Abstract
Rationale and objective
Various forms of Non-invasive respiratory support (NRS) have been used during COVID-19, to treat Hypoxemic Acute Respiratory Failure (HARF), but it has been suggested that the occurrence of strenuous inspiratory efforts may cause Self Induced Lung Injury(P-SILI). The aim of this investigation was to record esophageal pressure, when starting NRS application, so as to better understand the potential risk of the patients in terms of P-SILI and ventilator induced lung injury (VILI).
Methods and measurements
21 patients with early de-novo respiratory failure due to COVID-19, underwent three 30 min trials applied in random order: high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), and non-invasive ventilation (NIV). After each trial, standard oxygen therapy was reinstituted using a Venturi mask (VM). 15 patients accepted a nasogastric tube placement. Esophageal Pressure (ΔPes) and dynamic transpulmonary driving pressure (ΔPLDyn), together with the breathing pattern using a bioelectrical impedance monitor were recorded. Arterial blood gases were collected in all patients.
Main results
No statistically significant differences in breathing pattern and PaCO2 were found. PaO2/FiO2 ratio improved significantly during NIV and CPAP vs VM. NIV was the only NRS to reduce significantly ΔPes vs. VM (-10,2 ±5 cmH20 vs -3,9 ±3,4). No differences were found in ΔPLDyn between NRS (10,2±5; 9,9±3,8; 7,6±4,3; 8,8±3,6 during VM, HFNC, CPAP and NIV respectively). Minute ventilation (Ve) was directly dependent on the patient's inspiratory effort, irrespective of the NRS applied. 14% of patients were intubated, none of them showing a reduction in ΔPes during NRS.
Conclusions
In the early phase of HARF due to COVID-19, the inspiratory effort may not be markedly elevated and the application of NIV and CPAP ameliorates oxygenation vs VM. NIV was superior in reducing ΔPes, maintaining ΔPLDyn within a range of potential safety.
Keywords: Hypoxemic acute respiratory failure, COVID 19, Non invasive respiratory supports
1. Introduction
Non-invasive respiratory support (NRS), such as, Continuous Positive Airway Pressure (CPAP), High Flow Nasal Cannula (HFNC) or non-invasive ventilation (NIV), have been successfully applied to manage moderate to severe Hypoxemic Acute Respiratory Failure (HARF) [1,2,3,4,5]. A potential drawback associated to the use of NRS in patients with HARF, is the occurrence of strenuous spontaneous inspiratory efforts leading to large negative swings in intra-thoracic pressure and large swings of dynamic transpulmonary pressure (ΔPLDyn) that may cause Patient Self Induced Lung Injury (P-SILI) [6]. Tonelli et al. recently highlighted the importance of inspiratory effort and transpulmonary pressure during NIV. They showed that a 24 h trial of NIV failed leading to intubation when inspiratory effort remained unchanged and transpulmonary pressure increased within the first 2 h of ventilation [7].
Since the onset of the COVID-19 pandemic, several studies showed that the use of out-of-ICU, CPAP, HFNC or NIV, allowed to manage moderate to severe episodes of HARF, avoiding admission to the intensive care units for invasive mechanical ventilation [8,9,10]. All three NRS techniques have been indiscriminately applied, using different settings and timing of application. Some authors [11,12] pointed out that in patients with COVID-19, progressive deterioration of lung function might occur when respiratory drive is not reduced by oxygen administration and NRS. Persistent strong spontaneous inspiratory efforts simultaneously increase tissue stress and raise pulmonary transvascular pressures, vascular flows, and fluid leakage. Nevertheless, the COVID-19 syndrome causes unique lung injury that is not physiologically yet fully explored. Indeed, the physiological effects of the different NRS need to be better understood, to eventually minimize the risk of further lung damage.
A prompt evaluation of the patient's effort and the potential effects on transpulmonary pressure (PL) may be therefore desirable in these patients, in order to avoid harm. The only studies aimed to compare the physiological effects of NRS were performed in patients with de-novo HARF of various origins [2,13,14].
Therefore, we reasoned that the recording of esophageal pressure, when starting NRS application, may allow us to better understand the potential risk of the patients in terms of P-SILI and ventilator induced lung injury (VILI). We conducted a randomized short-term physiological investigation within the first 24 h of hospital admission to compare the effects of standard oxygen therapy, HFNC, CPAP and NIV on breathing pattern, gas exchange, inspiratory effort, and dynamic transpulmonary pressure (PLDyn) in patients with moderate-to-severe HARF due to COVID-19 pneumonia.
2. Methods
This pilot randomized short-term physiological study was conducted between February and May 2021, in the respiratory ICU (RICU) of Sant'Orsola Hospital, Bologna, Italy. The local Ethic Committee approved the study (691/2020/Sper/AOUBo) and written informed consent was obtained from all the patients. The study was prospectively registered at the Clinical Trial Registry (NCT04741659).
2.1. Patients
We considered eligible any adult patient (≥ 18 years old) with HARF and a PaO2/FiO2 ratio < 200 mmHg evaluated during spontaneous unassisted breathing trial (VM with FiO2 of at least 0.40), due to pneumonia and a confirmed molecular diagnosis of COVID-19 (positive real-time polymerase chain reaction for viral RNA performed on respiratory tract specimen). Exclusion criteria were: previous clinical, radiological and histological diagnosis of pneumopathy, body mass index > 30 kg/m2; known diagnosis of sleep-disorders, restrictive pulmonary/chest wall disease, cardiac arrest, severe haemodynamic instability (> 1 vasoactive amine for at least 24 h), acute coronary syndrome (unstable angina/IMA), severe arrhythmias, inability to protect the airway, respiratory arrest and need for intubation, pregnancy or suspected, use of sedative drugs, long-term home oxygen therapy.
This study was designed as a pilot study. A priori, standardized effect size comparing Venturi mask (VM), HFNC, CPAP, and NIV is not known. For this reason, we were not able to calculate the optimal sample size. To reach a statistical power of almost 80%, we assumed to enroll 20 subjects with a minimum of 12 subjects having full data available, [15,16,17]. Similar criteria were employed by other physiological studies [13,14].
2.2. Study Protocol
After enrolment, each patient underwent a 30 min spontaneous unassisted breathing trial with a VM. FiO2 was set to reach a SpO2 >92% <96% and it remained unmodified throughout the whole study period.
Thereafter, three 30 min trials (HFNC, CPAP and NIV) were randomly applied. The randomization sequence was established by a computer software (Research Randomizer 4.0). In order to minimize the carryover effect, a washout period of 30 min with VM between treatments was performed.
NIV was delivered by a non-invasive mechanical ventilator (Astral 150; ResMed, USA) through a single vented circuit and a full-face mask (Performax; Philips Respironics, USA). Investigators supervised the mask size and fitting in order to minimize leaks as much as possible.
All patients received HFNC through a cannula connected to a heated humidifier (AIRVO2; Fisher & Paykel Healthcare, Auckland, NZ). The cannula was selected to occlude patient's nostril of about 2/3. Flow rate and temperature were set at 60 L Min–1 and 34 °C, respectively. During the trial period, patients were encouraged to breath by the nose.
CPAP was performed with a helmet with an integral Venturi flow driver, which included an adjustable PEEP valve (StarMed VentuKit, Italia).
PEEP during CPAP and NIV was adjusted to 10 cmH2O. During NIV, two steps of pressure support (PS) were performed: 10 cmH2O (NIV PS10) and 5 cmH2O (NIV PS5). Initially, PS was set at 10 cmH2O and eventually decreased to 5 cmH2O if esophaegeal pressure swings (ΔPes) became positive during inspiration or less than -1 cmH2O (see measurements below), or if patient intolerance or asynchronies were present.
2.3. Physiological measurements
Patient demographics and clinical characteristics were collected at study entry.
A semi-quantitative scoring system was used to estimate the lung abnormalities based on the area involved at chest CT scan. Each of the 5 lung lobes was visually scored 18.
A bioelectrical impedance monitor and a single-use Pad Set sensor placed on the surface of the chest wall were used to monitor minute ventilation (Ve) and tidal volume (VT) (ExSpiron, Respiratory Motion Inc. USA) [19,20].
With the patient in a semi recumbent position, a nasogastric tube provided with an esophageal balloon (Marquat Genie Biomedical, France) was placed to measure esophageal pressure and register tidal change in esophageal pressure. The nasogastric tube was first measured outside the chest wall in order to estimate the distance from the nostril to the xyphoid, inserted though the nostril and then pushed down to the stomach, inflated and connected to a dedicated monitoring system (FluxMed, MBMed, Argentina). Once inserted, the esophageal balloon was filled with the minimum non-stress volume of air recommended by the manufacturer, to avoid over or underestimation of the measurements [21]. Due to the impossibility to do Baydur's occlusion test in this clinical condition, the proper position of the catheter at the 1/3 lower esophageal level was ascertained by the visualization of cardiac artefacts on Pes traces. [22,23]. During CPAP and NIV, a Fleisch-type pneumotacograph, placed at the end of the inspiratory limb of the circuit, measured inspiratory flow and airway pressure (Paw).
All patients underwent standard monitoring with 5-lead ECG and SpO2. Towards the end of each trial and during a stable period pattern of 5 minutes, VT, Ve, respiratory rate (RR), blood gases (ABG), heart rate (HR), arterial blood pressure (BP), and SpO2 were collected. Discomfort was also assessed for each device by using 0–10 visual analogue scales (VAS).
During all the stages of the study, Pes was recorded. Flow and airways pressure were also recorded during CPAP and NIV. When a stable breathing pattern was achieved, ΔPes was calculated as the maximum decrease or increase in Pes from the end-expiratory level [24]. We measured dynamic end inspiratory/expiratory transpulmonary pressure (PL= airway pressure minus Pes, at end inspiration/expiration) to calculate dynamic transpulmonary driving pressure (ΔPLDyn, the maximal positive variation of PL during inspiration). ΔPLDyn was taken as a surrogate for ΔPL, due to the impossibility of performing an inspiratory/expiratory pause during spontaneous breathing. ΔPLDyn is mainly affected by VT, and it also involves the resistive load due to airflow. Therefore, for the purposes of our study, we assumed that resistive pressure was not remarkably different within the same patient. Since it was impossible to monitor Paw during HFNC and standard oxygen, it was assumed constant and equivalent to 2.5 cmH2O, during HFNC and to 0 cmH2O during VM[25].
2.4. Outcome Measures
The changes in ΔPes and ΔPLdyn, recorded by esophageal pressure, and the changes in respiratory pattern recorded by respiratory inductance plethysmography, were the primary outocomes. Changes in ABG, Comfort score rated using a VAS, BP and HR measurements were assessed as secondary outcomes.
2.5. Statistical analysis
Continuous variables are presented as mean value and standard deviation (+SD). Median and interquartile range values were also calculated and reported in Tables 1 , 2 , and 3 . Differences in continuous variables between groups were analysed by one-way analysis of variance (ANOVA) and post-hoc t-tests. No adjustment was made for multiple statistical tests. Relationships between Ve, inspiratory effort expressed by ΔPes and ΔPLdyn were studied using the Spearman correlation. P values lower than 0.05 were considered statistically significant. The IBM SPSS Statistics software (version 21) was used for data analysis.
Table 1.
Baseline characteristics of the study group.
| N = 21 | |
|---|---|
| Age, years old; mean ±SD (median; iqr25-iqr75) | 63,57±9,04 (62; 57-72) |
| Weight, kg; mean ±SD (median; iqr25-iqr75) | 80,70±10,66 (81,5; 71,5-88) |
| Height, cm; mean ±SD (median; iqr25-iqr75) | 172,95±9,36 (176; 165-178,5) |
| PBW, kg; mean ± SD (median; iqr25-iqr75) | 67,84±9,78 (71,4; 60-73,5) |
| Smokers or ex smokers, n (% of N) | 12(57%) |
| Charlson Score, points; mean ± SD (median; iqr25-iqr75) | 2,19±1,25 (2; 1-3) |
| SAPSII, points; mean ± SD (median; iqr25-iqr75) | 22±6,36 (22;18-28) |
| APACHE score, points; mean ± SD (median; iqr25-iqr75) | 8,71±2,35 (9; 7-10) |
| Days from emergency ward to RICU, n; mean ± SD | 0,57±0,87 |
| Laboratory tests | |
| D-Dimer, mg/l; mean ± SD (median; iqr25-iqr75) | 1,90±3,57 (0,55; 0,6-1,5) |
| CPR, mg/dl; mean ± SD (median; iqr25-iqr75) | 7,97±8,30 (5,44; 0,42-11,6) |
| Ferritin, ng/ml; mean ± SD (median; iqr25-iqr75) | 979,90±985,63 (674; 529-1079) |
| IL-6, pg/ml; mean ± SD (median; iqr25-iqr75) | 97,18±72,52 (74; 52-134,87) |
| CT Radiographic features | |
| Bilateral involvement, n (% of N) | 21 (100%) |
| Pattern Mainly interstitial, n (% of N) 16 (76,2%) Mainly consolidative, n (% of N) 5 (23,8%) | |
| Total CT score of the pulmonary involvement; mean ± SD (min-max) |
9,38±3,25 (3-16) |
| Physiological parameters on RICU admission | |
| FiO2 mean ± SD (median; iqr25-iqr75) | 0,5±0,1 (0,5; 0,5-0,6) |
| pH mean ± SD (median; iqr25-iqr75) | 7,46±00,3 (7,47; 7,45-7,47) |
| PaCO2, mmHg; mean ± SD (median; iqr25-iqr75) | 34,46±4,02 (34;32-36,55) |
| PaO2, mmHg; mean ± SD (median; iqr25-iqr75) | 66,45±9,79 (66,4; 61,75-74,25) |
| PaO2/FiO2, mmHg; mean ± SD (median; iqr25-iqr75) | 126,90±34,06 (125; 104-158) |
| RR, breaths per minute; mean ± SD (median; iqr25-iqr75) | 24,62±5,44 (25; 20-28) |
| HR, beats per minute; mean ± SD (median; iqr25-iqr75) | 80,05±12,71 (83; 76-88) |
| SpO2 %; mean ±SD (median; iqr25-iqr75) | 93,40±2,70 (94; 92,7-95) |
| Comfort VAS; mean ± SD (median; iqr25-iqr75) | 2,29±1,59 (2; 1-3) |
| Mean VT, ml; mean ± SD (median; iqr25-iqr75) | 456,46±126,10 (420; 377,8-513,6) |
| VT/PBW, ml/KGPBW; mean ± SD (median; iqr25-iqr75) | 6,80±1,75 (6,56; 5,72-7,47) |
| Mean Ve, L/m; mean ± SD (median; iqr25-iqr75) | 10,92±3,08 (10; 8,8-12,56 |
Quantitative data are presented as mean ± SD, Median and interquartile range values, minimum and maximum value, while counting data are presented as count (percentage of the total).
(PBW=Predicted body weight; SAPSII =Simplified Acute Physiology Score; CPR= c-Reactive Protein; IL- 6=Interleukin 6; CT= Computer Tomography; RR =Respiratory Rate; HR = Heart Rate; RICU= Respiratory Intensive Care Unit; VAS= Visual Analogue Score; VT = Tidal Volume; Ve= Minute Ventilation).
Table 2.
Effects of different non-invasive respiratory supports on gas exchange, breathing pattern, inspiratory effort, and respiratory mechanics (fifteen patients).
| N = 15 | Venturi Mask | HFNC | CPAP | NIV |
|---|---|---|---|---|
| PaCO2, mmHg; mean ± SD mean ± SD (median; iqr25-iqr75) |
33,73±3,69 (34; 32-34,75) |
33,24±4,2 (34,5; 33-36,5) |
34,42±2,9 (34;32 -35,8) |
34,2±3,1 (35; 31,3- 37) |
| PaO2, mmHg; mean ± SD (median; iqr25-iqr75) |
64,41±9,2* (65; 59,8-72,7) |
83,11±40,8 (73; 59-91) |
90,78±26,1* (86,5; 76,5-95,2) |
112,33±49,5* (86; 76,7-113) |
| PaO2/FiO2, mmHg; mean ± SD (median; iqr25-iqr75) |
120,6±36,1† (112; 101-155) |
152,2±75,4 (139,5; 96-168,2) |
170,9±61,8† (166; 137,2-209) |
207,4±94,2† (180,5; 145,5-213,2) |
| RR, breaths per minute; mean ± SD (median; iqr25-iqr75) |
23,6±5,3 (24;20-28) |
20,4±5,6 (20,5; 16,2-23,7) |
23,2±4,3 (22; 21-26) |
22,8±5 (22; 21- 23,75) |
| MeanVT, ml mean ± SD (median; iqr25-iqr75) |
450,5±100,6 (419,2; 404-513) |
450,1±96,9 (452,2; 416-497) |
502,7±101,7 (526,5; 438,7-559,5) |
400,4±96,3 (391,6; 362,6-460,3) |
| VT/PBW, ml/KGPBW; mean ± SD (median; iqr25-iqr75) |
6,5±1,4 (6,6; 5,7-7,2) |
6,4±1,1 (6,4;5,7-7) |
7,3±1,7 (7,3; 6,1-8,1) |
5,8±1,4 (6,4; 4,8- 6,6) |
| Mean Ve, L/m; mean ± SD (median; iqr25-iqr75) |
10,6±2,6 (10; 8,9-11,9) |
9,5±3,2 (8,6; 7,7-12,3) |
11,5±3,6 (10,9; 9,1-13,8) |
9±2,1 (8,9; 8- 9,5) |
| Mean ΔPes, cmH20; mean ± SD (median; iqr25-iqr75) |
-10.2±5§ (-8,6; -7,4 - -13,5) |
-9,9±3,8§ (-10,9; -7,3- -12,7) |
-9,6±5,2§ (-9,43; -5,7- -11,9) |
-3,9±3,38§ (-4,9; -2,1- -5,9) |
| Mean ΔPLDyn, cmH20; mean ± SD (median; iqr25-iqr75) |
10,2±5,1 (8,6; 7,4 13,5) |
9, 9±3,8 (10,9; 7,3 -12,7) |
7,6±4,3 (7,1; 5- 10,9) |
8,8±3,6 (8,9; 7,3 – 11,1) |
(HFNC= high flow nasal cannula; CPAP= continuous positive airway pressure; NIV= non-invasive mechanical ventilation; RR =Respiratory Rate; Ve= minute ventilation, VT = inspiratory tidal volume; PBW=Predicted body weight; ΔPes = tidal change in esophageal pressure, ΔPLDyn= Dynamic tidal change in transpulmonary pressure)
p=0.001 NIV versus Venturi Mask; p=0.001 CPAP versus Venturi Mask
p=0.002 NIV versus Venturi Mask; p=0.012 CPAP versus Venturi Mask
p=0.001 NIV versus Venturi Mask; p=0.000 NIV versus HFNC; p=0.002 NIV versus CPAP
Table 3.
Effects of different pressure support levels on breathing pattern, inspiratory effort, and respiratory mechanics (fifteen patients).
| N = 15 | NIV PS5 | NIV PS10 | P value |
|---|---|---|---|
| RR, breaths per minute; mean ± SD (median; iqr25-iqr75) |
22,8±5 (22; 21- 23,75) |
21,4±7 (23,5; 18- 26) |
>0,05 |
| MeanVT, ml; mean ± SD (median; iqr25-iqr75) |
400,4±96,3 (391,6; 362,6-460,3) |
489,9±123,9 (458,2; 382,5-555,5) |
>0,05 |
| VT/PBW, ml/KGPBW; mean ± SD (median; iqr25-iqr75) |
5,8±1,4 (6,4; 4,8- 6,6) |
7±1,3 (7,1; 6 -7,8) |
>0,05 |
| Mean Ve, L/m; mean ± SD (median; iqr25-iqr75) |
9±2,1 (8,9; 8- 9,5) |
10,1±2,7 (9,4; 8,4-11,5) |
>0,05 |
| Mean ΔPes, cmH20; mean ± SD (median; iqr25-iqr75) |
-3,9±3,38 (-4,9; -2,1 - -5,9) |
-0,03±3,2 (1,4; -2,28 - +1,7) |
0,007 |
| Mean ΔPLDyn, cmH20; mean ± SD (median; iqr25-iqr75) |
8,8±3,6 (8,9; 7,3 – 11,1) |
10,6±2,2 (10,4; 8,6-12,1) |
>0,05 |
(NIV= non-invasive mechanical ventilation; RR =Respiratory Rate; Ve= minute ventilation, VT = tidal volume; PBW=Predicted body weight; ΔPes = tidal change in esophageal pressure, ΔPLDyn= Dynamic tidal change in transpulmonary pressure).
Patient consent: Obtained.
Ethics approval: Comitato Etico Indipendente dell'Azienda Ospedaliero-Universitaria di Bologna, Policlinico S. Orsola-Malpighi.
3. RESULTS
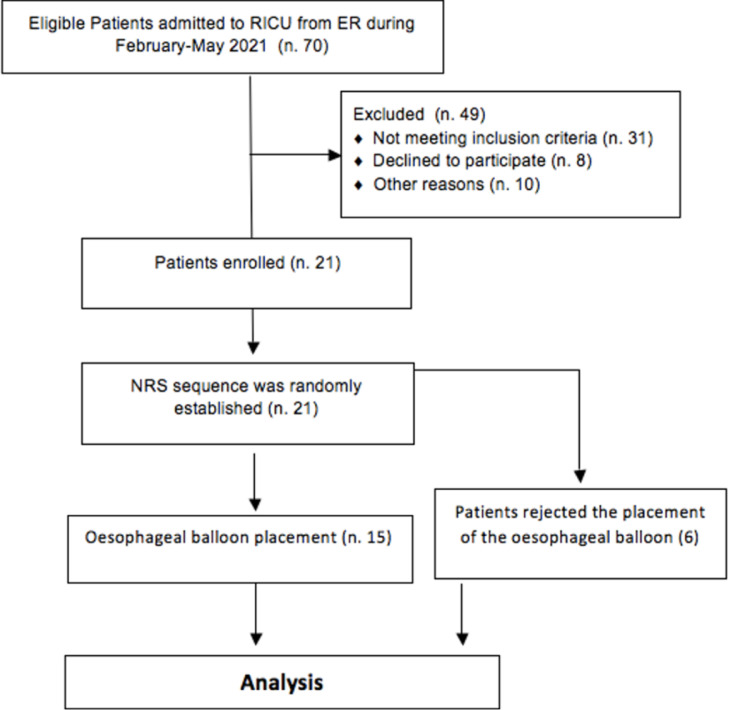
Table 1 shows the patients’ baseline characteristics. Twenty-one patients were studied within the first 24 h after RICU admission. Six of them rejected the placement of the esophageal balloon. The flowchart for patients in this study is shown in Fig. 1 .
Fig. 1.
Flowchart for patients. (ER=Emergency Room; n.= number; NRS= non invasive respiratory support).
The mean time-lapse between emergency ward and RICU admission was 0,57±0,87 days. Mean PaO2/FiO2 was 126,9 ±34,06 at enrolment. Quantitative analysis of lung CT revealed a low extend of infiltrates (total CT score 9,38±3,25).
3.1. Breathing pattern, respiratory mechanics and gas exchange Outcomes
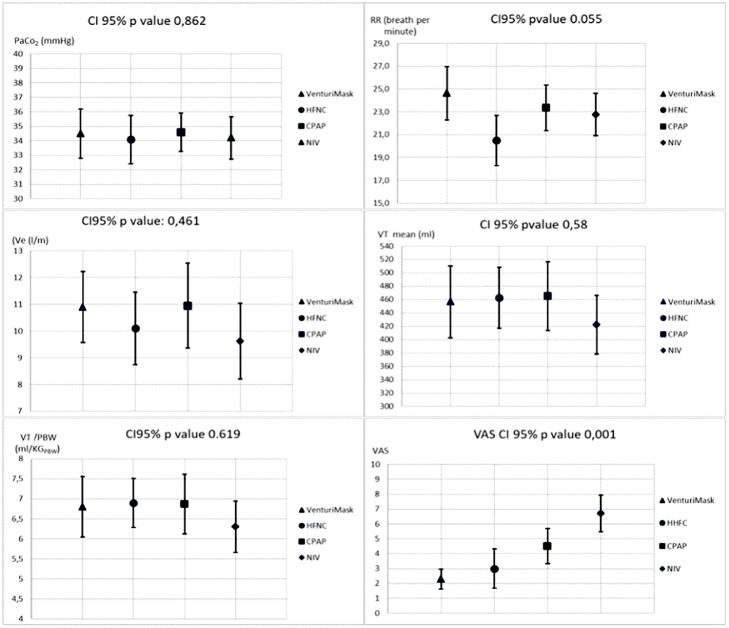
As shown on Fig. 2 , no statistically significant differences were found in RR, VT, VT normalized by predicted body weight (VT/PBW), Ve and PaCO2 between treatments in all study population. Differently, VM was judged more comfortable compared to the others.
Fig. 2.
Effects of different non-invasive respiratory supports on breathing pattern, ventilation, and comfort; (twenty-one patient). (HFNC= high flow nasal cannula; CPAP= continuous positive airway pressure; NIV= non-invasive mechanical ventilation; RR =Respiratory Rate; Ve= minute ventilation. VTi = inspiratory tidal volume; PBW=Predicted body weight; VAS= Visual Analogue Score).
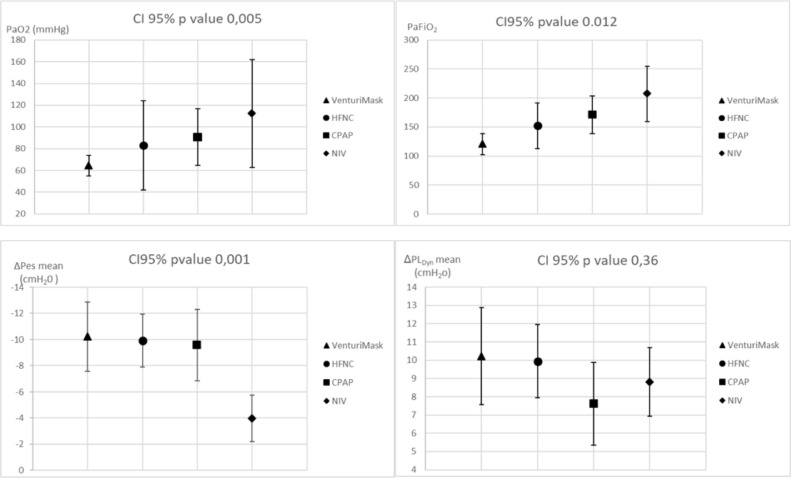
Pathophysiological features of the 15 patients undergoing respiratory mechanics measurements are reported in Table 2 and Fig. 3 . RR, PaCO2, VT, VT/PBW and Ve were similar in all trials. In contrast, PaO2/FiO2 improved using VM, HFNC, CPAP and NIV, respectively. In particular, a higher PaO2/FiO2 ratio was observed during NIV and CPAP trials compared to VM (p = 0,002 and p = 0,012 respectively).
Fig. 3.
Effects of different non-invasive respiratory supports on gas exchange and respiratory mechanics (fifteen patients). (HFNC= high flow nasal cannula; CPAP= continuous positive airway pressure; NIV= non-invasive mechanical ventilation; PaFiO2 = PaO2/FiO2 ratio; ΔPes = tidal change in oesophageal pressure, ΔPLDyn = Dynamic tidal change in transpulmonary pressure).
Inspiratory effort progressively decreased using VM, HFNC, CPAP and NIV (mean ΔPes of -10,2 ±5,0 cmH20 vs -9,9 ±3,9 vs -9,5 ±5,0 vs -3,98 ±3,4 respectively) (Fig. 3). However, NIV was the only support able to reduce significantly ΔPes compared the others [NIV vs VM (p = 0,001); NIV vs HFNC (p = 0,000) and NIV vs CPAP (p = 0,002)], while no difference was found in ΔPLDyn between treatments (10,2 ±5,1 vs 9,9 ±3,8 vs 7,6 ±4,3 vs 8,8 ±3,6 cmH2O; p value= 0,360)(Table 2).
ΔPes was significantly reduced from -3,98 ± 3,4 cmH2O to 0,03 ± 3,2 (p = 0,007) during NIV PS5 vs NIV PS10. No statistically significant differences were found in RR, VT, VT/PBW, Ve and ΔPLdyn, between NIV PS5 and NIV PS10 (Table 3). Because during NIV PS10 ΔPes was close to zero or even positive, we decided to compare only data during NIV PS5 with other NRS.
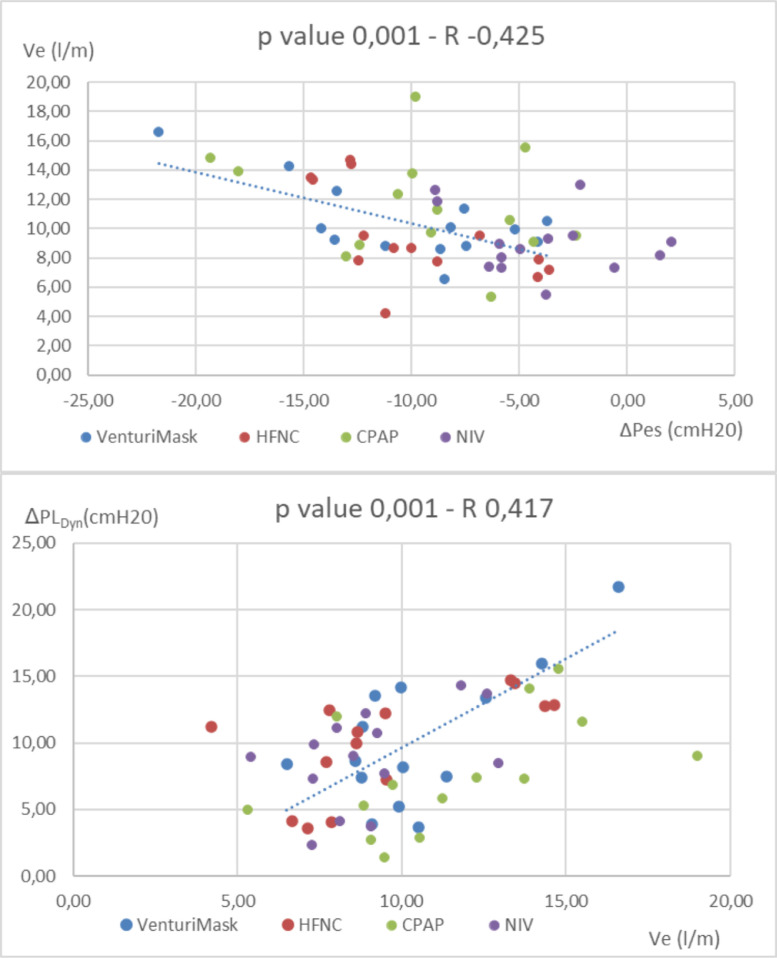
In addition, the correlation analysis showed that Ve was directly dependent on the patient's inspiratory effort, irrespective of the type of the respiratory support applied (p value =0,001): patients with higher ΔPes experienced an increase in Ve (Fig. 4 , upper part). Likewise, patients that increased Ve experienced higher ΔPLdyn (p value= 0,001) (Fig. 4, lower part).
Fig. 4.
Upper part: correlation analysis between minute ventilation and ΔPes (inspiratory effort). Lower pan: correlation analysis between minute ventilation and ΔPLCym. (HFNC; High flow nasal cannula, CPAP; continuous positive airway pressure, NIV; non invasive mechanical ventilation. Ve= minute ventilation, ΔPes = tidal change in oesophageal pressure. ΔPLDym = Dynamic tidal change in transpulmonary pressure).
3.2. Clinical outcomes
3 of 21 (14%) patients were intubated at 24, 43 and 84 h after the end of this investigation. They were all undergoing NIV at the time of intubation. These patients did not respond to the NIV trial with PS of 10 cmH2O in terms of ΔPes reduction (14,5±3,1 at baseline vs 11,2±2,3 cmH2O with NIV PS10).
4. Discussion
NRS are attractive treatment strategies that might avoid intubation and the risk of its complications. Although NRS have been largely used in COVID-19 population, the optimal initial respiratory support for these patients is controversial. In addition, some concerns have been raised regarding the use of NRS treatments including the risk of inappropriate delays in intubation and protective ventilation or exacerbation of lung injury by increasing VILI/P-SILI in spontaneous breathing patients.
To the best of our knowledge, this study is the first face-to-face comparison of changes in respiratory mechanics between all the 3 major forms of NRS applied in spontaneously breathing patients with COVID-19 developing HARF.
Even with the limit of the small sample size, our pilot study demonstrated that NIV might improve the inspiratory effort versus all the other devices, keeping transpulmonary pressure constant. NIV and CPAP were also associated with better gas exchange. RR remained stable among the different trials. Patients judged the VM the more comfortable device.
Three patients were intubated later in the course of the disease. Our results are in line with other studies that have applied NRS. For example, Brusasco [26] and coworkers and Oranger et al [27] showed in COVID-19 patients undergoing CPAP a failure rate of 17% and 23% respectively, while other recent studies reported NRS treatment failure of 24, 25 and 23, respectively [4,28,29]. HFNC, on the other hand, has been shown to be associated with a higher percentage of failure [8,30,31].
The pathophysiology of COVID-19 is still matter of investigation, but it has been suggested that at least in a subset of patients this form of lung injury is not similar to those with ARDS unrelated to COVID-19[32,33,34]. Ramin et al [35] demonstrated in the early stage (median time between admission and intubation was 2 days), a static lung elastance of 19 cmH2O/l and a low ΔPL (4 cmH2O) on day 1 of invasive mechanical ventilation. It has been shown that the lung weight in moderate and severe spontaneous breathing COVID-19 patients treated with helmet CPAP or NIV was approximately half of what has been described in typical ARDS with similar severity, confirming the dissociation between the gas exchange and the anatomical lung characteristics, typical of Covid-19 patients at early stage of disease [18]. However, more recent studies support that within 24 h from ICU admission, in invasive mechanically ventilated patients with COVID-19, respiratory mechanics are heterogeneous but similar to those reported for "classical" ARDS [36].
According to Coppola classification [28], our patients fit well the group of “moderate-severe COVID-ARDS”, characterized by a mean PaO2/FiO2 value of 126. Similarly, we observed low extend of infiltrates at CT scan, despite the moderate-severe impairment of gas exchange.
These results may explain the reason why the inspiratory effort was relatively low, as expected for other form of de-novo respiratory failure [37]. Coppola et al [28] also reported an average ΔPes at day 6 after hospital admission of 7.5 cmH2O without major differences between supported and unsupported breathing. Unluckily, Coppola, studied a very small number of patients on NIV (9 vs 131 on CPAP), and no data on respiratory mechanics were reported in this subset of patients. In contrast, we observed a marked decreased in the inspiratory effort during NIV.
However, one may claim that our trial lasted “only” 30 min, but it has been shown that a progressive increase in ΔPes is already evident at one minute in condition of respiratory distress, as during an unsuccessful weaning trial, whereas breathing pattern changes may not significantly vary [38].
Recently, Grieco and coworkers [9] compared NIV vs HFNC in critically ill patients with moderate to severe HARF due to COVID-19. NIV was delivered with a helmet, using a PEEP >10 cmH20 over a PS ranging between 10 and 15 cmH20. Helmet NIV was able to significantly reduce RR, ΔPes and comfort, while ΔPL was not statistically different, except for the subset of patients with low effort, where NIV increased ΔPL, compared to HFNC. Therefore, they concluded that in patients with low inspiratory effort, the use of a lower level of PS should be warranted. Similarly, we noted that the application of an inspiratory pressure of 10 cmH20 resulted in a reduction of ΔPes close to zero or even positive, suggesting that the majority of COVID-19 patients in the early phase of their disease may act differently to other forms of de-novo respiratory failure where higher inspiratory support is usually applied.
Interestingly enough, however, during NIV, the ΔPL remained on average below the threshold of 15 cmH20, which it has been suggested, but not proven yet, a safety limit to avoid deterioration in lung injury [39,40].
Pes swings were mostly reduced during NIV, that is the only NRS able to provide an inspiratory support, and therefore should be considered the only “true” form of ventilatory support, compared to HFNC or CPAP.
ΔPLDyn can be split into two components, the first is the part necessary to overcome the flow resistance, and second is the pressure generated to expand the alveoli. This latter is the component of the PL that is the product of volume and elastance. PL is the result of Paw (the ventilator inspiratory pressure) minus the pleural pressure, which is the ΔPes generated by inspiratory muscles. It represents the stress that alveoli are exposed to, and it has been considered, but not still firmly demonstrated, among the most important determinants of P-SILI/VILI. Like Tonelli et al (7), we saw that the VT/ΔPL ratio does not change significantly: VT did not modify significantly during any NRS trial, not even during NIV with PS10. Moreover, any NRS modify ΔPLDyn. ΔPL was kept constant: at expense of the ΔPes reduction during PS application (8.8 cmH2O, during NIV trial) or simply at equivalent ΔPes values without PS (10,2, 9,9, 7,6 cmH2O in VM, HFNC and CPAP trials, respectively). Therefore, if VT and ΔPL did not change, we assume that lung compliance (VT/ΔPL ratio) has not been modified by the application of positive pressure (not reduced by over-distension, nor increased by the fact of recruitment). Our findings are in line with the interstitial CT pattern described in our population.
On the other hand, one might think that “in clinical practice” applying NIV should increase VT. In this case, if VT increases, with a same ΔPL value, it might be related to an improvement of lung compliance due to the recruitment effect of the PEEP, as expected in classic forms of ARDS.
Finally, in our study CPAP and NIV seem to be the two NRS able to significantly improve oxygenation, while HFNC did not, despite a small increase in PaO2/FiO2 ratio vs. VM. These data confirm the results reported by Grieco et al [9] concerning the use of NIV and HFNC, and those of Coppola and coworkers [28] that found a statistical improvement in gas exchange when comparing CPAP with VM. Rather surprising, we did not observe changes in breathing pattern among the different trials, while other authors did. This might be related by the fact that our patients were enrolled in the early phase of their disease and despite a comparable PaO2/FiO2 ratio, their RR was on average lower than on other studies. As we previously described a "non-recruitable lung" condition in this population, one hypothesis to justify the P/F improvement during NIV and CPAP, might be that CPAP or PEEP effect during NIV could redistribute the blood flow of the alveoli with low ventilation perfusion ratio (V/Q), i.e. highly perfused, generating a redistribution effect and a new balance in V/Q [41].
This study has limitations. First, it represents an exploratory analysis with a small sample size. Second, because of the study design, we could not investigate possible changes in the respiratory mechanics during the time course of patient's hospital stay, especially for those patients requiring IMV. However, the 3 patients who were intubated showed a higher ΔPes compared to the overall group (14,5+3,1 cmH2O with VM) after the end of the study, even under NIV. Third, some data about respiratory mechanics are missing because 6 patients refused to swallow the esophageal balloon; nevertheless we could record these parameters in the remaining 15, which is the minimum sample size to reach a statistical power of almost 80%. Moreover, in this study it was impossible to measure Paw during the HFNC trial and during VM. For this reason, we assumed an existing reference value as in published literature. However, regardless of the actual absolute value of Paw, it was assumed to be constant during the respiratory cycle and should not modify the DPLdyn. Last, the helmet with an integral Venturi flow driver (the VentuKit) used in this study delivers a flow under the reported experimental conditions. It delivers a flow of approximately 48 L/min, which is not enough to perform a CPAP "high flow" device [42]. Studies [43,44] have shown that the esophageal pressure delta is lower when the device is able to deliver a higher flow and consequently a more stable airway pressure. However, in our study, the advantage of measuring flow and pressure through an external sensor (pneumotacograph mentioned in the article) allowed us to be aware that CPAP flows should not be less than 60 L/m, not only because of the fact that pressure becomes unstable, but also because of the undesirable effect of rebreathing. In addition, the “pressure variations” due to flow or the patient effort were taken into account for all calculations in this study.
In conclusion, our results showed that during the early phase of HARF due to COVID-19 pneumonia, the application of NIV and CPAP ameliorate gas exchange compared to VM, keeping breathing pattern unmodified. NIV can be superior to HFNC and CPAP in reducing the inspiratory effort, maintaining transpulmonary pressure similar to the other NRS devices and within a range of safety, concerning the potential risk of P-SILI or VILI. Further studies are needed to assess the clinical outcomes, the impact of NRS on SILI, and the potential changes in respiratory mechanics in more advanced stages of the disease .
Contributors
GS, LV, SN: study design, experimental procedure, writing and data analysis. LP: study design, experimental procedure, writing. IP: writing and data analysis. VC: experimental procedure and writing and data analysis. Vitt.C: experimental procedure. IB: experimental procedure and data analysis. MZ: data analysis. VMR: writing and data analysis
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2022.04.012.
Appendix. Supplementary materials
References
- 1.Brambilla AM, Aliberti S, Prina E, et al. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 2014;40(7):942–949. doi: 10.1007/s00134-014-3325-5. Erratum in: Intensive Care Med. 2014 Aug;40(8):1187. PMID: 24817030. [DOI] [PubMed] [Google Scholar]
- 2.Frat J-P, Thille AW, Mercat A, et al; FLORALI Study Group; REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015 ;372(23):2185-96. [DOI] [PubMed]
- 3.Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320(20):2099–2107. doi: 10.1001/jama.2018.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaber S, Lescot T, Futier E, et al. NIVAS Study Group. Effect of noninvasive ventilation on tracheal reintubation among patients with hypoxemic respiratory failure following abdominal surgery: a randomized clinical trial. JAMA. 2016;315(13): 1345-1353. [DOI] [PubMed]
- 5.He H, Sun B, Liang L, et al. ENIVA Study Group. A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome. Crit Care. 2019;23(1):300. doi: 10.1186/s13054-019-2575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 7.Tonelli R, Fantini R, Tabbì L, et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure. a pilot study. Am J Respir Crit Care Med. 2020;202(4):558–567. doi: 10.1164/rccm.201912-2512OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco C, Facciolongo N, Tonelli R, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020;56(5) doi: 10.1183/13993003.02130-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grieco DL, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325(17):1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202:1039–1042. doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020 9;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 12.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grieco DL, Menga LS, Raggi V, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2020;201(3):303–312. doi: 10.1164/rccm.201904-0841OC. [DOI] [PubMed] [Google Scholar]
- 14.Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195:1207–1215. doi: 10.1164/rccm.201605-0916OC. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead AL, Julious SA, Cooper CL, et al. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25(3):1057–1073. doi: 10.1177/0962280215588241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell ML, Whitehead AL, Julious SA. Guidance for using pilot studies to inform the design of intervention trials with continuous outcomes. Clin Epidemiol. 2018 18;10:153–157. doi: 10.2147/CLEP.S146397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julious S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut. Statist. 2005;4:287–291. [Google Scholar]
- 18.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodali BS, Choi L, Chau A, et al. Use of a novel non-invasive respiratory monitor to study changes in pulmonary ventilation during labor epidural analgesia. J Clin Monit Comput. 2020;34(3):567–574. doi: 10.1007/s10877-019-00349-1. [DOI] [PubMed] [Google Scholar]
- 20.Williams GW, 2nd, George CA, Harvey BC, et al. A comparison of measurements of change in respiratory status in spontaneously breathing volunteers by the exspiron noninvasive respiratory volume monitor versus the capnostream capnometer. Anesth Analg. 2017;124(1):120–126. doi: 10.1213/ANE.0000000000001395. [DOI] [PubMed] [Google Scholar]
- 21.Mojoli F, Chiumello D, Pozzi M, et al. Esophageal pressure measurements under different conditions of intrathoracic pressure: an in vitro study of second generation balloon catheters. Minerva Anestesiol. 2015;81:855–864. [PubMed] [Google Scholar]
- 22.Akoumianaki E, Maggiore SM, Valenza F, et al. PLUG Working Group (Acute Respiratory Failure Section of the European Society of Intensive Care Medicine). The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 23.Baydur A, Behrakis PK, Zin WA, et al. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126:788–791. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 24.Bellani G, Grasselli G, Teggia-Droghi M, Mauri T, Coppadoro A, Brochard L, Pesenti A. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care. 2016;20(1):142. doi: 10.1186/s13054-016-1290-9. PMID: 27160458; PMCID: PMC4862136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207–1215. doi: 10.1164/rccm.201605-0916OC. [DOI] [PubMed] [Google Scholar]
- 26.Brusasco C, Corradi F, Di Domenico A, et al. CPAP-COVID-19 study group; collaborators of the Galliera CPAP-COVID-19 study group are. Continuous positive airway pressure in COVID-19 patients with moderate-to-severe respiratory failure. Eur Respir J. 2021;57(2) doi: 10.1183/13993003.02524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oranger M, Gonzalez-Bermejo J, Dacosta-Noble P, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J. 2020;56(2) doi: 10.1183/13993003.01692-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppola S, Chiumello D, Busana M, et al. Role of total lung stress on the progression of early COVID-19 pneumonia. Intensive Care Med. 2021:1–10. doi: 10.1007/s00134-021-06519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan J, Chen B, Liu X, et al. Use of high-flow nasal cannula and noninvasive ventilation in patients with COVID-19: a multicenter observational study. Am J Emerg Med. 2021;46:276–281. doi: 10.1016/j.ajem.2020.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wendel Garcia PD, Aguirre-Bermeo H, Buehler PK, Alfaro-Farias M, Yuen B, David S, et al. Implications of early respiratory support strategies on disease progression in critical COVID-19: a matched subanalysis of the prospective RISC-19-ICU cohort. Crit Care. 2021;25(1):175. doi: 10.1186/s13054-021-03580-y. PMID: 34034782; PMCID: PMC8146172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie, et al. The RECOVERY-RS randomized clinical trial. JAMA. 2022;327(6):546–558. doi: 10.1001/jama.2022.0028. PMID: 35072713; PMCID: PMC8787685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gattinoni L, Busana M, Camporota L, et al. COVID-19 and ARDS: the baby lung size matters. Intensive Care Med. 2021;47(1):133–134. doi: 10.1007/s00134-020-06324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramin S, Charbit J, Dagod G, et al. Transpulmonary pressure in SARS-CoV-2-associated acute respiratory distress syndrome: a single-center observational study. Crit Care. 2020;24(1):408. doi: 10.1186/s13054-020-03129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grasselli G, Cattaneo E, Florio G, Ippolito M, Zanella A, Cortegiani A, Huang J, Pesenti A, Einav S. Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review. Crit Care. 2021;25(1):115. doi: 10.1186/s13054-021-03536-2. PMID: 33743812; PMCID: PMC7980724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tonelli R, Busani S, Tabbì L, et al. Inspiratory effort and lung mechanics in spontaneously breathing patients with acute respiratory failure due to COVID-19: a matched control study. Am J Respir Crit Care Med. 2021;204(6):725–728. doi: 10.1164/rccm.202104-1029LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jubran A, Grant BJ, Laghi F, et al. Weaning prediction: esophageal pressure monitoring complements readiness testing. Am J Respir Crit Care Med. 2005;171(11):1252–1259. doi: 10.1164/rccm.200503-356OC. [DOI] [PubMed] [Google Scholar]
- 39.Pelosi P, Ball L, Barbas CSV, et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2021;25(1):250. doi: 10.1186/s13054-021-03686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauri T, Yoshida T, Bellani G, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42(9):1360–1373. doi: 10.1007/s00134-016-4400-x. [DOI] [PubMed] [Google Scholar]
- 41.Oldani S, Ravaglia C, Bensai S, Bertolovic L, Ghirotti C, Puglisi S, et al. Pathophysiology of light phenotype SARS-CoV-2 interstitial pneumonia: from histopathological features to clinical presentations. Pulmonology. 2021;S2531-0437(21):00077–00078. doi: 10.1016/j.pulmoe.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brusasco C, Corradi F, De Ferrari A, Ball L, Kacmarek RM, Pelosi P. CPAP devices for emergency prehospital use: a bench study. Respir Care. 2015;60(12):1777–1785. doi: 10.4187/respcare.04134. [DOI] [PubMed] [Google Scholar]
- 43.Mistraletti G, Giacomini M, Sabbatini G, Pinciroli R, Mantovani ES, Umbrello M, et al. Noninvasive CPAP with face mask: comparison among new air-entrainment masks and the Boussignac valve. Respir Care. 2013;58(2):305–312. doi: 10.4187/respcare.01598. [DOI] [PubMed] [Google Scholar]
- 44.Sehlin M, To¨rnell SS, O¨ hberg F, Johansson G, Winso¨ O. Pneumatic performance of the Boussignac CPAP system in healthy humans. Respir Care. 2011;56(6):818–826. doi: 10.4187/respcare.01015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.