Abstract
The SARS-CoV-2 virus gains entry to cells by binding to angiotensin-converting enzyme 2 (ACE2). Since circumventricular organs and parts of the hypothalamus lack a blood–brain barrier, and immunohistochemical studies demonstrate that ACE2 is highly expressed in circumventricular organs which are intimately connected to the hypothalamus, and the hypothalamus itself, these might be easy entry points for SARS-CoV-2 into the brain via the circulation. High ACE2 protein expression is found in the subfornical organ, area postrema, and the paraventricular nucleus of the hypothalamus (PVH). The subfornical organ and PVH are parts of a circuit to regulate osmolarity in the blood, through the secretion of anti-diuretic hormone into the posterior pituitary. The PVH is also the stress response centre in the brain. It controls not only pre-ganglionic sympathetic neurons, but is also a source of corticotropin-releasing hormone, that induces the secretion of adrenocorticotropic hormone from the anterior pituitary. It is proposed that the function of ACE2 in the circumventricular organs and the PVH could be diminished by binding with SARS-CoV-2, thus leading to a reduction in the ACE2/Ang (1–7)/Mas receptor (MasR) signalling axis, that modulates ACE/Ang II/AT1R signalling. This could result in increased presympathetic activity/neuroendocrine secretion from the PVH, and effects on the hypothalamic–pituitary–adrenal axis activity. Besides the bloodstream, the hypothalamus might also be affected by SARS-CoV-2 via transneuronal spread along the olfactory/limbic pathways. Exploring potential therapeutic pathways to prevent or attenuate neurological symptoms of COVID-19, including drugs which modulate ACE signalling, remains an important area of unmet medical need.
Keywords: COVID-19, ACE2, Circumventricular organs, Subfornical organ, Area postrema, Pineal gland, OVLT, Paraventricular nucleus of the hypothalamus
COVID-19 and Brain Blood Vessels, Glial Cells, and Neurons
Although the main clinical manifestations of COVID-19 are associated with respiratory or intestinal symptoms, reports of neurological signs and symptoms are increasing. The primary neurologic symptoms include ‘brain fog’ (81%), headache (68%), numbness/tingling (60%), dysgeusia (59%), anosmia (55%), and myalgia (55%). Most patients (85%) also report fatigue (Graham et al., 2021). The virus could potentially enter the brain through the circumventricular organs, disrupted blood–brain barrier, or retrograde transport via peripheral nerves (Kumar et al., 2021). Immune activation with astrocytosis, axonal damage, and blood–brain barrier leakage has been observed together with viral antigen and angiotensin-converting enzyme 2 (ACE2)-positive cells, at the blood–brain interface (Schwabenland et al., 2021). In addition, CSF levels of the chemokines CCL2 and CXCL8, and the blood vessel marker, vascular endothelium growth factor A are greater in severe COVID-19 cases than milder cases suggesting damage to the neurovascular unit (Bernard-Valnet et al., 2021). SARS-CoV-2 can stimulate extracellular neutrophils traps (NETs) in a process called NETosis. This normally functions as a defence against pathogens, but in excess may lead to increased reactive oxygen species (ROS) production in neutrophils and thrombus formation (Arcanjo et al., 2020; Pramitasuri et al., 2021). The SARS-CoV-2 spike protein reportedly binds to brain endothelial cells, resulting in inflammatory changes and loss of blood–brain barrier integrity (Buzhdygan et al., 2020). Increased numbers of microglia and astrocytes, and elevated levels of pro-inflammatory markers are found in post-mortem specimens of the cerebral cortex in patients with COVID-19 (Boroujeni et al., 2021). Analysis of single-nucleus transcriptomes from the dorsolateral prefrontal cortex of patients with severe COVID-19 shows that transcriptional changes consistent with activated microglia are present, despite an absence of viral transcripts post-mortem (Fullard et al., 2021).
SARS-CoV-2 infection of cells occurs through the binding with ACE2. Virus binding to ACE2 induces conformational changes in the S1 subunit of its spike protein and exposes the S2’ cleavage site in the S2 subunit. The S2’ site is then cleaved by a protease (e.g. cathepsin or transmembrane protease serine 2 (TMPRSS2)) to expose a fusion peptide within the spike protein, that is able to attach to and induce fusion of the viral envelope with the host cell membrane, thus facilitating infection of the cell [for recent review, see (Jackson et al., 2022)]. Infection of neurons leads to activation of nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2), free radical formation, and release of ROS and inflammatory molecules (Sindona et al., 2021). Free radical attack alters the phospholipid composition of mitochondrial membranes (Clough et al., 2021). This could lead to a loss of mitochondrial membrane potential and activation of the NLRP3 inflammasome, resulting in increased expression of pro-inflammatory genes (Clough et al., 2021; Sita et al., 2021). Imaging mass spectrometry analysis indicates astrocytosis, axonal damage and blood–brain barrier leakage, together with the detection of viral antigen in ACE2-positive cells in vascular compartments in post-mortem cases of COVID-19 (Schwabenland et al., 2021). Oligodendrocytes could also be affected by the SARS-CoV-2 virus and/or activated microglia, and this might result in CNS demyelination (Pan et al., 2020).
It is possible that the same mechanisms of COVID-19-induced damage in glial or endothelial cells described above could also occur in neurons. Increased levels of serum neurofilament light, indicating central and/or peripheral neuronal damage, are found in hospitalized patients with COVID-19, whereas no elevation is detected in milder cases (Paterson et al., 2021). Likewise, greater levels of CSF neurofilament light are present in critical cases of COVID-19 cases, compared to less severe cases (Garcia et al., 2021). RNA sequencing analyses of the amygdala of severe COVID-19 cases show increases in neuroinflammatory genes, but decreases in neuronal genes including those related to synaptic function (Piras et al., 2021). In addition, network analyses reveal a close relationship between COVID-19-induced neuroinflammation and pathways involved in Alzheimer’s disease (Zhou et al., 2021). Together, these findings indicate increased involvement of microglia, brain microvessels, and neurons with greater severity of COVID-19 (Stefano et al., 2021).
The SARS-CoV-2 virus enters cells by binding to ACE2 (Hoffmann et al., 2020). In this situation, ACE2 functions as a receptor for the spike protein of the SARS-CoV-2 and facilitates internalization of the virus and infection of the host cell (Jackson et al., 2022). It is also important to note that infection of cells by SARS viruses including SARS-CoV-2 results in a decrease of ACE2 expression (Kuba et al., 2005; Triana et al., 2021) and loss of ACE2 activity (Glowacka et al., 2010; Haga et al., 2010). This could prevent ACE2 from performing its normal function, to regulate or act as a ‘brake’ against ACE/Ang II/AT1R signalling (see below). Diminished effect on reducing angiotensin II (Ang II) signalling is suggested to contribute to injury in patients with COVID-19 (Sriram & Insel, 2020).
ACE2 and the Hypothalamus
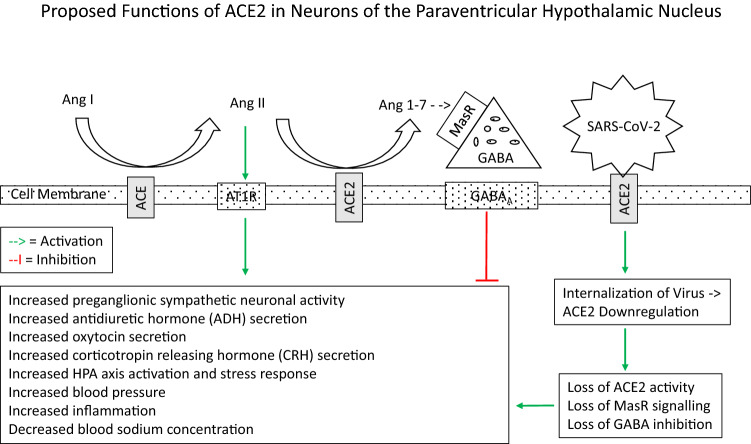
ACE helps in the formation of Ang II from angiotensin I, and ACE2 cleaves angiotensin 1 and angiotensin II into angiotensin (1–9) and angiotensin (1–7) (Ang (1–7)), respectively. The metabolism of Ang II to the vasodilatory peptide Ang(1–7) by ACE2 is part of the ACE2/Ang (1–7)/Mas receptor (MasR) axis which helps to reduce blood pressure, as opposed to the ACE/Ang II/AT1 Receptor (AT1R) axis, which increases blood pressure (Fig. 1). Surface-Enhanced Laser Desorption Ionization–Time of Flight mass spectroscopic analyses to study Ang processing in normal mice show that not only ACE2 activity is found in the brain and kidney but also that the hypothalamus is the part of the brain that contains the highest ACE2 activity. This is in contrast to ACE, which is most active in the plasma (Elased et al., 2008). Overexpression of ACE2 induced by injection of adenovirus encoding ACE2 in the paraventricular hypothalamic nucleus results in attenuation of Ang II-induced hypertension in rats (Sriramula et al., 2011). On the other hand, selective knockdown of ACE2 in the subfornical organ and paraventricular hypothalamic nucleus in mice results in partial loss of the ability to regulate deoxycorticosterone acetate-salt induced neurogenic hypertension (Xia et al., 2015). Studies also show lower protein expression of ACE2 in spontaneously hypertensive rats, compared to normotensive rats (Wang et al., 2017). Moreover, downregulation of components of the ACE2/Ang (1–7)/MasR axis is present in spontaneously hypertensive rats (Han et al., 2020). These findings highlight an important role of the hypothalamic ACE2/Ang (1–7)/MasR axis in regulation of blood pressure.
Fig. 1.
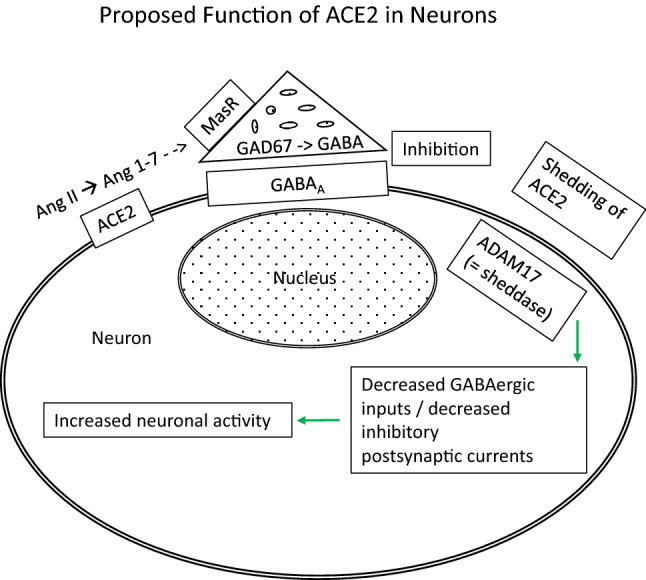
Proposed general functions of ACE2 in neurons. ACE2 catalyses the breakdown of Ang II to Ang (1–7). The latter is an agonist of the Mas receptor (MasR), which has been colocalised with GABAergic neurons (in the amygdala) (Wang et al., 2016). Ang (1–7) causes inhibition of principal neurons by promoting GABAergic transmission in a MasR-dependent manner (Wang et al., 2016). This could occur via facilitation of GABA release through a nitric oxide-mediated pathway (Stragier et al., 2005) and possibly supplemented by an increase in GABA production via upregulation of glutamate decarboxylase 67 (GAD67) expression (studied in the pancreas) (Ma et al., 2020). GABA binds to GABAA receptors to produce inhibition of the postsynaptic neuron. ACE2 is shed from the cell membrane, resulting in loss of activity through the action of another enzyme, ADAM17 (Xia et al., 2013). The latter is mostly expressed in glutamatergic projection neurons (Xu et al., 2019). In this manner, ACE2 and ADAM17 exert opposite effects on neuronal excitability (studied in presympathetic projection neurons of the paraventricular hypothalamic nucleus) (Mukerjee et al., 2019). It is possible that the above schema could also apply to neurons in other parts of the brain including those in the cerebral cortex and hippocampus, which express lower levels of ACE2 (Doobay et al., 2007)
Overexpression of ACE2 also significantly decreases anxiety-like behaviour in paradigms dependent on approach-avoidance conflict and novelty, but has no effect on basal and/or stress-induced hypothalamic–pituitary–adrenal (HPA) axis (de Kloet et al., 2020). In another study, mice with overexpression of ACE2 in corticotropin-releasing hormone (CRH) neurons of the hypothalamus show reduced plasma corticosterone level in response to restraint stress, and decreased anxiety-like behaviour in the elevated plus maze and open field test, compared to controls (Wang et al., 2018). The ACE2/Ang (1–7)/MasR axis attenuates stress-induced tachycardia, and this could contribute to reduced sympathetic load to the heart during emotional stress (Martins Lima et al., 2013). Adenovirus-mediated overexpression of ACE2 in the paraventricular hypothalamic nucleus results in reduction of sympathetic outflow (Zheng et al., 2011). In addition, direct microinjection of Ang (1–7) to the paraventricular hypothalamic nucleus results in modulation of sympathetic activity in renovascular hypertensive rats (Han et al., 2012). ACE2 is also known to have anti-inflammatory and anti-oxidative effects (Sriramula et al., 2011). This could occur via modulation of levels of ANG II which has both a pro-inflammatory effect via the production of tumour necrosis factor alpha (TNFα); and a pro-oxidative effect by increasing NADPH-oxidase expression and ROS production (Sriramula et al., 2013). Together, these findings indicate an important role of hypothalamic ACE2 in modulating the stress response.
ACE2 has an effect on regulating neuronal activity (Fig. 1). As noted above, ACE2 catalyses the breakdown of Ang II to Ang (1–7). The latter is an agonist of the Mas receptor (MasR), which has been colocalised with GABAergic neurons (in the amygdala) (Wang et al., 2016). Ang (1–7) causes inhibition of principal neurons by promoting GABAergic transmission in a MasR-dependent manner (Wang et al., 2016). This could occur via facilitation of GABA release through a nitric oxide-mediated pathway (Stragier et al., 2005) and possibly supplemented by an increase in GABA production via upregulation of glutamate decarboxylase 67 (GAD67) expression (studied in the pancreas) (Ma et al., 2020). GABA binds to GABAA receptors to produce inhibition of the postsynaptic neuron. ACE2 is shed from the cell membrane, resulting in loss of activity through the action of another enzyme, ADAM17 (Xia et al., 2013). The latter is mostly expressed in glutamatergic projection neurons (Xu et al., 2019). In this manner, ACE2 and ADAM17 exert opposite effects on neuronal excitability (studied in presympathetic projection neurons of the paraventricular hypothalamic nucleus) (Mukerjee et al., 2019). ADAM17 activity itself could be affected by oxidative stress. Mice that received an antioxidant, alpha-lipoic acid, showed modulation of oxidative stress-induced increased ADAM17 activity and decreased ACE2 activity in the hypothalamus (de Queiroz et al., 2015). It is possible that the above schema could also apply to neurons in other parts of the brain including those in the cerebral cortex and hippocampus, which express lower levels of ACE2 (Doobay et al., 2007).
In contrast to Ang (1–7), Ang II has an excitatory effect on neurons (Fig. 2). Both in vivo and in vitro studies show that Ang II stimulates PVN neuronal activity (Bains et al., 1992; Cato & Toney, 2005; Li et al., 2003). This is likely through a direct effect on AT1 receptors (AT1R) which has been localized to neurons in the PVH and the circumventricular organs, including the organum vasculosum of the lamina terminalis, subfornical organ, area postrema, and median eminence (Sumners et al., 2020). An indirect effect involving inhibition of the astrocyte glutamate transporter has also been proposed (Stern et al., 2016).
Fig. 2.
Proposed functions of ACE and ACE2 in neurons of the paraventricular hypothalamic nucleus. ACE catalyses the breakdown of Ang I to Ang II. The latter is further cleaved by ACE2 to the peptide Ang (1–7). In contrast to Ang (1–7) (see Fig. 1), Ang II has an excitatory effect on neurons. Both in vivo and in vitro studies show that Ang II stimulates PVN neuronal activity (Bains et al., 1992; Cato & Toney, 2005; Li et al., 2003). This is likely through a direct effect on AT1 receptors (AT1R) which has been localized to neurons in the PVH and the circumventricular organs, including the organum vasculosum of the lamina terminalis, subfornical organ, area postrema, and median eminence (Sumners et al., 2020). An indirect effect involving inhibition of the astrocyte glutamate transporter has also been proposed (Stern et al., 2016). SARS-CoV-2 binds to the ACE2 enzyme and is internalized into the neuron. Infection of cells by SARS viruses including SARS-Cov-2 results in a decrease of ACE2 expression (Kuba et al., 2005; Triana et al., 2021) and loss of ACE2 activity (Glowacka et al., 2010; Haga et al., 2010). This is postulated to produce an increase in neuronal excitation over neuronal inhibition, with resultant increased neuronal activity (including presympathetic neuronal activity), neuroendocrine secretion, and hormone release. These and other downstream effects are listed in the figure
ACE2 and Circumventricular Organs that are Intimately Connected to the Hypothalamus
The seven circumventricular organs are the organum vasculosum of the lamina terminalis (OVLT), subfornical organ, area postrema, pineal gland, subcommissural organ, median eminence, and neurohypophysis. Previous studies have shown high level of ACE2 expression in circumventricular organs (Doobay et al., 2007) and nuclei that are connected to the circumventricular organs. The latter includes the nucleus of the tractus solitarius, rostral ventrolateral medulla, and paraventricular nucleus of the hypothalamus (Doobay et al., 2007; Kar et al., 2010; Zucker et al., 2014). ACE2 is localised in neurons of the above nuclei (Doobay et al., 2007; Kar et al., 2010). The OVLT, subfornical organ, and area postrema are important in regulation of osmotic thirst, anti-diuretic hormone release, and blood pressure (Johnson et al., 1996; McKinley et al., 1992b). Their constituent neurons project not only to the paraventricular hypothalamic nucleus, but also the brainstem. Axons send collateral branches to the nucleus of the tractus solitarius, and rostral ventrolateral medulla, before terminating on pre-ganglionic sympathetic neurons in the intermediolateral horn of the spinal cord to increase sympathetic activity (Duan et al., 2020; Gu, 2021; Thomas, 2011).
Organum Vasculosum of the Lamina Terminalis (OVLT)
The OVLT is located in the anterior wall of the third ventricle, approximately midway between the optic chiasm and the anterior commissure (McKinley et al., 2004). Unlike other species, fenestrations in the capillary endothelial cells have not been observed in the human OVLT. Nevertheless, reports of imbibed silver suggest altered blood–brain barrier characteristics of the human OVLE (Landas et al., 1985). The major afferent inputs to the OVLE appear to come from several hypothalamic nuclei (median preoptic, lateral preoptic, anterior, lateral, dorsomedial, and ventromedial nuclei) and extrahypothalamic regions (subfornical organ, locus coeruleus, central grey) (Camacho & Phillips, 1981). Studies in the rat indicate that the OVLE project to the median preoptic nucleus immediately dorsal to the OVLT, the supraoptic nucleus (Camacho & Phillips, 1981), and the paraventricular hypothalamic nucleus (Sunn et al., 2001). The OVLT has a role as an osmoreceptor, a mediator of the febrile response, and a receptor for blood-borne Ang II and relaxin to stimulate the CNS [Reviewed in (McKinley et al., 2004)]. It is part of a neural network that is involved in the regulation of fluid balance. Dense binding of radioiodinated analogs of Ang II have been observed in the human OVLT, median preoptic nucleus, subfornical organ, median eminence, arcuate nucleus, and paraventricular nucleus (McKinley et al., 1987). This is consistent with the observation in animals of high concentrations of angiotensin AT1 receptors in the OVLT (McKinley et al., 1986; Mendelsohn et al., 1984). These findings support the notion that OVLT is one of the sites at which blood-borne Ang II may act on the CNS to affect fluid balance and arterial blood pressure (Camacho & Phillips, 1981).
Subfornical Organ
The subfornical organ is located in the midline anterior wall of the third ventricle at the level of the superior border of the interventricular foramen (McKinley et al., 2004) Capillaries in some regions of the subfornical organ are fenestrated and lack a blood–brain barrier (Shaver et al., 1990). Such permeability of the vasculature to circulating agents provides the basis for its function as a site of receptors for circulating angiotensin II (Giles et al., 1999). High-affinity binding of radiolabelled Ang II (McKinley et al., 1987; Mendelsohn et al., 1984; Speth et al., 1985), together with high expression of angiotensin AT1R (Giles et al., 1999) have been found in the subfornical organ. Efferents from the subfornical organ to the paraventricular hypothalamic nucleus and supraoptic nucleus change the excitability of anti-diuretic hormone and oxytocin neurons projecting to the posterior pituitary (Ferguson et al., 1984a); as well as that of anti-diuretic hormone containing neurons projecting to the dorsolateral medulla (Ferguson et al., 1984b). Studies using a combination of Fos staining and tract tracing show that neurons in the subfornical organ which project to the supraoptic and paraventricular nucleus, can be activated by hypertonicity or by circulating levels of Ang II or relaxin (Larsen & Mikkelsen, 1995; Oldfield et al., 1994; Sunn et al., 2001). Reduction in blood flow to the renal artery or increased sympathetic activity via renal nerves results in secretion of renin from juxtaglomerular cells in the tunica media of the renal arterioles as they enter the glomeruli. Renin converts angiotensinogen to angiotensin 1. Most of the angiotensin-converting enzyme is located in endothelial cells [Reviewed in (Barrett et al., 2016)], but some of the blood-borne angiotensin I reaches the subfornical organ where it is converted to Ang II. This leads to activation of neurons in the subfornical organ, induction of water drinking, anti-diuretic hormone secretion, and a central pressor response (McKinley et al., 1992a, 1997; Simpson, 1981).
Pineal Gland
The pineal gland in humans is a solid organ located in the midline roof of the third ventricle. It contains astrocytes and pinealocytes, which secrete melatonin. The central part of the gland highly vascularized by large sinusoid capillaries and its peripheral part poorly vascularized by small and fine blood vessels (Duvernoy et al., 2000). The major neural to the pineal gland is from the peripheral nervous system, i.e. sympathetic innervation from the superior cervical ganglion (Kappers, 1965). Signals from the retina are transmitted via the retinohypothalamic tract to the suprachiasmatic nucleus of the hypothalamus; from here, information is relayed via the paraventricular hypothalamic nucleus to the intermediolateral cell column of the spinal cord. The latter sends axons to synapse in the superior cervical ganglion; thereafter, postganglionic axons travel along the great cerebral vein of Galen to enter the pineal gland and innervate pinealocytes (Larsen et al., 1998; Teclemariam-Mesbah et al., 1999). It is through this pathway that information regarding changes in the day-night cycle and seasonal alterations in illumination is transmitted to the pineal gland to regulate melatonin secretion (Tamarkin et al., 1985). mRNA for angiotensinogen, angiotensin receptor type 1A (AT1aR) and 1B (AT1bR), and ACE have been detected in the pineal glands of rats. The presence of angiotensin receptors points to a role for renin-angiotensin system in the physiology of the pineal gland (Baltatu et al., 1998).
Area Postrema
The area postrema is located in the dorsomedial medulla oblongata as two prominences bulging into the most caudal aspect of the fourth ventricle (McKinley et al., 2004). This nucleus and the specialized region of the adjacent nucleus of the tractus solitarius have permeable capillaries (Gross et al., 1990). Ablation of the area postrema in animals interferes with taste aversion, due to nausea-inducing stimuli (Berger et al., 1973). The area postrema is long thought to be the ‘chemoreceptor trigger zone’ for vomiting. This nucleus receives afferents from the lateral parabrachial nucleus, the adjacent nucleus of the tractus solitarius (Shapiro & Miselis, 1985; van der Kooy & Koda, 1983), and the paraventricular hypothalamic nucleus (Shapiro & Miselis, 1985). In turn, it projects to the nucleus of the tractus solitarius, nucleus ambiguus, and noradrenergic neurons of the caudal ventrolateral medulla (McKinley et al., 2004). The unique position of the area postrema at the point of entry of visceral sensory information, as well as lack of a blood–brain barrier that exposes it to the circulation, enables it to integrate and modulate homeostatic responses in the body (Gross et al., 1990; Miselis et al., 1987; Shaver et al., 1991).
Median Eminence
The median eminence and arcuate nucleus of the hypothalamus have a deficient blood–brain barrier [Reviewed in (Haddad-Tovolli et al., 2017)]. Moreover, dense binding of radioiodinated analogs of Ang II have been observed in the human median eminence and arcuate nucleus (McKinley et al., 1987).
Subcommissural Organ
Unlike other mammals, the human subcommissural organ is only clearly evident in the foetus and new-born. In the adult it has almost completely disappeared (McKinley et al., 2004).
The Hypothalamus and COVID-19 (Fig. 2)
There has been increasing attention to the role of the hypothalamus and brainstem autonomic centres in the pathophysiology of COVID-19 (Chigr et al., 2020). The fact that circumventricular organs lack a blood–brain barrier (Haddad-Tovolli et al., 2017), together with the high levels of ACE2 expression in circumventricular organs and hypothalamus (Doobay et al., 2007), suggests that they could be easy entry points for SARS-CoV-2 into the brain via the circulation. On the corollary, the observation that some of the ACE2-positive regions of the brain lack a blood–brain barrier, could imply that they are amenable to treatment with antiviral agents/other therapeutic agents, that otherwise have difficulty crossing the barrier. Hypothalamic pathology is found in a case of COVID-19 (Pascual-Goni et al., 2020), and involvement of the hypothalamus/pituitary gland as well as other endocrine glands has been suggested to result in an ‘endocrine phenotype’ of the disease (Frara et al., 2021; Mussa et al., 2021; Puig-Domingo et al., 2021).
The paraventricular nucleus of the hypothalamus receives abundant connections from the circumventricular organs which contain high level of ACE2 expression, and itself expresses ACE2 protein (Doobay et al., 2007), as mentioned above. These are part of a circuit to regulate osmolarity in the blood through secretion of anti-diuretic hormone (ADH) from the paraventricular hypothalamic nucleus. It is interesting that hyponatremia has been found in a relatively high proportion of COVID-19 patients. The Health Outcome Predictive Evaluation for COVID-19 (HOPE) study found that 20.5% of COVID-19 patients had hyponatremia at admission (Ruiz-Sanchez et al., 2020), and greater morbidity and mortality are reported in hospitalized patients with hyponatremia (Tzoulis et al., 2021). Many of the cases of hyponatremia likely occur in the setting of Syndrome of Inappropriate Secretion of Antidiuretic Hormone (SIADH) (Frara et al., 2021; Habib et al., 2020; Yousaf et al., 2020). The latter is a disorder of impaired water excretion caused by the inability to suppress the secretion of anti-diuretic hormone. If water intake exceeds the reduced urine output, the ensuing water retention leads to the development of hyponatremia. This could be due to loss of function of ACE2 as a result of binding to SARS-CoV-2, and consequent increase in ACE signalling. Changes in the subfornical organ neurons likely affect its output to the paraventricular hypothalamic nucleus. Interference with ADH release into the posterior pituitary via an effect on the subfornical organ and paraventricular hypothalamic nucleus has been postulated to lead to hydroelectrolytic imbalance in some patients with COVID-19 (de Melo et al., 2021).
The paraventricular hypothalamic nucleus is also the stress response centre in the brain. Neurons in this nucleus coordinate this response through their neuronal connections, and via their neuroendocrine secretion of CRH (Kim et al., 2019). The latter has several actions, but its main role is the central driver of the HPA axis. It is proposed that inflammatory mediators released at the site of COVID-19 infection are transmitted as stress signals to cause dysfunction to the complex neurological circuit of the paraventricular hypothalamic nucleus, and result in interference with the modulation of stress (Mackay, 2021). Higher levels of cortisol have been detected in patients with severe COVID-19 compared to less severe cases (Guven & Gultekin, 2021; Tan et al., 2020). These findings are consistent with the findings of pre-clinical studies, that mice with overexpression of ACE2 in the hypothalamus show reduced plasma corticosterone level in response to restraint stress. These animals also exhibit decreased anxiety-like behaviour in the “elevated-plus maze” and open field test (Wang et al., 2018). High level of stress might induce downstream effects on hippocampal neurogenesis in the dentate gyrus, leading to deficits in the formation of new memories (McEwen, 1999). It is proposed that the function of ACE2-positive neurons in the paraventricular hypothalamic nucleus is affected by binding with the SARS-CoV-2 virus, resulting in interference with modulation of Ang II function and reduced modulation of stress/anxiety, in patients with COVID-19. Some of the other psychiatric consequences of COVID-19 patients such as post-viral fatigue states, aberrant daytime oscillation in alertness, disturbed sleep cycles, and significant fluctuating anxiety have also been postulated to be due to altered function of the paraventricular hypothalamic nucleus (Rosenzweig et al., 2020).
The paraventricular hypothalamic nucleus and several other hypothalamic nuclei are the source of releasing and inhibiting hormones, which control the secretion of hormones from the anterior pituitary. Gonadotropic-releasing hormone neurons are located primarily in the medial preoptic area; growth-hormone-inhibiting hormone (= somatostatin) secreting neurons are found in the periventricular nucleus; thyrotropin-releasing hormone (TRH) and corticotropin-releasing hormone (CRH) secreting neurons are located in the paraventricular nucleus; while growth hormone releasing (GRH) neurons are located in the arcuate nucleus [Reviewed in (Barrett et al., 2016)]. These hormones are released from nerve terminals in the median eminence of the hypothalamus and carried via the bloodstream to the anterior pituitary, where they cause secretion of anterior pituitary hormones. The median eminence and arcuate nucleus of the hypothalamus have a deficient blood–brain barrier [Reviewed in (Haddad-Tovolli et al., 2017)] and could be readily affected by circulating SARS-CoV-2. Damage to the hypothalamus and/or pituitary gland as a result of COVID-19 has been suggested to result in anomalies of the hypothalamus–pituitary–thyroid axis (Caron, 2020; Malik et al., 2021). This could result in a central hypothyroidism (Sandru et al., 2021), besides potential damage from the virus to the thyroid gland itself (Croce et al., 2021). COVID-19 injury of the hypothalamus is also proposed to lead to malfunction of the hypothalamic–pituitary–testicular axis (Ardestani Zadeh & Arab, 2021; Selvaraj et al., 2021) with possible effects on testicular function (Selvaraj et al., 2021).
Besides potential infection from the bloodstream, the hypothalamus might also be affected by transneuronal spread of SARS-CoV-2, through its neuronal connections with the olfactory and limbic systems. These pathways have been postulated to be a potential route of spread of SARS-CoV-2 from the olfactory epithelium (Baig & Sanders, 2020; Bougakov et al., 2021; Jiao et al., 2021; Mussa et al., 2021). After receiving inputs from the olfactory epithelium in the nasal cavity, the olfactory bulb projects via the medial olfactory stria to the septal nuclei, which in turn projects to the hypothalamus [Reviewed in (Getz, 2007)]. The olfactory bulb also projects via the lateral olfactory stria to the amygdala, which projects to the hypothalamus via the stria terminalis. Other parts of the lateral olfactory stria terminate in the entorhinal cortex, which projects to the hippocampus, and the latter projects to the mammillary bodies of the hypothalamus via the fornix [Reviewed in (Getz, 2007)]. Previous studies have shown the ability of SARS-CoV to induce neuronal death in mice by invading the brain via the olfactory epithelium (Netland et al., 2008). Direct infection of neurons in organoids by SARS-CoV-2 has also been demonstrated (Song et al., 2021). Decreased 18F-FDG PET metabolism in olfactory/limbic regions and the hypothalamus has been found in a pilot study of COVID-19 patients (Guedj et al., 2021), suggesting that olfactory and limbic regions are affected by the virus.
Conclusion
A better understanding of the role of ACE2 in circumventricular organs and the hypothalamus may help in appreciating the effects of neurological symptoms of COVID and its underlying mechanisms. Given the socio-economic impact and drain on healthcare resources from COVID and the scale and persistence of the pandemic, exploring potential therapeutic pathways to prevent or attenuate these long-lasting neurological symptoms, including drugs which modulate ACE signalling, remains an important area of unmet medical need.
Acknowledgements
This work was supported by grants from the Ministry of Education (NUHSRO/2019/051/T1/Seed-Mar/04) and the National Medical Research Council of Singapore (HLCA21Jan-0019).
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arcanjo, A., Logullo, J., Menezes, C. C. B., de Souza Carvalho Giangiarulo, T. C., Dos Reis, M. C., de Castro, G. M. M et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19) Science and Reports. 2020;10(1):19630. doi: 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardestani Zadeh A, Arab D. COVID-19 and male reproductive system: Pathogenic features and possible mechanisms. Journal of Molecular Histology. 2021;52(5):869–878. doi: 10.1007/s10735-021-10003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig AM, Sanders EC. Potential neuroinvasive pathways of SARS-CoV-2: Deciphering the spectrum of neurological deficit seen in coronavirus disease-2019 (COVID-19) Journal of Medical Virology. 2020;92(10):1845–1857. doi: 10.1002/jmv.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: Functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Research. 1992;599(2):223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- Baltatu O, Lippoldt A, Hansson A, Ganten D, Bader M. Local renin-angiotensin system in the pineal gland. Molecular Brain Research. 1998;54(2):237–242. doi: 10.1016/s0169-328x(97)00339-2. [DOI] [PubMed] [Google Scholar]
- Barrett, K. E., Barman, S. M., Boitano, S., & Brooks, H. L. (2016). Ganong's review of medical physiology. Mc Graw Hill.
- Berger BD, Wise CD, Stein L. Area postrema damage and bait shyness. Journal of Comparative and Physiological Psychology. 1973;82(3):475–479. doi: 10.1037/h0034112. [DOI] [PubMed] [Google Scholar]
- Bernard-Valnet, R., Perriot, S., Canales, M., Pizzarotti, B., Caranzano, L., Castro-Jimenez, M., et al. (2021). Encephalopathies associated with severe COVID-19 present neurovascular unit alterations without evidence for strong neuroinflammation. Neurology - Neuroimmunology Neuroinflammation. 10.1212/NXI.0000000000001029 [DOI] [PMC free article] [PubMed]
- Boroujeni ME, Simani L, Bluyssen HAR, Samadikhah HR, Zamanlui Benisi S, Hassani S, et al. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chemical Neuroscience. 2021;12(12):2143–2150. doi: 10.1021/acschemneuro.1c00111. [DOI] [PubMed] [Google Scholar]
- Bougakov D, Podell K, Goldberg E. Multiple neuroinvasive pathways in COVID-19. Molecular Neurobiology. 2021;58(2):564–575. doi: 10.1007/s12035-020-02152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, Bullock TA, McGary HM, Khan JA, et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiology of Diseases. 2020;146:105131. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A, Phillips MI. Horseradish peroxidase study in rat of the neural connections of the organum vasculosum of the lamina terminalis. Neuroscience Letters. 1981;25(3):201–204. doi: 10.1016/0304-3940(81)90391-8. [DOI] [PubMed] [Google Scholar]
- Caron P. Thyroid disorders and SARS-CoV-2 infection: From pathophysiological mechanism to patient management. Annales d'Endocrinologie (Paris) 2020;81(5):507–510. doi: 10.1016/j.ando.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: An in vitro patch-clamp study in brain slices. Journal of Neurophysiology. 2005;93(1):403–413. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigr F, Merzouki M, Najimi M. Autonomic brain centers and pathophysiology of COVID-19. ACS Chemical Neuroscience. 2020;11(11):1520–1522. doi: 10.1021/acschemneuro.0c00265. [DOI] [PubMed] [Google Scholar]
- Clough E, Inigo J, Chandra D, Chaves L, Reynolds JL, Aalinkeel R, et al. Mitochondrial dynamics in SARS-COV2 spike protein treated human microglia: Implications for neuro-COVID. Journal of Neuroimmune Pharmacology. 2021 doi: 10.1007/s11481-021-10015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce L, Gangemi D, Ancona G, Liboa F, Bendotti G, Minelli L, et al. The cytokine storm and thyroid hormone changes in COVID-19. Journal of Endocrinological Investigation. 2021;44(5):891–904. doi: 10.1007/s40618-021-01506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Cahill KM, Scott KA, Krause EG. Overexpression of angiotensin converting enzyme 2 reduces anxiety-like behavior in female mice. Physiology & Behavior. 2020;224:113002. doi: 10.1016/j.physbeh.2020.113002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo IS, Sabino-Silva R, Cunha TM, Goulart LR, Reis WL, Jardim ACG, et al. Hydroelectrolytic disorder in COVID-19 patients: Evidence supporting the involvement of subfornical organ and paraventricular nucleus of the hypothalamus. Neuroscience and Biobehavioral Reviews. 2021;124:216–223. doi: 10.1016/j.neubiorev.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Queiroz TM, Xia H, Filipeanu CM, Braga VA, Lazartigues E. alpha-Lipoic acid reduces neurogenic hypertension by blunting oxidative stress-mediated increase in ADAM17. American Journal of Physiology-Heart and Circulatory Physiology. 2015;309(5):H926–934. doi: 10.1152/ajpheart.00259.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2007;292(1):R373–381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Xue X, Zhang QQ, Wang SY, Gong PY, et al. ACE2 activator diminazene aceturate ameliorates Alzheimer's disease-like neuropathology and rescues cognitive impairment in SAMP8 mice. Aging. 2020;12(14):14819–14829. doi: 10.18632/aging.103544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM, Parratte B, Tatu L, Vuillier F. The human pineal gland: Relationships with surrounding structures and blood supply. Neurological Research. 2000;22(8):747–790. doi: 10.1080/01616412.2000.11740753. [DOI] [PubMed] [Google Scholar]
- Elased KM, Cunha TS, Marcondes FK, Morris M. Brain angiotensin-converting enzymes: Role of angiotensin-converting enzyme 2 in processing angiotensin II in mice. Experimental Physiology. 2008;93(5):665–675. doi: 10.1113/expphysiol.2007.040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AV, Day TA, Renaud LP. Subfornical organ efferents influence the excitability of neurohypophyseal and tuberoinfundibular paraventricular nucleus neurons in the rat. Neuroendocrinology. 1984;39(5):423–428. doi: 10.1159/000124015. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Day TA, Renaud LP. Subfornical organ stimulation excites paraventricular neurons projecting to dorsal medulla. American Journal of Physiology. 1984;247(6 Pt 2):R1088–1092. doi: 10.1152/ajpregu.1984.247.6.R1088. [DOI] [PubMed] [Google Scholar]
- Frara S, Allora A, Castellino L, di Filippo L, Loli P, Giustina A. COVID-19 and the pituitary. Pituitary. 2021;24(3):465–481. doi: 10.1007/s11102-021-01148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullard JF, Lee HC, Voloudakis G, Suo S, Javidfar B, Shao Z, et al. Single-nucleus transcriptome analysis of human brain immune response in patients with severe COVID-19. Genome Medicine. 2021;13(1):118. doi: 10.1186/s13073-021-00933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Barreras PV, Lewis A, Pinilla G, Sokoll LJ, Kickler T, et al. Cerebrospinal fluid in COVID-19 neurological complications: Neuroaxonal damage, anti-SARS-Cov2 antibodies but no evidence of cytokine storm. Journal of the Neurological Sciences. 2021;427:117517. doi: 10.1016/j.jns.2021.117517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz, S. (2007). Liebman's neuroanatomy made easy and understandable. Pro-Ed.
- Giles ME, Fernley RT, Nakamura Y, Moeller I, Aldred GP, Ferraro T, et al. Characterization of a specific antibody to the rat angiotensin II AT1 receptor. Journal of Histochemistry and Cytochemistry. 1999;47(4):507–516. doi: 10.1177/002215549904700409. [DOI] [PubMed] [Google Scholar]
- Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. Journal of Virology. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 "long haulers". Annals of Clinical Translational Neurology. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. American Journal of Physiology. 1990;259(6 Pt 2):R1131–1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Gu, C. (2021). Rapid and reversible development of axonal varicosities: A new form of neural plasticity. [Review]. Frontiers in Molecular Neuroscience. 10.3389/fnmol.2021.610857 [DOI] [PMC free article] [PubMed]
- Guedj E, Million M, Dudouet P, Tissot-Dupont H, Bregeon F, Cammilleri S, et al. (18)F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: Substrate for persistent/delayed disorders? European Journal of Nuclear Medicine and Molecular Imaging. 2021;48(2):592–595. doi: 10.1007/s00259-020-04973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven M, Gultekin H. Could serum total cortisol level at admission predict mortality due to coronavirus disease 2019 in the intensive care unit? A prospective study. Sao Paulo Medical Journal. 2021;139(4):398–404. doi: 10.1590/1516-3180.2020.0722.R1.2302021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib MB, Sardar S, Sajid J. Acute symptomatic hyponatremia in setting of SIADH as an isolated presentation of COVID-19. Idcases. 2020;21:e00859. doi: 10.1016/j.idcr.2020.e00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad-Tovolli R, Dragano NRV, Ramalho AFS, Velloso LA. Development and function of the blood–brain barrier in the context of metabolic control. Frontiers in Neuroscience. 2017;11:224. doi: 10.3389/fnins.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S, Nagata N, Okamura T, Yamamoto N, Sata T, Yamamoto N, et al. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Research. 2010;85(3):551–555. doi: 10.1016/j.antiviral.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Wang M, Zhai X, Gan Q, Guan S, Qu X. Chemical renal denervation-induced upregulation of the ACE2/Ang (1–7)/Mas axis attenuates blood pressure elevation in spontaneously hypertensive rats. Clinical and Experimental Hypertension. 2020;42(7):661–668. doi: 10.1080/10641963.2020.1772812. [DOI] [PubMed] [Google Scholar]
- Han Y, Sun HJ, Li P, Gao Q, Zhou YB, Zhang F, et al. Angiotensin-(1–7) in paraventricular nucleus modulates sympathetic activity and cardiac sympathetic afferent reflex in renovascular hypertensive rats. PLoS ONE. 2012;7(11):e48966. doi: 10.1371/journal.pone.0048966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M., Kleine-Weber, H., Schroeder, S., Kruger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280 e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed]
- Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nature Reviews Molecular Cell Biology. 2022;23(1):3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L, Yang Y, Yu W, Zhao Y, Long H, Gao J, et al. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal Transduction and Targeted Therapy. 2021;6(1):169. doi: 10.1038/s41392-021-00591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AK, Cunningham JT, Thunhorst RL. Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis. Clinical and Experimental Pharmacology and Physiology. 1996;23(2):183–191. doi: 10.1111/j.1440-1681.1996.tb02594.x. [DOI] [PubMed] [Google Scholar]
- Kappers JA. Survey of the innervation of the epiphysis cerebri and the accessory pineal organs of vertebrates. Progress in Brain Research. 1965;10:87–153. doi: 10.1016/s0079-6123(08)63448-2. [DOI] [PubMed] [Google Scholar]
- Kar, S., Gao, L., & Zucker, I. H. (2010). Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. Journal of Applied Physiology (1985), 108(4), 923–932. 10.1152/japplphysiol.00840.2009 [DOI] [PMC free article] [PubMed]
- Kim JS, Han SY, Iremonger KJ. Stress experience and hormone feedback tune distinct components of hypothalamic CRH neuron activity. Nature Communications. 2019;10(1):5696. doi: 10.1038/s41467-019-13639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nature Medicine. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Harilal S, Sabitha M, Pappachan LK, Roshni PR, Mathew B. Current perspective of COVID-19 on neurology: A mechanistic insight. Combinatorial Chemistry & High Throughput Screening. 2021 doi: 10.2174/1386207324666210805121828. [DOI] [PubMed] [Google Scholar]
- Landas S, Fischer J, Wilkin LD, Mitchell LD, Johnson AK, Turner JW, et al. Demonstration of regional blood-brain barrier permeability in human brain. Neuroscience Letters. 1985;57(3):251–256. doi: 10.1016/0304-3940(85)90500-2. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Enquist LW, Card JP. Characterization of the multisynaptic neuronal control of the rat pineal gland using viral transneuronal tracing. European Journal of Neuroscience. 1998;10(1):128–145. doi: 10.1046/j.1460-9568.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute "stress" to the hypothalamic paraventricular nucleus. Journal of Neuroscience. 1995;15(4):2609–2627. doi: 10.1523/jneurosci.15-04-02609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. Journal of Neuroscience. 2003;23(12):5041–5049. doi: 10.1523/jneurosci.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Gao F, Chen Q, Xuan X, Wang Y, Deng H, et al. ACE2 modulates glucose homeostasis through GABA signaling during metabolic stress. Journal of Endocrinology. 2020;246(3):223–236. doi: 10.1530/joe-19-0471. [DOI] [PubMed] [Google Scholar]
- Mackay A. A paradigm for post-covid-19 fatigue syndrome analogous to ME/CFS. Frontiers in Neurology. 2021;12:701419. doi: 10.3389/fneur.2021.701419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik J, Zaidi SMJ, Waqar AU, Khawaja H, Malik A, Ishaq U, et al. Association of hypothyroidism with acute COVID-19: A systematic review. Expert Review of Endocrinology and Metabolism. 2021;16(5):251–257. doi: 10.1080/17446651.2021.1968830. [DOI] [PubMed] [Google Scholar]
- Martins Lima A, Xavier CH, Ferreira AJ, Raizada MK, Wallukat G, Velloso EP, et al. Activation of angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis attenuates the cardiac reactivity to acute emotional stress. American Journal of Physiology-Heart and Circulatory Physiology. 2013;305(7):H1057–1067. doi: 10.1152/ajpheart.00433.2013. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual Review of Neuroscience. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Allen A, Clevers J, Denton DA, Mendelsohn FA. Autoradiographic localization of angiotensin receptors in the sheep brain. Brain Research. 1986;375(2):373–376. doi: 10.1016/0006-8993(86)90761-4. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Allen AM, Clevers J, Paxinos G, Mendelsohn FA. Angiotensin receptor binding in human hypothalamus: Autoradiographic localization. Brain Research. 1987;420(2):375–379. doi: 10.1016/0006-8993(87)91260-1. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Badoer E, Oldfield BJ. Intravenous angiotensin II induces Fos-immunoreactivity in circumventricular organs of the lamina terminalis. Brain Research. 1992;594(2):295–300. doi: 10.1016/0006-8993(92)91138-5. [DOI] [PubMed] [Google Scholar]
- McKinley, M. J., Bicknell, R. J., Hards, D., McAllen, R. M., Vivas, L., Weisinger, R. S., et al. (1992b). Efferent neural pathways of the lamina terminalis subserving osmoregulation. In A. Ermisch, R. Landgraf, & H.-J. Rühle (Eds.), Progress in brain research (Vol. 91, pp. 395–402). Elsevier. [DOI] [PubMed]
- McKinley, M. J., Clarke, I. J., & Oldfield, B. J. (2004). Circumventricular organs. In G. Paxinos, & J. K. Mai (Eds.), The human nervous system. (2nd ed.). Elsevier.
- McKinley MJ, Colvill LM, Giles ME, Oldfield BJ. Distribution of Fos-immunoreactivity in rat brain following a dipsogenic dose of captopril and effects of angiotensin receptor blockade. Brain Research. 1997;747(1):43–51. doi: 10.1016/s0006-8993(96)01178-x. [DOI] [PubMed] [Google Scholar]
- Mendelsohn FA, Quirion R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin II receptors in rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(5):1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miselis, R. R., Shapiro, R. E., & Hyde, I. M. (1987). The area postrema. In Circumventricualar organs and body fluids (Vol. 2, pp. 185–207). CRC Press.
- Mukerjee S, Gao H, Xu J, Sato R, Zsombok A, Lazartigues E. ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension. 2019;74(5):1181–1191. doi: 10.1161/HYPERTENSIONAHA.119.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussa BM, Srivastava A, Verberne AJM. COVID-19 and neurological impairment: Hypothalamic circuits and beyond. Viruses. 2021 doi: 10.3390/v13030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. Journal of Virology. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience. 1994;60(1):255–262. doi: 10.1016/0306-4522(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Pan R, Zhang Q, Anthony SM, Zhou Y, Zou X, Cassell M, et al. Oligodendrocytes that survive acute coronavirus infection induce prolonged inflammatory responses in the CNS. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(27):15902–15910. doi: 10.1073/pnas.2003432117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Goni E, Fortea J, Martinez-Domeno A, Rabella N, Tecame M, Gomez-Oliva C, et al. COVID-19-associated ophthalmoparesis and hypothalamic involvement. Neurology: Neuroimmunology & Neuroinflammation. 2020 doi: 10.1212/NXI.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, R. W., Benjamin, L. A., Mehta, P. R., Brown, R. L., Athauda, D., Ashton, N. J., et al. (2021). Serum and cerebrospinal fluid biomarker profiles in acute SARS-CoV-2-associated neurological syndromes. Brain Communications, 3(3), fcab099. 10.1093/braincomms/fcab099 [DOI] [PMC free article] [PubMed]
- Piras, I. S., Huentelman, M. J., Walker, J. E., Arce, R., Glass, M. J., Vargas, D., et al. (2021). Olfactory bulb and amygdala gene expression changes in subjects dying with COVID-19. medRxiv. 10.1101/2021.09.12.21263291
- Pramitasuri TI, Laksmidewi A, Putra IBK, Dalimartha FA. Neutrophil extracellular traps in coronavirus disease-19-associated ischemic stroke: A novel avenue in neuroscience. Experimental Neurobiology. 2021;30(1):1–12. doi: 10.5607/en20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Domingo, M., Marazuela, M., Yildiz, B. O., & Giustina, A. (2021). COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine, 72(2), 301–316. 10.1007/s12020-021-02734-w [DOI] [PMC free article] [PubMed]
- Rosenzweig I, Mitrecic D, Petanjek Z, Duffy B, Young AH, Nesbitt AD, et al. Does damage to hypothalamic paraventricular nucleus underlie symptoms of ultradian rhythm disorder and an increased anxiety in coronavirus disease 2019? Croatian Medical Journal. 2020;61(4):377–380. doi: 10.3325/cmj.2020.61.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Sanchez, J. G., Nunez-Gil, I. J., Cuesta, M., Rubio, M. A., Maroun-Eid, C., Arroyo-Espliguero, R., et al. (2020). Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia. A HOPE-COVID-19 (health outcome predictive evaluation for COVID-19) registry analysis. Frontiers in Endocrinology (Lausanne), 11, 599255. 10.3389/fendo.2020.599255 [DOI] [PMC free article] [PubMed]
- Sandru F, Carsote M, Petca RC, Gheorghisan-Galateanu AA, Petca A, Valea A, et al. COVID-19-related thyroid conditions (review) Experimental and Therapeutic Medicine. 2021;22(1):756. doi: 10.3892/etm.2021.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabenland, M., Salie, H., Tanevski, J., Killmer, S., Lago, M. S., Schlaak, A. E., et al. (2021). Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity, 54(7), 1594–1610 e1511. 10.1016/j.immuni.2021.06.002 [DOI] [PMC free article] [PubMed]
- Selvaraj K, Ravichandran S, Krishnan S, Radhakrishnan RK, Manickam N, Kandasamy M. Testicular atrophy and hypothalamic pathology in COVID-19: Possibility of the incidence of male infertility and HPG axis abnormalities. Reproductive Sciences. 2021;28(10):2735–2742. doi: 10.1007/s43032-020-00441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. The Journal of Comparative Neurology. 1985;234(3):344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- Shaver SW, Pang JJ, Wall KM, Sposito NM, Gross PM. Subregional topography of capillaries in the dorsal vagal complex of rats: I. Morphometric properties. The Journal of Comparative Neurology. 1991;306(1):73–82. doi: 10.1002/cne.903060106. [DOI] [PubMed] [Google Scholar]
- Shaver SW, Sposito NM, Gross PM. Quantitative fine structure of capillaries in subregions of the rat subfornical organ. The Journal of Comparative Neurology. 1990;294(1):145–152. doi: 10.1002/cne.902940111. [DOI] [PubMed] [Google Scholar]
- Simpson JB. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology. 1981;32(4):248–256. doi: 10.1159/000123167. [DOI] [PubMed] [Google Scholar]
- Sindona C, Schepici G, Contestabile V, Bramanti P, Mazzon E. NOX2 activation in COVID-19: Possible implications for neurodegenerative diseases. Medicina (Kaunas, Lithuania) 2021 doi: 10.3390/medicina57060604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sita G, Graziosi A, Hrelia P, Morroni F. NLRP3 and infections: Beta-amyloid in inflammasome beyond neurodegeneration. International Journal of Molecular Sciences. 2021 doi: 10.3390/ijms22136984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. Journal of Experimental Medicine. 2021 doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth RC, Wamsley JK, Gehlert DR, Chernicky CL, Barnes KL, Ferrario CM. Angiotensin II receptor localization in the canine CNS. Brain Research. 1985;326(1):137–143. doi: 10.1016/0006-8993(85)91392-7. [DOI] [PubMed] [Google Scholar]
- Sriram K, Insel PA. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. British Journal of Pharmacology. 2020;177(21):4825–4844. doi: 10.1111/bph.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS ONE. 2013;8(5):e63847. doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovascular Research. 2011;92(3):401–408. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Buttiker P, Weissenberger S, Martin A, Ptacek R, Kream RM. Editorial: The pathogenesis of long-term neuropsychiatric COVID-19 and the role of microglia, mitochondria, and persistent neuroinflammation: A hypothesis. Medical Science Monitor. 2021;27:e933015. doi: 10.12659/MSM.933015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Son S, Biancardi VC, Zheng H, Sharma N, Patel KP. Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension. 2016;68(6):1483–1493. doi: 10.1161/hypertensionaha.116.07747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier B, Hristova I, Sarre S, Ebinger G, Michotte Y. In vivo characterization of the angiotensin-(1–7)-induced dopamine and gamma-aminobutyric acid release in the striatum of the rat. European Journal of Neuroscience. 2005;22(3):658–664. doi: 10.1111/j.1460-9568.2005.04188.x. [DOI] [PubMed] [Google Scholar]
- Sumners C, Alleyne A, Rodríguez V, Pioquinto DJ, Ludin JA, Kar S, et al. Brain angiotensin type-1 and type-2 receptors: Cellular locations under normal and hypertensive conditions. Hypertension Research. 2020;43(4):281–295. doi: 10.1038/s41440-019-0374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunn N, McKinley MJ, Oldfield BJ. Identification of efferent neural pathways from the lamina terminalis activated by blood-borne relaxin. Journal of Neuroendocrinology. 2001;13(5):432–437. doi: 10.1046/j.1365-2826.2001.00650.x. [DOI] [PubMed] [Google Scholar]
- Tamarkin L, Baird CJ, Almeida OF. Melatonin: A coordinating signal for mammalian reproduction? Science. 1985;227(4688):714–720. doi: 10.1126/science.3881822. [DOI] [PubMed] [Google Scholar]
- Tan T, Khoo B, Mills EG, Phylactou M, Patel B, Eng PC, et al. Association between high serum total cortisol concentrations and mortality from COVID-19. The Lancet Diabetes and Endocrinology. 2020;8(8):659–660. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teclemariam-Mesbah R, Ter Horst GJ, Postema F, Wortel J, Buijs RM. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. The Journal of Comparative Neurology. 1999;406(2):171–182. doi: 10.1002/(SICI)1096-9861(19990405)406:2<171::AID-CNE3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Thomas GD. Neural control of the circulation. Advances in Physiology Education. 2011;35(1):28–32. doi: 10.1152/advan.00114.2010. [DOI] [PubMed] [Google Scholar]
- Triana S, Metz-Zumaran C, Ramirez C, Kee C, Doldan P, Shahraz M, et al. Single-cell analyses reveal SARS-CoV-2 interference with intrinsic immune response in the human gut. Molecular Systems Biology. 2021;17(4):e10232. doi: 10.15252/msb.202110232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. Journal of Clinical Endocrinology and Metabolism. 2021;106(6):1637–1648. doi: 10.1210/clinem/dgab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY. Organization of the projections of a circumventricular organ: The area postrema in the rat. The Journal of Comparative Neurology. 1983;219(3):328–338. doi: 10.1002/cne.902190307. [DOI] [PubMed] [Google Scholar]
- Wang K, Xu Y, Yang W, Zhang Y. Insufficient hypothalamic angiotensin-converting enzyme 2 is associated with hypertension in SHR rats. Oncotarget. 2017;8(12):20244–20251. doi: 10.18632/oncotarget.15666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, de Kloet AD, Pati D, Hiller H, Smith JA, Pioquinto DJ, et al. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central Mas receptors. Neuropharmacology. 2016;105:114–123. doi: 10.1016/j.neuropharm.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LA, de Kloet AD, Smeltzer MD, Cahill KM, Hiller H, Bruce EB, et al. Coupling corticotropin-releasing-hormone and angiotensin converting enzyme 2 dampens stress responsiveness in male mice. Neuropharmacology. 2018;133:85–93. doi: 10.1016/j.neuropharm.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, de Queiroz TM, Sriramula S, Feng Y, Johnson T, Mungrue IN, et al. Brain ACE2 overexpression reduces DOCA-salt hypertension independently of endoplasmic reticulum stress. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2015;308(5):R370–378. doi: 10.1152/ajpregu.00366.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Sriramula S, Chhabra KH, Lazartigues E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circulation Research. 2013;113(9):1087–1096. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Molinas AJR, Mukerjee S, Morgan DA, Rahmouni K, Zsombok A, et al. Activation of ADAM17 (a disintegrin and metalloprotease 17) on glutamatergic neurons selectively promotes sympathoexcitation. Hypertension. 2019;73(6):1266–1274. doi: 10.1161/hypertensionaha.119.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. COVID-19-associated SIADH: A clue in the times of pandemic! American Journal of Physiology-Endocrinology and Metabolism. 2020;318(6):E882–E885. doi: 10.1152/ajpendo.00178.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Liu X, Patel KP. Angiotensin-converting enzyme 2 overexpression improves central nitric oxide-mediated sympathetic outflow in chronic heart failure. American Journal of Physiology-Heart and Circulatory Physiology. 2011;301(6):H2402–2412. doi: 10.1152/ajpheart.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xu J, Hou Y, Leverenz JB, Kallianpur A, Mehra R, et al. Network medicine links SARS-CoV-2/COVID-19 infection to brain microvascular injury and neuroinflammation in dementia-like cognitive impairment. Alzheimer's Research & Therapy. 2021;13(1):110. doi: 10.1186/s13195-021-00850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clinical Science (London, England) 2014;126(10):695–706. doi: 10.1042/CS20130294. [DOI] [PMC free article] [PubMed] [Google Scholar]