Abstract
Background
Anastomotic leakage (AL) after oesophagectomy and oesophageal perforations are associated with significant morbidity and mortality. Minimally invasive endoscopy is often used as first-line treatment, particularly endoluminal vacuum therapy (EVT). The aim was to assess the performance of the first commercially available endoluminal vacuum device (Eso-Sponge®) in the management of AL and perforation of the upper gastrointestinal tract (GIT).
Methods
The Eso-Sponge® registry was designed in 2014 as a prospective, observational, national, multicentre registry. Patients were recruited with either AL or perforation within the upper GIT. Data were collected with a standardized form and transferred into a web-based platform. Twenty hospitals were enrolled at the beginning of the study (registration number NCT02662777; http://www.clinicaltrials.gov). The primary endpoint was successful closure of the oesophageal defect.
Results
Eleven out of 20 centres recruited patients. A total of 102 patients were included in this interim analysis; 69 patients with AL and 33 with a perforation were treated by EVT. In the AL group, a closure of 91 per cent was observed and 76 per cent was observed in the perforation group. The occurrence of mediastinitis (P = 0.002) and the location of the defect (P = 0.008) were identified as significant predictors of defect closure.
Conclusions
The Eso-Sponge® registry offers the opportunity to collate data on EVT with a uniform, commercially available product to improve standardization. Our data show that EVT with the Eso-Sponge® is an option for the management of AL and perforation within the upper GIT.
We present results of 102 patients recruited in a multicentre study assessing the performance of the first commercially available endoluminal vacuum device (Eso-Sponge®) in various indications (anastomotic insufficiency and perforation within the upper gastrointestinal tract). It seems to offer a safe, effective, and minimally invasive therapeutic approach with low mortality.
Introduction
The incidence of oesophageal carcinoma is increasing and the prognosis is poor, with a median survival of less than 2 years and long-term survival rates below 15 per cent1. Despite continuous innovations of surgical treatment, oesophagectomy is a highly complex procedure with comparatively significant rates of postoperative complications. The incidence of anastomotic leakage (AL) secondary to oesophagectomy or gastrectomy ranges from 5 to 30 per cent and is associated with a morbidity and mortality rate of 20–50 per cent2,3. AL is defined as a defect of the intestinal wall at the anastomotic site with communication between intra- and extraluminal compartments4.
Oesophageal perforation after trauma or iatrogenic injury can lead to a life-threatening situation with mortality rates of up to 20 per cent5,6. Perforation is defined as a defect of the oesophageal wall that is caused iatrogenically, traumatically, or due to Boerhaave syndrome7. In addition to the size and location of the defect, clinical outcome is significantly influenced by the general condition of the patient8,9.
Timely and appropriate treatment is crucial in the management of oesophageal injuries. Over time, management has shifted from radical surgical intervention to more conservative measures, including endoscopic interventions. Treatment options include conservative treatment for small leak, surgical debridement, closure of the defect, or revising the anastomosis with simultaneous drainage10. Procedures such as endoscopic closure with clips, injection of fibrin glue, endoluminal drainage through gastric probes, endoluminal sutures, and the installation of endoscopic stent systems are also options11,12.
Endoluminal vacuum therapy (EVT) for treating AL and perforations has been successfully established and is increasingly used. Several studies have demonstrated high closure rates of approximately 90 per cent with a mortality rate of 10 per cent13–16.
In 2014 the European Society of Gastrointestinal Endoscopy (ESGE) suggested using endoscopy as a first-line intervention in leakages of the upper gastrointestinal tract (GIT)17.
To date, several low-volume single-centre studies with varying methodology have been published15.
The first commercially available endoluminal vacuum sponge system (Eso-Sponge®) of B. Braun for the endoscopic treatment of transmural defects within the upper GIT has been available since July 2014. It is CE marked and implemented in many centres for the treatment of perforations and ALs. A multicentre, prospective, web-based online registry was initiated in 2015 to evaluate the performance of the Eso-Sponge® in the upper GIT.
The aim of this study is to present interim results of a standardized multicentre registry of Eso-Sponge® therapy.
Methods
Patient recruitment and study design
The study design was a prospective, national, multicentre, open registry. The registry was sponsored and funded by B. Braun, Aesculap AG, Tuttlingen, Germany. The Medical Scientific Affairs department of Aesculap AG was responsible for project management, data management, statistics, and study registration (registration number NCT02662777; http://www.clinicaltrials.gov).
The objective of the present research was to evaluate Eso-Sponge® as an E-VAC therapy for AL and perforations within the upper GIT. Patients presenting with clinical suspicion of AL after oesophagectomy, such as elevated levels of C-reactive protein and pathological drainage fluid, had confirmatory endoscopic examination. Clinical impression and suspicious drain contents triggered radiological investigation with a CT thorax/abdomen.
An interim analysis of the first 100 patients to report results was planned.
Hospitals applying Eso-Sponge® in their daily clinical routine for E-VAC treatment in the upper GIT were eligible to participate. Twenty hospitals in Germany are participating and 11 centres are actively treating and including patients.
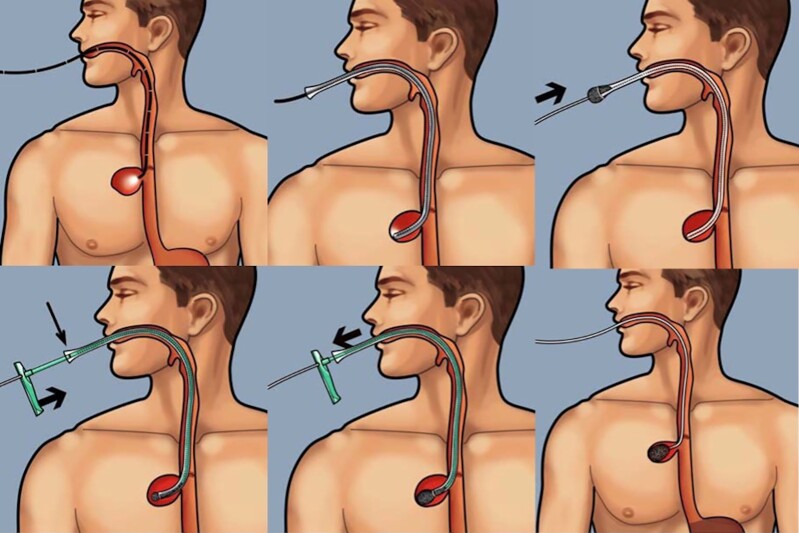
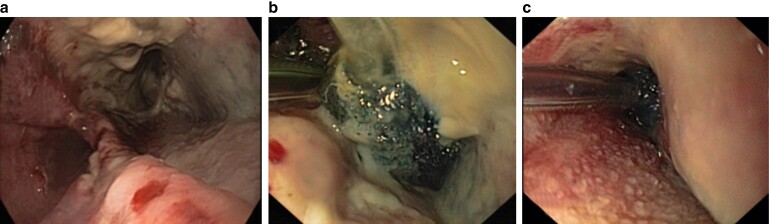
Patients were treated according to the local standards for E-VAC therapy and Eso-Sponge®, according to the manufacturer’s instructions. The Eso-Sponge® System (B. Braun Melsungen AG, Melsungen, Germany), is a minimally invasive method for E-VAC, consisting of a drainage tube with an attached open-pore sponge, a connection system, and an application system with a lavage set. Fig. 1 displays an image of the Eso-Sponge®-procedure. The Eso-Sponge®-therapy was performed as described in previous publications8. Depending on the size of the defect, EVT was carried out either by endoscopic insertion of an Eso-Sponge® into the abscess cavity with an extraluminal placement, or in case of a limited defect size and the absence of a cavity, by endoscopic insertion in the lumen of the oesophagus itself as a luminal covering of the leak (Fig. 2a–c). To better compare the different oesophageal leakages, they were divided into three groups. We based this classification exclusively on the depth and/or diameter of the leakage. For defects, a diameter and/or depth of 0–1 cm was described as small, 1–4 cm was described as medium, and more than 4 cm was described as large. Over the course of treatment, with a reduction in defect size, the sponge placement could be moved from its initial intracavitary location to an intraluminal positioning allowing for complete closure of the leakage.
Fig. 1.
Endoscopic vacuum therapy
Controlled continuous negative pressure (100–125 mmHg) was applied via a transnasal gastric tube using an electronic vacuum pump (Eso-Sponge® by B.Braun, Aesculap AG, Tuttlingen, Germany)
Fig. 2.
Endoscopic vacuum therapy
a,b Endoscopic insertion of an Eso-Sponge® into the abscess cavity by means of an extra luminal placement. c, In cases of limited defect size and the absence of a cavity, endoscopic insertion of a sponge in the lumen of the oesophagus itself as a luminal covering of the leak.
The primary endpoint was the successful closure of the oesophageal defect, defined as the point when the defect size became too small for further sponge placements, and the surface was epithelialized. Data were collected on a case report form and transferred to a web-based platform offered by the sponsor (Fig. S1). Table 1 displays the parameters used to describe the baseline, treatment details, outcomes, and factors influencing the clinical outcome. Patients were recruited consecutively, and informed consent was obtained before enrolment. The procedures were conducted in accordance with the ethical standards of the Committee on Human Experimentation of the institutions (A141/14).
Table 1.
Parameters that define the baseline, treatment details, outcome of the treatment and to identify influencing factors of the Eso-Sponge® therapy success
| Parameters to describe the baseline, the treatment details, and outcome of the treatment | Parameters to identify influencing factors of the ESO-Sponge® therapy success |
|---|---|
| Number of used sponges | Age |
| Mean replacement intervals | Sex |
| Duration of E-VAC treatment | Body mass index |
| Healing rate | Diabetes (insulin-dependent) |
| Complication rate | Smoking |
| Death rate | Alcohol consumption |
| Reoperation rate | Cancer diagnosis |
| Dysphagia score | Neoadjuvant therapy |
| Anastomosis (versus perforation) | |
| Defect localization | |
| Stent applied | |
| Days to leakage diagnosis | |
| Distance from the arch (cm) | |
| Defect volume (log ml) |
E-VAC, endoluminal vacuum.
Evaluated variables
The evaluation protocol was divided into three main sections: the screening/initial data collection, E-VAC treatment, and follow-up. Complications (bleeding, fistula, abscess, peritonitis, pneumonia, mediastinitis, sepsis, renal failure, pleural empyema, and stenosis), mortality, and reoperations until the end of E-VAC treatment were entered into the web-based platform. The rate of granulated cavity, the number of endoscopic procedures, the total number of used sponges, the average replacement intervals, the duration of sponge treatment, and the dysphagia score after treatment were listed. Postinterventional ingestion discomfort was determined by a dysphagia score system, which was modified with the scoring method described by Mellow and Pinkas18.
Statistics
All patients who had a surgical intervention with the product under investigation without any eligibility violation were included in the analysis.
Statistical analyses were performed for all subsets of clinical parameters. Kaplan–Meier survival estimation was used for calculation of complication rates if appropriate. For univariate analysis, the statistical significance was assessed by the log rank test. We used a Student’s t test and ANOVA for parametric data. Distribution and frequencies of categorical data were compared by Pearson’s chi-squared test. A P value < 0.05 was used for statistical tests to identify significance. Multivariate mixed effects logistic regression models were used to analyse predictors of response (granulated cavity). The backward elimination procedure with a ‘remain-in-model’ threshold of P < 0.10 was applied to detect covariates. Statistical calculation and testing were performed with SAS software version 9.4 (SAS, Cary, North Carolina, USA).
Results
Patient demographics and clinical characteristics
Patient recruitment started on 27 October 2014. A total of 102 patients had been recorded on the registry by 4 January 2019. In total, 69 (67.6 per cent) patients were diagnosed with an AL after oesophagectomy and 33 (32.4 per cent) with a perforation of the upper GIT.
The cohort comprised 72 (70.6 per cent) men and 30 (29.4 per cent) women. The mean patient age at the time of EVT was 60.9 years (23–82 years) in the AL group and 61.7 years (38–94 years) in the perforation group. Causes of iatrogenic perforation (27.3 per cent) were due to endoscopic procedures for biopsies or diagnostics, and fistula formation after chemoradiotherapy. The Boerhaave syndrome (21.2 per cent) was the most common cause of spontaneous perforation.
Oncological resection of distal oesophageal tumours was the most performed procedure (69.3 per cent), followed by bariatric procedures (11.8 per cent), and oncological surgery resecting the stomach (15.9 per cent) in the AL group. Histological examination demonstrated an adenocarcinoma in 73.9 per cent of cases, squamous cell carcinoma in 4.4 per cent, and no malignancy in 7.2 per cent of the subgroup. In these cases, the initial surgery was due to a non-malignant disease. Because of previous confirmatory histology/emergency presentations, histological specimen collection was not performed in 34.3 per cent of the patients. A full synopsis of the clinical data for each subgroup is given in Tables 2 and 3.
Table 2.
Patient and treatment characteristics of the anastomotic leakage group
| Anastomotic leakage n (%) | |
|---|---|
| 69 (100) | |
| Age (years) | |
| Under 70 | 53 (76.9) |
| 70 or older | 16 (23.1) |
| Sex | |
| Male | 53 (76.9) |
| Female | 16 (23.1) |
| Diagnosis | |
| Oesophageal cancer | 44 (63.8) |
| Obesity | 8 (11.6) |
| Cancer of the gastric cardia | 8 (11.6) |
| Other | 9 (13) |
| Histology | |
| Adenocarcinoma | 51 (73.9) |
| Squamous cell carcinoma | 3 (4.4) |
| No malignancy | 5 (7.2) |
| Not done | 10 (14.5) |
| Previous treatment | |
| Chemoradiotherapy | 7 (10.1) |
| Chemotherapy | 32 (46.4) |
| None | 30 (43.5) |
| Resection type | |
| Oesophagectomy | 48 (69.3) |
| Endoscopic procedure | 1 (1.5) |
| Gastrectomy | 11 (15.9) |
| Gastric sleeve resection | 3 (4.5) |
| Gastric bypass | 5 (7.3) |
| Others | 1 (1.5) |
| Reconstruction type | |
| Oesophagogastrostomy | 53 (76.9) |
| Oesophagojejunostomy | 6 (8.7) |
| Gastrojejunostomy | 6 (8.7) |
| No reconstruction | 4 (5.7) |
| Site of reconstruction | |
| Intrathoracic | 49 (71.1) |
| Abdominal | 15 (21.7) |
| Thoracoabdominal | 1 (1.5) |
| No reconstruction | 4 (5.7) |
Table 3.
Patient and treatment characteristics of the perforation group
| Perforation n (%) | |
|---|---|
| 33 (100) | |
| Age (years) | |
| Under 70 | 24 (72.7) |
| 70 or older | 9 (27.3) |
| Sex | |
| Male | 19 (57.6) |
| Female | 14 (42.4) |
| Diagnosis | |
| Oesophageal cancer-lower | 1 (3.1) |
| Obesity | 3 (9.1) |
| Iatrogenic perforation | 9 (27.3) |
| Boerhaave’s syndrome | 7 (21.2) |
| Other | 13 (39.3) |
| Histology | |
| Squamous cell carcinoma | 3 (9.1) |
| No malignancy | 5 (13.4) |
| Not done | 25 (77.5) |
| Previous treatment | |
| Chemoradiotherapy | 3 (9.1) |
| None | 30 (90.9) |
| Resection type | |
| Oesophagectomy | 3 (9) |
| Endoscopic procedure | 14 (42.5) |
| Gastrectomy | 2 (6) |
| Gastric sleeve resection | 2 (6) |
| Others | 12 (36.5) |
| Reconstruction type | |
| Oesophagogastrostomy | 2 (6) |
| Gastrojejunostomy | 1 (3.1) |
| No reconstruction | 30 (90.9) |
| Site of reconstruction | |
| Intrathoracic | 3 (6.1) |
| Abdominal | 6 (18.2) |
| Cervical | 1 (3.1) |
| No reconstruction | 23 (72.6) |
Surgical procedures and leakage characteristics for oesophageal AL and perforations
Oesophagectomy (50 per cent) was the most frequently performed surgery requiring E-VAC therapy, followed by endoscopic procedures (14.7 per cent), gastrectomy (12.7 per cent), and bariatric operations such as gastric sleeve resection (4.9 per cent) and gastric bypasses (4.9 per cent). In patients who underwent oesophageal resection, oesophagogastrostomy was performed in all patients. The site of reconstruction was localized in the thoracic, abdominal, and cervical region in 51 per cent, 20.6 per cent and 1 per cent of the patients respectively.
No reconstruction was performed in 34 patients (33.3 per cent), 4 of whom are recorded as AL. Three patients had a staple line leak from sleeve gastrectomy and one patient had an AL following an endoscopic dilatation of an anastomotic stenosis (Table 2).
Oesophageal defects were divided into three subsets based on the diameter and depth of the defect. A defect diameter/depth of 0–1 cm was described as small, a diameter/depth of 1–4 cm was described as medium, and a defect more than 4 cm was described as large. In the AL group, 62.35 per cent of patients presented with a defect of 1–4 cm, 26.1 per cent had a small defect and 11.6 per cent had a large defect. In the perforation group, 51.5 per cent had a small defect, 42.4 per cent had a medium defect, and 6 per cent had a large defect.
In the AL group the defect depth was small in 17.4 per cent of cases, medium in 47.8 per cent, and large in 34.8 per cent. In the perforation group the defect depth was small in 24.2 per cent of cases, medium in 45.4 per cent, and large in 30.3 per cent.
E-VAC therapy of the AL
In the AL group most patients (92.8 per cent) were treated within 24 h after diagnosis. After initial surgery the treatment started after a mean of 24 days (1–60 days) in the AL group. A granulated cavity was observed in 91 per cent of patients. The mean of the entire E-VAC treatment was 24.9 days (1–99 days). The placement of the Eso-Sponge® was 31 cm from the dental arch (15–50 cm, s.d. 8.3 cm). In 47.8 per cent of the AL population the Eso-Sponge® had an intraluminal and intracavitary placement. An intraluminal application on its own was performed in 20.3 per cent of cases and an intracavitary placement alone was reported in 31.9 per cent. The mean count of used Eso-Sponges® was 7.7 (1–32 sponges, s.d. 6 sponges). A mean of 7 sponge changes was reported (0–32 changes, s.d. 5.5 changes) with a mean interval of 3.1 days (2–7 days, s.d. 0.6 days). In three cases, E-VAC treatment was less than 3 days (one patient, 1 day and two patients, 2 days). In these cases, treatment with Eso-Sponge® was prophylactic for high inflammatory markers without a clearly visible oesophageal leak. A total of 75.4 per cent of the patients were treated in an ICU for the mean length of 19 days (1–141 days, s.d. 28.7 days). Additional thoracic drainage was necessary in 52 per cent of the patients. The incidence of jejunal enteral, trilumina probe (TLP) enteral, parental, and percutaneous endoscopic gastrostomy (PEG) enteral feeding was 68.1 per cent, 24.6 per cent, 4.4 per cent, and 1.5 per cent respectively.
A newly diagnosed abscess (4 per cent), fistula (9 per cent), or bleeding (6 per cent) was considered as a local complication. Systemic complications such as peritonitis (1.5 per cent), pneumonia (13 per cent), mediastinitis (13 per cent), pleural empyema (7.3 per cent), sepsis (10.1 per cent), renal failure (7.3 per cent), acute respiratory distress syndrome (4.4 per cent), or a mediastinal emphysema (5.8 per cent) were reported. A complication requiring intubation of the patient occurred in 3.9 per cent of the total cohort. Postinterventional stenosis was seen in 11.6 per cent of the patients and an additional stent was applied in 8.7 per cent. A dysphagia score of 0 was recorded for most of the patients (78 per cent), followed by a dysphagia score of 1 (14.5 per cent) and a score of 2 (6 per cent). There was only one patient (1.5 per cent) in the AL group with a dysphagia score of 3.
A reoperation was necessary in a total of 18 patients (26.1 per cent). Four reinterventions (3.9 per cent) were recorded with a definite relationship (n = 1) or with a suspected relationship (n = 3) caused by the Eso-Sponge®. The case in which a causal relationship was recorded involved an explorative laparoscopy to rule out a free perforation of the AL cavity. In two cases of the suspected causal relationship, a bronchial fistula was the reason for an operation and the third reason was a haemothorax. In the patient with a reoperation due to haemothorax, a bleeding intercostal vein was identified, which was worsened by anticoagulants. For the remaining reoperations in six cases the anastomosis had to be revised, in four cases a wound-healing disorder was diagnosed, in two cases a lavage was performed after a leakage had occurred, and in one patient each a splenic haemorrhage and a repositioning of a small bowel feeding tube were the reasons for the new operation. A clear definition of the assessment of the adverse event classification is listed when entering the events in the web-based portal (Table S1).
The overall mortality rate in the AL group was 6 per cent (n = 4 patients), and mainly due to the severe underlying disease and co-morbidities. Two patients died because of multiple organ failure. One patient died because of a malignant pleural effusion, and in another patient heart failure combined with a mediastinitis was the cause of death. All data are shown in Tables 4 and 5.
Table 4.
Treatment of the endoluminal vacuum therapy
| Total n (%) |
AL n (%) |
Perforation n (%) |
|
|---|---|---|---|
| 102 (100) | 69 (67.6) | 33 (33.4) | |
| Treatment started within 24 h | |||
| Yes | 92 (90.2) | 65.3 (92.8) | 28 (84.9) |
| No | 10 (8.9) | 5 (7.2) | 5 (15.1) |
| Treatment type | |||
| Intraluminal and intracavitary | 50 (49) | 33 (47.8) | 17 (51.5) |
| Intracavitary | 27 (26.5) | 22 (31.9) | 5 (15.2) |
| Intraluminal | 25 (24.5) | 14 (20.3) | 11 (33.3) |
| Intensive care unit | |||
| Yes | 74 (72.6) | 52 (75.4) | 22 (66.7) |
| No | 28 (27.5) | 17 (24.6) | 11 (33.3) |
| Additional thoracic/pleural drainage | |||
| None | 55 (53.9) | 33 (47.8) | 22 (66.7) |
| Thoracic drainage | 41 (40.2) | 34 (49.3) | 7 (21.2) |
| Thoracic drainage lavage | 6 (5.9) | 2 (2.9) | 4 (12.2) |
| Feeding type | |||
| Jejunal/enteral | 49 (48) | 47 (68.1) | 2 (6.1) |
| Enteral feeding tube/enteral | 28 (27.5) | 17 (24.6) | 11 (33.3) |
| Parenteral | 20 (19.6) | 3 (4.4) | 17 (51.5) |
| PEG/enteral | 3 (2.9) | 1 (1.5) | 2 (6.1) |
| Dysphagia score | |||
| 0, no dysphagia: able to eat normal diet | 77 (75.5) | 54 (78.3) | 23 (69.7) |
| 1, moderate passage: able to eat some solid foods | 18 (17.7) | 10 (14.5) | 8 (24.2) |
| 2, poor passage: able to eat semi-solid foods | 6 (5.9) | 4 (5.8) | 2 (6.1) |
| 3, very poor passage: able to swallow liquids only | 1 (1) | 1 (1.5) | n.a. |
AL, anastomotic leakage; PEG, percutaneous endoscopic gastrostomy.
Table 5.
Outcome of the endoluminal vacuum therapy
| Total n (%) |
AL n (%) |
Perforation n (%) |
||||
|---|---|---|---|---|---|---|
| 102 (100) | 69 (67.6) | 33 (33.4) | ||||
| Yes | No | Yes | No | Yes | No | |
| Granulated cavity | 88 (86.3) | 14 (13.7) | 63 (91.3) | 6 (8.7) | 25 (75.8) | 8 (24.2) |
| New abscess | 10 (9.8) | 92 (90.2) | 3 (4.4) | 66 (95.6) | 7 (21.2) | 26 (78.8) |
| Fistula | 7 (6.9) | 95 (93.1) | 6 (8.7) | 63 (91.3) | 1 (3) | 32 (97) |
| Bleeding, transfusion needed | 5 (4.9) | 97 (95.1) | 4 (5.8) | 65 (94.2) | 1 (3) | 32 (97) |
| Peritonitis | 4 (3.9) | 98 (96.1) | 1 (1.5) | 68 (98.5) | 3 (9.1) | 30 (91) |
| Pneumonia | 11 (10.8) | 91 (89.2) | 9 (13) | 59 (87) | 2 (6.1) | 31 (94) |
| Mediastinitis | 10 (10) | 92 (90.2) | 9 (13) | 60 (87) | 1 (3) | 32 (97) |
| Pleural empyema | 6 (5.9) | 96 (94.1) | 5 (7.3) | 64 (92.8) | 1 (3.1) | 32 (97) |
| Sepsis | 10 (9.8) | 92 (90.2) | 7 (10.1) | 62 (89.9) | 3 (9.1) | 30 (90.9) |
| Renal failure | 5 (4.9) | 97 (95.1) | 5 (7.3) | 64 (92.8) | n.a. | 33 (100) |
| ARDS | 3 (2.9) | 99 (97.1) | 3 (4.4) | 65 (94.2) | n.a. | 33 (100) |
| Mediastinal emphysema | 5 (4.9) | 97 (95.1) | 4 (5.8) | 65 (94.2) | 1 (3.1) | 32 (97) |
| Intubation | 4 (3.9) | 98 (96.1) | 2 (2.9) | 67 (97.1) | 2 (6.1) | 31 (93.9) |
| Stenosis | 10 (9.8) | 92 (90.2) | 8 (11.6) | 61 (88.4) | 2 (6.1) | 31 (93.9) |
| Stent applied | 9 (8.8) | 93 (91.2) | 6 (8.7) | 63 (91.3) | 3 (9.1) | 30 (90.9) |
| Reoperation | 26 (25.5) | 76 (74.5) | 18 (26.1) | 51 (73.9) | 8 (12.1) | 25 (75.8) |
| Death | 7 (6.9) | 95 (93.1) | 4 (5.8) | 65 (94.2) | 3 (9) | 30 (91) |
| Mean (s.d.) | Mean (range) | Mean (range) | ||||
| Therapy duration (days) | 26.41 (24.68) | 24.95 (1–99) | 30.09 (2–141) | |||
| Distance from the arch (cm) | 30.23 (9.22) | 30.91 (15–50) | 28.82 (15–45) | |||
| Defect diameter (cm) | 2.54 (2.16) | 2.77 (0–15) | 2.04 (0–8) | |||
| Defect depth (cm) | 4.49 (4.20) | 4.33 (0–15) | 4.8 (0–24) | |||
AL, anastomotic leakage; ARDS, acute respiratory distress syndrome.
E-VAC therapy of the perforations
Most patients in the perforation group were treated within the first 24 h (84.9 per cent). A sealed and granulated cavity was observed in 75.76 per cent of the treated patients. The mean of E-VAC treatment was 30.1 days (2–141 days, s.d. 32.8 days) in the perforation group.
The mean distance from the dental arch was 30 cm (15–45 cm, s.d. 10.9 cm). In 51.6 per cent of the cases, the Eso-Sponge® had an intraluminal and intracavitary placement. A mean of 9 Eso-Sponges® were used (1–37 sponges, s.d. 9.9 sponges) and changed a mean of 7.4 times (0–36 changes, s.d. 8.6 changes) with a change interval of 3 days (2–4 days, s.d. 0.5 days). A total of 66.7 per cent of the patients had to be treated in an ICU for a mean length of 22 days (1–114 days, s.d. 30.2 days). Additional thoracic drainage was necessary in 33 per cent of the patients. The incidence of jejunal enteral, TLP enteral, parental, and PEG enteral feeding was 6.1 per cent, 33.3 per cent, 51.5 per cent, and 6.1 per cent respectively.
A new abscess (21 per cent), fistula (3 per cent), or bleeding (3 per cent) was recorded within the group. Systemic complications such as peritonitis (9 per cent), pneumonia (6 per cent), mediastinitis (3 per cent), pleural empyema (3 per cent), sepsis (9 per cent), or mediastinal emphysema (3 per cent) were recorded. Procedural complications included bleeding (3 per cent), aspiration (3 per cent), and intubation (6 per cent). A stenosis was seen in 6 per cent of patients and an additional stent was applied in 9 per cent.
A dysphagia score of 0 was recorded for most of the patients (69.7 per cent), followed by dysphagia score of 1 (24 per cent), and a score of 2 (6 per cent).
A reoperation was needed in eight patients. In five cases an oesophageal resection had to be performed due to a persistent wound cavity at the site of the perforation. In three of the eight cases a potential causal relationship between the Eso-Sponge® and the reoperation was found. One operation was carried out to perform an abdominal lavage, one because of a mediastinal abscess, and the case with a possible causal relationship to Eso-Sponge® was a thoracotomy for bleeding.
The mortality rate was 9 per cent (n = 3 patients), mainly due to the underlying disease and co-morbidities of the patients. One patient died due to a myocardial infarction, one due to intraoperative pulmonary bleeding, and the third due to a fulminant pulmonary embolism. All data are shown in Tables 4 and 5.
Influencing variables on the Eso-Sponge® therapy success
Variables that might influence the outcome of the Eso-Sponge® treatment, and therefore the condition of the defect, were evaluated. The presence of a granulated cavity in the defect was considered a positive response. The start of therapy within 24 h (P = 0.07), previous neoadjuvant therapy (P = 0.09), and presence of pneumonia (P = 0.06) were not predictive.
The presence of mediastinitis was associated with significant failure of EVT (P = 0.002). EVT applied in patients with an AL had better outcomes than for oesophageal perforations (P = 0.026). In particular, the height from the dental arch of the leakage or perforation was identified as a highly significant risk factor (P = 0.0088). A success rate of 89 per cent was found for a distance of more than 20 cm.
Discussion
Oesophageal perforations and AL following oesophageal resections can lead to serious complications, resulting in high mortality7,19. Historically the only options for treatment were stents or surgical treatment20. A few years ago, EVT was established as an endoscopic option14 with potential advantages and reduced mortality compared with previous treatments14. Contrary to previous single-centre studies, a standardized protocol in a multicentre setting was used, which allows wider applicability of data concerning EVT. In general, EVT has been deemed a reliable and effective minimally invasive procedure21–24. It is well tolerated and at the same time the complication rates are relatively low25,26.
This study demonstrated a success rate of 91 per cent for EVT in patients, consistent with the literature. Loske et al. reported success rates of 60–100 per cent in a recent review of the available literature on endoscopic negative pressure therapy in the upper GIT27.
There was a low complication rate with stenosis in 10 per cent, comparable to previous studies28. Serious complications have rarely been described to date and include bleeding due to vascular erosion and the development of an oesophagobronchial fistula8,29. In this study seven patients developed a fistula after EVT. This is consistent with a previous study that demonstrated oesophagobronchial fistula in 2 out of 35 patients30.
The reoperation rate after EVT treatment was high at 26 per cent compared with the data of Brangewitz et al.13, where only one revisional surgery was performed on 32 patients. However, in another study the rate was four out of nine patients31. This demonstrates the challenging nature of this type of intervention in small single-centre studies to be able to accurately compare outcomes. The majority of reoperations in this study were due to secondary problems (such as wound infection, percutaneous endoscopic jejunostomy dislocation, and haemothorax). Only 9 of the 26 reoperations were performed for anastomosis revision or treatment of surgical perforation.
The mortality rate was 6 per cent, which was similar to published data (0–18 per cent)7,32,33. Further stratifying of patients into subgroups with AL and perforation, mortality rates were 6 per cent and 9 per cent respectively. These results seem favourable for Eso-Sponge® therapy, considering Schorsch et al. published a mortality rate for AL of 12–35 per cent and a perforation-related mortality rate of 11.7 per cent30.
Generally, the optimal time point for EVT is thought to be immediately after the diagnosis of an oesophageal AL. It improves clinical outcome in comparison with a postponed treatment, more than 24 h after detection, which is associated with a prolonged duration of hospital stay and a higher mortality rate21,25. In our study, commencement of EVT within 24 h could be achieved in 84 per cent of the perforation cohort and even in 91 per cent of the AL cohort. This factor did not, however, influence outcomes.
EVT can be safely and effectively used in patients who have been previously treated with chemoradiotherapy and those presenting with severe leaks. Several studies describe poor wound healing and AL rates after neoadjuvant chemotherapy34,35. Contrary to the findings of Min et al., no significant influence of neoadjuvant pretreatment on EVT was identified in this study36.
In this study only mediastinitis had a significant influence on the healing rate during EVT. The incidence of mediastinitis was low at 9.8 per cent of the overall cohort compared with previous studies with 43 per cent24. The lower healing rate for those patients could be explained by a delayed leak drainage and thus prevention of further contamination, which is the underlining concept of therapy with EVT37.
In our group of patients, sponges were changed at an interval of 3–4 days, which has proven effective in our clinical experience. It gives the sponge sufficient time to induce granulation of the wound cavity and yet still ensures easy removal. This finding is in keeping with the current literature16,24,28, although there are promising studies reporting an alternating cycle of 1–2 weeks38.
While the results of this study are promising, there are limitations. The major limitation of our study is the lack of a comparative cohort with alternative therapies (such as stent therapy). In the present study, however, no comparative analysis of the results was intended, but rather a descriptive characteristic of the present procedure in a uniquely large patient cohort, applying a highly standardized application of treatment. This important aspect is missing in the current literature39.
The lack of standardization of clinical conditions can directly affect the results. Data about fistula size, laboratory studies, sepsis, antibiotic regimen, or an objective assessment through a predictive score system (such as Acute Physiology and Chronic Health Evaluation score) could reduce the risk of bias. However, this study is a presentation of the EVT within the framework of a uniform standardized procedure.
This multicentre study confirms the promising results of standardized EVT for the management of upper gastrointestinal ALs and perforation.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge V. Breul, Biomedical Statistician, Aesculap AG, Medical Scientific Affairs, Tuttlingen, Germany, for the statistical analysis; C. Triller, Municipal Hospital Heinsberg, Heinsberg, Germany; K. Tüffers, St. Johannes-Hospital Dortmund, Medical Clinic I, Dortmund, Germany; F. Arnold, SRH Wald Klinikum Gera, Department of General, Visceral, and Pediatric Surgery, Gera, Germany; D. Kompa, Protestant Hospital Herne, Department of Internal Medicine, Herne, Germany; D. Wichmann, Department of General, Visceral and Transplantation Surgery, University Hospital of Tuebingen, Tuebingen, Germany; A. Calderoni, Department of General Internal Medicine, Gastroenterology, and Diabetology, Augusta-Kliniken, Bochum, Germany; and J. Höppner, Department of General and Visceral Surgery, University of Freiburg, Freiburg im Breisgau, Germany, for the recruitment of patients. F.R. and A.H. contributed equally to this work.
Disclosure. P.B. is an employee of Aesculap AG. C.S., B.S., N.H., and M.E. have worked for Aesculap AG as speakers. The authors declare no other conflicts of interest.
Funding
This registry was sponsored and funded by B. Braun, Aesculap AG, Tuttlingen, Germany. The Medical Scientific Affairs department of Aesculap AG was responsible for project management, data management, statistics, and study registration (NCT02662777).
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data that support the findings of this study are available from the sponsor of this study upon reasonable request.
References
- 1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013;381:400–412 [DOI] [PubMed] [Google Scholar]
- 2. Lang H, Piso P, Stukenborg C, Raab R, Jähne J. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol 2000;26:168–171 [DOI] [PubMed] [Google Scholar]
- 3. Ikeguchi M, Oka S, Gomyo Y, Tsujitani S, Maeta M, Kaibara N. Postoperative morbidity and mortality after gastrectomy for gastric carcinoma. Hepatogastroenterology 2001;48:1517–1520 [PubMed] [Google Scholar]
- 4. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich Aet al. . Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010;147:339–351 [DOI] [PubMed] [Google Scholar]
- 5. Chirica M, Champault A, Dray X, Sulpice L, Munoz-Bongrand N, Sarfati Eet al. . Esophageal perforations. J Visc Surg 2010;147:e117–e128 [DOI] [PubMed] [Google Scholar]
- 6. Pennathur A, Luketich JD. Resection for esophageal cancer: strategies for optimal management. Ann Thorac Surg 2008;85:S751–S756 [DOI] [PubMed] [Google Scholar]
- 7. Vallböhmer D, Hölscher AH, Hölscher M, Bludau M, Gutschow C, Stippel Det al. . Options in the management of esophageal perforation: analysis over a 12-year period. Dis Esophagus 2010;23:185–190 [DOI] [PubMed] [Google Scholar]
- 8. Ahrens M, Schulte T, Egberts J, Schafmayer C, Hampe J, Fritscher-Ravens Aet al. . Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy 2010;42:693–698 [DOI] [PubMed] [Google Scholar]
- 9. Crestanello JA, Deschamps C, Cassivi SD, Nichols FC, Allen MS, Schleck Cet al. . Selective management of intrathoracic anastomotic leak after esophagectomy. J Thorac Cardiovasc Surg 2005;129:254–260 [DOI] [PubMed] [Google Scholar]
- 10. Page RD, Shackcloth MJ, Russell GN, Pennefather SH. Surgical treatment of anastomotic leaks after oesophagectomy. Eur J Cardiothorac Surg 2005;27:337–343 [DOI] [PubMed] [Google Scholar]
- 11. Böhm G, Mossdorf A, Klink C, Klinge U, Jansen M, Schumpelick Vet al. . Treatment algorithm for postoperative upper gastrointestinal fistulas and leaks using combined Vicryl plug and fibrin glue. Endoscopy 2010;42:599–602 [DOI] [PubMed] [Google Scholar]
- 12. Hampe J, Schniewind B, Both M, Fritscher-Ravens A. Use of a NOTES closure device for full-thickness suturing of a postoperative anastomotic esophageal leakage. Endoscopy 2010;42:595–598 [DOI] [PubMed] [Google Scholar]
- 13. Brangewitz M, Voigtländer T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer Jet al. . Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013;45:433–438 [DOI] [PubMed] [Google Scholar]
- 14. Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose Tet al. . Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 2013;27:3883–3890 [DOI] [PubMed] [Google Scholar]
- 15. Bludau M, Fuchs HF, Herbold T, Maus MKH, Alakus H, Popp Fet al. . Results of endoscopic vacuum-assisted closure device for treatment of upper GI leaks. Surg Endosc 2018;32:1906–1914 [DOI] [PubMed] [Google Scholar]
- 16. Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes Det al. . Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 2017;31:2687–2696 [DOI] [PubMed] [Google Scholar]
- 17. Paspatis GA, Dumonceau JM, Barthet M, Meisner S, Repici A, Saunders BPet al. . Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2014;46:693–711 [DOI] [PubMed] [Google Scholar]
- 18. Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med 1993;329:1302–1307 [DOI] [PubMed] [Google Scholar]
- 19. Schmidt H, Manegold BC, Stuker D, Grund KE. [Anastomotic insufficiencies of the esophagus–early surgical endoscopy and endoscopic therapy]. Kongressbd Dtsch Ges Chir Kongr 2001;118:278–281 [PubMed] [Google Scholar]
- 20. Leers JM, Vivaldi C, Schäfer H, Bludau M, Brabender J, Lurje Get al. . Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent. Surg Endosc 2009;23:2258–2262 [DOI] [PubMed] [Google Scholar]
- 21. Heits N, Stapel L, Reichert B, Schafmayer C, Schniewind B, Becker Tet al. . Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann Thorac Surg 2014;97:1029–1035 [DOI] [PubMed] [Google Scholar]
- 22. Wedemeyer J, Brangewitz M, Kubicka S, Jackobs S, Winkler M, Neipp Met al. . Management of major postsurgical gastroesophageal intrathoracic leaks with an endoscopic vacuum-assisted closure system. Gastrointest Endosc 2010;71:382–386 [DOI] [PubMed] [Google Scholar]
- 23. Bludau M, Hölscher AH, Herbold T, Leers JM, Gutschow C, Fuchs Het al. . Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc 2014;28:896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuehn F, Schiffmann L, Janisch F, Schwandner F, Alsfasser G, Gock Met al. . Surgical endoscopic vacuum therapy for defects of the upper gastrointestinal tract. J Gastrointest Surg 2016;20:237–243 [DOI] [PubMed] [Google Scholar]
- 25. Möschler O, Nies C, Mueller MK. Endoscopic vacuum therapy for esophageal perforations and leakages. Endosc Int Open 2015;3:E554–E558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mennigen R, Senninger N, Laukoetter MG. Novel treatment options for perforations of the upper gastrointestinal tract: endoscopic vacuum therapy and over-the-scope clips. World J Gastroenterol 2014;20:7767–7776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loske G. Endoscopic negative pressure therapy of the upper gastrointestinal tract. Chirurg 2019;90:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuehn F, Loske G, Schiffmann L, Gock M, Klar E. Endoscopic vacuum therapy for various defects of the upper gastrointestinal tract. Surg Endosc 2017;31:3449–3458 [DOI] [PubMed] [Google Scholar]
- 29. Schniewind B, Schafmayer C, Both M, Arlt A, Fritscher-Ravens A, Hampe J. Ingrowth and device disintegration in an intralobar abscess cavity during endosponge therapy for esophageal anastomotic leakage. Endoscopy 2011;43:E64––E65. [DOI] [PubMed] [Google Scholar]
- 30. Schorsch T, Müller C, Loske G. [Endoscopic vacuum therapy of perforations and anastomotic insufficiency of the esophagus]. Chirurg 2014;85:1081–1093 [DOI] [PubMed] [Google Scholar]
- 31. Kuehn F, Schiffmann L, Rau BM, Klar E. Surgical endoscopic vacuum therapy for anastomotic leakage and perforation of the upper gastrointestinal tract. J Gastrointest Surg 2012;16:2145–2150 [DOI] [PubMed] [Google Scholar]
- 32. Eroglu A, Turkyilmaz A, Aydin Y, Yekeler E, Karaoglanoglu N. Current management of esophageal perforation: 20 years experience. Dis Esophagus 2009;22:374–380 [DOI] [PubMed] [Google Scholar]
- 33. Brinster CJ, Singhal S, Lee L, Marshall MB, Kaiser LR, Kucharczuk JC. Evolving options in the management of esophageal perforation. Ann Thorac Surg 2004;77:1475–1483 [DOI] [PubMed] [Google Scholar]
- 34. Klevebro F, Friesland S, Hedman M, Tsai JA, Lindblad M, Rouvelas Iet al. . Neoadjuvant chemoradiotherapy may increase the risk of severe anastomotic complications after esophagectomy with cervical anastomosis. Langenbeck’s Arch Surg 2016;401:323–331 [DOI] [PubMed] [Google Scholar]
- 35. Walle CV, Ceelen WP, Boterberg T, Vande Putte D, Van Nieuwenhove Y, Varin Oet al. . Anastomotic complications after Ivor Lewis esophagectomy in patients treated with neoadjuvant chemoradiation are related to radiation dose to the gastric fundus. Int J Radiat Oncol Biol Phys 2012;82:e513–e519 [DOI] [PubMed] [Google Scholar]
- 36. Min YW, Kim T, Lee H, Min BH, Kim HK, Choi YSet al. . Endoscopic vacuum therapy for postoperative esophageal leak. BMC Surg 2019;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bartels H, Siewert JR. [Therapy of mediastinitis in patients with esophageal cancer]. Chirurg 2008;79:30–37 [DOI] [PubMed] [Google Scholar]
- 38. Noh SM, Ahn JY, Lee JH, Jung HY, AlGhamdi Z, Kim HRet al. . Endoscopic vacuum-assisted closure therapy in patients with anastomotic leakage after esophagectomy: a single-center experience. Gastroenterol Res Pract 2018;2018:1697968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. do Monte Junior ES, de Moura DTH, Ribeiro IB, Hathorn KE, Farias GFA, Turiani CVet al. . Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: systematic review and meta-analysis. Dig Endosc 2021;33:892–902 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the sponsor of this study upon reasonable request.