Abstract
Allergic fungal rhinosinusitis (AFRS) is a highly resistant disease and is challenging to treat. Patients with recurrent attacks of the disease despite surgical management can benefit from biologics as adjunct therapies. Dupilumab has shown promising endpoints in patients with chronic rhinosinusitis with nasal polyposis (CRSwNP). This case series reports 4 patients with resistant AFRS concomitant with asthma, for which dupilumab therapy was administered. Long-term follow-ups showed that dupilumab improved the symptoms and improved the results of objective tools such as imaging and pulmonary function test.
Keywords: Biological therapies, Hypersensitivity, Nasal polyp, Sinusitis, Saudi Arabia
Key Points:
-
1.
Dupilumab is potentially effective in patients with allergic fungal rhinosinusitis. Both clinical evaluation and radiological assessment results appear to be promising in patients receiving dupilumab.
-
2.
In patients with allergic fungal rhinosinusitis and concomitant asthma, dupilumab has been found to improve asthma monitoring parameters.
-
3.
Only a few minor side effects pertaining to the use of dupilumab have been reported in the literature.
Introduction
Chronic rhinosinusitis (CRS) is classified into chronic rhinosinusitis with nasal polyposis (CRSwNP) and without nasal polyposis (CRSsNP). Allergic fungal rhinosinusitis (AFRS) is an allergic response characterized by type 2 inflammatory-mediated reactions to a colonizing fungus in the sinus that is not associated with tissue invasion.1 AFRS is a widely common and devastating disease accounting for up to 32% of CRS cases undergoing functional endoscopic sinus surgery (FESS).2,3 Patients with AFRS presenting with nasal polyps have a higher recurrence rate of polyps after surgery. In Saudi Arabia, a study estimated that 54.5% of AFRS patients had a recurrence of the disease after surgery.4 AFRS is a distinguishable form of CRSwNP and is characterized by primary localized disease with non-invasive fungal hyphae, which causes immunoglobulin E (IgE)-mediated mucosal hypersensitivity resulting in the formation of eosinophilic mucin5. Unlike other phenotypes of CRSwNP, studies showed that the IgE level is very high in AFRS.6,7
Although classical CRS can be treated medically, AFRS is highly resistant and surgery is the mainstay of treatment.5 It involves surgical debridement of the affected sinuses to remove the antigenic stimulation of AFRS and provide access for topical medical treatments. Corticosteroid use proved to decrease recurrence after surgery. Treatment with antifungals demonstrates some benefits. Other pharmacological agents, including allergen immunotherapy, have less evidence for their effectiveness.5
Biologics, as steroid-sparing agents, have recently become an emerging therapy and established a new era of treatment options for CRSwNP. Omalizumab, a recombinant humanized monoclonal antibody directed against IgE, is the first biologic approved for use in asthma. It has shown great efficacy in moderate-to-severe uncontrolled asthma and chronic spontaneous urticaria unresponsive to H1-antihistamine treatment.8,9 For the AFRS subtype, few cases in the literature received omalizumab to treat AFRS refractory to surgery and oral corticosteroids.10,11 The majority experienced a significant reduction in the Sino-Nasal Outcome Test-22 (SNOT-22) and polyp score. Dupilumab, a fully human monoclonal antibody to the interleukin 4 (IL-4) receptor a subunit, is effective in type 2 inflammatory-mediated diseases such as asthma and atopic dermatitis.12,13 It also has demonstrated clinical efficacy in CRSwNP.14
There is a paucity of evidence in the literature for the treatment of refractory AFRS with biologics. Most findings on the treatment of AFRS considered the CRSwNP population; therefore, it appears difficult to make a unified conclusion for the effectiveness of biologics in AFRS.5 There are very few numbers of exclusive cases reported in the literature concerning AFRS outcomes after dupilumab administration.15,16 In this report, the authors aimed to discuss 4 AFRS cases that had received dupilumab and resulted in favorable endpoints.
Case series
Case 1
A 29-year-old male with a long-standing history of bronchial asthma experienced symptoms of nasal congestion, rhinorrhea, sneezing, coughing, postnasal drip and itchy eyes and nose for 4 years. His quality of life was affected, and these symptoms caused poor and interrupted sleeping at night. He denied any history of atopic dermatitis, urticaria, or food or drug allergies. He reported a significant family history of allergy. The patient had a history of FESS with polyp removal (twice). Upon initial encounter, SNOT-22 score was 65, and Asthma Control Test (ACT) score was 16. Forced expiratory volume in 1 second (FEV1) was 69% predicted, and FEV1/Forced Vital Capacity (FVC) was 70% predicted. The specific IgE test was positive for cockroaches, Penicillium notatum (0.43 kU/L), and Aspergillus Fumigatus (0.43 kU/L). The allergy skin test showed a positive response to Aspergillus Fumigatus (5 mm × 5 mm). Computed Tomography (CT) of the sinuses revealed double-density sign, suggesting AFRS (Table 1). The disease was refractory despite using intranasal corticosteroid, nasal irrigation, and nasal decongestant. However, the asthma symptoms were controlled using inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA). Subsequently, dupilumab 300 mg (biweekly) was given over 5 months. The patient endorsed a dramatic improvement in his rhinosinusitis and asthma symptoms. On a follow-up encounter, SNOT-22 score improved to 3, and ACT score reached 22. The eosinophil count initially was 330 cells/μl and became approximately 1500 cells/μl after initiating the biologic. A rinoscopy examination showed clear sinuses and no signs of disease recurrence. No side effects were reported during the follow-up period. Informed consent for publishing the case was obtained from the patient in advance.
Table 1.
Characteristics of the reported cases fulfilling the diagnostic criteria of AFRS.
| Diagnostic criteria of AFRS26 | Case 1 | Case 2 | Case 3 | Case 4 | ||
|---|---|---|---|---|---|---|
| Symptoms (requires one or more): | Anterior and/or posterior nasal drainage | Present | Present | Present | Present | |
| Nasal obstruction | Present | Present | Present | Present | ||
| Decreased sense of smell | Present | Present | Present | Present | ||
| Facial pain-pressure-fullness | Present | Present | Present | Present | ||
| Objective findings (requires all): | Presence of allergic mucin (fungal hyphae with degranulating eosinophils) | Present | Present | Present | Present | |
| Evidence of fungal specific IgE (skin test of in vitro test) | Aspergillus Fumigatus: IgE (0.43 kU/L); Skin test (5 mm × 5 mm) | Aspergillus Fumigatus: IgE (8.62 kU/L); Skin test (N/A) | Aspergillus Fumigatus: IgE (N/A); Skin test (8 mm × 11 mm) | Aspergillus Fumigatus: IgE (N/A); Skin test (15 mm × 10 mm) | ||
| Penicillium notatum: IgE (0.43 kU/L); Skin test (N/A) | Cladiosporum: IgE (N/A); Skin test (5 mm × 5 mm) | Altenaria: IgE (N/A); Skin test (9 mm × 10 mm) | ||||
| Cladiosporum: IgE (N/A); Skin test (7 mm × 8 mm) | ||||||
| No histologic evidence of invasive fungal disease | No evidence of invasive fungal disease | No evidence of invasive fungal disease | No evidence of invasive fungal disease | No evidence of invasive fungal disease | ||
| Radiographic Findings (highly recommended): | Sinus CT demonstrating:
|
Diffuse obliteration of lateral, maxillary, ethmoidal sinuses, and sphenoethmoidal recess. Erosion of posterolateral wall of left maxillary sinus | Bilateral double-density sign in maxillary sinuses with obliteration of ethmoidal sinus and sphenoethmoidal recess | Diffuse opacification of right maxillary sinus with double-density sign | Mucosal thickening with double-density opacification involving both maxillary antra, ethmoid and sphenoid sinuses, and right frontal sinus | |
| Other diagnostic Measures (possible, but not required): | Fungal culture | No growth | No growth | Aspergillus flavus | No growth | |
| Total serum IgE | 702 kU/L | 1051 kU/L | 437 kU/L | 653 kU/L | ||
AFRS: Allergic Fungal Rhinosinusitis; IgE: Immunoglobulin E; N/A: not available
Case 2
A 31-year-old male with a history of childhood asthma and CRSwNP complained of nasal congestion, sneezing, anosmia, headache, and postnasal drip for the last 8 years. The patient endorsed a temporary resolution of asthma symptoms during puberty with return of illness after he started experiencing nasal symptoms. The patient had a severe systemic allergic reaction to shellfish as well. He underwent FESS with polyp removal (5 times and required 5–6 courses of systemic steroids per year. Initially, SNOT-22 and ACT scores were 64 and 14, respectively. FEV1 was 65% predicted, and FEV1/FVC was 82% predicted. The serum eosinophil counts ranged between 500 and 1200 (cells/μL). The specific IgE test was positive to D. farina (3.12 kU/L), D. pteronyssinus (2.56 kU/L), and Aspergillus Fumigatus (8.62 kU/L). A sinus CT revealed double-density sign, suggesting AFRS. The disease was refractory despite using maximum medical therapy, including intranasal corticosteroid and nasal irrigation. The asthma symptoms were partially controlled with the use of a high-dose combination of ICS and LABA. Given the disease phenotype, dupilumab was started. The patient received an initial loading dose of 600 mg then 300 mg biweekly. Six months later, the patient reported a dramatic improvement in the rhinosinusitis and asthma symptoms. Upon a follow-up encounter, SNOT-22 score improved to 7, and ACT score reached 21. The eosinophil counts went down to 100 (cells/μl). A rhinoscopy examination showed clear sinuses and no signs of disease recurrence (Fig. 1). The patient reported no side effects during the follow-up period, and he was off systemic steroids since the dupilumab treatment started. The patient provided informed consent for publishing his case presentation, including his treatment response.
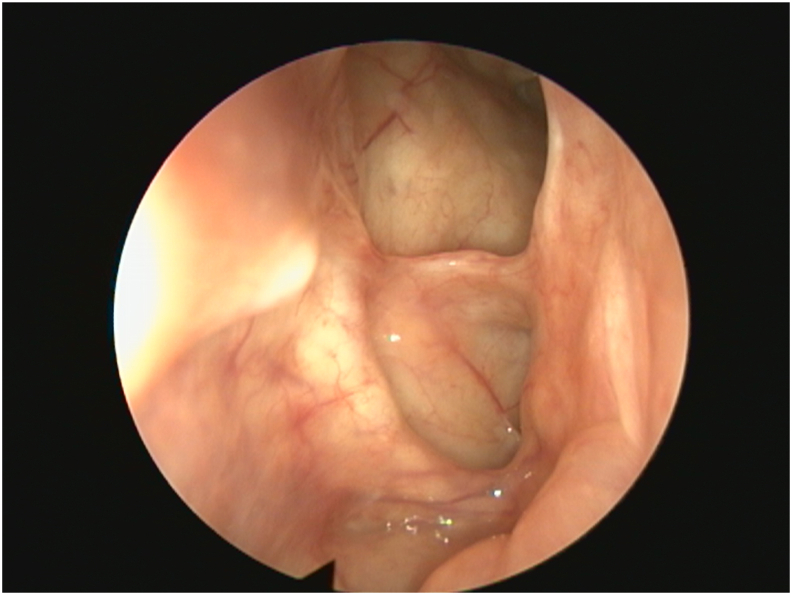
Fig. 1.
Post-treatment endoscopic view showing a wide patent frontal sinus and skull base
Case 3
A 31-year-old female with a history of food allergy and CRSwNP complained of nasal congestion, anosmia, sinus headache, postnasal drip, and ear fullness for the last 5 years. The patient had a history of FESS with polyp removal (3 times). Upon initial encounter, SNOT-22 score was 43, andserum eosinophil count was 320 (cells/μl) while she was on systemic steroids. Prick skin testing revealed reactivity to D. farina (5 mm × 7 mm), D. pteronyssinus (5 mm × 5 mm), Aspergillus fumigatus (8 mm × 11 mm), and Cladiosporum (5 mm × 5 mm). A CT of the sinuses revealed diffuse opacification of right maxillary sinus with double-density sign, suggesting AFRS. The patient was treated with intranasal corticosteroid and nasal irrigation. Sublingual immunotherapy targeting the positive allergens was added. The patient had initial improvement with sublingual immunotherapy; however, she discontinued it due to compliance issues. The patient underwent another FESS with a good clinical response. Culture from the sinus surgery was positive for Aspergillus. Despite the medical and surgical treatments, the symptoms recurred a few weeks later. Due to the disease recurrence and frequent surgeries, dupilumab was started at 300 mg biweekly. Six months later, the patient reported a dramatic improvement in her rhinosinusitis and asthma symptoms. A follow-up assessment revealed that SNOT-22 score improved to 5, and no side effects were reported during the follow-up period. Informed consent to publish the report was obtained from the patient.
Case 4
A 33-year-old female had a history of atopic dermatitis during childhood. She experienced nasal congestion, nasal itchiness, postnasal drip, and cough for the last 5 years, but no shortness of breath or wheezing was noted. She had occasional snoring with interrupted night sleep. She had no eye or nose itchiness, but her father had a history of atopy. The patient underwent FESS 11 times. The first FESS was at the age of 17, and the last surgery was 3 years ago. A physical exam revealed large inferior nasal turbinates and deviated nasal septum. Her skin prick test for allergies was positive for Dermatophagoides farinae (+3), D. pteronyssinus (+4), Aspergillus (+5), Altenaria (+4), and Cladiosporum (+3). On initial evaluation, the SNOT-22 score was 74, and the ACT score was 18. FEV1 was 82% predicted, and FEV1/FVC was 84% predicted. A postoperative sinus CT revealed mucosal thickening with double-density opacification involving both maxillary antra, ethmoid, sphenoid sinuses as well as the right frontal sinus (Fig. 2). In addition, diminished aeration with fluid obliteration of left mastoid air cells was found, suggesting acute on top of chronic mastoiditis. The disease was refractory despite the regular use of intranasal corticosteroids, oral steroids, and nasal irrigation. Subsequently, dupilumab was started, initially at a loading dose of 600 mg followed by 300 mg biweekly over four months. The patient reported a significant improvement in her rhinosinusitis symptoms with SNOT-22 score of 6. The ACT score improved to 21 and she had no asthma symptoms during the treatment period. CT scan was performed later and showed resolution of the aforementioned findings (Fig. 3). Fig. 4 presents the resultant endoscopic findings. The patient tolerated the medication well with no side effects being reported. The patient opted for utilizing her data for research purposes and informed consent was obtained.
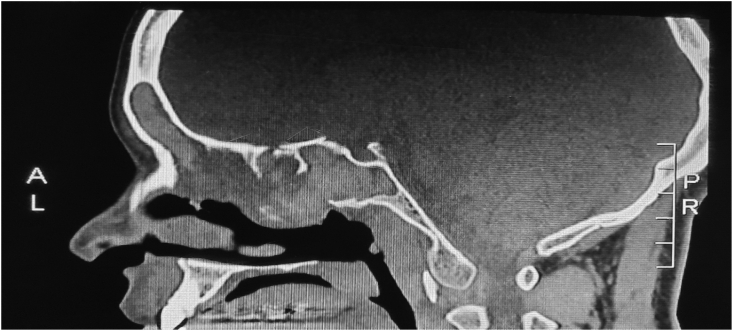
Fig. 2.
Sagittal view of the CT scan showing the characteristic double-density sign of AFRS involving all of the sinuses
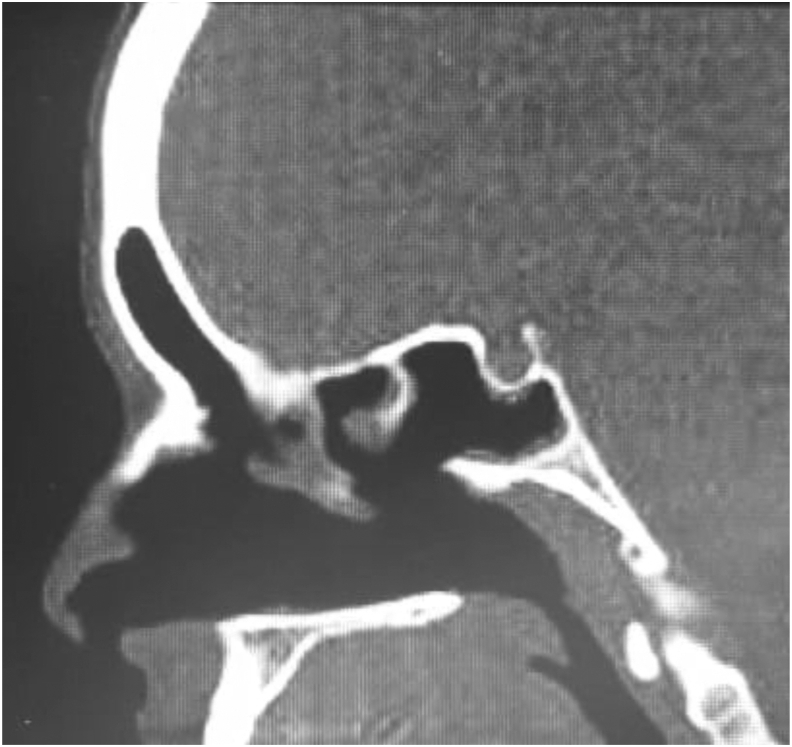
Fig. 3.
Post-treatment sagittal view of the CT scan showing resolution of the characteristic double-density sign of AFRS in the involved sinuses
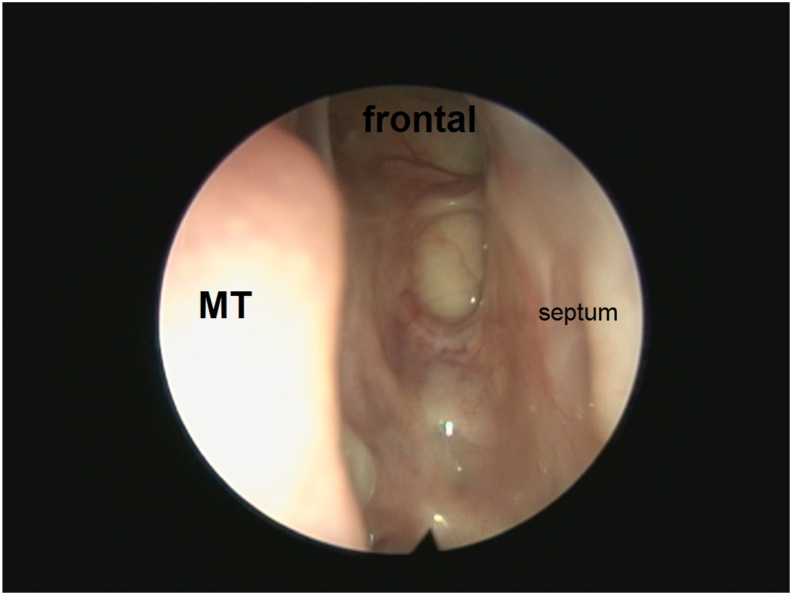
Fig. 4.
Post-treatment endoscopic view showing a wide patent frontal sinus and skull base. MT, middle turbinate
Discussion
The definition and diagnostic criteria of AFRS have evolved and become more solidified and distinct entirely. The traditional definition of AFRS included the detection of fungi and allergic mucin (mucus containing clusters of eosinophils) in the sample. However, researchers claimed that some healthy individuals had positive growth with no significant difference in positive growth rate when compared to CRS patients.17 In addition, mucin with or without fungi could be present in non-AFRS CRS.18, 19, 20 Accordingly, 2 closely related types of CRS were proposed, namely, eosinophilic fungal rhinosinusitis20 and eosinophilic mucin rhinosinusitis;18 yet there has been controversy on the distinctiveness of AFRS as a clinical phenotype of CRSwNP.21 Although fungal sensitization may exist in non-AFRS CRS, the elicited allergic reaction producing high levels of specific IgE is typically manifested in and may underlie the AFRS disease.22 Therefore, IgE-mediated type 1 hypersensitivity has been incorporated under the diagnostic umbrella of AFRS.23 The constellation of clinical, radiological, and immunological parameters has been of interest for many researchers in the development of a more reliable model for AFRS differentiation, and more importantly, for therapeutic approach guidance. Accordingly, high-quality guidelines and position papers have been implemented to tackle the aforementioned dilemma.5,24,25
The authors of this report strictly adopted the most conventional diagnostic method set by Bent and Kuhn23 and developed by Meltzer EO et al.26 The diagnostic criteria include (1) type I hypersensitivity; (2) nasal polyposis; (3) characteristic CT findings; (4) eosinophilic mucin without fungal invasion into the sinus tissue; and (5) positive fungal stain. A positive fungal culture, although desirable, is not necessary to make the diagnosis.23
The disease is mediated by type 2 inflammation and is characterized by a high level of IgE, IL-4, and IL-5.27 Dupilumab demonstrates its efficacy by hindering type 2 inflammation mediators such as IL-4, IL-13, and IgE via blocking the IL-4 α receptor subunit. Dupilumab's mechanism of action provides an excellent rationale for evaluating its potential efficacy in AFRS patients. In addition to CRSwNP, dupilumab is an effective treatment for other type 2 inflammation-mediated disorders, including atopic dermatitis, asthma, and eosinophilic esophagitis. Given the remarkable increase in IgE and eosinophils in our patients with type 2 inflammation, along with asthma and the suboptimal lung function test results, dupilumab was considered as a treatment option.
A CT scan of the paranasal sinuses is the initial tool used to aid in the AFRS diagnosis. The characteristic findings of CT imaging consist of heterogenous signal hyper-intensities within the affected sinuses filled with thick fungal mucin (hyper-attenuated substance), that are referred to as the "double-density" sign.28 This radiological sign is dominant in fungal sinusitis patients with an occurrence rate of 92%, which is relatively higher than other radiological features including expansion of the sinus and bony erosions (81% and 60%, respectively). In terms of its diagnostic accuracy, the "double-density" sign has a high diagnostic accuracy for AFRS identification (diagnostic accuracy = 89%), when the histopathology is taken as the reference “gold standard”.29 Furthermore, the 2020 European position paper on rhinosinusitis and nasal polyps 2020 (EPOS 2020) supports the criteria of CT findings for diagnosing AFRS.5 Accordingly, the authors utilized this hallmark, along with other diagnostic features, to make a proper diagnosis.
Our study found that clinical scoresremarkably improved after the dupilumab injection with a five-month follow-up time (Table 2). Moreover, our patients experienced an improvement in asthma symptoms and became less dependent on oral prednisolone and asthma medications. Iqbal et al studied the response of pulmonary function test after dupilumab and demonstrated a significant improvement in comparison to placebo or topical steroids.30 In our study, a remarkable improvement was observed in the results of pulmonary function tests as being delineated by FEV1 and FEV1/FVC.
Table 2.
Laboratory, immunological and respiratory parameters before and after the administration of dupilumab.
| Parameter | Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|---|
| SNOT-22 | Before | 65 | 64 | 43 | 74 |
| After | 3 | 7 | 5 | 6 | |
| ACT score | Before | 16 | 14 | N/A | 18 |
| After | 22 | 21 | N/A | 23 | |
| FEV1 | Before | 69% | 65% | N/A | 82% |
| After | 81% | 79% | N/A | N/A | |
| FEV1/FVC | Before | 70% | 82% | N/A | 84% |
| After | 85% | 83% | N/A | 88% | |
| Serum eosinophils counts (cells/μl) | Before | 330 | 500–1200 | 320 | 630 |
| After | 1500a | 100 | 480 | 123 |
N/A, not available; SNOT-22, SINO-NASAL OUTCOME TEST-22; ACT, asthma control test; FEV1, Forced Expiratory Volume in 1 s; FVC, Forced vital capacity.
Transient eosinophilia is a known effect of dupilumab
The optimal duration of dupilumab in CRSwNP is unknown. According to the LIBERTY NP SINUS-24 trial, the effect of dupilumab diminished after discontinuation at 24 weeks.14 Therefore, the continuation of the agent is mandated to have sustainable disease control. In our cases, the plan is to continue dupilumab for one year, then discuss whether continuation of the treatment is necessary.
Few adverse effects were reported among patients with CRSwNP receiving dupilumab. These include nasopharyngitis, injection-site reactions, and headache.31 The results of LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52 showed that the majority of side effects were reported in the placebo group.14 In our research, no adverse events were reported during the biologic agent course, and the patients tolerated the medication well.
Expert groups and advisory committees from countries of the Gulf Cooperation Council (GCC) support the use of dupilumab in resistant CRSwNP, providing there is an evidence of type 2 inflammation (eg, total IgE ≥100 or serum eosinophils ≥250), impaired quality of life (SNOT-22 ≥40), and, preferably, concomitant asthma.24,25 The present case series found promising outcomes of the dupilumab use in AFRS, as a subset of CRSwNP, among the Saudi population (as part of the Gulf countries ) who met the aforementioned criteria, with no significant adverse events. Future studies on a larger scale should be conducted evaluating the use of biologics in AFRS.
Abbreviations
AFRS, Allergic fungal rhinosinusitis; CRSwNP, Chronic rhinosinusitis with nasal polyposis; CRSsNP, Chronic rhinosinusitis without nasal polyposis; FESS, Functional endoscopic sinus surgery; IgE, immunoglobulin E; SNOT-22, Sino-nasal Outcome Test-22; IL-4, Interleukin 4; ACT, Asthma Control Test; FEV1, Forced expiratory volume in 1 second; FVC, Forced Vital Capacity; CT, Computed Tomography; LABA, Long-acting beta-agonist; EPOS 2020, 2020 European position paper on rhinosinusitis and nasal polyps.
Author contributions
Adeeb A. Bulkhi: Literature review, Writing – Original Draft; Ahmad A. Mirza: Literature review, Writing – Original Draft; Abdullah J. Aburiziza: Literature review, Writing – Original Draft; Osama A. Marglani: Novelty description, Validation, Writing – Review and Editing, Supervision.
Funding
This work was not supported or funded by any drug company.
Consent for publication
All authors approved for the final version to be published.
Availability of data and materials
Extra data and materials are available upon reasonable request.
Ethical approval
This is a case series where ethical approval was not required. Written informed consent to publish their cases was obtained from the patients.
Declaration of competing interests
All authors have no conflict of interests to declare.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Cho S.H., Hamilos D.L., Han D.H., Laidlaw T.M. Phenotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8(5):1505–1511. doi: 10.1016/j.jaip.2019.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laury A.M., Wise S.K. Allergic fungal rhinosinusitis. Am J Rhinol Allergy. 2013;27(3_suppl):S26–S27. doi: 10.2500/ajra.2013.27.3891. [DOI] [PubMed] [Google Scholar]
- 3.Wise S.K., Ghegan M.D., Gorham E., Schlosser R.J. Socioeconomic factors in the diagnosis of allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2008;138(1):38–42. doi: 10.1016/j.otohns.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Telmesani L.M. Prevalence of allergic fungal sinusitis among patients with nasal polyps. Ann Saudi Med. 2009;29(3):212–214. doi: 10.5144/0256-4947.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fokkens W.J., Lund V.J., Hopkins C., et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- 6.Bakhshaee M., Fereidouni M., Mohajer M.N., Majidi M.R., Azad F.J., Moghiman T. The prevalence of allergic fungal rhinosinusitis in sinonasal polyposis. Eur Arch Oto-Rhino-Laryngol. 2013;270(12):3095–3098. doi: 10.1007/s00405-013-2449-5. [DOI] [PubMed] [Google Scholar]
- 7.Bakhshaee M., Fereidouni M., Nourollahian M., Movahed R. The presence of fungal-specific IgE in serum and sinonasal tissue among patients with sinonasal polyposis. Eur Arch Oto-Rhino-Laryngol. 2014;271(11):2871–2875. doi: 10.1007/s00405-014-2882-0. [DOI] [PubMed] [Google Scholar]
- 8.Patel T.R., Sur S. IgE and eosinophils as therapeutic targets in asthma. Curr Opin Allergy Clin Immunol. 2017;17(1):42–49. doi: 10.1097/ACI.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 9.Bérard F., Ferrier Le Bouedec M.C., Bouillet L., et al. Omalizumab in patients with chronic spontaneous urticaria nonresponsive to H1-antihistamine treatment: results of the phase IV open-label SUNRISE study. Br J Dermatol. 2019;180(1):56–66. doi: 10.1111/bjd.16904. [DOI] [PubMed] [Google Scholar]
- 10.Evans M.O., 2nd, Coop C.A. Novel treatment of allergic fungal sinusitis using omalizumab. Allergy Rhinol (Providence) 2014;5(3):172–174. doi: 10.2500/ar.2014.5.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan E.C., Habib A.R., Rajwani A., Javer A.R. Omalizumab therapy for refractory allergic fungal rhinosinusitis patients with moderate or severe asthma. Am J Otolaryngol. 2015;36(5):672–677. doi: 10.1016/j.amjoto.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Corren J., Castro M., O'Riordan T., et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract. 2020;8(2):516–526. doi: 10.1016/j.jaip.2019.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Beck L.A., Thaçi D., Hamilton J.D., et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 14.Bachert C., Han J.K., Desrosiers M., et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi: 10.1016/S0140-6736(19)31881-1. [DOI] [PubMed] [Google Scholar]
- 15.Lo R.M., Liu A.Y., Valdez T.A., Gernez Y. Dupilumab use in recalcitrant allergic fungal rhinosinusitis. Ann Allergy Asthma Immunol. 2020;125(5):617–619. doi: 10.1016/j.anai.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Alotaibi N.H., Aljasser L.A., Arnaout R.K., Alsomaili S. A case report of allergic fungal rhinosinusitis managed with Dupilumab. Int J Surg Case Rep. 2021;88:106479. doi: 10.1016/j.ijscr.2021.106479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebbens F.A., Georgalas C., Fokkens W.J. Fungus as the cause of chronic rhinosinusitis: the case remains unproven. Curr Opin Otolaryngol Head Neck Surg. 2009;17(1):43–49. doi: 10.1097/MOO.0b013e32831de91e. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson B.J. Eosinophilic mucin rhinosinusitis: a distinct clinicopathological entity. Laryngoscope. 2000;110(5 Pt 1):799–813. doi: 10.1097/00005537-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Thrasher R.D., Kingdom T.T. Fungal infections of the head and neck: an update. Otolaryngol Clin North Am. 2003;36(4):577–594. doi: 10.1016/s0030-6665(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 20.Ponikau J.U., Sherris D.A., Kern E.B., et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74(9):877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 21.Braun H., Buzina W., Freudenschuss K., Beham A., Stammberger H. 'Eosinophilic fungal rhinosinusitis': a common disorder in Europe? Laryngoscope. 2003;113(2):264–269. doi: 10.1097/00005537-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Hutcheson P.S., Schubert M.S., Slavin R.G. Distinctions between allergic fungal rhinosinusitis and chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(6):405–408. doi: 10.2500/ajra.2010.24.3533. [DOI] [PubMed] [Google Scholar]
- 23.Bent J.P., 3rd, Kuhn F.A. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111(5):580–588. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 24.Al-Ahmad M., Alsaleh S., Al-Reefy H., et al. Expert opinion on biological treatment of chronic rhinosinusitis with nasal polyps in the Gulf region. J Asthma Allergy. 2022;15:1–12. doi: 10.2147/JAA.S321017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsaleh S., Alqahtani A., Alotaibi N., et al. The use of biologics in chronic rhinosinusitis with polyps: Saudi otorhinolaryngology society position statement. Saudi J Otorhinolaryngol Head Neck Surg. 2020;22(2):93–94. [Google Scholar]
- 26.Meltzer E.O., Hamilos D.L., Hadley J.A., et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(6 Suppl):155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ragab A., Samaka R.M. Immunohistochemical dissimilarity between allergic fungal and nonfungal chronic rhinosinusitis. Am J Rhinol Allergy. 2013;27(3):168–176. doi: 10.2500/ajra.2013.27.3882. [DOI] [PubMed] [Google Scholar]
- 28.Kopp W., Fotter R., Steiner H., Beaufort F., Stammberger H. Aspergillosis of the paranasal sinuses. Radiology. 1985;156(3):715–716. doi: 10.1148/radiology.156.3.4023231. [DOI] [PubMed] [Google Scholar]
- 29.Naz N., Ahmad Z., Malik S.N., Zahid T. Diagnostic accuracy of CT scan in fungal sinusitis, diagnosis and extent. Annals of PIMS ISSN. 2016;1815:2287. [Google Scholar]
- 30.Iqbal I.Z., Kao S.S., Ooi E.H. The role of biologics in chronic rhinosinusitis: a systematic review. Int Forum Allergy Rhinol. 2020;10(2):165–174. doi: 10.1002/alr.22473. [DOI] [PubMed] [Google Scholar]
- 31.Bachert C., Hellings P.W., Mullol J., et al. Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy. 2020;75(1):148–157. doi: 10.1111/all.13984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Extra data and materials are available upon reasonable request.