Abstract
Background
Aberrant activation of androgen receptor signalling following castration therapy is a common clinical observation in prostate cancer (PCa). Earlier, we demonstrated the role of MYB overexpression in androgen-depletion resistance and PCa aggressiveness. Here, we investigated MYB-androgen receptor (AR) crosstalk and its functional significance.
Methods
Interaction and co-localization of MYB and AR were examined by co-immunoprecipitation and immunofluorescence analyses, respectively. Protein levels were measured by immunoblot analysis and enzyme-linked immunosorbent assay. The role of MYB in ligand-independent AR transcriptional activity and combinatorial gene regulation was studied by promoter-reporter and chromatin immunoprecipitation assays. The functional significance of MYB in castration resistance was determined using an orthotopic mouse model.
Results
MYB and AR interact and co-localize in the PCa cells. MYB-overexpressing PCa cells retain AR in the nucleus even when cultured under androgen-deprived conditions. AR transcriptional activity is also sustained in MYB-overexpressing cells in the absence of androgens. MYB binds and promotes AR occupancy to the KLK3 promoter. MYB-overexpressing PCa cells exhibit greater tumorigenicity when implanted orthotopically and quickly regain growth following castration leading to shorter mice survival, compared to those carrying low-MYB-expressing prostate tumours.
Conclusions
Our findings reveal a novel MYB-AR crosstalk in PCa and establish its role in castration resistance.
Subject terms: Prostate cancer, Oncogenes
Background
Prostate cancer (PCa) is the most common non-cutaneous malignancy and the second leading cause of cancer-associated death in men in the United States [1]. Androgens play an important role in PCa growth and metastasis and therefore ablation of androgen synthesis, commonly referred to as androgen deprivation therapy, is mainstay of treatment for patients with advanced disease [2–5]. However, most patients show therapy failure after an initial clinical response and develop highly aggressive and lethal castration-resistant (CR) PCa (CRPC) [3]. Progression to CRPC is commonly associated with a rise in serum prostate-specific antigen (PSA) levels suggesting a restoration of AR signalling [6]. This is attributed to a variety of mechanisms including AR overexpression/mutations, increased sensitivity or altered specificity to ligands, deregulated expression of co-regulators and other compensatory mechanisms [3, 6]. Indeed, the progression of PCa from castration-sensitive (CS) to CR disease is a complex process, which likely involves multiple genetic and epigenetic alterations that yet remain to be clearly understood.
MYB is a proto-oncogene and a cellular progenitor of the v-myb oncogenes carried by the chicken retroviruses AMV and E26 [7]. It encodes for a transcription factor, which binds to specific upstream regulatory elements of the genes in the promoter regions leading to activated gene expression in most cases [7]. Earlier reports suggested a restricted expression of MYB in immature haematopoietic cells, where it played a role in the maintenance of an undifferentiated proliferative state [8]. Later, aberrant MYB expression was also reported in haematological and several other malignancies including PCa, where it exhibited increased amplification frequency in hormone-refractory disease [9–13]. MYB confers its oncogenic activity by regulating the expression of a wide array of target genes in a context-specific manner, thus influencing diverse biological functions [14–18]. The qualitative and functional diversity of MYB functions is suggested to result from its interactions with a wide variety of cellular proteins [11, 17–20].
We earlier demonstrated an association of MYB overexpression in PCa with androgen-depletion resistance and aggressive tumour phenotypes [11]. In this study, we attempted to investigate the underlying molecular mechanisms by investigating the functional interaction of MYB and AR. We demonstrate that MYB and AR interact and co-localize in PCa cells. Further, we show that MYB facilitates ligand-independent nuclear localisation of AR and sustains its transcriptional activity under androgen-deprived condition. We also show that MYB and AR engage in combinatorial regulation of androgen-responsive genes and present in vivo data to show an association of MYB overexpression with castration resistance, PSA relapse and mice survival. Thus, our findings provide a novel mechanism for the CR progression of PCa.
Materials and methods
Cell culture
We used stable forced MYB-overexpressing (LNCaP-MYB) and MYB-knockdown (C4-2-shMYB) cell lines along with their control transfectants (LNCaP-Neo and C4-2-Scr) that were generated in our previous study [11]. In brief, LNCaP cells were transfected with pCMV6-MYB or empty (pCMV6-Neo) plasmids, whereas C4-2 cells were stably transfected with pGFP-V-RS-shMYB or scrambled sequence expressing control (pGFP-V-RS-Scr) plasmids, using FuGENE (Promega, Madison, WI) as a transfection reagent. All the plasmids were procured from Origene (Rockville, MD). The stable selection was done by culturing cells in G418 (200 μg/ml; for MYB overexpression) or Puromycin (2 μg/ml; for MYB-short hairpin RNA (shRNA)) containing media. Cell lines were routinely tested for mycoplasma contamination. Short tandem repeat (STR) genotyping and intermittent testing for MYB, androgen receptor (AR) and PSA expression were used to authenticate the cell lines in-house or commercially.
Reagents and antibodies
The reagents used were Roswell Park Memorial Institute medium-1640; foetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA); charcoal-stripped serum (CSS, steroid-reduced) (Gemini Bio-Products, West Sacramento, CA); penicillin and streptomycin (Invitrogen, Carlsbad, CA); PSA (human)-ELISA (Enzyme-Linked Immunosorbent Assay) Kit (Sigma-Aldrich, St. Louis, MO); Nuclear Extraction Kit and ChIP (Chromatin Immunoprecipitation) Assay Kit (Active Motif, Carlsbad, CA); Pierce Crosslink IP Kit and SuperSignal West Femto Maximum Sensitivity Substrate Kit (Thermo Scientific, Logan, UT); MycoSensorPCR Assay Kit (Stratagene, La Jolla, CA); dihydrotestosterone was from Sigma-Aldrich; Dual-luciferase Assay Kit (Promega); Gaussia luciferase (GLuc)-ON™ Promoter Reporter construct (GeneCopoeia Inc., Rockville, MD); EZ-Dewax (Biogenex, Fremont, CA); polymer, probe and background sniper (Biocare Medical, Concord, CA); NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Logan, UT); High-Capacity Complementary DNA Reverse Transcription Kit and SYBR Green Master Mix (Applied Biosystems, Carlsbad, CA); VivoGlo Luciferin (Promega, Madison, WI, USA). The antibodies used were MYB (#ab109127), PSA (#ab76113), AR (#ab108341) (all rabbit monoclonal) (Abcam, Cambridge, MA), rabbit polyclonal cleaved caspase-3 (#9661), α-tubulin (#2144) (Cell Signalling Technology, Denver, MA) and rabbit polyclonal Ki-67 (#ab15580), lamin A (#ab26300) (Abcam, Cambridge, MA), mouse anti-β-actin peroxidase antibody (#A3854, 1:20,000; Sigma-Aldrich, St. Louis MO) and horseradish peroxidase-labelled secondary antibodies (#sc-2357 (anti-rabbit), #sc-2005 (anti-mouse), 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA). Four unique 29-mer MYB-shRNA constructs (pGFP-V-RS-shMYB) and 29-mer scrambled shRNA control vector (pGFP-V-RS-Scr) purchased from Origene (Rockville, MD) and MYB overexpression construct was generated through cloning of MYB insert from pCMV6-XL5-MYB plasmid (Origene) into pCMV6-NEO vector (Origene). These constructs were used as described earlier [11].
RNA-interference-mediated gene silencing
For the knockdown of AR in PCa cells, cells were cultured in 6-well plates and transfected with 100 nmol/L of non-targeted scrambled (siSCR) or ON-TARGETplus SMARTpool AR targeting small interfering RNAs (siAR) (Dharmacon Horizon Discovery, Lafayette, CO) for 48 h according to the manufacturer’s instruction.
Protein extraction and Western blot analysis
Cells were processed for protein extraction as described earlier [21]. For nuclear protein extraction, cells were washed with PBS/phosphatase inhibitors and protein was lysed in hypotonic buffer. The lysate was then centrifuged at 14,000 × g at 4 °C, and the nuclear pellet was resuspended in complete lysis buffer and centrifuged again at 14,000 × g for 10 min at 4 °C. Supernatants containing nuclear fractions were collected. For immunoblotting, 20–80 µg of total protein were resolved on 10% polyacrylamide gels and transferred onto PVDF membranes. Subsequently, blots were incubated with specific antibodies followed by washing and incubation with relevant enzyme-linked secondary antibodies. A signal was generated using SuperSignal West Femto Maximum Sensitivity Substrate Kit and bands were visualised and recorded using ChemiDoc Imaging Systems (Thermo Scientific, Logan, UT).
Immunofluorescence and immunohistofluorescence assays
For immunofluorescence, PCa cells (5 × 104) were grown on cover-glass bottom cell culture dishes, washed with 1× Tris-buffered saline (TBS), fixed with 4% paraformaldehyde for 10 min and blocked with antibody diluent for 30 m. For immunohistoflourescence, prostate tumour tissue sections were deparaffinised using EZ-Dewax, rehydrated and antigen retrieval was performed. Next, slides were separately incubated with anti-MYB (#ab62824, goat polyclonal, Abcam, Cambridge, MA) and anti-AR (#5153, rabbit monoclonal, Cell Signalling, Danvers, MA) antibodies (1:100) for 60 min at room temperature, followed by washing twice with 1× TBS. After that slides were incubated with tetramethylrhodamine (TRITC)-conjugated (#A16141, anti-goat) and fluorescein isothiocyanate-conjugated (# 31584, anti-rabbit) secondary antibodies (Thermo Scientific, Rockford, IL) for 60 min, washed twice with 1× TBS and mounted with antifade Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Immunofluorescence of cultured cells was imaged using a Nikon TE2000-E Automated Widefield Microscope equipped with a CoolSnap HQ CCD camera, and immunohistofluorescence was imaged using a Nikon A1rsi Laser Scanning Confocal Microscope (Nikon Instruments Inc., Melville, NY).
Reciprocal-immunoprecipitation analysis
Co-immunoprecipitation studies were performed in the MYB-overexpressing PCa cell lines as previously described by us [22]. Briefly, protein lysates were incubated with 1 μg of MYB or AR antibodies overnight at 4 °C. Thereafter, the bound antibody/protein conjugate was incubated with Protein A agarose beads (Thermo Scientific, Rockford, IL) for 2 h at room temperature. Immunoprecipitate was centrifuged and washed with a lysis buffer. The antigen was then eluted with elution buffer (primary amine, pH 2.8) and diluted with 6× sample loading buffer using 1 M Tris (1:5). Subsequently, 20% of the total protein used in pull-down as an input, and immunoprecipitated proteins were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted for MYB and AR.
ChIP assay
The binding of AR/MYB to the KLK3/PSA and FKBP5/FKBP51 promoter was analysed by ChIP assay using a ChIP-IT Enzymatic Kit as previously described. Reverse transcription-polymerase chain reaction (RT-PCR) was performed using specific primers sets (Table S1).
Promoter-reporter luciferase assay
PCa cells were transfected with GLuc-ON™ promoter and secreted alkaline phosphatase (SEAP) construct. After 24 h of transfection, media were replaced with which was steroid supplemented, depleted or enzalutamide and cultured for n additional 48 h, the supernatant was collected and GLuc and SEAP activity was monitored by Secrete-Pair Dual Luminescence Assay Kit. SEAP served as an internal control.
Site-directed mutagenesis
Site-directed mutagenesis was performed using QuikChange Lightning Multi Site-Directed Mutagenesis Kit from Agilent Technologies (Santa Clara, CA) according to the manufacturer’s instructions. The PSA luciferase reporter vector was used as a template and the predicted target site sequence 5′-AAAAGTTC-3′ was mutated to 5′-ACAAATTT-3′ by PCR using specific primer sets (Table S1). The mutagenised plasmids were isolated using the Qiagen Miniprep Kit (Qiagen, Germantown, MD) and DNA sequencing (Eurofins, Louisville, KY) confirmed mutations of the region containing the mutation.
In vivo monitoring of orthotopic prostate tumour growth, castration resistance and survival
Studies involving the use of animals were approved by the University’s Institutional Animal Care and Use Committee. Male immunocompromised mice (aged 4–6 weeks) were purchased from Harlan Laboratories (Prattville, AL, USA) and acclimatised for about 2 weeks in the animal facility. Food and water were given ad libitum. Luciferase-tagged 3 × 106 of LNCaP-Neo or LNCaP-MYB cells or 1 × 106 of C4-2-Scr or C4-2-shMYB prostate cell lines (1:1 with Matrigel) were orthotopically implanted into the dorsal lobe of mouse prostate mice and the tumour growth was monitored weekly after intraperitoneal injection of d-Luciferin (150 mg/kg body weight), followed by bioluminescence imaging using Xenogen-IVIS cooled CCD optical system (IVIS Spectrum). Ten male mice were randomly allocated to each experimental group. In one group, we continued to monitor tumour growth without castrating the mice, while in another group, mice were castrated when tumour volumes reached a comparable size. Blood was collected weekly by penetrating the retro-orbital sinus in mice with a capillary for PSA measurements. Acquisition of data of a group was terminated in the week when the number of mice per group became less than six. This study also served to assess thhe survival of the mice bearing high- or low-MYB-expressing tumours with or without castration. Tumour burden and/or >10% body weight loss in 1 week or poor general condition (hunched posture, not eating/drinking, extreme weakness, trouble in urination/defecation, laboured breathing or haematuria) as determined by the institutional veterinarian were considered as study endpoints and the study was terminated entirely on the 150th day. Kaplan–Meier curves were used to measure survival. All the measurements were taken in an unbiased manner regardless of the experimental group.
Enzyme-linked immunosorbent assay
Serum from the blood was separated following centrifugation at 1000 r.p.m. and PSA level was examined by using a human PSA ELISA Kit as per the manufacturer’s instructions.
Immunohistochemical analyses
Tumour sections were cut from orthotopic tumour xenograft and performed immunohistochemistry (IHC) to examine Ki-67 and cleaved caspase-3 expression. IHC was performed as described previously [23] and tumour tissue sections were visualised under a microscope and photographed.
Statistical analysis
All the experiments were performed at least three times and data were expressed as mean ± S.D. Experimental groups were compared with the control group using an unpaired two-tailed Student’s t test. Differences were considered to be significant at P < 0.05. Kaplan–Meier test was used for the survival analysis using GraphPad v.6 (GraphPad Software Inc., La Jolla, CA).
Results
MYB and AR interact and co-localize in prostate tumour cells
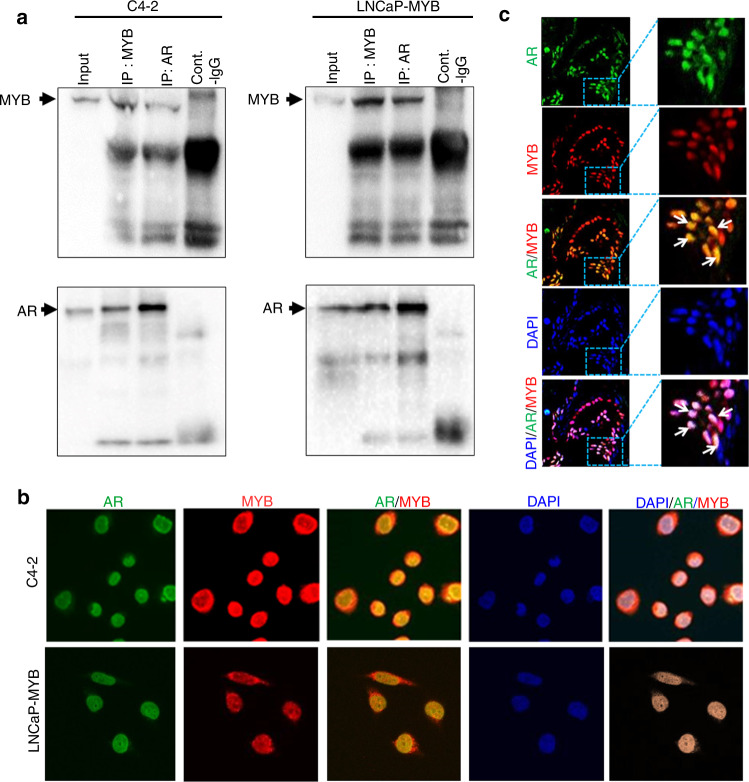
Since both MYB and AR serve as transcription factors and regulate an overlapping set of genes [7, 24], we set out to examine the possibility of physical interaction between them. For this, we used two well-established prostate tumour cell lines, LNCaP and C4-2, which are genetically related and express functional AR. LNCaP cells are CS and C4-2 cell line has been derived from it by in vivo selection in castrated mice and thus these cells are CR [25]. C4-2 cells also exhibit significantly greater endogenous expression of MYB relative to LNCaP [11], and therefore for interaction studies, we used its genetically engineered derived line, LNCaP-MYB, exhibiting forced overexpression of MYB. Immunoprecipitation of MYB and AR was performed using MYB- and AR-specific antibodies in total cell lysates followed by immunoblotting. We observed efficient reciprocal co-immunoprecipitation of both MYB and AR, suggesting that they interact with each other. The pulldown of MYB and AR and interaction between them was specific as we detected no or negligible pulldown with IgG controls (Fig. 1a). To further substantiate these findings and to examine their sub-cellular localisation, we performed a dual-immunofluorescence assay for MYB and AR in these cells. We observed predominant nuclear localisation of both MYB and AR, and digital overlay of images demonstrated that these proteins co-localise with each other (Fig. 1b and Supplementary Fig. 1). These findings were further confirmed in a small subset of FFPE human PCa samples (Fig. 1c), which further supported the interaction and co-localisation of MYB and AR inside the nucleus.
Fig. 1. MYB and AR interact and co-localize with each other in prostate cancer cells.
a Reciprocal co-immuno-precipitation assay was performed with anti-MYB (rabbit mAb) and anti-AR (rabbit pAb) antibodies using the total cell lysates from MYB- and AR-expressing C4-2 and LNCaP-MYB (ectopically MYB-expressing) PCa cells. Following reciprocal immunoprecipitation, proteins were resolved on polyacrylamide gel by electrophoresis and subjected to immunoblot analysis. A cross pulldown of both antigens (MYB and AR) was observed when immunoprecipitated with either antibody. b Immunofluorescence of cultured high-MYB-expressing prostate cancer cells was used to determine the localisation of MYB and AR. AR and MYB are shown in green (FITC-conjugated) and red (TRITC-conjugated), respectively. c Immunohistofluorescence assay was performed to determine the cellular localisation of MYB and AR in prostate cancer tissue samples (n = 5).
MYB-overexpressing PCa cells retain AR in the nucleus when cultured under androgen-depleted condition
Having observed the continued interaction of AR with MYB, we next determined the effect of MYB on AR sub-cellular localisation. High- and low-MYB-expressing control (C4-2-Scr and LNCaP-Neo, respectively) and their genetically engineered MYB-knockdown (C4-2-shMYB) and forced MYB-expressing (LNCaP-MYB) cell lines were cultured in media supplemented with either FBS or CSS. Following 48 h of culturing in respective media, cellular localisation of MYB and AR was examined by confocal immunofluorescence assay. As expected, we observed that AR was predominantly localised in the nucleus in PCa cells cultured in FBS-supplemented media and co-localised with MYB in MYB-expressing cells (Fig. 2a). However, when cultured in androgen-depleted condition (CSS-supplemented media), AR translocated to the cytoplasm in LNCaP-Neo and MYB-knockdown C4-2 cells, while it remained in the nucleus in MYB-expressing cells (C4-2-Scr and LNCaP-MYB) (Fig. 2a). To further support these findings, we prepared nuclear and cytosolic fractions of cell lysates from high- and low-MYB-expressing cells and examined MYB and AR expression by immunoblot analyses. In corroboration with our immunofluorescence data, we observed that nuclear levels of AR were largely retained in MYB-overexpressing (C4-2-Scr and LNCAP-MYB) cells when grown in androgen-depleted media, compared to those grown in androgen-supplemented condition compared to a cytosolic fraction (Fig. 2b). However, a significant reduction in nuclear AR while an increase in cytosolic expression was detected in low-MYB-expressing LNCaP-Neo and C4-2-shMYB cells when grown in androgen-depleted media. Together, these data suggest a role of MYB in nuclear retention of AR under androgen-depleted culture conditions.
Fig. 2. MYB-overexpressing cells are able to retain AR in the nucleus under androgen-deprived conditions.
a MYB-silenced C4-2 (shMYB) and ectopic MYB-expressing LNCaP (MYB) cells and their control cells, C4-2-Scr and LNCaP-Neo, respectively, were cultured on glass dishes in steroid-supplemented or steroid-depleted media. Subsequently, cells were fixed and subjected to immunofluorescence staining using anti-MYB (goat-primary) and anti-AR (rabbit-primary) antibodies, followed by incubation with TRITC-conjugated (anti-goat) and FITC-conjugated (anti-rabbit) secondary antibodies. b Cytosolic and nuclear protein fraction were prepared from C4-2 (Scr and shMYB) and LNCaP (Neo and MYB) cells cultured under FBS or CSS conditions. Proteins were resolved and subjected to immunoblotting for AR and MYB expression. α-Tubulin and lamin A were used as a loading control for cytoplasm and nuclear fractions, respectively.
MYB sustains the transcriptional activity of AR under androgen-depleted condition and regulate AR-responsive gene expression
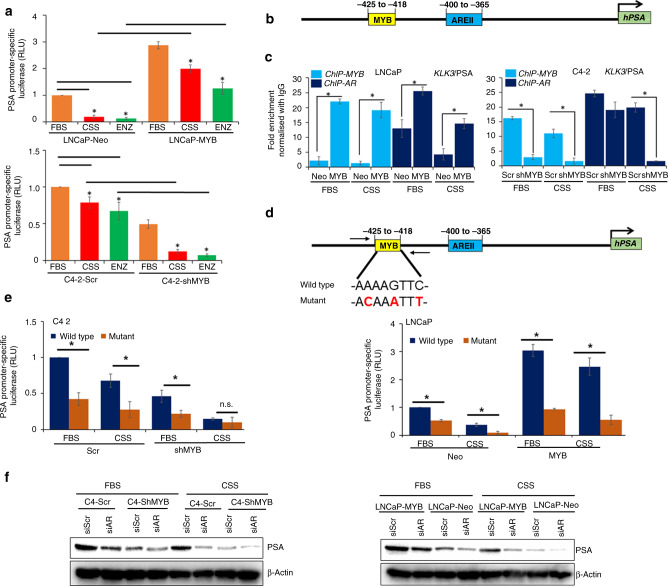
Next, we determined the impact of MYB on AR transcriptional activity in PCa cells when they were cultured under androgen-depleted conditions or treated with enzalutamide (a non-steroidal anti-androgen) by using a PSA (an AR-responsive gene) promoter-reporter assay. High- and low-MYB-expressing prostate tumour cells were transiently transfected with GLuc-ONTM PSA promoter-reporter plasmid under androgen-supplemented (FBS) and androgen-deprived (CSS) conditions or subjected to enzalutamide treatment. As expected, we observed a profound decrease in PSA promoter activity in LNCaP-Neo cells when subjected to CSS culture condition or treated with enzalutamide, whereas a modest decrease was observed in C4-2-Scr cells (Fig. 3a). In contrast, MYB-overexpressing LNCaP cells exhibited sustained PSA promoter activity, while it was decreased tremendously in MYB-knockdown C4-2 cells when they were cultured under CSS conditions or treated with enzalutamide (Fig. 3a). Intriguingly, we observed that MYB expression level, regardless of culture condition (androgen-supplemented or androgen-deprived) correlated with an increase in PSA promoter activity. This was further confirmed by examining the expression of PSA at the messenger RNA and protein levels (Supplementary Fig. 2). Together, these findings suggested a combinatorial regulation of PSA promoters by MYB and AR. To test this possibility, we analysed the PSA promoter for putative MYB-binding regions. In silico analysis identified an MYB-binding site (−425 to −418) adjacent to an AR binding region (−400 to −365) (Fig. 3b). To confirm MYB- binding to the KLK3/PSA promoter and its impact on AR binding, we performed ChIP using MYB- and AR-specific antibodies in both FBS and CSS culture conditions followed by RT-PCR amplification of the region containing the binding sites for MYB and AR. The data reveal that both AR and MYB bind to the KLK3/PSA promoter, and binding of AR to PSA promoter is reduced in low-MYB-expressing cells, while it is significantly increased in cells having high endogenous or forced MYB expression (Fig. 3c). To determine whether the predicted target site (−425 to −418) for MYB binding is a functional target site, we mutated the MYB-binding sequence 5′-AAAAGTTC-3′ to 5′-ACAAATTT-3′ (Fig. 3d) using Site-Directed Mutagenesis Kit, and mutation was confirmed by DNA sequencing of the region containing the mutation. The PCa cells, LNCaP and C4-2, were transfected with mutant or wild-type PSA promoter-reporter constructs. Reduced relative luciferase activity was observed in mutant construct-transfected cells, compared to the wild-type promoter-reporter constructs (Fig. 3e). Finally, we examined if AR remains indispensable for sustained PSA expression under CSS conditions. For this, we transiently silenced the expression of AR using siRNA in PCa cell lines exhibiting high or low MYB expression and cultured them under either FBS or CSS condition for another 48 h (Supplementary Fig. 3). Subsequently, PSA expression was analysed by immunoblotting. The data show that the silencing of AR led to a decrease in PSA expression in both high- and low-MYB-expressing cells; however, the PSA repression was more pronounced under CSS conditions with almost negligible expression reported in cells with reduced expression of both MYB and AR (Fig. 3f). To further ascertain a role of MYB in ligand-independent activation of AR, we analysed the expression of five additional androgen-responsive genes (ABCC4, KLF4, ALDH1A3, CAMKK2 and FKBP5). Significantly altered expression was reported for all of them except CAMKK2 in MYB-modulated cell lines (Supplementary Fig. 4). Further, we identified an MYB-binding region in the FKBP5 promoter and confirmed MYB binding and associated enrichment of AR by ChIP assays (Supplementary Fig. 5). Altogether, our data suggest that MYB sustains AR transcriptional activity and engage in combinatorial regulation of a selective set of androgen-responsive genes.
Fig. 3. Transcriptional activity of AR is increased in MYB-expressing prostate cancer cells and sustained under androgen-depleted conditions.
a C4-2 (Scr and shMYB) and LNCaP (Neo and MYB) cells were transfected with a promoter-reporter construct containing PSA/KLK3 promoter upstream of the luciferase gene and cultured under steroid-supplemented, steroid-depleted and enzalutamide (15 µM). Transcriptional activity was measured indirectly by performing a luciferase activity assay. b PSA/KLK3 promoter was analysed for transcription factor-binding regions and two MYB- and AR-binding sites were identified in proximity to each other. c Chromatin immunoprecipitation (ChIP) assay was performed using anti-MYB, anti-AR or normal IgG antibodies. Genomic fragments from the immunoprecipitates were isolated and RT-PCR was performed using a specific primer set for the region flanking MYB- and AR- binding sites within the KLK3/PSA promoter. d, e MYB-binding site (−418 to −425) sequence 5′-AAAAGTTC-3′ was mutated to 5′-ACAAATTT-3′ using a site-directed mutagenesis kit. The wild-type and mutated PSA promoter construct was transfected into prostate cancer cells and luciferase assay was performed 48 h after transfection by Secrete-Pair Dual Luminescence Assay Kit. SEAP served as an internal control. f C4-2 (Scr and shMYB) and LNCaP (Neo and MYB) were either transfected with siRNA against AR or scrambled non-targeting sequence, and subsequently cultured either in FBS- or CSS-supplemented media in 6-well dishes. PSA expression was analysed by immunoblotting. β-Actin served as a loading control. *P < 0.05; n.s. not significant.
MYB facilitates the castration-resistant progression of PCa
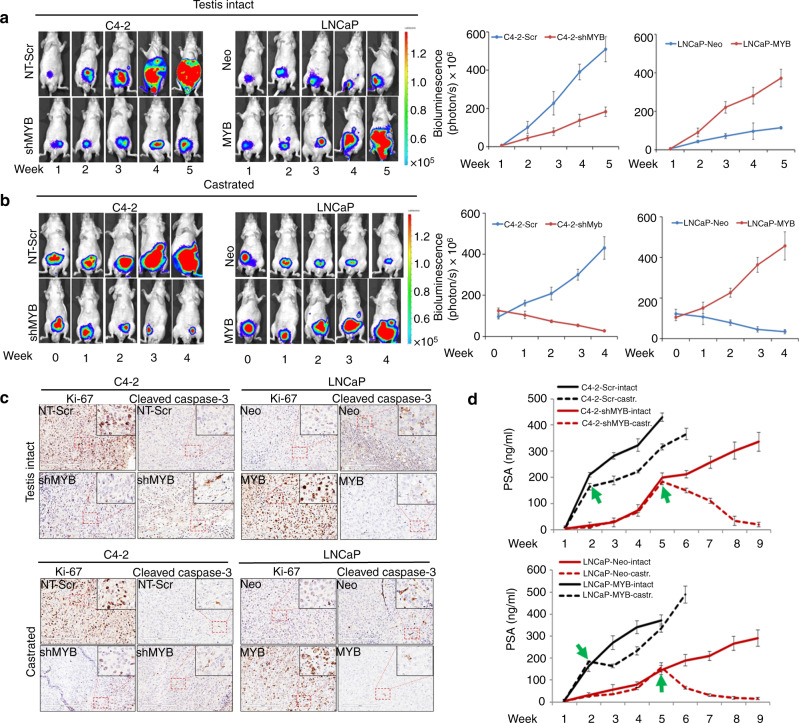
We examined the role of MYB in the castration-resistant progression of prostate tumour cells using an orthotopic mouse model. Luciferase-tagged MYB-silenced (C4-2-shMYB) or MYB-overexpressing (LNCaP-MYB) cell lines along with their respective control sublines (C4-2-Scr and LNCaP-Neo) were injected in the dorsal lobe of the prostate and their growth was monitored on alternate days by palpating and weekly by non-invasive in vivo bioluminescence imaging. In a subset, we castrated the mice when tumours reached comparable sizes in both low- (C4-2-shMYB and LNCaP-Neo; 5 weeks) and high-MYB-expressing (C4-2-Scr and LNCaP-MYB; 2 weeks) groups. We observed 100% tumour incidence in mice injected with prostate tumour cells; however, tumours in high-MYB-expressing group mice grew faster. However, despite forced MYB expression, LNCaP cells exhibited relatively slower growth as compared to control C4-2 cells (Fig. 4a). In the castrated mice, tumours in C4-2-shMYB and LNCaP-Neo groups exhibited a drastic decrease in growth, whereas tumours in C4-2-Scr and LNCaP-MYB groups continued to grow (Fig. 4b). We next performed immunohistochemical analyses of Ki-67 (cell proliferation marker) and cleaved caspase-3 (apoptosis marker) in PCa xenograft tumour sections. Increased and sustained expression of Ki67 in MYB-overexpressing PCa cells was detected in tumours derived from testis-intact and castrated mice, respectively, suggestive of higher cell proliferation. Moreover, we also observed a decreased staining of cleaved caspase-3 in MYB-overexpressing PCa cells suggestive of reduced apoptosis in orthotopic tumours from both castrated and non-castrated mice (Fig. 4c). Since relapse of serum PSA level is also reported in patients having a recurrence of castration-resistant disease, we also monitored PSA levels on a weekly basis in mice bearing prostate tumours. Our data show an increase in serum PSA levels in prostate tumour bearing mice in parallel with tumour growth. In castrated mice, a sharp decrease is observed in PSA levels in mice bearing low-MYB-expressing tumours, while mice bearing high-MYB-expressing tumours largely sustained serum PSA level following a slight initial decline following castration (Fig. 4d). We further confirmed these findings in the tumour tissues after the endpoint by conducting IHC analyses. Our IHC data also corroborated the observed serum PSA pattern (Supplementary Fig. 6). To study the effect of MYB expression on mice survival, we monitored tumour growth and mice survival for 150 days and a survival curve was plotted using Kaplan–Meier analysis. Data reveal that testis-intact animals bearing C4-2-Scr and LNCaP-MYB tumours had a median survival of 31 and 37.5 days, respectively, while increased median survival was observed in C4-2-shMYB (55 days) and LNCaP-Neo (62 days) group of mice (Fig. 5a, b). Castration of C4-2-Scr and LNCaP-MYB tumour-bearing mice could marginally increase their survival (6 and 13 days, respectively) as compared to testis-intact animals, while prolonged survival was observed in LNCaP-Neo- and C4-2-shMYB-castrated group as most mice remained alive and were sacrificed on the 150th day (Fig. 5a, b).
Fig. 4. MYB facilitates castration-resistant growth of PCa cells in the orthotopic mouse model.
Luciferase-tagged control (C4-2-Scr or LNCaP-Neo) or MYB-silenced (C4-2-shMYB) or MYB-overexpressing (LNCaP-MYB) sublines were orthotopically injected in nude mice (n = 20 per group). A subgroup of mice (n = 10) was left non-castrated (a), while mice in another subgroup (n = 10) were castrated (b) when tumours in control or treated (MYB-silenced or ectopically overexpressed) groups reached equivalent size as determined by non-invasive bioluminescence imaging. c Tumour tissues stained with Ki-67 and cleaved caspase-3 and representative images show predominantly nuclear (Ki-67) and cytoplasmic (cleaved caspase-3) staining. Images were taken at ×20 and ×40 (inset) magnification. d Serum PSA levels were determined every week in mice from all the groups. Broken lines indicate data from the castrated group with arrows demarcating the time of castration in the particular group.
Fig. 5. Mice carrying MYB-overexpressing tumours have poorer survival.
Mice from the castrated and un-castrated groups were followed for survival and the study was terminated at 150 days. Kaplan–Meier analysis was performed to assess the survival of testes-intact/castrated mice bearing a MYB-silenced (C4-2-shMYB) vs. control (C4-2-Scr) or b MYB-overexpressing (LNCaP-MYB) vs. control (LNCaP-Neo) PCa orthotopic xenograft tumours. Bars represent the median survival (days) of different groups of mice. Study endpoints were overall survival, disease progression and tumour burden.
Discussion
In the present study, we have identified MYB as a novel interacting partner of AR in PCa cells. Such interactions enable ligand-independent nuclear localisation and sustained transcriptional activity of AR. We also observe that MYB potentiates AR enrichment on the PSA promoter and is involved in its combinatorial induction. Finally, we present data in an orthotopic mouse model to show the association of MYB overexpression with castration resistance, PSA relapse and mice survival.
Androgen signalling plays important roles in the development of PCa and its sustained activation despite clinical interventions targeting this signalling node at various levels remains an important challenge in the effective therapy of PCa [24, 26, 27]. Relapsed castration-resistant prostate tumours are highly aggressive and metastatic, and do not respond well to other alternative therapies [26, 28]. Efforts to better understand the transition of PCa from CS to castration-resistant disease have identified multiple mechanisms and in consideration of the fact that PCa is a highly heterogeneous disease, it is likely that more than one mechanism with unique or overlapping functions is operative [29–33]. Our findings from this study suggest a novel mechanism where MYB overexpression promotes castration resistance via its interaction with AR. MYB is a transcription factor protein that regulates the expression of hundreds of genes [7, 18, 34]. Its conversion to oncogenic protein is suggested to involve genetic mutations that alter its activity and specificity; however, its aberrant overexpression resulting from changes in multiple signalling pathways can also significantly contribute to cancer pathogenesis [7, 35, 36]. MYB is shown to interact with a number of proteins, which define its context-dependent and cell-type-specific or disease-specific functions resulting from regulation of a differential set of genes [7, 37, 38]. In this regard, the novel interaction of MYB with AR is an important finding, which might have implications beyond the scope of this study and remain a subject of our continued investigations.
Canonical AR activation involves the binding of androgens to the ligand-binding domain, which causes a conformation change in the AR leading to its translocation from cytoplasm to the nucleus where it binds to androgen-responsive elements in gene promoters [4, 26, 39, 40]. Therefore, reducing the availability of androgens to prostate tumour cells as a therapeutic approach leads to the inhibition of AR nuclear translocation causing repressed expression of androgen-responsive genes [6, 40–43]. One important functional outcome of MYB-AR interactions in our findings was its role in sustained nuclear localisation of AR, which was transcriptionally active, even in the absence of an exogenous ligand. This is quite significant and provides another novel mechanism that could potentially be implicated in unrelenting AR signalling in PCa. However, it is important to delineate if sustained nuclear localisation of AR in the absence of androgens involves post-transcriptional changes in AR and/or its co-regulator proteins, and if so, how those changes are induced by MYB in PCa cells. Interestingly, we also observed MYB directly bound to at least two androgen-responsive genes (KLK3/PSA and FKBP5) promoters and enhanced AR recruitment to responsive elements to positively modulate its transcriptional activity. This supports the possibility that the interaction between MYB and AR may alter their genomic occupancy and together they may regulate a different set of genes compared to ones that they would regulate on an individual basis. This transcriptional reprogramming may alter gene expression in a way that not only favours castration resistance but may also be involved in the aggressive progression of prostate tumour cells and their resistance to other therapies as well, thus warranting future investigation. It will be interesting to investigate if MYB can promote castration resistance in an AR-independent manner as well, by cross-stimulating shared target genes. This notion is supported by a recent report showing that both MYB and AR regulate a similar subset of DNA damage response genes [44]. Similar co-opting of a pathway for resistance to anti-androgens has also been reported for glucocorticoid receptor (GR) [45]. Furthermore, MYB has been shown to interact with GR [46], raising the possibility that MYB may also support castration resistance by potentiating other alternative signalling mechanisms [11, 47, 48].
In corroboration with our in vitro findings, we observed that highly MYB-expressing prostate tumour cells exhibited increased tumorgenicity and a quick relapse following castration. However, despite forced MYB overexpression, LNCaP cells exhibited slower tumour growth and relapse as compared to C4-2 cells. This suggests that although MYB is an important determinant of aggressive and castration-resistant growth, other existing molecular differences in these two cell lines also play important roles. Indeed, studies from our lab and others have identified several other differentially expressed genes in these two cell types, many of which have also been associated with tumour aggressiveness and castration resistance [2, 26, 49, 50]. Therefore, it will be interesting to investigate the mechanistic association of MYB with these genes to identify their cooperative and/or compensatory functions. Furthermore, examining MYB-associated transcriptomic alterations in different tumour cell lines will also be the key to better understanding its context-specific functions and broader mechanisms of action. Another important observation in our study was that serum PSA levels paralleled prostate tumour growth prior to and after castration. This is significant as PSA relapse in PCa patients serves as an excellent marker for disease progression and therapy failure [51, 52]. Therefore, considering the functional involvement of MYB in prostate tumour pathobiology and its regulation of PSA, it will be logical to investigate if MYB, alone or in combination with other genes, can be used as a marker to predict disease aggressiveness and response to therapy. Moreover, considerable improvement in survival of mice bearing low-MYB-expressing prostate tumours suggests that the targeting of MYB also has significant potential to improve PCa therapy.
In summary, our study has identified novel MYB-AR crosstalk and unveiled its functional significance in the castration resistance of PCa. Future investigations are warranted to define the mechanisms underlying MYB overexpression in PCa, its crosstalk with other relevant signalling pathways, and to develop novel approaches for its direct or indirect targeting to achieve therapeutic benefit.
Supplementary information
Acknowledgements
We would like to acknowledge the support of veterinarian and vivarium staff in our animal studies.
Funding information
We would like to acknowledge the funding from NIH/NCI [R01CA224306, U01CA185490 (to APS) and R01CA204801, R01CA231925 (to SS)] and USAMCI (to APS and SS).
Author contributions
Conception and design: APS, SKS, MAK, SA, HZ and SS; development of methodology: APS, SKS, MAK, SA, HZ and SS; experiment and data generation: SKS, MAK, SA, HZ, SKD, GKP and JA; analysis and interpretation of data: APS, SKS, MAK, SA, HZ, SKD, JA, BW and JEC; writing original draft: SKS and APS; writing/reviewing and editing: MAK, APS, SKS, SA, SKD, HZ, GKP and JA; administrative and study supervision: APS and SS. All authors read and approved the final manuscript.
Data availability
We confirm that all the data in this manuscript is original and we have access to the raw data files.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study involved no human subjects. Only the de-identified and archived human prostate cancer tissues were used under an exempt (category 4) protocol as determined by the University of South Alabama Institutional Review Board.
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sanjeev Kumar Srivastava, Mohammad Aslam Khan.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01641-1.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Breijo S, Chantada-Abal V, Aller-Rodriguez M, Bohorquez-Cruz M, Sacristan-Lista F, Ponce-Diaz J, et al. Castration resistance mechanisms in prostate cancer. Arch Esp Urol. 2018;71:628–38. [PubMed] [Google Scholar]
- 3.Kita Y, Goto T, Akamatsu S, Yamasaki T, Inoue T, Ogawa O. Castration-resistant prostate cancer refractory to second-generation androgen receptor axis-targeted agents: opportunities and challenges. Cancers. 2018;10:345. doi: 10.3390/cancers10100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saranyutanon S, Deshmukh SK, Dasgupta S, Pai S, Singh S, Singh AP. Cellular and molecular progression of prostate cancer: models for basic and preclinical research. Cancers. 2020;12:2651. doi: 10.6084/m9.figshare.hgv.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharp A, Coleman I, Yuan W, Sprenger C, Dolling D, Nava Rodrigues D. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest. 2019;129:192–208. doi: 10.3390/cancers10100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinogen. 2011;10:20. doi: 10.4103/1477-3163.83937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Ness SA. Myb proteins: angels and demons in normal and transformed cells. Front Biosci. 2011;16:1109–31. doi: 10.2741/3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker SJ, Ma’ayan A, Lieu YK, John P, Reddy MV, Chen EY, et al. B-myb is an essential regulator of hematopoietic stem cell and myeloid progenitor cell development. Proc Natl Acad Sci USA. 2014;111:3122–7. doi: 10.1073/pnas.1315464111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Jin K, van Pelt GW, van Dam H, Yu X, Mesker WE, et al. c-Myb enhances breast cancer invasion and metastasis through the Wnt/beta-catenin/Axin2 pathway. Cancer Res. 2016;76:3364–75. doi: 10.1158/0008-5472.CAN-15-2302. [DOI] [PubMed] [Google Scholar]
- 10.Tichy M, Knopfova L, Jarkovsky J, Pekarcikova L, Veverkova L, Vlcek P, et al. Overexpression of c-Myb is associated with suppression of distant metastases in colorectal carcinoma. Tumour Biol. 2016;37:10723–9. doi: 10.1007/s13277-016-4956-7. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava SK, Bhardwaj A, Singh S, Arora S, McClellan S, Grizzle WE, et al. Myb overexpression overrides androgen depletion-induced cell cycle arrest and apoptosis in prostate cancer cells, and confers aggressive malignant traits: potential role in castration resistance. Carcinogenesis. 2012;33:1149–57. doi: 10.1093/carcin/bgs134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards J, Krishna NS, Witton CJ, Bartlett JM. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clin Cancer Res. 2003;9:5271–81. [PubMed] [Google Scholar]
- 13.Miree O, Srivastava SK, Khan MA, Sameeta F, Acharya S, Ndetan H, et al. Clinicopathologic significance and race-specific prognostic association of MYB overexpression in ovarian cancer. Sci Rep. 2021;11:12901. doi: 10.1038/s41598-021-92352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hijiya N, Zhang J, Ratajczak MZ, Kant JA, DeRiel K, Herlyn M, et al. Biologic and therapeutic significance of MYB expression in human melanoma. Proc Natl Acad Sci USA. 1994;91:4499–503. doi: 10.1073/pnas.91.10.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA. 2009;106:18740–4. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson MA, Rosenthal MA, Ellis SL, Friend AJ, Zorbas MI, Whitehead RH, et al. c-Myb down-regulation is associated with human colon cell differentiation, apoptosis, and decreased Bcl-2 expression. Cancer Res. 1998;58:5168–75. [PubMed] [Google Scholar]
- 17.Bhardwaj A, Srivastava SK, Singh S, Tyagi N, Arora S, Carter JE, et al. MYB Promotes desmoplasia in pancreatic cancer through direct transcriptional up-regulation and cooperative action of sonic hedgehog and adrenomedullin. J Biol Chem. 2016;291:16263–70. doi: 10.1074/jbc.M116.732651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azim S, Zubair H, Srivastava SK, Bhardwaj A, Zubair A, Ahmad A, et al. Deep sequencing and in silico analyses identify MYB-regulated gene networks and signaling pathways in pancreatic cancer. Sci Rep. 2016;6:28446. doi: 10.1038/srep28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava SK, Bhardwaj A, Arora S, Singh S, Azim S, Tyagi N, et al. MYB is a novel regulator of pancreatic tumour growth and metastasis. Br J Cancer. 2015;113:1694–703. doi: 10.1038/bjc.2015.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintana AM, Liu F, O’Rourke JP, Ness SA. Identification and regulation of c-Myb target genes in MCF-7 cells. BMC Cancer. 2011;11:30. doi: 10.1186/1471-2407-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel GK, Khan MA, Bhardwaj A, Srivastava SK, Zubair H, Patton MC, et al. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br J Cancer. 2017;116:609–19.. doi: 10.1038/bjc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhardwaj A, Srivastava SK, Singh S, Arora S, Tyagi N, Andrews J, et al. CXCL12/CXCR4 signaling counteracts docetaxel-induced microtubule stabilization via p21-activated kinase 4-dependent activation of LIM domain kinase 1. Oncotarget. 2014;5:11490–500. doi: 10.18632/oncotarget.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan MA, Srivastava SK, Zubair H, Patel GK, Arora S, Khushman M, et al. Co-targeting of CXCR4 and hedgehog pathways disrupts tumor-stromal crosstalk and improves chemotherapeutic efficacy in pancreatic cancer. J Biol Chem. 2020;295:8413–24. doi: 10.1074/jbc.RA119.011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma NL, Massie CE, Ramos-Montoya A, Zecchini V, Scott HE, Lamb AD, et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell. 2013;23:35–47. doi: 10.1016/j.ccr.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–81. [PubMed] [Google Scholar]
- 26.Bhardwaj A, Singh S, Srivastava SK, Honkanen RE, Reed E, Singh AP. Modulation of protein phosphatase 2A activity alters androgen-independent growth of prostate cancer cells: therapeutic implications. Mol Cancer Ther. 2011;10:720–31. doi: 10.1158/1535-7163.MCT-10-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisano C, Tucci M, Di Stefano RF, Turco F, Scagliotti GV, Di Maio M, et al. Interactions between androgen receptor signaling and other molecular pathways in prostate cancer progression: current and future clinical implications. Crit Rev Oncol Hematol. 2021;157:103185. doi: 10.1016/j.critrevonc.2020.103185. [DOI] [PubMed] [Google Scholar]
- 28.Merseburger AS, Bellmunt J, Jenkins C, Parker C, Fitzpatrick JM. European Treatment Practices G. Perspectives on treatment of metastatic castration-resistant prostate cancer. Oncologist. 2013;18:558–67. doi: 10.1634/theoncologist.2012-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, A’Hern R, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–8. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 30.Reyes EE, VanderWeele DJ, Isikbay M, Duggan R, Campanile A, Stadler WM, et al. Quantitative characterization of androgen receptor protein expression and cellular localization in circulating tumor cells from patients with metastatic castration-resistant prostate cancer. J Transl Med. 2014;12:313. doi: 10.1186/s12967-014-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen T, Wang LH, Farrar WL. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60:2132–5. [PubMed] [Google Scholar]
- 32.Culig Z, Hobisch A, Cronauer MV, Cato AC, Hittmair A, Radmayr C, et al. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993;7:1541–50. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- 33.Schroder FH. Progress in understanding androgen-independent prostate cancer (AIPC): a review of potential endocrine-mediated mechanisms. Eur Urol. 2008;53:1129–37. doi: 10.1016/j.eururo.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 34.Lang G, White JR, Argent-Katwala MJ, Allinson CG, Weston K. Myb proteins regulate the expression of diverse target genes. Oncogene. 2005;24:1375–84. doi: 10.1038/sj.onc.1208301. [DOI] [PubMed] [Google Scholar]
- 35.Sakura H, Kanei-Ishii C, Nagase T, Nakagoshi H, Gonda TJ, Ishii S. Delineation of three functional domains of the transcriptional activator encoded by the c-myb protooncogene. Proc Natl Acad Sci USA. 1989;86:5758–62. doi: 10.1073/pnas.86.15.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weston K, Bishop JM. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell. 1989;58:85–93. doi: 10.1016/0092-8674(89)90405-4. [DOI] [PubMed] [Google Scholar]
- 37.Ness SA. Myb binding proteins: regulators and cohorts in transformation. Oncogene. 1999;18:3039–46. doi: 10.1038/sj.onc.1202726. [DOI] [PubMed] [Google Scholar]
- 38.Kaspar P, Dvorakova M, Kralova J, Pajer P, Kozmik Z, Dvorak M. Myb-interacting protein, ATBF1, represses transcriptional activity of Myb oncoprotein. J Biol Chem. 1999;274:14422–8. doi: 10.1074/jbc.274.20.14422. [DOI] [PubMed] [Google Scholar]
- 39.Tan MH, Li J, Xu HE, Melcher K, Yong EL. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015;36:3–23. doi: 10.1038/aps.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masoodi KZ, Xu Y, Dar JA, Eisermann K, Pascal LE, Parrinello E, et al. Inhibition of androgen receptor nuclear localization and castration-resistant prostate tumor growth by pyrroloimidazole-based small molecules. Mol Cancer Ther. 2017;16:2120–9. doi: 10.1158/1535-7163.MCT-17-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gan L, Chen S, Wang Y, Watahiki A, Bohrer L, Sun Z, et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res. 2009;69:8386–94. doi: 10.1158/0008-5472.CAN-09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salami J, Alabi S, Willard RR, Vitale NJ, Wang J, Dong H, et al. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun Biol. 2018;1:100. doi: 10.1038/s42003-018-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, Tian G, Wang J, Zhao LY, Co O, Underill ZC, et al. Inhibition of androgen receptor transactivation function by adenovirus type 12 E1A undermines prostate cancer cell survival. Prostate. 2018;78:1140–56. doi: 10.1002/pros.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Chang W, Yang G, Ren C, Park S, Karantanos T, et al. Targeting poly(ADP-ribose) polymerase and the c-Myb-regulated DNA damage response pathway in castration-resistant prostate cancer. Sci Signal. 2014;7:ra47. doi: 10.1126/scisignal.2005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarvaiya PJ, Schwartz JR, Geng CD, Vedeckis WV. c-Myb interacts with the glucocorticoid receptor and regulates its level in pre-B-acute lymphoblastic leukemia cells. Mol Cell Endocrinol. 2012;361:124–32. doi: 10.1016/j.mce.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson TC, Li L. New targets for resistant prostate cancer. Oncotarget. 2014;5:8816–7. doi: 10.18632/oncotarget.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Todorova K, Metodiev MV, Metodieva G, Zasheva D, Mincheff M, Hayrabedyan S. miR-204 is dysregulated in metastatic prostate cancer in vitro. Mol Carcinogen. 2016;55:131–47. doi: 10.1002/mc.22263. [DOI] [PubMed] [Google Scholar]
- 49.Bhardwaj A, Singh S, Srivastava SK, Arora S, Hyde SJ, Andrews J, et al. Restoration of PPP2CA expression reverses epithelial-to-mesenchymal transition and suppresses prostate tumour growth and metastasis in an orthotopic mouse model. Br J Cancer. 2014;110:2000–10. doi: 10.1038/bjc.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansinho A, Macedo D, Fernandes I, Costa L. Castration-resistant prostate cancer: mechanisms, targets and treatment. Adv Exp Med Biol. 2018;1096:117–33.. doi: 10.1007/978-3-319-99286-0_7. [DOI] [PubMed] [Google Scholar]
- 51.Nonomura N, Takayama H, Nakayama M, Nakai Y, Kawashima A, Mukai M, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107:1918–22. doi: 10.1111/j.1464-410X.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 52.Pezaro C, Woo HH, Davis ID. Prostate cancer: measuring PSA. Intern Med J. 2014;44:433–40. doi: 10.1111/imj.12407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We confirm that all the data in this manuscript is original and we have access to the raw data files.