Summary
The in vitro production of stem-cell-derived islets (SC-islets) has brought forth the potential of transplanting these cells to restore glycemic control in people with diabetes. Nonetheless, alloimmune and autoimmune responses remain considerable challenges for a broad clinical implementation of β-cell replacement therapies. β-cell stress has been implicated in the onset of β-cell immunogenicity and death and is likely to contribute to β-cell failure following transplantation. We show that inducing stress and/or administering cytokines causes SC-islet apoptosis, cellular dysfunction, and an increased expression of β-cell stress- and immune-interaction-related genes. We then demonstrate that manipulating some of these genes results in enhanced protection of SC-islets from apoptosis in vitro.
Keywords: β-cell stress, cell repacement therapy, T1D disease modeling
Graphical abstract
Highlights
-
•
Stem-cell-derived islets (SC-islets) are vulnerable to stress in vitro
-
•
Genetic manipulation protects SC-islets from stress-mediated apoptosis and dysfunction
-
•
Genetic manipulation protects SC-islets from apoptosis mediated by allorecognition
Leite et al. describe that reduction in the expression of genes related to β-cell stress results in enhanced protection of SC-islets from apoptosis. The data presented here begins the understanding of the effects of stress on SC-islets function and viability in vitro. This information is a step forward toward the possibility of using SC-islets in long-term transplantation therapies without the need for lifelong and general immunosuppression.
Introduction
Type 1 diabetes (T1D) results from autoimmune-mediated destruction of pancreatic β cells (Katsarou et al., 2017). Genetic and environmental factors have been implicated in the emergence of β-cell immunogenicity and dysfunction; however, the initial signals that trigger autoimmunity, the intracellular mediators that result in β-cell destruction, and the cross talk between β cells and immune cells in T1D remain poorly understood (Engin, 2016). Advances in the production of insulin-secreting β cells from human embryonic stem cells (SC-islets) have brought forth the potential of restoring glycemic control in diabetic individuals by transplanting these in vitro produced cells (Melton, 2021). While utilizing human pluripotent stem cells to generate SC-islets addresses the issue of islet supply, transplantation will require immune protection with an encapsulation device, manipulation of the host immune response, and/or genetic modification of the transplanted cells (Siehler et al., 2021).
β cells have long been thought to be non-provoking victims of autoimmune destruction. However, recent studies have pointed to the possibility that β-cell stress may contribute to the T1D immune attack, suggesting dysfunction of both the immune system and the β cell (Roep et al., 2020). Thus, approaches to successful cell transplantation for T1D might be expanded beyond calcineurin inhibitor administration, regulatory T cell (Treg) manipulation, and other manipulations of the host immune system (Roep et al., 2020). Therapies that aim to induce selective immune tolerance to islet autoantigens, favoring the engagement of the immune system, are being tested to reverse the immunopathogenesis of T1D (Alhadj Ali et al., 2017). In combination with SC-islet therapy, these could improve β-cell stamina and vitality and protect these cells from metabolic and inflammatory stress.
Several studies on the effects of exposing islets to environmental stressors show reduced expression of key transcription factors as mediators of gluco- and lipotoxicity (Dai et al., 2016). But these experiments, typically done in the context of type 2 diabetes (T2D), may not reflect what happens when SC-islets are transplanted into T1D patients. One can anticipate the induction of β-cell stress by metabolic overload, exposure to the proinflammatory milieu, and/or glucotoxicity. As part of the goal to mitigate the responses to transplanted SC-islets, we characterized the stress response of SC-islets upon exposure to environmental triggers and use that information to protect SC-islets from stress-induced apoptosis.
Results
SC-islets and human islets are vulnerable to stress in vitro
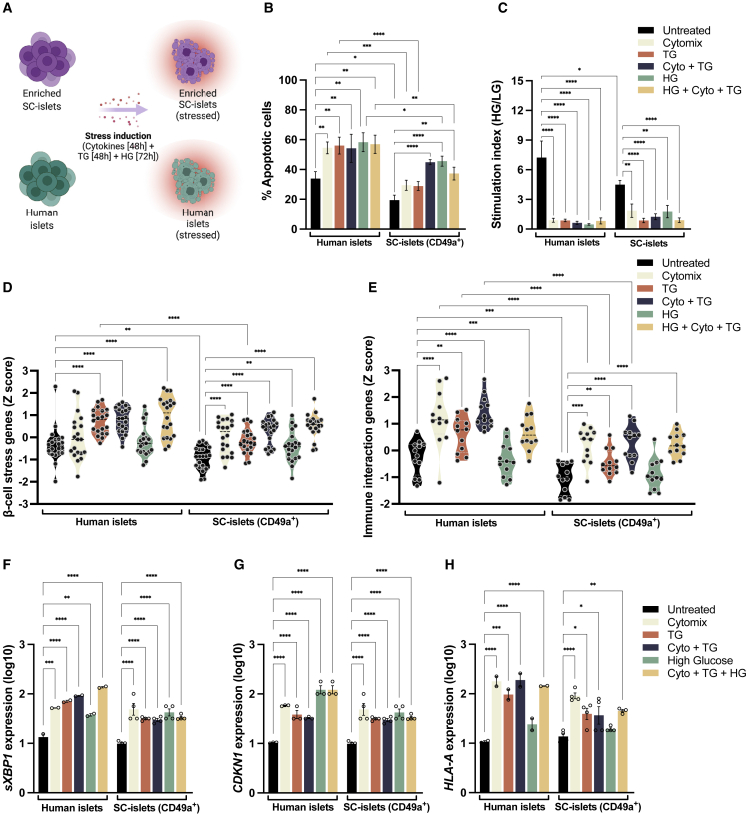
To examine the stress response of SC-islets and compare it with that of human islets (HIs), we investigated the level of apoptosis of SC-islets and HIs following treatment with stress-inducing factors. Embryonic stem cells were differentiated in vitro to SC-islets, which generates a mixed population of endocrine and non-endocrine cells (Figure S1A). To examine the β-cell effects, we used anti-CD49a to enrich β cells in the SC-islets for all experiments performed, unless otherwise indicated, and this enriches the β-cell population from ∼20% to ∼80% purity (Veres et al., 2019) (Figure S1B). Following enrichment, SC-islets and HI controls were treated with three different stress conditions, as previously described (Maxwell et al., 2020) (Figure 1A): cytomix (interleukin [IL]-1β—50 ng/mL, tumor necrosis factor [TNF]-α—100 ng/mL, and interferon [IFN]-γ—500 ng/mL; 48 h), thapsigargin (TG) (10 μM, 48 h), and high glucose (HG) (33 mM, 72 h), mimicking inflammatory, endoplasmic reticulum (ER), and metabolic stress, respectively. The gating strategy for the apoptosis assay is shown in Figure S1C.
Figure 1.
SC-islets are vulnerable to stress in vitro
(A) Experimental design: SC-islets and human islets (HIs) were treated with cytokines (IL-1β—50 ng/mL, TNF-α—100 ng/mL, and IFN-γ—500 ng/mL; 48 h), thapsigargin (TG, 10 μM, 4 8 h) and high glucose (HG, 33 mM, 72 h) to induce inflammatory, ER, and metabolic stress, respectively.
(B) Apoptosis in SC-islets and HIs treated with cytokines, TG, and HG.
(C) Stimulation index of HIs and SC-islets following treatment with stress-inducing factors as in (B).
(D and E) Relative mRNA expression of β-cell stress-associated genes (D) and immune-interaction-associated genes (E) in HIs and SC-islets treated as in (B). Each dot represents one gene from the heatmap in (S1E). Black dashed lines represent the median.
(F–H) mRNA expression level of spliced XBP1 (F), CDKN1A (G), and HLA-A (H) in HIs and SC-islets treated with stress-inducing factors as in (B).
(B–H) n = 4 SC-islets differentiations and n = 2 HI donors, for two independent experiments. Data are means ± SEMs. ∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005, and ∗∗∗∗p < 0.0001. Ordinary 1-way ANOVA.
HIs exhibited increased levels of apoptosis after treatment with all stressors (cytomix, TG, and HG) compared with untreated controls (Figures 1B and S1D). Similarly, SC-islets exhibited heightened apoptosis when treated with stressors; however, the percentage of apoptotic cells and dead cells in the treated conditions was reduced compared with that of HIs (Figures 1B and S1D). These results indicate that SC-islets are vulnerable to stress and apoptosis in vitro, albeit to a lesser degree than HIs in this assay.
An in vitro functional assay measuring glucose-stimulated insulin secretion (GSIS) was performed on native, non-enriched SC-islet populations and HI controls following treatment with stressors, as described above. Observed stimulation indices of the HIs and SC-islets treated with stressors revealed irregular patterns following glucose challenges (Figure 1C). These results show an impairment of the coupling of glucose metabolism and insulin secretion in HIs and SC-islets following exposure to environmental stressors. β-cell stress induced by cytokines and nutrients directly impairs insulin secretion and other aspects of β-cell function and survival, indicating the pathological consequences of excess glucose and/or inflammation (Eizirik et al., 2020; Sims et al., 2020).
In addition to cellular death and dysfunction, β-cell stress has been shown to result in reduced expression of genes related to cellular homeostasis and heightened expression of genes related to inflammation, peptide presentation, and stress (Eizirik et al., 2020). To determine whether similar gene expression changes are induced in SC-islets after stress, multiplex gene expression analysis was performed on stressed SC-islets and HIs. Both HIs and SC-islets exhibited increased expression of genes related to β-cell stress (Figures 1D and S1E) and immune interaction (Figures 1E and S1E). Expression of genes upregulated after stress seems to increase most when HIs and SC-islets are exposed to cytokines, TG, and HG combined. Stress induction did not impair SC-islet identity (Figure S1F). In conclusion, stress treatment causes SC-islet gene upregulation in vitro, albeit to a lesser level than HI.
Genetic manipulation is associated with protection of SC-islets from stress-mediated apoptosis and dysfunction
Considering the possible role of β-cell stress in instigating an immune rejection, we hypothesized that targeting genes related to β-cell stress, in addition to those related to immune interaction, might result in enhanced protection of SC-islets from apoptosis. The rationale for the dual approach, targeting stress and immune recognition genes, is that we believe both β-cell stress and islet autoimmunity can be harnessed as targets for intervention strategies. Thus, genes that were elevated upon treatment and involved in peptide presentation and stress were selected.
Noting that XBP1 (Figure 1F), CDKN1A (Figure 1G), and HLA class I expression (Figure 1H) (regulated by the genes β2M and NLRC5) are upregulated in most stress conditions, we targeted the genes β2M, CDKN1A, NLRC5, and XBP1 to study resistance to apoptosis. XBP1 is directly involved in the ER stress response and plays differing roles depending on the nature of the stress. During acute ER stress, as in our study, XBP1 is involved in the unfolded protein response (Eizirik et al., 2020). CDKN1A promotes apoptosis of β cells in response to various stressors (Kaneto et al., 1999). NLRC5 is a transcriptional regulator of HLA-ABC and β2M, responsible for orchestrating the expression of critical components in the HLA class I pathway (Meissner et al., 2010). NLRC5 deficiency impairs killing by cytotoxic T cells (Staehli et al., 2012), and knockdown in mesangial cells in HG conditions is associated with reduced inflammation (Luan et al., 2018). Lastly, β2M knockout results in the reduction of HLA class I expression and reduces T-cell-mediated immune responses in endothelial cells (Han et al., 2019) and HIs (Wang et al., 2012).
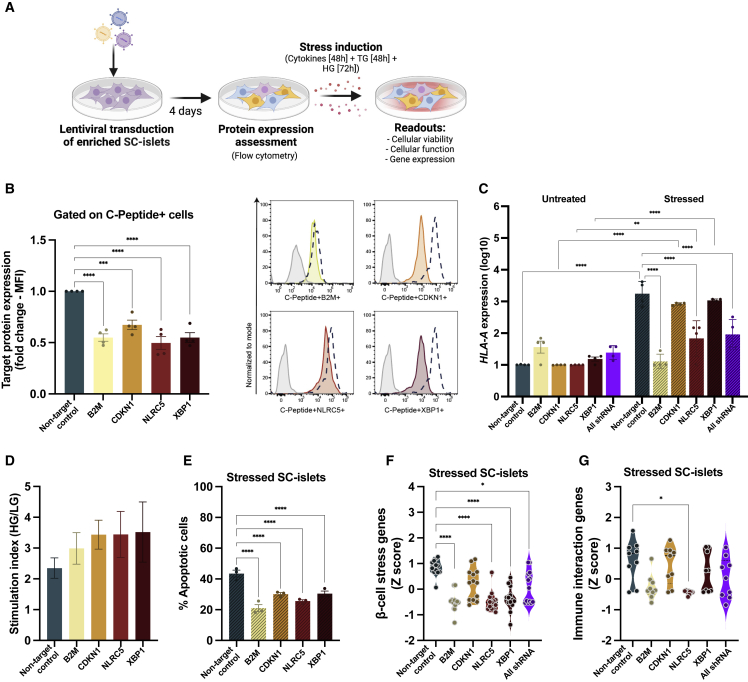
To study the effect of gene downregulation on SC-islets viability and function, we transduced SC-islets with lentiviral small hairpin RNA (shRNA) plasmids individually targeting each gene of interest (β2M, CDKN1A, NLRC5, and XBP1) or targeting all four genes together (all shRNA group). Following transduction, the SC-islets were treated with a combination of stress-inducing factors: cytomix (IL-1β—50 ng/mL, TNF-α—100 ng/mL, and IFN-γ—500 ng/mL; 48 h), TG (10 μM, 48 h), and HG (33 mM, 72 h) (Figure 2A). Efficacy of the shRNA plasmids was evaluated by quantification of GFP (Figures S2A and S2B) and target protein expression in the SC-islets four days after transduction (Figure 2B). Because treatment with stressors leads to upregulation of HLA class I in SC-islets (Demine et al., 2020; Leite et al., 2020), following transduction and stress induction of SC-islets, we assessed HLA-A expression (Figure 2C). Target protein expression revealed that CDKN1A and XBP1 shRNAs efficiently reduce individual gene expression in the untreated condition and that β2M and NLRC5 shRNAs inhibit HLA-A upregulation upon stressor treatment. Furthermore, genetic modifications did not impair SC-islet function, as measured by a GSIS assay (Figure 2D).
Figure 2.
Genetic manipulation increases protection of SC-islets from stress-mediated apoptosis and dysfunction
(A) Experimental design: SC-islets were transduced with lentiviral shRNA plasmids individually targeting each gene of interest or targeting all four genes together (all shRNA group). Following transduction, the SC-islets were treated with a combination of stress-inducing factors: cytomix (IL-1β—50 ng/mL, TNF-α—100 ng/mL, and IFN-γ—500 ng/mL; 48 h), TG (10 μM, 48 h), and HG (33 mM, 72 h).
(B) Representative fold-change target protein expression with values represented as adjusted mean fluorescence intensity (MFI). Data are means ± SEMs and representative of four experiments, each with one SC-islet differentiation (n = 4). Representative histogram plots are shown on the right. Dashed line represents the non-target control and solid colored plot represent the gene of interest. Isotype control is shown in gray.
(C) mRNA expression level of HLA-A in SC-islets following genetic modification and treated with stress-inducing factors as in (A).
(D) Stimulation index of SC-islets following genetic modification. n = 2 SC-islet differentiations.
(E) Apoptosis in SC-islets following genetic modification and treatment with stress-inducing factors as in (A).
(F and G) Relative mRNA expression of β-cell stress-associated genes (F) and immune-interaction-associated genes (G) in SC-islets stressed as in (A), shown as violin plots. Each dot represents one gene from the heatmap in (S2D). Black dashed lines represent the median.
(C–G) Data are means ± SEMs. n = 3 SC-islet differentiations, for three independent experiments. ∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005, and ∗∗∗∗p < 0.0001. Ordinary 1-way ANOVA.
We then evaluated the protective capacity of the genetic modifications against β-cell death as mediated by stress. Reduction in the expression of all target genes resulted in a decrease in the level of apoptotic SC-islets following treatment with stressors compared with the non-target control shRNA (Figures 2E and S2C). Next, we sought to determine whether our genetic modifications prevent aberrant gene expression following induction of stress. Genetically modified, stressed SC-islets exhibited decreased expression of genes related to β-cell stress (Figures 2F and S2D) and immune interaction (Figures 2G and S2D) compared with the non-target control group. In conclusion, knocking down the selected genes enhanced the protection of SC-islets from apoptosis while maintaining SC-islet identity and function.
Genetic manipulation increases protection of SC-islets from apoptosis mediated by allorecognition in vitro
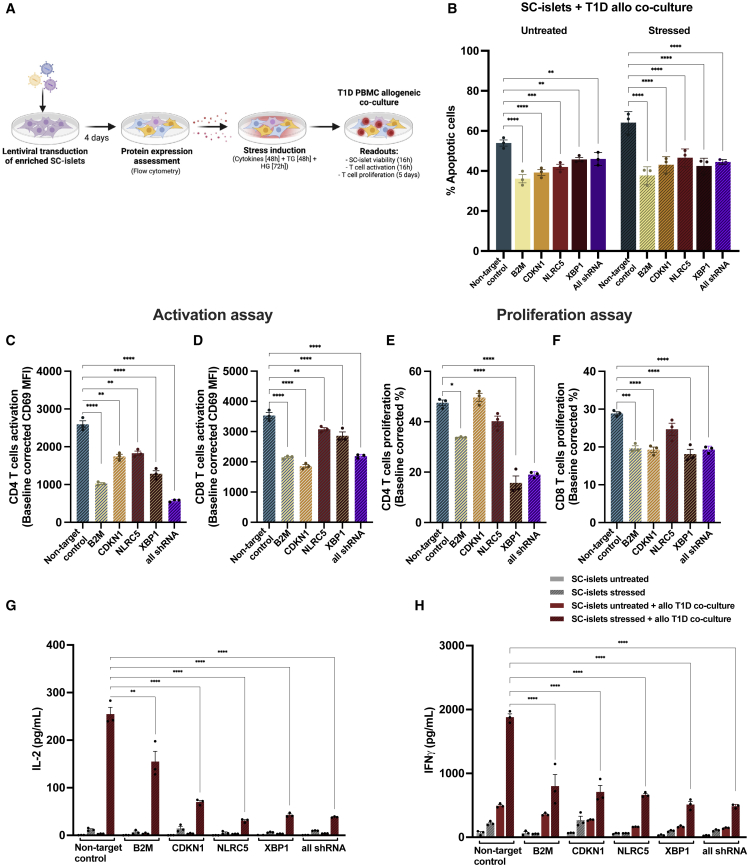
To study the protective capacity of the genetic modifications on apoptosis mediated by stress and allorecognition, following transduction of SC-islets with lentiviral shRNA plasmids individually targeting each gene of interest or targeting all four genes together (all shRNA group) and treatment with stress-inducing factors (cytomix [IL-1β—50 ng/mL, TNF-α—100 ng/mL, and IFN-γ—500 ng/mL; 48 h], TG [10 μM, 48 h], and HG [33 mM, 72 h]), SC-islets were co-cultured with allogeneic T1D peripheral blood mononuclear cells (PBMCs) (Figure 3A). We then assessed SC-islet viability and T cell activation after 16 h and T cell proliferation after 5 days of co-culture. Individually targeting each gene of interest (β2M, CDKN1A, NLRC5, and XBP1) or targeting all four genes together (all shRNA group), resulted in a decrease in the level of apoptotic SC-islets compared with the non-target control (Figures 3B and S3A–S3C). No changes were observed in the quantification of T cell subpopulations within PBMCs after co-culture (Figure S3D). Altogether, these data indicate that reduction in the expression of β2M, CDKN1A, NLRC5, and XBP1 provides some protection of SC-islets from apoptosis induced by stress and allorecognition.
Figure 3.
Genetic manipulation increases protection of SC-islets from apoptosis mediated by allorecognition in vitro
(A) Experimental design: following transduction of SC-islets with lentiviral shRNA plasmids individually targeting each gene of interest or targeting all four genes together (all shRNA group) and treatment with stress-inducing factors (cytomix [IL-1β—50 ng/mL, TNF-α—100 ng/mL, and IFN-γ—500 ng/mL; 48 h], TG [10 μM, 48 h], and HG (33 mM, 72 h]), SC-islets were co-cultured with allogeneic T1D PBMCs.
(B) Apoptosis in SC-islets following genetic modification and treatment with stress-inducing factors as in (A) and co-cultured with allogeneic T1D PBMCs for 16 h.
(C and D) T cell activation after 16 h of co-culture of PBMCs with SC-islets treated with stress-inducing factors as in (A). The values are represented as adjusted MFI and are baseline corrected by the average of each untreated condition.
(E and F) T cell proliferation after 5 days of co-culture of PBMCs with SC-islets treated with stress-inducing factors as in (A). The values are baseline corrected by the average of each untreated condition.
(G and H) Proinflammatory cytokine detection in supernatants collected after 16 h of co-culture of T1D PBMCs with SC-islets treated with stress-inducing factors as in (A).
(B–H) Data are means ± SEMs and representative of three experiments, each with one SC-islet differentiation (n = 3). ∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005, and ∗∗∗∗p < 0.0001. Ordinary 1-way ANOVA.
To further examine the protective capacity of the genetic modifications against β-cell death by immune-mediated killing, immune activation was assessed by surface staining of T cell activation and proliferation markers as well as quantification of proinflammatory cytokines IL-2 and IFN-γ, following 16 h of co-culture of genetically modified SC-islets with allogeneic T1D PBMCs.
In line with prior studies (Leite et al., 2020), co-culturing SC-islets with allogeneic PBMCs resulted in the upregulation of T cell activation marker CD69. However, reduction in the expression of all target genes within stressed SC-islets resulted in a significant decrease in the level of activation marker expression in both CD4+ and CD8+ T cells after co-culture compared with the non-target control (Figures 3C, 3D, and S3E). Following co-culture with SC-islets, the level of proliferation of both CD4+ and CD8+ T cells was measured (Figures 3E and 3F). Reduction in expression of β2M, XBP1, and the all shRNA group condition in stressed SC-islets resulted in a decrease in the level of CD4+ and CD8+ T cell proliferation after co-culture compared with the non-target control. As a positive control, PBMCs were stimulated with anti-CD3/CD28 (Figures S3F and S3G). The gating strategy for the co-culture assay is shown in Figure S3H.
Quantification of proinflammatory cytokines revealed a decrease in IL-2 and IFN-γ upon co-culturing PBMCs with SC-islets containing a reduced expression of the target genes individually or a combination of all shRNAs compared with the non-target control (Figures 3G and 3H). Together these results demonstrate that genetic manipulation of SC-islets results in decreased activation and proliferation of CD4+ and CD8+ T cells as well as reduced expression of proinflammatory cytokines, following co-culture with allogeneic T1D PBMCs.
Discussion
For decades, T1D has been viewed primarily as a T-cell-mediated autoimmune disease in which autoreactive T cells mistakenly destroy healthy, insulin-producing β cells. However, evidence accumulated over the past 15 years points to a role for the β cell as a contributor to the disease rather than an innocent bystander (Eizirik et al., 2020). Although the relative contributions of β-cell pathophysiology and autoimmunity remain unknown, β-cell stress has been shown to play an important role in T1D development. Prior studies on the exposure of HIs to stressors, such as chronic hyperglycemia and proinflammatory cytokines, have been extensively investigated (Brozzi and Eizirik, 2016; Dai et al., 2016; Abdullahi et al., 2017), but the effects of stress on SC-islets function and viability have not been thoroughly explored. As stress-inducing factors are likely to be present following transplantation, an initial understanding of the consequences of stress on SC-islets motivated the present study.
As had been shown with HIs, stress induction of SC-islets causes increased apoptosis, irregular GSIS, and heightened expression of stress and immune-interaction-related genes. The heightened expression of immune-interaction- and β-cell-stress-related genes upon stress induction in both HIs and SC-islets follows on prior studies that were performed on HIs exposed to proinflammatory cytokines in vitro (Eizirik et al., 2009) and studies that found stress-impaired transcription factor expression and insulin secretion in transplanted HIs (Dai et al., 2016). Furthermore, studies on induced pluripotent stem cell (iPSC)-islets showed that cytokine treatment induced a proinflammatory phenotype and stress (Demine et al., 2020). We see a similar pattern in our results in which untreated SC-islets express low levels of genes related to immune interaction and, following stress induction, increase the expression of HLA-ABC chemokines, such as CXCL8 and -10, and β-cell stress genes, XBP1 and Caspase 3. However, compared with HIs, SC-islets have reduced apoptotic cells and expression of genes related to peptide presentation and cytokine signaling in the treated conditions. These results support the idea that SC-islets may be less vulnerable to apoptosis mediated by cellular stress than HIs, although one cannot draw a strong conclusion from this in vitro assay.
Downregulation of the target genes with shRNAs was associated with protection of SC-islets from apoptosis mediated by stress while not altering the function of the cells. In addition, we observed unexpected results, namely the same level of protection from all target genes combined compared with targeting a single gene. This outcome demonstrates that protection against apoptosis is limited by methods of gene modification, such as shRNA-mediated gene silencing. Further examination of the protective capacities of the genetic modifications against immune-mediated β-cell death was determined through quantification of immune activation, proliferation, and SC-islets apoptosis. In line with previous studies (Leite et al., 2020), co-culturing stressed SC-islets with allogeneic PBMCs resulted in the upregulation of T cell activation and proliferation compared with co-culture with unstressed SC-islets. Reduction in the expression of target genes within stressed SC-islets, however, resulted in decreased levels of T cell activation and proliferation after co-culture compared with the non-target control. It is important to note that in these studies we quantified activation through the presence of only one activation marker (CD69) and one time point; therefore, it is possible that quantifying other activation markers that act through different pathways, at multiple time points, may lead to different levels of protection. Altogether, these results demonstrate that reduction in the expression of genes related to immune recognition and β-cell stress confers a modest level of protection of SC-islets from apoptosis mediated by stress and allorecognition in vitro and reduces the expression of other genes related to stress and immune recognition.
The findings of this research should be interpreted considering three significant limitations. First, we did not want to interfere with the homeostasis of β cells by deleting genes (Zhang et al., 2020; Bilekova et al., 2021) and instead performed a gene knockdown instead of knockout strategy. This approach would be strengthened by using techniques in which the expression of specific genes that do not interfere with β-cell homeostasis is permanently prevented. Second, we selected the target genes based on prior findings in the literature. An unbiased screening approach focusing on β-cell protection might provide novel targets that may have a more protective outcome. And third, we observed modest effects with our approach, and the pooled gene assays did not confer greater protection. To have complete protection of SC-islets, a different gene combination or approach might be necessary.
This is an initial study of a difficult problem. One genetic change is unlikely to solve the problem of protection from stress or immune rejection. The data presented here begin the understanding of the effects of stress on SC-islets function and viability in vitro. This information is a step forward toward the possibility of using SC-islets in long-term transplantation therapies without the need for lifelong and general immunosuppression.
Experimental procedures
An expanded section is available in the supplemental experimental procedures. All procedures were performed in accordance with the IRB guidelines at Harvard University under IRB and ESCRO protocols.
Cell culture
Human pluripotent stem cell maintenance and differentiation were carried out with Harvard University Embryonic Stem Cells 8 (HUES8), as previously described (Veres et al., 2019).
Magnetic enrichment using CD49a
Following SC-islets differentiation, the β-cell population was enriched using magnetic sorting, as previously described (Leite et al., 2020).
Apoptosis assay
SC-islets were plated at a density of ∼150,000 cells per well on 96-well round bottom plates and treated with IFN-γ, IL-1β, TNF-α, and TG for 48 h, and HG for 72 h. Following treatment, and co-culture in the case of the immune-protection assays, cells were washed to remove residual cytokines, TG, and glucose. Apoptosis was determined by staining with Annexin/SYTOX.
Glucose-stimulated insulin secretion assay
GSIS assay was performed as previously described (Blum et al., 2012).
T cell activation and proliferation assays
Transduced SC-islets were used as target cells. Approximately 200,000 target cells were plated on 96-well round bottom plates and treated with IFN-γ, IL-1β, TNF-α, and TG for 48 h and HG for 72 h. PBMCs (200,000 per well) were then added to SC-islets, with and without pre-treatment. After 16 h of co-culture, we analyzed T cell activation and, after 5 days of co-culture, T cell proliferation.
Antibodies used in this study can be found in Table S1.
Quantification and statistical analysis
Statistical analyses were carried out using GraphPad Prism software. Statistical assays were performed as described in each figure legend; n represents the number of biological replicates in all cases where reported. Biological replicates refer to unique donor-derived batches of HIs or unique differentiations of SC-islets produced from unique suspension cultures.
Author contributions
N.C.L. designed and conceived the study. Experiments were performed by N.C.L. and G.C.P. D.A.M gave technical support and conceptual advice and supervised the research.
Conflicts of interests
D.A.M. is a founder of Semma Therapeutics and advisor to Vertex Pharmaceuticals, which have licensed technologies developed in the Melton laboratory. All other authors declare no competing interests.
Acknowledgments
We thank J. Babon, E. Sintov, D. Gerace, and R. Pop for discussions and feedback on the manuscript and the UMass Diabetes Center of Excellence for blood sample donations. D.A.M. is an investigator of the Howard Hughes Medical Institute. N.C.L. is supported by American Diabetes Association grant#1-19-PMF-024. This work was supported by grants from the Harvard Stem Cell Institute (DP-0180-18-02), JDRF (5-COE-2020-967-M-N), and JPB Foundation (award #2695).
Published: March 3, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.01.018.
Supplemental information
References
- Abdullahi A., Stanojcic M., Parousis A., Patsouris D., Jeschke M.G. Modeling Acute ER stress in vivo and in vitro. Shock. 2017;47:506–513. doi: 10.1097/SHK.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadj Ali M., Liu Y.F., Arif S., Tatovic D., Shariff H., Gibson V.B., Yusuf N., Baptista R., Eichmann M., Petrov N., Heck S., et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci. Transl. Med. 2017;9:eaaf7779. doi: 10.1126/scitranslmed.aaf7779. [DOI] [PubMed] [Google Scholar]
- Bilekova S., Sachs S., Lickert H. Pharmacological targeting of endoplasmic reticulum stress in pancreatic beta cells. Trends Pharmacol. Sci. 2021;42:85–95. doi: 10.1016/j.tips.2020.11.011. [DOI] [PubMed] [Google Scholar]
- Blum B., Hrvatin S., Schuetz C., Bonal C., Rezania A., Melton D.A. Functional β-cells maturation is marked by an increase in the glucose threshold for insulin secretion and by expression of urocortin3. Nat. Biotechnol. 2012;30:261–264. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozzi F., Eizirik D.L. ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes. Ups. J. Med. Sci. 2016;121:133–139. doi: 10.3109/03009734.2015.1135217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C., Kayton N.S., Shostak A., Poffenberger G., Cyphert H.A., Aramandla R., Thompson C., Papagiannis I.G., Emfinger C., Shiota M., Stafford J.M., et al. Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J. Clin. Invest. 2016;126:1857–1870. doi: 10.1172/JCI83657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demine S., Schiavo A.A., Marín-Cañas S., Marchetti P., Cnop M., Eizirik D.L. Pro-inflammatory cytokines induce cell death, inflammatory responses, and endoplasmic reticulum stress in human iPSC-derived beta cells. Stem Cell Res. Ther. 2020;11:7. doi: 10.1186/s13287-019-1523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik D.L., Colli M.L., Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- Eizirik D.L., Pasquali L., Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat. Rev. Endocrinol. 2020;16:349–362. doi: 10.1038/s41574-020-0355-7. [DOI] [PubMed] [Google Scholar]
- Engin F. ER stress and development of type 1 diabetes. J Investig Med. 2016;64:2–6. doi: 10.1097/JIM.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Wang M., Duan S., Franco P.J., Kenty J.H., Hedrick P., Xia Y., Allen A., Ferreira L.M.R., Strominger J.L., Melton D.A., et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl. Acad. Sci. U S A. 2019;116:10441–10446. doi: 10.1073/pnas.1902566116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H., Kajimoto Y., Fujitani Y., Matsuoka T., Sakamoto K., Matsuhisa M., Yamasaki Y., Hori M. Oxidative stress induces p21 expression in pancreatic islet cells: possible implication in beta-cell dysfunction. Diabetologia. 1999;42:1093–1097. doi: 10.1007/s001250051276. [DOI] [PubMed] [Google Scholar]
- Katsarou A., Gudbjörnsdottir S., Rawshani A., Dabelea D., Bonifacio E., Anderson B.J., Jacobsen L.M., Schatz D.A., Lernmark Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers. 2017;3:17016. doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- Leite N.C., Sintov E., Meissner T.B., Brehm M.A., Greiner D.L., Harlan D.M., Melton D.A. Modeling type 1 diabetes in vitro using human pluripotent stem cells. Cell Rep. 2020;32:107894. doi: 10.1016/j.celrep.2020.107894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan P., Zhuang J., Zou J., Li H., Shuai P., Xu X., Zhao Y., Kou W., Ji S., Peng A., Xu Y., et al. NLRC5 deficiency ameliorates diabetic nephropathy through alleviating inflammation. FASEB J. 2018;32:1070–1084. doi: 10.1096/fj.201700511RR. [DOI] [PubMed] [Google Scholar]
- Maxwell K.G., Augsornworawat P., Velazco-Cruz L., Kim M.H., Asada R., Hogrebe N.J., Morikawa S., Urano F., Millman J.R. Gene-edited human stem cell-derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med. 2020;12:eaax9106. doi: 10.1126/scitranslmed.aax9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner T.B., Li A., Biswas A., Lee K.H., Liu Y.J., Bayir E., Iliopoulos D., van den Elsen P.J., Kobayashi K.S. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl. Acad. Sci. U S A. 2010;107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64:1030–1036. doi: 10.1007/s00125-020-05367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roep B.O., Thomaidou S., van Tienhoven R., Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?) Nat. Rev. Endocrinol. 2020;17:1–12. doi: 10.1038/s41574-020-00443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehler J., Blöchinger A.K., Meier M., Lickert H. Engineering islets from stem cells for advanced therapies of diabetes. Nat. Rev. Drug Discov. 2021;20:920–940. doi: 10.1038/s41573-021-00262-w. [DOI] [PubMed] [Google Scholar]
- Sims E.K., Mirmira R.G., Evans-Molina C. The role of beta-cell dysfunction in early type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2020;27:215–224. doi: 10.1097/MED.0000000000000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehli F., Ludigs K., Heinz L.X., Seguín-Estévez Q., Ferrero I., Braun M., Schroder K., Rebsamen M., Tardivel A., Mattmann C., MacDonald H.R., et al. NLRC5 deficiency selectively impairs MHC class I- dependent lymphocyte killing by cytotoxic T cells. J. Immunol. 2012;188:3820–3828. doi: 10.4049/jimmunol.1102671. [DOI] [PubMed] [Google Scholar]
- Veres A., Faust A.L., Bushnell H.L., Engquist E.N., Kenty J.H., Harb G., Poh Y.C., Sintov E., Gürtler M., Pagliuca F.W., Peterson Q.P., et al. Charting cellular identity during human in vitro β-cell differentiation. Nature. 2019;569:368–373. doi: 10.1038/s41586-019-1168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Yigit M.V., Ran C., Ross A., Wei L., Dai G., Medarova Z., Moore A. A theranostic small interfering RNA nanoprobe protects pancreatic islet grafts from adoptively transferred immune rejection. Diabetes. 2012;61:3247–3254. doi: 10.2337/db12-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang I.X., Raghavan M., Satin L.S. The endoplasmic reticulum and calcium homeostasis in pancreatic beta cells. Endocrinology. 2020;161:bqz028. doi: 10.1210/endocr/bqz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.