Summary
Background
Mortality among children with acute illness in low-income and middle-income settings remains unacceptably high and the importance of post-discharge mortality is increasingly recognised. We aimed to explore the epidemiology of deaths among young children with acute illness across sub-Saharan Africa and south Asia to inform the development of interventions and improved guidelines.
Methods
In this prospective cohort study, we enrolled children aged 2–23 months with acute illness, stratified by nutritional status defined by anthropometry (ie, no wasting, moderate wasting, or severe wasting or kwashiorkor), who were admitted to one of nine hospitals in six countries across sub-Saharan Africa and south Asia between Nov 20, 2016, and Jan 31, 2019. We assisted sites to comply with national guidelines. Co-primary outcomes were mortality within 30 days of hospital admission and post-discharge mortality within 180 days of hospital discharge. A priori exposure domains, including demographic, clinical, and anthropometric characteristics at hospital admission and discharge, as well as child, caregiver, and household-level characteristics, were examined in regression and survival structural equation models.
Findings
Of 3101 children (median age 11 months [IQR 7–16]), 1120 (36·1%) had no wasting, 763 (24·6%) had moderate wasting, and 1218 (39·3%) had severe wasting or kwashiorkor. Of 350 (11·3%) deaths overall, 234 (66·9%) occurred within 30 days of hospital admission and 168 (48·0%) within 180 days of hospital discharge. 90 (53·6%) post-discharge deaths occurred at home. The proportion of children who died following discharge was relatively preserved across nutritional strata. Numerically large high-risk and low-risk groups could be disaggregated for early mortality and post-discharge mortality. Structural equation models identified direct pathways to mortality and multiple socioeconomic, clinical, and nutritional domains acting indirectly through anthropometric status.
Interpretation
Among diverse sites in Africa and south Asia, almost half of mortality occurs following hospital discharge. Despite being highly predictable, these deaths are not addressed in current guidelines. A fundamental shift to a child-centred, risk-based approach to inpatient and post-discharge management is needed to further reduce childhood mortality, and clinical trials of these approaches with outcomes of mortality, readmission, and cost are warranted.
Funding
The Bill & Melinda Gates Foundation.
Introduction
Despite remarkable reductions in child mortality globally, more than 5 million children died in 2019, predominantly in low-income and middle-income countries (LMICs).1 Most seriously ill children access the health-care system at some point;2 however, the mortality risk in LMICs among children admitted to hospital and following discharge remains unacceptably high.3 Reported paediatric inpatient fatality varies from around 2% to more than 20%.4, 5 Although several risk scores based on clinical signs and anthropometry have been devised, none are in widespread use or have reduced case fatality at scale.6
Hospital admission in LMICs commonly occurs following a series of contacts with medical and traditional practitioners, and is influenced by beliefs, costs, and access.7, 8 Thus, variation in case fatality reflects how populations seek care at different health system levels, the staffing and resources available, and the local prevalence of malnutrition, HIV, and other comorbidities.
Between 2004 and 2008, children (aged <15 years) in Kenya were observed to have a more than seven times higher risk of mortality in the year following discharge from hospital than did their community counterparts.9 A subsequent systematic review of paediatric mortality following hospital discharge identified 24 studies, with post-discharge fatality ranging from 1–2% to more than 20%,3 reflecting diverse study designs, populations, and loss to follow-up.
In hospitals, syndrome-based protocols defined in national and WHO10 guidelines are widely adopted, although often not fully implemented. These guidelines provide some recommendations by severity for several common clinical syndromes (eg, pneumonia and dehydration). However, children with poor health commonly meet the criteria for multiple syndromes11 and can have concurrent undernutrition, chronic diseases, or challenging social circumstances.12, 13 Nevertheless, guidance for care and resource allocation does not consider these contexts11 or advise on post-discharge care and integration with community-based health services, apart from specialist services for some specific conditions (including malnutrition or HIV).
Research in context.
Evidence before this study
Between Aug 1, 2016, and Sept 1, 2021, we searched PubMed without date or language restrictions for studies published from date of database inception to Sept 1, 2021, using the following search terms: ((“infant”[MeSH Terms] OR “infant”[All Fields] OR “infants”[All Fields] OR “infant s”[All Fields] OR (“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields] OR “child s”[All Fields] OR “children s”[All Fields] OR “childrens”[All Fields] OR “childs”[All Fields])) AND (“hospital mortality”[MeSH Terms] OR (“hospital”[All Fields] AND “mortality”[All Fields]) OR “hospital mortality”[All Fields] OR (“mortality”[All Fields] AND “hospital”[All Fields]) OR “mortality hospital”[All Fields])) AND (“acute”[All Fields] OR “acutely”[All Fields] OR “acutes”[All Fields] OR (“inpatient s”[All Fields] OR “inpatients”[MeSH Terms] OR “inpatients”[All Fields] OR “inpatient”[All Fields]) OR “post-discharge”[All Fields])) AND (“africa”[MeSH Terms] OR “africa”[All Fields] OR “africa s”[All Fields] OR “africas”[All Fields] OR (“asia”[MeSH Terms] OR “asia”[All Fields])). Epidemiological studies of children presenting to hospitals in low-income and middle-income countries (LMICs) report inpatient case fatality ranging from 2% to more than 20%, and a case fatality of 5–8% among all children discharged from hospital. However, previous studies have addressed inpatient mortality without addressing specific criteria for discharge or acknowledging post-discharge mortality. Furthermore, studies reporting post-discharge mortality are highly heterogeneous, with wide variation in inclusion criteria and duration of follow-up. Some studies of selected groups have suggested that the number of post-discharge deaths could equal or exceed that of deaths observed in hospital. Several paediatric prognostic scores exist in LMICs, predominantly from single centres or specific patient groups, yet none have achieved widespread use. Hospital admission of children with acute illness complicated by severe wasting has been consistently reported to be the predominant factor associated with mortality, with reported inpatient case fatality ranging from 8% to 16%, despite implementation of current WHO and national guidelines. Prognostic models have not generally included household or psychosocial factors, or characteristics of children at the time of discharge.
Added value of this study
The Childhood Acute Illness and Nutrition (CHAIN) prospective cohort study provides comprehensive data from a cohort of 3101 children admitted to nine hospitals in six countries across sub-Saharan Africa and south Asia, using harmonised procedures. Despite support to implement WHO and national guidelines, an average of 11% of children died between hospital admission and 6 months later (weighted for enrolment strata). Overall, 168 (48·0%) of a total 350 deaths occurred following hospital discharge, with the highest rates in the first month after discharge and most occurring at home. Besides nutritional status and illness severity, structural equation models showed the direct effects of HIV infection, other underlying medical conditions, adverse caregiver characteristics, and access to health care on both 30-day mortality and post-discharge mortality within 180 days. Indirect effects of child-level nutritional risk exposures, underlying medical conditions, and access to health care on mortality operated through nutritional status rather than illness severity. Post-discharge mortality within 180 days was as predictable as 30-day mortality (area under receiver operating characteristic curve 0·81 [95% CI 0·78–0·84]).
Implications of all the available evidence
The results from this study, together with previous findings, suggest that a fundamental change is needed for international paediatric guidelines, away from a continued incremental adaptation of syndromic guidelines and towards a structured risk-based and child-centred approach. Risks of both acute mortality (within 30 days) and post-discharge mortality include the key roles of caregiver adversities and comorbidities, and household adversities. Although anthropometry captures the effects of these risks to some extent, it does not adequately target specific caregiver and household psychosocial interventions. A risk-based approach to acute childhood illness could allow for more rational allocation of treatment and resources, specific assignment of responsibility for post-discharge care, and facilitation of access to emergency care for recently discharged children at highest risk and vulnerable families to help reduce residual child mortality.
To inform the development of interventions, we aimed to explore the epidemiology of deaths among young children with acute illness across sub-Saharan Africa and south Asia and to identify the modifiable biomedical and social factors that influence mortality within 30 days of hospital admission and during the first 180 days following hospital discharge.
Methods
Study design and participants
In this prospective, stratified cohort study (the Childhood Acute Illness and Nutrition [CHAIN] cohort),14 we systematically enrolled children aged 2–23 months with acute illness who had been admitted to one of nine hospitals in six countries across sub-Saharan Africa and south Asia (Bangladesh, Burkina Faso, Kenya, Malawi, Pakistan, and Uganda) between Nov 20, 2016, and Jan 31, 2019 (appendix pp 4–7). The following nine participating hospitals were selected to reflect various rural and urban environments, and differing prevalences of malaria and HIV: Dhaka Hospital and Matlab Hospital (Dhaka and Matlab, Bangladesh); Banfora Referral Hospital (Banfora, Burkina Faso); Kilifi County Hospital, Mbagathi County Hospital, and Migori County Referral Hospital (Kilifi, Nairobi, and Migori, Kenya); Queen Elizabeth Central Hospital (Blantyre, Malawi); Civil Hospital (Karachi, Pakistan); and Mulago National Referral Hospital (Kampala, Uganda).15
Nutritional status defined by anthropometry has been consistently shown to be a strong predictor of mortality.16, 17, 18 To increase the inclusion of children at high risk of mortality, enrolment was stratified by anthropometry using mid-upper-arm circumference (MUAC), which is more easily measured in children with poor health and is less affected by dehydration than is anthropometry based on weight-to-length ratios. Children meeting one of the following three strata were enrolled in a 2:1:2 ratio: no wasting (MUAC ≥12·5 cm [age ≥6 months] or MUAC ≥12·0 cm [age <6 months]), moderate wasting (MUAC 11·5–12·5 cm [age ≥6 months] or MUAC 11·0–12·0 cm [age <6 months]), and severe wasting (MUAC <11·5 cm [age ≥6 months] or MUAC <11·0 cm [age <6 months], or bilateral pedal oedema [kwashiorkor] unexplained by other medical causes), thus deliberately over-representing children with moderate wasting and severe wasting or kwashiorkor. Children were screened for eligibility at hospital admission for non-surgical and non-traumatic conditions. The full list of exclusion criteria are provided in the appendix (p 3).
Ethical approval was obtained from the Oxford University Tropical Research Ethics Committee and the ethics committees at all participating institutions (appendix p 4). Written informed consent was obtained from parents or caregivers of all participants.
The protocol for this study is available online.14
Procedures
Standardised data on demographics, medical history, underlying medical conditions, and clinical examination were collected on paper forms by trained study clinicians and entered into a REDCap database (appendix p 8). A complete examination of blood cell count, plasma glucose concentration, rapid malaria test, and HIV testing were conducted systematically. Social, household-level, and primary caregiver information was collected within 48 h, including maternal mental health (Patient Health Questionnaire-9); education, income, employment, assets, water, and sanitation (Demographic and Health Survey questionnaires); food security (adapted Household Food Insecurity Access Scale); and means, time, and cost of travel to hospital.19, 20, 21, 22 Household coordinates were collected by GPS after discharge or death of inpatient, and distance to the study hospital and the nearest health facility was calculated. Comprehensive definitions of all study variables are given in the appendix (pp 6–7).
A pre-study audit was conducted to identify areas of divergence from national and WHO guidelines,5 and ongoing care audits were undertaken using the study database. CHAIN study staff were embedded within hospitals to support inpatient care, training, and adherence to national guidelines. Comprehensive internal quality control and external study monitoring were conducted with Westat (Rockville, MD, USA).
At discharge, full clinical examination and anthropometry were performed. Children with severe wasting or kwashiorkor were discharged from hospital when feeds were completed and were all linked to an outpatient therapeutic feeding programme. Although these children received therapeutic feeds as part of outpatient nutritional rehabilitation, children with moderate wasting did not routinely receive supplementary feeds following hospital discharge, reflecting current policy at the study sites. A single home visit was undertaken after discharge or death of the patient and study follow-up visits were scheduled at days 45, 90, and 180 after hospital discharge at the recruiting hospital, whereby vital status, anthropometry, and health were assessed. Non-attendees were traced through home visits or by telephone.
Further information on data collection tools and standard operating procedures can be found on the CHAIN Network website.
Outcomes
In line with best practices on reporting mortality23 and unaffected by differential discharge decisions, co-primary outcomes were 30-day mortality (ie, deaths within 30 days of hospital admission) and post-discharge mortality (ie, deaths within 180 days of hospital discharge).
Statistical analysis
Pearson's χ2 test suggested that 2600 participants were required after hospital discharge for 80% power to detect a difference in case fatality of 2·4% in children with no wasting versus 5·1% in children with moderate wasting (assuming α=0·05), allowing 10% loss to follow-up and a two-sided hypothesis. These values were based on previous data from Kenya.19
All analyses were done according to an a priori statistical analysis plan, which accounted for the stratified design, specified the handling of missing data and imputation methods, and followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (appendix pp 56–70).
We first examined the simple proportions of deaths: during the index hospital admission, during the first 30 days of admission, and following discharge; and the proportions of post-discharge deaths, at home or at a health facility. Incidence was estimated for 30-day mortality and post-discharge mortality within 180 days.
Exposures were investigated using two analytical methods. Standard survival models and structural equation modelling (SEM) were used to describe pathways according to an a priori conceptual framework (appendix p 46) based on the well established UNICEF conceptual framework linking immediate and underlying exposures to undernutrition, illness, and mortality. We grouped exposures a priori into domains that might influence mortality according to this framework (appendix p 46). We validated the selection of variables in domains using confirmatory factor analysis (appendix p 12). Domain scores were estimated as standalone latent variables using the underlying domain variables and were categorised into tertiles (appendix p 33).
For compatibility with previous studies, we first conducted a simple survival analysis using mixed-effects Weibull regression models (site as a random effect), inverse probability weighted to reflect estimated prevalence of nutritional strata among all paediatric admissions and loss to follow-up by site, and nutritional strata (27 groups). Children lost to follow-up were censored at the time last known to be alive. We reported adjusted hazard ratios (HRs) with 95% CIs. Sensitivity analyses examined various sampling weights that were plausible in similar settings; classification of nutritional status by weight-for-age and weight-for-length rather than MUAC; the inter-relationship of effects of the two central domains, MUAC, and illness severity on mortality; and characteristics associated with earlier and later deaths that occurred following discharge (appendix pp 38–42).
To generate a more comprehensive understanding of the relationships and mediation between domains and their direct and indirect effects on mortality, we used SEM for 30-day survival and 180-day post-discharge survival according to our a priori framework using a Weibull distribution, a random intercept for site, and weighted as per the survival regression analyses. Outcomes were adjusted for age and sex. In reporting, we distinguish direct pathways (from a domain to an outcome) and indirect pathways (from a domain to an outcome via another domain). SEM findings were considered to be the primary outputs on effects of exposures.
Multivariable model performance was assessed by bootstrapped area under receiver operating characteristic curves (AUCs; 1000 replications). Statistical analyses were done using Stata (version 15.1) and R (version 3.6.2). This study is registered at ClinicalTrials.gov, NCT03208725.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
3101 children, with a median age of 11 months (IQR 7–16), were enrolled between Nov 20, 2016, and Jan 31, 2019, of whom 1120 (36·1%) had no wasting, 763 (24·6%) had moderate wasting, and 1218 (39·3%) had severe wasting or kwashiorkor (appendix p 47). 1721 (55·5%) children were admitted to hospital after meeting criteria for diarrhoea, 667 (21·5%) for severe pneumonia, 440 (14·2%) for malaria, and 443 (14·3%) for severe anaemia. 182 (5·9%) children had been exposed to HIV and 106 (3·4%) had HIV infection. Overall, 175 (5·6%) children had been admitted to a hospital within the previous week. Among the 1218 individuals with severe wasting or kwashiorkor, eight (0·7%) were admitted to hospital due to a failed appetite test only, rather than clinical signs of illness. Participant, caregiver, and household characteristics are shown by strata in table 1 and by strata and site in the appendix (pp 15–23).
Table 1.
Baseline characteristics
| No wasting (n=1120) | Moderate wasting (n=763) | Severe wasting or kwashiorkor (n=1218) | All participants (n=3101) | ||
|---|---|---|---|---|---|
| Participant demographics | |||||
| Age, months | 11·0 (7·2–16·0) | 11·0 (7·0–15·0) | 10·0 (6·4–16·0) | 11·0 (6·8–16·0) | |
| Sex | |||||
| Female | 432 (38·6%) | 329 (43·2%) | 583 (47·9%) | 1344 (43·3%) | |
| Male | 688 (61·4%) | 434 (56·9%) | 635 (52·1%) | 1757 (56·7%) | |
| Clinical presentation at admission | |||||
| Signs of shock* | |||||
| None | 652 (58·2%) | 489 (64·1%) | 729 (59·9%) | 1870 (60·3%) | |
| Some (≥1) | 468 (41·8%) | 274 (35·9%) | 489 (40·1%) | 1231 (39·7%) | |
| Systemic inflammatory response syndrome† | 395 (35·3%) | 284 (37·2%) | 386 (31·7%) | 1065 (34·3%) | |
| Impaired consciousness (not alert, responds to voice or pain, or unresponsive) | 46 (4·1%) | 47 (6·2%) | 53 (4·4%) | 146 (4·7%) | |
| Severe pneumonia‡ | 286 (25·5%) | 179 (23·5%) | 202 (16·6%) | 667 (21·5%) | |
| Diarrhoea | 525 (46·9%) | 484 (63·4%) | 712 (58·5%) | 1721 (55·5%) | |
| Malaria (positive rapid diagnostic test) | 202 (18·0%) | 106 (13·9%) | 132 (10·8%) | 440 (14·2%) | |
| Anaemia§ | |||||
| None | 243 (21·7%) | 131 (17·2%) | 216 (17·7%) | 590 (19·0%) | |
| Mild | 279 (24·9%) | 164 (21·5%) | 241 (19·8%) | 684 (22·1%) | |
| Moderate | 463 (41·3%) | 345 (45·2%) | 576 (47·3%) | 1384 (44·6%) | |
| Severe | 135 (12·1%) | 123 (16·1%) | 185 (15·2%) | 443 (14·3%) | |
| Blood glucose concentration | |||||
| Normal | 1041 (92·9%) | 715 (93·7%) | 1101 (90·4%) | 2857 (92·1%) | |
| Abnormal¶ | 79 (7·1%) | 48 (6·3%) | 117 (9·6%) | 244 (7·9%) | |
| Anthropometry at admission | |||||
| Nutritional oedema (kwashiorkor) | 0 | 0 | 353 (29·0%) | 353 (11·4%) | |
| Mid-upper arm circumference, cm | 13·6 (1·0) | 11·9 (0·4) | 10·6 (1·4) | 12·0 (1·7) | |
| Weight-for-length Z score‖ | −0·6 (1·2) | −2·4 (1·0) | −3·1 (1·6) | −2·0 (1·7) | |
| Weight-for-age Z score‖ | −1·1 (1·1) | −2·7 (0·9) | −4·0 (1·4) | −2·6 (1·7) | |
| Length-for-age Z score | −1·1 (1·3) | −1·9 (1·3) | −3·1 (1·7) | −2·1 (1·7) | |
| HIV status | |||||
| Negative | 1010 (90·2%) | 693 (90·8%) | 1034 (85·0%) | 2737 (88·3%) | |
| Infected | 15 (1·3%) | 17 (2·2%) | 74 (6·1%) | 106 (3·4%) | |
| Exposed | 56 (5·0%) | 39 (5·1%) | 87 (7·1%) | 182 (5·9%) | |
| Refused testing or untested | 39 (3·5%) | 14 (1·8%) | 23 (1·9%) | 76 (2·5%) | |
| Underlying conditions | |||||
| Stunting | |||||
| None (Z score −2 and above) | 851 (76·0%) | 413 (54·1%) | 293 (24·1%) | 1557 (50·2%) | |
| Moderate (Z score −2 to −3) | 193 (17·2%) | 211 (27·7%) | 322 (26·4%) | 726 (23·4%) | |
| Severe (Z score up to −3) | 76 (6·8%) | 139 (18·2%) | 603 (49·5%) | 818 (26·4%) | |
| Small birth size** | 132 (11·8%) | 132 (17·3%) | 245 (20·1%) | 509 (16·4%) | |
| Chronic conditions†† | 59 (5·3%) | 51 (6·7%) | 93 (7·6%) | 203 (6·5%) | |
| Previous hospitalisation | |||||
| None | 868 (77·5%) | 580 (76·0%) | 860 (70·6%) | 2308 (74·4%) | |
| <1 week previously | 50 (4·5%) | 52 (6·8%) | 73 (6·0%) | 175 (5·6%) | |
| 1 week to 1 month previously | 56 (5·0%) | 42 (5·5%) | 109 (8·9%) | 207 (6·7%) | |
| >1 month previously | 146 (13·0%) | 89 (11·7%) | 176 (14·4%) | 411 (13·3%) | |
| Child-level nutritional risk exposures | |||||
| Recommended appropriate diet | 645 (57·6%) | 431 (56·5%) | 390 (32·0%) | 1466 (47·3%) | |
| Reported recent weight loss | 109 (9·7%) | 297 (38·9%) | 713 (58·5%) | 1119 (36·1%) | |
| Reported poor feeding | 80 (7·1%) | 91 (11·9%) | 366 (30·0%) | 537 (17·3%) | |
| Reported current breastfeeding | 927 (82·8%) | 631 (82·7%) | 674 (55·3%) | 2232 (72·0%) | |
| Caregiver characteristics | |||||
| Primary caregiver is biological mother | 1091 (97·4%) | 731 (95·8%) | 1134 (93·1%) | 2956 (95·3%) | |
| Primary caregiver currently ill | 152 (13·6%) | 138 (18·1%) | 158 (13·0%) | 448 (14·4%) | |
| Caregiver's attained education level | |||||
| None | 236 (21·1%) | 220 (28·8%) | 345 (28·3%) | 801 (25·8%) | |
| Primary (8 years in total) | 486 (43·4%) | 305 (40·0%) | 533 (43·8%) | 1324 (42·7%) | |
| Secondary or tertiary (12 years in total) | 398 (35·5%) | 238 (31·2%) | 340 (27·9%) | 976 (31·5%) | |
| Maternal mental health (Patient Health Questionnaire-9 score) | |||||
| None to mild (0–9) | 970 (86·6%) | 614 (80·5%) | 933 (76·6%) | 2517 (81·2%) | |
| Moderate to severe (≥10) | 150 (13·4%) | 149 (19·5%) | 285 (23·4%) | 584 (18·8%) | |
| Maternal employment status | |||||
| Self-employed | 245 (21·9%) | 133 (17·4%) | 258 (21·2%) | 636 (20·5%) | |
| Employed | 100 (8·9%) | 80 (10·5%) | 113 (9·3%) | 293 (9·4%) | |
| No reported income | 775 (69·2%) | 550 (72·1%) | 847 (69·5%) | 2172 (70·0%) | |
| Household-level exposures | |||||
| Population density, 1000 people per km2 | 4·4 (0·4–14) | 4·0 (0·7–19) | 5·1 (0·4–19) | 4·6 (0·5–16) | |
| Assets index | |||||
| Quintile 1 (least assets) | 256 (22·9%) | 169 (22·1%) | 208 (17·1%) | 633 (20·4%) | |
| Quintile 2 | 221 (19·7%) | 156 (20·4%) | 252 (20·7%) | 629 (20·3%) | |
| Quintile 3 | 213 (19·0%) | 153 (20·1%) | 268 (22·0%) | 634 (20·4%) | |
| Quintile 4 | 215 (19·2%) | 141 (18·5%) | 240 (19·7%) | 596 (19·2%) | |
| Quintile 5 (most assets) | 215 (19·2%) | 144 (18·9%) | 250 (20·5%) | 609 (19·6%) | |
| Household food insecurity | |||||
| Low | 713 (63·7%) | 473 (62·0%) | 659 (54·1%) | 1845 (59·5%) | |
| Medium | 302 (27·0%) | 186 (24·4%) | 333 (27·3%) | 821 (26·5%) | |
| High | 105 (9·4%) | 104 (13·6%) | 226 (18·6%) | 435 (14·0%) | |
| Improved toilet | 835 (74·6%) | 606 (79·4%) | 900 (74·0%) | 2341 (75·5%) | |
| Improved water source | 946 (84·5%) | 632 (82·8%) | 986 (81·0%) | 2564 (82·7%) | |
| Access to health care | |||||
| Distance to study hospital, km | 7·9 (3·9–18) | 9·7 (4·5–21) | 9·7 (5·2–22) | 9·1 (4·5–21) | |
| Distance to nearest health facility, km | 1·3 (0·5–3·1) | 1·2 (0·5–3·7) | 1·3 (0·5–2·8) | 1·3 (0·5–3·1) | |
| Means of travel to study hospital | |||||
| Bus, ambulance, car, or train | 505 (45·1%) | 315 (41·3%) | 606 (49·8%) | 1426 (46·0%) | |
| Walking, motorcycle, tuk tuk, or rickshaw | 615 (54·9%) | 448 (58·7%) | 612 (50·2%) | 1675 (54·0%) | |
| Travel cost to study hospital, US$ | |||||
| <1 | 463 (41·3%) | 269 (35·3%) | 439 (36·0%) | 1171 (37·8%) | |
| 1–5 | 573 (51·2%) | 402 (52·7%) | 669 (54·9%) | 1644 (53·0%) | |
| ≥5 | 84 (7·5%) | 92 (12·1%) | 110 (9·0%) | 286 (9·2%) | |
| Travel time to study hospital, h | |||||
| <1 | 526 (47·0%) | 316 (41·4%) | 447 (36·7%) | 1289 (41·6%) | |
| 1–2 | 414 (37·0%) | 272 (35·6%) | 457 (37·5%) | 1143 (36·9%) | |
| ≥2 | 180 (16·1%) | 175 (22·9%) | 314 (25·8%) | 669 (21·6%) | |
Data are median (IQR), n (%), or mean (SD).
Capillary refill time >3 s, upper limb temperature gradient, weak pulse.
Presence of two of the following four criteria: heart rate low (<90 bpm) or high (>180 bpm), temperature low (<36·0°C) or high (≥38·5°C), respiratory rate high (>34 breaths per min), and white blood cell count low (<5·0 cells per μL) or high (>17·5 cells per μL).
Cough or difficulty in breathing with oxygen saturation <90%, central cyanosis, or grunting; very severe chest indrawing or inability to breastfeed or drink; or lethargy, reduced level of consciousness, or convulsions.
Anaemia by haemoglobin defined as none (>110 g/L), mild (100–110 g/L), moderate (70–100 g/L), and severe (<70 g/L).
Defined as blood glucose concentration <3 mmol/L or >10 mmol/L.
Children with oedema excluded from these values.
Defined as reported low birthweight (<2·5 kg) or premature birth.
Chronic conditions include thalassaemia, cerebral palsy, sickle cell disease, congenital cardiac disease, and known tuberculosis.
116 (3·7%) children were lost to follow-up; 63 (5·6%) of 1120 children with no wasting, 25 (3·3%) of 763 with moderate wasting, and 28 (2·3%) of 1218 with severe wasting or kwashiorkor (p<0·0001; appendix pp 24, 49). Loss to follow-up also varied by site (p<0·0001), with the highest proportion among children with no wasting in Malawi due to a period of community myths nationally, which included theories about use of blood drawn for research studies.
Overall, 350 children died between hospital admission and 180 days following hospital discharge. Causes of death and mortality by clinical syndromes at admission, by location, and by site are provided in the appendix (pp 27–32).
During index hospitalisation, 182 (5·9%) of all 3101 children died (52·0% of all 350 deaths): 22 (2·0%) of 1120 children with no wasting, 32 (4·2%) of 763 with moderate wasting, and 128 (10·5%) of 1218 with severe wasting or kwashiorkor (table 2; appendix p 47). Median time to death from hospital admission was 1·5 days (IQR 0·0–4·0) among children with no wasting, 2·0 days (1·0–5·5) among those with moderate wasting, and 3·0 days (1·0–8·0) among those with severe wasting or kwashiorkor (p=0·15).
Table 2.
Mortality by time period and cohort strata
| No wasting (n=1120) | Moderate wasting (n=763) | Severe wasting or kwashiorkor (n=1218) | |
|---|---|---|---|
| All deaths | |||
| Number of deaths (%) | 39 (3·5%) | 62 (8·1%) | 249 (20·4%) |
| Risk ratio (95% CI)* | 1 (ref) | 2·54 (1·77–3·66) | 5·89 (3·68–9·43) |
| Deaths during index admission | |||
| Number of deaths (%) | 22 (2·0%) | 32 (4·2%) | 128 (10·5%) |
| Risk ratio (95% CI)* | 1 (ref) | 2·37 (1·64–3·41) | 5·43 (3·55–8·31) |
| 30-day mortality† | |||
| Number of deaths (%) | 26 (2·3%) | 40 (5·2%) | 168 (13·8%) |
| Mortality rate, deaths per 1000 child-months (95% CI) | 24·1 (16·5–36·4) | 55·0 (40·5–76·5) | 156 (134–183) |
| Rate ratio (95% CI)‡ | 1 (ref) | 2·37 (1·42–3·95) | 6·38 (4·16–9·78) |
| Post-discharge mortality§ | |||
| Number of deaths (%) | 17/1072 (1·6%) | 30/724 (4·1%) | 121/1078 (11·2%) |
| Mortality rate, deaths per 1000 child-months (95% CI) | 2·72 (1·72–4·57) | 7·32 (5·16–10·7) | 21·0 (17·6–25·3) |
| Rate ratio (95% CI)‡ | 1 (ref) | 2·73 (1·52–4·89) | 7·05 (4·32–11·5) |
Risk ratios from multi-level mixed effects log-binomial regression models adjusted for sex, age, and site and inverse probability weighted for selection and loss to follow-up.
Includes inpatient and post-discharge deaths within 30 days of hospital admission (fixed period).
Rate ratios estimated using the Mantel-Cox method adjusted for sex, age, and site and inverse probability weighted for selection and loss to follow-up.
Includes deaths within 180 days of index hospital discharge (fixed period). The number of children discharged alive were 1072 children with no wasting, 724 with moderate wasting, and 1078 with severe wasting or kwashiorkor.
234 deaths (66·9% of all 350 deaths) occurred within 30 days of hospital admission: 26 (2·3%) deaths among children with no wasting (24·1 deaths per 1000 child-months [95% CI 16·5–36·4]), 40 (5·2%) deaths among those with moderate wasting (55·0 [40·5–76·5]), and 168 (13·8%) among those with severe wasting or kwashiorkor (156·0 [134·0–183·0]; figure 1; table 2; appendix p 25).
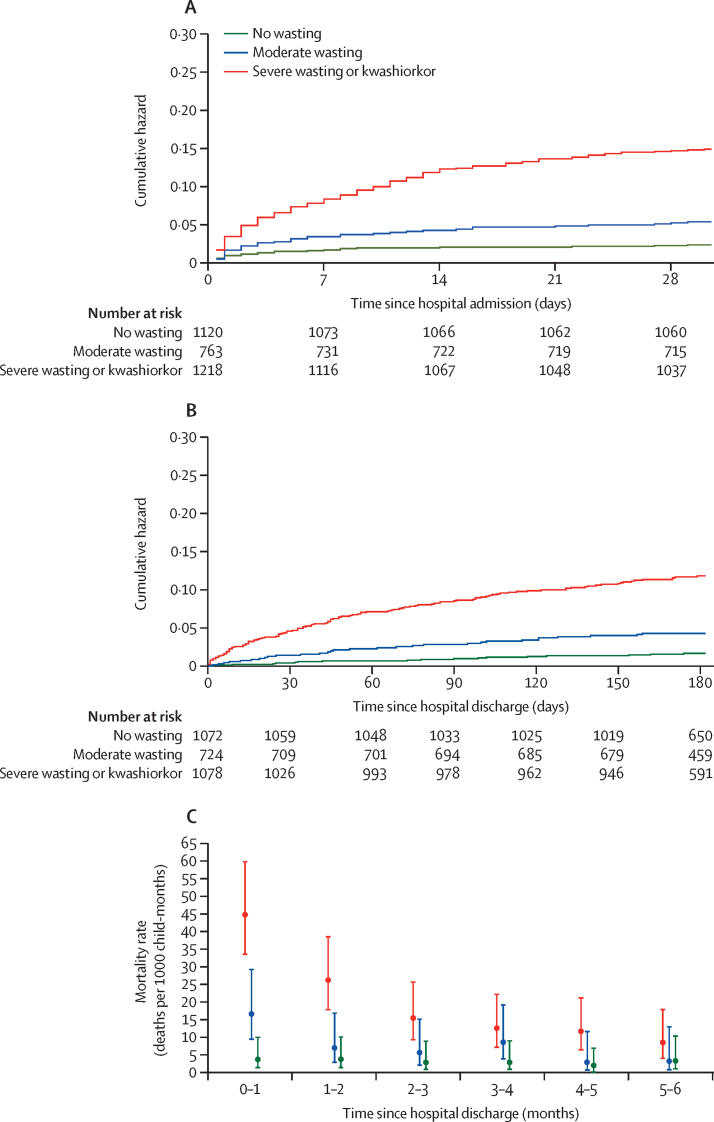
Figure 1.
Cumulative hazard curves, stratified by nutritional status at hospital admission
Data provided are adjusted for age, sex, and site. (A) Mortality in the first 30 days following hospital admission (2935 child-months). (B) Post-discharge mortality (15 999 child-months). (C) Monthly mortality rates following discharge from hospital.
Overall, 2874 children were discharged alive, did not withdraw from the study before discharge, and were followed up (figure 1; appendix p 47). Length of stay varied by site and strata: 3 days (IQR 2–5) among children with no wasting, 4 days (2–6) among those with moderate wasting, and 7 days (4–12) among those with severe wasting or kwashiorkor (p<0·0001; appendix p 50). 205 (7·1%) children left hospital against medical advice (appendix p 26). 22 (0·8%) discharges occurred after 30 days. Characteristics at discharge are shown in the appendix (p 26).
Overall, 168 (5·8%) of the 2874 children who were discharged from hospital alive died within 180 days of discharge (48·0% of all 350 deaths): 17 (1·6%) of 1072 children with no wasting (2·72 deaths per 1000 child-months [95% CI 1·72–4·57]), 30 (4·1%) of 724 with moderate wasting (7·32 [5·16–10·7]), and 121 (11·2%) of 1078 with severe wasting or kwashiorkor (21·0 [17·6–25·3]; figure 1; table 2; appendix pp 26, 47). Mortality was highest in the first month following hospital discharge, when 62 (36·9%) of 168 deaths occurred (figure 1). 90 (53·6%) post-discharge deaths occurred at home rather than in a health facility. Home deaths were more common among children with wasting than among those without (p=0·037; appendix p 33).
The distributions of children within exposure domain scores are shown in the appendix (p 33).
Lower nutritional strata at hospital admission (ie, moderate wasting and severe wasting or kwashiorkor), age per log month, higher signs of illness severity at admission, exposure to HIV, and HIV infection were associated with 30-day mortality (table 3). Other domains associated with 30-day mortality were underlying pre-existing medical conditions, adverse caregiver and household-level exposures, and access to health care (table 3).
Table 3.
Domains of exposures associated with 30-day and post-discharge mortality
|
30-day mortality |
Post-discharge mortality |
||||
|---|---|---|---|---|---|
| Adjusted HR (95% CI)* | p value | Adjusted HR (95% CI)* | p value | ||
| Base variables | |||||
| Nutritional stratum at hospital admission | |||||
| No wasting | 1 (ref) | .. | 1 (ref) | .. | |
| Moderate wasting | 2·03 (1·40–2·94) | <0·0001 | 2·58 (1·47–4·52) | 0·0012 | |
| Severe wasting or kwashiorkor | 4·51 (2·81–7·23) | <0·0001 | 4·36 (2·27–8·37) | <0·0001 | |
| Age, log months | 0·77 (0·65–0·91) | 0·0031 | 0·72 (0·55–0·94) | 0·017 | |
| Female | 1·19 (0·87–1·65) | 0·28 | 1·33 (0·86–2·06) | 0·19 | |
| Leaving against medical advice | .. | .. | 2·14 (1·33–3·43) | 0·0021 | |
| Admission duration, log days | .. | .. | 1·51 (0·95–2·39) | 0·083 | |
| Change in anthropometry at hospital discharge | |||||
| No change | .. | .. | 1 (ref) | .. | |
| Improved | .. | .. | 0·73 (0·57–0·93) | 0·010 | |
| Worsened | .. | .. | 0·81 (0·15–4·40) | 0·80 | |
| Exposure domains | |||||
| Signs of illness severity at admission | |||||
| Low | 1 (ref) | .. | 1 (ref) | .. | |
| Medium | 1·29 (0·76–2·19) | 0·34 | 1·36 (0·83–2·22) | 0·22 | |
| High | 3·82 (2·50–5·86) | <0·0001 | 1·76 (0·96–3·24) | 0·067 | |
| Signs of illness severity at discharge | |||||
| Low | .. | .. | 1 (ref) | .. | |
| Medium | .. | .. | 1·29 (0·91–1·84) | 0·15 | |
| High | .. | .. | 4·00 (1·66–9·64) | 0·0020 | |
| HIV status | |||||
| Negative | 1 (ref) | .. | 1 (ref) | .. | |
| Untested | 0·78 (0·37–1·65) | 0·52 | 1·88 (0·78–4·55) | 0·16 | |
| Exposed | 1·68 (1·29–2·18) | <0·0001 | 1·82 (1·19–2·80) | 0·0064 | |
| Infected | 2·45 (1·24–4·81) | 0·010 | 1·75 (0·91–3·37) | 0·094 | |
| Underlying medical conditions | |||||
| Low | 1 (ref) | .. | 1 (ref) | .. | |
| Medium | 2·45 (1·22–4·91) | 0·012 | 0·90 (0·61–1·33) | 0·60 | |
| High | 2·08 (1·25–3·45) | 0·0054 | 1·40 (0·99–1·97) | 0·058 | |
| Child-level nutritional risk exposures | |||||
| Low | 1 (ref) | .. | 1 (ref) | .. | |
| Medium | 0·59 (0·36–0·97) | 0·036 | 0·83 (0·41–1·67) | 0·61 | |
| High | 1·15 (0·78–1·69) | 0·47 | 1·20 (0·75–1·90) | 0·45 | |
| Caregiver characteristics | |||||
| Least adverse | 1 (ref) | .. | 1 (ref) | .. | |
| Moderately adverse | 2·03 (1·36–3·04) | 0·0010 | 1·37 (0·82–2·30) | 0·22 | |
| Most adverse | 1·53 (1·01–2·32) | 0·045 | 2·11 (1·18–3·77) | 0·012 | |
| Household-level exposures | |||||
| Least adverse | 1 (ref) | .. | 1 (ref) | .. | |
| Moderately adverse | 2·02 (1·35–3·03) | 0·0011 | 1·04 (0·78–1·38) | 0·80 | |
| Most adverse | 2·08 (1·38–3·13) | <0·0001 | 1·01 (0·68–1·50) | 0·95 | |
| Access to health care | |||||
| Least adverse | 1 (ref) | .. | 1 (ref) | .. | |
| Moderately adverse | 1·10 (0·74–1·64) | 0·64 | 1·09 (0·68–1·77) | 0·71 | |
| Most adverse | 1·77 (1·14–2·75) | 0·011 | 1·09 (0·64–1·85) | 0·74 | |
| Site variance (95% CI) | 0·05 (0·003–0·93) | .. | 0·02 (0·001–0·75) | .. | |
| Bootstrapped AUC (95% CI) | 0·80 (0·78–0·83) | .. | 0·81 (0·78–0·84) | .. | |
HR=hazard ratio. AUC=area under receiver operating characteristic curve.
Adjusted HR for all predictors in the multivariable model. All model results were weighted using sampling and lost to follow-up weights. HRs from a multi-level multivariable parametric (Weibull distribution) survival model with site as a random effect.
Lower nutritional stratum at hospital admission (ie, moderate wasting and severe wasting or kwashiorkor), most adverse caregiver characteristics, higher signs of illness severity at hospital discharge, discharge against medical advice, and HIV exposure, but not HIV infection, were significantly associated with post-discharge mortality within 180 days (table 3). Age per log month and improved anthropometry at discharge were associated with a lower post-discharge mortality rate within 180 days (table 3). Sex, signs of illness severity at admission, underlying medical conditions, child-level nutritional risk exposures, household-level exposures, access to health care, and duration of admission were not independently associated with post-discharge mortality (table 3).
Sensitivity analyses across various plausible sampling weights in similar settings (appendix pp 34–40) and using different anthropometric indices, rather than MUAC (appendix p 52), provided results similar to the primary models. Examining the effects of MUAC on a continuous scale and admission illness severity together, we observed that the relationship between MUAC and mortality is highly dependent on illness severity (appendix p 53). Unlike later post-discharge deaths, deaths during the first month following discharge were associated with age and leaving against medical advice (appendix pp 41–42).
At hospital admission, the lowest risk quintile predicted by the regression models had a 0·8% risk of 30-day mortality and the highest quintile had a 24·0% risk (appendix p 42). At discharge, the lowest two risk quintiles (40% of discharged children) had a 0·9–1·2% risk of post-discharge mortality, and the highest risk quintile (20% of discharged children) had an 18·0% risk (appendix pp 42–43).
SEM full estimation and model performance results are provided in the appendix (pp 44–45), as are the confirmatory factor analysis results (pp 12–13).
In the SEM, both lower nutritional strata (adjusted HR 2·07 [95% CI 1·42–3·04] for moderate wasting and 4·56 [2·63–7·90] for severe wasting or kwashiorkor vs no wasting) and high signs of illness severity at admission (3·67 [2·35–5·75]) at hospital admission were directly associated with 30-day mortality (figure 2A; appendix p 44). HIV infection (2·27 [1·25–4·12]), HIV exposure (1·51 [1·11–2·06]), underlying medical conditions, and child-level nutritional risk exposures directly influenced 30-day mortality, as well as acting through nutritional status (figure 2A; appendix p 44).
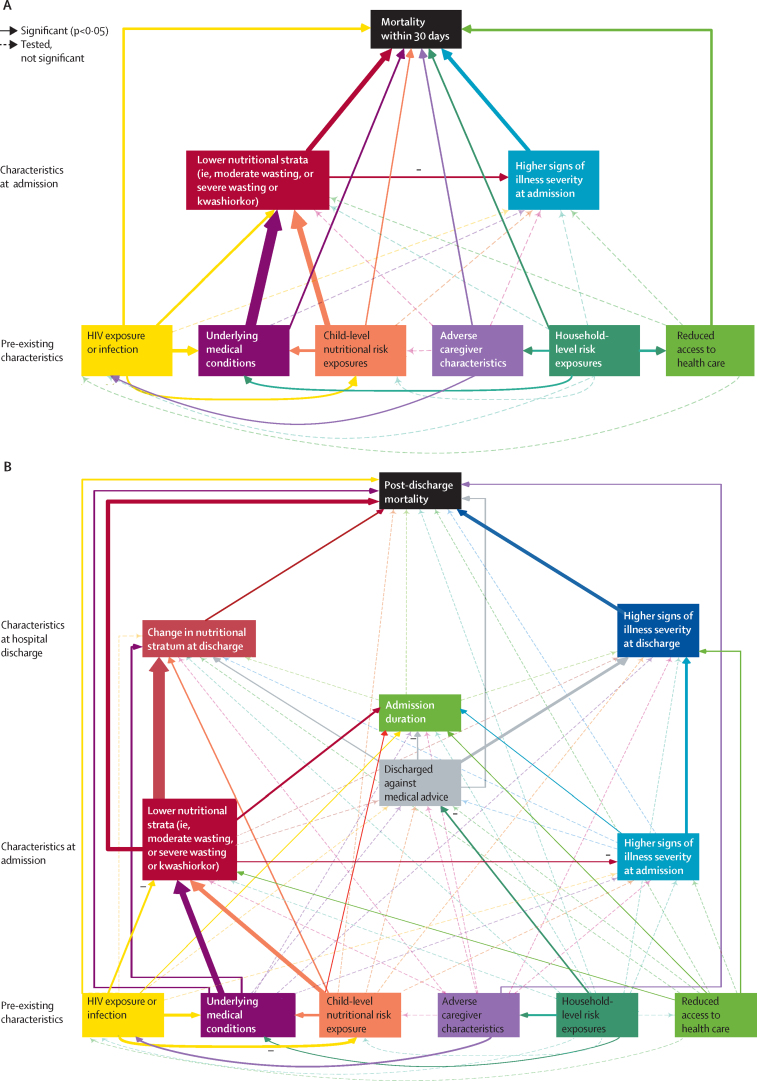
Figure 2.
Structural equation models of relationships between domains of characteristics and 30-day mortality and post-discharge mortality
(A) Relationships between domains of hospital admission and pre-existing characteristics and 30-day mortality. (B) Relationships between domains of hospital discharge, hospital admission, and pre-existing characteristics and post-discharge mortality. Significant associations (p<0·05) are indicated by solid arrows and non-significant associations by dashed arrows. Significant negative associations are indicated by the – symbol. For significant associations, the arrow thickness corresponds to the effect size. Each outcome was adjusted for age and sex and was modelled with a random intercept at the site level. The full estimation results are provided in the appendix (pp 44–45). Model results were weighted using sampling and lost to follow-up weights.
At hospital discharge, high signs of illness severity (adjusted HR 3·68 [95% CI 1·56–8·67]), improved anthropometry (0·70 [0·56–0·89]), and leaving against medical advice (2·05 [1·25–3·35]) were directly associated with post-discharge mortality within 180 days (appendix p 45). Many exposure domains directly affected duration of admission; however, duration of admission itself was not directly associated with post-discharge mortality, thus acting as a so-called sink (figure 2B; appendix p 45).
For the SEM models, bootstrapped AUCs (1000 replications) were 0·81 (95% CI 0·78–0·84) for 30-day mortality and 0·81 (0·77–0·84) for post-discharge mortality within 180 days. Removing all pre-existing characteristics from the models resulted in bootstrapped AUCs of 0·77 (0·74–0·79) for 30-day mortality and 0·78 (0·75–0·82) for post-discharge mortality.
Of the characteristics at hospital admission, nutritional stratum (adjusted HR 2·65 [95% CI 1·49–4·70] for moderate wasting and 4·45 [2·21–8·96] for severe wasting or kwashiorkor), but not signs of illness severity, directly affected post-discharge mortality within 180 days (figure 2B; appendix p 45). However, signs of illness severity at hospital admission influenced signs of illness severity at discharge, thereby being indirectly associated.
Of the six domains of pre-existing characteristics, HIV exposure (adjusted HR 1·63 [95% CI 1·09–2·44]), high underlying medical conditions (1·37 [1·04–1·82]), and most adverse caregiver characteristics (2·00 [1·16–3·46]) were directly and independently associated with post-discharge mortality within 180 days (figure 2B; appendix p 45).
Several important indirect effects were observed. Household-level exposures influenced leaving against medical advice, caregiver characteristics, and underlying medical conditions. Reduced access to health care, underlying medical conditions, and child-level nutritional risk exposures affected admission nutritional status, and child-level nutritional risk exposure was also associated with underlying medical conditions. Additionally, underlying medical conditions affected the change in nutritional status from hospital admission to discharge. Pre-existing characteristics were not associated with signs of illness severity at hospital admission or discharge. In the confirmatory factor analysis, caregiver employment, maternal mental health, and caregiver education were the main measures of the caregiver characteristics domain (appendix p 12). In both the 30-day mortality and post-discharge mortality models, lower nutritional strata were associated with less severe signs of illness at hospital admission.
Discussion
Many children in low-resource settings are at increased risk of morbidity and mortality, despite increasing (although inequitable) access to interventions, including vaccination and primary health care.24 In the children admitted to hospital in this study, we observed initially steep mortality curves, reducing in gradient after around 3 weeks, but not flattening over 6 months. Across different geographical settings, almost half of all deaths occurred after discharge from hospital, commonly within a few weeks of discharge, and around half of post-discharge deaths occurred at home. The ratio of inpatient to post-discharge deaths was preserved across diverse geographical settings and anthropometric strata, matching the highest estimates previously reported.3
Low nutritional status defined by anthropometry (ie, malnutrition) was associated with increased mortality, even though these children received medical and nutritional care in line with WHO and national guidelines. In the context of severe acute illness, our data show that low MUAC represents a high-risk state associated with mechanisms beyond reduced nutrient intake in the short term. Importantly, children with low MUAC admitted with low illness severity had a far lower risk of mortality, as seen in programmes for uncomplicated severe malnutrition, than did those with higher illness severity. These findings suggest that acutely ill children with wasting would benefit from greater risk stratification to target interventions beyond therapeutic and supplemental feeding in the short term, including before hospitalisation. General risk stratification among paediatric admissions might permit children at low risk to be identified according to formal criteria and discharged from hospital earlier, redirecting staff and resources to care for children at increased risk and saving costs to families.
Post-discharge mortality was as predictable as 30-day mortality using information at hospital admission, hospital discharge, and social circumstances. Clinical features at hospital discharge have not been previously reported17, 18 and, unsurprisingly, these were more strongly associated with post-discharge death than were admission features. Importantly, although multiple pathways converged on length of hospital stay, this was not associated with post-discharge mortality, suggesting that extending inpatient treatment is unlikely to reduce mortality for children who are judged to be ready for discharge. As expected, leaving hospital against medical advice was associated with an increased risk of mortality, suggesting a need to identify these families early to address concerns and provide support to avoid premature discharge. Biomarkers might provide additional information on risk at hospital admission and at the time of clinically apparent readiness for discharge,25 but this needs further research.
Caregiver characteristics, a domain in which adversity was dominated by maternal employment and mental health, directly influenced post-discharge mortality and was a more important domain than were household-level exposures or access to health care. These findings support earlier work investigating the roles, support, and finances of caregivers12, 26 and suggest that interventions specifically targeting maternal wellbeing, agency, and independent income are needed. These findings might also partly explain the observed inefficacy of individual interventions against post-discharge mortality in recent clinical trials.3, 27, 28, 29
Given that just over half of post-discharge deaths occur at home,3, 30 so-called down referral to the specific responsibility of a named community health worker could be a valuable strategy, such as the role of health visitors in high-income settings, which is not included in current WHO or national guidelines. Training for mothers and family members in recognising danger signs in infants and continued structured contact with a health professional could be provided at low cost by telephone and SMS to deliver advice and to allow for early recognition of children who need face-to-face evaluation or readmission. Facilitating access to emergency rooms for recently admitted children, avoiding queueing, and waiving hospital costs (including bed charges for the caregiver), could help to overcome hesitancy in re-presenting to hospital.12
Most findings of the simple regression analysis were replicated in the SEM analysis. Importantly, indirect effects of child-level nutritional risk exposures, underlying conditions, and household characteristics operated via anthropometric status rather than illness severity. Surprisingly, neither model found an association between access to health care and post-discharge mortality. The association between access to health care and 30-day mortality probably reflects delays in initial presentation and its correlates. HIV exposure and infection were associated with 30-day mortality in both models; however, HIV infection was not associated with post-discharge mortality in either model, potentially because of low numbers.
Strengths of the CHAIN cohort study include its size, geographical diversity, standardised care, and comprehensive data collection across a range of geographical and epidemiological settings. Limitations include potential bias by children admitted overnight and dying before enrolment in the morning, and the fact that weekend admissions were not targeted. We faced challenges in differentiating situational stressors and experiences from long-term psychosocial strain, not using a biochemical method to determine nutrient status, achieving full guideline adherence, and obtaining some data for enrolled children who died early on in the study. Study follow-up visits might have resulted in health service contact that would not normally occur. Causes of death are based on all available information, but might be unreliable. We chose to retain sepsis as a cause of death because a primary focus of infection was not always evident or children met the criteria for multiple syndromes, or both. Although the current analysis focuses on understanding broad pathways involved in mortality, prognostic models for individual clinical features and models of biological systems will be published in separate reports, as will analyses of post-discharge growth and readmission to hospital.
Acute illness and wasting in children represents a high-risk state, in which various biological and social vulnerabilities converge to drive child mortality independent of clinical syndrome at presentation to hospital, and despite support to provide treatment in line with guidelines. Almost half of all deaths observed in this study occurred following hospital discharge and were as predictable as deaths within 30 days of hospital admission. A fundamental shift towards a child-centred, risk-based approach to guidelines with a structured assessment at hospital admission and at discharge to inform appropriate care and social support care is needed to further reduce childhood mortality in low-resource settings.
Data sharing
The CHAIN cohort data and analysis code are deposited and may be requested at the Harvard Dataverse website.
Declaration of interests
All members of the writing group declare no competing interests.
Acknowledgments
Acknowledgments
We thank the CHAIN cohort participants and their families for their generous contribution to the study. We are indebted to the CHAIN teams at all sites, and the management and staff in hospitals and communities who kindly assisted in the conduct of the study. We thank Saskia van der Kam, Mark Manary, and Nigel Rollins for their key input as the scientific and policy advisory group, and Maureen Kelley for her valuable insights as the ethics advisor on research in vulnerable populations. The CHAIN Network is supported by the Bill & Melinda Gates Foundation (OPP1131320). JAB was supported by the Medical Research Council–Department for International Development–Wellcome Trust Joint Global Health Trials scheme (MR/M007367/1). Study staffing, facilities, and resources were contributed by the Wellcome Trust (203077_Z_16_Z). For the purpose of open access, the CHAIN Network has applied a CC BY public copyright license to any author-accepted manuscript version arising from this submission. No members of the writing group were paid to write this Article by any company, organisation, or agency.
The CHAIN Network
Writing group (not listed in any particular order): Daniella Brals (Department of Global Health, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands); Wieger P Voskuijl (Department of Paediatrics, Amsterdam Centre for Global Child Health, Emma Children's Hospital, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands); Robert H J Bandsma (Centre for Global Child Health, Hospital for Sick Children, Toronto, ON, Canada); Celine Bourdon (Translational Medicine, Hospital for Sick Children, Toronto, ON, Canada); James A Berkley, Priya Sukhtankar, Sassy Molyneux (Centre for Tropical Medicine and Global Health, University of Oxford, Oxford, UK); Christopher Maronga, Caroline Tigoi, James A Berkley, Julie Jemutai, Johnstone Thitiri, Moses Mburu, Moses Ngari, Molly Timbwa, Narshion Ngao, Priya Sukhtankar, Shalton Mwaringa (Clinical Research Department, KEMRI–Wellcome Trust Research Programme, Kilifi, Kenya); Sassy Molyneux (Health Systems and Research Ethics Department, KEMRI–Wellcome Trust Research Programme, Kilifi, Kenya); Judd L Walson, Kirkby D Tickell (Department of Epidemiology, University of Washington, Seattle, WA, USA); Christine J McGrath, Dorothy I Mangale, Donna M Denno, Joseph D Carreon, Judd L Walson, Kirkby D Tickell (Department of Global Health, University of Washington, Seattle, WA, USA); Judd L Walson (Department of Medicine, University of Washington, Seattle, WA, USA); Donna M Denno, Judd L Walson (Department of Pediatrics, University of Washington, Seattle, WA, USA); John Mukisa (Department of Immunology and Department of Molecular Biology Makerere University College of Health Sciences, Kampala, Uganda); Ezekiel Mupere (Department of Paediatrics and Child Health, Makerere University College of Health Sciences, Kampala, Uganda); Robert H J Bandsma (Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, ON, Canada); Christine Manyasi (Department of Paediatrics, Mbagathi Hospital, Nairobi, Kenya); Issaka Ouédraogo (Department of Pediatrics, Banfora Referral Regional Hospital, Banfora, Burkina Faso); Christina L Lancioni (Department of Pediatrics, Oregon Health and Science University, Portland, OR, USA); Ali Faisal Saleem, Syed Asad Ali, Zaubina Kazi (Department of Pediatrics and Child Health, Aga Khan University, Karachi, Pakistan); Emmanuel Chimwezi, Emmie Mbale, Jenala Njirammadzi, Macpherson Mallewa, Philliness Prisca Harawa (Department of Paediatrics and Child Health, Kamuzu University of Health Sciences, Blantyre, Malawi); Robert H J Bandsma (Department of Biomedical Sciences, College of Medicine, University of Malawi, Blantyre, Malawi); Abdoulaye Hama Diallo, Blaise Siézan Gnoumou, Roseline Maïmouna Bamouni (Department of Public Health, University Joseph Ki-Zerbo, Ouagadougou, Burkina Faso); Abdoulaye Hama Diallo (Department of Public Health, Centre Muraz Research Institute, Bobo-Dioulasso, Burkina Faso); Sayera Banu (Infectious Diseases Division, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh); Dilruba Ahmed, Syeda Momena Afsana (Laboratory Sciences and Services Division, International Centre for Diarrhoeal Disease Research Bangladesh, Dhaka, Bangladesh); Al Fazal Khan, Abu Sadat Mohammad Sayeem Bin Shahid, Dinesh Mondal, Gazi Md Salahuddin Mamun, Lubaba Shahrin, Md Iqbal Hossain, Mohammod Jobayer Chisti, Shamsun Nahar Shaima, Tahmeed Ahmed (Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research Bangladesh, Dhaka, Bangladesh); Benson O Singa, Catherine Achieng Otieno, Chris Oduol (Kenya Medical Research Institute, Nairobi, Kenya); Christopher Lwanga, Peace Aber (Uganda-Case Western Reserve University Research Collaboration, Kampala, Uganda).
Contributors
JAB and JLW contributed to funding. AFS, CLL, DMD, EMu, JAB, JLW, JJ, KDT, MMa, MJC, MMN, PS, RHB, SMo, SAA, TA, and WPV designed the study. AHD, AFS, BOS, CT, CAO, CB, COO, CLL, CMar, CL, DIM, DC, JAB, JN, JM, JT, JLW, KDT, MJC, MT, MMb, NN, PA, PS, RHB, RMB, SMo, WPV, and ZK coordinated the study. AHD, AFS, BOS, CB, CLL, CMan, CJM, DMD, EMb, EMu, GSM, JAB, JM, JT, JLW, KDT, MMa, MJC, MT, PS, RHB, SMo, SMw, SAA, TA, WPV, and ZK supervised the study. AMS, AFK, COO, CMan, CJM, CL, DA, DM, EMb, IO, JN, JM, LS, MH, MT, PPH, PS, SMw, SNS, SMA, and SB collected the data. AHD, BSG, CAO, CB, CMar, EC, JDC, JJ, MMb, MMN, NN, PA, and RMB managed the data. AHD, CT, RMB, and ZK did the laboratory analysis. DB, JAB, JLW, JJ, and MMN analysed the data. AHD, BSG, CAO, CB, CLL, DB, DMD, EMu, IO, JAB, JLW, KDT, MJC, MMN, PS, RHB, RMB, SMo, SAA, TA, and WPV interpreted the data. DB, JAB, and MMN wrote the first draft. AHD, AMS, AFK, AFS, BOS, BSG, CT, CAO, CB, COO, CLL, CMan, CJM, CMar, CL, DB, DA, DM, DMD, DIM, DC, EMb, EMu, GSM, IO, JAB, JN, JM, JT, JDC, JLW, JJ, KDT, LS, MMa, MH, MJC, MT, MMb, MMN, NN, PA, PPH, PS, RHB, RMB, SMo, SMw, SNS, SAA, SMA, SB, TA, WPV, and ZK critically reviewed the manuscript. JAB, MMN, and DB verified the underlying data reported in the manuscript. All members of the writing group had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
The Childhood Acute Illness and Nutrition (CHAIN) Network:
Abdoulaye Hama Diallo, Abu Sadat Mohammad Sayeem Bin Shahid, Ali Fazal Khan, Ali Faisal Saleem, Benson O Singa, Blaise Siezanga Gnoumou, Caroline Tigoi, Catherine Achieng Otieno, Celine Bourdon, Chris Odhiambo Oduol, Christina L Lancioni, Christine Manyasi, Christine J McGrath, Christopher Maronga, Christopher Lwanga, Daniella Brals, Dilruba Ahmed, Dinesh Mondal, Donna M Denno, Dorothy I Mangale, Emmanuel Chimezi, Emmie Mbale, Ezekiel Mupere, Gazi Md. Salauddin Mamun, Issaka Ouedraogo, James A Berkley, Jenala Njirammadzi, John Mukisa, Johnstone Thitiri, Joseph D Carreon, Judd L Walson, Julie Jemutai, Kirkby D Tickell, Lubaba Shahrin, MacPherson Mallewa, Md. Iqbal Hossain, Mohammod Jobayer Chisti, Molly Timbwa, Moses Mburu, Moses M Ngari, Narshion Ngao, Peace Aber, Philliness Prisca Harawa, Priya Sukhtankar, Robert H J Bandsma, Roseline Maimouna Bamouni, Sassy Molyneux, Shalton Mwaringa, Shamsun Nahar Shaima, Syed Asad Ali, Syeda Momena Afsana, Syera Banu, Tahmeed Ahmed, Wieger P Voskuijl, and Zaubina Kazi
Supplementary Material
References
- 1.UNICEF. WHO. World Bank Group. UN Levels and trends in child mortality 2019. Estimates developed by the UN Inter-agency group for child mortality estimation. September, 2019. https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2019
- 2.Price J, Lee J, Willcox M, Harnden A. Place of death, care-seeking and care pathway progression in the final illnesses of children under five years of age in sub-Saharan Africa: a systematic review. J Glob Health. 2019;9 doi: 10.7189/jogh.09.020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemetchek B, English L, Kissoon N, et al. Paediatric postdischarge mortality in developing countries: a systematic review. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-023445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JD, Sow SO, Levine MM, Kotloff KL. The causes of hospital admission and death among children in Bamako, Mali. J Trop Pediatr. 2004;50:158–163. doi: 10.1093/tropej/50.3.158. [DOI] [PubMed] [Google Scholar]
- 5.Gathara D, Malla L, Ayieko P, et al. Variation in and risk factors for paediatric inpatient all-cause mortality in a low income setting: data from an emerging clinical information network. BMC Pediatr. 2017;17:99. doi: 10.1186/s12887-017-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George EC, Walker AS, Kiguli S, et al. Predicting mortality in sick African children: the FEAST Paediatric Emergency Triage (PET) Score. BMC Med. 2015;13:174. doi: 10.1186/s12916-015-0407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price J, Willcox M, Kabudula CW, et al. Care pathways during a child's final illness in rural South Africa: findings from a social autopsy study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra K, Mohapatra I, Kumar A. A study on the health seeking behavior among caregivers of under-five children in an urban slum of Bhubaneswar, Odisha. J Family Med Prim Care. 2019;8:498–503. doi: 10.4103/jfmpc.jfmpc_437_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moisi JC, Gatakaa H, Berkley JA, et al. Excess child mortality after discharge from hospital in Kilifi, Kenya: a retrospective cohort analysis. Bull World Health Organ. 2011;89:725–732. doi: 10.2471/BLT.11.089235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . World Health Organization; Geneva: 2013. Pocket book of hospital care for children: second edition. Guidelines for the management of common childhood illnesses. [PubMed] [Google Scholar]
- 11.English M, Berkley J, Mwangi I, et al. Hypothetical performance of syndrome-based management of acute paediatric admissions of children aged more than 60 days in a Kenyan district hospital. Bull World Health Organ. 2003;81:166–173. [PMC free article] [PubMed] [Google Scholar]
- 12.Zakayo SM, Njeru RW, Sanga G, et al. Vulnerability and agency across treatment-seeking journeys for acutely ill children: how family members navigate complex healthcare before, during and after hospitalisation in a rural Kenyan setting. Int J Equity Health. 2020;19:136. doi: 10.1186/s12939-020-01252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bwakura-Dangarembizi M, Dumbura C, Amadi B, et al. Risk factors for postdischarge mortality following hospitalization for severe acute malnutrition in Zimbabwe and Zambia. Am J Clin Nutr. 2021;113:665–674. doi: 10.1093/ajcn/nqaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Childhood Acute Illness and Nutrition Network Childhood Acute Illness and Nutrition (CHAIN) Network: a protocol for a multi-site prospective cohort study to identify modifiable risk factors for mortality among acutely ill children in Africa and Asia. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-028454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tickell KD, Mangale DI, Tornberg-Belanger SN, et al. A mixed method multi-country assessment of barriers to implementing pediatric inpatient care guidelines. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madrid L, Casellas A, Sacoor C, et al. Postdischarge mortality prediction in sub-Saharan Africa. Pediatrics. 2019;143 doi: 10.1542/peds.2018-0606. [DOI] [PubMed] [Google Scholar]
- 17.Wiens MO, Kumbakumba E, Larson CP, et al. Postdischarge mortality in children with acute infectious diseases: derivation of postdischarge mortality prediction models. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngari MM, Fegan G, Mwangome MK, et al. Mortality after inpatient treatment for severe pneumonia in children: a cohort study. Paediatr Perinat Epidemiol. 2017;31:233–242. doi: 10.1111/ppe.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelaye B, Williams MA, Lemma S, et al. Validity of the Patient Health Questionnaire-9 for depression screening and diagnosis in East Africa. Psychiatry Res. 2013;210:653–661. doi: 10.1016/j.psychres.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coates J, Swindale A, Bilinsky P. Food and Nutrition Technical Assistance Project; Washington, DC: 2007. Household food insecurity access scale (HFIAS) for measurement of food access: indicator guide (version 3) [Google Scholar]
- 21.Ballard TJ, Kepple AW, Cafiero C. The food insecurity experience scale: development of a global standard for monitoring hunger worldwide. October, 2013. http://www.fao.org/economic/ess/ess-fs/voices/en
- 22.The Demographic and Health Surveys Program Wealth index construction. 2016. https://dhsprogram.com/topics/wealth-index/Wealth-Index-Construction.cfm
- 23.Medicare Medicare hospital compare: 30-day death (mortality) rates. 2020. https://www.medicare.gov/hospitalcompare/Data/Death-rates.html
- 24.Malik MA, Rohm LR, van Baal P, van Doorslaer EVD. Improving maternal and child health in Pakistan: a programme evaluation using a difference in difference analysis. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Njunge JM, Gwela A, Kibinge NK, et al. Biomarkers of post-discharge mortality among children with complicated severe acute malnutrition. Sci Rep. 2019;9 doi: 10.1038/s41598-019-42436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutherford ME, Dockerty JD, Jasseh M, et al. Access to health care and mortality of children under 5 years of age in The Gambia: a case-control study. Bull World Health Organ. 2009;87:216–224. doi: 10.2471/BLT.08.052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prendergast AJ, Walson JL. Seeking interventions to reduce post-discharge mortality among children in sub-Saharan Africa. Lancet Glob Health. 2019;7:e1306–e1307. doi: 10.1016/S2214-109X(19)30373-0. [DOI] [PubMed] [Google Scholar]
- 28.Berkley JA, Ngari M, Thitiri J, et al. Daily co-trimoxazole prophylaxis to prevent mortality in children with complicated severe acute malnutrition: a multicentre, double-blind, randomised placebo-controlled trial. Lancet Glob Health. 2016;4:e464–e473. doi: 10.1016/S2214-109X(16)30096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maitland K, Olupot-Olupot P, Kiguli S, et al. Co-trimoxazole or multivitamin multimineral supplement for post-discharge outcomes after severe anaemia in African children: a randomised controlled trial. Lancet Glob Health. 2019;7:e1435–e1447. doi: 10.1016/S2214-109X(19)30345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.English L, Kumbakumba E, Larson CP, et al. Pediatric out-of-hospital deaths following hospital discharge: a mixed-methods study. Afr Health Sci. 2016;16:883–891. doi: 10.4314/ahs.v16i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The CHAIN cohort data and analysis code are deposited and may be requested at the Harvard Dataverse website.