Summary
The intestine is one of the organs that relies on stem cell function for maintaining tissue homeostasis. Recent findings on intestinal aging show that intestinal architecture, such as villus length, crypt size, and cell composition changes in the aged crypts. The correspondent decline in the regenerative capacity of the intestine is mainly due to a decline in intestinal stem cell function upon aging, as the underlying mechanisms of aging intestinal stem cells are beginning to unravel. This review summarizes our current knowledge on stem cell-intrinsic mechanisms of aging of intestinal stem cells and their connection to extrinsic factors, such as niche cells and microbiota and will introduce recent approaches to attenuate or even revert the aging of intestinal stem cells.
Keywords: aging, intestinal stem cells, Wnt, Cdc42 activity
Graphical abstract
Highlights
-
•
Intestinal epithelial regeneration is impaired due to reduced ISC function upon aging
-
•
ISCs function is reduced due to ISC intrinsic factors upon aging
-
•
Extrinsic factors also play a major role regulating ISC function upon aging
-
•
Aged ISC function can be restored via therapeutic interventions
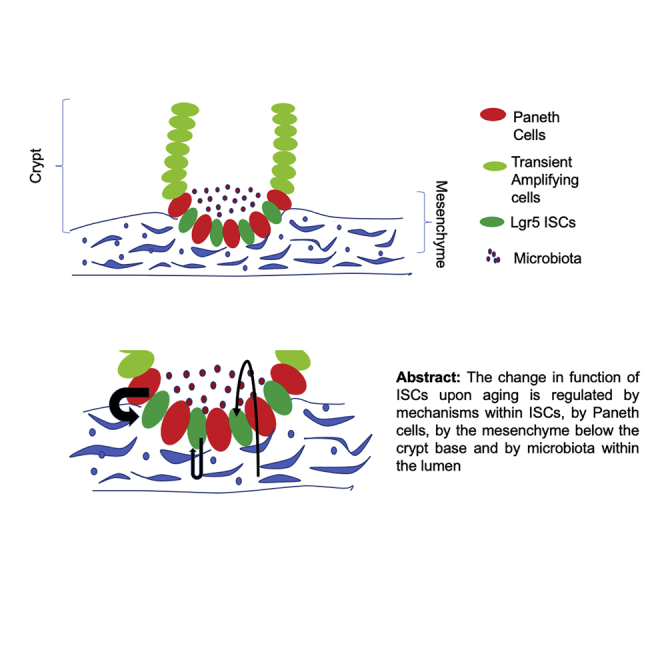
A decline in intestinal stem cell (ISC) function is one critical underlying mechanism for the reduced regeneration of aged intestinal epithelium. Factors both intrinsic and extrinsic to ISCs play a role in attenuating the function of aged ISCs. Therapeutic interventions are able to restore the function of aged ISCs to improve the regeneration of the intestinal epithelium upon aging.
Intestine and stem cells
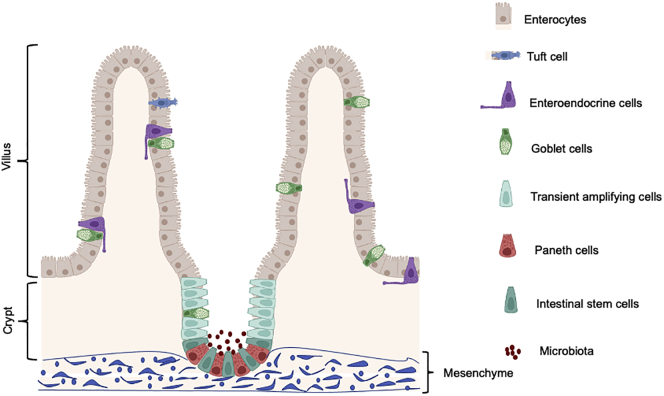
Stem cells are essential units of an organ for maintaining tissue homeostasis and organ function (Nalapareddy et al., 2008; Rando, 2006). Organs, such as the intestine, skeletal muscle, the hematopoietic system, and skin, are some of the major organs that depend on stem cell function for tissue homeostasis (Cheng and Leblond, 1974; Ge et al., 2020; Geiger et al., 2013; Nalapareddy et al., 2008; Rando, 2006). The intestinal epithelium consists of protrusions into the lumen called villi, and cup-shaped invaginations called crypts. Villi are the absorptive units of the intestine and crypts contain stem cells, and these stem cells give rise to all the cell lineages of intestinal epithelium, such as enterocytes, goblet cells, Paneth cells, enteroendocrine cells, and Tuft cells. The intestine relies on intestinal stem cells (ISCs) for tissue homeostasis and regeneration. ISCs, also called crypt base columnar cells (CBCs), reside at the base of the crypts (Barker et al., 2007; Cheng and Leblond, 1974; Potten et al., 1974). These stem cells are slowly dividing cells and can undergo asymmetric division, which gives rise to highly proliferative transit-amplifying (TA) cells (Sei et al., 2019). In mice, after four to five divisions, TA cells move upward toward the crypt-villus junction and start to differentiate into the other lineages of the intestine including Paneth cells, enterocytes, goblet cells, and enteroendocrine cells (Figure 1) (Barker et al., 2012). Within 2–3 days the enterocytes, goblet cells, and enteroendocrine cells move upward and reach the tip of the villus where they undergo apoptosis and are shed into the lumen of the gut. The Paneth cells, on the other hand, move toward the crypt bottom and stay there for approximately 6–8 weeks (Barker et al., 2012; Cheng and Leblond, 1974). The CBCs reside between the Paneth cells and express the Lgr5 gene. LGR5 (leucine-rich repeat containing, G protein-coupled receptor 5) functions as a receptor of R-spondins. R-spondins are agonists of Wnt signaling, and Wnt/β-catenin signaling plays an essential role in the development and maintenance of ISCs (Farin et al., 2012; Gregorieff and Clevers, 2005; Gregorieff et al., 2005). Signaling via LGR5 is therefore critical to potentiate Wnt/β-catenin signaling to regulate ISC survival and differentiation (Carmon et al., 2011). These LGR5-positive cells can generate all the cell lineages of the small intestine and can self-renew (Barker et al., 2007), the basic characteristic feature of a stem cell.
Figure 1.
Schematics of crypt and villus in intestinal epithelium, where ISCs reside at the crypt base flanked by Paneth cells (niche cells)
ISCs differentiate to transient amplifying cells that reach to the end of crypt and further differentiate to villus (i.e., the absorptive surface) that comprises enterocytes, goblet cells, and enteroendocrine cells.
The majority of tissues in humans and mice are affected upon chronological aging. Impaired regenerative response, loss of tissue homeostasis, and organ maintenance are the hallmark features of tissue aging (Geiger et al., 2013; Lopez-Otin et al., 2013; Nalapareddy et al., 2008; Rando, 2006). Upon aging, the intestine alters its architecture with increased crypt and villus sizes show changes in cell compositions such as Paneth cells, goblet cells, and stem cells, and manifests an impaired intestinal crypt regenerative function (Nalapareddy et al., 2017; Pentinmikko et al., 2019). A decline of ISC function is a major reason for reduced tissue regenerative capacity during aging (Nalapareddy et al., 2017). Molecular mechanisms regulating aging of mammalian ISCs have started to unravel more recently. Novel insights in these areas might provide additional rationale for therapeutic interventions. In this review, we discuss the aging of the intestine, ISCs, novel mechanisms regulating ISC aging, and the influence of the ISC niche on ISC aging.
Aging of the intestine and ISCs
The intestine and ISCs show a reduced regenerative capacity upon aging (Martin et al., 1998a, 1998b; Nalapareddy et al., 2017, 2021; Pentinmikko et al., 2019). In the late 1990s, studies from Martin. et al. revealed that the intestinal epithelium shows age-associated changes in phenotypes, such as a decrease in crypt numbers, increase in villus length, and a decline in regenerative potential upon irradiation in aged mouse intestine (Martin et al., 1998a, 1998b). Identification of LGR5 as an ISC marker further enhanced the perspective of the field to delineate the function and mechanisms underlying intestinal stem cells (Barker et al., 2007). Upon aging, intestinal crypt size, villus length, Paneth cell number, and goblet cell number all increase, but ISC number did not increase significantly upon aging, while the proliferative and regenerative potential of aged murine ISCs is reduced upon aging in mice (Nalapareddy et al., 2017). Data exist to support both a reduced number or an increased number of ISCs in aged mice, which explains why the topic is controversially discussed (Igarashi et al., 2019; Mihaylova et al., 2018; Moorefield et al., 2017; Pentinmikko et al., 2019); however, irrespective of the discrepancy in the reported change in ISC numbers these studies unequivocally confirm that ISC function is reduced upon aging. A change in ISC function upon aging is likely the critical aging-associated change in ISCs similar to what has already been reported for the aging of hematopoietic stem cells (Geiger et al., 2013; Nalapareddy et al., 2017). Reduced ISC function upon aging might also be one of the underlying mechanisms for age-associated intestinal disorders or even intestinal cancer due to an altered level of functional competition among aged ISCs.
The intestinal epithelium is a highly proliferative, self-renewing organ that continually self-renews every 3–5 days. The intestinal epithelium is highly sensitive to DNA damage agents, such as ionizing radiation and chemotherapy (Martin et al., 1998b; Metcalfe et al., 2014; Nalapareddy et al., 2017). To measure intestinal regeneration 10 or 12 grays (Gy) of radiation is the most widely used method to induce damage to the intestinal tissue and follow the regenerative phase post-injury. Radiation induces apoptosis in crypts, which leads to crypt shrinking followed by an eruption of proliferation by ISCs, resulting in transient enlargement of crypt followed by crypt fission to compensate for the crypt loss (Withers and Elkind, 1969). At doses of 14 GY or above, regeneration fails to happen due to severe loss of ISCs through apoptosis (Metcalfe et al., 2014). To measure self-renewal, we used a method called 10 + 10 Gy radiation (IR). With the first dose (day 1, 10 Gy), the existing ISCs succumb to apoptosis and the surviving ISCs start to regenerate to give the first generation of ISC after 10 GY IR. With the second dose (day 2, 10 Gy IR), the first-generation ISCs are damaged and the surviving ISCs give rise to the second generation that could further carry out the process of intestinal regeneration, which assists in the measurement of ISC self-renewal function. With the 10 + 10 Gy IR method, aged mouse intestinal epithelium showed reduced crypt regeneration compared with young mice measured by (transient) enlarged crypt size compared with the crypt size of aged mouse intestinal epithelium 3 days after 10 + 10 Gy IR, indicating a decline of ISC regenerative and self-renewing function of aged ISCs (Nalapareddy et al., 2017).
From the crypt base, the “+4” position that shows ISCs (Figure 1A) that are positive for markers LRIG1, HOPX1, BMI1, TERT, and SOX9 are considered to be “quiescent stem cells” (Barker et al., 2012). Quantitative gene expression analysis of these quiescent ISC-associated markers indeed showed a decline in expression in aged intestinal crypts (Nalapareddy et al., 2017). Interpretation of specifically +4 ISC data is difficult because all +4 ISCs are also positive for LGR5 (Munoz et al., 2012). However, during IR stress, Paneth cells that could dedifferentiate to ISCs due to radiation-induced stress (Buczacki et al., 2013) could also contribute to the process of regeneration upon aging, making the +4 reserve pool dispensable. In contrast, a more recent study demonstrated that BMI1-positive +4 murine ISCs exit quiescence upon radiation and contribute to intestinal epithelial regeneration after radiation injury (Orzechowska et al., 2020). Further studies are required to understand the function of “+4 quiescent intestinal stem cells” during homeostasis and upon aging.
In humans, reports exist to imply a decreased absorption of the intestine, reduced intestinal barrier function, increased inflammation, and changes in gut microbiota (Britton and McLaughlin, 2013). ISC function is also reduced upon aging in humans (Nalapareddy et al., 2017). Intestinal crypts can be isolated and cultured in 3D Matrigel to form organoids in vitro. It is known that the ability of crypts to form organoids in vitro and crypt regeneration after radiation injury in vivo is mainly stem cell driven (Metcalfe et al., 2014; Sato et al., 2009). Crypts derived from young mice can form organoids and can be passaged continuously, whereas aged crypts fail to form and/or the number of organoids formed is reduced after three to four passages indicating a decline of ISC function in vitro. Not only aged crypts but also individual aged ISCs, when put into the culture to form colonies, form less colonies compared with young ISCs (Nalapareddy et al., 2021; Pentinmikko et al., 2019). Aged ISCs from mice and aged crypts from both humans and mice, when put into the organoid culture in vitro, therefore showed impaired regeneration. Upon aging, expression of canonical Wnts, and especially Wnt3, which is secreted from Paneth cells and the underlying crypt mesenchyme, is reduced, along with reduced Wnt3 levels within ISCs (Nalapareddy et al., 2017). The addition of WNT3a to aged human and murine intestinal organoid cultures did indeed enhance ISC function and could ameliorate the aging-associated regenerative decline in the intestinal epithelium (Nalapareddy et al., 2017). Devising new treatments for ISCs with Wnts to improve the regenerative function of aged ISCs could form a novel basis for a therapeutic strategy to treat age-associated intestinal disorders. In this review, we will first explain changes in pathways and mechanisms upon aging that are intrinsic to ISCs (signaling pathways for which changes within ISCs alter ISC function) and will subsequently focus on extrinsic aging factors, such as changes in interactions with or factors secreted from the ISCs niche (Paneth cells, mesenchyme, gut microbiota) and how they affect signaling pathways within ISCs and thus ISC function.
Intrinsic mechanisms regulating aged ISC function
ISCs reside at the base of the crypt surrounded by differentiated Paneth cells that form a niche. Different signaling pathways (both intrinsic and extrinsic mechanisms), such as WNT, NOTCH, BMP, JAK/STAT1, and PI3K/AKT, play an important role in regulating ISC function, proliferation, and differentiation (Farin et al., 2012; Qi et al., 2017; Richmond et al., 2018; Tian et al., 2015; VanDussen et al., 2012). The cell-intrinsic signaling pathways regulate ISCs in response to extrinsic signals stemming from Paneth cells or mesenchyme that is present below intestinal crypts or from microbiota or immune cells within the gut. Canonical Wnt signaling controls cell behavior by activating the T cell factor/lymphoid enhancer factor 1 family of transcription factors via β-catenin (Clevers and van de Wetering, 1997). The proliferation of crypts is compromised in mice lacking canonical Wnts/Wnt signaling. At the same time, hyperactivation of canonical Wnt signaling, for example, adenomatous polyposis coli, a protein that is part of the β-catenin destruction complex, leads to hyperproliferation of ISCs and leads to intestinal tumorigenesis (Farin et al., 2012; Gregorieff and Clevers, 2005; van der Flier et al., 2009). Canonical Wnt signaling is crucial for intestinal development and homeostasis in young mice (Farin et al., 2012; Pinto et al., 2003). Upon aging, reduced canonical Wnt signaling is one of the causes of the impaired regenerative capacity and proliferation of ISCs (Nalapareddy et al., 2017; Pentinmikko et al., 2019). Signaling induced by canonical Wnts affects β-catenin, which in turn regulates the expression of Ascl2 (achaete-scute-like 2), a β-catenin-dependent transcription factor that controls ISC function. Furthermore, expression of ISC marker Lgr5 (van der Flier et al., 2009) is reduced in aged ISCs (Nalapareddy et al., 2017). It is known that organoid formation depends on ISC function (Sato et al., 2009). Activating β-catenin/ASCL2-mediated canonical Wnt signaling in organoids derived from both mouse and human aged crypts ameliorated the age-associated decline of organoid formation, indicating that canonical Wnt signaling in ISCs is causal for ISC function upon aging (Nalapareddy et al., 2017; Pentinmikko et al., 2019). A major reason for reduced canonical Wnt signaling upon aging might be to limit tumorigenesis due to the increased mutation rate upon aging in the canonical Wnt signaling cascade.
Along with Wnt signaling, Notch signaling is the other major pathway that affects ISC fate decisions by regulating helix-loop-helix transcription factor ATOH1 (Atonal homolog 1) (Tian et al., 2015). There are four Notch receptors, NOTCH1, 2, 3, and 4, and five Notch ligands, Delta-like 1, 3, and 5, and Jagged 1 and 2 (Kopan and Ilagan, 2009). Among the four Notch receptors, Notch1 gene expression was detected in ISCs and is reduced in aged ISCs (Nalapareddy et al., 2017). In line with reduced Notch1 gene expression, ISCs show an increase in Atoh1 gene expression in the aged ISCs (Nalapareddy et al., 2017). As ATOH1 regulates lateral inhibition through Delta-like notch ligand and secretory lineage genes, increases in Atoh1 in ISCs lead to differentiation to more secretory cells such as Paneth and goblet cells in the aged intestine (Nalapareddy et al., 2017). Olfactomedin-4 (OLFM4) is a Notch signaling target that regulates the proliferation and differentiation of ISCs (VanDussen et al., 2012). In line with the decline of Notch1 gene expression, Olfm4 gene expression in aged mouse intestine is also reduced compared with young mice. This lower level of expression could corroborate with reduced canonical Wnt signaling, leading to even further reduced proliferative potential of aged ISCs (Nalapareddy et al., 2017). Taken together, dysregulation of both canonical Wnt signaling and Notch signaling in ISCs leads to reduced regeneration and altered differentiation upon aging.
Extrinsic mechanisms regulating ISC aging
Paneth cells and mesenchymal cells that are found beneath intestinal crypts constitute the niche for ISCs (Farin et al., 2012; Sato et al., 2011). Canonical Wnt signaling in ISCs is regulated by Wnt ligands secreted from the niche (Smith et al., 2012). Wnt signaling is regulated by paracrine or autocrine Wnts secreted by niche cells (Farin et al., 2016). During organoid cultures, the presence of Paneth cells enhanced the organoid formation ability of ISCs in vitro, indicating the functional role of Paneth cells as a niche regulating ISC function in vitro (Sato et al., 2011). Gene expression changes between young and aged Paneth cells have also been reported (Nalapareddy et al., 2017; Pentinmikko et al., 2019). One of the reasons for the decline in canonical Wnt signaling in aged ISCs is mostly due to reduced Wnt secretion, especially WNT3 from niche cells upon aging (Nalapareddy et al., 2017). Reduced secretion of WNT3 by Paneth cells and/or mesenchyme present below intestinal crypts, in turn, leads to reduced canonical Wnt signaling in ISCs, compromising aged ISC function (Nalapareddy et al., 2017). Co-culture of ISCs with Paneth cells was shown to increase ISC colony formation in vitro (Sato et al., 2011). Upon aging, the increase in the number of Paneth cells is observed but still due to reduced Wnt3 expression in Paneth cells, canonical Wnt signaling and thus ISC function is also reduced in aged ISCs (Nalapareddy et al., 2017). It was recently shown that NOTUM, a Wnt deacetylase secreted from old Paneth cells, disengages Wnt ligands from frizzled receptors Lrp5 and Lrp6, which reduced Wnt activity and thus Wnt signaling in ISCs upon aging (Pentinmikko et al., 2019). NOTUM might thus be one of the underlying mechanisms for reduced canonical Wnt signaling in aged ISCs driven by the niche. It has also been shown that calorie restriction regulates mTORC1 in Paneth cells and enhances ISC function (Yilmaz et al., 2012). Although the presence of Paneth cells in vitro increases the organoid formation of ISCs, strong evidence exists for the dispensable nature of Paneth cells for the function of ISCs, as genetic ablation of Paneth cells in mouse models did not show any effect on ISC function in vivo (Durand et al., 2012). The dispensable nature of Paneth cell in vivo might be that mesenchyme found underneath crypts might compensate for the loss of Paneth cells by providing Wnts to ISCs. It is quite interesting to understand the mechanisms regulating ISC function in the absence of secretory cell lineage (mainly Paneth cells), which controls stem cell function and differentiation through Notch signaling in the intestinal epithelium as reduced Notch1 gene expression was also reported reduced in aged compared with young crypts (Nalapareddy et al., 2017). BMP signaling, mainly from the mesenchyme, is considered as a negative regulator of intestinal epithelial cell proliferation (Qi et al., 2017), but it remains unknown how BMP signaling may regulate ISC aging. There are also a few Wnts (e.g., WNT2, WNT2b, and WNT3) secreted by the mesenchyme (Nalapareddy et al., 2017) that could regulate Wnt signaling in ISCs but their functions have yet to be deciphered in the context of aging.
Although murine Paneth cells are dispensable for ISC function in vivo, in humans a change in the function of Paneth cells (mainly their antimicrobial peptide defensins secretion) leads to Crohn disease (Liu et al., 2016). Having altered or abnormal Paneth cells lead to an unwanted immune response affecting intestinal homeostasis, which differs from studies involving total loss of Paneth cells in mouse model systems (Durand et al., 2012; Liu et al., 2016). Environmental factors play a major role in the initiation of Crohn disease, and gut microbiota is one of many influential factors (Liu et al., 2016). Alterations in gut microbiota are observed upon aging (Claesson et al., 2011). Microbial dysbiosis leads to inflammation and is regarded as a primary cause of age-associated premature death in aged individuals (Thevaranjan et al., 2018). Evidence exists for increased inflammation due to alteration of metabolites, loss of integrity of the intestinal barrier, and gut leakiness, which further enhances systemic inflammation leading to an increase in age-associated disorders (Thevaranjan et al., 2018). Paneth cell numbers are also altered in the aged intestine (Nalapareddy et al., 2017), and Paneth cells secrete lysozyme and defensins which could alter gut microbiota (Liu et al., 2016). The increased Paneth cell numbers upon aging could be a compensatory mechanism to maintain Wnts and Wnt signaling and also to maintain the antimicrobial function of Paneth cells by secreting lysozyme and defensins to protect the intestine from pathogenic microbiota in the intestine. Thus, it will be highly significant to understand if altered microbiota and/or altered Paneth cells are a root cause of age-associated intestinal inflammatory diseases.
Intervening in the mechanisms of ISC aging
Attenuation of mechanisms that result in a decline of ISC function upon aging might contribute to maintaining intestinal homeostasis upon aging. Reduced canonical Wnt signaling is one central mechanism for a reduced function of aged ISC. Wnt3a, which increases canonical Wnt signaling within ISCs, when provided to organoid cultures increased canonical Wnt signaling within ISCs and could enhance the organoid forming ability of aged ISCs (Nalapareddy et al., 2017). Apart from Wnt signaling, it was recently reported that fasting activates the regenerative function of both young and aged ISCs by activating fatty acid oxidation (FAO) in ISCs (Mihaylova et al., 2018). However, upon aging FAO decreases. Pharmacological activation of FAO or the addition of an FAO substrate, such as palmitic acid, did enhance aged ISC organoid formation ability (Mihaylova et al., 2018). In addition, it was shown that the activity of the SIRT1/mTORC1 pathway was reduced upon aging, and that by reactivating the pathway via the NAD+ precursor nicotinamide riboside, gut regeneration was enhanced in the aged (Igarashi et al., 2019). These findings provide strong support that reduced aged ISC function is reversible by targeting mechanisms in aged ISCs.
Studies from hematopoietic stem cells (HSCs) indicate that there is a switch from preferentially canonical to non-canonical Wnt signaling in HSCs, with elevated expression of Wnt5a upon aging increasing CDC42GTP (Florian et al., 2012). CDC42 is a small RhoGTPase that regulates biological activity by shuttling between its inactive CDC42GDP form and an active CDC42GTP (Cdc42activity) form (Melendez et al., 2013). Cdc42 activity regulates actin and tubulin organization and cell polarity in HSCs. Increased Cdc42 activity in aged HSCs impairs HSC function (Florian et al., 2012). Cdc42 ablation in intestinal epithelium resulted in crypt hyperplasia, microvilli inclusion, and abnormal epithelial permeability. Cdc42-deficient intestinal crypts showed an increase in ISC proliferation, defective Paneth cell differentiation, and further displaced Paneth cells from the crypt base into the villi without affecting other cell lineages in the intestinal epithelium (Melendez et al., 2013). In the mouse, Cdc42 activity is increased in proliferating TA cells and ISCs in aged intestinal crypts (Nalapareddy et al., 2021). Pharmacological suppression of Cdc42 activity via CASIN (CDC42 activity-specific inhibitor) in aged intestinal crypts enhanced ISC regenerative function upon radiation-induced damage. CASIN treatment also enhanced the organoid forming ability of aged crypts and aged ISCs (Nalapareddy et al., 2021). These results suggest that it is possible to pharmacologically target aged ISC in vivo to improve ISC function. Delineating novel pathways governing ISC function might enhance therapeutic strategies to ameliorate the age-associated decline of ISC function.
Conclusions
It is widely accepted that, with chronological age, the function of ISCs declines. Mechanisms that affect ISC function upon aging have been recently identified, while additional research is needed to understand the relative contribution of intrinsic and extrinsic mechanisms regulating aged ISCs, and particularly on how various environmental factors influence intestinal aging. Future mechanistic studies will help to develop new therapeutic interventions to ameliorate age-associated intestinal diseases and to improve ISC regenerative potential for proper intestinal function upon aging.
Acknowledgments
Work in the laboratory was supported by grants from the NIH (R01DK104814, P30 DK078392, and RO1AG063937) and the Baden-Württemberg-Stiftung gGmbH, Project AMDA, Germany. Figures were created with BioRender.com and Microsoft PowerPoint.
Conflicts of interest
The authors declare no competing interests.
References
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N., van Oudenaarden A., Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452–460. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Britton E., McLaughlin J.T. Ageing and the gut. Proc. Nutr. Soc. 2013;72:173–177. doi: 10.1017/S0029665112002807. [DOI] [PubMed] [Google Scholar]
- Buczacki S.J., Zecchini H.I., Nicholson A.M., Russell R., Vermeulen L., Kemp R., Winton D.J. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Carmon K.S., Gong X., Lin Q., Thomas A., Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. U S A. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Leblond C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Claesson M.J., Cusack S., O'Sullivan O., Greene-Diniz R., de Weerd H., Flannery E., Marchesi J.R., Falush D., Dinan T., Fitzgerald G., et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. U S A. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., van de Wetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485–489. doi: 10.1016/s0168-9525(97)01305-x. [DOI] [PubMed] [Google Scholar]
- Durand A., Donahue B., Peignon G., Letourneur F., Cagnard N., Slomianny C., Perret C., Shroyer N.F., Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc. Natl. Acad. Sci. U S A. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin H.F., Van Es J.H., Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529 e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Farin H.F., Jordens I., Mosa M.H., Basak O., Korving J., Tauriello D.V., de Punder K., Angers S., Peters P.J., Maurice M.M., Clevers H. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- Florian M.C., Dorr K., Niebel A., Daria D., Schrezenmeier H., Rojewski M., Filippi M.D., Hasenberg A., Gunzer M., Scharffetter-Kochanek K., et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10:520–530. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y., Miao Y., Gur-Cohen S., Gomez N., Yang H., Nikolova M., Polak L., Hu Y., Verma A., Elemento O., et al. The aging skin microenvironment dictates stem cell behavior. Proc. Natl. Acad. Sci. U S A. 2020;117:5339–5350. doi: 10.1073/pnas.1901720117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger H., de Haan G., Florian M.C. The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- Gregorieff A., Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- Gregorieff A., Pinto D., Begthel H., Destree O., Kielman M., Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Igarashi M., Miura M., Williams E., Jaksch F., Kadowaki T., Yamauchi T., Guarente L. NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell. 2019;18:e12935. doi: 10.1111/acel.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R., Ilagan M.X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.C., Gurram B., Baldridge M.T., Head R., Lam V., Luo C., Cao Y., Simpson P., Hayward M., Holtz M.L., et al. Paneth cell defects in Crohn's disease patients promote dysbiosis. JCI Insight. 2016;1:e86907. doi: 10.1172/jci.insight.86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K., Kirkwood T.B., Potten C.S. Age changes in stem cells of murine small intestinal crypts. Exp. Cell Res. 1998;241:316–323. doi: 10.1006/excr.1998.4001. [DOI] [PubMed] [Google Scholar]
- Martin K., Potten C.S., Roberts S.A., Kirkwood T.B. Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. J. Cell Sci. 1998;111:2297–2303. doi: 10.1242/jcs.111.16.2297. [DOI] [PubMed] [Google Scholar]
- Melendez J., Liu M., Sampson L., Akunuru S., Han X., Vallance J., Witte D., Shroyer N., Zheng Y. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology. 2013;145:808–819. doi: 10.1053/j.gastro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe C., Kljavin N.M., Ybarra R., de Sauvage F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Mihaylova M.M., Cheng C.W., Cao A.Q., Tripathi S., Mana M.D., Bauer-Rowe K.E., Abu-Remaileh M., Clavain L., Erdemir A., Lewis C.A., et al. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell. 2018;22:769–778 e4. doi: 10.1016/j.stem.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorefield E.C., Andres S.F., Blue R.E., Van Landeghem L., Mah A.T., Santoro M.A., Ding S. Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging (Albany NY) 2017;9:1898–1915. doi: 10.18632/aging.101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J., Stange D.E., Schepers A.G., van de Wetering M., Koo B.K., Itzkovitz S., Volckmann R., Kung K.S., Koster J., Radulescu S., et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. EMBO J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapareddy K., Jiang H., Guachalla Gutierrez L.M., Rudolph K.L. Determining the influence of telomere dysfunction and DNA damage on stem and progenitor cell aging: what markers can we use? Exp. Gerontol. 2008;43:998–1004. doi: 10.1016/j.exger.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Nalapareddy K., Nattamai K.J., Kumar R.S., Karns R., Wikenheiser-Brokamp K.A., Sampson L.L., Mahe M.M., Sundaram N., Yacyshyn M.B., Yacyshyn B., et al. Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Rep. 2017;18:2608–2621. doi: 10.1016/j.celrep.2017.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapareddy K., Hassan A., Sampson L.L., Zheng Y., Geiger H. Suppression of elevated Cdc42 activity promotes the regenerative potential of aged intestinal stem cells. iScience. 2021;24:102362. doi: 10.1016/j.isci.2021.102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzechowska E.J., Katano T., Bialkowska A.B., Yang V.W. Interplay among p21(Waf1/Cip1), MUSASHI-1 and Kruppel-like factor 4 in activation of Bmi1-Cre(ER) reserve intestinal stem cells after gamma radiation-induced injury. Sci. Rep. 2020;10:18300. doi: 10.1038/s41598-020-75171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentinmikko N., Iqbal S., Mana M., Andersson S., Cognetta A.B., 3rd, Suciu R.M., Roper J., Luopajarvi K., Markelin E., Gopalakrishnan S., et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature. 2019;571:398–402. doi: 10.1038/s41586-019-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C.S., Kovacs L., Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7:271–283. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Qi Z., Li Y., Zhao B., Xu C., Liu Y., Li H., Zhang B., Wang X., Yang X., Xie W., et al. BMP restricts stemness of intestinal Lgr5(+) stem cells by directly suppressing their signature genes. Nat. Commun. 2017;8:13824. doi: 10.1038/ncomms13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando T.A. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- Richmond C.A., Rickner H., Shah M.S., Ediger T., Deary L., Zhou F., Tovaglieri A., Carlone D.L., Breault D.T. JAK/STAT-1 signaling is required for reserve intestinal stem cell activation during intestinal regeneration following acute inflammation. Stem Cell Rep. 2018;10:17–26. doi: 10.1016/j.stemcr.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sei Y., Feng J., Chow C.C., Wank S.A. Asymmetric cell division-dominant neutral drift model for normal intestinal stem cell homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;316:G64–G74. doi: 10.1152/ajpgi.00242.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N.R., Davies P.S., Silk A.D., Wong M.H. Epithelial and mesenchymal contribution to the niche: a safeguard for intestinal stem cell homeostasis. Gastroenterology. 2012;143:1426–1430. doi: 10.1053/j.gastro.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevaranjan N., Puchta A., Schulz C., Naidoo A., Szamosi J.C., Verschoor C.P., Loukov D., Schenck L.P., Jury J., Foley K.P., et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2018;23:570. doi: 10.1016/j.chom.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Biehs B., Chiu C., Siebel C.W., Wu Y., Costa M., de Sauvage F.J., Klein O.D. Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep. 2015;11:33–42. doi: 10.1016/j.celrep.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier L.G., van Gijn M.E., Hatzis P., Kujala P., Haegebarth A., Stange D.E., Begthel H., van den Born M., Guryev V., Oving I., et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- VanDussen K.L., Carulli A.J., Keeley T.M., Patel S.R., Puthoff B.J., Magness S.T., Tran I.T., Maillard I., Siebel C., Kolterud A., et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers H.R., Elkind M.M. Radiosensitivity and fractionation response of crypt cells of mouse jejunum. Radiat. Res. 1969;38:598–613. [PubMed] [Google Scholar]
- Yilmaz O.H., Katajisto P., Lamming D.W., Gultekin Y., Bauer-Rowe K.E., Sengupta S., Birsoy K., Dursun A., Yilmaz V.O., Selig M., et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]