Abstract
Macrolide antibiotics modulate the production of proinflammatory cytokines in vivo and in vitro. Transcription of the genes for these proinflammatory cytokines is regulated by nuclear factor κB (NF-κB). We examined whether or not clarithromycin inhibits the activation of NF-κB induced by tumor necrosis factor alpha (TNF-α) or staphylococcal enterotoxin A (SEA) in human monocytic U-937 cells, a T-cell line (Jurkat), a pulmonary epithelial cell line (A549), and peripheral blood mononuclear cells (PBMC). Flow cytometry revealed that clarithromycin suppresses NF-κB activation induced by TNF-α in U-937 and Jurkat cells in a concentration-related manner. Western blot analysis also demonstrated that clarithromycin inhibits NF-κB activation induced by TNF-α in U-937, Jurkat, and A549 cells and PBMC and by SEA in PBMC. Western blot analysis of cytoplasmic extracts of A549 cells revealed that this inhibition is not linked to preservation of expression of the IκBα protein. The chloramphenicol acetyltransferase assay indicated that NF-κB-dependent reporter gene expression is suppressed in U-937 cells pretreated with clarithromycin. These findings are consistent with the idea that clarithromycin suppresses the production of proinflammatory cytokines via inhibition of NF-κB activation.
Proinflammatory cytokines are important mediators in inflammation. Macrolide antibiotics exert anti-inflammatory effects through inhibition of the production of proinflammatory cytokines (25, 28, 35, 38, 40, 41). Clarithromycin is a 14-member lactone ring macrolide antibiotic which has been used for the treatment of infectious diseases. It is unclear how clarithromycin suppresses the production of proinflammatory cytokines, but it is not unreasonable to suspect that it inhibits the transcription of multiple cytokine genes.
Nuclear factor κB (NF-κB) is a ubiquitous and important transcription factor for genes that encode proinflammatory cytokines such as interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) (7, 12, 17, 19, 26). The prototype of NF-κB is a heterodimer consisting of p50 and p65 bound by members of the IκB family, including IκBα, in the cytoplasm (2, 3). NF-κB activation requires degradation of the IκB protein (10, 11). Phosphorylation of IκBα by drugs, cytokines, bacterial products, and viruses rapidly leads to IκB degradation and translocation of NF-κB to the nucleus (5, 16). Activation of NF-κB results in the binding of specific promoter elements and expression of mRNAs for proinflammatory cytokine genes (7, 12, 17, 19, 26). We tested the hypothesis that clarithromycin modulates inflammation by inhibiting NF-κB activation in experiments on human monocytic U-937 cells, a T-cell line (Jurkat), a pulmonary epithelial cell line (A549), and peripheral blood mononuclear cells (PBMC) stimulated by TNF-α or staphylococcal enterotoxin A (SEA).
MATERIALS AND METHODS
Cell culture, isolation, and stimulation conditions.
A549 cells were obtained from the American Type Culture Collection and maintained at 37°C under humidified 5% CO2 as a stationary culture. The cells were grown in Dulbecco's modified Eagle's medium containing 4.5 g of glucose/liter and supplemented with 10% fetal bovine serum (FBS), 10 mM l-glutamine, and 100 U of penicillin and 100 μg of streptomycin/ml. The day before each experiment, cells were seeded into six-well tissue culture dishes (Costar, Cambridge, Mass.) at the density of 106 cells/well.
U-937 cells, a human monocytic leukemia cell line, and Jurkat cells, a human T-cell leukemia line, were maintained at 37°C under humidified 5% CO2 as stationary cultures. Both types of cells were grown in RPMI 1640 medium containing 10% FBS and 100 U of penicillin and 100 μg of streptomycin/ml.
PBMC were obtained from heparinized blood by Histopaque 1077 (Sigma Chemical Co., St. Louis, Mo.) gradient centrifugation, and the mononuclear cells were resuspended in RPMI 1640 medium containing 10% FBS and 100 U of penicillin and 100 μg of streptomycin/ml.
Cells were exposed to 100 pM TNF-α (R&D Systems, Minneapolis, Minn.) or 10 μg of SEA (Sigma Chemical Co.)/ml with or without pretreatment with 3, 10, or 100 μg of clarithromycin (Taisho Pharmaceutical Co., Tokyo, Japan)/ml 30 min before incubation at 37°C for various times.
Flow cytometric analysis.
Flow cytometric analysis was performed by a modification of the previously published procedure (31). U-937 and Jurkat cells were permeabilized in 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.2, containing 0.1% saponin and 10 mM HEPES. The cells were then labeled with a mouse anti-NF-κB (nucleus-localized signal) antibody (immunoglobulin G3 [IgG3]; Boehringer GmbH, Mannheim, Germany) or a nonspecific mouse IgG3 antibody (Chemicon, Temecula, Calif.). The cells were then labeled with a fluorescein isothiocyanate-conjugated rat anti-mouse IgG3 monoclonal antibody (Pharmingen, San Diego, Calif.). After being washed, the cells were fixed with 1% paraformaldehyde in PBS and then stored at 4°C until flow-cytometric analysis. These experiments were repeated at least eight times.
Western blot analysis.
Nuclear extracts were harvested from U-937, Jurkat, and A549 cells and PBMC using a previously published procedure (15). The protein concentrations of the nuclear extracts were determined using Bio-Rad (Hercules, Calif.) protein concentration reagent. Nuclear extracts were stored at −80°C. To determine the IκBα levels, postnuclear (cytoplasmic) extracts were also stored at −80°C. Samples containing 10 μg of protein were separated in a denaturing 10% polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane. After three washings in TBST (40 mM Tris-HCl [pH 7.6], 300 mM NaCl, 0.5% Tween 20), the membranes were incubated in a 1:1,000 dilution of rabbit polyclonal anti-NF-κB-p65 antibodies or anti-IκBα antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.) in TBST containing 5% nonfat dry milk at room temperature for 1 h. After three washings in TBST, the membranes were incubated in a 1:2,500 dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad) for 1 h at room temperature. Immunoreactive proteins were detected using enhanced chemiluminescence (Amersham, Arlington Heights, Ill.) and analyzed by autoradiography. All experiments were repeated three times.
Plasmids, transfection, and CAT assay.
The plasmids containing the chloramphenicol acetyltransferase (CAT) gene with a human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR) with two NF-κB binding sites were kindly supplied by R. B. Gaynor of the Southwestern Medical Center (Dallas, Tex.). Characterization of the plasmids was described previously (36). U-937 cells were transfected with HIV-1 LTR CAT reporter plasmids using lipofection (FuGENE; Boehringer Mannheim, Indianapolis, Ind.). After 48 h of incubation, clarithromycin was added. Thirty minutes later, the cells were exposed to TNF-α for 2 h and then collected. The concentrations of CAT in cell extracts were determined with a sandwich-type enzyme-linked immunosorbent assay kit (Boehringer Mannheim). The CAT activities of samples were normalized to β-galactosidase activity (8). All experiments were repeated four times.
Statistical analysis.
The differences in the results between groups were analyzed by means of the Mann-Whitney U test.
RESULTS
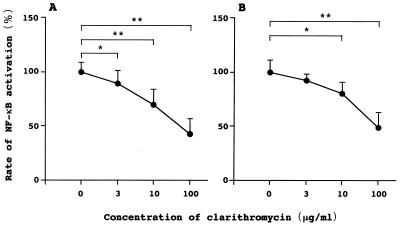
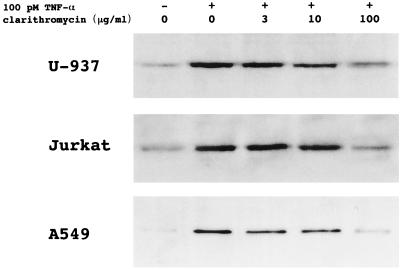
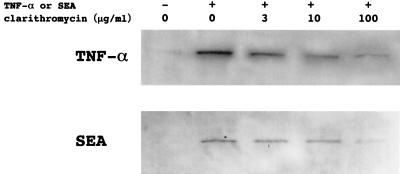
Flow cytometry of U-937 and Jurkat cells incubated with TNF-α for 30 min demonstrated that clarithromycin inhibited NF-κB activation in a concentration-related manner (Fig. 1). Western blot analysis of nuclear extracts of U-937, Jurkat, and A549 cells stimulated with TNF-α for 2 h revealed that pretreatment with clarithromycin decreased the expression of NF-κB p65 in a concentration-related manner (Fig. 2). Western blot analysis of nuclear extracts of PBMC stimulated with TNF-α or SEA for 2 h demonstrated that pretreatment with clarithromycin also decreased the expression of NF-κB p65 in a concentration-related fashion (Fig. 3).
FIG. 1.
Representative flow-cytometric analysis demonstrating that pretreatment with clarithromycin significantly inhibited NF-κB activation induced by TNF-α in U-937 (A) and Jurkat cells (B) in a concentration-related manner. ∗, P < 0.05; ∗∗, P < 0.01.
FIG. 2.
Representative Western blot of nuclear extracts of U-937, Jurkat, and A549 cells revealing that pretreatment with clarithromycin inhibited NF-κB activation induced by TNF-α in a concentration-dependent manner.
FIG. 3.
Representative Western blot of nuclear extracts of PBMC demonstrating that pretreatment with clarithromycin inhibited NF-κB activation induced by TNF-α or SEA in a concentration-dependent fashion.
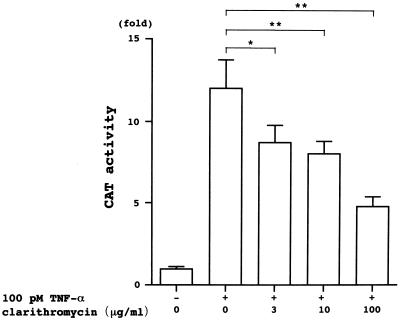
HIV-1 LTR containing NF-κB binding sites linked to the CAT gene was used to examine gene expression in U-937 cells 2 h after the addition of TNF-α. The CAT activity increased with the addition of TNF-α (Fig. 4). However, the activity was significantly inhibited in cells pretreated with clarithromycin (Fig. 4). The effect of clarithromycin was concentration related.
FIG. 4.
Representative CAT assay demonstrating clarithromycin-induced inhibition of NF-κB-mediated transcription by TNF-α in U-937 cells transfected with the HIV-1 LTR CAT gene. The clarithromycin-pretreated cells treated with TNF-α for 2 h expressed less activity than the non-clarithromycin-pretreated cells. The results are expressed as fold increases in activity over that in the cells treated with the medium alone (control). ∗, P < 0.05; ∗∗, P < 0.01.
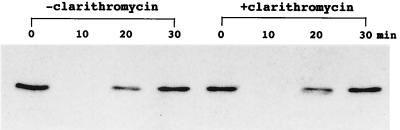
Western blot analysis of cytoplasmic extracts of A549 cells exposed to TNF-α revealed that expression of the IκBα protein exhibited decreased intensity within 10 min of the addition of TNF-α (Fig. 4). In A549 cells pretreated with clarithromycin, expression of the IκBα protein was not preserved (Fig. 5).
FIG. 5.
Representative Western blot demonstrating the effect of clarithromycin on TNF-α-induced IκBα degradation in A549 cells. Expression of the IκBα protein decreased within 10 min after the addition of TNF-α. The expression of the IκBα protein was not preserved in cells pretreated with 100 μg of clarithromycin/ml.
DISCUSSION
Inflammation is an important part of the pathogeneses of pulmonary diseases, not only infectious diseases due to bacteria, viruses, and fungi but also chronic obstructive pulmonary disease and neonatal chronic lung disease (32, 37). Inflammation mediated by proinflammatory cytokines is associated with and promotes the pathogeneses of these disorders. It is therefore important to modulate pulmonary inflammation in the treatment of patients with these lung disorders.
Macrolide antibiotics modulate inflammation in vitro and in vivo by inhibiting the production of proinflammatory cytokines and prostaglandin E2, neutrophil chemotactic activity, and elastase activities (14, 25, 27, 28, 34, 35, 38, 40, 41). Clarithromycin inhibits the production of IL-1, IL-6, IL-8, and TNF-α (25, 28). Clarithromycin also modulates antigen-specific T-cell proliferation (25) and improves IL-12-mediated anti-Mycobacterium avium activity (4). How does the clarithromycin action on peripheral blood immunocompetent and pulmonary epithelial cells result in the modulation of inflammation? Clarithromycin must modulate an event or process that is very basic to inflammation. One possibility is that clarithromycin modulates the transcription of genes for proinflammatory cytokines, the production of which is known to be modulated by clarithromycin.
Our results demonstrate that clarithromycin modulates TNF-α-induced NF-κB activation in U-937, Jurkat, and A549 cells and PBMC and modulates SEA-induced NF-κB activation in PBMC. The results of the CAT assay indicated that clarithromycin inhibits the transcription linked to NF-κB in U-937 cells. It is important to note that, while this report was in the final stage of preparation, Aoki and Kao published evidence consistent with the above observations (1). They noted that Jurkat T cells incubated with erythromycin and stimulated with phorbol 12-myristate 13-acetate and ionomycin showed reduced NF-κB activation. We proved that clarithromycin inhibited NF-κB activation in not only T cells but also monocytes/macrophages and pulmonary epithelial cells.
In infants administered a single oral dose of 5 or 10 mg/kg of body weight, the maximum concentrations of the drug in plasma were 2.26 ± 0.42 and 3.23 μg/ml, respectively (9). In adults administered an oral dose of 500 mg nine times at 12-h intervals, the concentration of clarithomycin in plasma was 3.29 ± 0.94 μg/ml at 4 h (30). The concentrations of clarithromycin in bronchopulmonary epithelial lining fluid (ELF) were 34.02 ± 5.16 μg/ml at 4 h, 20.63 ± 4.49 μg/ml at 8 h, 23.01 ± 11.9 μg/ml at 12 h, and 4.17 ± 0.29 μg/ml at 24 h in adults administered an oral dose of 500 mg nine times at 12-h intervals (30). The mean levels of clarithromycin at a mean time of 4.25 h were 4.0 μg/ml in serum, 20.5 μg/ml in ELF, and 372.7 μg/ml in alveolar cells in adults administered an oral dose of 500 mg seven times at 12-h intervals (13). Our results suggested that therapeutic clarithromycin administration has an anti-inflammatory effect by inhibition of NF-κB activation, because flow-cytometric analysis demonstrated that 3 and 10 μg of clarithromycin/ml significantly inhibited NF-κB activation in U-937 cells and Jurkat cells, respectively. Western blot analysis revealed that only 3 μg of clarithromycin/ml inhibited NF-κB activation in A549 cells.
Western blot analysis indicated that the inhibition of nuclear translocation of NF-κB was not linked to preservation of the IκBα protein. However, several inhibitors of NF-κB activation, such as aspirin, cyclosporin A, IL-10, IL-13, α-melanocyte-stimulating hormone, morphine, estrogen, and pyrrolidine dithiocarbamate, inhibit this translocation by preserving the IκBα protein (15, 18, 20, 22–24, 33, 39, 42). Thus clarithromycin, like IL-4, herbimycin A, and caffeic acid phenethyl ester, suppresses NF-κB activation without interfering with IκBα degradation (6, 21, 29). The precise mechanism underlying the inhibition of NF-κB activation by clarithromycin and these other agents remains unclear. It is possible that clarithromycin inhibits NF-κB activation through modulation of the binding of NF-κB with DNA or by affecting an unknown mechanism in the nuclear translocation of NF-κB.
In summary, our data extend the observation of the anti-inflammatory action of clarithromycin to lung and peripheral blood immunocompetent cells. We conclude that the modulation of NF-κB activation by clarithromycin results in inhibition of the production of proinflammatory cytokines.
REFERENCES
- 1.Aoki Y, Kao P N. Erythromycin inhibits transcriptional activation of NF-κB, but not NFAT, through calcineurin-independent signaling in T cells. Antimicrob Agents Chemother. 1999;43:2678–2684. doi: 10.1128/aac.43.11.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez L E, Petrofsky M, Wu M, Young L S. Clarithromycin significantly improves interleukin-12-mediated anti-Mycobacterium avium activity and abolishes toxicity in mice. J Infect Dis. 1998;178:896–899. doi: 10.1086/515351. [DOI] [PubMed] [Google Scholar]
- 5.Brown K, Gerstberger S, Carlson L, Franzoso L G, Siebenlist U. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 6.Clarke C J P, Taylor-Fishwick D A, Hales A, Chernajovsky Y, Sugamura K, Feldmann M, Foxwell B M J. Interleukin-4 inhibits κ light chain expression and NFκB activation but not IκBα degradation in 70Z/3 murine pre-B cells. Eur J Immunol. 1995;25:2961–2966. doi: 10.1002/eji.1830251037. [DOI] [PubMed] [Google Scholar]
- 7.Collart M A, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eustice D C, Feldman P A, Colberg-Poly A M, Buckery R M, Neubauer R H. A sensitive method for the detection of β-galactosidase in transfected mammalian cells. BioTechniques. 1991;11:739–742. [PubMed] [Google Scholar]
- 9.Fujii R, Iwata S, Satoh Y, Terashima I, Meguro H, Sunakawa K, Takeuchi Y, Aoyama T, Akita H, Yokota T, Nakamura H, Toyonaga Y, Ishihara T, Iwai N, Nakamura H, Nishimura T, Motohiro T. Clinical studies on clarithromycin dry syrup in the pediatric field. Pediatric Study Group of TE-031 Dry Syrup. Jpn J Antibiot. 1994;47:1283–1298. [PubMed] [Google Scholar]
- 10.Grimm S, Baeuerle P A. The inducible transcription factor NF-κB: structure-function relationship of its protein subunits. Biochem J. 1993;290:297–308. doi: 10.1042/bj2900297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henkel T, Machleidt T, Alkalay I, Krönke M, Ben-Neriah Y, Baeuerle P A. Rapid proteolysis of IκB-α is necessary for activation of transcriptional factor NF-κB. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 12.Hiscott J, Marois J, Garoufalis J, D'Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G, Fenton M. Characterization of a functional NF-κB site in the human interleukin 1β promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231–6240. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honeybourne D, Kees F, Andrews J M, Baldwin D, Wise R. The levels of clarithromycin and 14-hydroxy metabolite in the lung. Eur Respir J. 1994;7:1275–1280. doi: 10.1183/09031936.94.07071275. [DOI] [PubMed] [Google Scholar]
- 14.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, Iuvone T, D'Acquisto F, Di Rosa M. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292:156–163. [PubMed] [Google Scholar]
- 15.Ichiyama T, Zhao H, Catania A, Furukawa S, Lipton J M. α-Melanocyte-stimulating hormone inhibits NF-κB activation and IκBα degradation in human glioma cells and in experimental brain inflammation. Exp Neurol. 1999;157:359–365. doi: 10.1006/exnr.1999.7064. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Haque J, Lacoste J, Hiscott J, Williams B R G. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunsch C, Lang R K, Rosen C A, Shannon M F. Synergistic transcriptional activation of the IL-8 gene by NF-κB p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–164. [PubMed] [Google Scholar]
- 18.Lentsch A B, Shanley T P, Sarma V, Ward P A. In vivo suppression of NF-κB and preservation of IκBα by interleukin-10 and interleukin-13. J Clin Investig. 1997;100:2443–2448. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libermann T A, Baltimore D. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S F, Ye X, Malik A B. Pyrrolidine dithiocarbamate prevents I-κB degradation and reduces microvascular injury induced by lipopolysaccharide in multiple organs. Mol Pharmacol. 1999;55:658–667. [PubMed] [Google Scholar]
- 21.Mahon T M, O'Neill L A J. Studies into the effect of the tyrosine kinase inhibitor herbimycin A on NF-κB activation in T lymphocytes. J Biol Chem. 1995;270:28557–28564. doi: 10.1074/jbc.270.48.28557. [DOI] [PubMed] [Google Scholar]
- 22.Manna S K, Aggarwal B B. IL-13 suppresses TNF-induced activation of nuclear factor-κB, activation protein-1, and apoptosis. J Immunol. 1998;161:2863–2872. [PubMed] [Google Scholar]
- 23.Manna S K, Aggarwal B B. α-Melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-κB activation induced by various inflammatory agents. J Immunol. 1998;161:2873–2880. [PubMed] [Google Scholar]
- 24.Marienfeld R, Neumann M, Chuvpilo S, Escher C, Kneitz B, Avots A, Schimpl A, Serfling E. Cyclosporin A interferes with the inducible degradation of NF-κB inhibitor, but not with the processing of p105/NF-κB1 in T cells. Eur J Immunol. 1997;27:1601–1609. doi: 10.1002/eji.1830270703. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka N, Eguchi K, Kawakami A, Tsuboi M, Kawabe Y, Aoyagi T, Nagatani S. Inhibitory effect of clarithromycin on costimulatory molecule expression and cytokine production by synovial fibroblast-like cells. Clin Exp Immunol. 1996;104:501–508. doi: 10.1046/j.1365-2249.1996.46752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyajima M, Suga M, Nakagawa K, Ito K, Ando M. Effects of erythromycin on experimental extrinsic allergic alveolitis. Clin Exp Allergy. 1999;29:253–261. doi: 10.1046/j.1365-2222.1999.00430.x. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natarajan K, Singh S, Burke T R, Jr, Grunberger D, Aggarwal B B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel K B, Xuan D, Tessier P R, Russomanno J H, Quintiliani R, Nightingale C H. Comparison of bronchopulmonary pharmacokinetics of clarithomycin and azithromycin. Antimicrob Agents Chemother. 1996;40:2375–2379. doi: 10.1128/aac.40.10.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pyatt D W, Stillman W S, Yang Y, Gross S, Zheng J H, Irons R D. An essential role for NF-κB in human CD34+ bone marrow cell survival. Blood. 1999;93:3302–3308. [PubMed] [Google Scholar]
- 32.Rennard S I. Inflammation and repair processes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S33–S37. doi: 10.1164/ajrccm.160.supplement_1.5. [DOI] [PubMed] [Google Scholar]
- 33.Roy S, Cain K J, Chapin R B, Charboneau R G, Barke R A. Morphine modulates NFκB activation in macrophages. Biochem Biophys Res Commun. 1998;245:392–396. doi: 10.1006/bbrc.1998.8415. [DOI] [PubMed] [Google Scholar]
- 34.Sakata K, Yajima H, Tanaka K, Sakamoto Y, Yamamoto K, Yoshida A, Dohi Y. Erythromycin inhibits the production of elastase by Pseudomonas aeruginosa without affecting its proliferation in vitro. Am Rev Respir Dis. 1993;148:1061–1065. doi: 10.1164/ajrccm/148.4_Pt_1.1061. [DOI] [PubMed] [Google Scholar]
- 35.Schultz M J, Speelman P, Zaat S, van Deventer S J H, van der Poll T. Erythromycin inhibits tumor necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob Agents Chemother. 1998;42:1605–1609. doi: 10.1128/aac.42.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeler J S, Muchardt C, Podar M, Gaynor R B. Regulatory elements involved in Tax-mediated transactivation of the HTLV-1 LTR. Virology. 1993;196:442–450. doi: 10.1006/viro.1993.1500. [DOI] [PubMed] [Google Scholar]
- 37.Speer C P. Inflammatory mechanisms in neonatal chronic lung disease. Eur J Pediatr. 1999;158:S18–S22. doi: 10.1007/pl00014314. [DOI] [PubMed] [Google Scholar]
- 38.Sugiyama Y, Yanagisawa K, Tominaga S I, Kitamura S. Effects of long-term administration of erythromycin on cytokine production in rat alveolar macrophages. Eur Respir J. 1999;14:1113–1116. doi: 10.1183/09031936.99.14511139. [DOI] [PubMed] [Google Scholar]
- 39.Sun W H, Keller E T, Stebler B S, Ershler W B. Estrogen inhibits phorbol ester-induced IκBα transcription and protein degradation. Biochem Biophys Res Commun. 1998;244:691–695. doi: 10.1006/bbrc.1998.8324. [DOI] [PubMed] [Google Scholar]
- 40.Takizawa H, Desaki M, Ohtoshi T, Kawasaki S, Kohyama T, Sato M, Tanaka M, Kasama T, Kobayashi K, Nakajima J, Ito K. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;156:266–271. doi: 10.1164/ajrccm.156.1.9612065. [DOI] [PubMed] [Google Scholar]
- 41.Takizawa H, Desaki M, Ohtoshi T, Kitamura T, Okazaki H, Sato M, Akiyama N, Shoji S, Hiramatsu K, Ito K. Erythromycin suppresses interleukin 6 expression by human bronchial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun. 1995;210:781–786. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 42.Yin M, Yamamoto Y, Gaynor R B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]