Summary
Intestinal progenitor cells integrate signals from their niche, and the gut lumen, to divide and differentiate at a rate that maintains an epithelial barrier to microbial invasion of the host interior. Despite the importance of evolutionarily conserved innate immune defenses to maintain stable host-microbe relationships, we know little about contributions of stem-cell immunity to gut homeostasis. We used Drosophila to determine the consequences of intestinal-stem-cell immune activity for epithelial homeostasis. We showed that loss of stem-cell immunity greatly impacted growth and renewal in the adult gut. In particular, we found that inhibition of stem-cell immunity impeded progenitor-cell growth and differentiation, leading to a gradual loss of stem-cell numbers with age and an impaired differentiation of mature enteroendocrine cells. Our results highlight the importance of immune signaling in stem cells for epithelial function in the adult gut.
Keywords: Drosophila, intestinal stem cell, immunity, proliferation, differentiation, IMD
Highlights
-
•
The immune deficiency (IMD) pathway is active in Drosophila intestinal progenitors
-
•
Inhibition of IMD in progenitors impairs progenitor-cell proliferation
-
•
Blocking IMD in progenitors impairs generation of mature epithelial cells
In this article, Foley and colleagues show that activation of IMD in intestinal progenitor cells regulates stem-cell proliferation and differentiation in the adult Drosophila midgut. Blocking progenitor-cell IMD impairs epithelial renewal and impacts the generation of mature enteroendocrine cells.
Introduction
The intestine is an important contact point between animals and their environments. Intestinal epithelial cells regulate nutrient acquisition, microbiota tolerance, immune education, and pathogen elimination, and disruptions to epithelial homeostasis are linked to inflammatory diseases and cancers. As the epithelium contains a heterogenous population of specialist cell types, it is essential that we understand the mechanisms by which individual lineages regulate intestinal cell proliferation, differentiation, and renewal.
Intestinal epithelial cell (IEC) lineages vary by animal, but data from Drosophila, zebrafish, mice, and humans indicate evolutionary conservation of cell-type composition (Brugman, 2016; Buchon et al., 2013a; Lickwar et al., 2017; Miguel-Aliaga et al., 2018; Nguyen et al., 2015; Wallace et al., 2005). Typically, the epithelium is maintained by proliferative, multipotent intestinal stem cells (ISCs) that self-renew and generate all mature epithelial cell types. Most differentiated cells are columnar enterocytes, a cell type specializing in capture and digestion of lumenal nutrients. Secretory-cell-type complexity varies from animal to animal. In flies, the secretory lineage consists solely of hormone-producing enteroendocrine cells. Fish and mammals have mucus-secreting goblet cells in addition to the enteroendocrine population, and mammals also have long-lived, antimicrobial-peptide-producing Paneth cells that neighbor ISCs in basal crypts. ISCs integrate cues from their niche to divide and differentiate at a rate that replenishes dying epithelial cells. Notch, epidermal growth factor (EGF), Wnt, and bone morphogenetic protein (BMP) signal transduction pathways are important regulators of ISC proliferation and differentiation in vertebrates and invertebrates (Spit et al., 2018). Recent studies uncovered roles for immune signaling in ISC survival, growth, and differentiation. For example, vertebrate ISCs express major histocompatibility complex (MHC) class II molecules, and presentation of self-peptides by ISCs appears to be a critical aspect of intestinal invasion and epithelial destruction in graft-versus-host disease (Biton et al., 2018; Fu et al., 2019; Takashima et al., 2019). Likewise, ISCs are enriched for expression of the germline-encoded peptidoglycan receptor NOD2 (Nigro et al., 2014). NOD2 protects ISCs against reactive oxygen species toxicity (Levy et al., 2020), and mutations in NOD2 are associated with Crohn’s disease and intestinal tumorigenesis (Couturier-Maillard et al., 2013). Despite established requirements for immune-signaling pathways in the maintenance of intestinal health, it is unclear if innate defenses act specifically in progenitors to regulate epithelial homeostasis. We consider this an important knowledge gap given the central role of intestinal progenitors in building and maintaining the entire epithelium.
Drosophila melanogaster are widely used to characterize intestinal immunity and homeostasis. The adult fly intestine is a pseudostratified epithelium that is maintained by multipotent ISCs (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). The majority of ISC divisions are asymmetric, producing a new ISC, and are transient cell types that generate terminally differentiated epithelial cells. In most cases, ISC divisions generate a post-mitotic enteroblast that differentiates as an enterocyte in response to Notch signals (Bardin et al., 2010; Guo and Ohlstein, 2015; Ohlstein and Spradling, 2007). Collectively, ISCs and enteroblasts are classified as the intestinal progenitor compartment in flies. In the absence of cues from Notch, ISCs transition through a pre-enteroendocrine state to generate mature enteroendocrine cells that can be sub-classified into functional groups based on intestinal localization and hormone expression patterns (Biteau and Jasper, 2014; Guo and Ohlstein, 2015; Zeng and Hou, 2015). In the fly gut, bacterial diaminopimelic acid-type peptidoglycan (PGN) activates immune responses via the immune deficiency (IMD) pathway, a germline-encoded defense with similarities to vertebrate tumor necrosis factor receptor signaling (Buchon et al., 2009b, 2013a; Myllymäki et al., 2014). Detection of extracellular PGN by the PGN recognition protein LC (PGRP-LC) receptor, or intracellular PGN by the related PGRP-LE receptor, converges on a signaling complex that includes the Imd protein, the adaptor protein Fas-associated death domain (FADD), and the Caspase-8 homolog, Dredd. Dredd removes thirty N-terminal amino acids from Imd, initiating molecular events that activate c-Jun N-terminal kinase, and the p100/105 nuclear factor κB (NF-κB) ortholog Relish (Rel) (Leulier et al., 2000; Stoven et al., 2003; Stöven et al., 2000). Thus, Dredd-mediated processing of Imd is essential for IMD pathway activation, and expression of a non-cleavable Imd variant (ImdD30A) blocks host responses to PGN in cell culture and in vivo (Kim et al., 2014; Paquette et al., 2010). In the fly intestine, Rel and c-Jun N-terminal kinase initiate transcriptional responses that include regionalized expression of antimicrobial peptides, regulators of metabolism, and genes associated with growth and differentiation (Broderick et al., 2014; Buchon et al., 2009b, 2009a; Dutta et al., 2015; Hung et al., 2020). Earlier work uncovered significant differences between the responses of mature epithelial cell types to IMD activation. For example, infection-dependent activation of IMD in enterocytes results in antimicrobial peptide expression and extrusion of damaged cells (Buchon et al., 2009b; Dutta et al., 2015; Zhai et al., 2018). In contrast, activation of IMD in enteroendocrine cells by bacterial lactate modifies lipid metabolism in neighboring enterocytes (Kamareddine et al., 2018). Notably, genomic studies demonstrated expression of IMD pathway components in intestinal progenitor cells (Dutta et al., 2015; Hung et al., 2020). However, the contributions of progenitor-specific IMD activity to intestinal homeostasis are unexplored.
We took advantage of the genetic accessibility of flies to ask if progenitor-specific IMD affects intestinal homeostasis in Drosophila. Specifically, we used genomic and physiological assays to determine the consequences of blocking IMD in intestinal progenitors. We found that inhibition of progenitor-cell IMD had significant effects on ISC proliferation, progenitor compartment composition, and generation of mature enteroendocrine cells. As germline-encoded immune responses are known modifiers of vertebrate intestinal epithelial growth, we believe our findings are of general relevance to understanding how host immune responses control stem-cell function in the intestine.
Results
IMD regulates the intestinal progenitor-cell transcriptome
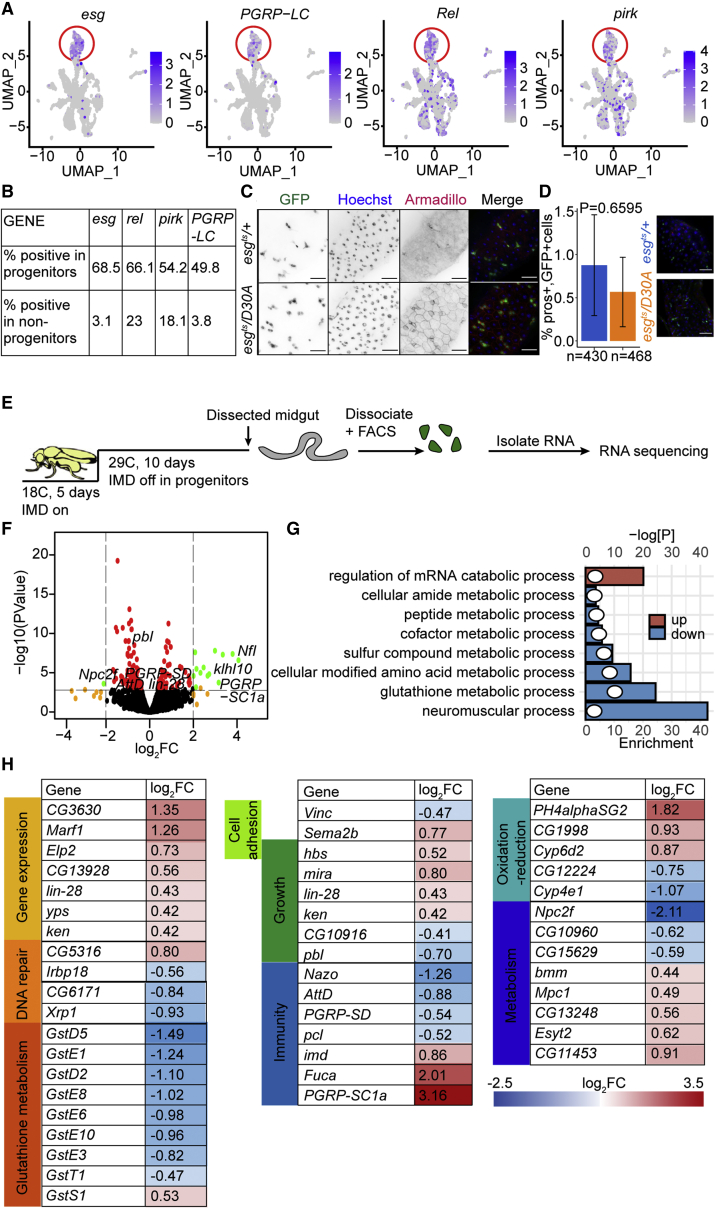
In a single-cell RNA sequencing profile of adult female Drosophila intestines, we identified 620 cells that expressed the progenitor-cell markers esg, Dl, and N (Figure S1). Within the progenitors, we also observed enriched expression of key IMD pathway components, including the PGN sensor pgrp-lc, the NF-kB transcription factor relish, and the IMD pathway target pirk (Figures 1A and 1B). Our data match earlier reports of IMD pathway gene expression in progenitors (Dutta et al., 2015; Hung et al., 2020) and raise the possibility that immune signals contribute to gut-progenitor-cell function.
Figure 1.
IMD regulates the intestinal progenitor-cell transcriptome
(A) Uniform manifold approximation and projection (UMAP) plot of intestinal epithelial cells isolated from 10-day-old esgts flies showing expression of the progenitor marker esg and the IMD pathway components PGRP-LC, Rel, and pirk. Progenitors are circled in red.
(B) Quantification of the percentage of progenitors and non-progenitors that express the indicated genes.
(C) Visualization of GFP, DNA (Hoechst), and the beta-catenin ortholog in intestines of 10-day-old esgts/+ and esgts/UAS-imdD30A (esgts/D30A) flies. Scale bars represent 25 μm.
(D) Quantification of the percentage of GFP-positive cells that express the enteroendocrine cell marker prospero in esgts/+ (n = 20) and esgts/D30A (n = 22) flies. esgts/+ (n = 430) and esgts/D30A (n = 468) n = number of GFP+ cells. Representative images are shown with DNA in blue, esg+ cells in green, and prospero-positive enteroendocrine cells in red. Significance measured using Student’s t test. Scale bars represent 25 μm.
(E) Schematic representation of an experimental strategy to quantify gene expression in progenitor cells purified from esgts/+ and esgts/D30A flies. Each experiment was performed in triplicate.
(F) Volcano plot showing relative changes (x axis) and significance (y axis) in gene expression of purified progenitors from esgts/D30A flies compared with age-matched esgts/+ controls. Genes are color-coded to indicate significance and relative gene expression changes.
(G) Gene Ontology analysis of processes significantly affected by inhibition of IMD in progenitors. Column size indicates the degree of enrichment for each term, and dots indicate the log-transformed significance of the respective enrichment.
(H) Representative sample of genes with affected expression upon inhibition of IMD in progenitors. See also Figures S1 and S2.
To test IMD activity in progenitors, we used the esgGAL4, GAL80ts, UASGFP (esgts) fly line to express a dominant inhibitory IMD protein (ImdD30A) in Drosophila progenitors (esgts/D30A) for 10 days. Blocking IMD in progenitors did not stop infection-mediated expression of IMD-responsive antimicrobial peptides in enterocytes, demonstrating that inhibition of progenitor-cell IMD does not affect IMD activity in differentiated progeny (Figure S2). We then asked if blocking IMD had direct effects on the progenitor population. Both esgts/D30A and control esgts/+ intestines had similar distributions of small, GFP-positive cells (Figure 1C) that rarely expressed the enteroendocrine cell marker prospero (Figure 1D), confirming that GFP exclusively marked progenitors in both lines. To measure effects of IMD on progenitors, we performed RNA sequencing (RNA-seq) analysis of gene expression in fluorescence-activated cell sorting (FACS)-purified GFP-positive cells from esgts/D30A and esgts/+ flies (Figure 1E). We found that inactivation of IMD disrupted expression of 154 genes in progenitors (Figure 1F; Table S1), including key IMD pathway regulators (pgrp-sd, pgrpsc1a), glutathione metabolism genes required for detoxification of xenobiotic substances, and 24 genes known to respond to the commensal microbiome (Broderick et al., 2014) (Figures 1F–1H). Notably, the impacts of IMD inhibition extended beyond conventional antimicrobial responses and included diminished expression of genes associated with stem-cell growth and adhesion to the niche, such as the growth regulator Xrp1, the asymmetric cell division regulator miranda (mira), and the effector of extracellular matrix adhesion Vinculin (Vinc; Figures 1F–1H), suggesting potential growth-regulatory roles for IMD in progenitors.
IMD modifies ISC division
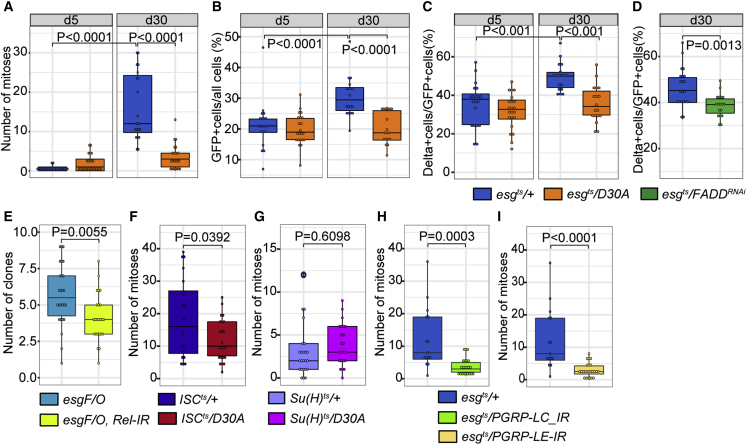
As IMD inhibition affected the progenitor transcriptome, we asked if IMD also affects progenitor homeostasis. Specifically, we measured ISC mitoses by quantifying phospho-histone H3, the percentage of midgut epithelial cells that expressed the progenitor marker esg, and the percentage of progenitors that expressed the ISC marker Delta (Dl) in 5- and 30-day-old esgts/D30A and esgts/+ intestines. In young esgts/+ intestines, we observed few ISC divisions (Figure 2A), approximately 20% esg+ cells in the posterior midgut (Figure 2B), and 40% Dl+ ISCs per progenitor (Figure 2C). Consistent with reports of age-related decline in gut function, we detected significantly increased numbers of ISC divisions, esg+ cells, and Dl+ cells in aged esgts/+ intestines relative to their 5-day-old counterparts (Figures 2A–2C). In contrast, we did not detect age-dependent increases in mitoses, progenitor numbers, or ISC numbers in 30-day-old esgts/D30A intestines (Figures 2A–2C). Instead, 30-day-old esgts/D30A intestines were characterized by significantly fewer mitoses, Dl+ ISCs, and progenitors than 30-day-old esgts/+ control flies. Importantly, these results are not an artifact of imdD30A expression, as progenitor-specific, RNAi-mediated depletion of the IMD pathway adaptor FADD caused a significant decline in the amounts of Dl-positive stem cells among intestinal progenitors of 30-day-old flies (Figure 2D). Furthermore, progenitor-specific inactivation of relish significantly impaired generation of mitotic clones in the posterior midgut (Figure 2E), confirming that genetic inhibition of IMD blocks intestinal epithelial proliferation. ISC-specific inactivation of IMD was sufficient to block proliferation (Figure 2F), whereas EB-specific inhibition of IMD had no effect (Figure 2G), suggesting a cell-autonomous role for IMD in controlling ISC division. Finally, we discovered that inhibition of the PGN sensors PGRP-LC or PGRP-LE (Figures 2H–2I) was sufficient to inhibit progenitor-cell proliferation. Collectively, our data indicate that inactivation of IMD in progenitors significantly impairs age-dependent accumulation of mitotically active progenitors in the adult midgut. We consider these findings particularly interesting as increased epithelial immune responses are a hallmark of the aging intestine (Broderick et al., 2014; Buchon et al., 2009a; Guo et al., 2014; Ren et al., 2007).
Figure 2.
Progenitor-cell IMD activity modifies stem-cell proliferation
(A) Quantification of mitoses per gut in intestines from esgts/+ and esgts/D30A flies of the indicated ages.
(B) Percentage of intestinal epithelial cells that express the progenitor marker esg in esgts/+ and esgts/D30A flies.
(C) Percentage of progenitors that express the stem-cell marker Delta in esgts/+ and esgts/D30A flies.
For (A)–(C), n = 20 at day 5 and 18 at day 30 in esgts/+ flies and n = 22 at day 5 and 18 at day 30 in esgts/D30A flies.
(D) Percentage of progenitors that express the stem-cell marker Delta in esgts/+ (n = 21) and esgts/FADDRNAi (n = 18) flies.
(E) Quantification of GFP-marked mitotic clones in the posterior midgut of esgF/O (n = 26) and esgF/O, rel-IR (n = 24) flies 9 days after marking of mitotic clones.
(F) Quantification of mitoses per gut in ISCts/+ ( n = 21) and ISCts/D30A (n = 24) 27-day-old flies.
(G) Quantification of mitoses per gut in 27-day-old Su(H)ts/+ (n = 25) and Su(H)ts/D30A (n = 24) flies.
(H and I) Quantification of mitoses per gut in 27-day-old esgts/+ (n = 15), esgts/PGRP-LC-IR (n=22) (H) and esgts/PGRP-LE-IR (n = 24) (I) flies.
Statistical significance for (A)–(C) was calculated using an ANOVA followed by pairwise Tukey comparisons, and significance for (D)– (I) was calculated using a Student's t test.
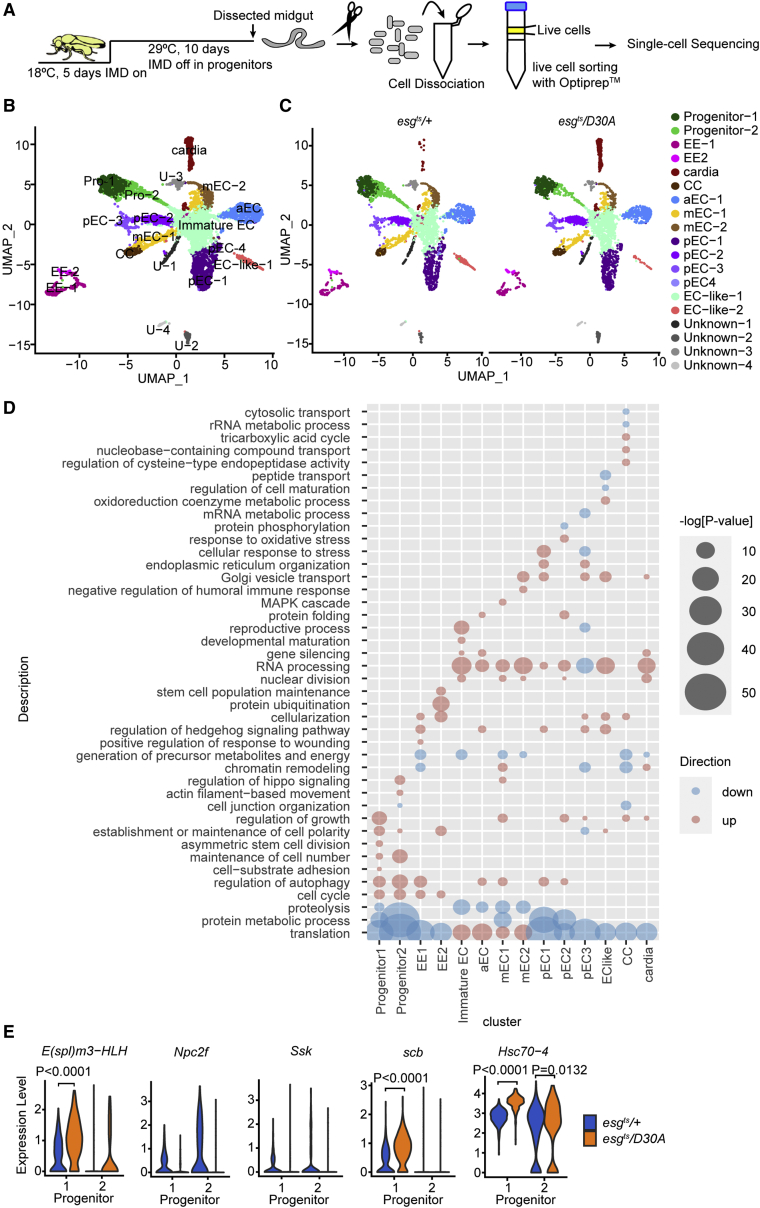
Single-cell analysis uncovers impacts of progenitor-cell IMD on the intestinal epithelium
Similar to vertebrates, the Drosophila intestine is a highly heterogenous tissue. Multipotent stem cells generate distinct epithelial lineages that control nutrient acquisition, hormone production, and responses to intestinal microbes in a regionally specialized fashion. Thus, although our data implicate IMD in progenitor-cell division, we do not yet understand the consequences of blocking IMD in progenitors for the entire intestine. To determine the effects of progenitor-specific IMD inhibition on all epithelial cell types, we resolved the transcriptomes of 10-day-old esgts/+ and esgts/D30A intestines at the single-cell level (Figures S3A and S3B). After excluding dead cells and doublets, we prepared RNA sequencing profiles of 3,675 cells from esgts/+ intestines and 3,654 cells from esgts/D30A intestines. Using unsupervised graph-based clustering of data from esgts/+ intestines, we identified all cell types previously described in the adult gut, including progenitors that expressed growth and differentiation regulators, enteroendocrine cells that produced peptide hormones, and enterocytes dedicated to digestion (Figure S3). A more detailed examination of single-cell transcriptomes from esgts/+ intestines uncovered clear signs of specialization among the individual cell types. Specifically, we discovered regionalized and cell-type-specific expression patterns for regulators of metabolism, growth, differentiation, PGN sensing and scavenging, and oxidative stress responses (Figure S4). Thus, our profile of esgts/+ guts accurately recapitulated known features of spatial and functional specialization within the fly gut (Dutta et al., 2015; Hung et al., 2020), providing a reliable control for analysis of intestines with impaired progenitor-cell IMD.
To determine if blocking IMD in progenitors affects mature epithelial cells, we used the integrated data analysis workflow in Seurat to identify cell-type-specific differences in gene-expression patterns between esgts/+ and esgts/D30A intestines. Unsupervised clustering of the integrated data resolved progenitors, enterocytes, and enteroendocrine cells, as well as cardia, copper cells, an enterocyte-like cluster, a cluster of immature enterocytes, and three lineages of unknown function (Figure 3B). Comparisons between the two genotypes suggested mild effects of blocking IMD in progenitors on the generation of mature IECs. For example, we noted fewer enterocyte (EC)-like cells and considerably more cardia in intestines from esgts/D30A than in esgts/+ flies (Figure 3B). Furthermore, blocking IMD in progenitors affected the expression of genes associated with critical regulatory functions in the gut. For example, intestines from esgts/D30A flies were characterized by shifts in RNA processing and translation in ECs, diminished precursor metabolite generation in enteroendocrine cells, and increased expression of genes involved in autophagy, cell polarity, and adhesion in progenitors (Figure 3C). Notably, blocking IMD in progenitors did not affect expression of antimicrobial peptides, or PGN recognition proteins, in differentiated ECs (Table S2), further arguing that expression of imdD30A in progenitor cells does not inhibit immune activity in progeny. Instead, we observed substantial effects of inhibiting IMD on expression of genes with essential roles in progenitor-cell division and polarity (Figure 3D), including the Notch signaling modifier Npc2f, the Notch pathway target E(spl)m3-HLH, and Snakeskin (Ssk) a key regulator of intestinal-stem-cell activity (Figure 3E). Thus, and consistent with data presented in Figures 1 and 2, our results indicate that inhibition of IMD in progenitors has significant effects on progenitor-cell homeostasis.
Figure 3.
Inactivating IMD in progenitors affects transcriptional activity in all intestinal epithelial cell types
(A) Schematic representation of an experimental strategy for single-cell transcriptomic analysis of purified intestinal epithelial cells from esgts/+ and esgts/D30A flies.
(B) UMAP plot visualizing cell types in integrated data from esgts/+ and esgts/D30A flies based on the expression of marker genes. Cells are color-coded by cell type.
(C) The same data from (B), split into the labeled genotypes. EE, enteroendocrine cells; CC, copper cells; EC, enterocytes subdivided according to anterior-posterior distribution along the intestine (a, anterior; m, middle; p, posterior).
(D) Gene Ontology term analysis of cell-type-specific processes significantly affected by progenitor-restricted inhibition of IMD. Bubble size indicates the log-transformed significance of the respective enrichments. Pink bubbles indicate enhanced terms, and blue bubbles indicate underrepresented terms.
(E) Representative violin plots of expression levels for the indicated genes in progenitors of esgts/+ and esgts/D30A flies. p values indicate significantly different expression levels. For Npc2f and Snakeskin (Ssk), no expression was observed in progenitors of esgts/D30A flies. See also Figures S3 and S4.
As we believe our gene expression data are likely of value to the community outside the scope of the current study, we have deposited both sets on the Broad Institute Single Cell Portal (see experimental procedures for further details).
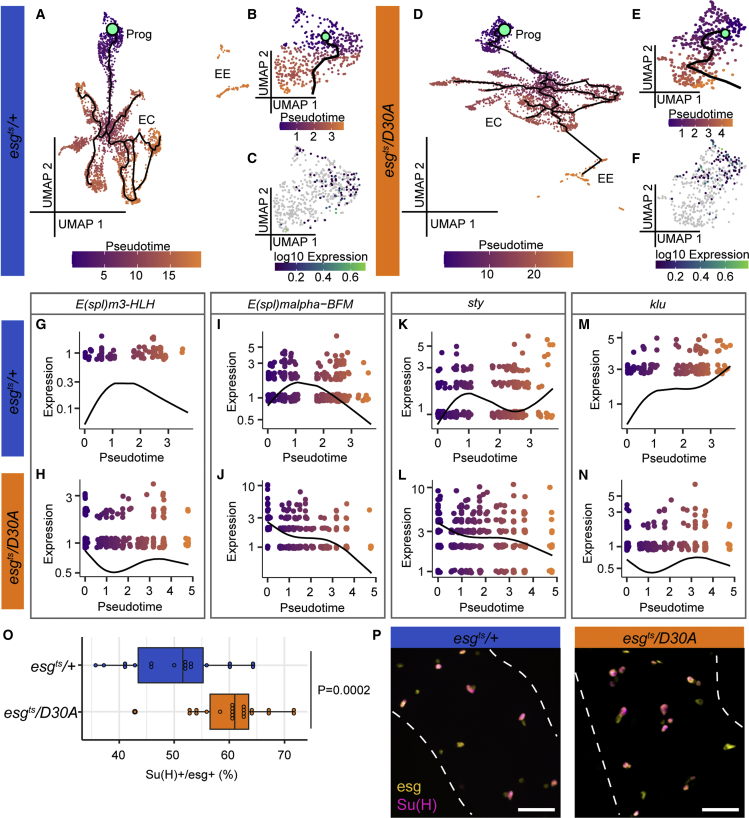
IMD affects developmental trajectories within the progenitor compartment
We were intrigued by our observation that blocking IMD in progenitors significantly affected expression of genes required for progenitor-niche interactions and progenitor differentiation. Therefore, we used Monocle to prepare pseudotime developmental trajectories for esgts/+ and esgts/D30A intestines. Analysis of the respective datasets successfully re-created developmental transitions from multipotent progenitors to differentiated lineages in both lines (Figures 4A and 4D). To directly examine effects of IMD on progenitors, we created subsets of the progenitor population for each genotype and analyzed gene expression in pseudotime for the respective subsets. Examination of the progenitor population from both genotypes revealed gene-expression patterns characteristic of developmental transitions along a pseudotime trajectory (Figures 4B and 4E). For example, both progenitor populations were characterized by expression of the ISC marker Dl in early stages of pseudotime (Figures 4C and 4F). However, IMD inhibition resulted in premature and prolonged pseudotime expression of the Notch targets E(spl)m3-HLH (Figures 4G and 4H) and E(spl)malpha-BFM (Figures 4I and 4J), the EGF regulator sprouty (sty; Figures 4K and 4L), the EC fate regulator klumpfuss (klu; Figures 4M and 4N), and numerous markers of EC maturation (Figure S5), indicating effects of progenitor-cell IMD on EC differentiation. To test if blocking IMD in progenitors impacts the transition from ISC to enteroblast, we monitored expression of fluorescent markers in esgGAL4, UAS-CFP, and Su(H)-GFP; GAL80ts flies that expressed ImdD30A. In these flies, ISCs are visible as CFP-positive cells (pseudocolored as yellow), and enteroblasts are visible as CFP and GFP double-positive cells (pseudocolored as magenta). Consistent with putative interactions between IMD and Notch, we found that blocking IMD significantly increased the percentage of progenitors that expressed the enteroblast marker Su(H)-GFP (Figures 4O and 4P). Thus, in agreement with the loss of ISCs noted in esgts/D30A intestines (Figure 2), our data argue that IMD activity influences progenitor-cell composition in the fly intestinal epithelium.
Figure 4.
Inhibition of IMD affects developmental trajectories within the progenitor compartment
Single-cell datasets from Seurat for each individual genotype were loaded into Monocle3, and pseudotime analysis was performed on midgut epithelial cells.
(A and D) esgts/+ midguts with wild-type progenitors (A) and esgts/D30A midguts with IMD-deficient progenitors (D). Mint green circles denote the root node and beginning of the intestinal trajectories. Dark purple marks cells at the beginning of pseudotime, while orange marks cells late in pseudotime. Black lines show trajectories. Prog, progenitors; EC, enterocytes; EE, enteroendocrine cells.
(B and E) Pseudotime within progenitor subsets of (A) and (D), respectively.
(C and F) Delta (Dl) expression patterns within esgts/+ progenitors (C) and esgts/D30A progenitors (F). Gray dots are cells with no detectable expression.
(G–N) Expression of Notch target genes E(spl)m3-HLH, E(spl)malpha-BFM, the EGF inhibitor sprouty (sty), and the EC fate regulator klumpfuss (klu) over pseudotime within progenitor subsets of the indicated genotypes.
(O) Percent of esg+ progenitors that are positive for the enteroblast marker Su(H)+ in esgts, UAS-CFP, Su(H)-GFP/+ (n = 18) and esgts, UAS-CFP, Su(H)-GFP /D30A (n = 22) posterior midguts 14 days after transgene expression. Significance found using Student’s t test.
(P) Representative images of intestines used to gather data for (O). Scale bars represent 25 μm. See also Figure S5.
Progenitor IMD affects generation of mature enteroendocrine cells
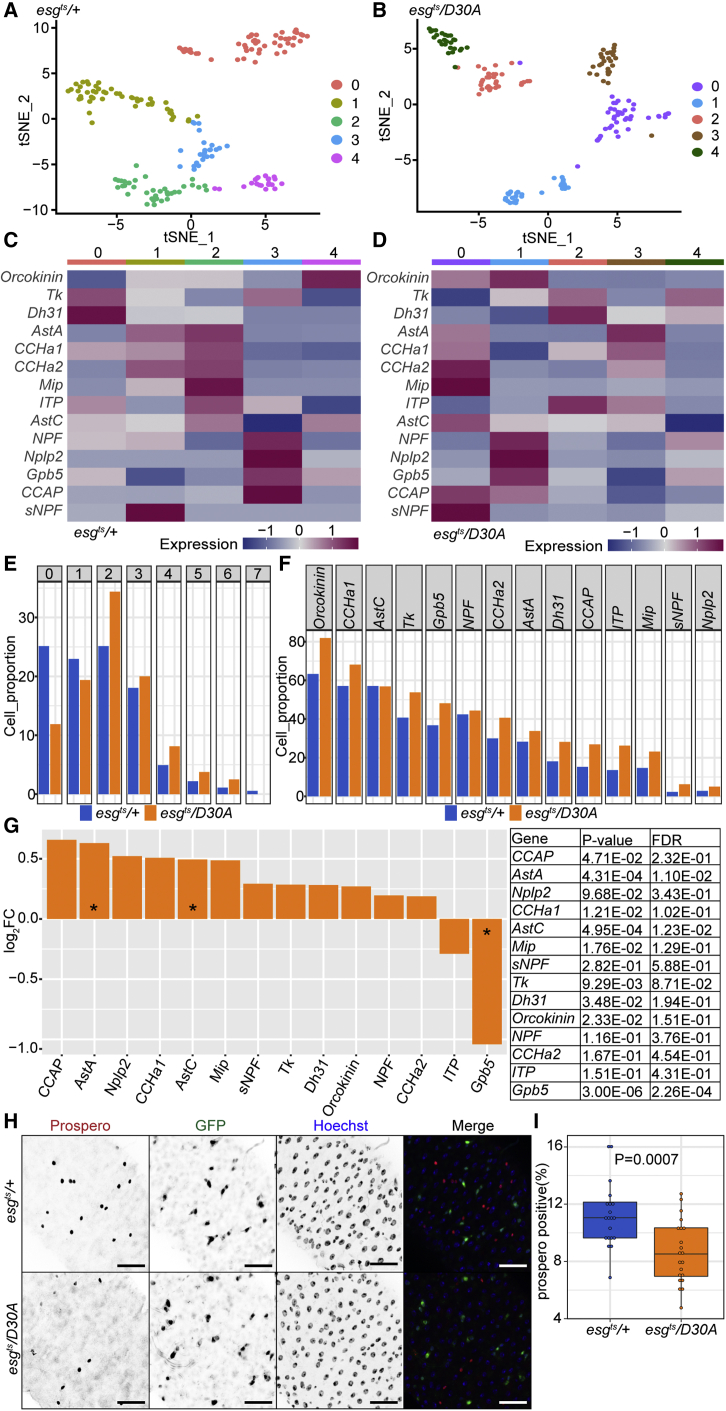
IMD inhibition impaired ISC proliferation, diminished ISC numbers, and impacted cell-type composition within the progenitor compartment, suggesting possible effects of progenitor-cell IMD on the development of mature epithelial cells. To determine if inhibition of IMD in progenitors affects epithelial differentiation, we monitored the prospero-positive enteroendocrine (EE) cell population in esgts/+ and esgts/D30A intestines. We focused on EE cells, as fly EE cells have been characterized to a single-cell resolution (Guo et al., 2019; Hung et al., 2020), permitting detailed comparisons between esgts/+ and esgts/D30A intestines. Drosophila EE cells can be divided into subsets with distinct peptide-hormone expression profiles that are stable during homeostasis or after recovery from infection (Beehler-Evans and Micchelli, 2015). Therefore, we tested if inhibition of IMD in progenitors affected the representation of EE subsets in the intestine. Using unsupervised clustering, we found that EE cells clustered into five subsets in both genotypes (Figures 5A and 5B). In both genotypes, each EE subset had a signature hormone-expression pattern (Figures 5C and 5D). In some cases, subset-restricted expression patterns were conserved between esgts/D30A and esgts/+ EE cells. For example, matching an earlier characterization of EE subsets (Beehler-Evans and Micchelli, 2015), we found that cells from esgts/+ subset zero, and from esgts/D30A subset two, expressed Tk and Dh31. Likewise, esgts/+ subset three cells and esgts/D30A subset one cells were characterized by enhanced expression of NPF and by partial expression of Gbp5 and CCAP. In contrast, we did not detect a counterpart of esgts/D30A subset zero cells in esgts/+ controls, and we saw minimal conservation of esgts/+ subset zero gene expression patterns in esgts/D30A EE cells, suggesting functional differences between EE cells in esgts/D30A flies compared with esgts/+ flies. When we classified EE cells based on the number of peptides they expressed, we noted further differences between esgts/+ and esgts/D30A intestines. In particular, fewer EE cells expressed zero or one peptide in esgts/D30A guts than in esgts/+ guts, and a greater proportion expressed two or more peptides (Figure 5E). Likewise, for thirteen of fourteen peptides examined, a greater percentage of esgts/D30A EE cells expressed the respective peptide than esgts/+ controls (Figure 5F), indicating enhanced peptide expression in esgts/D30A flies. To directly test the effects of blocking IMD in progenitors on peptide-expression levels, we performed an RNA-seq analysis of dissected midguts from esgts/D30A and esgts/+ flies. With the exceptions of ITP and Gpb5, we found that blocking IMD in progenitors resulted in increased expression of the remaining twelve peptides (Figure 5G), confirming a link between IMD inhibition and peptide-hormone expression. Finally, we quantified EE numbers in posterior midguts of esgts/+ and esgts/D30A flies. We found that inhibition of IMD in progenitors decreased the proportion of mature EE cells by roughly 20% relative to esgts/+ controls (Figure 5H). Combined, our results establish that inhibition of progenitor-cell IMD disrupts peptide-hormone expression patterns in mature EE cells, decreases the amount of total EE cells, and increases the expression of most peptide hormones, confirming a link between progenitor-cell IMD activity and EE-cell development.
Figure 5.
Inhibition of progenitor-cell IMD affects generation of mature enteroendocrine cells
(A and B) t-distributed stochastic neighbor embedding (tSNE) plot visualizing subsets of prospero-positive enteroendocrine cells in esgts/+ (A) and esgts/D30A intestines (B) based on the expression of marker genes. Cells are color-coded by cell subset.
(C and D) Heatmap showing relative expression of fourteen peptide hormones in each enteroendocrine cell subset in esgts/+ (C) and esgts/D30A (D) intestines.
(E) Quantification of the percentage of enteroendocrine cells that express the indicated numbers of peptide hormones. Genotypes are color-coded as indicated.
(F) Quantification of the percentage of enteroendocrine cells that express the indicated peptide hormone. Genotypes are color-coded as indicated.
(G) Quantification of the relative expression of each peptide hormone in isolated posterior midguts from esgts/D30A flies relative to esgts/+ flies based on bulk RNA-seq analysis.
(H) Visualization of Prospero, GFP, and DNA (Hoechst) in intestines of 10-day-old esgts/+ and esgts/D30A flies. Scale bars represent 25 μm.
(I) Quantification of the percentage of intestinal epithelial cells that express the enteroendocrine cell marker prospero in the intestines of 10-day-old flies as indicated. Statistical significance was calculated using Student’s t test. For esgts/+ flies n = 20, and for esgts/D30A flies n = 21.
Discussion
Notch, BMP, and WNT pathways regulate intestinal progenitor-cell growth and differentiation in vertebrates and invertebrates (Barker, 2014; Casali and Batlle, 2009; Miguel-Aliaga et al., 2018; Sancho et al., 2015; Vooijs et al., 2011; Xu et al., 2011). In contrast, it is less clear what effects progenitor-specific activation of germline-encoded immune responses has on epithelial homeostasis. Several studies indicated survival and growth-regulatory effects of host immunity on intestinal progenitors. For example, the PGN receptor NOD2 is enriched in ISCs of mice (Nigro et al., 2014) and protects ISCs from irradiation-induced cytotoxicity (Levy et al., 2020), while mutations in NOD2 are linked to Crohn’s disease (Hugot et al., 2001; Ogura et al., 2001). Likewise, TLR4 is expressed to higher levels in intestinal crypts (Price et al., 2018), where its activation promotes apoptosis and inhibits proliferation (Naito et al., 2017; Neal et al., 2012). In contrast, epithelium-wide activation of TLR4 promotes epithelial repair by activating EGF and JAK/STAT pathways in mice challenged with dextran sulfate sodium (Fukata et al., 2006; Hsu et al., 2010). These studies support roles for immune pathways in proliferative responses to extrinsic challenges. However, we do not know if progenitor-specific immune activity impacts homeostatic growth and differentiation. We consider this an important question, as intestines contain dense microbial communities that promote growth and influence cell-fate choices in intestinal epithelia (Ferguson and Foley, 2021).
We measured growth and differentiation in adult Drosophila midguts that we engineered to lack IMD activity in progenitors. The IMD pathway is highly similar to mammalian tumor necrosis factor receptor signaling, and IMD exerts broad regulatory effects on intestinal transcription (Broderick et al., 2014). In flies, IMD has context-dependent effects on ISC proliferation. IMD-pathway mutants have elevated rates of mitoses that are driven by the microbiome (Buchon et al., 2009b; Guo et al., 2014; Paredes et al., 2011), but IMD is not required for the proliferative burst observed after challenges with Ecc15 (Zhai et al., 2018). In contrast, infection with Vibrio cholerae blocks ISC proliferation in an IMD-dependent manner (Fast et al., 2020; Wang et al., 2013), whereas Herpetomonas muscarum induces IMD-dependent proliferation (Wang et al., 2019). In the absence of infection, persistent activation of IMD in progenitors increases ISC division frequency and skews differentiation towards elevated numbers of EE cells (Petkau et al., 2017). Similarly, overexpression of PGRP-LC in ECs induces Rel-dependent proliferation (Zhai et al., 2018). There are conflicting data on consequences of IMD inactivation in progenitors, with one study suggesting decreased proliferation (Wang et al., 2019), and a separate study indicating increased proliferation (Wang et al., 2013). We found that progenitor-specific inactivation of IMD diminished ISC proliferation, impaired age-dependent accumulation of esg-positive and Dl-positive progenitors, elevated the number of Su(H)-positive enteroblasts, and resulted in differentiation defects that included both fewer EE cells and shifts in gene-expression patterns associated with EC subtypes. Thus, our work suggests that progenitor-specific immunity contributes to epithelial homeostasis in flies and raises several questions about effects of progenitor-cell IMD on the adult gut.
Which progenitor cell requires IMD to regulate differentiation? The fly progenitor compartment consists of undifferentiated ISCs and post-mitotic enteroblasts that are committed to EC cell fate. Progenitors express IMD-pathway components, and genomic studies, including data presented here, show that ISCs and enteroblasts have highly similar gene-expression profiles (Dutta et al., 2015; Hung et al., 2020), suggesting that both cell types are likely equally competent at IMD activation. ISCs are basally situated within the midgut epithelium and are not expected to make frequent, direct contacts with the intestinal lumen. Enteroblasts are the apical daughters of ISC divisions that occur at oblique angles to the basement membrane. Thus, it seems more plausible that enteroblasts directly contact the lumen where they can detect PGN. However, it is important to note that gut-derived PGN is not strictly confined to the intestinal lumen in flies or vertebrates. PGN crosses the epithelial barrier, even in the absence of detectable breaches, and several mechanisms are in place to prevent accumulation of PGN in the fly hemolymph (Capo et al., 2017; Gendrin et al., 2009; Paredes et al., 2011; Troha et al., 2019; Zaidman-Rémy et al., 2006). Thus, we cannot exclude the possibility that passive, or active, transport mechanisms allow diffusion of PGN across the gut barrier to ISCs, which in turn activate IMD and modulate differentiation responses in the hosts. In this regard, we consider it interesting that several vertebrate pattern-recognition receptors have cell-type-specific, apicobasal distribution patterns. For instance, TLR4 is enriched apically in villi and basolaterally in crypts of the human colon (Fusunyan et al., 2001). Furthermore, apical stimulation of TLR9 promotes JNK activation, whereas basolateral stimulation of TLR9 leads to NF-kB activation, and IL-8 production (Lee et al., 2006). In future assays, it will be interesting to determine if basolateral detection of PGN influences fate choices within the fly progenitor compartment.
Is progenitor-specific IMD necessary to generate growth-regulatory ECs? Inhibition of progenitor-cell IMD did not block IMD-dependent immune responses in ECs, confirming that the phenotypes reported here are not a consequence of ImdD30A perdurance in differentiated epithelia. However, we also found that inhibition of IMD in progenitors had consequences for epithelial differentiation, including effects on gene-expression patterns within mature ECs. As ECs produce paracrine regulators of progenitor proliferation and differentiation, we cannot exclude the possibility that blocking IMD in progenitors disrupts enteroblast differentiation in a manner that modifies the ability of ECs to transduce growth and differentiation cues to progenitors. This may be particularly important in the context of epithelial damage, where secreted factors from dying ECs accelerate ISC proliferation to maintain the epithelial barrier and regenerate a mature gut. In this scenario, IMD activity in progenitors is important to establish homeostatic intercellular communications between ECs and the progenitor compartment, and loss of progenitor-cell IMD interrupts a developmental loop between progenitors and ECs. Consistent with requirements for IMD in the control of epithelial differentiation, we noticed that IMD in progenitors affected the generation of mature EE cells. Specifically, inhibition of progenitor-cell IMD led to a decline in EE numbers but a general increase in the expression of peptide hormones, possibly as a compensatory mechanism. Notably, flies raised in an axenic environment have fewer enteroblasts and more EE cells (Broderick et al., 2014), indicating non-overlapping contributions of progenitor-cell immunity and gut microbes to developmental trajectories of ISCs.
Does IMD have regional effects on intestinal progenitors? Intestines are functionally specialized along the rostro-caudal axis, with distinct partitions governing various aspects of food digestion and absorption. IMD also displays clear signs of regional specialization. The foregut is characterized by enriched expression of PGRP-LC, and Rel regulates expression of chitin-binding proteins that contribute to peritrophic matrix construction (Buchon et al., 2009b; Neyen et al., 2012). In the midgut, PGN detection primarily relies on PGRP-LE, particularly in the posterior midgut (Bosco-Drayon et al., 2012; Neyen et al., 2012). Activation of IMD in the anterior midgut results in expression of antimicrobial peptides that protect the fly from ingested microbes (Buchon et al., 2013b). In contrast, IMD activation leads to delamination of damaged cells in the midgut of flies infected with pathogenic bacteria, most notably in the R4 region of the posterior midgut (Zhai et al., 2018). In the posterior midgut, the transcription factor caudal prevents IMD-dependent antimicrobial peptide expression (Ryu et al., 2008). Instead, IMD induces expression of molecules that dampen immune signaling, including the PGRP-LC inhibitor pirk, and amidases that scavenge PGN (Bosco-Drayon et al., 2012). As a result, posterior midgut IMD establishes a tolerogenic environment for commensal bacteria. Notably, loss of IMD pathway inhibitors or expression of PGRP-LC in ECs, increases proliferation in the posterior midgut (Paredes et al., 2011; Zhai et al., 2018), raising the possibility that suppression of posterior midgut IMD activity is required to prevent excess proliferation in the absence of infection. With a large collection of genetic reagents, and accessible genomic methods, the fly is an excellent system to systematically characterize regional effects of immune responses on progenitor-cell function. Given the evolutionary conservation of immune responses, we believe the findings reported in this study to be of relevance for understanding fundamental principles of immune-regulated intestinal homeostasis.
Experimental procedures
Fly husbandry
Flies were raised on corn meal medium (Nutri-Fly Bloomington Formulation, https://bdsc.indiana.edu/information/recipes/bloomfood.html; Genesse Scientific) at 18°C or 25°C. All experimental flies were adult virgin females kept under a 12h:12h light:dark cycle and maintained at 18°C during collection then shifted to 29°C to express downstream genes as indicated. We used w1118 as a wild-type strain, backcrossed UAS-imdD30A transgenic lines into the w1118 background for eight generations prior to use, and used standard husbandry methods to ensure that esgts (esg-GAL4, tub-GAL80ts, UAS-GFP) flies had the same first and third chromosomes as our w1118 line. Fly lines used in this study were: w;esg-GAL4,tubGAL80ts,UAS-GFP (referred to as esgts); UAS-FADDRNAi (VDRC ID# 7926); w1118 (VDRC ID# 60000); w;esg-GAL4,UAS-CFP, Su(H)-GFP;tubGal80ts (esgts,UAS-CFP,Su(H)-GFP); GS 5961 (Mathur et al., 2010); dpt-GFP, esg-GAL4, tubGAL80ts, UAS-GFP;UAS-flp, Act>CD2>GAL4 (referred to as esgF/O); Esg[ts], Su(H) Gal80 (referred to as ISCts); Su(H)GBE-Gal4ts (referred to as Su(H)ts); PGRP-LE RNAi (VDRC ID# 108199); PGRP-LC RNAi (VDRC ID# 101636); Rel-RNAi (VDRC ID# 49413); and 40D-UAS (control for VDRC KK lines, VDRC ID# 60101) . To induce GFP-marked mitotic clones using the esgF/O system, flies of the indicated genotype were raised at 18°C for 3 days after eclosion, shifted to 29°C for 16 h, then raised at 25°C for an additional 9 days.
Data availability
The accession number for the gene expression data reported in this paper is GEO: SuperSeries GSE141897 (GSE171001 and GSE141896). The accession number for the Single cell gene expression data reported in this paper is Broad Institute Single Cell Portal: for esgts/+ flies (https://singlecell.broadinstitute.org/single_cell/study/SCP1696/single-cell-expression-data-for-d-melanogasterwild-type-intestines) and for esgts/D30A flies (https://singlecell.broadinstitute.org/single_cell/study/SCP1699/single-cell-expression-data-for-d-melanogasterintestines-with-immune-deficient-progenitor-cells#study-summary).
Author contributions
Conceptualization: M.S. and E.F.; methodology: M.S. and E.F.; formal analysis: M.S., M.F., and E.F.; investigation: M.S., M.F., R.J.W., L.O.J., and K.P.; data curation: M.S. and M.F.; writing – original draft: M.S. and E.F.; writing – review & editing: M.S., M.F., and E.F.; visualization: M.S. and M.F.; supervision: E.F.; project administration: E.F.; funding acquisition: E.F.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
Drosophila lines were provided by Drs. Shelagh Campbell, Lucy O’Brien, Bruce Edgar, Heinrich Jasper, and Bruno Lemaitre. We acknowledge microscopy support from Dr. Steven Ogg and Gregory Plummer at the Faculty of Medicine and Dentistry Imaging core, flow cytometry support from Dr. Aja Rieger at the Faculty of Medicine and Dentistry Flow Cytometry Core, and support with single-cell library preparation from Dr. Joaquin Lopez-Orozco. We are grateful to Dr. David Fast for assistance with analysis of the RNA-seq data. The authors wish to thank Kin Chan at the Network Biology Collaborative Centre for the RNA-seq service. Network Biology Collaborative Centre is a facility supported by Canada Foundation for Innovation, the Ontarian government, and Genome Canada and Ontario Genomics (OGI-139). This work was supported by a grant from the Canadian Institute of Health Research (grant no. PJT 159604). M.S. was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A3A0303955511). M.F. has funding through Alberta Innovates Graduate Student Scholarships and an NSERC PGS-D.
Published: March 17, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.02.009.
Supplemental information
References
- Bardin A.J., Perdigoto C.N., Southall T.D., Brand A.H., Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- Beehler-Evans R., Micchelli C.A. Generation of enteroendocrine cell diversity in midgut stem cell lineages. Dev. Camb. Engl. 2015;142:654–664. doi: 10.1242/dev.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B., Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 2014;7:1867–1875. doi: 10.1016/j.celrep.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton M., Haber A.L., Rogel N., Burgin G., Beyaz S., Schnell A., Ashenberg O., Su C.-W., Smillie C., Shekhar K., et al. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell. 2018;175:1307–1320.e22. doi: 10.1016/j.cell.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco-Drayon V., Poidevin M., Boneca I.G., Narbonne-Reveau K., Royet J., Charroux B. Peptidoglycan Sensing by the Receptor PGRP-LE in the Drosophila Gut Induces Immune Responses to Infectious Bacteria and Tolerance to Microbiota. Cell Host Microbe. 2012;12:153–165. doi: 10.1016/j.chom.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Broderick N.A., Buchon N., Lemaitre B. Microbiota-induced changes in drosophila melanogaster host gene expression and gut morphology. mBio. 2014;5 doi: 10.1128/mBio.01117-14. e01117–01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016;64:82–92. doi: 10.1016/j.dci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Buchon N., Broderick N.A., Chakrabarti S., Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Broderick N.A., Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat. Rev. Microbiol. 2013;11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- Buchon N., Broderick N.A., Poidevin M., Pradervand S., Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Buchon N., Osman D., David F.P.A., Fang H.Y., Boquete J.-P., Deplancke B., Lemaitre B. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 2013;3:1725–1738. doi: 10.1016/j.celrep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Capo F., Chaduli D., Viallat-Lieutaud A., Charroux B., Royet J. Oligopeptide Transporters of the SLC15 Family Are Dispensable for Peptidoglycan Sensing and Transport in Drosophila. J. Innate Immun. 2017;9:483–492. doi: 10.1159/000475771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali A., Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Couturier-Maillard A., Secher T., Rehman A., Normand S., Arcangelis A.D., Haesler R., Huot L., Grandjean T., Bressenot A., Delanoye-Crespin A., et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Invest. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D., Dobson A.J., Houtz P.L., Gläßer C., Revah J., Korzelius J., Patel P.H., Edgar B.A., Buchon N. Regional Cell-Specific Transcriptome Mapping Reveals Regulatory Complexity in the Adult Drosophila Midgut. Cell Rep. 2015;12:346–358. doi: 10.1016/j.celrep.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Fast D., Petkau K., Ferguson M., Shin M., Galenza A., Kostiuk B., Pukatzki S., Foley E. Vibrio cholerae-Symbiont Interactions Inhibit Intestinal Repair in Drosophila. Cell Rep. 2020;30:1088–1100.e5. doi: 10.1016/j.celrep.2019.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M., Foley E. Microbial recognition regulates intestinal epithelial growth in homeostasis and disease. FEBS J. 2021 doi: 10.1111/febs.15910. [DOI] [PubMed] [Google Scholar]
- Fu Y.-Y., Egorova A., Sobieski C., Kuttiyara J., Calafiore M., Takashima S., Clevers H., Hanash A.M. T Cell Recruitment to the Intestinal Stem Cell Compartment Drives Immune-Mediated Intestinal Damage after Allogeneic Transplantation. Immunity. 2019;51:90–103.e3. doi: 10.1016/j.immuni.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Chen A., Klepper A., Krishnareddy S., Vamadevan A.S., Thomas L.S., Xu R., Inoue H., Arditi M., Dannenberg A.J., et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusunyan R.D., Nanthakumar N.N., Baldeon M.E., Walker W.A. Evidence for an Innate Immune Response in the Immature Human Intestine: Toll-Like Receptors on Fetal Enterocytes. Pediatr. Res. 2001;49:589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- Gendrin M., Welchman D.P., Poidevin M., Hervé M., Lemaitre B. Long-Range Activation of Systemic Immunity through Peptidoglycan Diffusion in Drosophila. PLOS Pathog. 2009;5:e1000694. doi: 10.1371/journal.ppat.1000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Karpac J., Tran S.L., Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156:109–122. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Yin C., Yang F., Zhang Y., Huang H., Wang J., Deng B., Cai T., Rao Y., Xi R. The Cellular Diversity and Transcription Factor Code of Drosophila Enteroendocrine Cells. Cell Rep. 2019;29:4172–4185.e5. doi: 10.1016/j.celrep.2019.11.048. [DOI] [PubMed] [Google Scholar]
- Guo Z., Ohlstein B. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science. 2015;350 doi: 10.1126/science.aab0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D., Fukata M., Hernandez Y.G., Sotolongo J.P., Goo T., Maki J., Hayes L.A., Ungaro R.C., Chen A., Breglio K.J., et al. Toll-like receptor 4 differentially regulates epidermal growth factor-related growth factors in response to intestinal mucosal injury. Lab. Investig. J. Tech. Methods Pathol. 2010;90:1295–1305. doi: 10.1038/labinvest.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot J.P., Chamaillard M., Zouali H., Lesage S., Cézard J.P., Belaiche J., Almer S., Tysk C., O’Morain C.A., Gassull M., et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Hung R.-J., Hu Y., Kirchner R., Liu Y., Xu C., Comjean A., Tattikota S.G., Li F., Song W., Sui S.H., et al. A cell atlas of the adult Drosophila midgut. Proc. Natl. Acad. Sci. 2020;117:1514–1523. doi: 10.1073/pnas.1916820117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamareddine L., Robins W.P., Berkey C.D., Mekalanos J.J., Watnick P.I. The Drosophila Immune Deficiency Pathway Modulates Enteroendocrine Function and Host Metabolism. Cell Metab. 2018;28:449–462.e5. doi: 10.1016/j.cmet.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.-H., Paik D., Rus F., Silverman N. The caspase-8 homolog Dredd cleaves Imd and Relish but is not inhibited by p35. J. Biol. Chem. 2014;289:20092–20101. doi: 10.1074/jbc.M113.544841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Mo J.-H., Katakura K., Alkalay I., Rucker A.N., Liu Y.-T., Lee H.-K., Shen C., Cojocaru G., Shenouda S., et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat. Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- Leulier F., Rodriguez A., Khush R.S., Abrams J.M., Lemaitre B. The Drosophila caspase Dredd is required to resist gram-negative bacterial infection. EMBO Rep. 2000;1:353–358. doi: 10.1093/embo-reports/kvd073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A., Stedman A., Deutsch E., Donnadieu F., Virgin H.W., Sansonetti P.J., Nigro G. Innate immune receptor NOD2 mediates LGR5+ intestinal stem cell protection against ROS cytotoxicity via mitophagy stimulation. Proc. Natl. Acad. Sci. 2020;117:1994–2003. doi: 10.1073/pnas.1902788117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickwar C.R., Camp J.G., Weiser M., Cocchiaro J.L., Kingsley D.M., Furey T.S., Sheikh S.Z., Rawls J.F. Genomic dissection of conserved transcriptional regulation in intestinal epithelial cells. PLoS Biol. 2017;15:e2002054. doi: 10.1371/journal.pbio.2002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli C.A., Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Miguel-Aliaga I., Jasper H., Lemaitre B. Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics. 2018;210:357–396. doi: 10.1534/genetics.118.300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllymäki H., Valanne S., Rämet M. The Drosophila Imd Signaling Pathway. J. Immunol. 2014;192:3455–3462. doi: 10.4049/jimmunol.1303309. [DOI] [PubMed] [Google Scholar]
- Naito T., Mulet C., De Castro C., Molinaro A., Saffarian A., Nigro G., Bérard M., Clerc M., Pedersen A.B., Sansonetti P.J., et al. Lipopolysaccharide from Crypt-Specific Core Microbiota Modulates the Colonic Epithelial Proliferation-to-Differentiation Balance. mBio. 2017;8 doi: 10.1128/mBio.01680-17. e01680–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M.D., Sodhi C.P., Jia H., Dyer M., Egan C.E., Yazji I., Good M., Afrazi A., Marino R., Slagle D., et al. Toll-like Receptor 4 Is Expressed on Intestinal Stem Cells and Regulates Their Proliferation and Apoptosis via the p53 Up-regulated Modulator of Apoptosis. J. Biol. Chem. 2012;287:37296–37308. doi: 10.1074/jbc.M112.375881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyen C., Poidevin M., Roussel A., Lemaitre B. Tissue- and ligand-specific sensing of gram-negative infection in drosophila by PGRP-LC isoforms and PGRP-LE. J. Immunol. 2012;189:1886–1897. doi: 10.4049/jimmunol.1201022. [DOI] [PubMed] [Google Scholar]
- Nguyen T.L.A., Vieira-Silva S., Liston A., Raes J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro G., Rossi R., Commere P.-H., Jay P., Sansonetti P.J. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15:792–798. doi: 10.1016/j.chom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Ogura Y., Bonen D.K., Inohara N., Nicolae D.L., Chen F.F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R.H., et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Paquette N., Broemer M., Aggarwal K., Chen L., Husson M., Ertürk-Hasdemir D., Reichhart J.-M., Meier P., Silverman N. Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-kappaB signaling. Mol. Cell. 2010;37:172–182. doi: 10.1016/j.molcel.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes J.C., Welchman D.P., Poidevin M., Lemaitre B. Negative Regulation by Amidase PGRPs Shapes the Drosophila Antibacterial Response and Protects the Fly from Innocuous Infection. Immunity. 2011;35:770–779. doi: 10.1016/j.immuni.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Petkau K., Ferguson M., Guntermann S., Foley E. Constitutive Immune Activity Promotes Tumorigenesis in Drosophila Intestinal Progenitor Cells. Cell Rep. 2017;20:1784–1793. doi: 10.1016/j.celrep.2017.07.078. [DOI] [PubMed] [Google Scholar]
- Price A.E., Shamardani K., Lugo K.A., Deguine J., Roberts A.W., Lee B.L., Barton G.M. A Map of Toll-like Receptor Expression in the Intestinal Epithelium Reveals Distinct Spatial, Cell Type-Specific, and Temporal Patterns. Immunity. 2018;49:560–575.e6. doi: 10.1016/j.immuni.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C., Webster P., Finkel S.E., Tower J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007;6:144–152. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Ryu J.-H., Kim S.-H., Lee H.-Y., Bai J.Y., Nam Y.-D., Bae J.-W., Lee D.G., Shin S.C., Ha E.-M., Lee W.-J. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- Sancho R., Cremona C.A., Behrens A. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep. 2015;16:571–581. doi: 10.15252/embr.201540188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spit M., Koo B.-K., Maurice M.M. Tales from the crypt: intestinal niche signals in tissue renewal, plasticity and cancer. Open Biol. 2018;8:180120. doi: 10.1098/rsob.180120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöven S., Ando I., Kadalayil L., Engström Y., Hultmark D. Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage. EMBO Rep. 2000;1:347–352. doi: 10.1093/embo-reports/kvd072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoven S., Silverman N., Junell A., Hedengren-Olcott M., Erturk D., Engstrom Y., Maniatis T., Hultmark D. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc. Natl. Acad. Sci. U S A. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S., Martin M.L., Jansen S.A., Fu Y., Bos J., Chandra D., O’Connor M.H., Mertelsmann A.M., Vinci P., Kuttiyara J., et al. T cell-derived interferon-γ programs stem cell death in immune-mediated intestinal damage. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aay8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troha K., Nagy P., Pivovar A., Lazzaro B.P., Hartley P.S., Buchon N. Nephrocytes Remove Microbiota-Derived Peptidoglycan from Systemic Circulation to Maintain Immune Homeostasis. Immunity. 2019;51:625–637.e3. doi: 10.1016/j.immuni.2019.08.020. [DOI] [PubMed] [Google Scholar]
- Vooijs M., Liu Z., Kopan R. Notch: architect, landscaper, and guardian of the intestine. Gastroenterology. 2011;141:448–459. doi: 10.1053/j.gastro.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace K.N., Akhter S., Smith E.M., Lorent K., Pack M. Intestinal growth and differentiation in zebrafish. Mech. Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Wang L., Sloan M.A., Ligoxygakis P. Intestinal NF-κB and STAT signalling is important for uptake and clearance in a Drosophila-Herpetomonas interaction model. PLOS Genet. 2019;15:e1007931. doi: 10.1371/journal.pgen.1007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Hang S., Purdy A.E., Watnick P.I. Mutations in the IMD Pathway and Mustard Counter Vibrio cholerae Suppression of Intestinal Stem Cell Division in Drosophila. mBio. 2013;4 doi: 10.1128/mBio.00337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Wang S.Q., Tan D., Gao Y., Lin G., Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev. Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Zaidman-Rémy A., Hervé M., Poidevin M., Pili-Floury S., Kim M.-S., Blanot D., Oh B.-H., Ueda R., Mengin-Lecreulx D., Lemaitre B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Zeng X., Hou S.X. Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development. 2015;142:644–653. doi: 10.1242/dev.113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z., Boquete J.-P., Lemaitre B. Cell-Specific Imd-NF-κB Responses Enable Simultaneous Antibacterial Immunity and Intestinal Epithelial Cell Shedding upon Bacterial Infection. Immunity. 2018;48:897–910.e7. doi: 10.1016/j.immuni.2018.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the gene expression data reported in this paper is GEO: SuperSeries GSE141897 (GSE171001 and GSE141896). The accession number for the Single cell gene expression data reported in this paper is Broad Institute Single Cell Portal: for esgts/+ flies (https://singlecell.broadinstitute.org/single_cell/study/SCP1696/single-cell-expression-data-for-d-melanogasterwild-type-intestines) and for esgts/D30A flies (https://singlecell.broadinstitute.org/single_cell/study/SCP1699/single-cell-expression-data-for-d-melanogasterintestines-with-immune-deficient-progenitor-cells#study-summary).