Summary
Gametogenesis requires close interactions between germ cells and somatic cells. Derivation of sperm from spermatogonial stem cells (SSCs) is hampered by the inefficiency of spermatogonial transplantation technique in many animal species because it requires a large number of SSCs and depletion of endogenous spermatogenesis. Here we used mouse testis primordia and organoids to induce spermatogenesis from SSCs. We microinjected mouse SSCs into embryonic gonads or reaggregated neonatal testis organoids, which were transplanted under the tunica albuginea of mature testes. As few as 1 × 104 donor cells colonized both types of transplants and produced sperm. Moreover, rat embryonic gonads supported xenogeneic spermatogenesis from mouse SSCs when transplanted in testes of immunodeficient mice. Offspring with normal genomic imprinting patterns were born after microinsemination. These results demonstrate remarkable flexibility of the germ cell-somatic cell interaction and raise new strategies of SSC manipulation for animal transgenesis and analysis of male infertility.
Keywords: Gonad, Microinsemination, organoid, Spermatogenesis, transplantation
Graphical abstract

Highlights
-
•
SSCs can be injected into embryonic gonads or reaggregated neonatal testes
-
•
Spermatogenesis occurs in the gonads or reaggregated testes after transplantation
-
•
Offspring are born from SSC-derived sperm using microinsemination
-
•
Offspring show normal DNA methylation in imprinted genes
Spermatogonial transplantation has been a standard functional assay for spermatogonial stem cells (SSCs). However, preparation of recipient animals and low efficiency of obtaining sperm from transplanted SSCs have restricted its application. In this article, Kanatsu-Shinohara and colleagues show that only a small number of SSCs are sufficient to produce fertile sperm after microinjection into embryonic gonads or testis reaggregates.
Introduction
Spermatogonial stem cells (SSCs) are the only stem cells in the germline. These cells have enormous capacity to undergo self-renewal division and continuously produce sperm throughout the lifespan of male animals. The progression of spermatogenesis from SSCs to haploid sperm occurs in a stepwise, synchronous manner (Meistrich and van Beek, 1993; de Rooij, 2017). SSCs on the basement membrane continuously undergo self-renewal division in special microenvironments called niches, which are probably composed of several somatic cell types, including Sertoli cells. Committed progenitor spermatogonia gradually leave the niche and initiate differentiation. Spermatocytes then enter meiosis as they migrate from the basal compartment of the seminiferous tubules toward the adluminal compartment through the blood-testis barrier (BTB) between Sertoli cells, resulting in the formation of spermatozoa (Griswold, 2018). The whole process of spermatogenesis from SSCs to spermatozoa takes ∼35 days in mice.
In 1994, a spermatogonial transplantation technique was developed (Brinster and Zimmermann, 1994). In this technique, SSCs from donor testis cells were microinjected into the seminiferous tubules of infertile mouse testes. Donor SSCs transmigrate through the BTB and settle on the basement membrane of the seminiferous tubules. SSCs reinitiate spermatogenesis and produce germ cell colonies. In the most successful case, offspring from donor SSCs were born by natural mating. Although the spermatogonial transplantation technique has been used widely for functional analysis of SSCs, there are at least two problems with the current technique. The first problem is the number of SSCs in the donor cell population. Due to the low concentration of SSCs in the testis (0.02%–0.03% in mice) (Tegelenbosch and de Rooij, 1993; Meistrich and van Beek, 1993), donor cell colonization is typically poor; only 4%–10% of transplanted SSCs colonize the seminiferous tubules (Nagano et al., 1999; Ogawa et al., 2003). Therefore, more than 90% of transplanted SSCs are lost after microinjection. The second problem is the recipient preparation. SSC colonization requires depletion of endogenous spermatogenesis by hazardous treatment of recipient animals. Depletion of endogenous SSCs requires one cycle of spermatogenesis and also damages the testis microenvironment. For example, the imbalance caused by the removal of germ cells from rat testes causes severe edema (Ogawa et al., 1999a). Although spermatogonial transplantation is a promising technique for animal transgenesis and human infertility treatment, these two problems need to be resolved for practical application.
Seminiferous tubules gradually develop soon after colonization by primordial germ cells (PGCs) of the genital ridges at around 10.5 days postcoitum (dpc) (Ross and Capel, 2005; Koopman, 2016). Although complex cell-cell interactions are required for subsequent testis organogenesis, their interactions appear to be remarkably flexible. For example, PGCs from as early as 8.5 dpc embryos can produce fertile sperm when transplanted in postnatal testes (Chuma et al., 2005). Moreover, the seminiferous tubules have a remarkable capacity of regeneration. When embryonic or postnatal newborn testes are dissociated into single cells by enzymatic digestions, the dissociated cells can reorganize to form seminiferous tubule-like structures. This occurs both in vitro (Zenzes and Engel, 1981; Hadley et al., 1985; Van der Wee and Hofmann, 1995; Yokonishi et al., 2013) and in vivo (Shinohara et al., 2003; Kita et al., 2007; Honaramooz et al., 2007). Although most of the tubules are empty and tubules with haploid cells are rarely found after reaggregation, regeneration of testis organoids raises new possibilities for analysis of germ cell-Sertoli cell interactions and developing new techniques for germline manipulation.
Microinsemination has revolutionized the treatment of male infertility, because it allows offspring production from a small number of sperm (Kimura and Yanagimachi, 1995). We hypothesized that immature gonads or testis organoids might serve as a test tube to produce sperm. Because colonization of postnatal testis by PGCs suggests that PGCs and SSCs have similar requirements for survival, we microinjected SSCs into immature gonads or neonatal testis organoids, which were transplanted under the tunica albuginea of mature host animals to allow complete maturation. Transplanted donor cells colonized these fragments and offspring were born using germ cells that developed in the transplants.
Results
Preparation of testis primordia and organoids
To induce the differentiation of SSCs, we employed two strategies (Figure 1). In the first set of experiments, we focused on testis primordia. Embryonic gonads have immature seminiferous tubules, called testis cords. Embryonic gonads secrete glial cell line-derived neurotrophic factor (GDNF) and fibroblast growth factors (FGFs) as early as 12.5 dpc (Nef et al., 2005). Because these cytokines can stimulate self-renewal (Kanatsu-Shinohara and Shinohara, 2013), we reasoned that SSCs survive and proliferate in the embryonic gonads. Testis primordia can be readily identified from 12.5 dpc or later stages of embryos by the formation of testis cords. Microinjection can be carried out using gonads of any stage before the seminiferous tubules become highly convoluted in the later stages of gestation. In this study, we focused on 13.5 dpc embryos (Figure 1B). Although histological analysis showed poor development of the luminal cavity (Figure 1C), testis cords were apparent under a stereomicroscope (Figure 1B). Moreover, we could inject a larger number of donor cells at this stage versus using smaller 12.5 dpc gonads, which have a less distinctive tubule structure.
Figure 1.
Preparation of testis primordia and organoids for transplantation
(A) Experimental procedure. Germline stem (GS) cells were microinjected into the tubules in vitro and the fragments were transplanted into testes of mature mice.
(B and C) Macroscopic (B) and histological (C) appearance of male gonads from a 13.5 dpc embryo.
(D) Neonatal testis cells aggregated in a 96-well plate on the next day after plating.
(E and F) Testis aggregates on agarose gel at 2 (E) or 13 (F) days after testis dissociation.
(G) Histological appearance of testis organoid 2 weeks after culture initiation, showing a convoluted seminiferous tubule-like structure.
(H) Immunostaining of the testis aggregates by peritubular cell (ACTA2), Sertoli cell (GATA4), or Leydig cell (HSD3B) markers. Bar, 500 μm (B), 100 μm (C–G). Stains, H&E stain (C and G) and Hoechst 33342 (H).
In the second set of experiments, we evaluated the potential of testis organoids for supporting spermatogenesis. Testis organoids were prepared by aggregating neonatal testis cells by plating in a 96-well plate with minimum cell adhesion. On the day after plating, Matrigel was added to promote tubule formation (Figure 1D). On the second day after culture initiation, cell aggregates were picked up using a large pipette and transferred onto an agarose gel soaked in culture medium (Figure 1E). After 10–14 days, these aggregates gradually developed into a flat cell mass that contained convoluted seminiferous tubules (Figure 1F). Although necrotic areas were occasionally found in the center of the aggregates, possibly due to poor oxygenation, all organoids developed tubule-like structures. Histological sections showed the seminiferous tubule-like structures (Figure 1G). Because individual tubules differed in diameter and had irregular configurations, we carried out immunostaining to confirm the cell identity. Although the position in the cell aggregates was not completely normal, GATA4+ Sertoli cells formed the seminiferous tubule structure, which was surrounded by ACTA2+ peritubular cells. HSD3B+ Leydig cells were found in the interstitial area (Figure 1H). Consistent with a previous study (Yokonishi et al., 2013), germ cells were generally absent from most of the tubules, which could not be reversed by GDNF or FGF2 supplementation. These results suggested that SSCs were gradually lost during organoid formation.
Microinjection of GS cells and transplantation of testis organoids
Donor SSCs were prepared from germline stem (GS) cells, cultured spermatogonia with enriched SSC activity (Kanatsu-Shinohara et al., 2003). These cells were derived from pup testis cells by adding GDNF and FGF2, which results in the formation of grape-like clusters of spermatogonia. GS cells used in the present experiments express Egfp gene as a donor cell marker (Figure 2A). Logarithmically growing GS cells were dissociated into single cells by trypsin digestion. Testis primordia or organoids were placed in a microinjection dish containing ∼200–300 μL of DMEM supplemented with 10% fetal bovine serum (FBS). Using a glass needle, approximately 0.1 μl of GS cell suspension was microinjected into the tubule-like structures (Figure 2B). Fine forceps were used to gently hold the testis primordia during microinjection. When these primordia or organoids were examined under UV light, EGFP fluorescence in the tubule lumen was evident in all cases (Figures 2C and 2D). Immunostaining of injected gonads or aggregates showed GS cells in the lumen of the seminiferous tubules (Figure 2E). In contrast, when GS cells were aggregated with neonatal testis cells at the time of plating, some of the tubules initially contained EGFP+ cells. However, they gradually disappeared, and very few cells showed fluorescence after several weeks (Figure 2F).
Figure 2.
Microinjection and transplantation of testis primordia and organoids
(A) GS cells growing logarithmically in vitro.
(B) Microinjection of GS cells into testis organoid.
(C and D) Embryonic gonad (C) or testis organoid (D) injected with green GS cells showing fluorescence under UV light.
(E) Immunostaining of gonad or aggregate immediately after GS cell microinjection by Sertoli cell marker (GATA4).
(F) Aggregation of GS cells with neonatal testis cells 2 (left) and 24 (right) days after culture.
(G and H) Macroscopic (G) and histological (H) appearance of nude mouse recipient testis transplanted with gonad (left) or organoid (right) 3 months after microinjection of green GS cells.
(I and J) Immunostaining of gonad (I) and organoid (J) that developed in the recipient testes by spermatocyte (SYCP3) or haploid (PNA) cell markers. Arrowheads indicate donor cells expressing the marker antigens. Bars: 50 μm (A, E, I, and J), 200 μm (C, D, and F), 1 mm (G), and 100 μm (H). Stain, Hoechst 33342.
The testis organoids microinjected with GS cells were then transplanted under the tunica of busulfan-treated mice. We previously reported that orthotopic transplantation allows fertile sperm production in isogenic and xenogeneic immature testis fragments (Shinohara et al., 2002). A small incision was made in the tunica albuginea using a 21G needle, and two to three pieces of fragments were transplanted into different parts of each testis to prevent them merging with each other. In cases of testis organoid experiments, a total of three to four recipients with testis organoids were transplanted in a single experiment using three to five donor mice.
Recipient mice were analyzed 3 months after transplantation, which corresponds to more than two cycles of spermatogenesis. When the testis fragments were exposed to UV light, donor organoid grafts with green fluorescence were evident (Figure 2G). Of the total of 12 recipient mice transplanted with testis primordia, we found donor fragments in 11 of them. It was possible to distinguish the germ cell colonies in the recipient testis. Overall, 16/23 (69.6%) primordia organoids exhibited green fluorescence under UV light (Figure 2G). Likewise, we found testis organoids in all 12 recipient mice (Figure 2G). However, EGFP fluorescence patterns were more irregular in testis organoids than testis primordia, likely reflecting abnormal tubule morphogenesis in the original organoids. Nevertheless, the efficiency of donor cell colonization was similar, with 14/21 (66.7%) fragments exhibiting EGFP fluorescence.
Because green fluorescence was strong in many areas of the seminiferous tubules, it was likely that GS cells differentiated beyond meiosis. We made histological sections of the testes to examine the degree of spermatogenesis. We identified multiple seminiferous tubules with spermatogenic cells of various stages (Figure 2H). Although some tubules contained only premeiotic germ cells, many tubules with complete spermatogenesis were readily found despite the abnormal architecture of the seminiferous tubules. Immunostaining of the donor cell fragments revealed SYCP3+ spermatocytes as well as peanut agglutinin (PNA)+ haploid cells (Figures 2I and 2J). Because these cells also expressed EGFP protein, these results confirmed that GS cells transplanted into the testis grafts were able to differentiate into sperm after transplantation under the testis capsule of recipient mice.
Xenogeneic spermatogenesis in testis primordia
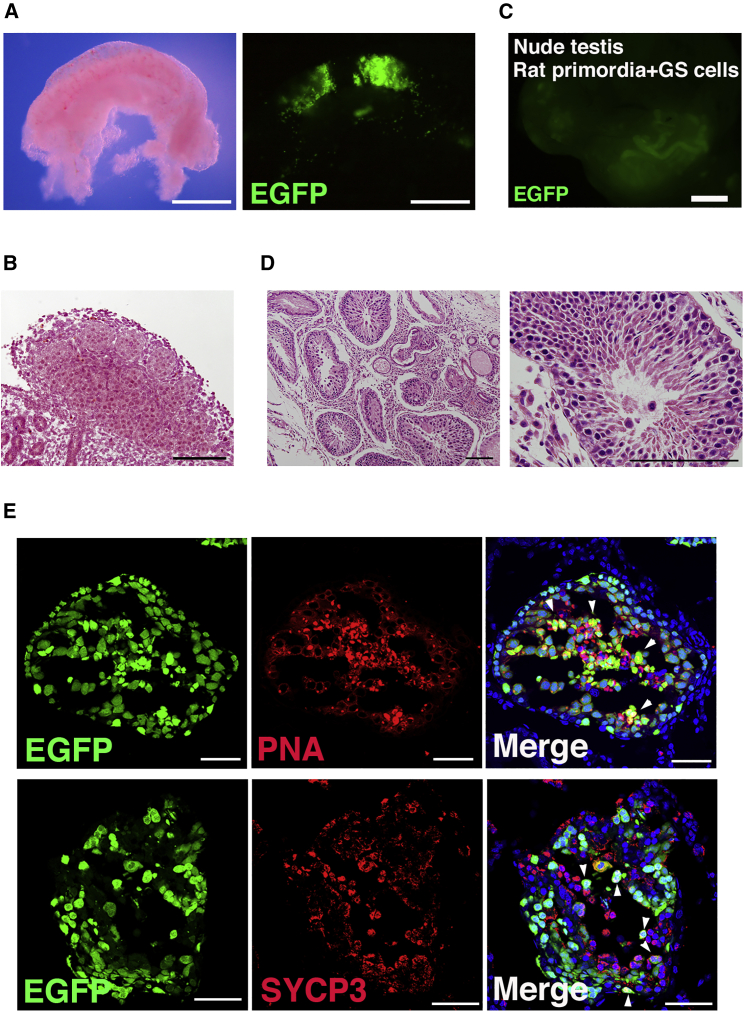
Mouse SSCs can undergo spermatogenesis in rat seminiferous tubules (Ogawa et al., 1999a). To test whether mouse GS cells can differentiate in xenogeneic embryonic gonads, we microinjected green mouse GS cells into male gonads of 14.5 dpc rat embryos (Figure 3A). Although rat gonads at this stage are significantly larger than those of mice, histological analysis showed that the adluminal cavity is barely formed at this stage (Figure 3B). Nevertheless, fluorescence of mouse GS cells in the tubule lumen was detected when the gonads were exposed to UV light after GS cell microinjection (Figure 3A). These gonads were then transplanted into the testes of three nude mouse testes for subsequent development.
Figure 3.
Xenogeneic spermatogenesis within testis primordia
(A and B) Macroscopic (A) and histological (B) appearance of male gonad from 14.5 dpc rat embryo. Donor mouse GS cells transplanted into the rat gonad can be visualized under UV light.
(C) Macroscopic appearance of recipient testis transplanted with rat testis primordia 3 months after microinjection of mouse green GS cells.
(D) Histological appearance of rat gonad in mouse testis.
(E) Immunostaining of rat testis primordia transplanted with mouse GS cells by spermatocyte (SYCP3) or haploid (PNA) cell markers. Arrowheads indicate donor cells expressing the marker antigens. Bars: 500 μm (A), 100 μm (B and D), 1 mm (C), and 50 μm (E). Stain, Hoechst 33342.
Three months after transplantation, we analyzed the recipients for the presence of xenogeneic spermatogenesis. All recipient tubules exhibited green fluorescence (Figure 3C), indicating that donor mouse GS cells colonized and regenerated spermatogenesis. Of the 12 gonads transplanted, 4 showed green fluorescence. Because of the high intensity of the fluorescence signal, it was likely that SSCs differentiated into haploid cells. Histological analysis showed spermatogenesis in the rat seminiferous tubules (Figure 3D). Because it was not possible to distinguish mouse and rat germ cells, we carried out immunostaining to confirm the presence of mouse spermatogenesis. Both SYCP3 and PNA signals were colocalized in EGFP-expressing tubules (Figure 3E), indicating that mouse GS cells not only underwent meiosis but also differentiated into PNA+ haploid cells. These results suggested that xenogeneic spermatogenesis can occur in testis primordia.
Offspring production by microinsemination using germ cells in testis fragments
Because we found normal appearing spermatogenesis in both types of transplanted testis fragments, we carried out a series of microinsemination experiments to test whether the germ cells were fertile. Testis samples were recovered after sacrificing the recipient mice and refrigerated overnight. On the next day after sample recovery, seminiferous tubules with EGFP fluorescence were dissected and punctured with a fine-tipped metal needle to release spermatogenic cells and spermatozoa into the medium. Because host testis cells can also contain endogenous spermatogenesis, we searched for donor cells under UV light and identified spermatids or sperm in the cell suspension. These cells were microinjected into oocytes for offspring production.
In experiments with testis primordia, 85 embryos were constructed and 48 embryos developed to the two-cell stage; these were transferred into oviducts of three pseudopregnant mothers. A total of 22 offspring were born, with 12 exhibiting green fluorescence (Figure 4A). Not all offspring showed green fluorescence, probably because the donor cells were hemizygous for the transgene but shared EGFP via cytoplasmic bridges (Braun et al., 1989). For testis organoids, 54 embryos were constructed and cultured in vitro (Figure 4B). On the next day, 50 embryos survived, 34 of which developed to the two-cell stage. All two-cell embryos were transferred into the oviducts of two pseudopregnant mothers. Two female offspring were born and both showed green fluorescence under UV light (Figure 4C). These results showed that the germ cells in the testis organoids are fertile.
Figure 4.
Offspring production by microinsemination
(A) Offspring born after microinsemination using germ cells that developed in gonad transplants.
(B) Round (arrows) and elongated spermatid (arrowhead) identified in cell suspension from testis organoid transplants.
(C) Offspring born after microinsemination using sperm that developed in testis organoid transplants.
(D) Rat seminiferous tubule fragment with mouse cells.
(E) Round spermatid (arrow) and sperm (arrowhead) identified in cell suspensions from rat gonad.
(F) Offspring born after microinsemination using germ cells that developed in rat gonad transplants.
Finally, based on the successful mouse-to-mouse transplantation experiments, we carried out microinsemination using mouse germ cells that developed in rat gonads. A testis was collected from one of the recipients 111 days after transplantation. After dissection of the seminiferous tubules with donor green fluorescence (Figure 4D), round spermatids and sperm were microinjected into mouse oocytes (Figure 4E). For round spermatid and sperm injection, 26 and 7 embryos were constructed, respectively. A total of 21 embryos (16 embryos from round spermatids and 5 embryos from sperm) that progressed to the two-cell stage were transferred into the oviducts of three pseudopregnant mothers on the day after oocyte injection. Two offspring were born from round spermatid injection, and one was born from sperm injection. All of these three offspring exhibited green fluorescence under UV light (Figure 4F), indicating a donor cell origin. These results confirmed the full developmental potential of the mouse germ cells that developed in rat seminiferous tubules.
DNA analysis of the offspring
Because genomic imprinting in the male germline starts during embryonic development (Sasaki and Matsui, 2008), the microenvironment in immature gonads or testis organoids could affect the epigenetic status of transplanted GS cells, which have androgenetic genomic imprinting patterns. To test whether germ cells that developed in immature testes or testis organoids affect genomic imprinting, we first carried out combined bisulfite restriction analysis (COBRA) on the tail DNA of the offspring (Figure S1). Control DNA from GS cell cultures showed complete androgenetic DNA methylation patterns. Although the differentially methylated region (DMR) in H19 was heavily methylated, no apparent methylation occurred in the DMR of Igf2r. In contrast, offspring born from immature testis or testis organoid grafts showed somatic cell methylation patterns, and both H19 and Igf2r DMRs were partially digested by methylation-specific restriction enzymes, similar to those found in offspring born after natural mating (Figure S1). Bisulfite sequencing of the H19 and Igf2r DMRs in representative offspring from each experiment confirmed the somatic cell-type imprinting patterns (Figures 5A–5C). These results suggested that immature testes and testis organoids did not induce apparent abnormalities in their offspring.
Figure 5.
DNA analysis of the offspring. Bisulfite sequencing of DMRs in H19 and Igf2r
Offspring from mouse gonad (A), mouse organoid (B) and rat gonad (C) microinjecction were analyzed. Black circles indicate methylated cytosine-guanine sites (CpGs), and white ovals indicate unmethylated CpGs. See also Figure S1.
Discussion
Embryonic cells from several tissues can form tissue-specific associations in vitro (Moscona, 1957). Recent studies employ this unique property to understand organogenesis of various organs (Iwasaka and Takebe, 2021). Although a gonadal reaggregation techniques were described several decades ago (Davis, 1978; Ohno et al., 1978), gametogenesis in testis organoids is hampered by the exclusion of germ cells from the aggregates and failure to achieve full maturation in vitro. Although spermatogenesis in testis aggregates was thus limited, spermatogenesis in surrogate animals occurred successfully by spermatogonial transplantation (Brinster and Zimmermann, 1994). The most important aspect of spermatogonial transplantation is the production of offspring (Brinster and Avarbock, 1994). However, low frequency of offspring production still limits the practical application of the spermatogonial transplantation technique to a wide range of animal species.
In this study, we describe new techniques for deriving fertile sperm from SSCs by using immature embryonic gonads or testis organoids. The most important factor in the success of spermatogenesis was the immaturity of the tubule-like structure. Spermatogonial transplantation was originally based on the direct microinjection of donor cells into the seminiferous tubules (Brinster and Zimmermann, 1994). However, later studies demonstrated the utility of microinjection via the efferent duct or rete testis (Ogawa et al., 1997). Efferent duct injection is probably more widely used than the other two methods because the wall of the efferent duct is more resistant than the seminiferous tubules and rete testis. However, because testis primordia and organoids lack an efferent duct and rete testis, cells must be directly injected into the seminiferous tubules in such cases. Because tubule components are well established by late gestation, microinjection into testes of later gestational stages (∼16.5 dpc) is more challenging than microinjection into adult seminiferous tubules, which have a larger diameter. However, this is easier with immature tubules because the tubule structure is incomplete and Sertoli cells can restore damaged tubules.
Endogenous germ cell removal is a prerequisite for spermatogonial transplantation. This is usually performed by treating recipient animals with toxic chemicals, such as busulfan (Brinster and Zimmermann, 1994). Alternatively, genetic mutants that lack endogenous germ cells, such as Kit mutants, may be used (Brinster and Zimmermann, 1994). However, such mutants are not readily available in all genetic backgrounds, and the efficiency of getting homozygous mutants is very low (12.5%). Our findings revealed that donor cells can colonize both immature primordia and testis organoids without removing endogenous germ cells. In vivo, PGCs migrated into the gonad prior to testis cord formation during embryogenesis at around 10.5 dpc. However, we previously showed that PGCs from day 8.5 dpc embryos can colonize postnatal testes (Chuma et al., 2005). The successful colonization by GS cells of testis primordia suggests that they have a competitive advantage over endogenous germ cells. This may be because gonocytes become mitotically quiescent within several days. By contrast, exclusion of germ cells from the reconstructed tubules is a problem in testis organoids (Yokonishi et al., 2013). However, this property was useful for donor cell injection, because it precludes busulfan treatment for recipient preparation.
Another factor that contributed to the success was the transplantation of testis fragments. Although in vitro spermatogenesis can occur in intact organ culture (Sato et al., 2011), immature gonads and reaggregated testis organoid failed to produce haploid cells in previous studies (Kojima et al., 2016). This could be due to poor development or loss of some cell types during dissociation and reaggregation. Although improving the culture medium composition or dissociation protocol may overcome this problem, the in vitro environment is apparently not optimal for gametogenesis due to the higher oxygen concentration and difficulty in determining the optimal nutritional requirements of both somatic and germ cells. Therefore, we chose testes as the site of transplantation, because they support the development of immature testis fragments for fertile sperm production (Shinohara et al., 2002). We reasoned that factors or cells missing in vitro may be present in the testis microenvironment. Although it is impossible to avoid ischemia and damage associated with transplantation, the transplanted grafts probably can receive sufficient blood supply for complete maturation.
Our technique has several advantages over the conventional spermatogonial transplantation technique. First, it is possible to produce sperm from a small number of SSCs. Because the colonization efficiency in adult testis is limited by the BTB (Kanatsu-Shinohara et al., 2020), it is typically necessary to inject a large number of donor cells for successful colonization. Because the size of the testis is significantly larger in farm animals or humans, preparation of sufficient number of SSCs is a dauting task. Second, it provides appropriate species-specific Sertoli cells for donor SSCs. Although mouse Sertoli cells can support spermatogenesis of hamster SSCs, rabbit SSCs cannot produce sperm in mouse testes (Dobrinski et al., 1999; Ogawa et al., 1999a, 1999b). Therefore, it is considered that the genetic distance between SSCs and Sertoli cells is critical for successful spermatogenesis. However, it is now possible to combine testes and SSCs of different species to test sperm developmental potential. Third, the techniques do not require in vivo endogenous germ cell removal for recipient preparation. Although this is easily performed in rodents, which have relatively short cycles of spermatogenesis, preparation of non-rodent recipients takes longer time and requires the administration of large amounts of toxic chemicals. Finally, gonad injection may allow colonization of germ cells from earlier stages of PGCs. This may facilitate gamete production from pluripotent stem cells.
We developed a novel strategy for inducing spermatogenesis from SSCs. Although studies in the last two decades provided opportunities for male germline manipulation, its practical application for animal transgenesis or human infertility treatment is still limited. It is necessary to develop long-term culture conditions for SSCs from various animal species and to induce sperm efficiently from them. While requirements for better SSC culture conditions are now being discovered (Kanatsu-Shinohara et al., 2018; Morimoto et al., 2021), finding an efficient strategy for sperm derivation from genetically manipulated SSCs is an urgent problem. Strategy based on conventional spermatogonial transplantation is not easily applicable because anatomical structures of testes are significantly different among species with a large number of SSCs and toxic host animal treatment. In this sense, in vitro SSC microinjection and microinsemination into premature gonads or testis organoids provide an attractive solution to this problem. Because microinsemination is applicable to a number of animal species (Ogura et al., 2005), our technique may enable xenogeneic spermatogenesis in various animal species, including endangered species for animal conservation. The technique may also be useful for analyzing defective human spermatogenesis for infertility studies. Our primary goal is to develop a universal strategy for deriving sperm in the most efficient way from SSCs in a variety of animal species.
Experimental procedures
Organ culture
To produce testis organoids, we dissociated newborn testis collected from 2–5-day-old WBB6F1 mice (Japan SLC, Shizuoka, Japan). Testis cells were digested by a two-step enzymatic procedure using collagenase type IV and trypsin (both from Sigma-Aldrich, St. Louis, MO), as described previously (Ogawa et al., 1997). After centrifugation, the cells were suspended in αMEM/10% KnockOut Serum Replacement (KSR) (Invitrogen, Carlsbad, CA), and 2 × 105 cells in 100 μl of medium were plated in a 96-well plate (PrimeSurface 96V; Sumitomo Bakelite, Tokyo, Japan). After overnight incubation, 20 μl of diluted growth factor-reduced Matrigel (Corning, NY) was added to each well to promote aggregation. The final concentration of Matrigel was 2%. The cell clump was transferred to agarose and cultured in αMEM/10% KSR and cultured under 5% CO2 at 34 °C, as described previously (Sato et al., 2011).
Microinjection procedure
Donor cells were prepared by trypsin digestion of green GS cells (Kanatsu-Shinohara et al., 2003). Cells were suspended in DMEM/10% FBS. For preparation of testis primordia, gonads were collected from 13.5 dpc Institute for Cancer Research mouse or 14.5 dpc Sprague-Dawley (SD) rat embryos (Japan SLC). Mesonephros was removed by a tungsten needle. For microinjection into organoids, cultured fragments were recovered ∼10–15 days after plating on agarose. A large pipette was used to recover the fragments from 96-well plates. For microinjection, three to four testis fragments were used in a watch glass filled with αMEM/10% KSR under a stereomicroscope. By holding each organoid with a set of fine forceps, the glass pipette was advanced using a micromanipulator. Approximately 0.1 μl of donor cells was microinjected into the tubule structure. Injection was confirmed by adding a small volume of trypan blue to the donor cell suspension.
Transplantation into nude mice
For recipient preparation, 4-week-old KSN nude mice received intraperitoneal injection of busulfan (44 mg/kg) (Ogawa et al., 1997) (Japan SLC). We used nude mice as recipients because donor GS cells (DBA/2) and rat gonads (SD rat) could be rejected by the host immune system. The testes were exteriorized through a midline incision in their abdomen after anesthetizing the mice. Using a 21G needle, a small cut was made in the tunica albuginea. Two to three grafts were inserted under the tunica albuginea. The Institutional Animal Care and Use Committee of Kyoto University approved all of the animal experimentation protocols.
Microinsemination
Testes were refrigerated overnight and used for microinsemination on the next day after recovery (Ogonuki et al., 2006). Seminiferous tubules with green fluorescence were collected under the UV light and dissociated with a fine-tipped metal needle. Round spermatids or spermatozoa were collected and microinjected into oocytes of C57BL/6 × DBA/2 F1 (BDF1) mice using a piezo-driven micropipette, as described previously (Ogonuki et al., 2006). Embryos were cultured for 24 h and transferred into the oviducts of ICR pseudopregnant mothers. Offspring were recovered by cesarean section.
Author contributions
T. S. and M.K.-S. conceived the idea and designed the study. T.S., N.O., S.M., H.M., Y.S., A.O., and M.K.-S. performed the experiments and analyzed data. T.S. and M.K.-S. wrote the manuscript.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
We thank J. Yang for technical assistance. Financial support for this research was provided by AMED (17933225 and JP21gm1110008) and MEXT (19K22512, 19H05750, 19H04906, 18H04882, 18H05281, and 18H02935).
Published: March 24, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.02.013.
Supplemental information
References
- Braun R.E., Behringer R.R., Peschon J.J., Brinster R.L., Palmiter R.D. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337:373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Brinster R.L., Zimmermann J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R.L., Avarbock M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuma S., Kanatsu-Shinohara M., Inoue K., Ogonuki N., Miki H., Toyokuni S., Hosokawa M., Nakatsuji N., Ogura A., Shinohara T. Spermatogenesis from epiblast and primordial germ cells following transplantation into postanal mouse testis. Development. 2005;132:117–122. doi: 10.1242/dev.01555. [DOI] [PubMed] [Google Scholar]
- Davis J.C. Morphogenesis by dissociated immature rat testicular cells in primary culture. J. Embryol. Exp. Morph. 1978;44:297–302. [PubMed] [Google Scholar]
- de Rooij D.G. The nature and dynamics of spermatogonial stem cells. Development. 2017;144:3022–3030. doi: 10.1242/dev.146571. [DOI] [PubMed] [Google Scholar]
- Dobrinski I., Avarbock M.R., Brinster R.L. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol. Reprod. 1999;61:1331–1339. doi: 10.1095/biolreprod61.5.1331. [DOI] [PubMed] [Google Scholar]
- Griswold M.D. 50 years of spermatogenesis: Sertoli cells and their interactions with germ cells. Biol. Reprod. 2018;99:87–100. doi: 10.1093/biolre/ioy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley M.A., Byers S.W., Suarez-Quian C.A., Kleinman H.K., Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 1985;101:1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A., Megee S.Q., Rathi R., Dobrinski I. Building a testis: formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biol. Reprod. 2007;76:43–47. doi: 10.1095/biolreprod.106.054999. [DOI] [PubMed] [Google Scholar]
- Iwasaka K., Takebe T. Organogenesis in vitro. Curr. Opin. Cell Biol. 2021;73:84–91. doi: 10.1016/j.ceb.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S., Shinohara T. Long-term proliferation and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Shinohara T. Spermatogonial stem cell self-renewal and development. Annu. Rev. Cell Dev. Biol. 2013;29:163–187. doi: 10.1146/annurev-cellbio-101512-122353. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Morimoto H., Watanabe S., Shinohara T. Reversible inhibition of the blood-testis barrier protein improves stem cell homing in mouse tetstes. J. Reprod. Dev. 2018;64:511–522. doi: 10.1262/jrd.2018-093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Ogonuki N., Matoba S., Ogura A., Shinohara T. Autologous transplantation of spermatogonial stem cells restores fertility in congenitally infertile mice. Proc. Natl. Acad. Sci. USA. 2020;117:7834–7844. doi: 10.1073/pnas.1914963117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Yanagimachi R. Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development. 1995;121:2397–2405. doi: 10.1242/dev.121.8.2397. [DOI] [PubMed] [Google Scholar]
- Kita K., Watanabe T., Ohsaka K., Hayashi H., Kubota Y., Nagashima Y., Aoki I., Taniguchi H., Noce T., Inoue K.I., et al. Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biol. Reprod. 2007;76:211–217. doi: 10.1095/biolreprod.106.056895. [DOI] [PubMed] [Google Scholar]
- Kojima K., Sato T., Naruse Y., Ogawa T. Spermatogenesis in explanted fetal mouse testis tissues. Biol. Reprod. 2016;95:63. doi: 10.1095/biolreprod.116.140277. [DOI] [PubMed] [Google Scholar]
- Koopman P. The curious world of gonadal development in mammals. Curr. Top. Dev. Biol. 2016;116:537–545. doi: 10.1016/bs.ctdb.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Meistrich M.L., van Beek M.E.A.B. In: Cell and Molecular Biology of the Testis. Desjardins C., Ewing L.L., editors. Oxford University Press; 1993. Spermatogonial stem cells; pp. 266–295. [Google Scholar]
- Moscona A. Development in vitro of chimaric aggregates of dissociated embryonic and mouse cells. Proc. Natl. Acad. Sci. U S A. 1957;43:184–194. doi: 10.1073/pnas.43.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto H., Yamamoto T., Miyazaki T., Ogonuki N., Ogura A., Tanaka T., Kanatsu-Shinohara M., Yabe-Nishimura C., Zhang H., Pommier Y., et al. An interplay of NOX1-derived ROS and oxygen determines the spermatogonial stem cell self-renewal efficiency under hypoxia. Genes Dev. 2021;35:250–260. doi: 10.1101/gad.339903.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M., Avarbock M.R., Brinster R.L. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol. Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef S., Schaad O., Stallings N.R., Cederroth C.R., Pitetti J.L., Schaer G., Malki S., Dubois-Dauphin M., Boizet-Bonhoure B., Descombes P., et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev. Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Aréchaga J.M., Avarbock M.R., Brinster R.L. Transplantation of testis germinal cells into mouse seminiferous tubules. Int. J. Dev. Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- Ogawa T., Dobrinski I., Avarbock M.R., Brinster R.L. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol. Reprod. 1999;60:515–521. doi: 10.1095/biolreprod60.2.515. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Dobrinski I., Brinster R.L. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–472. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Ohmura M., Yumura Y., Sawada H., Kubota Y. Expansion of murine spermatogonial stem cells through serial transplantation. Biol. Reprod. 2003;68:316–322. doi: 10.1095/biolreprod.102.004549. [DOI] [PubMed] [Google Scholar]
- Ogonuki N., Mochida K., Miki H., Inoue K., Fray M., Iwaki T., Moriwaki K., Obata Y., Morozumi K., Yanagimachi R., et al. Spermatozoa and spermatids retrieved from frozen reproductive organs or frozen whole bodies of male mice can produce normal offspring. Proc. Natl. Acad. Sci. U S A. 2006;103:13098–13103. doi: 10.1073/pnas.0605755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura A., Ogonuki N., Miki H., Inoue K. Microinsemination and nuclear transfer using male germ cells. Int. Rev. Cytol. 2005;246:189–229. doi: 10.1016/S0074-7696(05)46005-2. [DOI] [PubMed] [Google Scholar]
- Ohno S., Nagai Y., Ciccarese S. Testicular cells lysostripped of H-Y antigen organize ovarian follicle-like aggreagtes. Cytogenet. Cell Genet. 1978;20:351–364. doi: 10.1159/000130863. [DOI] [PubMed] [Google Scholar]
- Ross A.J., Capel B. Signaling at the crossroads of gonad development. Trends Endocrinol. Metab. 2005;16:9–25. doi: 10.1016/j.tem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat. Rev. Genet. 2008;9:129–140. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- Sato T., Katagiri K., Gohbara A., Inoue K., Ogonuki N., Ogura A., Kubota Y., Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Inoue K., Ogonuki N., Kanatsu-Shinohara M., Miki H., Nakata K., Kurome M., Nagashima H., Toyokuni S., Kogishi K., et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum. Reprod. 2002;17:3039–3045. doi: 10.1093/humrep/17.12.3039. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Orwig K.E., Avarbock M.R., Brinster R.L. Restoration of spermatogenesis in infertile mice by Sertoli cell transplantation. Biol. Reprod. 2003;68:1064–1071. doi: 10.1095/biolreprod.102.009977. [DOI] [PubMed] [Google Scholar]
- Tegelenbosch R.A., de Rooij D.G. A quantitative study of spermatogonial multiplication and stem cell renewal in the F1 hybrid mouse. Mutat. Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Van der Wee K., Hofmann H.C. An in vitro tubule assay identifies HGF as a morphogen for the formation of seminiferous tubules in the postnatal mouse testis. Exp. Cell Res. 1995;252:175–185. doi: 10.1006/excr.1999.4630. [DOI] [PubMed] [Google Scholar]
- Yokonishi T., Sato T., Katagiri K., Komeya M., Kubota Y., Ogawa T. In vitro reconstruction of mouse seminiferous tubules supporting germ cell differentiation. Biol. Reprod. 2013;89:15. doi: 10.1095/biolreprod.113.108613. [DOI] [PubMed] [Google Scholar]
- Zenzes M.T., Engel W. The capacity of testicular cells of the postnatal rata to reorganize into histotypic structures. Differentiation. 1981;20:157–161. doi: 10.1111/j.1432-0436.1981.tb01170.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.