Abstract
Microvascular haemodynamic alterations are associated with coronary artery disease (CAD). The conjunctival microcirculation can easily be assessed non-invasively. However, the microcirculation of the conjunctiva has not been previously explored in clinical algorithms aimed at identifying patients with CAD. This case–control study involved 66 patients with post-myocardial infarction and 66 gender-matched healthy controls. Haemodynamic properties of the conjunctival microcirculation were assessed with a validated iPhone and slit lamp-based imaging tool. Haemodynamic properties were extracted with semi-automated software and compared between groups. Biomarkers implicated in the development of CAD were assessed in combination with conjunctival microcirculatory parameters. The conjunctival blood vessel parameters and biomarkers were used to derive an algorithm to aid in the screening of patients for CAD. Conjunctival blood velocity measured in combination with the blood biomarkers (N-terminal pro-brain natriuretic peptide and adiponectin) had an area under receiver operator characteristic curve (AUROC) of 0.967, sensitivity 93.0%, specificity 91.5% for CAD. This study demonstrated that the novel algorithm which included a combination of conjunctival blood vessel haemodynamic properties, and blood-based biomarkers could be used as a potential screening tool for CAD and should be validated for potential utility in asymptomatic individuals.
Subject terms: Biomarkers, Cardiology, Health care, Medical research
Introduction
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in developed countries1. It accounts for 17.9 million deaths globally, with up to 80% of these deaths resulting from myocardial infarction (MI) and stroke1. Coronary artery disease (CAD) is the most common type of CVD and results from the accumulation of lipid deposits (atherosclerosis) in the coronary arteries2. CAD is the leading cause of death in developed countries3.
CVD not only carries a significant morbidity and mortality burden but has major economic implications. The World Heart Foundation estimated the total cost of CVD was US$863 billion in 2010, with a predicted increase to approximately US$1,044 billion by 20304. The total expenditure on CVD in the EU is €210 billion annually with 53% (€111 billion) due to direct health care expenditures, productivity losses cost 26% (€54 billion), and informal care of patients costs 21% (€45 billion)5.
Atherosclerosis is the key pathophysiological process that underlies CAD6. This process develops over a prolonged period of time and is influenced by a variety of medical and behavioural risk factors5. Atherosclerotic plaques in general do not produce symptoms until the affected vessel has significant luminal narrowing and the resulting ischaemic cascade can be silent in its early stages. Prior to atherosclerotic plaque rupture and resultant MI, patients may be asymptomatic. Many predictive approaches, screening and diagnostic methods for atherosclerotic diseases have evolved. These include utilization of independent traditional and biochemical risk factors, general CVD risk scores, and assessment of cardiac microvasculature6–11.
Current European Society of Cardiology (ESC) clinical guidelines recommend the systematic assessment of cardiovascular (CV) risk in men > 40 years and in women > 50 years or post-menopausal with no CV risk factors10. ESC assessment of risk for asymptomatic individuals utilises the Systematic Coronary Risk Estimation (SCORE) assessment tool, whilst National Institute for Clinical Excellence (NICE) advocates the use of QRISK312,13. Both tools take into consideration conventional vascular risk factors to estimate long-term CV risk. The results of these estimates of risk are then used to inform GPs’ decisions with regards to primary preventative therapies and overall long-term risk. The risk score obtained may be modified by the presence of pre-existing medical conditions that were not taken into consideration during the initial risk calculation (e.g. chronic kidney disease or diabetes mellitus); or with the detection of preclinical asymptomatic vascular damage using imaging modalities (e.g. computed tomography (CT) coronary calcium score, carotid ultrasound or ankle brachial pressure index13). All pre-existing screening methods are limited either by the exposure of the patient to ionizing radiation (CT); or by the need for expensive imaging modalities requiring operator expertise to perform and interpret (carotid ultrasound)11,14. Due to the global and regional health and socioeconomic implications of CAD, there is a need to identify novel factors for screening asymptomatic individuals for early detection of CAD.
Previously heart-type fatty acid-binding protein (H-FABP), for example, has been shown to be an early blood diagnostic biomarker of MI15. H-FABP is detected in blood within 30 min of an ischaemic event. Measurement of H-FABP with troponin proved to be a reliable diagnostic tool for the early diagnosis of MI and a valuable rule out test at presentation to the emergency department15,16. Cholesterol is usually measured in screening > 40-year-old males and > 50-year-old females. Measurement of biomarkers in addition to cholesterol may provide further information to identify individuals at risk of CAD in asymptomatic individuals as a screening tool.
Microcirculatory studies have demonstrated the association of inflammation with atherosclerosis17–20. In addition, it has been shown that endothelial dysfunction in the microcirculation is an early manifestation and marker of vascular disease21. Microcirculatory changes have been observed in patients with hypertension, diabetes, sickle cell disease and sepsis17,21–23. A change in microcirculation is a potential marker for determining risk of potential major adverse cardiovascular events (MACE). These changes may manifest in changes in blood biomarker levels associated with these processes [e.g. NT-proBNP, interleukin-6 (IL-6), C-reactive protein (CRP), H-FABP, high density lipoprotein (HDL), fibrinogen, apolipoproteins, adiponectin, HDL-3 and monocyte chemoattractant protein-1 (MCP-1)].
In addition, the networks of the human eye allow examination of the microcirculation at the anterior (ocular adnexum) and posterior (retina) aspect that can easily be visualised and accessed non-invasively24. Changes to the retinal vessel diameters show evidence of diseases that are conventional risk factors for CAD (e.g. diabetes and hypertension)25–27 and hence could play a pathophysiological role in CVD risk stratification and prediction28. Blood flow within the conjunctival vasculature can be directly visualised and hence assessment of microcirculatory parameters (e.g. velocity (V), flow (Q), wall shear rate (WSR)) can also be measured non-invasively29–31. The study by Brennan et al.31 assessed groups of subjects with and without hypertension, diabetes mellitus, dyslipidaemia, reduced left ventricular ejection fraction (< 50%), as well as smokers vs. non-smokers. The results show only the blood flow rate (pl/s) increased significantly in diabetics vs. non-diabetics respectively (181 ± 61 vs. 151 ± 39 pl/s, p = 0.04). Previous studies have identified conjunctival microvascular changes in diseases such as sickle cell, hypertension and diabetes mellitus32,33.
The aim of this study was to develop a screening tool that could be used to identify patients at risk of CAD. Our previous study detected changes in conjunctival microvascular measurements between control and post-MI patients31. As part of the study, blood samples were collected to determine if blood biomarkers could be used in conjunction with microvascular measurements to improve the diagnostic potential of the test.
Methods
Study population
In this case–control MACE study, we compared a cohort of inpatients with severe cardiovascular phenotype after an acute MI with a healthy gender-matched patient cohort. Participants < 18 years were excluded from the study. All study participants were recruited between January 2018 and November 2019. Participants were eligible for inclusion in the ‘healthy’ cohort if they had no previous history of CAD.
Patients were eligible for inclusion in the post-MI cohort if they had been admitted to hospital with a diagnosis of MI that fulfilled the European Society of Cardiology (ESC) 4th Universal definition of type 1 MI. This is defined as acute myocardial injury with clinical evidence of acute myocardial ischaemia. A fall and/or rise of cardiac troponin (cTn) with 1 or more values beyond the 99th percentile of the Upper Reference Limit (URL), in addition to one or more of the following: evidence of new ischaemic ECG changes; pathological Q waves; current loss of myocardium or regional wall motion abnormality in line with an ischaemic aetiology; and coronary thrombus34.
The study complied with ethical principles on human experimentation (Declaration of Helsinki) and was approved by the office for Research Ethics Committee Northern Ireland (ORECNI) (IRAS Reference-166742), and Research Governance Committee of Belfast Trust (Trust Reference-15144TM-AS). Written informed consent was obtained from each participant prior to recruitment. The study was conducted in accordance with Standard for Reporting Diagnostic Accuracy (STARD) guidelines35.
Clinical data collection
All participants were asked to complete a clinical questionnaire detailing baseline demographics, past medical history, current medications, family history of medical conditions and lifestyle information. Electronic healthcare records were reviewed following ethical approval and participants’ consent for medical history to ensure accuracy. Baseline measurement of blood pressure, heart rate, oxygen saturations, height, and weight were also performed.
Blood sampling and laboratory methods
For biomarker assessment, 24mls of venous blood was sampled. Serum and plasma samples were analysed by Randox Clinical Laboratory Services (RCLS), (Antrim, UK) on cytokine arrays (Randox Laboratories Ltd, Crumlin, UK); using an Evidence Investigator analyser (Randox Laboratories Ltd, Crumlin, UK) for the following proteins: Cytokine array 1: Interleukin (IL)-1α, -1β, -2, -4, -6, -8, -10, vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), tumour necrosis factor alpha (TNF-α), interferon gamma (IFNɣ) and MCP-1. H-FABP, adiponectin, homocysteine and HDL-3 were measured on the RX Imola analyser (Randox, Crumlin, UK). Folate and vitamin B12 were measured on a Cobas8000 (Roche, Basel, Switzerland).
Asymmetric dimethylarginine (ADMA) and leucine-rich alpha-2-glycoprotein-1 (LRG-1) were assessed by ELISA, according to manufacturer’s instructions (ELISAgenie, Ireland). The limits of detection (LOD) for the biomarkers were as follows: IL-1α 0.19 pg/ml; IL-1β 0.26 pg/ml; IL-2 2.97 pg/ml; IL-4 2.12 pg/ml; IL-6 0.12 pg/ml; IL-8 0.36 pg/ml; IL-10 0.37 pg/ml, VEGF 3.24 pg/ml; EGF 1.04 pg/ml; TNF-α 0.59 pg/ml; IFNɣ 0.44 pg/ml; MCP-1 3.53 pg/ml; H-FABP < 2.94 ng/ml; adiponectin 0.18 µg/ml; folate 2.72 nmol/l; homocysteine 1.74 µmol/l; vitamin B12 73.8 pmol/l; HDL-3 4 mg/dl; plasma ADMA < 0.938 µmol/L; plasma LRG-1 < 0.0047 µg/ml. Results below the LOD were inputted as 90% of the LOD36.
Troponin, lipids, NT-proBNP, urate, urea and electrolytes and all other biochemical measurements, were analysed by clinical staff at the Kelvin Building Laboratories, Royal Victoria Hospital, Belfast. Apo-lipoprotein A and B were analysed by the Biochemistry Department, Cardiff and Vale University Hospital, UK as reported previously31.
Conjunctival vessel imaging
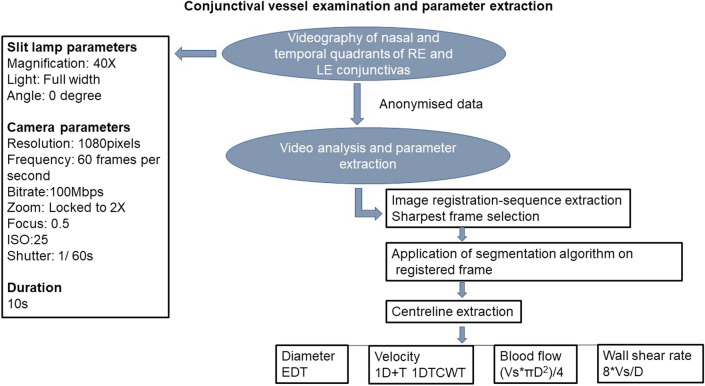
Conjunctival microvascular imaging was performed using our previously described non-invasive imaging tool (iPhone 6s with slit lamp biomicroscope) and semi-automated software; developed by the VAMPIRE centre (Dundee) and NIBEC (Ulster University) for quantifying vessel haemodynamic properties (vessel diameter (D), axial velocity (Va), cross-sectional velocity (Vs), Q, and WSR)30,37,38. The process is summarised as a flow diagram in Fig. 1.
Figure 1.
Vessel parameter data collection. RE Right Eye, LE Left Eye, EDT Euclidean Distance Transform, 1D + T 1 Dimension + Time, CWT Continuous Wavelet Transform, Vs Cross Sectional Velocity, D Diameter.
Briefly, the imaging tool consisted of a 2X magnification device (Apple iPhone 6s) adapted as an eye piece of a slit-lamp biomicroscope (Topcon SL-D4) with a 40 × magnification lens. A third-party camera application (ProMovie Recorder) was used for controlling camera settings (focus, shutter speed and ISO). Conjunctival imaging may take around 5 to 10 min per patient. Videos were obtained from conjunctival vessels in the nasal and temporal bulbar conjunctivas while the participants focused on a fixation target attached to the slit lamp. A total of 4 videos per participant were obtained in 40 s; each video was captured at 60 frames per second for 10 s. While all videos were captured under the same, or similar, lighting conditions in a hospital setting, the brightness of the light from the slit-lamp source rendered any small fluctuations in ambient lighting insignificant. Anonymised videos taken during the eye examinations were processed and analysed by researchers blinded to the participant baseline demographics and cohort allocation at Dundee University and Ulster University. They were analysed with set-up specific semi-automated software38. Measurements from vessels of both eyes were combined for the estimation of overall mean of each parameter per patient (patient’s mean).
Estimation of conjunctival microcirculatory parameters
Estimation of blood vessel parameters has been previously described30,37–39. Video sequences with minimal motion artefact were selected. The sharpest frame in the sequence was selected as a reference frame and all other frames registered to it. A segmentation algorithm was applied to segment vessels before vessel centrelines were extracted for estimating the dynamic properties of the vessels. Euclidean Distance Transformation was used for estimation of vessel diameter. The axial velocity (Va) was estimated based on spatial–temporal image (STI) via applying one dimension of space plus time (1D + T) continuous wavelet transform (1DTCWT)40. Blood flow was calculated from the product of the cross-sectional velocity (Vs) and diameter (D), using the formula, (Vs.πD2)/4. Wall shear rate was calculated by the formula 8Vs/D30,37,38.
Statistical analysis
Statistical analyses were performed using IBM SPSS v25 and R version 4.1.241. The following statistics were analysed on the appropriate data: descriptive statistics (mean ± SD) and percentages for summarizing parametric and nominal data, respectively; Chi square and Pearson’s tests for determining the association and correlation between variables; and independent t-test for differences between group means for parametric data. Where the assumptions of t-test were unmet, the Mann–Whitney U test or Wilcoxon rank-sum test was used. Variable medians were compared when distributions were similar but mean ranks were compared when distributions were dissimilar. Binary logistic regression and receiver operator characteristic (ROC) curves were used to test sensitivity and specificity. Normality was assessed by Shapiro Wilk test (p > 0.05). Data transformation was applied when the assumption of normality was unmet. Statistical significance was set at an α-level p < 0.05.
Results
Demographic characteristics
A total of n = 132 participants were recruited to the MACE study. There was an equal number of participants in each group; post-MI n = 66 and healthy controls n = 66. Post MI cases were recruited 2.53 ± 3.44 days (range 0–21 days) after hospital admission following type 1 MI. There was a significant difference between the mean ages of the control and MI groups (p = 0.039) but no significant difference between gender distribution (χ2(1) = 1.91, p = 0.168). Demographic and clinical characteristics are described in Table 1.
Table 1.
Demographic and clinical characteristics (mean ± SD).
| Variables | Control n = 66 | Post-MI n = 66 | p value |
|---|---|---|---|
| Age (years) | 52.50 ± 9.73 | 56.73 ± 11.41 | 0.039 |
| Gender (males) | 45/66 (68.2%) | 52/66 (78.8%) | 0.168 |
| Height (cm) | 168.21 ± 10.68 | 170.77 ± 9.34 | 0.262 |
| Weight (kg) | 81.40 ± 22.72 | 83.59 ± 14.93 | 0.186 |
| Body mass index (kg/m2) | 28.71 ± 7.66 | 28.62 ± 4.68 | 0.306 |
| Systolic BP (mmHg) | 128 ± 16 | 120 ± 16 | 0.006 |
| Diastolic BP (mmHg) | 76 ± 10 | 73 ± 11 | 0.066 |
| Heart rate (beats/min) | 71 ± 10 | 73 ± 12 | 0.299 |
| Hypertension | 8/66 (12.1%) | 31/66 (47.0%) | 0.001 |
| Diabetes | 2/66 (3.0%) | 15/66 (22.7%) | 0.001 |
| COPD | 6/66 (9.1%) | 8/66 (12.1%) | 0.572 |
| Hypercholesterolemia | 17/64 (26.6%) | 36/66 (54.5%) | 0.001 |
| Previous MI | 0/66 (0%) | 9/66 (13.6%) | 0.002 |
| Heart failure | 0/66 (0%) | 6/65 (9.2%) | 0.012 |
| Stroke | 0/66 (0%) | 1/66 (1.5%) | 0.315 |
| Smoking (yes) | 29/66 (43.9%) | 43/66 (65.2%) | 0.014 |
| Family history of IHD | 21/66 (31.8%) | 38/66 (57.6%) | 0.003 |
COPD chronic obstructive pulmonary disease, IHD ischaemic heart disease, BP blood pressure.
Clinical and lifestyle characteristics
None of the participants in the control group had previous MI (control: 0/66 (0.00%) vs. post-MI: 9/66 (13.6%), heart failure (control: 0/66 (0.00%) vs. post-MI: 6/66 (9.2%) or stroke (control 0/66 (0.00%) vs. post-MI:1/66 (1.5%), p < 0.05 for all variables). There were more smokers in the post-MI group; 43/66 (65.2%) vs. 29/66 (43.9%); p = 0.014, respectively. No significant differences in height were found between the cohorts. The mean and standard deviation values of height (cm) for both cohorts, controls vs. MI respectively were 168.21 ± 10.68 cm vs. 170.77 ± 9.34 cm, p = 0.262.
Comparison of microvascular parameters and biomarkers
Results of the comparison for microvascular parameters between groups are described in Table 2.
Table 2.
Ocular microvascular parameters (mean ± SD).
| Variables | Control n = 66 | Post-MI n = 66 | p value |
|---|---|---|---|
| Diameter (D, μm) | 21.45 ± 3.03 | 22.79 ± 3.07 | 0.012 |
| Axial velocity (Va, mm/s) | 0.54 ± 0.05 | 0.50 ± 0.06 | 0.001 |
| Cross-sectional velocity (Vs, mm/s) | 0.38 ± 0.04 | 0.35 ± 0.04 | 0.001 |
| Blood flow (Q, pl/s) | 159.66 ± 47.27 | 161 ± 48.12 | 0.457 |
| Wall shear rate (WSR, s−1) | 167.78 ± 34.37 | 143.59 ± 28.56 | 0.001 |
A summary of biomarker results between the control and post-MI group are described in Table 3.
Table 3.
Biomarker results (mean ± SD).
| Variables | Control n = 66 | Post MI n = 66 | p value |
|---|---|---|---|
| HbA1c (mmol/l) | 39.25 ± 8.29 | 45.27 ± 16.89 | 0.053 |
| Sodium (mmol/l) | 140.28 ± 1.77 | 138.95 ± 2.52 | 0.001 |
| Potassium (mmol/l) | 4.40 ± 0.31 | 4.33 ± 0.36 | 0.401 |
| Urea (mmol/l) | 5.38 ± 1.18 | 5.47 ± 2.18 | 0.541 |
| Creatinine (µmol/l) | 79.28 ± 16.66 | 84.05 ± 22.21 | 0.272 |
| Creatinine clearance (ml/min) | 110 ± 42.42 | 104.80 ± 34.93 | 0.733 |
| HGB (g/l) | 146.38 ± 11.00 | 142.39 ± 15.44 | 0.095 |
| Haematocrit (l/l) | 0.43 ± 0.03 | 0.42 ± 0.04 | 0.145 |
| White cell count (109 µl/l) | 6.76 ± 1.73 | 8.90 ± 3.01 | 0.001 |
| Platelet count (109/l) | 263.17 ± 46.54 | 262.59 ± 68.75 | 0.426 |
| MCV (fl) | 88.93 ± 4.30 | 88.20 ± 5.93 | 0.438 |
| CRP (mg/l) | 3.24 ± 6.39 | 20.96 ± 42.49 | 0.001 |
| NT-proBNP (ng/l) | 74.70 ± 186.68 | 1049.11 ± 1663.65 | 0.001 |
| Total cholesterol (mmol/l) | 5.11 ± 0.89 | 4.65 ± 1.44 | 0.005 |
| Triglyceride (mmol/l) | 1.67 ± 0.96 | 1.97 ± 1.35 | 0.217 |
| HDL (mmol/l) | 1.48 ± 0.48 | 1.13 ± 0.30 | 0.001 |
| LDL (mmol/l) | 2.85 ± 0.72 | 2.69 ± 1.33 | 0.124 |
| Non-HDL (mmol/l) | 3.62 ± 0.97 | 3.52 ± 1.41 | 0.404 |
| Chol-HDL ratio | 3.79 ± 1.37 | 4.31 ± 1.58 | 0.048 |
| Prothrombin Time (s) | 10.52 ± 0.50 | 10.94 ± 0.70 | 0.001 |
| APTT (s) | 26.47 ± 2.19 | 35.17 ± 29.28 | 0.468 |
| Fibrinogen (g/l) | 3.12 ± 0.61 | 3.89 ± 1.05 | 0.001 |
| Urate (mmol/l) | 0.32 ± 0.07 | 0.35 ± 0.11 | 0.280 |
| Apolipoprotein A (g/l) | 1.54 ± 0.29 | 1.34 ± 0.25 | 0.001 |
| Apolipoprotein B (g/l) | 1.02 ± 0.23 | 1.04 ± 0.31 | 0.949 |
| Folate (nmol/l) | 8.23 ± 6.06 | 6.45 ± 4.15 | 0.088 |
| Homocysteine (µmol/l) | 14.35 ± 6.96 | 15.81 ± 7.15 | 0.084 |
| Vitamin B12 (pmol/l) | 436.97 ± 210.39 | 396.92 ± 174.68 | 0.348 |
| Adiponectin (µg/ml) | 11.15 ± 6.20 | 8.58 ± 5.92 | 0.001 |
| H-FABP (ng/ml) | 3.62 ± 2.04 | 13.75 ± 29.73 | 0.001 |
| HDL-3 (mg/dl) | 20.17 ± 4.51 | 17.73 ± 4.75 | 0.001 |
| Serum IL-1α (pg/ml) | 0.48 ± 0.13 | 0.47 ± 0.13 | 0.320 |
| Serum IL-1β (pg/ml) | 2.38 ± 2.77 | 1.98 ± 0.50 | 0.550 |
| Serum IL-2 (pg/ml) | 4.25 ± 5.62 | 3.46 ± 1.37 | 0.673 |
| Serum IL-4 (pg/ml) | 2.80 ± 1.77 | 2.43 ± 0.47 | 0.332 |
| Serum IL-6 (pg/ml) | 1.92 ± 2.36 | 8.17 ± 16.77 | 0.001 |
| Serum IL-8 (pg/ml) | 10.87 ± 8.40 | 13.37 ± 13.56 | 0.082 |
| Serum IL-10 (pg/ml) | 1.38 ± 0.71 | 1.39 ± 0.46 | 0.469 |
| Serum MCP-1 (pg/ml) | 236.54 ± 100.32 | 201.79 ± 124.48 | 0.006 |
| Serum TNF-α (pg/ml) | 2.96 ± 2.17 | 2.75 ± 0.87 | 0.594 |
| Serum VEGF (pg/ml) | 120.62 ± 102.64 | 162.15 ± 123.21 | 0.043 |
| Serum IFNɣ (pg/ml) | 0.69 ± 0.67 | 0.98 ± 2.91 | 0.523 |
| Serum EGF (pg/ml) | 80.79 ± 41.54 | 60.89 ± 38.96 | 0.011 |
| Plasma IL-1α (pg/ml) | 0.44 ± 0.14 | 0.43 ± 0.13 | 0.796 |
| Plasma 1L-1β (pg/ml) | 2.42 ± 4.35 | 1.83 ± 0.52 | 0.366 |
| Plasma IL-2 (pg/ml) | 4.03 ± 5.23 | 3.31 ± 1.08 | 0.982 |
| Plasma IL-4 (pg/ml) | 2.82 ± 1.81 | 2.30 ± 0.49 | 0.031 |
| Plasma IL-6 (pg/ml) | 1.99 ± 3.00 | 7.06 ± 10.88 | 0.001 |
| Plasma IL-8 (pg/ml) | 4.74 ± 4.04 | 5.20 ± 8.14 | 0.564 |
| Plasma IL-10 (pg/ml) | 1.29 ± 0.46 | 1.43 ± 0.52 | 0.094 |
| Plasma MCP-1 (pg/ml) | 90.41 ± 37.16 | 87.13 ± 70.29 | 0.103 |
| Plasma TNF-α (pg/ml) | 2.95 ± 5.48 | 2.33 ± 0.87 | 0.347 |
| Plasma VEGF (pg/ml) | 24.92 ± 21.48 | 20.22 ± 9.97 | 0.468 |
| Plasma IFNɣ (pg/ml) | 0.63 ± 0.57 | 1.04 ± 2.88 | 0.478 |
| Plasma EGF (pg/ml) | 21.09 ± 16.39 | 16.04 ± 15.29 | 0.060 |
| ADMA (µmol/l) | 3.15 ± 1.68 | 3.63 ± 11.93 | 0.258 |
| LRG-1 (µg/ml) | 14.79 ± 9.44 | 11.93 ± 4.92 | 0.048 |
HbA1c glycated haemoglobin, HGB haemoglobin, MCV corpuscular volume, HDL high-density lipoprotein, LDL low-density lipoprotein, Chol-HDL cholesterol-high-density lipoprotein, NT-proBNP N-terminal pro brain natriuretic peptide, H-FABP heart-type fatty acid-binding protein, CRP C-reactive protein, IL interleukin, MCP-1 monocyte chemoattractant protein-1, VEGF vascular endothelial growth factor, TNF-α tumour necrosis factor alpha, IFNɣ interferon gamma, EGF endothelial growth factor, ADMA asymmetrical dimethylarginine, LRG-1 leucine-rich alpha-2-glycoprotein-1.
Ocular-biomarker algorithm derivation
Combining biomarker and ocular measurements may provide a novel method of identifying individuals at risk of CAD. Using forward and backward Wald logistic regression identified the combination of Vs, NT-proBNP and adiponectin as having the highest predictive ability to discriminate the post-MI group from control; with the fewest variables suitable for the patient cohort size for the study (χ2(3) = 111.74, p < 0.05). The model explained 80.4% (Nagelkerke R2) of the variance in CAD and correctly classified 90.1% of cases. Hosmer and Lemeshow test for goodness of fit was χ2(8) = 6.83, p = 0.55; sensitivity 93.0%, specificity 91.5%, positive predictive value (PPV) 91.4%, negative predictive value (NPV) 93.1%; area under receiver operator characteristic curve (AUROC) 0.967 (Table 4 and Fig. 2). In comparison, the QRISK3 score had a reduced sensitivity (71.7%), specificity (64.9%), PPV = 68.3% and NPV = 68.5% as shown in Table 4 and Supplementary Fig. 1.
Table 4.
Multivariate logistic regression with biomarkers and ocular parameters for detecting CAD.
| Biomarkers/ocular parameters | AUROC | Sensitivity % | Specificity % | PPV% | NPV% |
|---|---|---|---|---|---|
| Log10 NT-proBNP, adiponectin, log10 H-FABP, Va | 0.977 | 94.7 | 91.5 | 91.5 | 94.7 |
| Log10 NT-proBNP, log10 adiponectin, log10 H-FABP, Vs | 0.976 | 93.0 | 93.2 | 93.0 | 93.2 |
| Log10 NT-proBNP, log10 adiponectin, Vs | 0.967 | 93.0 | 91.5 | 91.4 | 93.1 |
| Log10 NT-proBNP, log10 adiponectin, Va | 0.966 | 94.7 | 93.2 | 93.1 | 94.8 |
| Log10 H-FABP, log10 NT-proBNP, Va | 0.953 | 88.1 | 91.9 | 91.2 | 89.1 |
| Log10 H-FABP, log10 NT-proBNP, Vs | 0.953 | 86.4 | 90.3 | 89.5 | 87.5 |
| Log10 H-FABP, log10 NT-proBNP, log10 WSR | 0.936 | 84.7 | 88.7 | 87.7 | 85.9 |
| Log10 NT-proBNP, Vs | 0.926 | 85.0 | 83.9 | 83.6 | 85.2 |
| Log10 NT-proBNP, Va | 0.925 | 83.3 | 85.5 | 84.7 | 84.1 |
| IL-6, Vs | 0.840 | 76.2 | 76.2 | 76.2 | 76.2 |
| Log10 H-FABP, Vs | 0.830 | 73.0 | 76.6 | 75.4 | 74.2 |
| Log10 H-FABP, Va | 0.815 | 73.0 | 71.9 | 71.9 | 73.0 |
| CRP, D | 0.810 | 79.4 | 67.2 | 71.4 | 75.9 |
| Log10 adiponectin, log10 WSR | 0.769 | 76.3 | 60.3 | 66.2 | 71.4 |
| Log10 adiponectin, Va | 0.721 | 76.2 | 53.1 | 61.5 | 69.4 |
| Log10 adiponectin, D | 0.702 | 72.1 | 54.2 | 62.0 | 65.3 |
| Vs | 0.706 | 63.1 | 73.4 | 70.7 | 66.2 |
| Log10 adiponectin | 0.674 | 65.6 | 62.5 | 63.6 | 64.5 |
| Log10 NT-proBNP | 0.897 | 78.7 | 90.6 | 88.9 | 81.7 |
| QRISK3 score | 0.732 | 71.7 | 64.9 | 68.3 | 68.5 |
| Age (years) | 0.604 | 59.1 | 57.6 | 58.2 | 58.5 |
Significant values are in bold.
CRP C-reactive protein, H-FABP heart-type fatty acid-binding protein, IL-6 interleukin-6, NT-proBNP N-terminal pro brain natriuretic peptide, D vessel diameter, Va axial velocity, Vs cross-sectional velocity, WSR wall shear rate, AUROC area under receiver operator characteristic curve, PPV positive predictive value, NPV negative predictive value.
Figure 2.
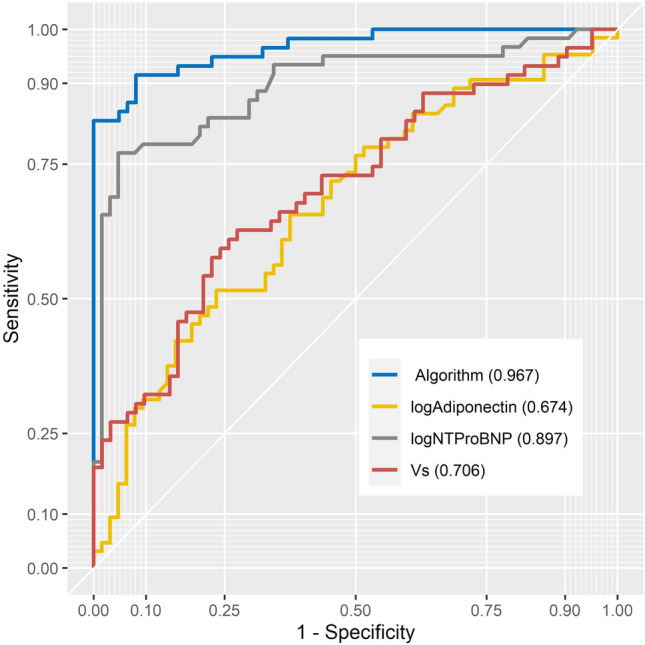
ROC curve of ocular-biochemical score.
Discussion
The aim of this case–control study was to evaluate the feasibility for using conjunctival microvascular parameters assessed non-invasively, in combination with biomarkers as a potential screening modality for CAD. This was achieved by comparing conjunctival imaging parameters and blood-based biomarkers in patients with established CAD and severe CVD phenotype (post-MI) with a control group of healthy volunteers.
Of the five conjunctival microvascular parameters assessed, four (diameter, axial velocity, cross-sectional velocity and wall shear rate) were significantly different between the post-MI and controls. Conjunctival vessel diameter was significantly higher in the post-MI group, however axial and cross-sectional velocities were lower compared with the control group. In addition, wall shear rate was lower in the post-MI group. An algorithm which included both conjunctival and biomarker measurements (microvascular velocity, adiponectin and NT-proBNP) was identified that differentiated the post-MI from control (AUROC 0.967, sensitivity 93.0%, specificity 91.5%) (Table 4 and Fig. 2). Application of this novel algorithm could be used to screen asymptomatic individuals for atherosclerotic disease.
Reduced blood velocity, elevated NT-proBNP and lower adiponectin reflect pathological cardiovascular conditions. The main function of the microcirculation is regulation of vascular resistance and oxygen perfusion of tissues. Therefore, decreased velocity and increased vessel diameter in the post-MI group, opposes the normal Poiseuille's law of blood flow dynamics, suggesting underlying microvascular dysfunction42.
Adiponectin and NT-proBNP are implicated in the pathogenesis and pathophysiology of CAD, for example, low levels of adiponectin (< 4.0 µg/mL) have been reported to be associated with an increased risk of CAD43. Low levels of adiponectin reflect underlying abnormal lipid metabolism, inflammation and is a contributor of atherosclerosis and subsequent vascular events43–45.
This study evaluated biomarkers of inflammation and endothelial dysfunction associated with CAD. A reduced conjunctival wall shear rate was observed in post-MI compared with control. Potential screening algorithms for CAD incorporating biomarkers and conjunctival blood vessel parameters have been identified (Table 4). These data suggest that changes in ocular microvascular parameters e.g. cross-sectional velocity, combined with biomarkers, have potential benefits for detecting atherosclerotic heart disease.
Detecting inflammation and endothelial dysfunction may offer the earliest opportunity to institute primary preventative therapies, lowering the risk of major adverse cardiac events. Endothelial dysfunction occurs in the initial stages of the ischemic cascade by promoting the development of atherosclerosis46. Reduction in wall shear rate and stress are associated with atherosclerotic plaque development and progression in coronary microvascular dysfunction42,47. Endothelial cells are regulators of inflammation; chronic inflammation is also an etiology of endothelial dysfunction. Hence, an understanding of the relationship and underlying pathophysiological mechanisms of endothelial dysfunction and inflammation is vital.
The authors of this report acknowledge that MI triggers inflammation aimed at enhancing healing and tissue repair which resolves between 2 and 4 weeks post-infarction48–50. Considering the recruitment interval of the post-MI group, post-infarct inflammation potentially could contribute to the inflammatory mediators released to promote healing51. However, the mediators have the potential of aggravating the existing atherosclerotic plaques leading to recurrent MI. Assessment of the relationship between cross-sectional velocity and biomarkers of endothelial dysfunction has potential utility in the screening of both asymptomatic and symptomatic CVD for stratifying risk of future events. This report suggests that the proposed algorithm identified in this study is tested and validated in a follow up study.
Study limitations
The small sample size had a tendency of generating false-positive errors (type 1)52. Hemorheological parameters respond differently to the cardiac cycle53. Hence, lack of differentiation of vessels has the potential of concealing true parameter values leading to biases in results and conclusions. Another study limitation relates to known confounding factors of vascular alteration including, but not limited to, age, lifestyle and medical factors5. Underlying medical factors with known effects on microvasculature include systemic blood pressure, hypercholesterolaemia, and diabetes. Heart failure and lifestyle factors such as smoking have known effects on the microvasculature. We acknowledge the sample size as the major study limitation on statistics on confounding factors, comorbidities, routine medication and hence recommend a larger study population for validation.
Conclusion
This study demonstrated the potential for a non-invasive ocular screening modality to detect conjunctival microvascular dysfunction in patients with overt atherosclerotic heart disease. The novel algorithm identified, combines ocular parameters with biomarkers that could distinguish between control and post-MI groups. The assessment of the conjunctival microcirculation is a rapid non-invasive test that can easily compliment blood investigations and existing lifestyle factors for CVD screening and risk stratification; however, this would need further validation as a screening tool in a larger sample of asymptomatic people.
Supplementary Information
Author contribution
Designing studies: T.C.B.M., M.A.N., M.W.R., M.S.S., E.T., J.A.D.M., D.D.F., A.A., P.B., M.J.K., J.L., P.F: Data Gathering: A.A., J.S.M., P.B., A.J.M, M.J., J.A.M: Research Database Preparation and Auditing: A.A., J.S.M., M.A.N., M.W.R., T.C.B.M. J.W.; Research Data Analysis: A.A., J.S.M., M.W.R., J.W; Manuscript Preparation: A.A., J.S.M., M.W.R., M.A.N., T.C.B.M., P.B., J.A.M., M.S.S., J.A.D.M, M.J.K., J.W., J.L., P.F: Editing of final version of manuscript: A.A., J.S.M., M.W.R., M.J.K., J. W, M.A.N., T.C.B.M, J.A.M., P.B.
Competing interests
Mark Ruddock, Mary Jo Kurth, Joanne Watt, and John Lamont are employees of Randox Laboratories Ltd but hold no shares in the Company. Peter Fitzgerald is the Managing Director and owner of Randox Laboratories Ltd. A patent has been filed to protect the biomarkers and biomarker combinations disclosed in this study.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-10491-7.
References
- 1.Institute of Health Metrix and Evaluations [IHME]. Global burden of disease study 2017. Lancet (2017).
- 2.Braun MM, Stevens WA. Stable coronary artery disease: Treatment. Am. Fam. Physician. 2018;97:376–384. [PubMed] [Google Scholar]
- 3.British Heart Foundation. UK Factsheet, 1–21 (British Heart Foundation, 2020).
- 4.World Economic Forum. The Global Economic Burden of Non-communicable Diseases, Harvard School of Public Health. (2011).
- 5.Wilkins E, et al. European Cardiovascular Disease Statistics 2017 edition. European Heart Network; 2017. p. 192. [Google Scholar]
- 6.Vasan RS. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 7.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): A case-control study. Lancet. 2008;373:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos JLL. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;17:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 9.Karmali K, et al. Risk scoring for the primary prevention of cardiovascular disease (review) summary of findings for the main comparison. Cochrane Database Syst. Rev. 2017;1:1–127. doi: 10.1002/14651858.CD006887.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piepoli MF, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representative) Eur. J. Prev. Cardiol. 2016;23:1–96. doi: 10.1177/2047487316653709. [DOI] [PubMed] [Google Scholar]
- 11.Pries AR, et al. A review of methods for assessment of coronary microvascular disease in both clinical and experimental settings. Cardiovasc. Res. 2008;80:165–174. doi: 10.1093/cvr/cvn136. [DOI] [PubMed] [Google Scholar]
- 12.NICE. Impact Cardiovascular Disease Prevention.
- 13.Piepoli MF, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int. J. Cardiovasc. Imaging. 2017;33:1021–1031. doi: 10.1007/s10554-017-1084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mcmahon CG, et al. Diagnostic accuracy of heart-type fatty acid-binding protein for the early diagnosis of acute myocardial infarction. Am. J. Emerg. Med. 2012;30:267–274. doi: 10.1016/j.ajem.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Navarro-Paredes C, et al. Diagnostic performance of a combination biomarker algorithm for rule-out of acute myocardial infarction at time of presentation to the emergency department, using heart-type fatty acid-binding protein and high-sensitivity troponin T tests. J. Clin. Exp. Cardiol. 2018;9:1–9. [Google Scholar]
- 17.Lockhart CJ, Hamilton PK, Quinn CE, Mcveigh GE. End-organ dysfunction and cardiovascular outcomes: the role of the microcirculation. Clin. Sci. 2009;116:175–190. doi: 10.1042/CS20080069. [DOI] [PubMed] [Google Scholar]
- 18.Stokes KY, Granger DN. The microcirculation: A motor for the systemic inflammatory response and large vessel disease induced by hypercholesterolaemia? J. Physiol. 2005;562:647–653. doi: 10.1113/jphysiol.2004.079640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 20.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: Molecular mechanisms and therapeutic interventions. Clin. Sci. 2007;112:375–384. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- 21.Abularrage CJ, et al. Evaluation of the microcirculation in vascular disease. J. Vasc. Surg. 2005;42:574–581. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Charlton M, Sims M, Coats T, Thompson JP. The microcirculation and its measurement in sepsis. J. Intensive Care Soc. 2017;18:221–227. doi: 10.1177/1751143716678638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menaa F, Khan BA, Uzair B, Menaa A. Sickle cell retinopathy: Improving care with a multidisciplinary approach. J. Multidiscip. Healthc. 2017;10:335–346. doi: 10.2147/JMDH.S90630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, et al. Human conjunctival microvasculature assessed with a retinal function imager (RFI) Microvasc. Res. 2013;85:134–137. doi: 10.1016/j.mvr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Jong F, et al. Retinal vascular caliber and risk of dementia. Neurology. 2011;76:816–821. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikram MK, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam study. Investig. Ophthalmol. Vis. Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 27.Ikram MK, et al. Retinal vessel diameters and risk of hypertension. Hypertension. 2006;47:189–194. doi: 10.1161/01.HYP.0000199104.61945.33. [DOI] [PubMed] [Google Scholar]
- 28.Rim TH, et al. Deep-learning-based cardiovascular risk stratification using coronary artery calcium scores predicted from retinal photographs. Lancet Digit. Heal. 2021;3:e306–e316. doi: 10.1016/S2589-7500(21)00043-1. [DOI] [PubMed] [Google Scholar]
- 29.Khansari MM, et al. Automated fine structure image analysis method for discrimination of diabetic retinopathy stage using conjunctival microvasculature images. Biomed. Opt. Express. 2016;7:2597. doi: 10.1364/BOE.7.002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan PF, et al. Assessment of the conjunctival microcirculation in adult patients with cyanotic congenital heart disease compared to healthy controls. Microvasc. Res. 2021;136:104167. doi: 10.1016/j.mvr.2021.104167. [DOI] [PubMed] [Google Scholar]
- 31.Brennan PF, et al. Assessment of the conjunctival microcirculation for patients presenting with acute myocardial infarction compared to healthy controls. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-020-79139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung ATW, et al. Microvascular abnormalities in sickle cell disease: A computer-assisted intravital microscopy study. Sci. Rep. 2011;99:3999–4005. doi: 10.1182/blood.v99.11.3999. [DOI] [PubMed] [Google Scholar]
- 33.Wong TY, et al. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam eye study: Methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111:1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 34.Thygesen K, et al. Fourth universal definition of myocardial infarction (2018) Eur. Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 35.Cohen JF, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open. 2016;6:1–17. doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurth MJ, et al. Acute kidney injury risk in orthopaedic trauma patients pre and post surgery using a biomarker algorithm and clinical risk score. Sci. Rep. 2020;10:20005–20005. doi: 10.1038/s41598-020-76929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brennan PF, et al. Cardiac-gated slit lamp videography as a novel approach to assessing a microcirculatory. Netw. R. Vict. Hosp. 2017;44:1–4. [Google Scholar]
- 38.Brennan PF, et al. Quantitative assessment of the conjunctival microcirculation using a smartphone and slit-lamp biomicroscope. Microvasc. Res. 2019;126:103907. doi: 10.1016/j.mvr.2019.103907. [DOI] [PubMed] [Google Scholar]
- 39.Jerman T, Pernus F, Likar B, Spiclin Z. Enhancement of vascular structures in 3D and 2D angiographic images. IEEE Trans. Med. Imaging. 2016;35:2107–2118. doi: 10.1109/TMI.2016.2550102. [DOI] [PubMed] [Google Scholar]
- 40.Duval-Destin M. Spatio-temporal wavelet: Appication to the analysis of moving. Prog. Wavelet Anal. Appl. 1993;1:1–10. [Google Scholar]
- 41.R Core Team. R: A language and environment for statistical computing. https://www.R-project.org/ (R Foundation for Statistical Computing, Vienna, Austria, 2021).
- 42.Koutsiaris AG, Tachmitzi SV, Batis N. Wall shear stress quantification in the human conjunctival pre-capillary arterioles in vivo. Microvasc. Res. 2013;85:34–39. doi: 10.1016/j.mvr.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Kumada M, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler. Thromb. Vasc. Biol. 2003;23:85–89. doi: 10.1161/01.ATV.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 44.Coughlin CC, et al. Effect of marked weight loss on adiponectin gene expression and plasma concentrations. Obesity. 2007;15:640–645. doi: 10.1038/oby.2007.556. [DOI] [PubMed] [Google Scholar]
- 45.Madeira IR, et al. Impact of obesity on metabolic syndrome components and adipokines in prepubertal children. J. Pediatr. 2009;85:261–268. doi: 10.2223/JPED.1873. [DOI] [PubMed] [Google Scholar]
- 46.Corban MT, Lerman LO. Endothelial dysfunction cardiovascular disease pathophysiology hidden in plain sight. Arter. Thromb Vasc. Biol. 2019;7:1272–1274. doi: 10.1161/ATVBAHA.119.312836. [DOI] [PubMed] [Google Scholar]
- 47.Samady H, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779–788. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 48.Kandat T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Hear. J. 2004;45:183–193. doi: 10.1536/jhj.45.183. [DOI] [PubMed] [Google Scholar]
- 49.Szekely Y, Arbel Y. A review of interleukin-1 in heart disease: Where do we stand today? Cardiol. Ther. 2018;7:25–44. doi: 10.1007/s40119-018-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanhaverbeke M, et al. C-reactive protein during and after myocardial infarction in relation to cardiac injury and left ventricular function at follow-up. Clin. Cardiol. 2018;41:1201–1206. doi: 10.1002/clc.23017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hackshaw A. Small studies: Strengths and limitations. Eur. Respir. J. 2008;32:1141–1143. doi: 10.1183/09031936.00136408. [DOI] [PubMed] [Google Scholar]
- 53.Chen HC, Patel V, Chen J, Rassam SM, Kohner EM. Vessel diameter changes during the cardiac cycle. Eye. 1994;8:97–103. doi: 10.1038/eye.1994.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.