Abstract
Objectives
This study aimed to evaluate the efficacy and adverse events of favipiravir in patients with COVID-19.
Methods
Our protocol was registered on PROSPERO (CRD42020206305). Fourteen databases were searched until February 8th, 2021. An update search for new RCTs was done on March 2nd, 2022. Meta-analysis was done for randomized controlled trials (RCTs) and non-RCTs.
Results
Overall, 157 studies (24 RCTs, 1 non-RCT, 21 observational studies, 2 case series, and 106 case reports) were included. On hospitalized patients, in comparison to standard of care, favipiravir showed a higher rate of viral clearance at day 5 (RR = 1.60, p = 0.02), defervescence at day 3–4 (RR = 1.99, p <0.01), chest radiological improvement (RR = 1.33, p <0.01), hospital discharge at day 10–11 (RR = 1.19, p <0.01), and shorter clinical improvement time (MD = –1.18, p = 0.05). Regarding adverse events, favipiravir groups had higher rates of hyperuricemia (RR = 9.42, p <0.01), increased alanine aminotransferase (RR = 1.35, p <0.01) but lower rates of nausea (RR = 0.42, p <0.01) and vomiting (R R= 0.19, p=0.02). There were no differences regarding mortality (RR=1.19, p=0.32), and increased aspartate aminotransferase (RR = 1.11, p = 0.25). On nonhospitalized patients, no significant differences were reported.
Conclusions
Adding favipiravir to the standard of care provides better outcomes for hospitalized patients with COVID-19. Pregnant, lactating women, and patients with a history of hyperuricemia should avoid using favipiravir.
Keywords: Favipiravir, COVID-19, SARS-CoV-2; Efficacy, side effects
Abbreviation: AIC, akaike information criterion; CCA, Cancer Council Australia; CI, confidence interval; CT, computed tomography; FEM, fixed-effect model; GRADE, The Grading of Recommendations Assessment, Development and Evaluation; MD, mean difference; NIH, National Institutes of Health; OR, odds ratio; PRISMA, preferred reporting Items for systematic reviews and meta-analyses; RCT, randomized controlled trial; RdRp, RNA-dependent-RNA-polymerase; REM, random-effect model; RoB 2, cochrane collaboration's risk of bias tool for randomized trials; ROBIN-I, cochrane collaboration's risk of bias tool for nonrandomized studies of interventions; RR, risk ratio; RNA, ribonucleic acid; SD, standard deviation; WHO, World Health Organization
Introduction
COVID-19 was declared a worldwide pandemic by the World Health Organization (WHO) on March 11, 2020 because of its widespread prevalence and life-altering consequences (World Health Organization 2020). The disease is caused by the SARS-CoV-2 virus, an enveloped positive-sense RNA virus that relies primarily on RNA-dependent RNA polymerase (RdRp) for viral gene transcription and replication (Zhu et al., 2020). It is well recognized for its unprecedented speed of transmission in asymptomatic and presymptomatic carriers (Gandhi et al., 2020). Until April 10, 2022, nearly 500,000,000 cases of SARS-CoV-2 infection have been confirmed and over 6,000,000 people have died worldwide as a result (Johns Hopkins, 2020). This had stretched intensive care units to their maximum capacity and beyond. Consequently, there is an urgent need for efficient and safe drugs to combat this pandemic.
Currently, the recommended treatments for COVID-19 are limited. Favipiravir is a promising antiviral medication (Agrawal et al., 2020), which was initially used as an anti-influenza agent in Japan in 2014 and was also used to treat Ebola and other viral diseases (Guedj et al., 2018; Shiraki and Daikoku, 2020). As a nucleoside analog, it inhibits the RdRp complex of SARS-CoV-2 through binding to its catalytic domain and preventing the inclusion of nucleotides for viral RNA replication, leading to an increased mutation frequency, and perhaps lethal mutagenesis (Shannon et al., 2020). RdRp has been shown to have an exceptionally high polymerization rate in combination with low fidelity and is highly error-prone in SARS-CoV-2, which makes it the target of choice with polymerase inhibitors like favipiravir (Shannon et al., 2020). Additionally, favipiravir does not affect the human DNA or proteins because RdRp has no host cell homolog (Zhu et al., 2020).
There have been many trials and observational studies that report the efficacy and adverse events of favipiravir in the management of patients with COVID-19 (Hase et al., 2020, Kocayiğit et al., 2021, Udwadia et al., 2021, Pushkar, 2020). Early similar meta-analyses were done to summarize this evidence (Hassanipour et al., 2021; Manabe et al., 2021; Prakash et al., 2020; Shrestha et al., 2020). However, as the number of new reports increased day by day, we conducted this systematic review and meta-analysis to provide more clinical evidence for the efficacy and adverse effects of favipiravir in patients with COVID-19.
Methods
Our systematic review and meta-analysis followed a previously published method (Tawfik et al., 2021) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Page et al., 2021) (Supplemental Table 1). The protocol was published on PROSPERO, the International Prospective Register of Systematic Reviews, under identification number CRD42020206305 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020206305).
Literature search
A total of 14 electronic databases were searched on February 8, 2021. The detailed databases and search terms can be found in Supplemental Table 2. An additional manual search on March 20 was done. All search results were imported into EndNote X9 to remove duplicated entries.
Study selection
The titles and abstracts were screened first, then the full texts of eligible studies were downloaded and checked for final inclusion. Any primary studies in which favipiravir was used on patients with COVID-19 would be eligible. Nonpeer-reviewed articles and data from trial registries were included. We excluded nonhuman studies, duplicated articles, and studies with unextractable data or missing full texts.
Data extraction
Data were extracted into an Excel form. For randomized controlled trials (RCTs) and non-RCTs, we extracted age, sex, follow-up day, and any quantity data relating to favipiravir efficacy and side effects. For categorical data, sample size, number of concerned events, and time points were extracted to calculate the risk ratio (RR) and their 95% confidence interval (95% CI). For continuous data, mean, standard deviation (SD), and sample size were extracted or estimated from median and range/interquartile range (Wan et al., 2014) to calculate the mean difference (MD) and 95% CI. Outcomes regarding favipiravir efficacy and side effects from observational studies and case reports were reported in quality review.
Quality assessment
The RCTs and non-RCTs were assessed by the Cochrane Collaboration's second version of the risk of bias tool for randomized trials (RoB 2) (Higgins et al., 2011) and the risk of bias in nonrandomized studies of interventions (ROBIN-I) (Sterne et al., 2016). Observational, cross-sectional studies, and case series were assessed using the National Institutes of Health (NIH) quality assessment tool (Quality assessment tool for observational cohort and cross-sectional studies, 2021). Case reports were assessed as recommended by the Clinical Guidelines Network Cancer Council Australia (CCA) handbook (Sydney: Cancer Council Australia, 2014). For important outcomes in the meta-analyses, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to assess the certainties of evidence (Atkins et al., 2004).
The study selection, data extraction, and quality assessment were done by at least 2 independent authors who engaged in a formal discussion later to resolve any disagreements. A difficult decision would be consulted with a senior member to resolve.
Statistical analysis
Meta-analysis using inverse variance method was conducted by the meta package in R 4.0.4 (Deeks, 2021). The heterogeneity was assessed using I2 and Cochrane Q test. If I2 was higher than 50% or the p-value less than 0.10, heterogeneity would be considered statistically significant, and the random-effect model and Hartung-Knapp-Sidik-Jonkman method would be used. Otherwise, a fixed-effect model would be applied. For each outcome, all trials comparing favipiravir to the standard of care (SOC) were pooled. Sensitivity analysis was conducted by removing studies using another antiviral drug as comparison. The pooled results were considered statistically significant if the p-value was below 0.05. A multivariate time series meta-analysis using the method by Musekiwa et al with the metafor package (Musekiwa et al., 2016) was performed on viral clearance rate at multiple timepoints; all described models were tried and 1 best-fitting model with the least Akaike Information Criterion (AIC) was visualized using Microsoft Excel. If 10 or more studies were found for any outcomes, we would use Egger regression test to assess for publication bias and visualize with Begg funnel plot. A p-value less than 0.10 would be considered significant, and the Duvall and Tweedie method of trim and fill would be performed.
Results
Search results and study baseline characteristics
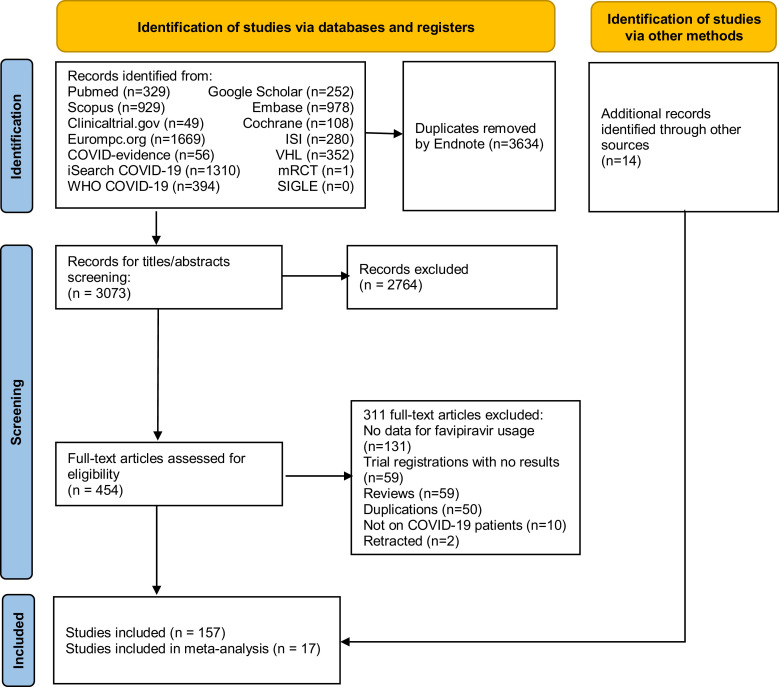
On February 8, 2021, database search identified 6707 papers. A manual search on March 2, 2022 for RCTs found an additional 14 papers. Using Endnote, 3637 duplications were removed. Later, 2764 records were removed after title and abstract screening. After full-text assessment, a total of 157 publications (24 RCTs, 1 non-RCT, 21 observational studies, 2 case series, and 106 case reports) entered the extraction and analysis stage. The detailed progress is presented in the PRISMA flow diagram in Figure 1 .
Figure 1.
PRISMA flow diagram of the literature search and screening for eligibility steps.
Meta-analysis for the effectiveness of favipiravir
We performed meta-analysis for 16 RCTs (Balykova et al., 2020a; Balykova et al., 2020b; Balykova et al., 2020c; Bosaeed et al., 2021; Chen et al., 2020a; Chuah et al., 2021; Doi et al., 2020; Finberg et al., 2021; Ivashchenko et al., 2021; Pushkar, 2020; Ruzhentsova et al., 2021; Shenoy et al., 2021; Shinkai et al., 2021; Solaymani-Dodaran et al., 2021; Tabarsi et al., 2021; Udwadia et al., 2021) and 1 non-RCTs (Cai et al., 2020), which studied the effect of favipiravir on hospitalized patients. The baseline characteristics of the controlled trials can be found in Supplemental Table 3.
Viral clearance
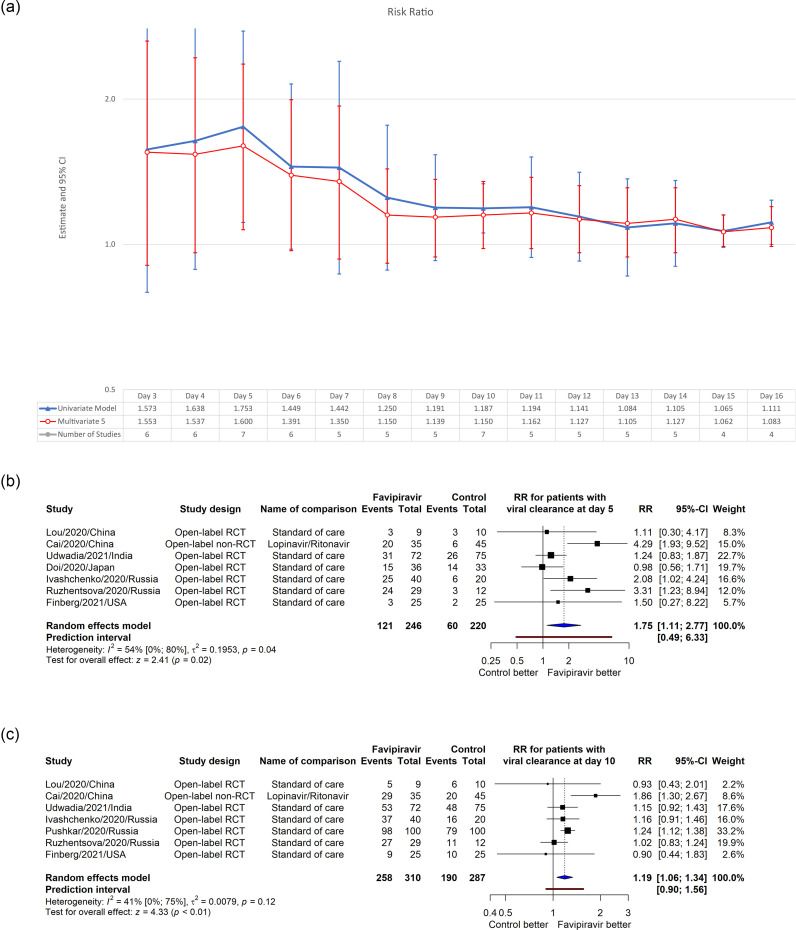
Using data from 8 studies, we plotted RR for the number of patients having the viral clearance from day 3–16 using the univariate and multivariate model by Musekiwa et al (Musekiwa et al., 2016). Multivariate model 5 had the least AIC and was used for the analysis. In multivariate model, although all RR values were greater than 1, significant better outcomes were found only at day 5 (RR = 1.60, 95% CI [1.07, 2.36], p = 0.02) (Figure 2 ). In the sensitivity analysis, multivariate model 2 fitted better (smallest AIC); however, significant results were still found on day 5 (Supplemental Figure 1). Detailed forest plots for univariate analysis at day 5 and 10 were given in the same figures; detailed numbers can be found in Supplemental Table 4.
Figure 2.
Multivariate and univariate meta-analysis for the effect of favipiravir on viral clearance rate at different timepoints (a) and 2 detailed forest plots for the univariate analysis at day 5 (b) and day 10 (c).
Despite insignificance, mean viral clearance time was shorter in patients who received favipiravir (MD = –2.62, 95% CI [–7.28, 2.03], p = 0.21, I2 = 74%) (Supplemental Figure 3a). Sensitivity analysis made heterogeneity plummet (I2 = 25%), with a smaller MD and 95% CI (MD = –1.08, 95% CI [–2.25, –0.08], p = 0.07) (Supplemental Figure 3b).
Clinical improvement, fever cessation, and chest radiological improvement
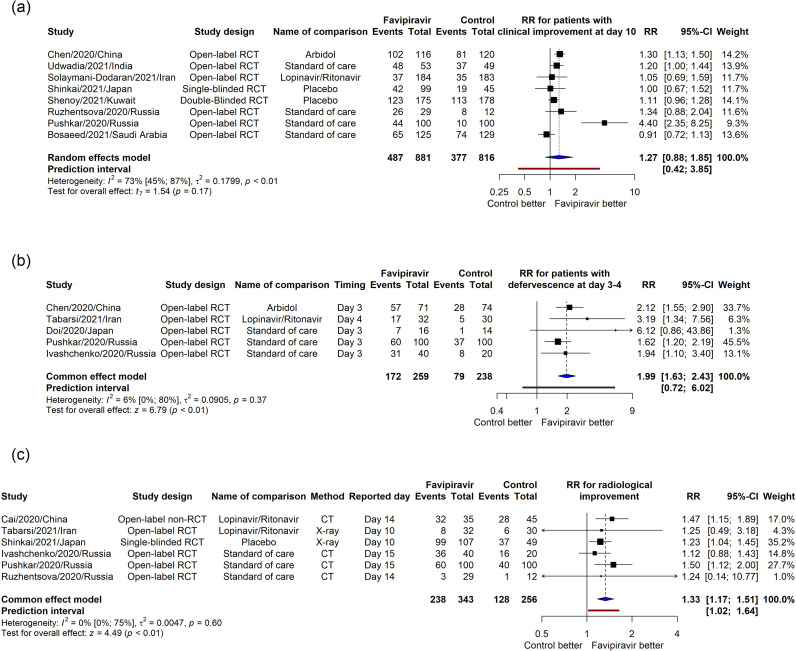
The pooled RR for clinical improvement rate from 8 RCTs was higher but not statistically significant (RR = 1.27, 95% CI [0.88, 1.85], p = 0.17), with high heterogeneity (I2 = 73%) (Figure 3 a). On the other hand, the pooled RR from 5 reports for defervescence at day 3–4 was significantly better in the favipiravir group (RR=1.99, 95% CI [1.63, 2.43], p<0.01, I2=6%) (Figure 3b). Favipiravir group also showed better chest radiological improvement rate (RR=1.33, 95% CI [1.17, 1.51], p<0.01, I2=0%) from 6 papers (Figure 3c). Sensitive analysis showed the same patterns for these 3 outcomes (Supplemental Figure 2a,b,c).
Figure 3.
Meta-analysis for the effect of favipiravir on the rate of patients having clinical improvement at day 10 (a), defervescence at day 3–4 (b) and radiological improvement (c).
The mean clinical improvement time pooled from 9 studies was shorter in favipiravir group (MD = –1.18, 95% CI [–2.34, –0.02], p = 0.05, I2 = 60%). The sensitivity analysis increased the difference and narrowed the 95% CI (MD = –1.69, 95% CI [–2.31, –1.07], p<0.01, I2 = 37%) (Supplemental Figure 3c and 3d). The pooled mean difference in defervescence time from 4 RCTs also showed a significant result (MD = –1.33, 95% CI [–1.59, –1.07], p <0.01, I2 = 4%) (Supplemental Figure 3e).
The definitions for these outcomes differed between studies and are given in Supplemental Table 5.
Discharge rates and length of hospitalization
Regarding the discharge rate, significant pooled results from 5 studies were found for favipiravir at day 10–11 (RR = 1.19, 95% CI [1.06, 1.33], p <0.01, I2 = 43%) but no difference was found at day 14–15 (RR = 1.00, 95% CI [0.93, 1.09], p = 0.92, I2 = 0%). Furthermore, the difference was insignificant in length of hospitalization (MD = –0.19, 95% CI [–0.67, 0.29], p = 0.44, I2 = 0%) as well as in the sensitivity analysis (Supplemental Figure 4a–d).
Mortality
No differences were found for mortality when pooling 12 studies with RR = 1.19, 95% CI (0.85, 1.66), p = 0.32, I2 = 0%. Furthermore, sensitivity analysis did not change the outcome (Supplemental Figure 5a and b).
Qualitative synthesis for the effectiveness of favipiravir
A summary of baseline characteristics and outcomes from observational studies and case series is given in Supplemental Table 6.
Time of favipiravir administration
Administrating favipiravir early (at the time of diagnosis) and late (few days later) were compared in some studies. Although an RCT showed insignificant differences (Doi et al., 2020), a case-control study showed better outcomes on viral negativity (p <0.001) and disease progression (p <0.05) (Uçan et al., 2021), and another retrospective cohort showed a lower mortality rate in early favipiravir treatment group (p = 0.002) (Karatas et al., 2021).
Dosage of favipiravir
A higher favipiravir dosage was suggested to be more effective in 2 investigations. A retrospective study reported that a low loading dose of favipiravir (≤45 mg/kg/day) was a poor predictive factor for clinical improvement on day 7 (p = 0.006) (Rattanaumpawan et al., 2020). In a noncontrolled study, most of the 49 blood samples from 13 patients, who received 1600 mg twice on the first day, followed by 600 mg twice from day 2, had favipiravir concentration less than the lower limit of quantification (1 μg/mL) and lower than the in vitro half-maximal effective concentration (Irie et al., 2020). In an RCT on mild to moderate patients, ivermectin plus doxycycline show better results than the favipiravir group even though they were not significant.
Favipiravir versus other antiviral drugs
For nonhospitalized patients, favipiravir did not affect the outcomes in 2 double-blind RCTs (Holubar et al., 2021; Lowe et al., 2022). Regarding hospitalized patients, a non-RCT reported favipiravir to be superior to lopinavir/ritonavir regarding viral clearance and chest imaging (p <0.01) (Cai et al., 2020). Favipiravir could also shorten the time of having pyrexia and cough compared with umifenovir in an RCT (p <0.01) (Chen et al., 2020b). In a cross-sectional survey, favipiravir group had a considerably decreased death rate in comparison to remdesivir (p <0.01) (Ara Perveen et al., 2021). No significant data was found comparing favipiravir to baloxavir marboxil (Lou et al., 2021). In combination with tocilizumab, favipiravir showed better lung lesion remissions compared with favipiravir alone in an RCT (p <0.05) (Zhao et al., 2021b). In patients with recurrent positive, the favipiravir group achieved negative PCR in a significantly shorter period than SOC (p = 0.038) (Zhao et al., 2021a).
Adverse events
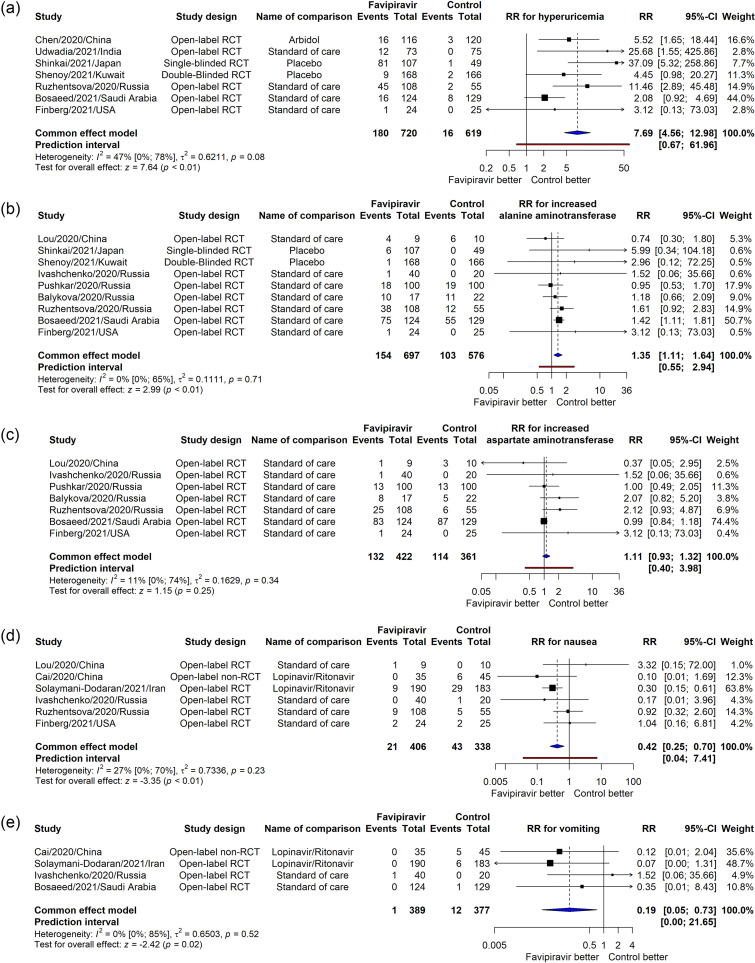
By pooling 14 RCTs and 1 non-RCT, 17 adverse events ranging in severity were meta-analyzed. Because no significant heterogeneity was found, the fixed-effect model was applied to all adverse events (Supplemental Figure 6). The adverse events that had higher risks after favipiravir usage compared to the control groups were hyperuricemia (RR = 7.69, 95% CI [4.56, 12.98], p <0.01, I2 = 47%) (Figure 4 a) and increased alanine aminotransferase (RR = 1.35, 95% CI [1.11, 1.64], p <0.01, I2 = 0%) (Figure 4b). Regarding hyperuricemia, Balykova et al and Ruzhentsova et al detected increased levels of uric acid after 15 and 5 days of the initiation of favipiravir therapy, respectively; whereas the latter reported that most patients’ uric acid levels normalized at day 28 (Balykova et al., 2020c; Ruzhentsova et al., 2020). Additionally, a case wherein acute gouty arthritis was triggered by the hyperuricemia effect of favipiravir was described (Hase et al., 2020). Finally, when combined with tocilizumab, a significant increase in uric acid level was detected compared with favipiravir or tocilizumab alone (Zhao et al., 2021b).
Figure 4.
Meta-analysis for the effect of favipiravir on the risk of hyperuricemia (a), increased alanine aminotransferase (b), increased aspartate aminotransferase (c), nausea (d), and vomiting (e).
There were no significant differences in the RR for increased aspartate aminotransferase (RR = 1.11, 95% CI [0.93, 1.32], p = 0.25, I2 = 11%) (Figure 4c). On the other hand, favipiravir reduced the risk for nausea (RR = 0.42, 95% CI [0.25, 0.70], p <0.01, I2 = 27%) (Figure 4d) and vomiting (RR = 0.19, 95% CI [0.05, 0.73], p = 0.02, I2 = 0%) (Figure 4e).
Various observational studies have addressed the safety profiles of favipiravir. In comparison to pretreatment values, although the drug seemed not to affect the QT interval on electrocardiogram (Çap et al., 2020), it was reported to be associated with increased liver enzymes (Yılmaz et al., 2021), increased platelet and lymphocyte count, and decreased neutrophil and red blood cell count (Yaylaci et al., 2020). Significant retinol depletions were also reported (Sarohan et al., 2021). A summary table for these outcomes is presented in Supplemental Table 7.
Case reports
A total of 106 studies with 142 cases were found (Supplemental Table 8). The mean age was 52.70 ± 17.13 years (range: 1–85) with a male-female ratio of 101 : 41. After favipiravir administration, the clinical status improved in 74 cases, worsened in 48 cases (with 21 deaths), and was unclear in 20 cases. The reported side effects were hyperuricemia (Hase et al., 2020, Hosoba et al., 2020, Ono et al., 2020, Takoi et al., 2020), fever (Koba et al., 2020; Kurita et al., 2020; Takoi et al., 2020), lymphocytosis (Dauby et al., 2021), eosinophilia (Takoi et al., 2020), lymphocytopenia (Atallah et al., 2020), hepatotoxicity (Hosoba et al., 2020; Takumida et al., 2021; Yamazaki et al., 2021), nephrotoxicity (Nasa et al., 2021), acute pancreatitis (Khan et al., 2021), wood's lamp fluorescence on nails and hair, and nausea (Aslan Kayiran et al., 2021).
Quality assessment and grading the evidence
Regarding the overall risk of bias judgment, all RCTs were of some concern except 3 papers that had low risk of bias due to proper blinding. A single non-RCT study had moderate overall risk of bias. Among 21 observation studies, 7 reports had a good rating, and the other 14 had a fair rating. A total of 2 case series were given a fair rating and all 53 case reports had a high overall risk of bias. The detailed results of the quality assessment were provided in Supplementary Tables S9–S13.
Using GRADE, there was a high certainty that patients who received favipiravir had faster recovery from fever and higher rate of hyperuricemia; moderate certainties for better virological, clinical, and radiological responses; and lower nausea rate (Table 1 ). No differences in mortality rate were found with a moderate certainty.
Table 1.
Summary of important findings and certainty of evidence using GRADE.
| Outcomes | Relative effect (95% CI) | Number of studies (Total patients) | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|
| Patients received favipiravir had better virological response than SOC | RR for viral clearance at day 5: 1.60 (1.07, 2.36) | 7 (466) | Moderate* | High heterogeneities were found in the forest plot between the RCTs and 1 non-RCTs. Removing the non-RCTs in the sensitive analysis caused the I2 reduced to 0%, therefore we grade down the quality by 1. |
| Patients received favipiravir had sooner clinical improvement than SOC | MD for mean clinical improvement time: –1.18 (–2.34, –0.02) | 9 (1499) | Moderate | High heterogeneities were found for the definitions of clinical improvement between papers, which also explain a high I2 in the forest plot. Therefore, we grade down the quality by 1. |
| Patients received favipiravir recovered from fever faster than SOC | RR for defervescence at day 3–4: 1.99 (1.63, 2.43) | 5 (497) | High | NA |
| Patients received favipiravir had better radiological imaging than SOC | RR for radiological improvement: 1.33, (1.17, 1.51) | 6 (599) | Moderate* | Different definitions for radiological improvement were found between papers. Therefore, we grade down the quality by 1. |
| No differences were found in mortality between favipiravir and SOC | RR 1.19 (0.85, 1.66) | 12 (2428) | Moderate* | Data were sparse and imprecise because most studies reported a low prevalence of mortality. Therefore, we grade down the quality by 1. |
| Favipiravir increase the risk of hyperuricemia | RR 7.69 (4.56, 12.98) | 7 (1339) | High* | We grade down the quality by 1 because the data were sparse and imprecise due to low prevalence of the event. However, the RR was high therefore the evidence was upgrade by 1. |
One non-RCTs entered these meta-analyses, however, no differences were found in the baseline characteristics in this study, removing the paper from the meta-analysis also did not significantly alter the results, therefore we still decided to still start the GRADE with high.
Discussion
In hospitalized patients, favipiravir showed a superior trend compared to SOC. In our multivariate time series meta-analysis analysis, the differences in viral clearance rate peaked on day 5 then decreased in the follow-up days. This could be explained by the fact that most of the patients in the included trials recovered at the end of the studies and therefore shrunk the differences between the 2 groups as time passed. It was consistent with the fact that there was a significant difference in the number of patients being discharged on days 10–11 but no difference on dasy 14–15 and no difference in the pooled RR for mortality at the end of the trials. Overall, it seemed that favipiravir could induce a better virological response in patients with COVID-19 as well as reduce the need for medical care, but more evidence would be needed to confirm this.
Chest radiological imaging, which had slower recovery rate (Rong et al., 2021), still showed fibrotic-like changes on more than 1/3 of severe patients with COVID-19 after 6 months (Han et al., 2021), and therefore, in our analysis, this outcome was found to be significantly better after 10 days on chest x-ray and 14–15 days on computerized tomography imaging. This suggested a favorable outcome in the long term for favipiravir groups. Accordingly, future RCTs for moderate to severe patients should consider it as a main outcome and also monitor long-term differences on imaging as it is a potential indicator for the effectiveness.
Regarding clinical improvement, the pooled RR showed that clinical improvement rate at day 10 was higher in favipiravir groups (despite being insignificant with p-value of 0.17). The mean clinical improvement time was significantly shorter in the favipiravir group in both the main and the sensitive analysis. Significant differences that favored favipiravir were also found in defervescence rate at day 3–4 and mean defervescence time in both the main and sensitive analysis. Taking everything into account, favipiravir could induce a better clinical improvement; however, more data would be needed for a concrete conclusion.
The similarity in the mortality rates between those taking favipiravir and not taking the drug can lead to the assumption that favipiravir can decrease the time until clinical, laboratory, or radiologic recovery but not an overall reduction in the morbidity or mortality. However, it is important to highlight that most of the included studies reported near-zero mortality rate and therefore, it was still too soon to make this conclusion.
In nearly all the included RCTs, favipiravir was used early (at day 1 of diagnosis). The benefit of early usage of favipiravir was reported in some observational studies (Karatas et al., 2021; Uçan et al., 2021). The viral clearance was also reported to be directly related to the early initiation of antiviral therapy and before the peak of viremia, leading some practitioners to start antiviral medications in the presymptomatic phase of COVID-19 (Goyal et al., 2020). Accordingly, even though no strong evidence was found, early usage of favipiravir could provide better outcomes for patients with COVID-19.
Regarding nonhospitalized mild patients, 2 double-blind RCTs showed that favipiravir had no effect compared to SOC on (Holubar et al., 2021; Lowe et al., 2022). This suggests that favipiravir should not be used on mild cases with low risk and should focus on moderate and severe cases.
A wide variety of adverse events have also been linked to favipiravir intake. Our meta-analysis strongly concluded that favipiravir usage significantly increases the risk of hyperuricemia compared with control, which is consistent with other reports (Hase et al., 2020; Koseki et al., 2022). Increased levels of liver enzymes has been associated with COVID-19 (Kaneko et al., 2020) as well as many antiviral drugs (Vitiello et al., 2021), including favipiravir. Our analysis detected a significant increase in alanine aminotransferase but not aspartate aminotransferase. However, these were not the main outcomes of the RCTs, and the effect sizes were small. The pooled RR were greatly contributed by 1 report owing to its high number of events. Moreover, on studies that focus on increase liver enzymes, no significant differences were found (Bayram et al., 2021). Therefore, more studies should be conducted before concluding the effect of favipiravir on liver enzymes. Nausea and vomiting turned out to be lower in favipiravir group. However, the pooled RR were mainly contributed by 2 reports comparing favipiravir versus lopinavir/ritonavir, which was known for nausea and vomiting adverse events (Hurst and Lopinavir, 2000). Therefore, this conclusion should not be concrete. Having potential teratogenic effects in some animal studies, favipiravir is contraindicated during pregnancy and lactation as well as in men seeking conception (Agrawal et al., 2020). High attention should be paid in these situations.
A total of 2 dosage regimens with a dissimilar duration (varying from 2–14 days) were found in most of the included studies. They all started with a loading dose of 1600 or 1800 mg doses twice a day on the first day and continued by 600 or 800 mg twice daily. However, 2 included observational studies suggested that higher doses than these could improve the efficacy of favipiravir (Irie et al., 2020; Uçan et al., 2021). In Ebola virus disease, the doses were amplified to 6000 mg loading dose and 2400 mg maintenance dose daily; however, it was not associated with a major enhancement in the outcomes but might yield higher rates of side effects (Nguyen et al., 2017). Overall, more trials are needed to investigate how much can we increase the dose to maximize the efficacy without causing extra harm to our patients.
Remdesivir, an antiviral drug that also targets the RdRp complex (Aleem and Remdesivir, 2021), received approval for use from the Japanese government in severe COVID-19 cases on May 7, 2020 (Lamb, 2020). It was also the first FDA-approved treatment on October 22, 2020 (Rubin et al., 2020). Previous studies found better results for remdesivir on clinical improvement and discharge rate compared to SOC (Enoki et al., 2021; Jiang et al., 2021; Piscoya et al., 2020; Yokoyama et al., 2020). Despite being marginal, similar conclusions were also made regarding favipiravir. Moreover, favipiravir also proved to be better than SOC regarding viral clearance, defervescence, and chest radiological improvement, which were lacking in evidence for remdesivir. Although some low level of evidence suggested that favipiravir is better than remdesivir (Ara Perveen et al., 2021), more RCTs comparing the 2 drugs would be required before making any conclusions.
Earlier systematic reviews and meta-analyses detected better viral clearance and clinical improvement in the favipiravir group compared to SOC, which is consistent with our conclusions (Hassanipour et al., 2021; Manabe et al., 2021; Prakash et al., 2020; Shrestha et al., 2020). Compared to these studies, our search was conducted later which resulted in more reports included in the meta-analysis. We all applied a similar approach: pooling all trials that compared favipiravir versus SOC or any antiviral drugs used in these SOC. However, because we had a wider selection, we also performed a sensitivity analysis by removing any trials comparing favipiravir versus single antiviral drugs. Additionally, we provided meta-analyses for defervescence, chest radiological improvement, and hospital discharge outcomes. Our analyses for adverse events were also more thorough and revealed the differences in the risk of hyperuricemia, vomiting, and nausea. Meta-analyses should be performed in the near future for higher quality insights about favipiravir as the number of RCTs keeps increasing.
Limitations
Owing to the small number of the published studies, some outcomes were borderline as well as the data that compared favipiravir versus other drugs was limited. Subgroup meta-analyses were not possible for different severities, different doses, and nonhospitalized patients. All reports entered in the meta-analysis were open-label except for 1 single-blind RCT and 1 double-blind RCT; therefore, future RCTs with appropriate blinding are needed. In our meta-analysis, because of ethical issues, the control groups in most RCTs were the national SOC, which differed a lot from 1 trial to another (Supplemental Table 3); however, favipiravir was found to be more effective regardless of the treatment plan. Another issue with the included reports was that the definition of chest radiological imaging and clinical improvement statuses differed according to the protocol that each country followed. These caused higher heterogeneity and increased the 95% CI in some analyses. The fact that even RCTs comparing favipiravir versus other antiviral drugs were pooled in the main meta-analyses could make us underestimate the effect size.
Conclusions
In conclusion, adding favipiravir to the SOC provides faster viral clearance and clinical improvement and better radiological imaging improvement for hospitalized patients. Nonhospitalized patients might not receive the benefit from favipiravir. Hyperuricemia should be noted when using favipiravir for prompt cessation of drug intake. In contrast, elevated hepatic enzymes might not be a side effect for using favipiravir in COVID-19, considering other published studies. Further cautions should be applied when administrating favipiravir to pregnant or lactating women owing to its probable teratogenic effects. Larger RCTs with proper blinding are required to detect the best dosage and administration time of favipiravir as well as to fortify the borderline results.
Contributions
NTH, KH, KK, and TI accounted for the idea. AMM, JMAA, and DTH searched the databases. All members contributed to the eligibility screening, extraction, and manual search. DTH, AMM, AF, GMT, and AHA performed the analysis and data visualization. All authors wrote the manuscript and designed the tables. NTH, KH, KK, and TI supervised the study.
Ethical approval
This study is exempt from ethical approval.
Acknowledgments
We would like to thank Timothy Waive (School of Medicine, The University of Buckingham, Buckingham, UK) for early joining and contributing to the study. This study was funded by the Japan Agency for Medical Research and Development (AMED) under Grant Asian clinical trial network construction project (Number JP20LK0201001J0001). The funders had no role in the preparation of the manuscript.
Footnotes
Conflict of interest: We declare no competing interests.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.04.035.
Appendix. Supplementary materials
References
- Agrawal U, Raju R, Udwadia ZF. Favipiravir: a new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020;76:370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem A, Remdesivir KJ. StatPearls [internet]; 2021. StatPearls (updated 2021 Jan 10).
- Ara Perveen R, Nasir M, Murshed M MM, Naznin R, Ahmed SN. Remdesivir and favipiravir changes hepato-renal profile in COVID-19 patients: a cross sectional observation in. Bangladesh. ijmsci. 2021;8:5196–5201. [Google Scholar]
- Aslan Kayıran M, Cebeci F, Erdemir VA, Aksoy H, Akdeniz N, Gürel MS. Fluorescence of nails and hair on Wood's lamp examination in Covid Pandemic; undefined effect of favipiravir in humans. Dermatol Ther. 2021;34:e14740. doi: 10.1111/dth.14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah B, Hamour I, Mallah SI, Bonilla MF, Bader F. Traveling for heart transplantation and returning with COVID-19: a logistical, clinical, and pharmacotherapeutic challenge from the Middle East. Drugs Ther Perspect. 2020:1–6. doi: 10.1007/s40267-020-00792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ (Clin Res Ed) 2004;328:1490–. [DOI] [PMC free article] [PubMed]
- Balykova LA, Govorov AV, Vasilyev AO, Simakina EN, Agafyina AS, Ivanova AY, et al. Characteristics of covid-19 and possibilities of early causal therapy. Results of favipiravir use in clinical practice. Infekc bolezni. 2020;18:30–40. [Google Scholar]
- Balykova LA, Granovskaya MV, Zaslavskaya KY, Simakina EN, Agaf'ina AS, Ivanova AY, et al. New possibilities for targeted antiviral therapy for COVID-19. Results of a multi center clinical study of the efficacy and safety of using the drug Areplivir. Infect Dis News Opin Train. 2020;9:16–29. [Google Scholar]
- Balykova LA, Pavelkina VF, Shmyreva NV, Pyataev NA, Selezneva NM, Shepeleva OI, et al. Efficacy and safety of some etiotropic therapeutic schemes for treating patients with novel coronavirus infection (COVID-19) Pharm Amp Pharmacol-Farm Farmakol. 2020;8:150–159. [Google Scholar]
- Bayram M, Yildirim O, Ozmen RS, Soylu B, Dundar AS, Koksal AR, et al. Elevation of serum transaminase levels due to favipiravir use in the treatment of COVID-19. Cureus. 2021;13:e18166. doi: 10.7759/cureus.18166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosaeed M, Mahmoud E, Alharbi A, Altayib H, Albayat H, Alharbi F, et al. Favipiravir and hydroxychloroquine combination therapy in patients with moderate to severe COVID-19 (FACCT trial): an open-label, multicenter, randomized, controlled trial. Infect Dis Ther. 2021;10:2291–2307. doi: 10.1007/s40121-021-00496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020;6:1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çap M, Bilge Ö, Işık F, Burak C, Karagöz A, İnci Ü, et al. The effect of favipiravir on QTc interval in patients hospitalized with coronavirus disease 2019. J Electrocardiol. 2020;63:115–119. doi: 10.1016/j.jelectrocard.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv 2020a .03.17.20037432.
- Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical. Trial. 2020 [Google Scholar]
- Chuah CH, Chow TS, Hor CP, Cheng JT, Ker HB, Lee HG, et al. Efficacy of early treatment with favipiravir on disease progression among high risk COVID-19 patients: a randomized, open-label clinical trial. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab962. [DOI] [PubMed] [Google Scholar]
- Dauby N, Van Praet S, Vanhomwegen C, Veliziotis I, Konopnicki D, Roman A. Tolerability of favipiravir therapy in critically ill patients with COVID-19: A report of four cases. J Med Virol. 2021;93:689–691. doi: 10.1002/jmv.26488. [DOI] [PubMed] [Google Scholar]
- Deeks JJ HJ. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2021. Analysing data and undertaking meta-analyses. DG A, editor. version 6.2 Cochrane, editor. [Google Scholar]
- Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, Hirose M, et al. A Prospective, Randomized, Open-Label Trial of Early versus Late favipiravir Therapy in Hospitalized Patients with COVID-19. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki Y, Igarashi Y, Watabe Y, Honma K, Suzuki Y, Hayashi Y, et al. Remdesivir for the treatment of coronavirus COVID-19: a meta-analysis of randomised controlled trials. J Glob Antimicrob Resist. 2021;24:81–82. doi: 10.1016/j.jgar.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg RW, Ashraf M, Julg B, Ayoade F, Marathe JG, Issa NC, et al. US201 study: a Phase 2, randomized proof-of-concept trial of favipiravir for the treatment of COVID-19. Open Forum Infect Dis. 2021;8:ofab563. doi: 10.1093/ofid/ofab563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control COVID-19. The COVID-19 reader. 2020:36–39. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Cardozo-Ojeda EF, Schiffer JT. Potency and timing of antiviral therapy as determinants of duration of SARS-CoV-2 shedding and intensity of inflammatory response. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj J, Piorkowski G, Jacquot F, Madelain V, Nguyen THT, Rodallec A, et al. Antiviral efficacy of favipiravir against Ebola virus: a translational study in cynomolgus macaques. PLOS Med. 2018;15 doi: 10.1371/journal.pmed.1002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase R, Kurata R, Ishida K, Kurita T, Muranaka E, Mito H. Acute gouty arthritis during favipiravir treatment for coronavirus Disease 2019. Intern Med. 2020;59:2327–2329. doi: 10.2169/internalmedicine.5377-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. sci rep. 2021;11(1):11022. doi: 10.1038/s41598-021-90551-6. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holubar M, Subramanian A, Purington N, Hedlin H, Bunning B, Walter KS, et al. Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial. Clin Infect Dis. 2021 doi: 10.1093/cid/ciac312. 11.22.21266690. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoba R, Makita S, Shiotsuka M, Kobayashi O, Nakano K, Muroya M, et al. COVID-19 pneumonia in a patient with adult T-cell leukemia-lymphoma. J Clin Exp Hematop. 2020;60:174–178. doi: 10.3960/jslrt.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst M, Lopinavir Faulds D. Drugs. 2000;60:1371–1379. doi: 10.2165/00003495-200060060-00009. discussion 80–1. [DOI] [PubMed] [Google Scholar]
- Irie K, Nakagawa A, Fujita H, Tamura R, Eto M, Ikesue H, et al. Pharmacokinetics of favipiravir in critically ill patients with COVID-19. Clin Transl Sci. 2020;13:880–885. doi: 10.1111/cts.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN, et al. AVIFAVIR for treatment of patients with moderate coronavirus Disease 2019 (COVID-19): interim results of a Phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2021;73:531–534. doi: 10.1093/cid/ciaa1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins. Coronavirus resource center; 2020, https://coronavirus.jhu.edu/map.html; (accessed April 10 2022).
- Jiang Y, Chen D, Cai D, Yi Y, Jiang S. Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: a network meta-analysis. J Med Virol. 2021;93:1171–1174. doi: 10.1002/jmv.26443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Kurosaki M, Nagata K, Taki R, Ueda K, Hanada S, et al. Liver injury with COVID-19 based on gastrointestinal symptoms and pneumonia severity. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatas E, Aksoy L, Kilic PE, Dogru A, Ozaslan E. Early onset favipiravir saves lives; 2021.
- Khan AEA, Hoque MM, Mallik MU, Basu KC, Hasan MM, Mahmud I, et al. Favipiravir induced acute pancreatitis in a COVID-19 patient. J Medicine. 2021;22:69–71. [Google Scholar]
- Koba H, Yoneda T, Kaneda T, Ueda T, Kimura H, Kasahara K. Severe coronavirus disease 2019 (COVID-19) pneumonia patients treated successfully with a combination of lopinavir/ritonavir plus favipiravir: case series. Clin Case Rep. 2020 doi: 10.1002/ccr3.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocayiğit H, Özmen Süner K, Tomak Y, Demir G, Yaylacı S, Dheir H, et al. Observational study of the effects of favipiravir vs lopinavir/ritonavir on clinical outcomes in critically Ill patients with COVID-19. J Clin Pharm Ther. 2021;46:454–459. doi: 10.1111/jcpt.13305. [DOI] [PubMed] [Google Scholar]
- Koseki T, Nakajima K, Iwasaki H, Yamada S, Takahashi K, Doi Y, et al. Baseline uric acid levels and steady-state favipiravir concentrations are associated with occurrence of hyperuricemia among COVID-19 patients. Int J Infect Dis. 2022;115:218–223. doi: 10.1016/j.ijid.2021.12.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Ishida K, Muranaka E, Sasazawa H, Mito H, Yano Y, et al. A favipiravir-induced fever in a patient with COVID-19. Intern Med. 2020;59:2951–2953. doi: 10.2169/internalmedicine.5394-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb YN. Remdesivir: first approval. Drugs. 2020;80:1355–1363. doi: 10.1007/s40265-020-01378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Liu L, Yao H, Hu X, Su J, Xu K, et al. Clinical outcomes and plasma concentrations of Baloxavir Marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci. 2021;157 doi: 10.1016/j.ejps.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DM, Brown L-AK, Chowdhury K, Davey S, Yee P, Ikeji F, et al. Favipiravir, lopinavir-ritonavir or combination therapy (FLARE): a randomised, double blind, 2×2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. medRxiv 2022:2022.02.11.22270775. [DOI] [PMC free article] [PubMed]
- Manabe T, Kambayashi D, Akatsu H, Kudo K. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:489. doi: 10.1186/s12879-021-06164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musekiwa A, Manda SO, Mwambi HG, Chen DG. Meta-analysis of effect sizes reported at multiple time points using general linear mixed model. PLOS One. 2016;11 doi: 10.1371/journal.pone.0164898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasa P, Shrivastava P, Kulkarni A, Vijayan L, Singh A. Favipiravir induced nephrotoxicity in two patients of COVID-19. J Assoc Phys India. 2021;69:88. [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute . and Blood Institute; 2021. Quality assessment tool for observational cohort and cross-sectional studies. available https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Google Scholar]
- Nguyen TH, Guedj J, Anglaret X, Laouénan C, Madelain V, Taburet AM, et al. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLOS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Kishimoto M, Shimasaki T, Uchida H, Kurai D, Deshpande GA, et al. Reactive arthritis after COVID-19 infection. RMD Open. 2020;6 doi: 10.1136/rmdopen-2020-001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscoya A, Ng-Sueng LF, Parra Del Riego A, Cerna-Viacava R, Pasupuleti V, Roman YM, et al. Efficacy and harms of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0243705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Singh H, Kaur H, Semwal A, Sarma P, Bhattacharyya A, et al. Systematic review and meta-analysis of effectiveness and safety of favipiravir in the management of novel coronavirus (COVID-19) patients. Indian J Pharmacol. 2020;52:414–421. doi: 10.4103/ijp.ijp_998_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkar D. Study of favipiravir compared to standard of care in hospitalized patients with COVID-19. ClinicalTrials.gov: United States National Library of Medicine; 2020.
- Rattanaumpawan P, Jirajariyavej S, Lerdlamyong K, Palavutitotai N, Saiyarin J. Real-world experience with favipiravir for treatment of COVID-19. In: Thailand: results from a multi-center observational study. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- Rong Y, Wang F, Tian J, Liang X, Wang J, Li X, et al. Clinical and CT features of mild-to-moderate COVID-19 cases after two sequential negative nucleic acid testing results: a retrospective analysis. BMC Infect Dis. 2021;21:333. doi: 10.1186/s12879-021-06013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D, Chan-Tack K, Farley J, Sherwat A. FDA approval of Remdesivir - a step in the right direction. N Engl J Med. 2020;383:2598–2600. doi: 10.1056/NEJMp2032369. [DOI] [PubMed] [Google Scholar]
- Ruzhentsova T, Chukhliaev P, Khavkina D, Garbuzov A, Oseshnyuk R, Soluyanova T, et al. Phase 3 trial of Coronavir (favipiravir) in patients with mild to moderate COVID-19. SSRN Electron J. 2020 [PMC free article] [PubMed] [Google Scholar]
- Ruzhentsova TA, Oseshnyuk RA, Soluyanova TN, Dmitrikova EP, Mustafaev DM, Pokrovskiy KA, et al. Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19. Am J Transl Res. 2021;13:12575–12587. [PMC free article] [PubMed] [Google Scholar]
- Sarohan AR, Akelma H, Arac E, Aslan O. Retinol depletion in severe COVID-19; 2021. [DOI] [PMC free article] [PubMed]
- Shannon A, Selisko B, Le NT, Huchting J, Touret F, Piorkowski G, et al. Rapid incorporation of favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat Commun. 2020;11:4682. doi: 10.1038/s41467-020-18463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S, Munjal S, Al Youha S, Alghounaim M, Almazeedi S, Alshamali Y, et al. Favipiravir in adults with moderate to severe COVID-19: a Phase 3 multicentre, randomized, double-blinded. placebo-controlled Trial. 2021 [Google Scholar]
- Shinkai M, Tsushima K, Tanaka S, Hagiwara E, Tarumoto N, Kawada I, et al. Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, Phase III clinical trial. Infect Dis Ther. 2021;10:2489–2509. doi: 10.1007/s40121-021-00517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209 doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha DB, Budhathoki P, Khadka S, Shah PB, Pokharel N, Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J. 2020;17:141. doi: 10.1186/s12985-020-01412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaymani-Dodaran M, Ghanei M, Bagheri M, Qazvini A, Vahedi E, Hassan Saadat S, et al. Safety and efficacy of favipiravir in moderate to severe SARS-CoV-2 pneumonia. Int Immunopharmacol. 2021;95 doi: 10.1016/j.intimp.2021.107522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. Robins-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydney: Cancer Council Australia. Clinical guidelines network Cancer Council Australia. Development of clinical practice Guidelines Using Cancer Council Australia's Cancer Guidelines Wiki. Handbook for section authors and the guideline working party; 2014.
- Tabarsi P, Vahidi H, Saffaei A, Hashemian SMR, Jammati H, Daraei B, et al. Favipiravir effects on the control of clinical symptoms of hospitalized COVID-19 cases: an experience with Iranian formulated dosage form. Iran J Pharm Res. 2021;20:1–8. doi: 10.22037/ijpr.2021.115510.15401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takoi H, Togashi Y, Fujimori D, Kaizuka H, Otsuki S, Wada T, et al. Favipiravir-induced fever in coronavirus disease 2019: a report of two cases. Int J Infect Dis. 2020;101:188–190. doi: 10.1016/j.ijid.2020.09.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumida H, Izumi S, Sakamoto K, Hashimoto M, Ishii S, Suzuki M, et al. Sustained coronavirus disease 2019-related organizing pneumonia successfully treated with corticosteroid. Respir Investig. 2021;59:377–381. doi: 10.1016/j.resinv.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik GM, Dila KAS, Mohamed MYF, Tam DNH, Kien ND, Ahmed AM, et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health. 2022;47:46. doi: 10.1186/s41182-019-0165-6. 1348–8945 (Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uçan A, Çerçi P, Efe S, Akgün H, Özmen A, Yağmuroğlu A, et al. Benefits of treatment with favipiravir in hospitalized patients for COVID-19: a retrospective observational case-control study. Virol J. 2021;18:102. doi: 10.1186/s12985-021-01577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udwadia ZF, Singh P, Barkate H, Patil S, Rangwala S, Pendse A, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71. doi: 10.1016/j.ijid.2020.11.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A, La Porta R, D'Aiuto V, Ferrara F. The risks of liver injury in COVID-19 patients and pharmacological management to reduce or prevent the damage induced. Egypt Liver J. 2021;11:11. doi: 10.1186/s43066-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Director-General's opening remarks at the mission briefing on COVID-19; 2020, https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020; (accessed August 4th 2021).
- Yamazaki S, Suzuki T, Sayama M, Nakada TA, Igari H, Ishii I. Suspected cholestatic liver injury induced by favipiravir in a patient with COVID-19. J Infect Chemother. 2021;27:390–392. doi: 10.1016/j.jiac.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaylaci S, Dheir H, Şenocak D, Genc AB, Kocayigit H, Çekiç D, et al. The effects of favipiravir on hematological parameters of covid-19 patients. Rev Assoc Med Bras. 2020;(2):65–70. doi: 10.1590/1806-9282.66.S2.65. (1992)[Suppl.]66 Suppl:65–70. [DOI] [PubMed] [Google Scholar]
- Yılmaz H, Güner AE, Altuntaş M. Results of favipiravir combined treatment in intensive care patients with Covid-19. BMB. 2021;6:339–345. [Google Scholar]
- Yokoyama Y, Briasoulis A, Takagi H, Kuno T. Effect of remdesivir on patients with COVID-19: A network meta-analysis of randomized control trials. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhang C, Zhu Q, Chen X, Chen G, Sun W, et al. Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: A multicenter, open-label, randomized trial. Int Immunopharmacol. 2021;97 doi: 10.1016/j.intimp.2021.107702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhu Q, Zhang C, Li J, Wei M, Qin Y, et al. Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size. Biomed Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Chen CZ, Gorshkov K, Xu M, Lo DC, Zheng W. RNA-dependent RNA polymerase as a target for COVID-19 drug discovery. SLAS Discov. 2020;25:1141–1151. doi: 10.1177/2472555220942123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.