Abstract
The soxRS regulon is activated by redox-cycling drugs such as paraquat and by nitric oxide. The >15 genes of this system provide resistance to both oxidants and multiple antibiotics. An association between clinical quinolone resistance and elevated expression of the soxRS regulon has been observed in Escherichia coli, but this association has not been explored for other enteropathogenic bacteria. Here we describe a soxRS-constitutive mutation in a clinical strain of Salmonella enterica (serovar Typhimurium) that arose with the development of resistance to quinolones during treatment. The elevated quinolone resistance in this strain derived from a point mutation in the soxR gene and could be suppressed in trans by multicopy wild-type soxRS. Multiple-antibiotic resistance was also transferred to a laboratory strain of S. enterica by introducing the cloned mutant soxR gene from the clinical strain. The results show that constitutive expression of soxRS can contribute to antibiotic resistance in clinically relevant S. enterica.
Antibiotic resistance is an increasing problem in clinical treatment of infectious disease (10, 13, 20). The acquisition of resistance has been linked to plasmid-borne genes that specify resistance to individual antibiotics (9), chromosomal mutations that alter the cellular targets (33), and activation of bacterial gene expression that confers resistance to diverse agents (1, 25, 27).
Two groups of coregulated genes (regulons) have been associated with chromosomally based resistance to multiple antibiotics in Escherichia coli and Salmonella enterica serovar Typhimurium: the marRAB and soxRS regulons (1, 6, 24, 25, 34; E. A. Martins, P. J. Pomposiello, and B. Demple, unpublished data). In the soxRS system, SoxR protein is activated by oxidation (18) or nitrosylation (11) to trigger transcription of the soxS gene. In the marRAB system, MarR-mediated repression is relieved in response to environmental agents to activate synthesis of MarA (1, 25), a close homolog of SoxS. The SoxS and MarA proteins are the direct activators of genes for resistance to oxidants and antibiotics (1, 16). Recent studies indicate that SoxS controls more than 15 genes in S. enterica and 39 genes in E. coli (31; P. J. Pomposiello, M. H. J. Bennick, and B. Demple, unpublished data) and that MarA in E. coli controls as many as 60 genes (4). There is some overlap between the soxRS and marRAB regulons, but most of the newly discovered genes are regulated uniquely by one or the other system (4; Pomposiello et al., unpublished data).
Antibiotic resistance mediated by soxRS and marRAB depends both on down-regulation of the outer membrane porin OmpF, mediated by the antisense RNA micF gene (6, 7), and on activation of the acrAB-encoded efflux pump (30, 35). Some evidence links the expression of the soxRS or marRAB regulons and resistance to antibiotics in pathogenic bacteria. One study correlated clinical quinolone resistance in E. coli with constitutive expression of either marA or soxS mRNA (∼15% of cases [29]). Mutations in the marR gene derepressed marA expression in these strains, but the mechanism of high soxS expression was not investigated. In the present work, we have investigated the contribution of soxRS to clinical quinolone resistance in S. enterica serovar Typhimurium. We show that constitutive expression of soxRS contributed significantly to the drug resistance of an S. enterica strain and that a constitutive mutation in soxR evidently arose during the treatment.
MATERIALS AND METHODS
The strains and plasmids used in these studies, along with their relevant properties and source descriptions, are listed in Table 1. Bacteria cultured overnight in Luria-Bertani broth (23) were diluted 1/100 in fresh Luria-Bertani broth and incubated with aeration for 120 min at 37°C. Inducing treatments with paraquat (PQ) were as described previously (31).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant propertiesa | Source or reference |
|---|---|---|
| Strain | ||
| ATCC 14028 | S. enterica serovar Typhimurium soxRS+ | American Type Culture Collection |
| EM1 | (ΔsoxRS)::tet derivative of ATCC 14028 | Martins et al., unpublished data |
| PP120 | ΔsoxRS derivative of ATCC 14028 | 31 |
| SL4213 | S. enterica, restriction deficient/modification proficient | J. Mekalanos, Harvard Medical School |
| St45 | S. enterica P185645, clinical isolate | 19 |
| St46 | S. enterica P185646, quinolone-resistant clinical isolate | 19 |
| Plasmid | ||
| pAK45 | 1-kb S. enterica St45 soxRS fragment in pACYC177 (ApR) | This work |
| pAK46 | 1-kb S. enterica St46 soxRS fragment in pACYC177 (ApR) | This work |
| pEM45 | 1-kb S. enterica St45 soxRS fragment in Bluescript (ApR) | This work |
| pEM46 | 1-kb S. enterica St46 soxRS fragment in Bluescript (ApR) | This work |
| pEM300 | 1-kb S. enterica LT2 soxRS fragment in Bluescript (CmR) | Martins et al., unpublished data |
Abbreviations, ApR, resistant to ampicillin; CmR, resistant to chloramphenicol.
Standard procedures (3) were used to prepare cell-free extracts by sonication for electrophoresis using sodium dodecyl sulfate-polyacrylamide gels and for blotting to membrane filters. The anti-SoxS antiserum was prepared in New Zealand rabbits using purified, recombinant SoxS protein (21). The antibody titer and specificity were estimated by immunoblotting against known amounts of purified SoxS protein. The optimal serum was obtained at 2 weeks after the second booster inoculation and was partially immunopurified by depleting nonspecific antibodies using filters containing whole-cell extracts of strains not expressing SoxS protein (15).
To clone soxRS from the clinical strains of S. enterica serovar Typhimurium a 1-kb fragment corresponding to just the soxRS region was amplified from bacterial genomic DNA using PCR with a forward primer (5′-TCAGTATTGTCAGGGATGGCA-3′; base pairs 208 through 228) and a reverse primer (5′-GTAGAGAGAAAGACAAAGACC-3′ [the underlined region corresponding to soxRS base pairs 1,266 through 1,254]). The amplified products were purified by agarose gel electrophoresis and blunt-ended using T4 DNA polymerase (3). The products from strains LT2, St45, and St46 were cloned into the EcoRV site of pBluescript (Stratagene) to yield plasmids pEM300, pEM45, and pEM46 (Table 1). The plasmid constructs were first selected in E. coli DH5α (3) and then passed through S. enterica SL4213, which is a restriction-deficient, modification-proficient strain (26), before transfer into S. enterica.
For testing the multiple-antibiotic-resistance phenotype associated with soxR-constitutive mutations, a 1-kb fragment corresponding to just the soxRS region was amplified from the genomic DNA of St45 and St46 (Table 1) using PCR. The forward primer was 5′-CGCGGATCCGCGTCAGTATTGTCAGGGATGGCA-3′ and the reverse primer 5′-CCATCGATGGGTAGAGAGAAAGACAAAGACC-3′ (regions corresponding to soxRS base pairs 208 through 228 and 1,266 through 1,254, respectively, are underlined) and contained BamHI (forward primer) and ClaI (reverse primer) restriction sites. The PCR products were purified using the QiaQuick kit (Qiagen), cut with BamHI and ClaI, repurified by agarose gel electrophoresis and cloned into the BamHI-ClaI sites of the low-copy-number pACYC177 plasmid to yield plasmids pAK45 and pAK46 (Table 1). After selection in E. coli DH5α and passage through S. enterica SL4213, these plasmids or the empty vector was transferred into the S. enterica ΔsoxRS strain PP120 (Table 1).
Northern blotting and antibiotic gradient plate assays were performed as described elsewhere (Martins et al., unpublished data). The degree of resistance to each antibiotic was determined by scoring for growth along an antibiotic gradient after 12 to 24 h of incubation at 37°C (6, 14).
RESULTS
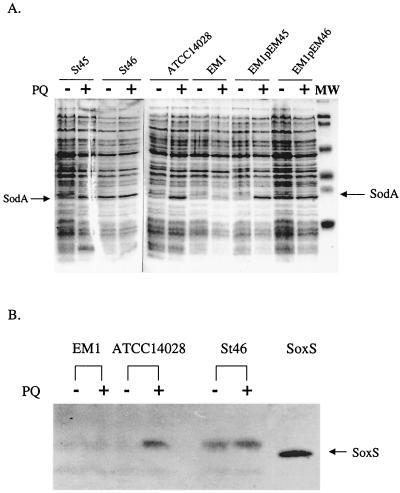
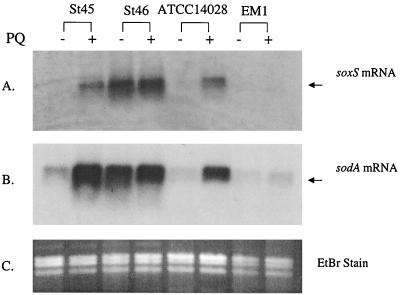
Laboratory strains of S. enterica serovar Typhimurium have a soxRS regulatory mechanism that is essentially identical to that of E. coli. Exposure to PQ (for example) induces Mn-containing superoxide dismutase (SOD), the sodA gene product, and confers resistance to multiple antibiotics (Martins et al., unpublished data). In examining samples of various pathogenic Enterobacteriaceae expressing multiple-antibiotic resistance, we discovered one S. enterica strain, St46, with high expression of an Mr ∼25,000 protein corresponding to SodA even without PQ treatment (Fig. 1A). This strain also showed high basal SOD activity (18 U/mg in St46 versus 6 U/mg in ATCC 14028). PQ treatment elicited only a small further increase in the SOD activity of St46 (to 21 U/mg) compared to a threefold increase in laboratory strain ATCC 14028 (to 18 U/mg). Elevated Mn-containing SOD expression could reflect the activation of any one of at least six different regulatory pathways, including marRAB and soxRS (8, 16). However, Northern analysis (data not shown) did not indicate elevated marRAB expression in strain St46. Instead, St46 constitutively expressed high levels of a 13-kDa protein that cross-reacted with antiserum against E. coli SoxS, and PQ treatment of the cells produced only a small further increase in the level of this SoxS-cross-reactive protein (Fig. 1B). The slight mobility difference between SoxS in S. enterica cell extracts and the purified, recombinant E. coli protein may have arisen from the composition of the cell lysis buffer (see Fig. 1 legend), perhaps in combination with the five amino acid differences between the S. enterica and E. coli proteins. Consistent with the apparent expression of SoxS and SodA proteins, St46 expressed exceptionally high levels of both soxS mRNA and sodA mRNA (Fig. 2).
FIG. 1.
Expression of SodA and SoxS proteins in antibiotic-resistant S. enterica. Laboratory strains ATCC 14028, EM1, EM1(pEM45), and EM1(pEM46) and clinical isolates St45 and St46 of S. enterica were cultured and treated for 60 min with 100 μM PQ where indicated (+). Cell-free extracts were prepared and analyzed by gel electrophoresis and Coomassie staining (A) or by Western blotting with anti-E. coli SoxS antiserum (B). For each type of analysis, 10 μg of total protein was loaded per lane. For panel B, 0.5 μg of purified E. coli SoxS protein (21) was loaded. The mobility difference between purified SoxS and the protein in crude cell extracts is likely related to the relatively high-salt lysis buffer present in the latter samples (100 mM HEPES and 100 mM NaCl). The position of SodA protein indicated in panel A was determined from separate experiments using an E. coli sodA strain.
FIG. 2.
Levels of soxS and sodA mRNA in antibiotic-resistant S. enterica. Total RNA was extracted using the RNeasy Mini Kit (Qiagen), electrophoresed (2 μg per lane), blotted, and probed with an S. enterica-specific probes for soxS (A) or an E. coli sodA probe (B). The ethidium bromide-stained (EtBr) gel prior to blotting is also shown (C) to demonstrate consistent loading in all lanes.
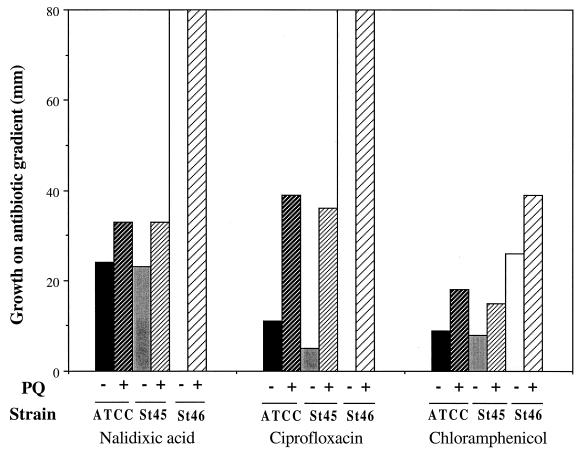
Strain St45 was isolated from a patient with salmonellosis, and St46 was obtained from the same patient after increased quinolone resistance had developed (19). Resistance to two quinolones and to chloramphenicol was PQ inducible in St45 (Fig. 3), as it is in laboratory strains of S. enterica (Martins et al., unpublished data). In contrast, St46 exhibited considerably higher constitutive resistance than St45 to all of these drugs (Fig. 3). The elevated chloramphenicol resistance of St46 was further increased in the presence of PQ (Fig. 3). The resistance of St46 to the quinolones was so high that a possible PQ-mediated increase in resistance could not be determined in this assay. In another set of experiments, inclusion of 50 μM PQ in the gradient plates with much higher levels of nalidixic acid (up to 4,000 μg/ml) or ciprofloxacin (up to 4 μg/ml) gave no significant increase in quinolone resistance in St46 (data not shown). Strain St46 also displayed elevated resistance to PQ compared to both St45 and laboratory strain ATCC 14028 (data not shown). This latter observation was also consistent with the possibility of constitutive activation of the soxRS regulon in St46.
FIG. 3.
PQ-inducible antibiotic resistance in S. enterica. Overnight cultures of laboratory S. enterica ATCC 14028 (ATCC) and clinical isolates St45 and St46 were plated on antibiotic gradient plates (14) in the presence (+) or absence (−) of 50 μM PQ. Growth was measured after 18 to 24 h of incubation at 37°C. The maximum antibiotic concentrations in the gradients were nalidixic acid, 15 μg/ml; ciprofloxacin, 0.25 μg/ml; and chloramphenicol, 40 μg/ml. The results shown are representative of four independent determinations.
Collectively, the foregoing results point to constitutive activation of soxRS in St46. To test this possibility directly, we transferred the soxRS region from St46 into EM1 (ΔsoxRS) and examined whether the St46 phenotype was transferred with it. The soxRS regions from St46 and the nonresistant strain St45 were cloned via PCR to generate plasmids pEM46 and pEM45, respectively (Table 1). In fact, SodA protein was expressed to a high constitutive level in EM1 carrying plasmid pEM46 but not in EM1 carrying pEM45 (Fig. 1A). PQ treatment induced SodA significantly in the EM1(pEM45) strain, exactly as it did in St45 itself and in the laboratory wild-type strain ATCC 14028 (Fig. 1A). However, PQ treatment did not further increase the already high expression of SodA in either St46 or EM1(pEM46) (Fig. 1A). Thus, the soxRS region from St46 determines the constitutive expression of SodA.
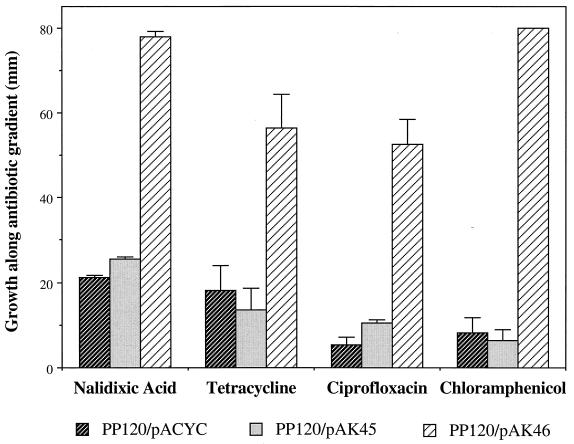
We also showed that increased resistance to multiple antibiotics was directed by the soxRS region from St46. For this purpose, we generated low-copy plasmids bearing the soxRS loci from St45 or St46 (plasmids pAK45 and pAK46, respectively). We introduced these plasmids into the S. enterica ΔsoxRS strain PP120 (31) to assay for antibiotic resistance. In strain EM1, general antibiotic resistance was elevated due to the tet allele replacing soxRS (Martins et al., unpublished data) and prevented using this strain for resistance experiments. While pAK45 had no effect on basal antibiotic resistance in PP120, pAK46 strongly increased the resistance to tetracycline, chloramphenicol, nalidixic acid, and ciprofloxacin (Fig. 4). Inclusion of PQ in the plates strongly increased the resistance of PP120(pAK45) to all of the antibiotics, but it had only a small additional effect on PP120(pAK46) (data not shown). The St46 soxRS region therefore determined a multiple-antibiotic-resistance phenotype that does not require activation by oxidative stress.
FIG. 4.
Multiple antibiotic resistance conferred by the St46 soxRS locus. The ΔsoxRS S. enterica strain PP120 (31) containing pAK45 (soxRS region from St45), pAK46 (soxRS region from St46), or the empty vector pACYC177 was tested for resistance using antibiotic gradient plates containing 100 μg of ampicillin per ml to select for the plasmids. Growth was measured after 18 to 24 h of incubation at 37°C. The maximum antibiotic concentrations in the gradients were nalidixic acid, 15 μg/ml; ciprofloxacin, 0.25 μg/ml; tetracycline, 15 μg/ml; and chloramphenicol, 40 μg/ml. The bars represent the means and standard deviations of six determinations.
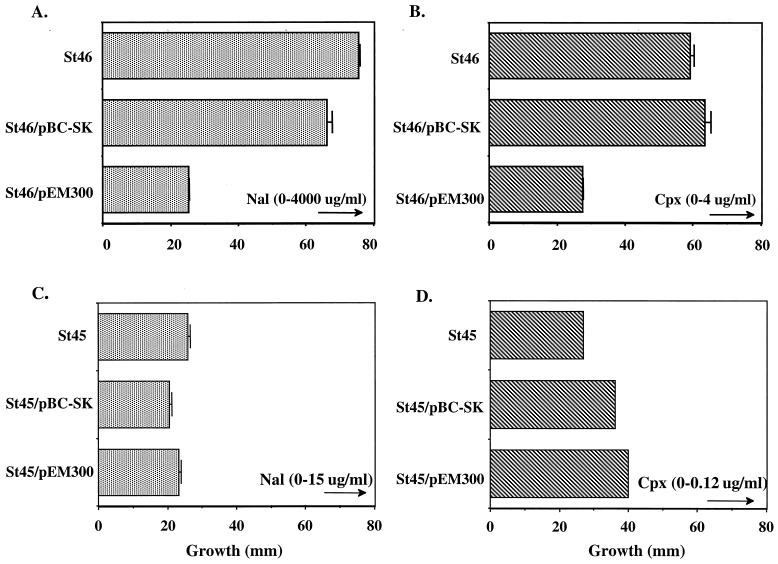
Compared to the soxRS sequence of St45 (which was identical to that of the laboratory strain LT2; GenBank accession number U61147 and updated sequence [Martins et al., unpublished data]), the DNA sequence of the entire soxRS region from St46 revealed only a single mutation—a G-to-A mutation at position 1,092. This change would convert glycine-121, which is located within the cysteine cluster that anchors the [••] centers to SoxR (5), to an aspartic acid. Several attempts to replace the mutant soxRS locus in St46 with a ΔsoxRS allele were unsuccessful. We therefore pursued another approach, predicated on the ability of nonactivated wild-type SoxR to compete with the mutant-activated protein (12, 17). We transformed St45 and St46 with a multicopy plasmid (pEM300) carrying the wild-type soxRS region and assayed the antibiotic resistance in these strains. The multicopy plasmid bearing wild-type soxRS dramatically reduced the resistance of St46 to both nalidixic acid and ciprofloxacin, while the control plasmid (vector only) showed no significant effect (Fig. 5). Plasmid pEM300 had little effect on the already lower antibiotic resistance of strain St45 (Fig. 5). Thus, an important part of the antibiotic resistance of St46 is due to constitutive activation of the soxRS regulon caused by the mutant SoxR protein in this strain.
FIG. 5.
Multicopy suppression of St46 antibiotic resistance by wild-type soxRS. (A and B) Specific suppression of quinolone resistance in St46. The multicopy soxRS plasmid pEM300 or the empty vector was transformed into S. enterica St46 (soxRC), and the resulting strains were tested in gradient plates for resistance to nalidixic acid (Nal [panel A]) or ciprofloxacin (Cpx [panel B]). Growth was measured after incubation at 37°C for 18 to 24 h. (C and D) Multicopy soxRS+ does not decrease quinolone resistance in St45. St45 was transformed with plasmids and tested for resistance as described for panels A and B. The maximum level of antibiotic in each plate is indicated above an arrow (μg, micrograms), indicating the direction of the gradient.
DISCUSSION
The occurrence of a soxRC mutation in the antibiotic-resistant clinical S. enterica isolate St46 is noteworthy. This strain arose in a chronic-renal-failure patient with salmonellosis, and the treatment was complicated by the development of quinolone resistance during the infection (19). Strain St45, isolated from the same patient before the quinolone resistance developed, carried only plasmid determinants for resistance to tetracycline and ampicillin. The additional resistance of strain St46 to quinolones depends significantly on its soxRC allele (Fig. 5).
The SoxRS- and MarRAB-regulated genes that contribute to broad antibiotic resistance include the acrAB operon encoding a multidrug efflux pump (22, 25, 30) and the micF gene encoding an antisense RNA that inhibits synthesis of the OmpF outer membrane porin (6, 7, 32). The higher antibiotic resistance of St46, in the absence of PQ, than of either St45 or ATCC 14028 in the presence of PQ could reflect various differences. One possibility is that the constitutive SoxR protein encoded in St46 is simply more active than wild-type SoxR following PQ activation under our conditions. Alternatively, multiple cell divisions may be needed for some changes conferring resistance, such as the clearance of OmpF protein from the outer membrane. Other resistance components (such as AcrA and its efflux partner TolC [2]) may accumulate to higher levels due to the constant activity of the SoxR-constitutive protein. Indeed, the level of soxS mRNA was higher in untreated St46 than in St45 exposed to 250 μM PQ for 30 min, but the amount of sodA mRNA was about the same in these two cases (Fig. 2). Another possible difference is that additional time might be required for maximal expression of the marRAB operon, the soxRS-dependent expression of which produces an extra increment of resistance (1, 25). Finally, additional mutations in St46 might elevate resistance to specific antibiotics (e.g., gyrA mutations for quinolones).
Constitutive mutations in soxRS or in marRAB have been correlated with fluoroquinolone resistance in clinical E. coli infections (29). To our knowledge, this is the first report of a soxRC mutation in an antibiotic-resistant S. enterica infection. Clearly, further studies are warranted to establish the frequency with which soxRC mutations accompany antibiotic resistance in other cases of salmonellosis.
The soxRS and marRAB regulons may contribute significantly to antibiotic resistance for which specific plasmid- or transposon-borne genes do not exist, e.g., quinolones (1). Although resistance provided by these pathways is typically lower than that produced by highly specific resistance determinants (9), its general nature could contribute a first step in the development of higher-level resistance (1, 25). Transient soxRS or marRAB activation (as opposed to constitutive mutations as described here and elsewhere [29]) may aid in the spread of antibiotic resistance, but such activation would have gone undetected in most studies performed thus far. The activation of these systems by immune attack and inflammatory responses (for soxRS [28]) or by antibiotics themselves (for marRAB [1, 25]) would provide multiple-antibiotic resistance similar to that observed for the constitutive strains, but this resistance would exist only while the regulons are activated. In this fashion, transient expression of soxRS- or marRAB-regulated resistance functions could allow for increased opportunities for the spread of other antibiotic-resistance determinants by increasing the probability of survival of cells lacking these determinants.
ACKNOWLEDGMENTS
We are grateful to P. Pomposiello for the ΔsoxRS strain PP120. We are indebted to members the Demple laboratory for invaluable advice and help.
This work was supported by grants from the U.S. National Institutes of Health (CA37831 to B.D. and GM51661 to S.B.L.). E.A.M. was supported by a fellowship from the São Paulo State Research Support Fund. A.K. was partially supported by a scholarship from the Alexander S. Onassis Public Benefit Foundation.
REFERENCES
- 1.Alekshun M N, Levy S B. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aono R, Tsukagoshi N, Yamamoto M. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1996. [Google Scholar]
- 4.Barbosa T M, Levy S B. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J Bacteriol. 2000;182:3467–3474. doi: 10.1128/jb.182.12.3467-3474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley T M, Hidalgo E, Leautaud V, Ding H, Demple B. Cysteine-to-alanine replacements in the Escherichia coli SoxR protein and the role of the [2Fe-2S] centers in transcriptional activation. Nucleic Acids Res. 1997;25:1469–1475. doi: 10.1093/nar/25.8.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou J H, Greenberg J T, Demple B. Posttranscriptional repression of Escherichia coli OmpF protein in response to redox stress: positive control of the micF antisense RNA by the soxRS locus. J Bacteriol. 1993;175:1026–1031. doi: 10.1128/jb.175.4.1026-1031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S P, McMurry L M, Levy S B. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J Bacteriol. 1988;170:5416–5422. doi: 10.1128/jb.170.12.5416-5422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compan I, Touati D. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J Bacteriol. 1993;175:1687–1696. doi: 10.1128/jb.175.6.1687-1696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 10.Dennesen P J, Bonten M J, Weinstein R A. Multiresistant bacteria as a hospital epidemic problem. Ann Med. 1998;30:176–185. doi: 10.3109/07853899808999401. [DOI] [PubMed] [Google Scholar]
- 11.Ding H, Demple B. Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proc Natl Acad Sci USA. 2000;97:5146–5150. doi: 10.1073/pnas.97.10.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudu P, Weiss B. SoxR, a [2Fe-2S] transcription factor, is active only in its oxidized form. Proc Natl Acad Sci USA. 1996;93:10094–10098. doi: 10.1073/pnas.93.19.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glynn M K, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo F J. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg J T, Chou J H, Monach P A, Demple B. Activation of oxidative stress genes by mutations at the soxQ/cfxB/marA locus of Escherichia coli. J Bacteriol. 1991;173:4433–4439. doi: 10.1128/jb.173.14.4433-4439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 16.Hidalgo E, Demple B. Adaptive responses to oxidative stress: the soxRS and oxyR regulons. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Austin, Tex: Landes Co.; 1996. pp. 435–452. [Google Scholar]
- 17.Hidalgo E, Demple B. An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 1994;13:138–146. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidalgo E, Ding H, Demple B. Redox signal transduction: mutations shifting [2Fe-2S] centers of the SoxR sensor-regulator to the oxidized form. Cell. 1997;88:121–129. doi: 10.1016/s0092-8674(00)81864-4. [DOI] [PubMed] [Google Scholar]
- 19.Howard A J, Joseph T D, Bloodworth L L, Frost J A, Chart H, Rowe B. The emergence of ciprofloxacin resistance in Salmonella typhimurium. J Antimicrob Chemother. 1990;26:296–298. doi: 10.1093/jac/26.2.296. [DOI] [PubMed] [Google Scholar]
- 20.Levy S B. The challenge of antibiotic resistance. Sci Am. 1998;278:46–53. doi: 10.1038/scientificamerican0398-46. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Demple B. SoxS, an activator of superoxide stress genes in Escherichia coli: purification and interaction with DNA. J Biol Chem. 1994;269:18371–18377. [PubMed] [Google Scholar]
- 22.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- 24.Miller P F, Gambino L F, Sulavik M C, Gracheck S J. Genetic relationship between soxRS and mar loci in promoting multiple antibiotic resistance in Escherichia coli. Antimicrob Agents Chemother. 1994;38:1773–1779. doi: 10.1128/aac.38.8.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller P F, Sulavik M C. Overlaps and parallels in the regulation of intrinsic multiple-antibiotic resistance in Escherichia coli. Mol Microbiol. 1996;21:441–448. doi: 10.1111/j.1365-2958.1996.tb02553.x. [DOI] [PubMed] [Google Scholar]
- 26.Neidhardt F C, Curtiss III R, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaecter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. [Google Scholar]
- 27.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 28.Nunoshiba T, DeRojas-Walker T, Tannenbaum S R, Demple B. Roles of nitric oxide in inducible resistance of Escherichia coli to activated murine macrophages. Infect Immun. 1995;63:794–798. doi: 10.1128/iai.63.3.794-798.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oethinger M, Podglajen I, Kern W V, Levy S B. Overexpression of the marA or soxS regulatory gene in clinical topoisomerase mutants of Escherichia coli. Antimicrob Agents Chemother. 1998;42:2089–2094. doi: 10.1128/aac.42.8.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pomposiello P J, Demple B. Identification of SoxS-regulated genes in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182:23–29. doi: 10.1128/jb.182.1.23-29.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pratt L A, Hsing W, Gibson K E, Silhavy T J. From acids to osmZ: multiple factors influence synthesis of the OmpF and OmpC porins in Escherichia coli. Mol Microbiol. 1996;20:911–917. doi: 10.1111/j.1365-2958.1996.tb02532.x. [DOI] [PubMed] [Google Scholar]
- 33.Spratt B G. Resistance to antibiotics mediated by target alterations. Science. 1994;264:388–393. doi: 10.1126/science.8153626. [DOI] [PubMed] [Google Scholar]
- 34.Sulavik M C, Dazer M, Miller P F. The Salmonella typhimurium mar locus: molecular and genetic analyses and assessment of its role in virulence. J Bacteriol. 1997;179:1857–1866. doi: 10.1128/jb.179.6.1857-1866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White D G, Goldman J D, Demple B, Levy S B. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J Bacteriol. 1997;179:6122–6126. doi: 10.1128/jb.179.19.6122-6126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]