Abstract
Metazoan organisms have many tRNA genes responsible for decoding amino acids. The set of all tRNA genes can be grouped in sets of common amino acids and isoacceptor tRNAs that are aminoacylated by corresponding aminoacyl-tRNA synthetases. Analysis of tRNA alignments shows that, despite the high number of tRNA genes, specific tRNA sequence motifs are highly conserved across multicellular eukaryotes. The conservation often extends throughout the isoacceptors and isodecoders with, in some cases, two sets of conserved isodecoders. This study is focused on non-Watson–Crick base pairs in the helical stems, especially GoU pairs. Each of the four helical stems may contain one or more conserved GoU pairs. Some are amino acid specific and could represent identity elements for the cognate aminoacyl tRNA synthetases. Other GoU pairs are found in more than a single amino acid and could be critical for native folding of the tRNAs. Interestingly, some GoU pairs are anticodon-specific, and others are found in phylogenetically-specific clades. Although the distribution of conservation likely reflects a balance between accommodating isotype-specific functions as well as those shared by all tRNAs essential for ribosomal translation, such conservations may indicate the existence of specialized tRNAs for specific translation targets, cellular conditions, or alternative functions.
INTRODUCTION
The set of all tRNA genes can be grouped into sets of isoacceptor tRNAs that are aminoacylated by the corresponding aminoacyl-tRNA synthetase, one per amino acid. The number of tRNA genes for the sets of tRNA isoacceptors can vary widely (1). Within each set of tRNA isoacceptors, the number of tRNA genes with the same anticodon triplet, the isodecoders (2,3), also varies (2,3). The central role of tRNAs in protein translation necessitates interactions with several other entities within the cell (4). tRNA transcription requires sequence-specific binding of transcription factors to their A- and B-box regions (5–10), and tRNA maturation requires interactions with RNases P and Z (11–13) plus a host of RNA modification enzymes (14). Fundamentally, native and mature tRNAs interact with the ribosome, mRNA codon, and corresponding aminoacyl tRNA synthetase during translation (15,16). Overall a multitude of factors act to shape or restrict tRNA sequences: the folding process, the 3D architecture (17–20), the interactions with enzymes involved in tRNA maturation (21–25), modification (26–29) and degradation (30–33), aminoacyl tRNA synthetases (34,35); initiation (36) and elongation factors (37), and ribosomal translation recognition sites (38–40), besides the non-canonical functions of tRNAs (41). To ensure that these interactions are not disrupted, tRNA gene sequences and structures are exceptionally well-conserved, even in the face of elevated mutation rates (42). However, in addition to pan-tRNA conservation, we observe isotype- and clade-specific motifs that are also strongly conserved. While these motifs likely play important structural or regulatory roles, the reasons for their isotype-specificity and conservation are unknown and are ripe for exploration.
Non-Watson–Crick base pairs frequently occur in RNA helical stems, especially GoU pairs (guanine paired with uridine). GoU pairs are structurally and functionally of singular interest (43). They display distinguishable molecular recognition features, especially the movement of the U in the major or minor groove (44). This movement leads to a change in helical twist between the framing base pairs from the normal helical angles (45). That helical twist variation can propagate away to the least constrained end of the helical region that contains the GoU pair (for overviews, see (45,46)). Thus, a GoU pair does not need to directly contact the protein or RNA ligand to exert an action on binding efficiency. In tRNAs, GoU pairs are important for well-established tertiary contacts that maintain tRNA fold and function, throughout their interactions with the aminoacyl-tRNA synthetases and the ribosomal machinery. We therefore seek to extend the analysis of tRNA sequences beyond the recently published study on tRNA-Ala and tRNA-Gly in eukaryotes (47).
Here, we attempt to identify base pairing signatures specific to each tRNA isotype that are conserved across several major clades of multicellular eukaryotes, and to relate these observations to known tRNA structures and interactions. To identify specific targets for experimental study in genetically pliable model metazoans, we leverage the broader distribution of tRNA genes currently known across hundreds of related species to ask: (i) are GoU pairs biased for specific stems and positions, and if so, for which amino acids or isodecoders? (ii) when a GoU pair is present, is the orientation, GoU versus UoG, also conserved? We have extracted and structurally aligned Homo sapiens, Mus musculus and Bombyx mori tRNA genes from the Genomic tRNA Database (48). We chose these three genomes as they represent well-studied model organisms from three distinct eukaryotic clades, namely primates, rodents, and insects, and therefore enable us to explore tRNA genomics across these clades in a simple and efficient manner. We generalized to other genomes within Mammalia and Insecta (especially Drosophila species) by tRNAviz (49). It is known that tRNA modifications are central to tRNA functions and that many uridines are replaced by pseudouridines (14,50,51). However, such a modification does not prevent the formation of a wobble pair (40–41) and, since these potential modifications are unknown in a large number of instances, they will not be discussed here.
MATERIALS AND METHODS
The analyses presented here are based on the genomic database of transfer RNA genes, GtRNAdb 2.0 (48). The database contains alignments of tRNA genes based on the tRNAscan-SE prediction algorithm (52). This is the most used tRNA gene identifier, using covariance models to classify potential tRNA genes, assigning a bit score to each. The bit score can be understood as a measure of how much each tRNA resembles a prototypical tRNA, with higher scoring tRNAs more likely to be transcribed and functional in translation, and lower scoring tRNAs more likely to be non-functional or pseudogenes. The covariance model score can be broken down into components representing the primary sequence conservation and secondary structure conservation (52). Overall scores below 55.0 bits may indicate the presence of a pseudogene, increasing in likelihood of a non-functional gene as the score decreases. Indeed, low-scoring tRNA genes often display non-complementary Watson–Crick pairs in the stem regions, or lack highly conserved residues involved in the architectural fold of the tertiary structure. The sequences are organized according to this overall bit score. For our analyses in this study, we focus on tRNA genes with bit scores of at least 55.
There are generally several isodecoders for each isoacceptor tRNA, but the number varies among species and isotypes (2). For most genomes, a fraction of the predicted isodecoder tRNA gene transcripts have been experimentally observed, and the tRNA modifications are known for still a smaller fraction of those based on the MODOMICS database (8). We extracted the tRNA alignments from the GtRNAdb 2.0 and ensured known tertiary structures were aligned for three species: Homo sapiens, Mus musculus, and Bombyx mori. The tRNA structural alignments for H. sapiens, M. musculus and B. mori are given in Supplementary Supplementary Figure S2 together with the consensus cloverleaf structures of tRNAs of the Mammalia and Insecta (Supplementary_Data_1 and _2). These observations were supported by analyses of additional genomes of Insecta and Mammalia using tRNAviz (49). The observations using tRNAviz are provided in the supplementary material, organized and annotated by the types of residues, amino acids and anticodon triplets derived from tRNAs in Insecta, Mammalia, or both (Supplementary_Data_3). Here, the pairing positions are indicated by ‘:’ (e.g. 1:72), Watson–Crick pairs by ‘ = ’ for G = C and ‘-’ for A-U, and non-Watson–Crick pairs by ‘o’ (e.g. GoU or AoG).
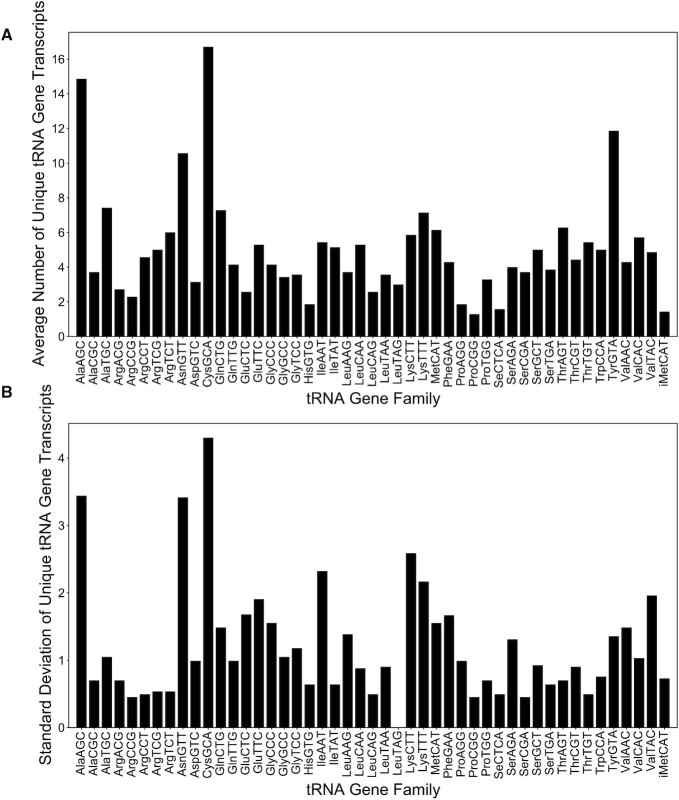
For all analyses regarding gene counts in primate species, we used a whole-genome alignment containing 7 primate species (human, chimpanzee, gorilla, orangutan, rhesus macaque, grey mouse lemur, Nancy Ma's night monkey), among other species, from our previous work (53). We used tRNAscan-SE 2.0 on these seven genomes to count the number of high-confidence tRNA genes with each anticodon in each species, excluding those in segmental duplications. We then counted the number of unique sequences across these gene sets, and calculated the mean and standard deviations across these genomes for depiction in Figure 2.
Figure 2.
Averages and standard deviations of the number of unique tRNA gene transcripts in primates as deduced from tRNAscan-SE (52). The mean (A) and standard deviation (B) of the unique high-confidence tRNA gene transcript counts across the reference genomes of seven primate species: Homo sapiens (human), Pan troglodytes (chimpanzee), Gorilla gorilla (gorilla), Pongo abelii (orangutan), Macaca mulatta (rhesus macaque), Microcebus murinus (gray mouse lemur), and Aotus nancymaae (Nancy Ma's night monkey). On these plots, only the 47 anticodons expected to be functional in primates are shown, including tRNASeC.
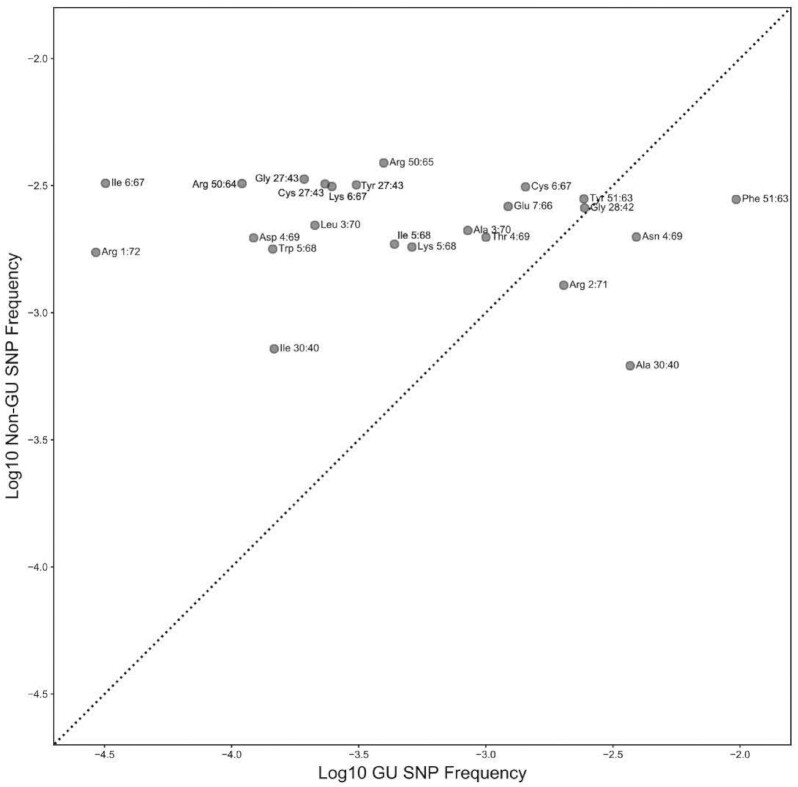
For the analyses in Figure 4, we first aligned all high-confidence tRNA genes from the hg19 human reference genome and generated an alignment using cmalign. We then assigned each nucleotide a Sprinzl position based on these alignments (54). We then downloaded data from dbSNP release 153 (55–57) using the UCSC Table Browser (58). For each position in the genome corresponding to a GoU or UoG base pair in a tRNA in the reference genome, we compared the allele frequency of the most common SNP disrupting this base pair, to the allele frequency of the most common SNP disrupting a non-GoU or UoG base pair at an equivalent position in a different tRNA. We found for 20 of 24 comparisons that the minor allele frequency for the allele disrupting the GoU or UoG base pair was lower, and used a sign test to find P < 6.3 × 10–4. Similarly, we also collected phyloP data (59) for all positions within tRNAs across seven primate species using a Cactus graph from a previous study (53,60), and compared the minimum phyloP score across the positions contributing to a GoU or UoG base pair in a tRNA to the minimum phyloP score across the equivalent positions in tRNAs without GoU or UoG base pairs. We found that for 14 of 23 comparisons, the GoU base pairs had higher phyloP scores than equivalent positions in other tRNA genes, but this was not statistically significant based on the sign test (P = 0.202).
Figure 4.
Polymorphisms disrupting GoU base pairs reach lower frequencies than other SNPs at identical positions. At each GoU position listed in Figure 3, we compare the average allele frequency for SNPs disrupting GoU base pairs to the average allele frequency of SNPs at the same Sprinzl positions across all other tRNAs. We find that for 20 of 24 comparisons, SNPs disrupting GoU base pairs reach a lower frequency (sign test, P < 6.3 × 10–4) and are above the diagonal line shown here. We used data from dbSNP for these comparisons (see Methods) (55).
RESULTS
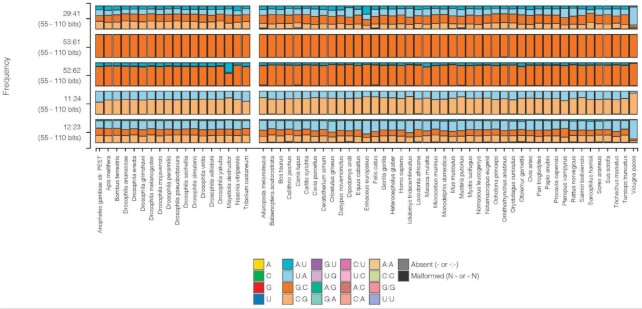
The long-established nucleotide conservations, or semi-conservations, imposed on tRNA sequences appear primarily in the loops and the portions of the A- and B-boxes in the D- and T-stems (5–8). Much of the variation in tRNAs occurs in the helical stems, but maintains the secondary structure (see Supplementary Figure S1 for some description of the code wheel with some general conservations in tRNA secondary structures). At each base pair position in the stems, four pairs (or six pairs considering GoU pairs) are possible. Thus, for the seven base pair AA-stem, there are close to 16 384 possible combinations (or 280 000 with GoU pairs) and, for the AC-stem with only five base pairs, the possibilities number 1024 (or 7776). Examples where the four types of base pairs occur can be seen on Figure 1 (29:41 or 12:23). The conservation constraints imposed by the B-box (5,6) and the tRNA fold are apparent in base pairs 52:62 and 53:61 (Figure 1).
Figure 1.
Examples of different levels of Watson–Crick base pair diversity in Insecta and Mammalia. Each column represents the distribution of nucleotides at the indicated stem position across all tRNAs for the species listed at the bottom (insects on left, mammals on right). This figure was generated using the Compare By Species tool in tRNAviz (49). Only tRNA genes with scores higher than 55 are considered. At the top, the 29:41 base pair in the AC-stem displays all four standard Watson–Crick pairs. Below, the last base pair of the T-stem is invariant and always G53 = C61. The 52:62 T-stem base pair prefers a purine:pyrimidine pair RoY. The pair 11:24 in the D-stem is either U-A or C = G. Finally, the base pair 12:23 in the D-stem is distributed among the four basic Watson–Crick pairs. Notably, these are the only four tRNA base pairs that do not exhibit GoU or UoG pairs in these species.
An important characteristic of tRNA gene families is their diversity in number of loci, even across closely related species and across isoacceptors for the same amino acid (61). Those tRNA genes that share the same anticodon triplet can vary in complexity between species and for different amino acids – some may contain a unique sequence with many multiple exact copies throughout the genome, and others may have many genes with variable sequence differences, each of which may occur at a single or multiple copies. Because all of these share the same anticodon, it is unclear if these variations offer biologically advantageous traits, or are just benign evolutionary noise. We will try to discuss these variations according to their locations, since changes in single-stranded or double-stranded regions, in tertiary pairs or in conserved positions are not expected to have the same impact.
Number and variations of tRNA genes
Before focusing on individual tRNA nucleotide features, we first performed a top-level statistical analysis of gene variation among multiple clades to gain context on overall variation among the different isotypes. Within primates, the number of unique tRNA gene transcripts varies significantly with the amino acid type and anticodon, as illustrated in Figure 2A (average counts across species) and 2B (standard deviation of counts across species). Four tRNA isodecoders stand out for the large number of unique genes: tRNA-Cys-GCA (16.7), tRNA-Ala-AGC (14.9), tRNA-Tyr-GUA (11.9) and tRNA-Asn-GUU (10.5). In terms of standard deviation of unique tRNA genes, tRNA-Cys-GCA (4.6) is the highest and tRNA-Tyr-GUA (1.5) the lowest. This analysis shows that even for a fairly closely related group of metazoans such as primates, there is an ever constant, but variable amount of mutational and selective pressure at work.
Alternatively, one may examine total gene copy number, irrespective of the uniqueness of the transcripts they produce. This can capture cases where high gene dosage effect is needed for certain tRNAs to amplify the protein production capacity of the cell. For example, in the silkworm B. mori, the numbers of tRNA genes for the tRNAs Ala-AGC, Gly-GCC, Gly-UCC, Asp-GUC are conspicuously high compared to other insects like Drosophila species (see Supplementary Table S1). These amino acids are among the main components of silk (62).
Sequence conservation in the helical stems
Two tRNA families have a conspicuously high number of tRNA genes with the same anticodon triplet in the analyzed phylogeny: tRNA-Ala-AGC and tRNA-Cys-GCA (see Supplementary Table S1). A molecular explanation for such redundancy is as yet unclear. For both tRNA gene families, the 5′ end of the amino acid acceptor (AA) stem is 5′-GGGGR, which is unique among metazoan tRNAs (Table 1). It has been shown that such G-rich sequences promote the formation of intermolecular G quadruplexes at high concentrations for stable small RNAs derived from tRNA-Ala and tRNA-Cys (63). tRNA-Gly-GCC also forms homodimers (64). Additional unknown clade-specific factors should be investigated given that the number of tRNA genes for Cys in Mammalia is twice as high as in Insecta (see Supplementary Table S1).
Table 1.
A map of conserved motifs for each stem in each tRNA isotype. The summary is based on the sequence alignments of tRNAs of H. sapiens, M. musculus, and B. mori shown in Supplementary Figure S1. The four main stems of the tRNA secondary structure are abbreviated as follows: the amino acid (AA), the dihydrouridine (D), the anticodon (AC), and the thymine (T) stems. Motifs are shown in the 5′ to 3′ direction for the first strand encountered in the secondary structure, in monospaced font for ease of alignment across rows. All bases follow standard IUPAC conventions, but K is intended to signify ‘G or U’, rather than the standard ‘G or T’. The GGGG(R) motif, specific to only Ala- and Cys-tRNAs, is highlighted in bold. (M) denotes that the motifs are relatively constant across Mammalia. Only five tRNAs have four nucleotides in the variable loop: Gly, Glu, Gln, Asp, and His. Leu (with a preference for 5′YYY…GGG3′) and Ser (with a preference for 5′GGG…CCC3′) have a long variable loop with an additional helix (long-arm tRNAs) (see Supplementary Figure S2 where tRNAs with introns are also indicated, the insertion site is always between nucleotides 37 and 38 (1). The amino acids are organized by decreasing number of isoacceptor families with the color code indicating the strength of the codon-anticodon triplet (blue, high GC-content, red, high AU-content, and black in-between). Note that in the 2-codon boxes NNY only one tRNA is used for decoding (with G34 in the anticodon triplet) and in the 2-codon boxes NNR two tRNAs are used (with both C34 and U34 in the anticodon triplet)
| Amino acid with anticodon triplet | Isoacceptor families | AA motif | D motif | AC motif | T motif |
|---|---|---|---|---|---|
| Ala | 3 | GGGGUUG | GCUC | C..GC | CCGGG |
| Ala AGC (2 states) | GGGGRAU | GYGGG | |||
| Gly | 3 | GCRYUGG | GU.C | …GC | CCGGG |
| Pro | 3 | GGCKCGU | GUCU | CUCGC | CCGGG |
| Val | 3 | GUUUCCG | GUGU | UYYGC | CCCGG |
| CUGGG | |||||
| Thr | 3 | GGCGCCG | GCY. | YYKGU | CUGGG |
| RCGGG | |||||
| Arg | 3 + 2 | G.CC..G | GC.Y | YYKGM | ..GGG |
| Arg ACG | G.GC..G | CYAGG | |||
| Leu UAA (M) | 3 + 2 | ACC.G.A | GCCG | UUGGA | GUGGG |
| Leu (all others) | G..AG.R | GCCG | YYR.. | GUGGG | |
| Ser | 3 + 1 | G..G..R | GCCG | WUGGA | GYRGG |
| Glu | 2 | UCCCW.R | GUCU | CCUGG | CGGCG |
| Gln | 2 | GGYYCCA | GUGU | CUGGA | CCGAG |
| Ile AAU | 2 | GGGCC.R | GCUC | UGGUG | GCGGG |
| Ile UAU | GCUCCAG | GCGC | CGGUA | GUGAG | |
| Lys CUU | 2 | GCCCGGC | GCUC | UGAGA | GUGGG |
| Lys UUU | GCCCGGA | GCUC | UYRGA | CAGGG | |
| Asp GUC | 1 | UCYUCGU | GUAU | CCCGC | CGGGG |
| His GUG | 1 | GCCGUGA | GUMU | CURCG | CYMGG |
| Cys GCA | 1 | GGGGRUA | GCUC | WUYGA | CCCGG |
| Trp CCA | 1 | GACYYCG | GCGC | UCUGA | GCGUG |
| Asn GUU | 1 | GYCUCYG | GCGC | UUCGG | GGUGG |
| Met CAU | 1 | GCCYYSK | GCGC | UMAGU | SUGAG |
| Meti CAU | 1 | AGCAGAG | GCGC | CUGGG | GRUGG |
| Phe GAA | 1 | GCCGAAA | GCUC | UUAGA | CCYGG |
| Tyr GUA | 1 | CCUUCGA | GCUC | GWGGA | GCUGG |
The first base pair of the amino acid stem is often a recognition element of tRNA aminoacyl synthetases (34,35) and participates in the anchoring of the pre-tRNA to the RNase P complex (65). As expected, only four specific tRNAs lack G1: Asp and Glu have U1-A72, Tyr has C1 = G72 (66), and Met has A1-U72 (67) (Supplementary Figure S3). However, tRNA-Leu-UAA has A1-U72 in Mammalia but G1 = C72 for the other four Leu isodecoders, excluding this as a possible aminoacyl transferase recognition element; conversely, Insecta uniformly has G1-C72 for all Leu tRNAs (Supplementary Figure S4). In Bacteria, Asn and Gln frequently have U1-A72 and Trp has A1-U72 (not shown). Interestingly, the 1:72 base pair is recognized by a direct contact with the RNase P RNA in Bacteria (64) and via the N-terminal segment of the POP1 protein (a protein subunit of RNase P) in Eukarya (65). It is possible that the protein-rich eukaryotic RNase P has a greater latitude in recognition of the 1:72 base pair thus allowing greater sequence drift (66). Because tRNA-Leu-UAA decodes one of the least-used Leu codons (TTA), this may hint at U1-A72 as a distinguishing regulatory feature for this isodecoder. Regardless, the biological basis for the transition to U1-A72 for Leu-UAA in mammals and other vertebrates (data not shown) is an intriguing question.
While the above sequence motifs are unique to specific isotypes or clades, other tRNA families have highly conserved stem motifs (Table 1). These are found in isoacceptor families with variations within isoacceptor families (e.g. Ala, Arg, Leu, Ile) or between isodecoders (as marked in Table 1 by Y, R, M, K, W, S). However, the conservation between human and silkworm is striking: 5′-GUUUCCG for the AA-stem in all isoacceptors of tRNA-Val and 5′-GGYYCCA for all isoacceptors of tRNA-Gln. The restricted variations in the dihydrouridine (D) stem can be in part explained by the A-box internal promoter for RNA polymerase III (Pol III) (5,6) and the tertiary pairs (see below). Even in the AC-stem, there are several conserved motifs associated with specific isodecoder families. For example, 5′-CCCGC is specific to tRNA-Asp and 5′-CCUGG is specific to tRNA-Glu. The last pair of the T-stem (always G53 = C61) is constrained by the B-box internal promoter and the three-dimensional fold of the T-loop (17), but the first four nucleotides of the 5′ strand of the T-stem are not constrained and again they are typical of a given amino acid and highly conserved. For example, in tRNA-Asn, the T-stem motif is 5′-GGUGG and, in tRNA-Tyr, 5′-GCUGG (Table 1). Also, the last three residues of the 5′-strand of the T-stem are often a series of three Gs in the strong anticodon-codon pairs (the Northern side of the genetic code wheel) and more often a series of two Gs in the Southern part. Even the additional helix in long-arm tRNAs (YYY…GGG in Leu and GGG…CCC in Ser) present unusually strong conservation throughout the genomes and clades of the three species analyzed in depth (Table 1, Supplementary Figure S2), relative to analogous observations in bacteria and yeast.
Non-Watson–Crick pairs in helical stems
The most frequently observed non-Watson–Crick pair in helical stems of structured RNAs is the wobble GoU. Each of the four helical stems may contain a GoU pair. Of the total 21 base pairing positions found in helical stems (7 + 4 + 5 + 5, for the amino acid (AA), the dihydrouridine (D), the anticodon (AC), the thymine (T) stems, respectively), 15 base pairing positions present GoU pairs (Figure 3A, B). Thus, out of 30 possibilities for a GoU or UoG pair, we found 10 positions with GoU in one or more tRNAs and 11 positions with at least one UoG; and of these, six positions had both GoU or UoG pairs (Figure 3). Some base pair positions do not show any GoU pairs: 11:24 and 12:23 in the D-stem, 29:41 in the AC-stem, and 53:61 in the T-stem (Figure 1). Notably, position 29:41 interacts with the rRNA in the P-state, and a Watson–Crick base pair is highly favored (68–70). Note that uridines in the AC-stem are generally modified into pseudouridines and this modification does not prevent formation of the wobble pair (37,71). The pair 53:61 in the T-stem does not form a GoU pair, presumably due to structural (17) and B-box constraints (5,6). Two other positions present a GoU pair rarely and, then, not specifically attached to a tRNA type: 52:62 and importantly the last base pair of the AC-stem, 31:39.
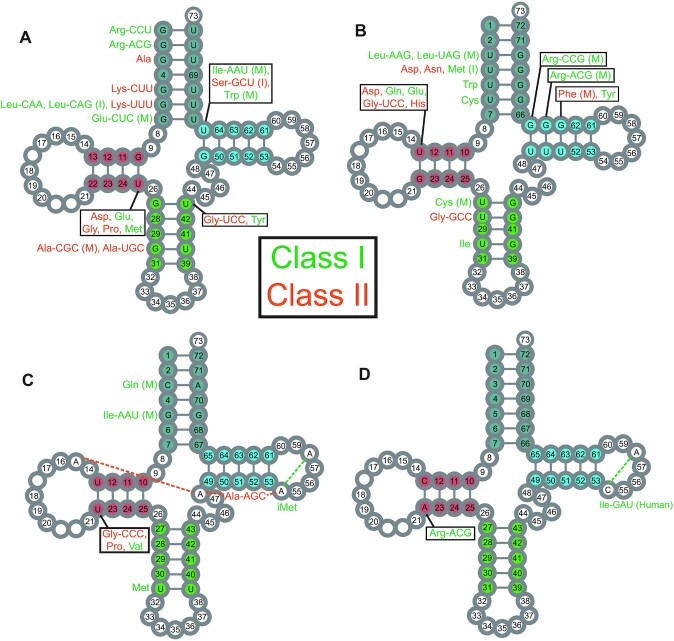
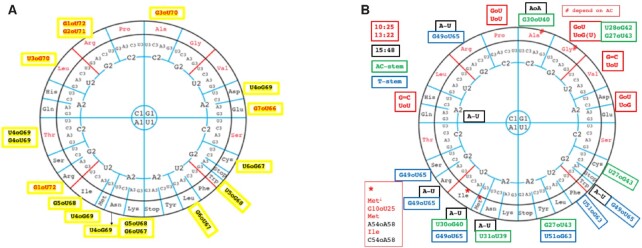
Figure 3.
Summary of GoU base pairs and other non-Watson–Crick base pairs in helical stems of tRNAs. Isotype- and position-specific conserved GoU (A) and UoG (B) base pairs in tRNA stems, as well as other non-Watson–Crick pairs (C, D) are shown by Sprinzl position. The long-arm tRNAs are characterized by a G13oA22. (C) In the AA-stem, Gln-UUG tRNAs present often in Mammalia a CoA opposition at positions 3:70 and Ile-AAU tRNAs have GoG at positions 5:68. Gly-CCC, Pro, and Val tRNAs have a UoU at positions 13:22 in the D-stem. In addition, Ala-AGC tRNAs have an RoA tertiary interaction at positions 15:48 together with A54oA58 (47), and Met tRNAs have AoA at positions 54:58. (D) Arg-ACG tRNAs have a CoA at positions 13:22 in the D-stem, and H. sapiens Ile-GAU tRNAs have the unusual CoA tertiary interaction at positions 54:58 in the T-loop. Numbers in stem positions indicate that no non-Watson–Crick interactions are observed at that position in insect or mammalian tRNAs (except for tRNA-Thr at positions 4:69 where both GoU and UoG are observed). Amino acids are colored based on aminoacyl tRNA synthetase class (Class I in green and Class II in orange). (M) indicates that the base pair is observed in Mammalia and (I) indicates that the base pair is observed in Insecta (all valid for Drosophila species). Isodecoders without either letter are conserved in both insects and mammals.
Structurally, a GoU pair is not equal to a UoG pair (45) and, depending on the amino acid, the occurrence and orientation of a GoU pair may be conserved throughout an isoacceptor family. Importantly, a GoU pair may be conserved in position and orientation in only a subset of the isodecoders of an isoacceptor family. Nine base-pairing positions occur in a single orientation (Figure 3A, B): these comprise four in the D-stem (G10oU25, U13oG22) and T-stem (U50oG64, U51oG63) with one in the AC-stem (U28:G42) and four in the AA-stem (G1oU72, G2oU71, U4oG69, G7oU66). Six pairs show both orientations of GoU pairs: three in the AA-stem (3:70, 5:68, 6:67), one in the T-stem (49:65), and two in the AC-stem (27:43, 30:40).
For the D-stem, the internal A-box promoter for Pol III transcription may restrict the alternate GoU pairs (5,6). In the T-stem, interactions with elongation factor Tu may also restrict alternate GoU pairs (72). The GoU pairs in the D- and T-stems are shared among several amino acids. Interestingly, U51oG63 is found in mammalian tRNA-Phe and tRNA-Tyr, two close amino acids not easily distinguished (73). Conversely, in the AA- and AC-stems, a given GoU orientation is attached to a specific amino acid. For example, in the AA-stem, there is the well-known identity element G3oU70 for Ala (74,75), as well as U3oG70 for Leu-NAG and G6oU67 for Leu-YAA, U5oG68 for Trp, and U6oG67 for Cys. In the AC-stem, we observe G27oU43 for Tyr and tRNA-Gly-UCC, U27oG43 for Cys, G30oU40 for Ala and tRNA-Arg-UCU(with intron), and U30oG40 for Ie (Figure 3A). In bacteria, the nature of the 27:43 pair has been correlated with the accommodation of a non-Watson–Crick base pair at the first codon:anticodon triplet position (76). Finally, in the AC-stem, two pairs, 29:41 and 30:40, are recognized by the ribosome during translocation at the P-state (68–70). As shown in Figure 3A and B, 29:41 is overwhelmingly a complementary Watson–Crick, while 30:40 does occur as either a GoU or UoG pair in tRNA-Ala-YGC and tRNA-Ile. Also, Arg-UCU tRNAs present a G30oU40 pair when the transcript contains an intron (in absence of intron, like in B. mori, it is a G30 = C40 like in all Drosophila). Because the pairs 29:41 and 30:40 interact with ribosomal elements in the P-state (68), a GoU pair at 30:40 may play a role during translocation (see discussion in (47)). However, a comparison between yeast tRNA-Asp free and complexed with its cognate aminoacyl synthetase show that deviations between the tRNAs occur at a hinge point formed by the yeast-specific G30oU40 pair in the AC-stem (77,78). Interestingly, the suppression efficiency of the yeast amber tRNA-Ile in E. coli is modulated by the presence of U30oG40 (79). Further, the yeast amber tRNA-Ile is charged by bacterial glutaminyl and lysyl tRNA synthetases and the G30oU40 mutant only by LysRS (79).
Other non-Watson–Crick pairs are conserved in the tRNA stems (Figure 3C, D), with none in the T-stem, one at one end of the D- and AC-stems, and two in the AA-stem. Except for U13oU22 (which occurs in Gly-CCC, Pro, Val), these non-Watson–Crick pairs are attached to a family or sub-family of isodecoders. Homo sapiens tRNA-Ile-GAU displays the very unusual C54oA58 opposition in the T-loop (other cases include D. willistoni and five among eight genomes in Primates). tRNA-Ile-AAU also presents a G5oG68 pair in Mammalia. Met-tRNAs present a U31oU39 pair while Meti-tRNAs have a A54oA58 pair with a C33 residue instead of the highly conserved U33 (80–82).
GoU base pairs in tRNAs are unlikely to be evolutionary intermediates
The occurrence of non-Watson–Crick base pairs in helical stems of functional RNAs is not surprising in itself, and such pairs are regularly observed in sequence alignments of many RNAs, as in ribosomal RNAs (see for analysis (83,84)). In the evolution of RNA molecules, GoU pairs are often considered as intermediates between G = C and A–U pairs (or vice versa). However, this is not likely to be the case for the GoU pairs described here in tRNAs. When conserved throughout a large phylogeny, a given non-Watson–Crick pair most likely harbors a folding constraint or a key point of contact with an interacting partner molecule. Consistent with this idea, we find that single nucleotide polymorphisms (SNPs) in human tRNA genes that disrupt GoU base pairs for each isotype and position shown in Figure 3A and B reach lower frequencies on average than SNPs disrupting non-GoU base pairs at the same positions in other isotypes (sign test, P < 6.3 × 10–4, Figure 4), based on human population data from dbSNP (55) (see Materials and Methods). Similarly, isotype-specific GoU base pairs in tRNA stems have higher phyloP scores across seven primate genomes than non-GoU base pairs at the same positions (sign test, P = 0.202) (53,59,85). Although this test is not statistically significant, the observation that GoU base pairs are more conserved than non-GoU pairs at the same positions indicates that these GoU base pairs are unlikely to be transient.
Are the GoU pairs correlated with other tertiary or critical pairs?
To analyze structural consequences of the molecular signatures associated with each isotype, we suggested an organization of the genetic code according to the strength (or free energy of the triplet minihelix) of the codon/anticodon triplet that must form in the ribosomal decoding site translation (86). The code is represented as a wheel with the strong triplets in the North and the weak triplets in the South regions. Such a representation displays the ‘oldest’ amino acids in the North and the more highly modified tRNA anticodons in the South region. This representation stresses the point that the free energy of triplet formation encompasses several complex interactions and contributes to our understanding of decoding in translation.
Figure 5A shows the distribution of the GoU pairs, for the AA-stem and the other three stems respectively, around the code wheel (Figure 5B). For the AA-stem, there are many more variations in the South part than in the North. Also, GoU pairs specifically attached to an amino acid are all in the North region (Arg is a six-codon box with a single aminoacyl tRNA synthetase (aaRS)). The GoU pairs in the D-stem occur only in the North or GC-rich region. The variations in the other stems are more frequent in the South part, with more diversity for Ala and Gly in the North. Interestingly, the tertiary A15oU48 pair occurs frequently with G49oU65, with both being very close to each other in the folded tRNA (Figure 5B).
Figure 5.
Distribution along the code wheel of GoU pairs. The triplets are organized from the center to the periphery so that the GC-rich codon:anticodon triplets are at the top (North) and the AU-rich codon:anticodon triplets at the bottom (South) of the wheel. Along the (red-line) diagonals are those triplets with intermediate energies (86). (A) The GoU pairs occurring in the AA-stem are shown next to the amino acid and those in red occur only in tRNAs specific for that amino acid. (B) The GoU pairs occurring in the D- (red), AC- (green) and T-stems (blue). Positions 15:48 (black) are G = C, except where indicated. When the specificity is attached to an isodecoder subfamily it is indicated in red.
Several positions in the tRNA architecture are key for tRNA folding, recognition of protein cofactors, or stability of the codon-anticodon triplet in the decoding site. Exceptions to these conservations can be observed in annotated tRNA genes, but they generally occur together with other point mutations, have low tRNAscan-SE bit scores, and most likely correspond to pseudogenes. We note some exceptions, such as C33 instead of U33 in tRNA-Meti. Residue 9 is always a purine (R9), except in tRNA-His where it is a C9 (residue 9 precedes the invariant G10 that starts the D-stem and forms a triple with 12:23 of the D-stem).
Several non-Watson–Crick pairs are key to the maintenance of the function or folding of the tRNA (17) and conservations are expectedly observed. In the D-loop, A14 interacts with U8 and A21. The loop-loop interactions between the D- and T-hairpins are maintained through the conserved G18G19 in the D-loop and U54, Ψ55, C56 in the T-loop. All these nucleotides and contacts are conserved in all analyzed tRNAs. Interestingly, other tertiary contacts where variations may occur stay identical for each tRNA isotype, as observed above for the secondary structure pairs (see Sup_Data_1_Align.docs and Sup_data_2_2D_Struct). Some exceptions are: (i) the pair 13:22 varies between the isoacceptor families of tRNA-Arg, tRNA-Gly, and tRNA-Thr; (ii) the long-range pair between residues 15 and 48 is always either G15oC48 or A15oU48 in all tRNAs of an isoacceptor family, except in the subclass of tRNA-Ala-AGC containing a A54oA58 where A15oA48 is also present (Figure 3C, D), and in tRNA-Ile-RAU (G15oC48) and tRNA-Ile-UAU (A15oU48). Also, there are three exceptions to the internal trans Watson–Crick/Hoogsteen pair U(T)54oA58: there is an A54oA58 in a class of tRNA-Ala-AGC and in all tRNA-Meti sequences, and a C54oA58 pair in Ile-GAU for several primates.
DISCUSSION
Here, starting from a systematic alignments of tRNA genes in H. sapiens, M. musculus, and B. mori, we extended the analysis to Mammalia and Insecta, although the great majority of what is known biochemically and molecularly is based on studies on Bacteria and Archaea (e.g. (82)). We have also excluded eukaryotic microbial species. There is a great diversity in tRNA and code variations in microorganisms (87–89), in contrast to the conservation among mammals and insects described here. We have observed several conserved isotype-specific motifs in these genomes that were extended and often generalized to other genomes within Mammalia and Insecta (especially Drosophila species), indicating that sequence motifs discussed here are not species-specific but often clade-specific or more deeply conserved.
We show that each of the four tRNA stems contains at least one GoU pair with conservation in positions that depends on the amino acid specificity of the tRNA and that, of the total 21 usual stem pairs, only four pairs never present a GoU pair: the two middle ones in the D-stem; the middle one in the AC-stem; and the last pair in the T-stem. Of the seven GoU pair positions that are amino acid specific, four are attached to a single amino acid (G3oU70 for Ala, U5oG68 for Trp, U6oG67 for Cys, U30oG40 for Ile), which could point to a role as a molecular identity element for their cognate amino acid tRNA synthetases, as is established for the G3oU70 in the Ala system (74,90). The U4oG69 pair occurs in tRNA-Asp and tRNA-Asn could also be part of the synthetase identity elements. The three other pairs are shared between some amino acids (G10oU25, U13oG22, U4oG69). For the first two in the D-stem, only a single orientation for each of the two GoU pairs is found (G10oU25 and U13oG22); they occur in tRNAs with specific amino acids corresponding to class II tRNA synthetases (Gly, Pro, Asp, His) or class I tRNA synthetases (Glu, Gln, Meti)), which includes the five tRNAs with 4 nts in the V-loop (Gly, Asp, Glu, His, Gln). This type of conservation could be instead related to the tight structural fold of the core of the tRNAs. However, for the six pairs that show both orientations of the GoU pairs (AA-stem, 3:70, 5:68, 6:69, T-stem, 49:65, and AC-stem, 27:43, 30:40), each orientation of the GoU pair is attached to a different amino acid, and such GoU pairs could be synthetase identity elements. Indeed, the position of a GoU pair at the ends of a helix maximizes the long-distance effect from the change in twist introduced by the GoU pair. In addition, the angle of change is different for GoU and UoG, which leads to additional molecular discrimination. Further, in mammals nine aminoacyl tRNA synthetases (Arg, Asp, Gln, Glu, Ile, Leu, Lys, Met, Pro) form a multi-synthetase complex (91,92) and the corresponding tRNAs are among the most frequent ones carrying a conserved GoU pair.
Interestingly, some GoU pairs are anticodon-specific throughout Mammalia and Insecta, while some are restricted to a clade. Among the first category are Arg and Leu which, as they belong to the 6-codon boxes, demand subtle recognition by their cognate synthetases (34,93). However, some anticodons of Ala, Gly, and Lys are also in that category. Additional conservation of GoU pairs occurs in an anticodon-specific fashion in Mammalia. The roles of such conservations are difficult to understand but they could indicate that some tRNAs have specialized functions either within the translation process or outside like aminoacyl tRNA synthetases do (41,94–96). Still, some tRNAs, specific for certain amino acids, do not present any conserved GoU pairs: Gly-CCC, Val of class I (but both with U13oU22), the Ser 4-codon box YCN of class II (but with G13oA22 and long variable loop, Supplementary Figure S2), and Arg-ACG with C13oU22.
Conservation in tRNA sequences is driven by the free energy of all intra- and inter-molecular interactions made by tRNAs during their biological functions. These functional contacts occur sequentially: the maturation and modification enzymes, the aminoacyl tRNA synthetases, the elongation factors, and the ribosomal grips in the three states of the translation process. The sequentiality of the interactions could imply that a particular tRNA-protein complex forms an interaction bottleneck (like the rate-limiting step in enzyme kinetics) that shapes some elements of the tRNA sequence. However, importantly, the molecular recognition modes are different in the various sequential states. This is especially noticeable when similar tRNA regions are recognized by different interactants: the whole range of physico-chemical interactions is taken advantage of and in a differential manner. The A- and B-boxes of the Pol III promoters are transcribed as linear (DNA) sequences by the polymerase but fold in the conserved T-loop with precise contacts between conserved nucleotides of the T- and D-loops in the three-dimensional tRNA architecture (17,37). Within the RNase P complex, the 1:72 base pair is recognized by a direct RNA-RNA contact in Bacteria (64) and via a protein subunit in Eukarya (65). In the -CCA end or the anticodon loop regions, the molecular recognition modes by the synthetases and the ribosomal recognition sites are not identical: sequence-guide base pairing dominates in the ribosome (61), while multiple and various contacts with amino acid side chains and peptide linkages occur in the synthetases (93). Similarly, the ribosome interacts with pre-organized anticodon loops and with -CCA ends where base stacking is extensive (38–40,68), while the synthetases often distort and destructure the anticodon loop to access directly the identity elements of the bases (34,93,97–99). Further, the pair 30:40 interacts with ribosomal elements in the P-state, and could play a role in translocation (68–70) and it has been involved in recognition by aminoacyl tRNA synthetases (77,79). Additionally, as discussed above, because a GoU pair induces a variation in helical twist within a helical fragment, direct contacts between the GoU pair and the protein or RNA ligand are not required for measurable effects on the binding efficiency (as in the paradigmatic case of Ala (74,90)). In the end, the variation in the distribution of conserved nucleotides and GoU pairs point to necessary but subtle trade-offs between the multiple and diverse tRNA molecular recognition events (19), which suggests that despite the singularity of each tRNA, globally they all tend to behave similarly, as shown for Pol III (100).
Conversely, deviations in tRNA sequences compared to standard conservations may indicate an alternative pathway, or a specific bias, during the translation processes in which tRNAs are involved. To assess that possibility, the establishment of ‘expected conservation’, with restraints on the lineages, is therefore a prerequisite. As discussed above, the many cases of conservation described here reflect the many contacts between tRNA molecules and their multiple interacting partners during maturation and translation, as well as for additional biological functions (41,101). Indeed, some deviations, especially among isodecoders, stand out (e.g. Ala, Gly) and may indicate the existence of ‘specialized tRNAs’ for specific translation or alternative functions, such as one tRNA-Arg that is involved in N-terminal arginylation (102), tRNAs that produce regulatory tRNA-derived small RNAs (103–105) or the brain-essential tRNA-Arg-UCU which is impaired by a G50 = C64 mutation to a GoU pair, causing ribosome stalling that can lead to neurodegeneration in mice (106). In bacteria, aminoacyl tRNAs are involved in nonribosomal biosynthesis (peptidoglycan (107), natural products (108), lipid modification (109,110), or protein degradation (111)); however, these unfrequently require special dedicated tRNA sequences (107). Finally, considering the high mutation rates of tRNA genes (42,53), the GoU conservations described here may be also useful for identifying differential tRNA gene expression during normal cell differentiation (112) or better recognizing damaging and disease-prone variants in humans (113–115).
DATA AVAILABILITY
The datasets generated during and/or analyzed during the current study are in the Supplementary Data or available from the corresponding author.
Supplementary Material
ACKNOWLEDGEMENTS
E.W. would like to thank for discussions V. Gopalan (Columbus, OH) on RNAse P, C. Müller (Heidelberg, Germany) on Pol III, and M. Szymanski (Poznan, Poland) on available aaRS structures.
Contributor Information
Eric Westhof, Université de Strasbourg, Institut de Biologie Moléculaire et Cellulaire, Architecture et Réactivité de l’ARN, CNRS UPR 9002, 2, allée Konrad Roentgen, F-67084 Strasbourg, France.
Bryan Thornlow, Department of Biomolecular Engineering, Baskin School of Engineering, University of California Santa Cruz, Santa Cruz, CA 95064, USA; UCSC Genomics Institute, University of California Santa Cruz, Santa Cruz, CA 95064, USA.
Patricia P Chan, Department of Biomolecular Engineering, Baskin School of Engineering, University of California Santa Cruz, Santa Cruz, CA 95064, USA; UCSC Genomics Institute, University of California Santa Cruz, Santa Cruz, CA 95064, USA.
Todd M Lowe, Department of Biomolecular Engineering, Baskin School of Engineering, University of California Santa Cruz, Santa Cruz, CA 95064, USA; UCSC Genomics Institute, University of California Santa Cruz, Santa Cruz, CA 95064, USA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
French National Program Investissement d’Avenir (Labex NetRNA) administered by the Agence Nationale de la Recherche [ANR-10-LABX-0036_NETRNA]; B.T. was funded by the National Institutes of Health (National Human Genome Research Institute) [F31HG010584]; P.C. and T.L. were funded by the National Institutes of Health (National Human Genome Research Institute) [R01HG006753 to T.L.]. Funding for open access charge: ANR.
Conflict of interest statement. Eric Westhof is Executive Editor of Nucleic Acids Research.
REFERENCES
- 1. Grosjean H., de Crécy-Lagard V., Marck C.. Deciphering synonymous codons in the three domains of life: co-evolution with specific tRNA modification enzymes. FEBS Lett. 2010; 584:252–264. [DOI] [PubMed] [Google Scholar]
- 2. Goodenbour J.M., Pan T.. Diversity of tRNA genes in eukaryotes. Nucleic Acids Res. 2006; 34:6137–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saks M.E., Conery J.S.. Anticodon-dependent conservation of bacterial tRNA gene sequences. RNA. 2007; 13:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shepherd J., Ibba M.. Bacterial transfer RNAs. FEMS Microbiol. Rev. 2015; 39:280–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paule M.R., White R.J.. Survey and summary: transcription by RNA polymerases i and III. Nucleic Acids Res. 2000; 28:1283–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geiduschek E.P., Kassavetis G.A.. The RNA polymerase III transcription apparatus. J. Mol. Biol. 2001; 310:1–26. [DOI] [PubMed] [Google Scholar]
- 7. Marck C., Kachouri-Lafond R., Lafontaine I., Westhof E., Dujon B., Grosjean H.. The RNA polymerase III-dependent family of genes in hemiascomycetes: comparative RNomics, decoding strategies, transcription and evolutionary implications. Nucleic Acids Res. 2006; 34:1816–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitra S., Das P., Samadder A., Das S., Betai R., Chakrabarti J.. Eukaryotic tRNAs fingerprint invertebrates vis-à-vis vertebrates. J. Biomol. Struct. Dyn. 2015; 33:2104–2120. [DOI] [PubMed] [Google Scholar]
- 9. Noma K.-I., Cam H.P., Maraia R.J., Grewal S.I.S.. A role for TFIIIC transcription factor complex in genome organization. Cell. 2006; 125:859–872. [DOI] [PubMed] [Google Scholar]
- 10. Galli G., Hofstetter H., Birnstiel M.L.. Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature. 1981; 294:626–631. [DOI] [PubMed] [Google Scholar]
- 11. Ziehler W.A., Day J.J., Fierke C.A., Engelke D.R.. Effects of 5′ leader and 3′ trailer structures on pre-tRNA processing by nuclear RNase P. Biochemistry. 2000; 39:9909–9916. [DOI] [PubMed] [Google Scholar]
- 12. Hopper A.K. Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast saccharomyces cerevisiae. Genetics. 2013; 194:43–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maraia R.J., Lamichhane T.N.. 3′ processing of eukaryotic precursor tRNAs. Wiley Interdiscip. Rev. RNA. 2011; 2:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Crécy-Lagard V., Boccaletto P., Mangleburg C.G., Sharma P., Lowe T.M., Leidel S.A., Bujnicki J.M.. Matching tRNA modifications in humans to their known and predicted enzymes. Nucleic Acids Res. 2019; 47:2143–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korostelev A., Trakhanov S., Laurberg M., Noller H.F.. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006; 126:1065–1077. [DOI] [PubMed] [Google Scholar]
- 16. Yarus M., Berg P.. Recognition of tRNA by isoleucyl-tRNA synthetase. Effect of substrates on the dynamics of tRNA-enzyme interaction. J. Mol. Biol. 1969; 42:171–189. [DOI] [PubMed] [Google Scholar]
- 17. Quigley G.J., Rich A.. Structural domains of transfer RNA molecules. Science. 1976; 194:796–806. [DOI] [PubMed] [Google Scholar]
- 18. Roovers M., Droogmans L., Grosjean H.. Post-transcriptional modifications of conserved nucleotides in the T-loop of tRNA: a tale of functional convergent evolution. Genes. 2021; 12:140–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giegé R., Jühling F., Pütz J., Stadler P., Sauter C., Florentz C.. Structure of transfer RNAs: similarity and variability. Wiley Interdiscip. Rev. RNA. 2012; 3:37–61. [DOI] [PubMed] [Google Scholar]
- 20. Rich A., RajBhandary U.L.. Transfer RNA: molecular structure, sequence, and properties. Annu. Rev. Biochem. 1976; 45:805–860. [DOI] [PubMed] [Google Scholar]
- 21. Altman S. Ribonuclease P. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011; 366:2936–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hough L.E., Dutta K., Sparks S., Temel D.B., Kamal A., Tetenbaum-Novatt J., Rout M.P., Cowburn D. The molecular mechanism of nuclear transport revealed by atomic-scale measurements. Elife. 2015; 4:e10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakanishi K., Nureki O.. Recent progress of structural biology of tRNA processing and modification. Mol. Cells. 2005; 19:157–166. [PubMed] [Google Scholar]
- 24. Mann H., Ben-Asouli Y., Schein A., Moussa S., Jarrous N.. Eukaryotic RNase P: role of RNA and protein subunits of a primordial catalytic ribonucleoprotein in RNA-based catalysis. Mol. Cell. 2003; 12:925–935. [DOI] [PubMed] [Google Scholar]
- 25. Lai L.B., Lai S.M., Szymanski E.S., Kapur M., Choi E.K., Al-Hashimi H.M., Ackerman S.L., Gopalan V.. Structural basis for impaired 5′ processing of a mutant tRNA associated with defects in neuronal homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2022; 119:e2119529119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suzuki T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 2021; 22:375–392. [DOI] [PubMed] [Google Scholar]
- 27. Pan T. Modifications and functional genomics of human transfer RNA. Cell Res. 2018; 28:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krutyhołowa R., Zakrzewski K., Glatt S.. Charging the code - tRNA modification complexes. Curr. Opin. Struct. Biol. 2019; 55:138–146. [DOI] [PubMed] [Google Scholar]
- 29. Agris P.F., Narendran A., Sarachan K., Väre V.Y.P., Eruysal E.. Chanfreau G.F. Chapter one - The Importance of being modified: the role of RNA modifications in translational fidelity. The Enzymes. 2017; 41:Academic Press; 1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peng G., He Y., Wang M., Ashraf M.F., Liu Z., Zhuang C., Zhou H.. The structural characteristics and the substrate recognition properties of RNase ZS1. Plant Physiol. Biochem. 2021; 158:83–90. [DOI] [PubMed] [Google Scholar]
- 31. Wellner K., Betat H., Mörl M.. A tRNA’s fate is decided at its 3′ end: collaborative actions of CCA-adding enzyme and RNases involved in tRNA processing and degradation. Biochim. Biophys. Acta (BBA) - Gene Regul. Mech. 2018; 1861:433–441. [DOI] [PubMed] [Google Scholar]
- 32. Phizicky E.M., Hopper A.K.. tRNA biology charges to the front. Genes Dev. 2010; 24:1832–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guimarães A.R., Correia I., Sousa I., Oliveira C., Moura G., Bezerra A.R., Santos M.A.S.. tRNAs as a driving force of genome evolution in yeast. Front. Microbiol. 2021; 12:634004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giegé R., Sissler M., Florentz C.. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998; 26:5017–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schimmel P., Giegé R., Moras D., Yokoyama S.. An operational RNA code for amino acids and possible relationship to genetic code. Proc. Natl. Acad. Sci. U.S.A. 1993; 90:8763–8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hinnebusch Alan G. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol. Mol. Biol. Rev. 2011; 75:434–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Auffinger P., Westhof E.. An extended structural signature for the tRNA anticodon loop. RNA. 2001; 7:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Voorhees R.M., Ramakrishnan V.. Structural basis of the translational elongation cycle. Annu. Rev. Biochem. 2013; 82:203–236. [DOI] [PubMed] [Google Scholar]
- 39. Noller H.F., Yusupov M.M., Yusupova G.Z., Baucom A., Cate J.H.D.. Translocation of tRNA during protein synthesis. FEBS Lett. 2002; 514:11–16. [DOI] [PubMed] [Google Scholar]
- 40. Yusupova G., Jenner L., Rees B., Moras D., Yusupov M.. Structural basis for messenger RNA movement on the ribosome. Nature. 2006; 444:391–394. [DOI] [PubMed] [Google Scholar]
- 41. Katz A., Elgamal S., Rajkovic A., Ibba M.. Non-canonical roles of tRNAs and tRNA mimics in bacterial cell biology. Mol. Microbiol. 2016; 101:545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thornlow B.P., Hough J., Roger J.M., Gong H., Lowe T.M., Corbett-Detig R.B.. Transfer RNA genes experience exceptionally elevated mutation rates. Proc. Natl. Acad. Sci. USA. 2018; 115:8996–9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Varani G., McClain W.H.. The g x u wobble base pair. A fundamental building block of RNA structure crucial to RNA function in diverse biological systems. EMBO Rep. 2000; 1:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rozov A., Demeshkina N., Khusainov I., Westhof E., Yusupov M., Yusupova G.. Novel base-pairing interactions at the tRNA wobble position crucial for accurate reading of the genetic code. Nat. Commun. 2016; 7:10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Masquida B., Westhof E.. On the wobble GoU and related pairs. RNA. 2000; 6:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Westhof E., Yusupov M., Yusupova G.. The multiple flavors of GoU pairs in RNA. J. Mol. Recognit. 2019; 32:e2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Westhof E., Liang S., Tong X., Ding X., Zheng L., Dai F.. Unusual tertiary pairs in eukaryotic tRNAAla. RNA. 2020; 26:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chan P.P., Lowe T.M.. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 2016; 44:D184–, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin B.Y., Chan P.P., Lowe T.M.. tRNAviz: explore and visualize tRNA sequence features. Nucleic Acids Res. 2019; 47:W542–W547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boccaletto P., Machnicka M.A., Purta E., Piatkowski P., Baginski B., Wirecki T.K., de Crécy-Lagard V., Ross R., Limbach P.A., Kotter A.et al.. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018; 46:D303–D307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Crécy-Lagard V., Jaroch M.. Functions of bacterial tRNA modifications: from ubiquity to diversity. Trends Microbiol. 2021; 29:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chan P.P., Lin B.Y., Mak A.J., Lowe T.M.. tRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. Nucleic Acids Res. 2021; 49:9077–9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thornlow B.P., Armstrong J., Holmes A.D., Howard J.M., Corbett-Detig R.B., Lowe T.M.. Predicting transfer RNA gene activity from sequence and genome context. Genome Res. 2020; 30:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R.. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989; 26:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sherry S.T., Ward M.H., Kholodov M., Baker J., Phan L., Smigielski E.M., Sirotkin K.. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001; 29:308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Holmes J.B., Moyer E., Phan L., Maglott D., Kattman B.. SPDI: data model for variants and applications at NCBI. Bioinformatics. 2020; 36:1902–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sayers E.W., Barrett T., Benson D.A., Bolton E., Bryant S.H., Canese K., Chetvernin V., Church D.M., DiCuccio M., Federhen S.et al.. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2011; 39:D38–D51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Karolchik D., Hinrichs A.S., Furey T.S., Roskin K.M., Sugnet C.W., Haussler D., Kent W.J.. The UCSC table browser data retrieval tool. Nucleic Acids Res. 2004; 32:D493–D496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pollard K.S., Hubisz M.J., Rosenbloom K.R., Siepel A.. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2010; 20:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Armstrong J., Hickey G., Diekhans M., Deran A., Fang Q., Xie D., Feng S., Stiller J., Genereux D., Johnson J.et al.. Progressive alignment with cactus: a multiple-genome aligner for the thousand-genome era. Nature. 2020; 587:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bermudez-Santana C., Attolini C.S.-O., Kirsten T., Engelhardt J., Prohaska S.J., Steigele S., Stadler P.F.. Genomic organization of eukaryotic tRNAs. BMC Genomics. 2010; 11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fournier A. Quantitative data on the bombyx mori l. silkworm: a review. Biochimie. 1979; 61:283–320. [DOI] [PubMed] [Google Scholar]
- 63. Ivanov P., O’Day E., Emara M.M., Wagner G., Lieberman J., Anderson P.. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc. Natl. Acad. Sci. U.S.A. 2014; 111:18201–18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tosar J.P., Gámbaro F., Darré L., Pantano S., Westhof E., Cayota A.. Dimerization confers increased stability to nucleases in 5′ halves from glycine and glutamic acid tRNAs. Nucleic Acids Res. 2018; 46:9081–9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Phan H.-D., Lai L.B., Zahurancik W.J., Gopalan V.. The many faces of RNA-based RNase p, an RNA-world relic. Trends Biochem. Sci. 2021; 46:976–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yaremchuk A., Kriklivyi I., Tukalo M., Cusack S.. Class i tyrosyl-tRNA synthetase has a class II mode of cognate tRNA recognition. EMBO J. 2002; 21:3829–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Åström S.U., von Pawel-Rammingen U., Byström A.S.. The yeast initiator tRNAMet can act as an elongator tRNAMetIn vivo. J. Mol. Biol. 1993; 233:43–58. [DOI] [PubMed] [Google Scholar]
- 68. Selmer M., Dunham C.M., Murphy F.V., Weixlbaumer A., Petry S., Kelley A.C., Weir J.R., Ramakrishnan V.. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006; 313:1935–1942. [DOI] [PubMed] [Google Scholar]
- 69. Watson Z.L., Ward F.R., Méheust R., Ad O., Schepartz A., Banfield J.F., Cate J.H.. Structure of the bacterial ribosome at 2 Å resolution. Elife. 2020; 9:e60482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shalev-Benami M., Zhang Y., Rozenberg H., Nobe Y., Taoka M., Matzov D., Zimmerman E., Bashan A., Isobe T., Jaffe C.L.et al.. Atomic resolution snapshot of leishmania ribosome inhibition by the aminoglycoside paromomycin. Nat. Commun. 2017; 8:1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Charette M., Gray M.W.. Pseudouridine in RNA: what, where, how, and why. IUBMB Life. 2000; 49:341–351. [DOI] [PubMed] [Google Scholar]
- 72. Schrader J.M., Uhlenbeck O.C.. Is the sequence-specific binding of aminoacyl-tRNAs by EF-Tu universal among bacteria. Nucleic Acids Res. 2011; 39:9746–9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Raina M., Moghal A., Kano A., Jerums M., Schnier P.D., Luo S., Deshpande R., Bondarenko P.V., Lin H., Ibba M.. Reduced amino acid specificity of mammalian tyrosyl-tRNA synthetase is associated with elevated mistranslation of tyr codons. J. Biol. Chem. 2014; 289:17780–17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hou Y.M., Schimmel P.. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature. 1988; 333:140–145. [DOI] [PubMed] [Google Scholar]
- 75. McClain W.H., Chen Y.M., Foss K., Schneider J.. Association of transfer RNA acceptor identity with a helical irregularity. Science. 1988; 242:1681–1684. [DOI] [PubMed] [Google Scholar]
- 76. Schultz D.W., Yarus M.. tRNA structure and ribosomal function. I. tRNA nucleotide 27-43 mutations enhance first position wobble. J. Mol. Biol. 1994; 235:1381–1394. [DOI] [PubMed] [Google Scholar]
- 77. Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J.C., Moras D.. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science. 1991; 252:1682–1689. [DOI] [PubMed] [Google Scholar]
- 78. Sauter C., Lorber B., Cavarelli J., Moras D., Giegé R.. The free yeast aspartyl-tRNA synthetase differs from the tRNA(Asp)-complexed enzyme by structural changes in the catalytic site, hinge region, and anticodon-binding domain. J. Mol. Biol. 2000; 299:1313–1324. [DOI] [PubMed] [Google Scholar]
- 79. Büttcher V., Senger B., Schumacher S., Reinbolt J., Fasiolo F.. Modulation of the suppression efficiency and amino acid identity of an artificial yeast amber isoleucine transfer RNA in escherichia coli by a G-U pair in the anticodon stem. Biochem. Biophys. Res. Commun. 1994; 200:370–377. [DOI] [PubMed] [Google Scholar]
- 80. Basavappa R., Sigler P.B.. The 3 a crystal structure of yeast initiator tRNA: functional implications in initiator/elongator discrimination. EMBO J. 1991; 10:3105–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kolitz S.E., Lorsch J.R.. Eukaryotic initiator tRNA: finely tuned and ready for action. FEBS Lett. 2010; 584:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marck C., Grosjean H.. tRNomics: analysis of tRNA genes from 50 genomes of eukarya, archaea, and bacteria reveals anticodon-sparing strategies and domain-specific features. RNA. 2002; 8:1189–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mokdad A., Krasovska M.V., Sponer J., Leontis N.B.. Structural and evolutionary classification of G/U wobble basepairs in the ribosome. Nucleic Acids Res. 2006; 34:1326–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hosseini M., Roy P., Sissler M., Zirbel C.L., Westhof E., Leontis N.. How to fold and protect mitochondrial ribosomal RNA with fewer guanines. Nucleic Acids Res. 2018; 46:10946–10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hubisz M.J., Pollard K.S., Siepel A.. PHAST and RPHAST: phylogenetic analysis with space/time models. Brief. Bioinform. 2011; 12:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Grosjean H., Westhof E.. An integrated, structure- and energy-based view of the genetic code. Nucleic Acids Res. 2016; 44:8020–8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vargas-Rodriguez O., Badran A.H., Hoffman K.S., Chen M., Crnković A., Ding Y., Krieger J.R., Westhof E., Söll D., Melnikov S.. Bacterial translation machinery for deliberate mistranslation of the genetic code. Proc. Natl. Acad. Sci. U.S.A. 2021; 118:e2110797118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ling J., O’Donoghue P., Söll D.. Genetic code flexibility in microorganisms: novel mechanisms and impact on physiology. Nat. Rev. Microbiol. 2015; 13:707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mukai T., Vargas-Rodriguez O., Englert M., Tripp H.J., Ivanova N.N., Rubin E.M., Kyrpides N.C., Söll D.. Transfer RNAs with novel cloverleaf structures. Nucleic Acids Res. 2017; 45:2776–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Naganuma M., Sekine S.-I., Chong Y.E., Guo M., Yang X.-L., Gamper H., Hou Y.-M., Schimmel P., Yokoyama S.. The selective tRNA aminoacylation mechanism based on a single G•U pair. Nature. 2014; 510:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kaminska M., Havrylenko S., Decottignies P., Le Maréchal P., Negrutskii B., Mirande M.. Dynamic organization of Aminoacyl-tRNA synthetase complexes in the cytoplasm of human cells. J. Biol. Chem. 2009; 284:13746–13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu R.-J., Long T., Li H., Zhao J., Li J., Wang M., Palencia A., Lin J., Cusack S., Wang E.-D.. Molecular basis of the multifaceted functions of human leucyl-tRNA synthetase in protein synthesis and beyond. Nucleic Acids Res. 2020; 48:4946–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Giegé R., Springer M.. Aminoacyl-tRNA synthetases in the bacterial world. EcoSal Plus. 2016; 7: 10.1128/ecosalplus.ESP-0002-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hausmann C.D., Ibba M.. Aminoacyl-tRNA synthetase complexes: molecular multitasking revealed. FEMS Microbiol. Rev. 2008; 32:705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wakasugi K., Yokosawa T.. Non-canonical functions of human cytoplasmic tyrosyl-, tryptophanyl- and other aminoacyl-tRNA synthetases. Enzymes. 2020; 48:207–242. [DOI] [PubMed] [Google Scholar]
- 96. Guo M., Schimmel P.. Essential nontranslational functions of tRNA synthetases. Nat. Chem. Biol. 2013; 9:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shen N., Guo L., Yang B., Jin Y., Ding J.. Structure of human tryptophanyl-tRNA synthetase in complex with tRNATrp reveals the molecular basis of tRNA recognition and specificity. Nucleic Acids Res. 2006; 34:3246–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yang X.-L., Otero F.J., Ewalt K.L., Liu J., Swairjo M.A., Köhrer C., RajBhandary U.L., Skene R.J., McRee D.E., Schimmel P.. Two conformations of a crystalline human tRNA synthetase-tRNA complex: implications for protein synthesis. EMBO J. 2006; 25:2919–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Deng X., Qin X., Chen L., Jia Q., Zhang Y., Zhang Z., Lei D., Ren G., Zhou Z., Wang Z.et al.. Large conformational changes of insertion 3 in human Glycyl-tRNA synthetase (hGlyRS) during catalysis. J. Biol. Chem. 2016; 291:5740–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kutter C., Brown G.D., Gonçalves A., Wilson M.D., Watt S., Brazma A., White R.J., Odom D.T.. Pol III binding in six mammals shows conservation among amino acid isotypes despite divergence among tRNA genes. Nat. Genet. 2011; 43:948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Avcilar-Kucukgoze I., Kashina A.. Hijacking tRNAs from translation: regulatory functions of tRNAs in mammalian cell physiology. Front. Mol. Biosci. 2020; 7:610617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kwon Y.T., Kashina A.S., Davydov I.V., Hu R.-G., An J.Y., Seo J.W., Du F., Varshavsky A.. An essential role of N-terminal arginylation in cardiovascular development. Science. 2002; 297:96–99. [DOI] [PubMed] [Google Scholar]
- 103. Shen Y., Yu X., Zhu L., Li T., Yan Z., Guo J.. Transfer RNA-derived fragments and tRNA halves: biogenesis, biological functions and their roles in diseases. J. Mol. Med. 2018; 96:1167–1176. [DOI] [PubMed] [Google Scholar]
- 104. Boskovic A., Bing X.Y., Kaymak E., Rando O.J.. Control of noncoding RNA production and histone levels by a 5′ tRNA fragment. Genes Dev. 2020; 34:118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Goodarzi H., Liu X., Nguyen H.C.B., Zhang S., Fish L., Tavazoie S.F.. Endogenous tRNA-derived fragments suppress breast cancer progression via YBX1 displacement. Cell. 2015; 161:790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ishimura R., Nagy G., Dotu I., Zhou H., Yang X.-L., Schimmel P., Senju S., Nishimura Y., Chuang J.H., Ackerman S.L.. RNA function. Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration. Science. 2014; 345:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rietmeyer L., Fix-Boulier N., Le Fournis C., Iannazzo L., Kitoun C., Patin D., Mengin-Lecreulx D., Ethève-Quelquejeu M., Arthur M., Fonvielle M.. Partition of tRNAGly isoacceptors between protein and cell-wall peptidoglycan synthesis in staphylococcus aureus. Nucleic Acids Res. 2021; 49:684–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Moutiez M., Belin P., Gondry M.. Aminoacyl-tRNA-utilizing enzymes in natural product biosynthesis. Chem. Rev. 2017; 117:5578–5618. [DOI] [PubMed] [Google Scholar]
- 109. Fields R.N., Roy H.. Deciphering the tRNA-dependent lipid aminoacylation systems in bacteria: novel components and structural advances. RNA Biol. 2018; 15:480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yakobov N., Mahmoudi N., Grob G., Yokokawa D., Saga Y., Kushiro T., Worrell D., Roy H., Schaller H., Senger B.et al.. RNA-dependent synthesis of ergosteryl-3β-O-glycine in ascomycota expands the diversity of steryl-amino acids. J. Biol. Chem. 2022; 298:101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Watanabe K., Toh Y., Suto K., Shimizu Y., Oka N., Wada T., Tomita K.. Protein-based peptide-bond formation by aminoacyl-tRNA protein transferase. Nature. 2007; 449:867–871. [DOI] [PubMed] [Google Scholar]
- 112. Rak R., Polonsky M., Eizenberg-Magar I., Mo Y., Sakaguchi Y., Mizrahi O., Nachshon A., Reich-Zeliger S., Stern-Ginossar N., Dahan O.et al.. Dynamic changes in tRNA modifications and abundance during t cell activation. Proc. Natl. Acad. Sci. U.S.A. 2021; 118:e2106556118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Parisien M., Wang X., Pan T.. Diversity of human tRNA genes from the 1000-genomes project. RNA Biol. 2013; 10:1853–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lant J.T., Berg M.D., Heinemann I.U., Brandl C.J., O’Donoghue P. Pathways to disease from natural variations in human cytoplasmic tRNAs. J. Biol. Chem. 2019; 294:5294–5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kapur M., Ackerman S.L.. mRNA translation gone awry: translation fidelity and neurological disease. Trends Genet. 2018; 34:218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are in the Supplementary Data or available from the corresponding author.