy sessions.
Strengths and Limitations
The strength of our study was the high success rate in a homogeneous cohort of patients with severe and long-persisting vulvodynia. However, some limitations must be mentioned:
No Control Arm. In complex interventions, such as acupuncture or manual therapy, as well as TLA, building a control arm is a major challenge. The difficulty of control arms in observational research with complex interventions has been discussed widely, and not yet sufficiently resolved.39 The placebo effect according to placebo research studies40,41 counts for an average of 20% of the therapeutic success.41 Taking this into account, we still observed an effect far beyond just placebo. A study with a control group could further elucidate this issue. Taken TLA as an add-on therapy, it can take the credit of the therapeutic success, provided none of the ongoing therapy has been changed in the protocol. Although previous therapies varied among patients, a synergistic effect cannot be completely excluded. This may be evaluated in future controlled studies.
Sample Size. We observed a relatively small sample size in a monocentric setting. A sample with 45 patients is rather heterogenous concerning the comorbidities, such as HSV, and lichen sclerosus, and yields small subgroups of primary versus secondary vulvodynia. Yet the sample is larger than the studies of McDonald & Rapkin23 with 32 patients, and Rey-Novea31 with five patients. In our study, we prospectively included all consecutive cases of vulvodynia in the respective period. Nevertheless, there was a selection bias due to our strict inclusion criteria towards more severe cases of vulvodynia. We assume we can transfer these results to lighter forms of vulvodynia (<6 months duration, < NAS 6), as well. This should be proven in further studies with broader inclusion criteria.
Strict Definition of Success. Our definition of therapy success was very strict: NAS improvement to NAS ≤4 within 12 TLA sessions. Due to this strict definition, other patients who experienced limited therapeutic success were not counted as responders even if they were satisfied with the improvement to their complaints.
NAS as Outcome Measure. In order to measure outcome, questionnaires as the McGill Pain Questionnaire,23 a four-digit scale,11 or relative measures as in42 were used. Measuring treatment effectiveness in vulvodynia has been addressed by Andrews and coworkers.3 In congruence with these authors, we also used the NAS scale. Many pain research groups believe, in the light of the lack of objective pain parameters, NAS self-judging is the best measure for pain, compromising the sum of psychological and physical comfort.11 Therefore, we also adopted this measure in this pilot study on vulvodynia.3
Interpretation in Light of Other Evidence
Vulvodynia is a chronic condition hard to treat.43 Even after five years of multimodal therapy, 2/3 patients are not symptom-free.8 A multitude of therapeutic approaches have been proposed: Antibacterial, antifungal, analgesics (eg, opiates), anticonvulsives (pregabalin), antidepressants, and surgical excision,3,6,7 all with limited success. Vestibulectomy seems to have short-term success.3 In a retrospective study, long-term satisfaction was also reported,7 however this operation is debilitating, and non-reversible. The ISSVD therefore advocates not only focusing on the primary site of pain but a more holistic approach.44
Advantage of TLA in Vulvodynia. Therapeutic regimes of TLA in previous studies were based on difficult and expensive techniques.21,26,45 Recently, we developed a novel yet simplistic TLA approach for pudendal therapy, applicable without elaborate tools, and easy-to-learn for gynecologists and other physicians.32 The protocol in the present study is based on this technique for LA injections.
Most studies investigated a regimen with a single injection technique, such as dorsal,22 or trans-gluteal21 infiltration of the pudendal nerve, and infiltration of the impar ganglion.42 They reported a success rate of 41–43% after one month. The success rate after three months was not reported.42 Therefore, injection to a single, specific nerve does not seem to be the solution.
Rapkin et al used a standardized protocol with three different injections and reported on a reduction of NAS from 5.5 ± 0.5 to 3.5 ± 0.5 after five visits.23 This confirms the findings of our study, that inclusion of other nerves after unsuccessful treatment with pudendal nerve infiltration can increase the success rate from ∼68–80%. In contrast to the repeated application of injections on defined nerves, for example the impar ganglion42 or the pudendal nerve,23,24 we performed an individual-based regimen, with different nerve blockades depending on the progress of therapy effects, and on the individual symptoms of the respective patient. Rey-Novea et al31 successfully treated women with localized provoked vulvodynia using a simple technique of local injections. There needs to be further investigation to see if this technique is also applicable in a mixed cohort with women with generalized, or spontaneous pain as in our patient group. TLA was used as an additional therapy. Therefore, it is highly probable that including TLA in the therapy markedly improved the therapeutic success. However, a synergistic effect cannot completely be excluded and should be investigated in further studies.
In summary, TLA is an easy-to-learn, low-risk, low-cost, and effective therapy with little discomfort and no known long-term adverse effects to the patient. Up to 12 therapy sessions may seem like a burden on the patient; however, according to our experience, patients are often willing to tolerate these efforts after experiencing the first pain relieving effects early on in treatment, especially after having suffered from vulvodynia for years.
Analysis of Non-Response. We used a very strict definition of “response”— improvement to NAS ≤4 within a maximum of 12 TLA sessions. It is therefore important to notice that patients allocated to the non-responder group also experienced improvement to their complaints. However, this occurred later than in responders, and to a lesser extent. Nevertheless, the therapeutic effect was also significant in non-responders (P = .03), with a median NAS reduction of 2.25 (range 0–3.5). Possible reasons for non-response are psychological, previous trauma, intoxication, and chronic silent inflammation. We are currently analyzing co-morbidities and covariates for success vs non-success with this therapeutic approach. However, an extensive discussion of risk factors would go beyond the scope of this study.
Functional Rather Than Surgical Approach. This study, comparable to previous findings,19,23,24,30 used therapeutic blockades of the pudendal nerve, revealing sufficient therapeutic effects. Our data support the hypothesis of Labat et al46 that a substantial number of vulvodynia patients suffer from pudendal neuralgia. The success rate of TLA brings into question the theory of a mechanical nerve compression (entrapment) as a main cause of complaints. Surgery to release nerve entrapment had a success rate of only 36% over a long period of time,6,7 suggesting that surgery is not the first choice to treat this neural disease, which has been stated by the ISSVD as well.1 We prefer a functional approach with repeated anesthesia of the nerves involved.
LA address a multitude of receptors far beyond sodium channels alone, such as Gq-proteins,47 N-methyl-D-aspartate receptor,48 transient receptor potential (TRP) channel,49 and other ion channels. Therefore, LA infiltration of nerves seems to be more than just a blockade of the nerve conduction, but rather a “reset” of neural function and pain memory using their anti-inflammatory properties.
Vulvodynia – A Form of Neuralgia?. The ISSVD classification of vulvodynia2 divides it into different subgroups. This nomenclature does not provide an explanation of the causes, but identifies primary (idiopathic), and secondary forms (associated with previous events). We did not see a difference between these two groups. Therefore, we agree with the hypothesis46 that the common denominator of vulvodynia is the neuralgiform reaction of nerves to an unknown stimulus, independent from the cause. This concurs with other researchers identifying vulvodynia as pudendal neuralgia.19,50,51 Pelvic floor dysfunction12 then may be a sequela, not a cause of the neurogenic disorder. We postulate that repeated analgesia of the nerves involved is a major key to reducing this form of neuralgia, independent from its original etiology.
Vulvodynia – A Complex Pain Syndrome?. The long-term effects of TLA may be based on reset mechanisms in the periphery by local anesthesia (extinction of the pain memory?).50, 51, 52 Further studies to elucidate this question will be necessary. This pilot study provides data which suggests TLA could be a viable treatment for vulvodynia. Within this pilot study, we can preliminarily point to TLA which can substantially improve the treatment options of vulvodynia. We can conclude from our data that vulvodynia is associated with a peripheral or central sensitization, associated with neurogenic inflammation of the pudendal nerve's target region.50,51 But vulvodynia seems to be more complex than just pudendal neuralgia. The individual regimen in our study including further neural structures of the genital region from the 4th session on suggests that vulvodynia is a pain syndrome affecting several nerves of the pelvic region24,30, comparable to the chronic regional pain syndrome (CRPS) in the extremities.53 Therefore, injections to a single nerve are sufficient to treat some, but not all patients. Some forms with a more ventral vulvar localization may be due to other forms of neuralgia, for instance of the genitofemoral nerve,36 or the hypogastric plexus.37 In the present study, we used all these techniques, especially if symptoms of the respective nerve region were found. We conclude that repeated infiltrations of different neural structures induce increasingly pain-free intervals better than with pudendal injection only, probably reducing peripheral and/or central sensitization in vulvodynia.
Up to now, the cause of vulvodynia is often considered to be sexual and/or psychological. Our high rate of success, however, may be better explained by the theory of a silent, neurogenic inflammation.54, 55, 56
CONCLUSIONS
In this study a new therapy for vulvodynia was investigated. Therapy was successful in the majority (80%) of patients. The long-term therapeutic effectiveness of TLA suggests that some subgroups of vulvodynia may be based on pudendal neuralgia; others resemble regional pain syndromes affecting several nerves of the pelvic floor. This view may open new perspectives for treating this debilitating disease. We postulate that this individual and symptom-oriented form of TLA, provides a highly effective and easy-to-apply approach to treating vulvodynia, especially if integrating the novel perineal pudendal injection technique. This individual form of TLA can be adopted for all vulvodynia types.
STATEMENT OF AUTHORSHIP
Stefan Weinschenk: Conceptualization, data curation, investigation, project administration, formal analysis, validation, interpretation, writing: original draft, review & editing, final approval; Justus Benrath: Methodology, formal analysis, interpretation, writing: review & editing, final approval; Eugen Kessler: Formal analysis, investigation, data curation, validation, writing: review & editing, final approval; Thomas Strowitzki: Supervision; writing: review & editing, final approval; Manuel Feisst: Methodology, analysis, data validation and interpretation, writing: review & editing, final approval.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: None.
REFERENCES
- 1.Bornstein J, Goldstein AT, Bergeron S, et al. ISSVD ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. J Low Genit Tract Dis. 2015;2016:126–130. doi: 10.1097/LGT.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 2.Feldhaus-Dahir M. The causes and prevalence of vestibulodynia. Urol Nurs. 2011;31:51–54. [PubMed] [Google Scholar]
- 3.Andrews JC. Vulvodynia interventions–systematic review and evidence grading. Obstet Gynecol Surv. 2011;66:299–315. doi: 10.1097/OGX.0b013e3182277fb7. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron S, Brown C, Lord MJ, et al. Physical therapy for vulvar vestibulitis syndrome: a retrospective study. J Sex Marital Ther. 2002;28:183–192. doi: 10.1080/009262302760328226. [DOI] [PubMed] [Google Scholar]
- 5.Powell J, Wojnarowska F. Acupuncture for vulvodynia. J R Soc Med. 1999;92:579–581. doi: 10.1177/014107689909201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haefner HK. Critique of new gynecologic surgical procedures: surgery for vulvar vestibulitis. Clin Obstet Gynecol. 2000;43:689–700. doi: 10.1097/00003081-200009000-00028. [DOI] [PubMed] [Google Scholar]
- 7.David A, Bornstein J. Evaluation of long-term surgical success and satisfaction of patients after vestibulectomy. J Low Genit Tract Dis. 2020;24:399–404. doi: 10.1097/LGT.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann D, Strauhal MJ, Nelson CA. Treatment of women in the United States with localized, provoked vulvodynia: Practice survey of women's health physical therapists. J Reprod Med. 2007;52:48–52. [PubMed] [Google Scholar]
- 9.Morgan TK, Allen-Brady KL, Monson MA, et al. Familiality analysis of provoked vestibulodynia treated by vestibulectomy supports genetic predisposition. Am J Obstet Gynecol. 2016;214:609 e1–7. doi: 10.1016/j.ajog.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Masheb RM, Kerns RD, Lozano C, et al. A randomized clinical trial for women with vulvodynia: cognitive-behavioral therapy vs. supportive psychotherapy. Pain. 2009;141:31–40. doi: 10.1016/j.pain.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventolini G. Measuring treatment outcomes in women with vulvodynia. J Clin Med Res. 2011;3:59–64. doi: 10.4021/jocmr526w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reissing ED, Brown C, Lord MJ, et al. Pelvic floor muscle functioning in women with vulvar vestibulitis syndrome. J Psychosom Obstet Gynaecol. 2005;26:107–113. doi: 10.1080/01443610400023106. [DOI] [PubMed] [Google Scholar]
- 13.Thibault-Gagnon S, McLean L, Goldfinger C, et al. Differences in the biometry of the levator hiatus at rest, during contraction, and during valsalva maneuver between women with and without provoked vestibulodynia assessed by transperineal ultrasound imaging. J Sex Med. 2016;13:243–252. doi: 10.1016/j.jsxm.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Westrom LV, Willen R. Vestibular nerve fiber proliferation in vulvar vestibulitis syndrome. Obstet Gynecol. 1998;91:572–576. [PubMed] [Google Scholar]
- 15.Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148:1021–1027. doi: 10.1046/j.1365-2133.2003.05308.x. [DOI] [PubMed] [Google Scholar]
- 16.Bohm-Starke N, Hilliges M, Falconer C, et al. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1999;48:270–275. doi: 10.1159/000010198. [DOI] [PubMed] [Google Scholar]
- 17.Tympanidis P, Casula MA, Yiangou Y, et al. Increased vanilloid receptor VR1 innervation in vulvodynia. Eur J Pain. 2004;8:129–133. doi: 10.1016/S1090-3801(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 18.Bohm-Starke N, Hilliges M, Brodda-Jansen G, et al. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain. 2001;94:177–183. doi: 10.1016/S0304-3959(01)00352-9. [DOI] [PubMed] [Google Scholar]
- 19.Labat JJ, Robert R, Delavierre D, et al. Symptomatic approach to chronic neuropathic somatic pelvic and perineal pain. Prog Urol. 2010;20:973–981. doi: 10.1016/j.purol.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 20.Zolnoun DA, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol. 2003;102:84–87. doi: 10.1016/s0029-7844(03)00368-5. [DOI] [PubMed] [Google Scholar]
- 21.Prat-Pradal D, Metge L, Gagnard-Landra C, et al. Anatomical basis of transgluteal pudendal nerve block. Surg Radiol Anat. 2009;31:289–293. doi: 10.1007/s00276-008-0445-z. [DOI] [PubMed] [Google Scholar]
- 22.Hough DM, Wittenberg KH, Pawlina W, et al. Chronic perineal pain caused by pudendal nerve entrapment: anatomy and CT-guided perineural injection technique. AJR Am J Roentgenol. 2003;181:561–567. doi: 10.2214/ajr.181.2.1810561. [DOI] [PubMed] [Google Scholar]
- 23.McDonald JS, Rapkin AJ. Multilevel local anesthetic nerve blockade for the treatment of generalized vulvodynia: A pilot study. J Sex Med. 2012;9:2919–2926. doi: 10.1111/j.1743-6109.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 24.Rapkin AJ, McDonald JS, Morgan M. Multilevel local anesthetic nerve blockade for the treatment of vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2008;198:41 e1-5. doi: 10.1016/j.ajog.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Rigaud J, Riant T, Delavierre D, et al. Somatic nerve block in the management of chronic pelvic and perineal pain. Prog Urol. 2010;20:1072–1083. doi: 10.1016/j.purol.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs P, Gruber H, Piegger J, et al. New, simple, ultrasound-guided infiltration of the pudendal nerve: Ultrasonographic technique. Dis Colon Rectum. 2001;44:1381–1385. doi: 10.1007/BF02234802. [DOI] [PubMed] [Google Scholar]
- 27.Weinschenk, S, Handbuch Neuraltherapie - Diagnostik und Therapie mit Lokalanästhetika; Stefan Weinschenk (Hrsg.). 1. edn. ed. 2010, München: Elsevier Urban & Fischer. XIII, 1106 S. Ill.
- 28.Weinschenk, S, Handbuch Neuraltherapie - Diagnostik und Therapie mit Lokalanästhetika [Textbook of Therapy with Local Anesthetics]. 2. edn. ed. 2020, Stuttgart: Thieme Verlag. 1030 S.
- 29.Weinschenk S. Neural therapy - a review of the therapeutic use of local anaesthetics. Acupunct Rel Ther. 2012;4:25–29. [Google Scholar]
- 30.Weinschenk S, Brocker K, Hotz L, et al. Successful therapy of vulvodynia with local anaesthetics. A case report. Forsch Komplementmed. 2013;20:138–143. doi: 10.1159/000350023. [DOI] [PubMed] [Google Scholar]
- 31.Rey Novoa M, Munoz-Sellart M, Catalan Soriano M, et al. Treatment of localized vulvar pain with neural therapy: a case series and literature review. Complement Med Res. 2021:1–7. doi: 10.1159/000514945. [DOI] [PubMed] [Google Scholar]
- 32.Weinschenk S, Hollmann MW, Strowitzki T. New perineal injection technique for pudendal nerve infiltration in diagnostic and therapeutic procedures. Arch Gynecol Obstet. 2016;293:805–813. doi: 10.1007/s00404-015-3812-0. [DOI] [PubMed] [Google Scholar]
- 33.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD initiative. BMJ. 2003;326:41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinschenk S, Mergenthaler C, Armstrong C, et al. Local anesthetics, procaine, lidocaine, and mepivacaine show vasodilatation but no type 1 allergy: A double-blind, placebo-controlled study. Biomed Res Int. 2017;2017 doi: 10.1155/2017/9804693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picardi S, Cartellieri S, Groves D, et al. Local anesthetic-induced inhibition of human neutrophil priming: The influence of structure, lipophilicity, and charge. Reg Anesth Pain Med. 2013;38:9–15. doi: 10.1097/AAP.0b013e31827a3cbe. [DOI] [PubMed] [Google Scholar]
- 36.Weinschenk S. In: Handbook Therapy with Local Anesthetics (Neural Therapy) Weinschenk S., editor. Thieme Publ.; Stuttgart: 2020. Genitofemoral and Ilioinguinal Nerve Injection Technique; pp. 482–483. [German Ed.] [German Ed.]Editor. [Google Scholar]
- 37.Weinschenk, S, Uterovaginal plexus injection technique, in Handbook Therapy with Local Anesthetics (Neural Therapy) [German Ed.], S. Weinschenk, Editor. 2020, Thieme Publ.: Stuttgart. p. 432-435.
- 38.Kupke T. Injection to the vesical trigone, a new injection technique for the treatment of urge incontinence and sexual disorders. Dtsch Zschr Akupunktur. 2006;49:36–39. [Google Scholar]
- 39.Frieden TR. Evidence for health decision making - beyond randomized, controlled trials. N Engl J Med. 2017;377:465–475. doi: 10.1056/NEJMra1614394. [DOI] [PubMed] [Google Scholar]
- 40.Walach H, Jonas WB. Placebo research: The evidence base for harnessing self-healing capacities. J Altern Complement Med. 2004;10:S103–S112. doi: 10.1089/1075553042245773. Suppl 1: p. [DOI] [PubMed] [Google Scholar]
- 41.Biller-Andorno N. The use of the placebo effect in clinical medicine–ethical blunder or ethical imperative? Sci Eng Ethics. 2004;10:43–50. doi: 10.1007/s11948-004-0061-1. [DOI] [PubMed] [Google Scholar]
- 42.Labat JJ, Riant T, Lassaux A, et al. Adding corticosteroids to the pudendal nerve block for pudendal neuralgia: A randomised, double-blind, controlled trial. BJOG. 2017;124:251–260. doi: 10.1111/1471-0528.14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moyal-Barracco M, Lynch PJ. ISSVD terminology and classification of vulvodynia: A historical perspective. J Reprod Med. 2003;49:772–777. 2004. [PubMed] [Google Scholar]
- 44.Nunns D, Mandal D, Byrne M, et al. Guidelines for the management of vulvodynia. Br J Dermatol. 2010;162:1180–1185. doi: 10.1111/j.1365-2133.2010.09684.x. [DOI] [PubMed] [Google Scholar]
- 45.Calvillo O, Skaribas IM, Rockett C. Computed tomography-guided pudendal nerve block. A new diagnostic approach to long-term anoperineal pain: a report of two cases. Reg Anesth Pain Med. 2000;25:420–423. doi: 10.1053/rapm.2000.7620. [DOI] [PubMed] [Google Scholar]
- 46.Robert R, Prat-Pradal D, Labat JJ, et al. Anatomic basis of chronic perineal pain: role of the pudendal nerve. Surg Radiol Anat. 1998;20:93–98. doi: 10.1007/BF01628908. [DOI] [PubMed] [Google Scholar]
- 47.Hollmann MW, McIntire WE, Garrison JC, et al. Inhibition of mammalian Gq protein function by local anesthetics. Anesthesiology. 2002;97:1451–1457. doi: 10.1097/00000542-200212000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Hahnenkamp K, Durieux ME, Hahnenkamp A, et al. Local anaesthetics inhibit signalling of human NMDA receptors recombinantly expressed in Xenopus laevis oocytes: role of protein kinase C. Br J Anaesth. 2006;96:77–87. doi: 10.1093/bja/aei271. [DOI] [PubMed] [Google Scholar]
- 49.Leffler A, Lattrell A, Kronewald S, et al. Activation of TRPA1 by membrane permeable local anesthetics. Mol Pain. 2011;7:62. doi: 10.1186/1744-8069-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDonald DM, Bowden JJ, Baluk P, et al. Neurogenic inflammation. A model for studying efferent actions of sensory nerves. Adv Exp Med Biol. 1996;410:453–462. [PubMed] [Google Scholar]
- 51.Wesselmann U. Neurogenic inflammation and chronic pelvic pain. World J Urol. 2001;19:180–185. doi: 10.1007/s003450100201. [DOI] [PubMed] [Google Scholar]
- 52.Zieglgänsberger W, Plastizität Neuronale. In: Handbuch Neuraltherapie - Therapie mit Lokalanästhetika. Weinschenk S., editor. Thieme Verlag: Stuttgart; 2020. Schmerzgedächtnis und chronischer Schmerz; pp. 68–70. Editor. [Google Scholar]
- 53.Pfister M, Fischer L. [The treatment of the complex regional pain syndrome (CRPS 1 and CRPS 2) of the upper limb with repeated local anaesthesia to the stellate ganglion.] Praxis Bern. 2009;98:247–257. doi: 10.1024/1661-8157.98.5.247. (1994) [DOI] [PubMed] [Google Scholar]
- 54.Kronenberg RM, Ludin SM, Fischer L. Severe Case of chronic pelvic pain syndrome: Recovery after injection of procaine into the vesicoprostatic plexus-case report and discussion of pathophysiology and mechanisms of action. Case Rep Urol. 2018;2018 doi: 10.1155/2018/9137215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Littlejohn G. Neurogenic neuroinflammation in fibromyalgia and complex regional pain syndrome. Nat Rev Rheumatol. 2015;11:639–648. doi: 10.1038/nrrheum.2015.100. [DOI] [PubMed] [Google Scholar]
- 56.Hornick L, Slocumb JC. Treating chronic pelvic pain. Focus on pain triggers and neurogenic inflammation. Adv Nurse Pract. 2008;16:44–53. quiz 54. [PubMed] [Google Scholar]