Abstract
Purpose
To describe evolution and severity of radiographic findings and assess association with disease severity and outcomes in critically ill COVID-19 patients.
Materials and Methods
This retrospective study included 62 COVID-19 patients admitted to the intensive care unit (ICU). Clinical data was obtained from electronic medical records. A total of 270 chest radiographs were reviewed and qualitatively scored (CXR score) using a severity scale of 0-30. Radiographic findings were correlated with clinical severity and outcome.
Results
The CXR score increases from a median initial score of 10 at hospital presentation to the median peak CXR score of 18 within a median time of 4 days after hospitalization, and then slowly decreases to a median last CXR score of 15 in a median time of 12 days after hospitalization. The initial and peak CXR score was independently associated with invasive MV after adjusting for age, gender, body mass index, smoking, and comorbidities (Initial, odds ratio [OR]: 2.11 per 5-point increase, confidence interval [CI] 1.35-3.32, P= 0.001; Peak, OR: 2.50 per 5-point increase, CI 1.48-4.22, P= 0.001). Peak CXR scores were also independently associated with vasopressor usage (OR: 2.28 per 5-point increase, CI 1.30-3.98, P= 0.004). Peak CXR scores strongly correlated with the duration of invasive MV (Rho = 0.62, P< 0.001), while the initial CXR score (Rho = 0.26) and the peak CXR score (Rho = 0.27) correlated weakly with the sequential organ failure assessment score. No statistically significant associations were found between radiographic findings and mortality.
Conclusions
Evolution of radiographic features indicates rapid disease progression and correlate with requirement for invasive MV or vasopressors but not mortality, which suggests potential nonpulmonary pathways to death in COVID-19.
Introduction
The COVID-19 pandemic has changed daily life like no other disease in the past 100 years, yet it has similarities with several viral pneumonias that have caused diseases of epidemic proportions.1 In COVID-19, cases are typically detected by reverse transcription-polymerase chain reaction using nasopharyngeal swabs.2 However, in individuals with pneumonia or severe acute respiratory illness related to COVID-19, imaging can aid in detection, management, and prognostication.3 , 4 Chest CT has several advantages over radiography, but the use of CT is hampered by difficult scanning logistics, including the need to sanitize equipment between patients, the potential for infection of healthcare workers, the lack of portability, and a relatively high radiation dose.5 At the same time, chest radiography has advantages in all of these areas, although its sensitivity is lower than that of CT, and it is more difficult to achieve uniform quality compared to CT.6 , 7 In addition to case finding (as opposed to screening), radiographs are a convenient means of assessing the evolution of disease.7 While these roles seem intuitive, there are few if any studies that provide insight on the radiographic evolution of disease severity over the course of COVID-19 related pneumonia.6, 7, 8, 9 Approximately 5% of the COVID-19 patients become critically ill with a high mortality and mobility and require intensive care treatment.10 Conventional chest radiographs are important and recommended for monitoring the lung disease progression in critically ill patients.10 However, there are only few studies reporting the radiographic trajectory and its association with clinical severity or outcomes in critically ill COVID-19 patients.11
The purpose of this retrospective study was to evaluate longitudinal evolution of radiographic patterns and radiographic severity in individuals admitted to the intensive care unit (ICU) during their hospital stay for COVID-19 related lung disease. Secondary purposes are to identify association of the chest radiographic patterns and severity scores with clinical severity including the need for invasive mechanical ventilation (MV) or vasopressors, duration of invasive MV, sequential organ failure assessment (SOFA) scores,12 and mortality.
Materials and Methods
Patients and Study Design
We studied a cohort of patients who were confirmed to have COVID-19 infection and admitted to ICUs in 2 hospitals (Harborview Medical Center, UW Medical Center–Montlake) in Seattle, Washington between March 5 and April 14 2020. Inclusion criteria were as follows: (1) Patients at 18 years of age or older; (2) Patients presented with symptoms consistent with COVID-19 and were confirmed by reverse transcription-polymerase chain reaction assay. (3) Patients were admitted to an ICU during their hospitalization. Patients without chest radiographs were excluded.
The UW institutional review board approved this study and written informed consent form from the patients was waived.
Clinical Features
Clinical data from the electronic medical record were obtained through chart abstraction using a research form in Research Electronic Data Capture software (REDCap, Vanderbilt University, Nashville, TN). Clinical data elements included demographic data, comorbidities, survival, uses of invasive MV, or vasopressors, the duration of invasive MV and SOFA score.12
Longitudinal Radiographic Patterns and Severity Score Assessment
Chest radiographs were reviewed by two experienced cardiothoracic radiologists (S.P., more than 20 years of experience; W.W., 6 years of experience) on GE Centricity PACS (GE Healthcare, Waukesha, WI). The radiologists were blinded to all clinical outcomes. All chest radiographs obtained during the course of hospitalization for each patient were assessed. Radiographic patterns (Fig 1 ) and severity of pulmonary abnormalities were recorded for hospital presentation (first) chest radiographs and ICU admission radiographs (immediately before or after ICU admission).
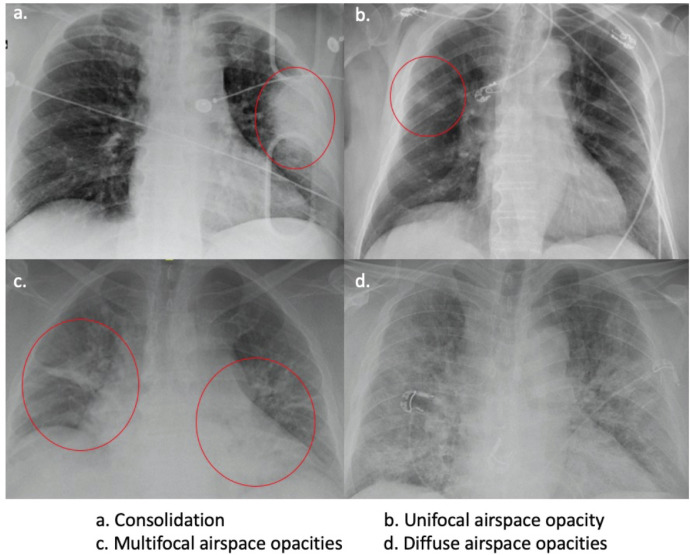
FIG 1.
Examples of CR predominant patterns.
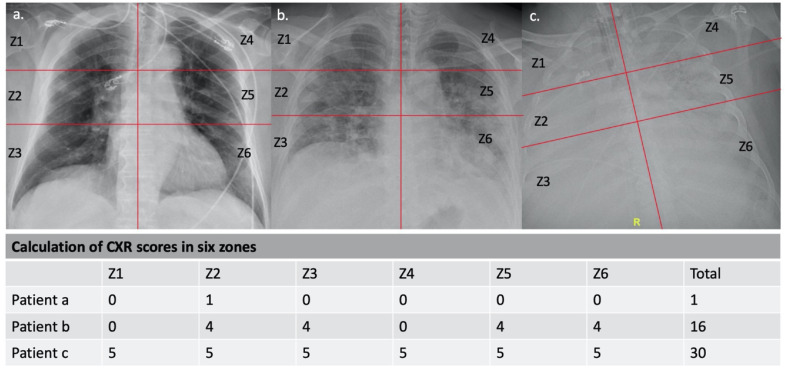
All chest radiographs obtained during the course of hospitalization were qualitatively scored for severity of disease. A modified chest radiograph (CXR) severity score was used within six zones: right and left lungs, each with upper, mid and lower lung zones that were visually divided evenly on the basis of right lung capacity. Each zone was scored for severity, based on the radiographic assessment of lung edema (RALE) score,13 and was assigned a score ranging from 0 to 5 depending on the degree and extent of opacification (0, none; 1, minimal; 2, mild; 3, moderate; 4, extensive; 5, complete). Scores from all of the zones were combined to yield a composite score that ranged from a minimum score of 0 to a maximum score of 30 (Fig 2 ).
FIG 2.
Examples of CR scoring. (a) The initial presentation chest radiograph showed unifocal airspace opacity in right middle zone with the total CR score of 1 in a 90-year-old female patient with COVID-19. (b) The chest radiograph on hospital day 9 showed multifocal airspace opacities with a total CR score of 16 in a 53-year-old female patient with COVID-19. (c) The chest radiograph shows a complete opacification from diffuse airspace disease with a total CR score of 30 in a 59-year-old male patient with COVID-19.
For each patient, initial CXR score at hospital presentation and peak CXR score during hospitalization were further selected for analyzing the correlation with clinical severity including the use of invasive MV and vasopressors, the duration of invasive MV and SOFA score. One radiologist (S.P.) reviewed the CXRs for all patients and the interpretations were used for the primary analysis. Another radiologist (W.W.) reviewed CXRs from 31 randomly selected patients to assess inter-rater agreement.
Statistical Analysis
Continuous data were presented as median (range) and categorical data were presented as counts (percentages). Clinical demographics or chest radiographic features between two groups were compared using Fisher's exact test for categorical variables or Wilcoxon rank-sum test for continuous variables. McNemar's or Bowker's test was used to compare the radiographic features at hospital presentation with those at ICU admission. Multivariate logistic regression was used to evaluate whether the CXR score was independently associated with disease severity including mechanical ventilation and requirement for vasopressors after adjusting for age, gender, body mass index (BMI), smoking status, and the presence of any comorbidities. Spearman's rank correlation coefficient was used to examine the relationships between the CXR scores and clinical or laboratory parameters. Kappa statistics and interclass correlation coefficients (ICC) were calculated to assess inter-rater agreement. Data analysis was performed using the PASW Statistics23.0 TM analysis package (SPSS Inc., Chicago, IL) and R (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
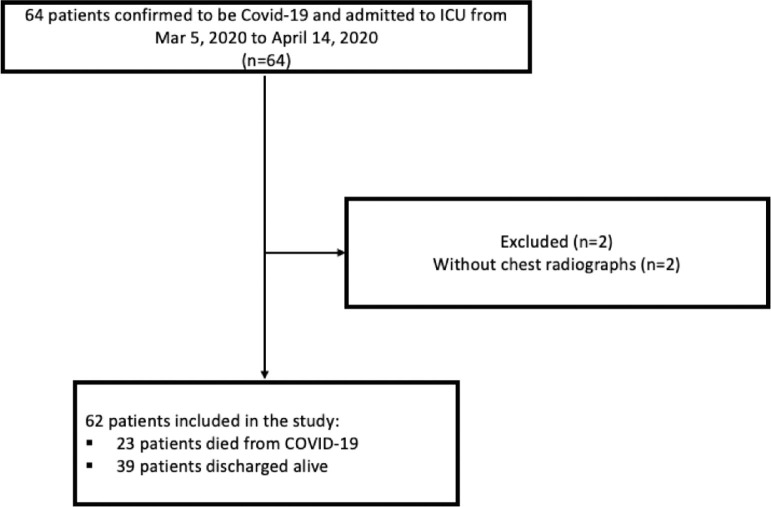
Sixty-four eligible adults were identified from two hospitals in the University of Washington (UW) Medicine system. Among 64 eligible patients, 2 subjects without chest radiographs were excluded and 62 hospitalized patients with ICU admission were included in our study (Fig 3 ). The clinical characteristics of some of these patients have been previously reported.14 Demographic features and comorbidities are shown in Table 1 . The median age was 59 years (range, 23-90), and 71% were men. The majority of patients were overweight or obese (BMI >25, 89%). Smoking status was not available in one quarter of patients (27%), but among those whose smoking status was known, 69% of the patients were never smokers while 31% were current or ever smokers. More than two-thirds (68%) had one or more comorbidities. Diabetes mellitus (40%) and chronic kidney disease (21%) were the most common comorbidities. Thirty-nine (63%) patients were discharged from hospital, and 23 (37%) patients died. Comorbidities were more often seen in the deceased subgroup than in the survivor subgroup (91.3% vs 53.8%, P = 0.002). Thirty-nine patients (63%) received invasive MV, and 35 patients (57%) received vasopressor therapy. There were more male patients in the group that received invasive MV (82.1% vs 52.2%, P = 0.02) and those requiring vasopressors (82.9% vs 53.8%, P = 0.02). The SOFA score was higher in those who died than survived (median: 9 vs 5, P = 0.003) as well as in those who needed invasive MV (median: 9 vs. 2, P ≤ 0.001) or vasopressors (median: 9 vs 2, P ≤ 0.001) than those who did not.
FIG 3.
Patients enrollment in the study.
TABLE 1.
Demographics by outcome and interventions
| Demographics | Total (N = 62) | Outcome |
Requirement of invasive mechanical ventilation (MV) |
Requirement of vasopressors^ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survived (N = 39) | Deceased (N = 23) | P-value | No invasive MV (N = 23) | Invasive MV (N = 39) | P-value | No Vasopressors (N = 26) | Vasopressors (N = 35) | P-value | ||
| Age | 59 (23-90) | 56 (29-90) | 65 (23-79) | 0.16 | 56 (34-90) | 60 (23-88) | 0.35 | 54 (29-90) | 65 (23-88) | 0.02 |
| Gender | ||||||||||
| Female | 18 (29.0 %) | 13 (33.3%) | 5 (21.7%) | 0.40 | 11 (47.8%) | 7 (17.9%) | 0.02 | 12 (46.2%) | 6 (17.1%) | 0.02 |
| Male | 44 (71.0 %) | 26 (66.7%) | 18 (78.3%) | 12 (52.2%) | 32 (82.1%) | 14 (53.8%) | 29 (82.9%) | |||
| BMI | 0.29 | 0.23 | 0.87 | |||||||
| <25 | 7 (11.3%) | 3 (7.7%) | 4 (17.4%) | 4 (17.4%) | 3 (7.7%) | 2 (7.7%) | 5 (14.3%) | |||
| 25-30 | 23 (37.1%) | 17 (43.6%) | 6 (26.1%) | 10 (43.5%) | 13 (33.3%) | 10 (38.5%) | 12 (34.3%) | |||
| >30 | 32 (51.6%) | 19 (48.7%) | 13 (56.5%) | 9 (39.1%) | 23 (59.0%) | 14 (53.8%) | 18 (51.4%) | |||
| Smoking status* | 0.74 | 0.33 | 0.53 | |||||||
| Current/former smoker | 14 (31.1%) | 8 (28.6%) | 6 (35.3%) | 7 (41.2%) | 7 (25.0%) | 5 (25.0%) | 9 (36.0%) | |||
| Never smoker | 31 (68.9%) | 20 (71.4%) | 11 (64.7%) | 10 (58.8%) | 21 (75.0%) | 15 (75.0%) | 16 (64.0%) | |||
| Known comorbidities | 0.002 | 0.78 | 0.16 | |||||||
| None | 20 (32.3%) | 18 (46.2%) | 2 (8.7%) | 8 (34.8%) | 12 (30.8%) | 11 (42.3%) | 8 (22.9%) | |||
| Present | 42 (67.7%) | 21 (53.8%) | 21 (91.3%) | 15 (65.2%) | 27 (69.2%) | 15 (57.7%) | 27 (77.1%) | |||
| SOFA score | 6 (0-16) | 5 (0-15) | 9 (1-16) | 0.003 | 2 (0-12) | 9 (1-16) | <0.001 | 2 (0-11) | 9 (1-16) | <0.001 |
Note: Continuous data are presented as median (range); binary and categorical data are presented as count (percentage). Comparisons between groups were performed using Fisher's exact test (categorical variables) or the Wilcoxon rank-sum test (continuous variables).
Bolded numbers indicate P < 0.05.
There were 45 patients with known smoking status while data was missing for 17 patients. ^ one missing data in the vasopressors usage, total N = 61.
Longitudinal Radiographic Patterns and Severity Score Assessment
In 28 (45%) patients, the CXRs at the time of initial presentation to the hospital were same as the CXRs at ICU admission. For the remaining 34 patients, the median time interval between the hospital presentation radiograph and ICU admission radiograph was 3.0 (range, 0.2-27) days. The predominant radiographic patterns at ICU admission were statistically different from those at initial presentation to the hospital, with more diffuse airspace disease (50% vs 36%) and less unifocal airspace disease (5% vs 19%) (P = 0.006) on the ICU admission radiographs. At hospital presentation, of those with unifocal disease, the majority (69%) were in the lower lung zones. Among those with multifocal or diffuse lung disease, 27% (13 of 49) had lower lung predominance and 73% (36 of 49) had diffuse distribution. At ICU admission, among those with multifocal or diffuse lung disease on CXRs, 24% (14 of 58) had lower lung predominance and 74% (43 of 58) had diffuse distribution. The details of the radiographic features are summarized in Table 2 .
TABLE 2.
Comparison of radiological features between hospital presentation CXR and ICU admission CXR
| Radiological findings | Hospital presentation | ICU admission | P-value* |
|---|---|---|---|
| All patients (N = 62) | All patients (N = 62) | ||
| Predominant pattern | 0.006 | ||
| Consolidation | 1 (1.6%) | 1 (1.6%) | |
| Airspace (unifocal) | 12 (19.4%) | 3 (4.8%) | |
| Diffuse airspace | 22 (35.5%) | 31 (50%) | |
| Multifocal (patchy) airspace | 26 (41.9%) | 26 (41.9%) | |
| Others | 1 (1.6%) | 1 (1.6%) | |
| Consolidation presence | 32 (51.6%) | 36 (58.1%) | 0.29 |
| Distribution (Unifocal/Multifocal) | 0.004 | ||
| Unifocal | 13 (21.0%) | 4 (6.5%) | |
| RUZ | 1 (1.6%) | 0 | |
| RMZ | 1 (1.6%) | 0 | |
| RLZ | 7 (11.3%) | 3 (4.8%) | |
| LUZ | 0 | 0 | |
| LMZ | 2 (3.2%) | 1 (1.6%) | |
| LLZ | 2 (3.2%) | 0 | |
| Multifocal | 49 (79.0%) | 58 (93.5%) | |
| Upper lung | 0 | 0 | |
| Mid lung | 0 | 1 (1.6%) | |
| Lower lung | 13 (21.0%) | 14 (22.6%) | |
| Diffuse | 36 (58.1%) | 43 (69.4%) | |
Bolded numbers indicate P < 0.05.
Comparisons between groups were performed using McNemar test or Bowker test.
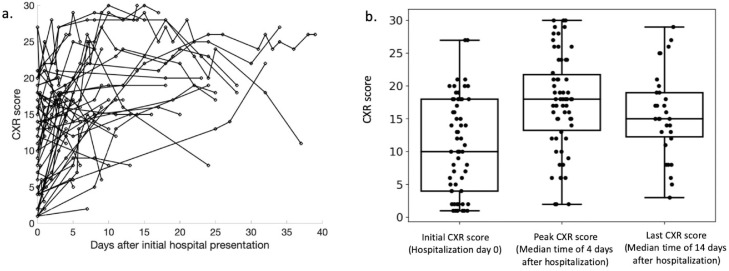
In total, 270 chest radiographs were reviewed and qualitatively scored. The median number of CXR exams performed per patient was 4 (range 1-16). Spaghetti plot showing evolution of CXR scores for each patient among hospitalization are provided (Fig 4 a). The median initial score on hospital presentation was 10 (range 1-27) while median peak score during the hospital stay was 18 (range, 2-30). The median time to reach the peak CXR score was 4 days (range 0-32). For those patients (N = 29) with follow up CXRs after their peak score, the median score of the last CXR during hospitalization was 15 (range 3-29) while the median time of the last CXR exam was at day 12 during hospitalization (range 0.23-47). Boxplots showing CXR scores at different time points are presented in Figure 4b.
FIG 4.
Spaghetti plot and boxplots demonstrating the evolution of radiographic severity during hospitalization. (a) Spaghetti plot presented shows evolution of CXR scores for each patient. (b) Boxplots summarized the median and IQR, of CXR scores at initial hospital presentation, peak CXR score during hospitalization, and CXR score of the last CXR exam for patients with follow-up CXRs after peak score (N = 29).
In total, 134 chest radiographs from 31 patients were reviewed independently by 2 radiologists. The inter-rater agreement for predominant patterns on initial hospital presentation CXRs (n = 31) and ICU admission CXRs (n = 31) was very good (weighted kappa 0.83, 95% confidence interval [CI]: 0.72-0.95). The concordance between the readers for CXR severity score was excellent (ICC = 0.94, 95% CI: 0.91-0.96).
Association of Chest Radiographic Findings and CXR Scores With Clinical Severity and Outcome
The association of radiographic findings with invasive MV and the need for vasopressors are presented in Tables 3 and 4 , respectively. Comparing patients who received invasive MV during hospitalization and those who did not, the radiographic patterns at initial hospital presentation were statistically different (P = 0.024) with less unifocal opacities (7.7% vs 39.1%), more multifocal airspace opacities (46.2% vs 34.8%) and diffuse lung disease (41.0% vs 26.1%) among patients who received invasive MV. The radiographic patterns at ICU admission also showed more diffuse lung disease (59.0% vs 33.3%) in the patients receiving invasive MV during hospitalization.
TABLE 3.
Radiographic imaging findings stratified by invasive mechanical ventilation
| Radiological findings | Hospital presentation |
ICU admission |
||||||
|---|---|---|---|---|---|---|---|---|
| Total(N = 62) | No invasive MV (N = 23) | Invasive MV (N = 39) | P-value* | Total(N = 62) | No invasive MV (N = 23) | Invasive MV(N = 39) | P-value* | |
| Predominant pattern | 0.024 | 0.044 | ||||||
| Consolidation (unifocal) | 1 (1.6%) | 0 | 1 (2.6%) | 1 (1.6%) | 0 | 1 (2.6%) | ||
| Airspace (unifocal) | 12 (19.4%) | 9 (39.1%) | 3 (7.7%) | 3 (4.8%) | 3 (13.0%) | 0 | ||
| Diffuse airspace | 22 (35.5%) | 6 (26.1%) | 16 (41.0%) | 31 (50.0%) | 8 (34.8%) | 23 (59.0%) | ||
| Multifocal (patchy) airspace | 26 (41.9%) | 8 (34.8%) | 18 (46.2%) | 26 (41.9%) | 12 (52.2%) | 14 (35.9%) | ||
| Others | 1 (1.6%) | 0 | 1 (2.6%) | 1 (1.6%) | 0 | 1 (2.6%) | ||
| Consolidation presence | 32 (51.6%) | 8 (34.8%) | 24 (61.5%) | 0.065 | 36 (58.1%) | 11 (47.8 %) | 25 (64.1%) | 0.288 |
| Distribution | 0.01 | 0.14 | ||||||
| Unifocal | 13 (21.0%) | 9 (39.1%) | 4 (10.3%) | 4 (6.5%) | 3 (13.0%) | 1 (2.6%) | ||
| Multifocal | 49 (79.0%) | 14 (60.9%) | 35 (89.7%) | 58 (93.5%) | 20 (87.0%) | 38 (97.4%) | ||
| Initial CXR score at presentation† | 10.0(1.0, 27.0) | 5.0(1.0, 19.0) | 14.0(1.0, 27.0) | 0.001 | ||||
| Peak CXR score during hospitalizationa | 18.0(2.0, 30.0) | 14.0(2.0, 22.0) | 19.0(6.0, 30.0) | <0.001 | ||||
Note: Continuous data are presented as median (range); binary and categorical data are presented as count (percentage);
Comparisons between groups were performed using Fisher's exact test (categorical variables) or the Wilcoxon rank-sum test (continuous variables). Bolded numbers indicate P < 0.05.
N = 61, CXR score was not applicable in one patient with pre-existed diffuse lung fibrosis.
TABLE 4.
Radiographic imaging findings stratified by vasopressor therapy
| Radiological findings | Hospital presentation |
ICU admission |
||||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 61) | No Vasopressors (N = 26) | Vasopressors (N = 35) | P-value* | Total (N = 61) | No Vasopressors (N = 26) | Vasopressors (N = 35) | P-value* | |
| Predominant pattern | 0.112 | 0.015 | ||||||
| Consolidation (unifocal) | 1 (1.6%) | 0 | 1 (2.9%) | 1 (1.6%) | 0 | 1 (2.9%) | ||
| Airspace (unifocal) | 11 (18%) | 8 (30.8%) | 3 (8.6%) | 3 (4.9%) | 3 (11.5%) | 0 | ||
| Diffuse airspace | 22 (36.1%) | 7 (26.9%) | 15 (42.9%) | 31 (50.8%) | 9 (34.6%) | 22 (62.9%) | ||
| Multifocal (patchy) airspace | 26 (42.6%) | 11 (42.3%) | 15 (42.9%) | 25 (41.0%) | 14 (53.8%) | 11 (31.4%) | ||
| Others | 1 (1.6%) | 0 | 1 (2.9%) | 1 (1.6%) | 0 | 1 (2.9%) | ||
| Consolidation presence | 32 (52.5%) | 13 (50%) | 19 (54.3%) | 0.799 | 35 (57.4%) | 14 (53.8%) | 21 (60.0%) | 0.79 |
| Distribution | 0.102 | 0.303 | ||||||
| Unifocal | 12 (19.7%) | 8 (30.8%) | 4 (11.4%) | 4 (6.6%) | 3 (11.5%) | 1 (2.9%) | ||
| Multifocal | 49 (80.3%) | 18 (69.2%) | 31 (88.6%) | 57 (93.4%) | 23 (88.5%) | 34 (97.1%) | ||
| Initial CXR score at presentation† | 10.0(1.0, 27.0) | 10.0(1.0, 19.0) | 13.5(1.0, 27.0) | 0.054 | ||||
| Peak CXR score during hospitalizationa | 18.0(2.0, 30.0) | 15.5(2.0, 26.0) | 20(6.0, 30.0) | 0.006 | ||||
Note: Continuous data was presented as median (range); binary and categorical data are presented as count (percentage).
Comparisons between groups were performed using Fisher's exact test (categorical variables) or the Wilcoxon rank-sum test (continuous variables). Bolded numbers indicate P < 0.05.
Total N = 60, CXR score was not applicable in one patient with pre-existed diffuse lung fibrosis.
The assessment of CXR scores revealed statistically significant differences in the scores at initial presentation and the peak score during the course of hospitalization when comparing those who did and did not receive invasive MV. The initial presentation CXR scores and the peak CXR scores in those who did not receive invasive MV were significantly lower than those who did (median: 5.0 vs 14.0, P = 0.001, and 14.0 vs 19.0, P < 0.001). After adjusting for age, gender, BMI, smoking status, and the presence of any comorbidities, the logistic regression analysis showed that the CXR score at initial presentation (odds ratio [OR]: 2.11 per 5-point increase, CI 1.35-3.32, P = 0.001) and the peak CXR score (OR: 2.50 per 5-point increase, CI 1.48-4.22, P = 0.001) were both independently associated with invasive MV. In addition, we found that the peak CXR score correlated strongly (positively) with the number of days on invasive MV (Rho = 0.62, P < 0.001).
Between patients with vasopressor usage and those without during hospitalization, the radiographic patterns at ICU admission were statistically different as well (P = 0.015) with less unifocal opacities (0% vs 11.5%), less multifocal airspace opacities (31.4% vs 53.8%) and more diffuse lung disease (62.9% vs 34.6%) in the patients requiring vasopressors. The peak CXR score was also higher in those requiring vasopressor therapy than those not requiring such therapy (median: 15.5 vs 20, P < 0.006). The peak CXR score remained significantly associated with the requirement for vasopressors, even after adjusting for age, gender, BMI, smoking status, and the presence of comorbidities (OR: 2.28 per 5-point increase, CI 1.30-3.98, P = 0.004). In addition, the SOFA score correlates weakly with the initial CXR score (Rho = 0.26, P = 0.044) and the peak CXR score during the hospital stay (Rho = 0.27, P = 0.033).
The association of CXR findings with survival is presented in Table 5 . There were no significant differences in the radiographic features, predominant pattern, and distribution of abnormalities between the cohort who survived and those who died. Both the median CXR score at the time of presentation and peak CXR score were similar in survivors compared to non-survivors (10.0 vs 11.0 and 18.0 vs 17.0).
Table 5.
Radiographic imaging findings stratified by death
| Radiological findings | Hospital presentation |
ICU admission |
||||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 62) | Survived (N = 39) | Deceased (N = 23) | P-value* | Total (N = 62) | Survived (N = 39) | Deceased (N = 23) | P-value* | |
| Predominant pattern | 0.473 | 0.4 | ||||||
| Consolidation (unifocal) | 1(1.6%) | 0 | 1 (4.3%) | 1 (1.6%) | 0 | 1 (4.3%) | ||
| Airspace (unifocal) | 12 (19.4%) | 9 (23.1%) | 3 (13.0%) | 3 (4.8%) | 3 (7.7%) | 0 | ||
| Diffuse airspace | 22 (35.5%) | 12 (30.8%) | 10 (43.5%) | 31 (50.0%) | 18 (46.2%) | 13 (56.5%) | ||
| Multifocal (patchy) airspace | 26 (41.9%) | 17 (43.6%) | 9 (39.1%) | 26 (41.9%) | 17 (43.6%) | 9 (39.1%) | ||
| Others | 1 (1.6%) | 1 (2.6%) | 0 | 1 (1.6%) | 1 (2.6%) | 0 | ||
| Consolidation presence | 32 (51.6%) | 20 (51.3%) | 12 (52.2%) | 1 | 36 (58.1%) | 24 (61.5%) | 12 (52.2%) | 0.596 |
| Distribution | 0.751 | 1 | ||||||
| Unifocal | 13 (21.0%) | 9 (23.1%) | 4 (17.4%) | 4 (6.5%) | 3 (7.7%) | 1 (4.3%) | ||
| Multifocal | 49 (79.0%) | 30 (76.9%) | 19 (82.6%) | 58 (93.5%) | 36 (92.3%) | 22 (95.7%) | ||
| Initial CR score at presentation† | 10.0(1.0, 27.0) | 10.0 (1.0, 21.0) | 11.0 (1.0, 27.0) | 0.934 | ||||
| Peak CR score during hospitalization† | 18.0 (2.0, 30.0) | 18.0 (2.0, 30.0) | 17.0 (6.0, 30.0) | 0.816 | ||||
Note: Continuous data are presented as median (range); binary and categorical data are presented as count (percentage).
Comparisons between groups were performed using Fisher's exact test (categorical variables) or the Wilcoxon rank-sum test (continuous variables).
Total N = 61, CXR score was not applicable in one patient with pre-existed diffuse lung fibrosis.
Discussion
In summary, we have evaluated the progression of disease on CXRs from hospital presentation to ICU admission, assessed the evolution of chest radiograph severity scores throughout hospitalization as well as the correlation with clinical severity and outcome for critically ill COVID-19 patients. Our results indicate that diffuse lung disease was more common on radiographs at ICU admission than at hospital presentation and seen more frequently in patients with invasive MV and vasopressors. The CXR score increases from a median initial score of 10 at hospital presentation to the median peak CXR score of 18 within a median time of 4 days after hospitalization, and then slowly decreases to a median last CXR score of 15 in a median time of 12 days after hospitalization. The CXR scores correlate with requirement for invasive MV or vasopressors, invasive MV duration and SOFA score but not the mortality.
In our study, 61% of patients received invasive MV during their hospital course indicating an overall high proportion with severe disease, when comparing to the need of invasive MV in 13% of the hospitalized COVID-19 patients in a large cohort of 10,021 patients.15 There was a high percentage (68%) of individuals with comorbidities in our study when compared to the presence of comorbidities in 24% of critical COVID-19 patients in a recently published study on critically ill COVID-19 patients.16 As reported in the literature, there was a male preponderance, two thirds of our cohort was men.17 , 18 In addition, male patients required mechanical ventilation or vasopressors more often than female patients. About 9 in 10 patients in our study were overweight, similar to other large case series17 , 18 which showed that obesity was strongly correlated with disease severity.
We studied all CXRs obtained during the hospital course of 62 patients and investigated the evolution of imaging patterns on CXRs between initial hospital presentation and ICU admission. Multifocal lung disease and diffuse lung disease were the most common patterns on CXRs of individuals hospitalized with COVID-19. The current study possibly had more diffuse distribution compared to lower lung distribution reported elsewhere6 because of the higher clinical severity of the disease. Approximately 45% of patients in this study were admitted directly to the ICU when they presented to the hospital. There was more diffuse airspace disease (50% vs 36%) and less unifocal airspace disease (5% vs 19%; P= 0.006) on the ICU admission radiographs compared to initial hospital presentation radiographs in our study. A unifocal presentation progressed to diffuse airspace disease in 6% of the patients in a median time of 3 days, indicating a rapid evolution of pulmonary damage.
Qualitative CT severity scores during hospitalization and their ability to predict clinical outcomes in COVID-19 have been reported.19 , 20 For CXRs, the RALE score system was commonly used to predict clinical outcomes in ARDS patients.13 , 19 , 20 The RALE score and modified RALE score were also reported to evaluate the disease severity of COVID-19 or to predict clinical outcomes in COVID-19 patients.6 , 21 , 22 In our study, we assessed modified CR severity scores based on RALE scores for all radiographs throughout hospitalization and presented the evolution of the scores for each patient as spaghetti plot (Fig 4a). In order to summarize the key information, we then presented the scores at three most important time points during hospitalization in the boxplot to facilitate understanding of the evolution of disease (Fig 4b). A recent study on time course of lung changes at chest CT23 reported disease severity peak to be between 10 and 13 days from the time of onset of the symptoms. In our study, the median time to reach the peak CXR severity score on the radiographs was 4 days after hospitalization (14 days after onset of symptoms), which is 1-4 days later than the previous CT study.24 Difference in selection criteria between our study and the previously reported study23 may explain the difference in time to peak disease severity, as they excluded patients with severe disease in their study while we only included critically ill patients. Nevertheless, the median time of 4 days after hospitalization to reach the peak score on the radiographs indicates rapid progression of the disease and can inform providers about the timing of severe lung damage.
While it may seem intuitive that higher severity of lung disease would predict the need for ventilation, to assume this would be to simplify what often tends to be a complex situation with several important variables that determine management in the ICU. The variables that we focus on including invasive MV, vasopressor usage, and SOFA score are important parameters to assess disease severity and predict clinical outcomes. The need for mechanical ventilation has been associated with death in COVID-19.24 We found that the predominant radiographic patterns at both initial hospital presentation and ICU admission were associated with the need for invasive MV. The CXR score at initial presentation and the peak CXR score were both independently associated with invasive MV after adjusting for age, gender, BMI, smoking status, and the presence of any comorbidities (OR: 2.11 per 5-point increase, CI 1.35-3.32, P= 0.001; OR: 2.50 per 5-point increase, CI 1.48-4.22, P= 0.001, respectively), a finding similar to that reported by Toussie et al.21 The peak CXR score also correlated strongly (positively) with the number of days on invasive MV (Rho = 0.62, P< 0.001). Usage of vasopressors indicates severity of disease and has been shown to be a predictor of poor outcomes.25 26 In our study, the peak CXR score during the hospital stay was independently associated with vasopressor requirement and was accordingly higher in those requiring vasopressors. The SOFA score based on the severity of organ dysfunction in respiratory, cardiovascular, renal, hepatic, coagulation and neurological systems, is useful in assessing and tracking the acute morbidity for critically ill patients. SOFA score was reported to be associated with increased odds of in-hospital death in COVID-19.27 Consistently, in our study, the SOFA score was higher in the deceased subgroup when compared to the survived subgroup (median: 9 vs 5, P= 0.003). Our study also showed that peak and initial radiographic CXR scores weakly correlated with SOFA score, indicating some association between CXR scores (or respiratory disease severity) and the acute morbidity in these critically ill patients. The lung is the organ most vulnerable to and affected by SARS-COV-2 infection, however, the CXR scores in this study and other previously reported parameters like CT severity scores and oxygenation index to measure hypoxemic respiratory failure can only partially reflect the extent of organ dysfunction in COVID-19 or overall disease severity.28
Several studies have shown radiological features like CT or CXR scores can predict mortality in COVID-1929,.30 Similar to a study by Smet et al, which did not find an association between CT score and mortality in older adults31, our study did not show an association of CXR patterns or CXR severity scores with mortality, although they were associated with disease severity including the need for mechanical ventilation or vasopressors. Similar to Smet et al’s study, the small sample size may have limited the ability to detect an association between CXR scores and mortality. Another possible explanation of lack of correlation with mortality might be the potential selection bias of all subjects requiring an ICU admission.
Respiratory failure is considered a major cause of death in COVID-19 pneumonia. However, in modern ICUs many patients with respiratory failure are supported by invasive mechanical ventilation allowing time for the lungs to recover. In a study on COVID-19 ICU ARDS patients, the authors also found that CT lung involvement scores are not sufficient to predict mortality in critically ill COVID-19 patients, while SOFA score seems to assess fatal disease course more accurately and comprehensively.28 In many cases, there may be additional non-pulmonary contributors to death in COVID-19 patients, including shock, multiorgan dysfunction, or acute kidney injury.32 In our study, this may be another reason that we found no significant association between CXR severity and survival. The pathways leading to death in COVID-19 are likely multifactorial and complex, with factors such as age and comorbidities playing an important role.30 In our study, 67.7% of patients had comorbidities, including diabetes and chronic kidney disease, and there were statistically more patients with comorbidities among non-survivors than survivors (91.3% vs 53.8%, P= 0.002). Although invasive MV supports adequate gas exchange without damage to lungs in patients with acute respiratory failure, the complications from invasive MV can also reduce survivorship. There were significantly more patients receiving invasive MV in non-survivors when compared to survivors in our study (82.6% vs 51.3%, P= 0.016), which indicates the association between invasive MV and mortality. The higher frequency of invasive MV in non-survivors reflected more severe lung disease in this group, on the other hand, the complications from invasive MV can also increase patients’ risk for mortality. In our study, shock was associated with intubation in 11 patients. Two patients who died from respiratory failure were also diagnosed with possible ventilator-associated pneumonia, indicating the roles of ventilator-associated pneumonia in the respiratory failure of these patients. We further analyzed the cause of death among non-survivors in our study and found that 10 of 23 non-survivors had non-pulmonary factors that contributed to death, including cardiac arrhythmias, shock, sepsis, cardiovascular collapse, multiorgan dysfunction, or acute renal failure. Our analysis was limited in that cause of death was determined only by chart abstraction. Nonetheless, it suggests that there may be several other pathways to death in critically ill patients with COVID-19 rather than isolated respiratory failure. This may be one reason that CXR scores were associated with disease severity in COVID-19, but not with mortality.
There were several limitations to our study: a small sample size; retrospective methodology; selection bias (only patients admitted to an ICU were included in this study); and some missing data in variables including smoking status. Though our study had limited statistical power, we were able to identify several important associations. Specifically, we found differences in radiographic patterns and severity scores in subgroups of patients with more severe clinical disease. Future prospective studies comparing those who were admitted to ICU due to other respiratory causes such as influenza pneumonia or those COVID-19 patients who were not in ICU admission are warranted to validate and complement our findings.
In conclusion, multifocal (patchy) lung disease and diffuse lung disease were the most common patterns on CXRs of individuals hospitalized with COVID-19 pneumonia. Evolution of radiographic patterns and severity scores demonstrate the rapid progression of pulmonary damage. Radiographic findings correlate with disease severity, including the requirement for mechanical ventilation and vasopressors. However, there is no association between radiographic findings and mortality, which may suggest potential non-pulmonary pathways to death in critically ill patients with COVID-19.
Footnotes
Conflicts of interest: All the authors have no conflicts of interest to disclose. No funding is related to this article.
References
- 1.Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin G D, Ryerson C J, Haramati L B, et al. The Role of Chest Imaging in patient management during the COVID-19 Pandemic: A multinational consensus statement from the Fleischner Society. Chest. 2020;158(1):106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li T, Cheng GS, Pipavath SNJ, et al. The novel coronavirus disease (COVID-19) complicated by pulmonary embolism and acute respiratory distress syndrome. J Med Virol. 2020 doi: 10.1002/jmv.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mossa-Basha M, Meltzer C C, Kim D C, et al. Radiology department preparedness for COVID-19: Radiology scientific expert review panel. Radiology. 2020;296(2) doi: 10.1148/radiol.2020200988. E106-E112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong H Y F, Lam H Y S, Fong A H T, et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology. 2020;296(2) doi: 10.1148/radiol.2020201160. E72-E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litmanovich DE, Chung M, R Kirkbride R, et al. Review of chest radiograph findings of COVID-19 pneumonia and suggested reporting language. J Thorac Imaging. 2020;35(6):354–360. doi: 10.1097/RTI.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 8.Stephanie S, Shum T, Cleveland H, et al. Determinants of chest X-ray sensitivity for COVID-19: A multi-institutional study in the United States. Radiol Cardiothorac Imaging. 2020;2(5) doi: 10.1148/ryct.2020200337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M D, Arun N T, Gidwani M, et al. Automated assessment and tracking of COVID-19 pulmonary disease severity on chest radiographs using convolutional siamese neural networks. Radiol Artif Intell. 2020;2(4) doi: 10.1148/ryai.2020200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluge S, Janssens U, Welte T, et al. German recommendations for critically ill patients with COVID19. Med Klin Intensivmed Notfmed. 2020;115(3):111–114. doi: 10.1007/s00063-020-00689-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valk CM, Zimatore C, Mazzinari G, et al. The prognostic capacity of the radiographic assessment for lung edema score in patients with COVID-19 acute respiratory distress syndrome—An international multicenter observational study. Front. Med. 2021;8:772056. doi: 10.3389/fmed.2021.772056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent J L, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 13.Warren M A, Zhao Z, Koyama T, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73(9):840–846. doi: 10.1136/thoraxjnl-2017-211280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobb N L, Sathe N A, Duan K I, et al. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann Am Thorac Soc. 2021;18(4):632–640. doi: 10.1513/AnnalsATS.202007-805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schalekamp S, Huisman M, van Dijk R A, et al. Model-based prediction of critical illness in hospitalized patients with COVID-19. Radiology. 2021;298(1):E46–E54. doi: 10.1148/radiol.2020202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson S, Hirsch J S, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palaiodimos L, Kokkinidis D G, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colombi D, Bodini F C, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296(2) doi: 10.1148/radiol.2020201433. E86-E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toussie D, Voutsinas N, Finkelstein M, et al. Clinical and chest radiography features determine patient outcomes in young and middle-aged adults with COVID-19. Radiology. 2020;297(1) doi: 10.1148/radiol.2020201754. E197-E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabio C, Antonella C, Patrizia R Q, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: A longitudinal study. Radiology. 2020;296(2) doi: 10.1148/radiol.2020200843. E55-E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma B, Sharma M, Majumder M, et al. Thrombocytopenia in septic shock patients–a prospective observational study of incidence, risk factors and correlation with clinical outcome. Anaesth Intensive Care. 2007;35(6):874–880. doi: 10.1177/0310057X0703500604. [DOI] [PubMed] [Google Scholar]
- 26.Gosling P, Czyz J, Nightingale P, et al. Microalbuminuria in the intensive care unit: Clinical correlates and association with outcomes in 431 patients. Crit Care Med. 2006;34(8):2158–2166. doi: 10.1097/01.CCM.0000228914.73550.BD. [DOI] [PubMed] [Google Scholar]
- 27.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puhr-Westerheide D, Reich J, Sabel BO, et al. Sequential organ failure assessment outperforms quantitative chest CT imaging parameters for mortality prediction in COVID-19 ARDS. Diagnostics. 2022;12(1):10. doi: 10.3390/diagnostics12010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borghesi A, Zigliani A, Golemi S, et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: A study of 302 patients from Italy. Int J Infect Dis. 2020;96:291–293. doi: 10.1016/j.ijid.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan M, Yin W, Tao Z, et al. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Smet R, Mellaerts B, Vandewinckele H, et al. Frailty and mortality in hospitalized older adults with COVID-19: Retrospective observational study. J Am Med Dir Assoc. 2020;21(7):928–932. doi: 10.1016/j.jamda.2020.06.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent J L, Taccone F S. Understanding pathways to death in patients with COVID-19. Lancet Respir Med. 2020;8(5):430–432. doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]