Abstract
Hereditary Angioedema (HAE) is a rare and disabling disease for which early diagnosis and effective therapy are critical. This revision and update of the global WAO/EAACI guideline on the diagnosis and management of HAE provides up-to-date guidance for the management of HAE. For this update and revision of the guideline, an international panel of experts reviewed the existing evidence, developed 28 recommendations, and established consensus by an online DELPHI process. The goal of these recommendations and guideline is to help physicians and their patients in making rational decisions in the management of HAE with deficient C1-inhibitor (type 1) and HAE with dysfunctional C1-inhibitor (type 2), by providing guidance on common and important clinical issues, such as: 1) How should HAE be diagnosed? 2) When should HAE patients receive prophylactic on top of on-demand treatment and what treatments should be used? 3) What are the goals of treatment? 4) Should HAE management be different for special HAE patient groups such as children or pregnant/breast feeding women? 5) How should HAE patients monitor their disease activity, impact, and control? It is also the intention of this guideline to help establish global standards for the management of HAE and to encourage and facilitate the use of recommended diagnostics and therapies for all patients.
Keywords: Hereditary angioedema, C1-inhibitor, diagnosis, GRADE therapy, management, disease control, DELPHI, guideline, prophylaxis, quality of life, recommendations, self-administration
Introduction
Hereditary angioedema (HAE) is a rare genetic disease that manifests with episodes of cutaneous or submucosal edema, most commonly affecting the skin, the abdomen, and the upper respiratory tract. HAE is a serious global health problem, for patients and their families. Evidence-based recommendations are needed to inform and guide clinical decision makers.
The most common cause of HAE involves either a deficiency (type 1) or dysfunction (type 2) of C1 inhibitor (C1–INH),1,2 which leads to the overproduction of bradykinin and activation of bradykinin B2 receptors. This increases vascular permeability and results in angioedema attacks.3
This is the second revision and update of the international guideline for the diagnosis and management of HAE,4,5 which was developed by the World Allergy Organization (WAO) in collaboration with the European Academy of Allergy and Clinical Immunology (EAACI). This revised and updated WAO/EAACI guideline on the diagnosis and management of HAE differs from previous versions and other guidelines, consensus reports, and position papers6, 7, 8, 9, 10, 11, 12 in that it builds on the most recently published evidence on HAE and the expertise and experience of a global panel of experts. Published evidence was identified by a structured and incremental review and assessed systematically and transparently for its quality, considering the Appraisal of Guidelines Research and Evaluation (AGREE II) Instrument and the methods suggested by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group.13 In line with the GRADE approach, this revision and update of the guideline acknowledges that evidence alone is insufficient to guide treatment decisions, and it incorporates values and preferences as well as clinical circumstances and expertise.
A global and diverse panel of expert clinicians, scientists, HAE patients and patient advocates was assembled for the development of this update and revision of the guideline. The expert panel composition reflects the global nature of this guideline, with geographical and gender balance of its members. Given that the management of patients with angioedema requires an interdisciplinary approach, specialists from different fields were involved including allergology, dermatology, emergency medicine, gastroenterology, hematology, immunology, internal medicine, otolaryngology, and pediatrics.
The efforts of the expert panel were coordinated by the members of the steering committee (MMau, MMag, SB, TC). All physician/clinician panel members needed to be actively treating patients with angioedema and/or be involved in research directly related to angioedema. All expert panel members provided financial disclosure. This guideline is unique in that global involvement was ensured by the participation of international experts from 5 continents and 28 different countries. All expert panel members obtained a mandate to be the delegate of a national or international scientific society, which confirmed in writing that it nominated the expert as its delegate and that it endorsed the guideline and will help with its dissemination. Most of these experts were nominated by Allergy and Immunology Associations of different countries affiliated to WAO.
The goal of this guideline is to provide clinicians and their patients with guidance that will assist them in making rational decisions in the management of HAE, primarily HAE type 1 and type 2 (HAE-1/2). The reason to not focus upon HAE with normal C1-inhibitor is that for most patients there is not a diagnostic test. In addition, genetic testing can only identify a small subset of those suspected to have the disease. Lastly, in some of the newly identified genetic abnormalities bradykinin's role as the main mediator of the edema is questionable. This suggests re-evaluation of HAE with normal C1-inhibitor is required. To this end, 28 recommendations (numbered and given in framed boxes) were developed, of which 7 are new, 13 are revised, and 8 are unchanged, compared to the previous version of this guideline. Key questions covered by these recommendations include: 1) How should HAE be defined and classified?, 2) How should HAE be diagnosed?, 3) How should HAE patients be treated, and what treatment options should be used?,4) What are the goals of treating HAE?, 5) Should HAE management be different for special patient groups such as children and pregnant/breast feeding women?, and 6) How should HAE patients monitor their disease activity, impact, and control? It is important to note that access to modern diagnostics and therapies for HAE patients is limited in certain areas of the world.14 It is the intention of this revision and update of the guideline to help change this and to encourage and facilitate the global use of recommended diagnostics and therapies for all patients.
Methods
Selection of key questions, wording of recommendations
All authors were assigned to one of 4 taskforces, each dedicated to a defined HAE subject area: 1) nomenclature and diagnosis, 2) on demand therapy, 3) prophylaxis, and 4) special populations and management considerations. First, the taskforces were asked to review the existing recommendations from the previous WAO/EAACI guideline on HAE in their subject area and to assess these recommendations for accuracy and relevance to current practice.4,5 The taskforce members were then asked to critically review the wording and to update it if necessary. The taskforces were also asked to consider if new recommendations were needed and to develop them accordingly. The taskforces searched and reviewed the literature related to each recommendation.
The recommendations provided by this guideline use standardized wording, ie,. “we recommend” or “we suggest”. “We recommend” reflects a strong recommendation, implying: 1) that all or almost all informed people would make that choice, 2) that less time is needed for health care providers to make decisions and more time is available for overcoming barriers to their implementation and adherence, and 3) that, in most clinical situations, the recommendation may be adopted as policy. “We suggest” reflects a weak recommendation, implying: 1) that most informed people would make that choice, but a substantial number would not, 2) that health care providers and patients will need to devote more time on the process of decision-making as compared to strong recommendations, and 3) that policy making may require the use of further resources. Importantly, this guideline acknowledges and aims to mitigate the disparity in health-care resources for the management of HAE between countries. The recommendations in this guideline are meant to inform an optimal approach to HAE, by developing global standards for the diagnosis and treatment of HAE. This guideline will be in force for the next 4 years after its publication, when it will be updated and revised. Novel insights and improved tools, diagnostics and treatments that materialize before the next revision and update of this guideline should be used to improve the management of HAE as soon as they become available.
Literature search and review
For the update and revision of recommendations from the previous version of the guideline, a systematic search of the literature from June 1, 2016 was performed. For new and additional recommendations (recommendations 3, 11,13, 16, 17, 19, 25), as well as for pre-existing recommendations with a revised wording (recommendations 1, 2, 4, 5, 7, 9, 10, 12, 14, 18, 21, 22, 28), a complete search from January 1, 1985 was performed. Relevant information was extracted from the publications identified, and their quality was assessed with the help of a standardized worksheet as described before.4,5 Each manuscript/trial included in the guideline was evaluated with regard to its methodological quality (Table 1), and the literature search and evaluation process continued during the review process and manuscript development and was continuously updated until July 19, 2021.
Table 1.
Evidence grades
| A. Randomized, double-blind clinical trial of high quality (for example, sample size calculation, flow chart of patient inclusion, intention-to-treat (ITT) analysis, sufficient sample size) |
| B. Randomized clinical trial of lesser quality (for example, only single-blind, limited sample size: at least 15 patients per study arm) |
| C. Comparative trial with severe methodological limitations (for example, not blinded, very small sample size, no randomization) or large retrospective observational study, large open-label-study, registry data |
| D. Adapted from existing consensus document or statement based on expert opinion voting during consensus conference, evidence non A-C |
Consensus procedures
Consensus was established as described in the previous revision of the WAO/EAACI guideline for HAE, with the exception that an online DELPHI process was used rather than a consensus conference, due to the COVID-19 pandemic. The DELPHI process was facilitated by KW, with the help of dedicated software (Welphi®; decisioneyes, Lisbon, Portugal). The DELPHI process is a validated approach to evaluate and to refine group opinion through iterative rounds of questioning. The anonymity of this process is key and enables views to be changed over the course of the process, while ensuring that opinions are considered equally.15
The DELPHI panel comprised the voting members of the expert panel including the members of the steering committee. The Delphi process was performed in 2 rounds, the final round of which achieved consensus on all 28 recommendations of the guideline. In round 1 (22 days from November 11 to December 3, 2020) and round 2 (15 days from January 29 to February 11, 2021), 53 and 52 participants took part, respectively. All participants voted on all suggested recommendations provided by the taskforces; accordingly, the absolute number of voters was 53 or 52 for all recommendations. In round one, recommendations developed by the 4 task forces were evaluated by all participants with the two options "I agree to the text and the strength of the recommendation" or "I do not agree". In case participants did not agree, they were asked to make a specific suggestion for an alternative recommendation wording together with a justification for the suggested change. All participants had the opportunity to provide additional feedback with free text responses. Respondents were asked to consider their clinical experience, the patient management approach followed in their practice, and their broader knowledge on HAE. The steering committee members revised the recommendations based on the results and feedback obtained in round one and provided feedback to all comments made.
In round two, to gain consensus, respondents were asked if they agree or disagree with each revised recommendation. Strong consensus and consensus were defined, a priori, as agreement by at least >90% and >75% of respondents, and percentage agreement was recorded for each recommendation.16 Again, the steering committee members addressed the comments made by expert panel members and provided to all of them. The input of expert panel members from both DELPHI rounds was used to develop the final version of the manuscript, which was consented for publication by all.
Presentation of recommendations
Each recommendation is shown in a box, which contains 1) the recommendation, 2) the level of consensus reached, and 3) the level of quality of the data that supports the recommendation.
Definitions, nomenclature, and classification
Angioedema is characterized by a transient vascular reaction of deep dermal/subcutaneous tissues or mucosal/submucosal tissues with localized increased permeability of blood vessels resulting in tissue swelling.17, 18, 19, 20, 21 Angioedema can be mediated by bradykinin and/or mast cell-mediators including histamine (Table 2).22, 23, 24, 25, 26 Bradykinin-mediated angioedema is either hereditary or acquired. Hereditary angioedema (HAE) can be due to a deficiency/defect of C1 inhibitor (C1–INH) or other mechanisms (Table 2).27, 28, 29 Different forms of hereditary angioedema (HAE) are currently recognized and genetically identifiable: 1) HAE due to C1–INH deficiency (Type 1 HAE, HAE-1), characterized by low antigenic and functional C1–INH levels; 2) HAE due to C1–INH dysfunction (Type 2 HAE, HAE-2), characterized by normal (or elevated) antigenic but low functional C1–INH levels;1,2 3) HAE with mutation in the factor XII gene (HAE-FXII);30,31 4) HAE with mutation in the angiopoietin-1 gene (HAE-ANGPT1);32 5) HAE with mutation in the plasminogen gene (HAE-PLG);33 6) HAE with mutation in the kininogen 1 gene (HAE-KNG1);34 7) HAE with mutation in the myoferlin gene (HAE-MYOF);35 and 8) HAE with mutation in the heparan sulfate 3-O-sulfotransferase 6 gene (HAE-HS3ST6).36 In addition, some patients have HAE due to unknown mutations (HAE-UNK). The different forms of HAE share some clinical features and, possibly, therapeutic options.37,38
Table 2.
Classification of angioedema.
| Bradykinin-induced AE |
Mast cell mediator-induced AE |
Unknown mediator | ||||
|---|---|---|---|---|---|---|
| C1–INH deficiency/defect |
C1–INH normal |
IgE mediated | non-IgE mediated | |||
| Inherited | Acquired | Inherited | Acquired | |||
| HAE-1 HAE-2 | AAE-C1-INH | HAE-nC1-INH (HAE-FXII, HAE-PLG, HAE-KNG1, HAE- HS3ST6, HAE-ANGPTI+, HAE-MYOF+, HAE-UNK) | ACEI-AE Other drug-induced AE∗ | Angioedema with Anaphylaxis Angioedema with or without wheals (Urticaria) | Angioedema with or without wheals (Urticaria) | Idiopathic AE |
HAE-1: Hereditary angioedema due to C1-Inhibitor deficiency; HAE-2: Hereditary angioedema due to C1-Inhibitor dysfunction; AAE-C1-INH: acquired angioedema due to C1-Inhibitor deficiency; HAE-nC1-INH: Hereditary angioedema with normal C1-Inhibitor levels, either due to a mutation in FXII (Factor 12), ANGPT1 (angiopoietin-1), PLG (plasminogen), KNG1 (kininogen), MYOF (myoferlin), and HS3ST6 (heparan sulfate-glucosamine 3-O-sulfotransferase 6) or unknown (UNK). + HAE-ANGPT1 and HAE-MYOF are due to mutations involving the vascular endothelium and the role of bradykinin as mediator of angioedema symptoms seems to be an indirect or conditional one. ACEI-AE angiotensin converting enzyme inhibitor-induced angioedema, ∗ other drugs like Angiotensin II receptor blockers, gliptins, neprilysin inhibitors or tissue plasminogen activators are thought to potentially induce bradykinin-mediated AE
There are several types of bradykinin-mediated acquired angioedema. Underlying causes include acquired C1–INH deficiency with low C1-inhibitor (AAE-C1-INH), and angiotensin converting enzyme (ACE)-inhibitors induced angioedema (ACEI-AE) (Table 2).39, 40, 41, 42, 43, 44 These types of angioedema share some clinical features and treatment options with HAE.
The pathophysiology of HAE
The pathophysiology of HAE-1 and HAE-2
HAE-1/2 is a rare autosomal dominant disease that is estimated to affect, globally, 1 in 50,000 individuals.45, 46, 47, 48 HAE-1/2 is caused by a mutation in the SERPING1 gene, which codes for C1–INH.49 Currently, more than 700 different SERPING1 variants are known to be linked to HAE-1/2.50 In approximately 20–25% of patients, a de novo mutation of SERPING1 is responsible for the disease.51, 52, 53
C1–INH is a serine protease inhibitor (SERPIN) and the major inhibitor of several complement proteases (C1r, C1s, and mannose-binding lectin–associated serine protease [MASP] 1 and 2) and contact-system proteases (plasma kallikrein and coagulation factor XIIa) as well as a relatively minor inhibitor of the fibrinolytic protease plasmin.54, 55, 56
The primary mediator of swelling in HAE-1/2 is bradykinin,3 a low molecular weight nonapeptide that is generated when active plasma kallikrein cleaves high molecular weight kininogen (HMWK). Bradykinin is rapidly metabolized by endogenous metalloproteases including angiotensin-converting enzyme (ACE). Plasma kallikrein is activated from its inactive zymogen prekallikrein by the protease factor XII, which autoactivates upon contact with negatively charged surfaces. Both, plasma kallikrein and factor XII are inhibited by C1–INH. Increased vascular permeability and swelling induced by bradykinin are primarily mediated through the bradykinin B2 receptor.56, 57, 58, 59, 60, 61
The pathophysiology of HAE with normal C1 inhibitor
HAE with normal C1–INH (HAE-nC1-INH) is a group of very rare diseases. The clinical appearance of HAE-nC1-INH largely resembles that of HAE-1/2.38 Six types of HAE-nC1-INH are currently recognized, based on underlying mutations of: 1) factor XII (FXII), 2) angiopoietin 1 (ANGPT1), 3) plasminogen (PLG), 4) kininogen 1 (KNG1), 5) myoferlin (MYOF), and 6) heparan sulfate-glucosamine 3-O-sulfotransferase 6 (HS3ST6).30, 31, 32, 33, 34, 35, 36 However, in many patients with HAE-nC1-INH, no gene mutation can be found, and the pathogenesis remains to be characterized in detail. There is clinical evidence that bradykinin may play a major role in most types of HAE-nC1-INH, primarily in patients with HAE-FXII and HAE-PLG.62,63 HAE-ANGPT1 and HAE-MYOF are due to mutations involving the vascular endothelium.32,35 Although HAE-nC1-INH shares some clinical features and, possibly, therapeutic options with HAE-1/2, this guideline focuses on HAE-1/2.
The diagnosis of HAE
HAE-1/2 should be suspected when a patient presents with a history of recurrent swelling of the skin (extremities, face, genitals), gastrointestinal attacks (painful abdominal cramps, and/or laryngeal edema). Suspicion of HAE-1/2 is further suspected when patients report any or all of the following: 1) a positive family history (although this may not be present in up to 25% of patients), 2) onset of symptoms in childhood/adolescence, 3) recurrent and painful abdominal symptoms, 4) occurrence of upper airway edema, 5) failure to respond to antihistamines, glucocorticoids, omalizumab, or epinephrine, 6) presence of prodromal signs or symptoms before swellings, and 7) the absence of wheals. Suspicion of HAE-1/2 should prompt laboratory investigations to support the diagnosis of HAE-1/2.10,64,65
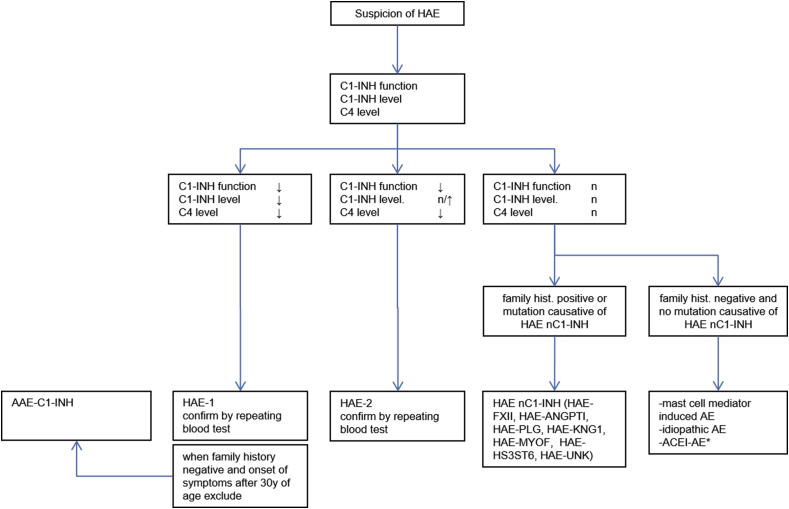
Measurements of serum/plasma levels of C1–INH function, C1–INH protein, and C4 are used to diagnose HAE-1/2 (Recommendation 1, Fig. 1). With the combined use of these 3 tests, the diagnostic accuracy for identifying HAE-1/2 is very high, higher than with the use of any of the 3 alone.66, 67, 68, 69 This guideline acknowledges that the availability and quality of tests for C1–INH function, C1–INH protein, and C4 vary throughout the world, necessitating physicians in some countries to adapt their own diagnostic approach (for example, the sensitivity of a C4 test can be increased by drawing blood during an emerging attack, but without improving specificity). In countries where recommended diagnostic tests are not available these guidelines should be used to advocate to health authorities to fund the appropriate diagnostic testing to decrease mortality and morbidity associated with HAE.14
Fig. 1.
Diagnostic work up in patients suspected to have HAE. Abbreviations: HAE-1: Hereditary angioedema due to C1-Inhibitor deficiency; HAE-2: Hereditary angioedema due to C1-Inhibitor dysfunction; AAE-C1-INH: acquired angioedema due to C1-Inhibitor deficiency; HAE-nC1-INH: Hereditary angioedema with normal C1-Inhibitor levels, either due to a mutation in ANGPT1 (angiopoietin1), PLG (plasminogen), KNG1 (kininogen), MYOF (myoferlin), and HS3ST6 (heparan sulfate-glucosamine 3-O-sulfotransferase 6) or unknown (UNK). ACEi-AE angiotensin converting enzyme inhibitor-induced angioedema, ∗ other drugs like angiotensin II receptor blockers, gliptins, neprilysin inhibitors or tissue plasminogen activators might induce bradykinin-mediated
In HAE-1, which comprises about 85% of HAE-1/2 patients, both, the concentration and function of C1–INH are low (<50% of normal) (Table 3). In HAE-2, C1–INH concentrations are either normal or elevated, whereas C1–INH function is reduced (<50% of normal). C4 levels are usually low in HAE-1/2 patients, but the sensitivity and specificity of C4 as a marker for HAE are limited.66,67,69, 70, 71 Because of this, its use for screening patients and its use as only parameter to diagnose HAE-1/2 are not recommended. Complement C3 levels are expected to be normal in HAE, and testing is not helpful. Sequencing of the SERPING1 gene can be supportive in the diagnostic workup of some HAE-1/2 patients (including prenatal diagnosis); however, biochemical C1–INH testing is effective and less expensive than genetic testing.70 DNA sequencing may miss mutations such as those creating cryptic splice sites. Genetic testing may be relevant in particular cases such as mosaicisms in order to allow for correct genetic counselling.72,73
| RECOMMENDATION 1 |
| We recommend that all patients suspected to have HAE are assessed for blood levels of C1INH function, C1–INH protein, and C4. |
| 92% agreement, evidence level D |
Table 3.
Typical diagnostic laboratory profile of HAE-1 and HAE-2 patients.
| C1–INH function | C1–INH protein level | C4 protein level | |
|---|---|---|---|
| HAE-1 | ↓ | ↓ | ↓ |
| HAE-2 | ↓ | N/↑ | ↓ |
The most straightforward parameter is the assessment of C1–INH function, which is low in HAE-1 and 2. For daily routine diagnostic purposes, three commercial test kits are available. The read outs are either by chromogenic substrates or the formation of C1–INH–C1s complexes. For apparent C1–INH function the read out matters. Only skilled laboratories can provide correct interpretation of results. In HAE-1 the concentration of the inhibitory protein is low (<50% of the normal mean), while in HAE-2 the concentration is normal or elevated
Test results that point to HAE-1/2 should be confirmed, ie, testing should be repeated, ideally in a certified laboratory (Recommendation 2). The same is true for inconsistent results or results in contradiction to the phenotype.68 HAE implicates numerous and lifelong consequences, for patients and their families, so its diagnosis should be based on confirmed test results (Fig. 1). Tests for C1–INH, by many laboratories, are performed infrequently, which runs the risk of false positive and false negative results, which is mitigated by repeat testing. More robust tests are under development.71 The recommendation to repeat testing for C1–INH function, C1–INH protein, and C4 refers only to the initial diagnosis of HAE. There is no indication for repeated testing once the diagnosis has been established. Of note, confirmation of HAE by repeat testing, in patients who tested positive, must not delay effective treatment.
| RECOMMENDATION 2 |
| We suggest that testing for C1–INH function, C1–INH protein, and C4 is repeated in patients who test positive, to confirm the diagnosis of HAE-1/2. |
| 87% agreement, evidence level D |
The differential diagnosis of HAE
The differential diagnoses of HAE-1/2 include HAE with normal C1 inhibitor, bradykinin-mediated types of acquired angioedema such as AAE-C1-INH and ACEI-AE, and mast cell-mediated types of acquired angioedema, such as angioedema in patients with chronic spontaneous urticaria without wheals and allergic angioedema, as well as idiopathic acquired angioedema (Table 2).52,74 Because the pathophysiology and the management of these diseases are different from those of HAE-1/2, it is important to determine the correct diagnosis.
Using laboratory tests, HAE with normal C1 inhibitor can currently only be diagnosed by genetic testing, which is becoming increasingly available. In patients with normal C1–INH levels and function suspected to have HAE, genetic testing should be performed (Recommendation 3). Currently, this should include testing for HAE with mutation in the factor XII gene (HAE-FXII); HAE with mutation in the angiopoietin-1 gene (HAE-ANGPT1); HAE with mutation in the plasminogen gene (HAE-PLG); HAE with mutation in the kininogen 1 gene (HAE-KNG1); HAE with mutation in the myoferlin gene (HAE-MYOF); and HAE with mutation in the heparan sulfate 3-O-sulfotransferase 6 gene (HAE-HS3ST6).30, 31, 32, 33, 34, 35, 36 Additional mutations are likely to be identified in the future and should be included in the genetic diagnostic workup for HAE.75 This guideline works with the intention that recommended diagnostic procedures, eg, genetic testing, should be used where available and that other options should be considered where recommended procedures are not available. Family history is an important tool for identifying patients with HAE-nC1-INH.76
| RECOMMENDATION 3 |
| We recommend that patients who are suspected to have HAE and have normal C1–INH levels and function are assessed for known mutations underlying HAE-nC1-INH. |
| 91% agreement, evidence level D |
AAE-C1-INH occurs less frequently than HAE-1/2. AAE-C1-INH symptoms are similar to those of HAE-1/2 and the basic diagnostic laboratory profile (C1–INH function, C1–INH protein and C4) is indistinguishable from HAE-1. Differences include onset at later age, underlying diseases such as lymphoma or benign monoclonal gammopathy (MGUS), occasional constitutional symptoms (B symptoms), and often decreased C1q levels.39,44,77,78 C1q level should be measured to investigate patients for AAE-C1-INH, especially those with new onset of angioedema after the age of 30 years and a negative family history. C1q is nearly always normal in HAE.44 C1q is low in 75% of patients with AAE-C1-INH.44,79 C1q may be normal in AAE-C1-INH, particularly in patients taking anabolic androgens. Many patients with AAE-C1-INH have autoantibodies that inactivate C1–INH.77,80
Patients who are diagnosed with ACEI-AE should be tested for HAE-1/2, as the occurrence of angioedema attacks after the initiation of treatment with an ACE-inhibitor may point to previously asymptomatic HAE.81 Angioedema attacks in patients with ACEI-AE inhibitors are thought to be bradykinin-mediated.38,82, 83, 84, 85
Recurrent mast cell-mediated angioedema is frequently associated with intensely pruritic wheals (hives) in patients with chronic spontaneous urticaria (CSU). Some CSU patients do not develop wheals and exclusively have angioedema.24,86 Importantly, CSU is a common disease, which can also affect HAE patients.87,88 The occurrence of wheals, therefore, does not necessarily exclude HAE, and the absence of wheals does not exclude mast cell-mediated angioedema.89 Non-sedating antihistamines, at standard or higher than standard doses, alone or in combination with omalizumab or immune modulators such as cyclosporine can prevent wheals and angioedema in CSU patients.86,90 Because mast cell-mediated angioedema is far more common than HAE-1/2, on demand therapy with antihistamines and, if necessary, with epinephrine and corticosteroids, is indicated when the diagnosis is not yet determined and the history seems to be inconsistent with HAE.91, 92, 93
On-demand treatment of HAE attacks
HAE attacks of the upper airways can result in asphyxiation.94, 95, 96, 97 Abdominal attacks are painful and debilitating.98, 99, 100 Peripheral attacks such as those of hands or feet result in impaired function.101 All of these consequences of HAE attacks can be minimized by on-demand treatment,102,103 and on-demand treatment should, therefore, be considered to be used to treat all attacks (Recommendation 4).
| RECOMMENDATION 4 |
| We recommend that all attacks are considered for on-demand treatment. |
| 98% agreement, evidence level D |
On demand treatment of attacks that affect or that may affect the upper airway is mandatory (Recommendation 5).104, 105, 106
| RECOMMENDATION 5 |
| We recommend that any attack affecting or potentially affecting the upper airway is treated. |
| 100% agreement, evidence level C |
Early on-demand treatment of HAE attacks with intravenous-C1-INH, ecallantide, or icatibant provides a better treatment response than late treatment, and HAE attacks should, therefore, be treated as early as possible (Recommendation 6).107, 108, 109, 110 Early treatment is associated with a shorter time to resolution of symptoms and shorter total attack duration regardless of attack severity.110,111 As early treatment is facilitated by self-administration, all patients with HAE-1/2 should be considered for home therapy and self-administration training. In many patients, a significant number of attacks are preceded by prodromal symptoms, and in some this may be an opportunity to treat before an attack occurs. The specificity of prodromes is still not known, and this may lead to over usage of on demand therapy.112, 113, 114, 115 All C1–INH concentrates and icatibant are licensed for self-administration, although approved product indications vary around the world.116, 117, 118, 119, 120
| RECOMMENDATION 6 |
| We recommend that attacks are treated as early as possible. |
| 100% agreement, evidence level B |
For HAE-1/2, icatibant, ecallantide, and intravenous C1–INH are the recommended on-demand treatments of choice (Recommendation 7).121, 122, 123, 124, 125, 126 Where these first-line therapies are not available, attacks should be treated with solvent detergent-treated plasma (SDP). If SDP is not available, attacks should be treated with fresh frozen plasma (FFP), where safe supply is available.127, 128, 129 We advise against the use of antifibrinolytics (eg, tranexamic acid) or androgens (eg, danazol) for on-demand treatment of HAE attacks,130 as these drugs show no or only minimal benefit.
On-demand treatment with C1 inhibitor
Treatment with plasma-derived or recombinant C1–INH replaces the deficient/dysfunctional protein in HAE-1/2 patients. Exogenous C1–INH acts on the same targets as endogenous C1–INH. Treatment results in an increase of the plasma levels of C1–INH and helps to regulate all cascade systems involved in the production of bradykinin during attacks.105,122,131, 132, 133 One unit of C1–INH-concentrate corresponds to the mean quantity of C1–INH present in 1 mL of fresh normal plasma. For on-demand treatment, only the intravenous application of C1–INH is effective.134, 135, 136
Plasma-derived C1–INH (pdC1-INH) is obtained by separating C1–INH from cryodepleted human plasma by adsorption and precipitation, purification, pasteurization, and virus filtration. Two pdC1-INH concentrates are available for on-demand treatment of HAE-1/2, Berinert (CSL Behring) and Cinryze (Takeda). Approved product indications vary around the world. The mean plasma half-life of pdC1-INH is longer than 30 h.134, 135, 136, 137, 138 The safety and tolerability of all available pdC1-INH are good, and few adverse events have been reported. The risk of allergic reactions is negligible. Modern pdC1-INH use has not been associated with transmission of hepatitis B nor C nor human immunodeficiency viruses.139, 140, 141, 142
Ruconest (Pharming) is the only available recombinant human C1–INH (rhC1-INH). Its mode of action is identical to that of pdC1-INH. RhC1–INH is indicated for on-demand treatment of all types of HAE attacks in adults and children (2 years or older).125,143 It is derived from the milk of transgenic rabbits using a 3-step purification procedure including cation exchange chromatography, anion exchange chromatography, and affinity chromatography. It appears that differential glycosylation of Ruconest relative to the human protein decreases the plasma half-life to approximately 3 h.144, 145, 146 Safety data from controlled and uncontrolled studies with rhC1-INH support a favorable safety profile. Transmission of human viruses is not a concern.147, 148, 149
On-demand treatment with the kallikrein-inhibitor ecallantide
The kallikrein inhibitor ecallantide (Kalbitor; Takeda) is licensed only in the United States and a few Latin American countries for the on-demand treatment of all types of HAE attacks in HAE-1/2 patients aged 12 years and older.118,150 Inhibition of kallikrein activity inhibits the cleavage of high-molecular weight kininogen to bradykinin as well as the further activation of FXIIa, halting the positive feedback mechanism leading to additional kallikrein production. Ecallantide is a 60-amino acid recombinant protein produced by expression in the yeast Pichia pastoris and has a plasma half-life of 2 h. The main safety concern is potentially serious hypersensitivity reactions, including anaphylaxis, which was reported in 3%–4% of treated patients. The drug, therefore, should only be administered by a health care professional with appropriate medical support to manage anaphylaxis.118,123,151,152
On-demand treatment with the bradykinin B2 receptor antagonist icatibant
Bradykinin binds to and stimulates the bradykinin B2 receptor, thereby mediating vasodilatation and increased capillary permeability.153, 154, 155 Icatibant (Firazyr; Takeda), a 10-amino acid synthetic peptide, is a specific and selective competitive antagonist of the bradykinin B2 receptor and prevents binding of bradykinin to its receptor. Icatibant is indicated for self-administered on-demand treatment of all types of HAE attacks in adults and children.120 It has a plasma half-life of 1–2 h. By far the most attacks resolve with one injection of icatibant.156 The safety and tolerability of icatibant are good, although transient local injection site reactions (erythema, wheal, pruritus, and burning sensation) may occur. Allergic reactions have not been reported.121,157, 158, 159
| RECOMMENDATION 7 |
| We recommend that attacks are treated with either intravenous C1 inhibitor, ecallantide, or icatibant. |
| 96% agreement, evidence level A |
The clinical course of HAE attacks is unpredictable. Mortality due to laryngeal angioedema occurs, and extreme caution is essential.97,104, 105, 106 Laryngeal HAE attacks should be considered as medical emergencies. Rapid treatment with an effective HAE on-demand medication is essential in addition to preparing for emergency airway management procedures if respiratory compromise develops. Intubation or surgical intervention, after the injection of on demand medication, should be considered early in all progressive HAE attacks affecting the upper airway (Recommendation 8).160,161
| RECOMMENDATION 8 |
| We recommend that intubation or surgical airway intervention is considered early in progressive upper airway edema. |
| 100% agreement, evidence level D |
Providing HAE patients with on-demand medication
HAE is unpredictable, and any attack may be followed by another one in short succession. It is essential that patients have on-demand medication to treat all attacks. We, therefore, recommend that all patients have and carry on-demand medication for the treatment of at least two attacks (Recommendation 9).162 In patients with frequent attacks, the time it takes to obtain more on-demand medication should be taken into consideration in the provision of treatment, so that they never run out of on-demand medication.
| RECOMMENDATION 9 |
| We recommend that all patients have sufficient medication for on-demand treatment of at least two attacks and carry on-demand medication at all times. |
| 100% agreement, evidence level D |
Short-term prophylactic treatment of HAE
The treatment of HAE patients with the intent of minimizing the consequent risk of angioedema during exposure situations where there is an increased risk of an attack is referred to as short-term prophylaxis, sometimes also called situational prophylaxis.
It is well recognized that surgical trauma, dental surgery and other interventions associated with mechanical impact to the upper aerodigestive tract (eg, endotracheal intubation, bronchoscopy or esophagogastroduodenoscopy), may precipitate angioedema near the site of intervention. Angioedema associated with these procedures usually occurs within 48 h. Following tooth extraction, more than one-third of patients without pre-procedural prophylaxis may develop local angioedema, and 50% of the swellings occur within 10 h, and 75% start within 24 h.163, 164, 165, 166, 167, 168 Pre-procedural prophylaxis reduces the risk of angioedema associated with these interventions. We, therefore, recommend short-term prophylactic treatment before medical, surgical, or dental procedures as well as exposure to other angioedema attack-inducing events (Recommendation 10).165,168, 169, 170, 171 Expert clinical judgement is needed, and individualized risk assessment should be used in the identification of angioedema-inducing events that warrant short-term prophylaxis.
| RECOMMENDATION 10 |
| We recommend considering short-term prophylaxis before medical, surgical, or dental procedures as well as exposure to other angioedema attack-inducing events. |
| 94% agreement, evidence level C |
We recommend the use of intravenous pdC1-INH as first line short-term prophylaxis (Recommendation 11),165,166,168,170,172 although evidence for its efficacy is scarce. Case reports and series suggest that angioedema may occur even after relatively minor procedures despite prophylaxis.163,166 However, several reports document a reduction in the incidence of angioedema for both adults and children with pdC1-INH used intravenously as pre-procedural prophylaxis, and the response appears to be dose related.165,166,169,170165,166,169,170 Pre-procedural prophylaxis with intravenous pdC1-INH concentrate is therefore recommended for all medical, surgical, and dental procedures associated with any mechanical impact to the upper aerodigestive tract. Intravenous pdC1-INH concentrate should be used for pre-procedural prophylaxis, as close as possible to the start of the procedure. Dosage has yet to be fully established. Product approved indication may vary by country.116,117 Most experts use either 1000 units or a dose of 20 units/kg of pdC1-INH. Some recent evidence suggests benefit with rhC1-INH short-term prophylaxis as it reduced the rate of post-procedure HAE attacks compared with control procedures without prophylaxis.171 This could be considered if intravenous pdC1-INH is not available.
Fresh frozen plasma (FFP) may be used for short-term prophylaxis, but it is not as safe as intravenous pdC1-INH concentrate and is a second-line agent because of the greater risk of blood borne disease transmission and allosensitization.7,11,169,173, 174, 175, 176
Attenuated androgens (eg, danazol) have been recommended in the past for pre-procedural prophylaxis as an alternative to intravenous pdC1-INH concentrates,170 but intravenous pdC1-INH concentrate is considered the prophylactic agent of choice.166 Frequent short courses of attenuated androgens may lead to side effects associated with long-term use. For scheduled pre-procedural prophylaxis, androgens are used for 5 days before and 2–3 days post event. Tranexamic acid has been used for pre-procedural prophylaxis in the past, however it is not recommended by most guideline experts7,11,169,173, 174, 175
With all pre-procedural prophylactic treatments, break-through attacks can occur, so patients and treating physicians should be aware of this increased risk and understand the treatment plan, and on demand treatment needs to be available.11,165,166,173,176
It is possible that short-term prophylaxis should be handled differently in patients with complete response to effective long-term prophylaxis, for example subcutaneous C1–INH, lanadelumab or berotralstat. No recommendation on this can be given at this time, as data are lacking. We encourage studies and reports on the need for short-term prophylaxis in patients on long-term prophylaxis.
| RECOMMENDATION 11 |
| We recommend the use of intravenous plasma-derived C1 inhibitor as first line short-term prophylaxis. |
| 91% agreement, evidence level C |
In addition to specific medical procedures, there can be patient-specific angioedema-inducing situations such as emotional stressors that can precipitate attacks. Currently, there are no controlled clinical trials in this area, and data come from personal experience, retrospective reviews, and surveys. Nevertheless, a similar approach to short-term prophylaxis should be considered when patients are exposed to specific situations known to increase the risk of attacks for a given patient (Recommendation 12).4,176,177
| RECOMMENDATION 12 |
| We suggest considering prophylaxis prior to exposure to patient specific angioedema inducing situations. |
| 90% agreement, evidence level D |
Long-term prophylactic treatment of HAE
The goals of treatment, in HAE, are to achieve complete control of the disease and to normalize patients’ lives (Recommendation 13).178 This can currently only be achieved by long-term prophylactic treatment, also referred to as long term prophylaxis (LTP), ie, the regular use of medication that reduces the burden of the disease by preventing attacks.
| RECOMMENDATION 13 |
| We recommend that the goals of treatment are to achieve total control of the disease and to normalize patients' lives. |
| 100% agreement, evidence level D |
Complete control of their disease, for HAE patients, translates to no longer having attacks. Over the past years, several new long-term prophylactic treatments have become available. These treatments significantly reduce attack rates, and many patients achieve complete response. In addition to achieving complete disease control, treatment of HAE should aim at normalizing the patient's life. The impact of HAE on health-related quality of life (QoL) is well documented, as is the reduction of QoL impairment by modern treatment options.126,179, 180, 181, 182, 183 The availability of modern prophylactic treatments, personalized disease management, and instruments for measuring its outcome means that complete control of HAE is now a realistic possibility for many patients.184, 185, 186, 187, 188, 189, 190, 191, 192, 193
Long-term prophylaxis should be individualized and considered in all HAE-1/2 patients taking into consideration the disease activity, patient's quality of life, availability of health-care resources, and failure to achieve adequate control by appropriate on-demand therapy. We, therefore, recommend evaluating patients with HAE for LTP at every visit, taking disease activity, burden, and control as well as patient preference into consideration (Recommendation 14).182,194, 195, 196, 197, 198, 199, 200, 201 As all of these factors can vary over time, all patients should be evaluated for LTP at least once a year. The goal of LTP is to achieve full control of disease burden while attempting to minimize treatment burden and side effects. Successful LTP requires a high degree of compliance; therefore, the patient's preferences should be taken into consideration. This requires appropriate and comprehensive physician patient communication and allocating time for this.
Patients who use LTP should be assessed regularly for efficacy and safety of the therapy, and dosage and/or treatment interval should be adapted according to the clinical response. Upper airway edema and other attacks may occur despite the use of long-term prophylaxis.195,197,202, 203, 204, 205, 206, 207 Therefore, all patients using long-term prophylaxis should also have on-demand medication (intravenous-C1-INH, ecallantide, or icatibant) as per recommendation 7.6, 7, 8,208, 209, 210, 211, 212
| RECOMMENDATION 14 |
| We recommend that patients are evaluated for long-term prophylaxis at every visit, taking disease activity, burden, and control as well as patient preference into consideration. |
| 96% agreement, evidence level D |
Long-term prophylaxis with plasma-derived C1–INH
Plasma-derived C1–INH is currently a preferred LTP agent for the prevention of HAE attacks, and we recommend its use as first-line long-term prophylaxis (Recommendation 15).126,197,205,213, 214, 215, 216 Approved product indications vary around the world. Dosing should be twice a week based upon the half-life of pdC1-INH. Dose and/or frequency may need adjustment for optimum efficacy.126,217, 218, 219, 220
Recent studies show that subcutaneous twice-weekly administration of pdC1-INH at a dose of 60 U per kilogram bodyweight provided very good and dose-dependent preventive effects on the occurrence of HAE attacks.205 The subcutaneous route may provide more convenient administration as well as maintain improved steady-state plasma concentrations of C1–INH compared to LTP with intravenous C1–INH, allowing for better symptom control.221, 222, 223, 224
| RECOMMENDATION 15 |
| We recommend the use of plasma-derived C1 inhibitor as first-line long-term prophylaxis. |
| 87% agreement, evidence level A |
Appropriate vaccination for hepatitis A and B should be generally considered for patients in regular/repeated administration of human plasma-derived products including C1 inhibitor.140,141 Routine prophylaxis with pdC1-INH has been shown to be safe and effective, and it improves quality of life in patients with relatively frequent HAE attacks compared with acute treatment of individual HAE attacks.210,222,223,225, 226, 227
Thromboembolic events due to C1–INH concentrate use in HAE are rare, and patients who experience such events often have underlying thromboembolic risk factors (eg, implanted central venous catheters.228, 229, 230, 231, 232, 233 There are no known interactions with other medicinal products. Tachyphylaxis seems rare with only 1 report of increasing doses required to prevent attacks when C1–INH concentrate is used regularly for prophylaxis.234
Long-term prophylaxis with lanadelumab
Lanadelumab is a subcutaneously injectable, fully human, anti-active plasma kallikrein monoclonal antibody (IgG1/κ-light chain). It is a preferred LTP agent for the prevention of HAE attacks due to its efficacy and the fact it is administered subcutaneously. We, therefore, recommend the use of lanadelumab as first-line LTP (Recommendation 16).195,235, 236, 237 It is typically administered as 300 mg every 2 weeks; however, a dosing interval of 300 mg every 4 weeks may be considered if a patient is well controlled (eg, attack free).196,238 It appears safe with the rate of adverse events not appreciably higher among patients who received lanadelumab than among those who received placebo.195,204
| RECOMMENDATION 16 |
| We recommend the use of lanadelumab as first-line long-term prophylaxis. |
| 89% agreement, (strong recommendation), evidence level A |
Long-term prophylaxis with berotralstat
Berotralstat is a plasma kallikrein inhibitor that binds to plasma kallikrein and inhibits its proteolytic activity. It is a preferred LTP agent for the prevention of HAE attacks due to its efficacy and the fact it is an oral medication (Recommendation 17).206,239,240 It is typically administered as 150 mg orally with food with dose reductions to 110 mg in some regions where it is licensed based on if there is hepatic impairment, use of P- glycoprotein or BCRP inhibitors (drug interactions) or patients experience gastrointestinal symptoms on the 150 mg dose.241 Berotralstat appears safe, with the most common side effects being gastrointestinal reactions, including abdominal pain, vomiting, and diarrhea which occurred more frequently in patients receiving 150 mg versus 110 mg or placebo.240 These reactions generally occurred early after initiation of treatment with Berotralstat, became less frequent with time and typically self-resolved.242,243
| RECOMMENDATION 17 |
| We recommend the use of berotralstat as first-line long-term prophylaxis. |
| 81% agreement, evidence level A |
Taken together, this guideline recommends any of the 3 medications for the first-line long-term prophylactic treatment of patients with HAE-1/2, ie, plasma-derived C1–INH, lanadelumab, berotralstat, based on the results of randomized controlled clinical trials.126,205,235,240 Where all 3 first-line LTP medications are available, the choice of which one to use should be made by shared decision making.244 This guideline encourages studies that compare the efficacy and safety of first-line LTP medications and the identification of predictors of treatment responses. Currently, there is not enough evidence to recommend any of these three treatment options over each other. Where none of the three recommended first-line LTP treatments are available, efforts should aim to change this. Alternative options for LTP, in the absence of all 3 first-line LTP treatments, include the off-label use of intravenous recombinant C1–INH.245
Importantly, first-line LTP treatments should be initiated as approved. For lanadelumab, and to some extent for C1–INH, adapting the dose and/or treatment interval, after achieving complete response, can decrease treatment burden.196,219,220 Changes in the dose or the treatment intervals should be based on data obtained using patient-reported outcome measures. Poor control should prompt treatment optimization including consideration of switching LTP medication to improve efficacy.198,201,246,247
Long-term prophylaxis with androgens
Attenuated androgens have traditionally been used for long-term prophylaxis of HAE-1/2.248, 249, 250, 251, 252 Androgen derivatives have been demonstrated to be effective in HAE-1/2, and the oral administration facilitates their use.248,249,253 However, androgens must be regarded critically, especially in light of their adverse androgenic and anabolic effects, drug interactions, and contraindications. Side effects are numerous and involve most patients; in other words, the absence of side effects is exceptional.250,254 Side effects appear to be dose related. Virilization is the most feared complication in women; menstrual disorders and even amenorrhea as well as diminished libido and hirsutism are also common,255, 256, 257 as are weight gain, headache, myalgia, depression, and acne. Androgens may lead to virilization of the female fetus and are, therefore, absolutely contraindicated during pregnancy.258,259 In children and adolescents, therapy with androgens may interfere with the natural growth and maturation process. In addition, androgens are subject to numerous contraindications and show interactions with many other drugs (eg, statins, antidepressants).211,260,261 Careful surveillance is imperative in long-term prophylaxis with androgens. In addition to clinical tests and examinations and questioning of the patient, semiannual blood and urine tests (standard urine test strip) are needed, and at least once a year, an ultrasound of the liver should be performed.211,260,262,263 Because of this, androgens should not be used as first-line LTP, and we recommend using them only as second-line long-term prophylactic treatment (Recommendation 18).252,264
| RECOMMENDATION 18 |
| We recommend the use of androgens only as second-line long-term prophylaxis. |
| 89% agreement, evidence level C |
The dose of androgens needed to control HAE attacks can vary between the equivalent of 100 mg every other day and 200 mg of danazol 3 times a day. The minimal effective dose should be used.7,174 Dosages above 200 mg of danazol daily for extended periods of time are not recommended, because of side effects.265,266 The response to androgens varies considerably, and the dose required for long-term prophylaxis is variable. For this reason, the dosage should be adjusted according to clinical response and not C4 or C1–INH levels.6,7,267 It is unclear if stopping long term prophylaxis with attenuated androgens should be done by tapering off gradually over time.262,268,269 This guideline encourages studies that will help to guide physicians and patients on how to best discontinue androgen treatment.
Long-term prophylaxis with antifibrinolytics
Antifibrinolytics such as tranexamic acid are not recommended for long-term prophylaxis. Data for their efficacy are largely lacking, but some patients may find them helpful.270, 271, 272, 273, 274 They are primarily used where first-line prophylactic treatment options are not available and androgens are contraindicated. The safety profile of antifibrinolytics is good. The most common side effect is gastrointestinal upset. Contraindications/precautions include the presence of thrombophilia or increased thrombotic risk or acute thrombosis, eg, deep venous thrombosis, pulmonary embolism. The doses of tranexamic acid used range from 30 to 50 mg/kg body weight daily divided into 2 or 3 doses to a maximum of 6 g per day. Dose ranging studies and comparisons with other prophylactic medications have not been performed.6,7,272,275,276
Monitoring of long-term prophylaxis
Patients with HAE should monitor their disease activity, impact, and control, and this is especially important in patients who use long-term prophylactic treatment (Recommendation 19).184,246,277, 278, 279 Validated patient-reported outcome measures such as the angioedema activity score (AAS),192,280 the hereditary angioedema activity score (HAE-AS),186 the angioedema quality of life questionnaire (AE-QoL),191,193,281 the hereditary angioedema quality of life questionnaire (HAE-QoL),187,188 and the angioedema control test (AECT)189,190 are available in a wide range of languages and should be used for this purpose277,282 The aims of effective HAE treatment, ie, the absence of attacks, normalization of QoL, and complete control, are best achieved when assessed by appropriate tools.
Monitoring of HAE disease activity is based on the regular assessment and documentation of attacks by the patient. As HAE activity can change frequently, it is best measured by advising patients to document their attacks continuously, for example with the help of the AAS.26,192 The AAS has been translated into more than 80 languages for use in more than 50 countries, and is a valid and reliable instrument, with high convergent and known-groups validities, excellent internal consistency, and good test-retest reliability280 The AAS and other disease activity scores are widely used in clinical studies and routine clinical practice.283,284
High HAE disease activity often comes with low QoL. However, some patients with low attack rates also have markedly impaired QoL, possibly linked to the unpredictability of HAE and continuous fear of attacks, the need to avoid triggers of attacks, psychological distress due to chronic disease burden, and the presence of comorbid diseases, such as depression and anxiety, which are common in HAE patients.247 It is, therefore, important for patients and their physicians to assess the impact of HAE on QoL, in addition to disease activity. Validated PROMs for the evaluation of HAE-driven QoL impairment include the HAE-QoL and AE-QoL.187,188,191,193,281 Both are used in clinical practice and trials of HAE therapies.195,240,285, 286, 287
The assessment of HAE disease control is done with the AECT. The 4 questions of the AECT address the frequency of symptoms, QoL impairment, the unpredictability of episodes, and the level of control achieved by current therapy. Responses use a 5-point Likert scale and are scored from 0 to 4 points, with a minimum and maximum total score of 0 (no control) and 16 (complete control), respectively. The higher the AECT score the better the control of HAE. The AECT comes with high levels of internal consistency and test-retest-reliability and a cut-off value of 10 points to distinguish patients with poorly controlled and well controlled HAE.189,190 The AECT is available in 2 versions, 1 with a recall period of 4 weeks the other with a recall period of 3 months. Both yield largely similar results, are easy to administer, complete, and score, and can help to guide treatment decisions in HAE.
| RECOMMENDATION 19 |
| We suggest all patients who are using long-term prophylaxis be routinely monitored for disease activity, impact, and control to inform optimization of treatment dosages and outcomes. |
| 98% agreement, evidence level A |
Management of HAE-1/2 in children
Course and clinical picture in children with HAE
The gene defect (SERPING1 mutation) of HAE-1/2 is already present at birth, but symptoms are uncommon in neonates and infants. Attacks can first occur at any age, but usually start in childhood or adolescence. Half of all female HAE patients develop first attacks before the age of 12, and 90% by the time they are 23 years old. Of male patients with HAE, 50% and 90% have first attacks before the age of 13 and 25, respectively.288 Most attacks and most first attacks, in children, manifest with angioedema of the skin. Abdominal attacks may often go unrecognized in children, as abdominal pain is common in childhood. With angioedema of the upper airway, asphyxia can ensue rapidly in children, probably because of the small airway diameter.289 The earliest occurrence was described in a 4-week-old boy.161 The frequency and the severity of attacks may increase during puberty and adolescence. The earlier the onset of symptoms, the more severe the subsequent course of HAE-1/2.290,291 Erythema marginatum as a prodromal sign is more frequent in the pediatric population and occurs in 42%–58% of cases. It is often misdiagnosed as urticaria, which can lead to incorrect or insufficient treatment.87,275,276,292, 293, 294, 295, 296, 297, 298
Diagnosis of HAE in children
With autosomal dominant inheritance, the offspring of a patient with HAE-1/2 stands a 50% chance of inheriting the disease. Newborns with a positive family history are considered potentially affected until HAE-C1-INH is excluded and must be well observed and tested as early as possible, ideally before the onset of clinical manifestations, to ensure optimal management of the disease.299 Therefore, we recommend testing children of parents with HAE-1/2 as early as possible (Recommendation 20).70,297,300, 301, 302 Until a full investigation for HAE-1/2 is complete, all offspring of parents with HAE-1/2 should be considered to also have the disease.
| RECOMMENDATION 20 |
| We recommend testing children from HAE-affected families be carried out as soon as possible and all offspring of an affected parent be tested |
| 98% agreement, evidence level D |
Complement levels measured in the umbilical cord blood of full-term neonates are lower than maternal levels. Antigenic and functional C1–INH levels correspond to 70% and 62% of adult values, respectively.297,301,303 Therefore, using umbilical cord blood for complement measurements may produce false positive (low) results. The assessment of complement in peripheral venous blood (serum/plasma) in children lacks reference values. However, C1–INH antigenic levels and/or functional activity in children with HAE-1/2, who are less than 1 year old, are low, with exceptions.70,300 In contrast, the measurement of C4 was found not to be useful for diagnosing of HAE-1/2 in children below the age of 12 months, as C4 levels are frequently low in healthy infants.70,300 Genetic testing increases the diagnostic reliability in children and may be helpful where biochemical measurements are inconclusive and the genetic mutation of the parent is known.70,297,300 All early complement testing performed in offspring of HAE-1/2 patients should be repeated after the age of 1 year.70,275,297,300,304
Prenatal diagnosis of HAE-1/2 is not common in clinical practice.299 Reasons include that 1) mutations in affected parent C1–INH gene are not detected in up to 10% of cases, 2) identical mutations may be associated with substantially different phenotypes, and 3) advances in therapy have significantly improved the QoL and disease control of patients with HAE-1/2.49,72,275,305,306
Measurements of C1–INH antigen (protein) level, C1–INH functional (activity) level, and C4 level are advisable in children with angioedema without wheals.
Therapy of HAE in children
Like adults, all pediatric HAE-1/2 patients need to have a treatment action plan (see below) and on-demand therapy (Recommendation 21).143,214,307, 308, 309 C1–INH and icatibant are the only approved on-demand treatments for children with HAE-1/2.116,117,119,120 Both are effective, well tolerated and show a good safety profile. For abdominal attacks, parenteral fluid replacement may be required as children are more susceptible to hypovolemia and dehydration, and extravasation into the peritoneal cavity and the intestinal lumen can be substantial. When C1–INH and icatibant are not available, SDP is preferred over FFP, but both are considered second-line treatment. Ecallantide is licensed for the use in adolescents in the United States.118
| RECOMMENDATION 21 |
| We recommend C1 inhibitor or icatibant be used for the treatment of attacks in children under the age of 12. |
| 94% agreement, evidence level A |
As in adults, pre-procedural prophylaxis is recommended for medical, surgical, and dental procedures associated with any mechanical impact to the upper aerodigestive tract.165,166 Plasma-derived C1–INH is the first-line pre-procedural prophylactic option, and short courses of attenuated androgens should only be used second line, when C1–INH concentrate is not available. With either option, on-demand therapy should be available because short-term prophylaxis is not 100% effective.168
The indications for long-term prophylaxis in adolescents are the same as in adults (see above). The preferred therapy in children younger than 12 years of age for long-term prophylaxis is pdC1-INH. The dosing interval and dose may need to be adjusted according to the individual response. When C1–INH concentrate is not available for long-term prophylaxis, antifibrinolytics (ie, tranexamic acid 20–50 mg/kg) are preferred to androgens because of their better safety profile; however, efficacy is questioned by many, and data in support of its use are not available. Epsilon aminocaproic acid is less well tolerated than tranexamic acid. Androgens are not recommended for long-term prophylaxis in children and adolescents prior to Tanner Stage V. The administration of androgens requires careful safety monitoring. The continued need for regular prophylaxis with androgens and the dosing should be reviewed on a regular basis. Initial danazol dose for children is 2.5 mg/kg per day with subsequent adjustment, until symptom suppression or the maximum tolerated, or maximum recommended dose is reached, with a maximum single dose of 200 mg per day. Androgens result in masculinization and hypogonadism in boys and menstruation irregularities in girls. Unfavourable effects on behaviour are possible. Reduction in ultimate body height may occur owing to the premature closure of epiphyseal growth plates.6,7,297, 298, 299,310,311
Primary prevention and other management considerations in children with HAE
As in adults, most attacks in children with HAE-1/2 occur without an obvious trigger.312 Infections seem to be more common triggers of attacks in childhood. Compulsory and recommended vaccinations for children are safe, and the prevention of infections (eg, throat infections) may reduce the frequency of attacks. Medicinal products that can cause edema as an adverse effect are less frequently used in children. Treatment with an ACE inhibitor is less often necessary during childhood. However, early initiation of oral estrogen-containing contraceptives is increasingly common, may trigger attacks, and should be avoided. Hormonal contraception with progesterone-only pills may benefit many young women with HAE-1/2275,313,314 or at least should not increase attack frequency. Other triggers like strenuous physical activities involving mechanical trauma and emotional challenges (stress) are essential elements of childhood and adolescence.315 Restrictions of suspected triggers should be individualized and sensibly applied, along with use of prophylaxis where necessary, with the aim of avoiding any limitations in activities and lifestyle. The aim of HAE-1/2 management at all ages is to normalize the lives of patients.297,316
Providing pediatric patients and their families with appropriate information is indispensable to support them to adopt a suitable lifestyle and to avoid complications. Educators, teachers, and health care personnel responsible for the child at day care or school should receive written information on the disease, with advice on the management of HAE attacks, including the urgency of treatment for airway attacks. C1–INH or icatibant for emergency use should be available at home, school, and travel including school field trips. An action plan is necessary, and the family and local hospital should have therapies available for emergency treatment, and this should be included in the treatment plan. All HAE patients have a potential for receiving human blood products. Vaccinations for hepatitis A and B are recommended by many experts.295,297 All patients should be considered to receive influenza vaccine and other routine vaccinations.
Management of HAE-1/2 in pregnant and breast-feeding patients
Course and clinical picture in pregnant and breast-feeding patients with HAE
The anatomical, physiological, and hormonal changes during pregnancy may influence the manifestations and affect the course and treatment of HAE-1/2. Pregnancy can mitigate or aggravate HAE disease activity or have no effect. Infrequently, the manifestations of HAE-1/2 first occur during pregnancy. Attack frequency observed during previous pregnancies is only in part predictive of that in subsequent ones.317, 318, 319, 320, 321 Pregnant HAE-1/2 patients require vigilant care and meticulous monitoring by an HAE expert. Patients should be managed in close cooperation by professionals from relevant medical specialties. Labour and delivery only rarely induce an attack, which may occur either during labour or within 48 h of delivery. Close follow-up is recommended for at least 72 h postpartum after uncomplicated vaginal delivery. Breastfeeding may be associated with an increased number of maternal attacks, with abdominal symptoms and facial edema, but is recommended based on benefits provided to the infant.275,317,318,322 Care for C-section, especially if intubation is necessary, should proceed as in any other surgical procedure performed on a patient with HAE-1/2 as covered below.
Diagnosis of HAE in pregnant and breast-feeding patients with HAE
In healthy women, the plasma levels of C1–INH decrease during pregnancy and return to normal after delivery.323,324 Therefore, measurements of levels of C1–INH function, C1–INH protein and C4 for the purpose of diagnosing HAE-1/2 during pregnancy should be interpreted with caution. It is recommended to repeat the measurements after childbirth to confirm the diagnosis of HAE.275,322
Therapy of HAE in pregnant and breast-feeding patients with HAE
C1–INH is recommended as first-line therapy for pregnant or breast-feeding HAE-1/2 patients as it is safe and effective (Recommendation 22).222,325, 326, 327, 328, 329 The use of ecallantide, lanadelumab and berotralstat in pregnancy is off label and not recommended as no published experience is available as of now. Although contraindicated by label, there are isolated case reports about the administration of icatibant during pregnancy with no maternal or fetal adverse effects reported.330, 331, 332 SDP may be used when C1–INH is not available and fresh frozen plasma when SDP is not available.275,317, 318, 319,333, 334, 335, 336, 337
| RECOMMENDATION 22 |
| We recommend plasma-derived C1 inhibitor as the preferred therapy during pregnancy and lactation. |
| 100% agreement, evidence level D |
Pre-procedural prophylaxis in pregnancy is recommended, preferably with C1–INH, for interventions that come with a risk of attacks such as chorionic villus sampling, amniocentesis, and induced surgical abortion. Alternatively, C1–INH should be available and administered immediately at the onset of an attack. It is recommended to manage childbirth in the hospital setting unless robust measures for the prompt and effective treatment of HAE attacks are available. Although mechanical trauma and stress are known to trigger attacks, few women develop angioedema during labour and delivery.275,319 Therefore, routine administration of pre-procedural prophylaxis before uncomplicated natural delivery is not mandatory, but C1–INH concentrate should be available for immediate on-demand use. Administering C1–INH concentrate as pre-procedural prophylaxis is recommended before labour and delivery when symptoms have been recurring frequently during the third trimester and the patient's history includes genital edema caused by mechanical trauma, during forceps delivery or vacuum extraction. Vaginal delivery is preferred because surgery or general anaesthesia may involve endotracheal intubation. Pre-procedural prophylaxis with C1–INH and epidural anaesthesia is recommended before a caesarean section, and intubation should be avoided if possible. If intubation is planned, pre-procedural prophylaxis is mandatory (see recommendation 10 and 11).
LTP may become indicated during pregnancy, especially in women experiencing an increase of attack frequency. In these women, C1–INH is considered a safe and effective prophylactic treatment option.318 Antifibrinolytics may be considered if C1–INH concentrate is unavailable, but efficacy is not proven.275,322,337,338 Androgens are contraindicated, as these drugs cross the placenta. The most common adverse effects is masculinization of the female fetus.258,259 Breast-feeding should be discontinued before androgens are introduced. Terminating lactation itself may reduce attack frequency.318
Plasma-derived C1–INH is considered the best therapy for on-demand treatment, short-term prophylaxis and long-term prophylaxis when indicated during lactation. Androgens and antifibrinolytics are secreted in breast milk. In contrast to androgens, tranexamic acid was found to be safe during breastfeeding.339
Patient support, home therapy and self-administration, and other management considerations
Patient support
Patient organizations and support groups provide help and support for HAE patients, caregivers, and family members. They endorse that all patients worldwide should have sufficient resources to control their HAE symptoms and fulfil their potential at school, at work, and in their relationships. HAEi, the international umbrella organization for the world's HAE patient groups, and national HAE associations have active informative web sites for patients and health care providers. HAEi has launched a “call to action” aimed at increasing the awareness and knowledge on HAE with governments, health authorities, and health care professionals and to achieve recognition of HAE as a serious, disabling, potentially life-threatening, and chronic condition that must receive timely accurate diagnosis and effective treatment.
Patient organizations also work toward identifying and addressing unmet needs in HAE management, which include the development of safe and well-tolerated new prophylactic and on-demand therapies, the optimization of existing long-term prophylactic and on-demand therapies (eg, by dose-ranging studies, paediatric studies), increasing the availability of modern treatment options worldwide, especially in low income countries, emphasizing the need for self-care, individual action plans, early therapy, and research. Information obtained from the internet is not always accurate and reliable; however, HAEi provides reviewed, updated, and scientifically sound information and is a quality source for patient education.
Individualized action and treatment plans for patients with HAE
Because HAE-1/2 is an unpredictable, painful, and life-threatening condition that can incur a huge stressful burden on patients and their families, an individualized treatment plan should be carefully developed by shared decision making (Recommendation 23).4,176,226,279,340, 341, 342, 343, 344, 345, 346 Individ-ualized treatment plans should address preven-tive measures as well as home care and self-administration. It should include an effective emergency (on-demand) treatment plan, with clear instructions on how to best use medications to treat HAE attacks. Patients should carry on-demand medication and an HAE identification card with instructions on how to manage an HAE attack. Patients on long-term prophylaxis also require an action plan and available therapy for on-demand use.347, 348, 349, 350
Patients should be appropriately prepared for surgery, dental work and procedures, and also surgeons, dentists, and proceduralists should be informed about the need of a short term prophylaxis if the procedure is in proximation of the airway. Co-management with an HAE expert is recommended.344
| RECOMMENDATION 23 |
| We recommend that all patients have an action plan. |
| 98% agreement, evidence level D |
HAE is a rare, complex, unpredictable life-long, and devastating disease, with impact on life. Effective HAE management requires comprehensive and integrated care, which should be available for all patients (Recommendation 24).176,297,346,351, 352, 353, 354 Integrated HAE management aims to achieve improved patient care through optimized coordination of services provided. It helps improve patient outcomes and allows for a proactive approach to the identification, prevention, and management of potential complications.
| RECOMMENDATION 24 |
| We recommend that HAE-specific comprehensive, integrated care is available for all patients. |
| 100% agreement, evidence level D |
The need for specialist care in HAE
HAE-1/2 patients are encouraged to find a health care provider with HAE-specific knowledge, interest, expertise, and experience. All patients with HAE should be treated by a specialist with specific expertise in HAE (Recommendation 25).4,176,244,297,346,347,355
There are several barriers for HAE patients to obtain optimal care. They include long delays in obtaining the correct diagnosis, physicians with little HAE knowledge and experience, not enough time allocated for their visits and communication with their physician, disconnects between patients’ beliefs, expectations, and priorities and those of their physicians, administrative and payer-related requirements for obtaining appropriate treatment, and the lack of therapies in their country. Cost and access may also be an issue for patients. HAE expert physicians can help to overcome these barriers. When and where possible, care should be provided by comprehensive angioedema centers with expertise in HAE. This guideline acknowledges the fact that there are not enough HAE expert physicians and angioedema centers, globally, and supports all efforts to change this, for example through the GA2LEN/HAEi network of angioedema centers of reference and excellence (ACARE).356
It is recommended that HAE patients have a medical evaluation at least annually. Newly diagnosed patients and those on long-term prophylaxis should be seen in shorter intervals, until control is achieved. Patients on androgens should continue to be seen twice a year.262 Evaluation at follow-up visits should include a review of patient-documented disease activity, impact, and control and of the frequency of use and effectiveness of on-demand treatment for swelling attacks. A physical examination and appropriate laboratory evaluation should be conducted.177,343
Emergency departments and other medical treatment facilities that provide acute care are strongly advised to develop and implement angioedema management algorithms and train their staff to effectively recognize and treat laryngeal and abdominal HAE attacks.357, 358, 359, 360
| RECOMMENDATION 25 |
| We recommend that patients are treated by a specialist with specific expertise in managing HAE. |
| 100% agreement, evidence level D |
Home therapy and self-administration
Self-administration is crucial for an effective on-demand therapy as early treatment of an attack. This effect is independent of the on-demand medication used and facilitated by the skill of the self-administrator or home therapy partner.9,110,340,352,361, 362, 363 Similarly, self-administration facilitates long-term prophylaxis. Every patient with HAE should be considered for home therapy and self-administration. All patients who are provided with on-demand treatment licensed for self-administration should be taught to self-administer (Recommendation 26).111,363,364
Having to attend a medical facility to receive on-demand medication may result in delayed treatment, prolonged observation, and inappropriate therapy. Self-administration training should ideally include a home therapy partner, ie, a family member or friend who can provide support, advice, and administration of therapy when the patient is compromised or unable or uncomfortable with self-treatment. Home therapy decreases the severity and duration of HAE attacks, reduces morbidity and disability, and can improve quality of life and productivity. In addition, the cost of care is reduced considerably using home and self-therapy.9,210,350,365, 366, 367, 368, 369, 370, 371, 372, 373
| RECOMMENDATION 26 |
| We recommend that all patients who are provided with on-demand treatment licensed for self-administration should be taught to self-administer. |
| 98% agreement, evidence level C |
Home therapy is also suitable for children with HAE, where a responsible adult is available and willing to undertake training. Experience with haemophilia suggests that it is beneficial for children to be encouraged to take an active part early in their treatment, and subcutaneous and intravenous self-administration has been demonstrated to be possible and safe in patients as young as 8 years.365,374 Advanced age is not a contraindication for home therapy if patients and/or home therapy partners can safely and effectively administer the treatment. The subcutaneous route may provide more convenient administration in all age groups.
Early treatment is crucial in cases of upper airway involvement (eg, tongue, posterior pharyngeal, uvula, larynx and vocal cords). Patients should self-administer treatment while awaiting transfer to the hospital. It is extremely important to encourage all patients to seek further care immediately after the administration of therapy. Upper airway swelling may progress or rebound and repeat dosing may be necessary. Seeking emergency care after therapy is essential to reduce the risk of asphyxia.
Avoidance of triggers of HAE attacks
A variety of conditions and events are known to trigger HAE attacks. Trauma, whether accidental or associated with dental, medical, and surgical procedures, may result in a swelling attack. The use of estrogen-containing oral contraceptive agents and estrogen hormone replacement therapy may trigger attacks and should be avoided. Hormonal contraception with progesterone-only pills may be beneficial for many women with HAE-1/2.275,313,314 Antihypertensive agents containing ACE inhibitors may increase the frequency or precipitate HAE attacks and should therefore be strictly avoided. Other reported triggers include psychological stress, fatigue, febrile illness, and the menstrual cycle. All patients with HAE should be educated about triggers that may induce attacks (Recommendation 27).4,176,342,349,375, 376, 377
| RECOMMENDATION 27 |
| We recommend that all patients should be educated about triggers that may induce attacks. |
| 100% agreement, evidence level D |
Patients should be made aware of potentially relevant triggers of symptoms to reduce precipitation of attacks. However, most attacks are unpredictable and not prompted by triggers. Therefore, physicians should not support excessive avoidance of suspected triggers, in order not to limit the patient's normal life. Influenza vaccine may reduce upper airway infections and possibly reduce upper airway swelling. Good dental care can reduce extractions, need for aggressive dental procedures, and prevent acute or chronic intraoral inflammation, which may reduce the threshold for attacks.164,167,312,315,378,379
The severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) disease COVID-19 pandemic has raised many questions and created uncertainty among patients with HAE and their healthcare providers. Questions are primarily related to the risk of infection, the risk of increased severity of COVID-19 disease, the risk of an increase in activity of HAE disease due to SARS-CoV-2 infection, modification of COVID-19 by therapies approved for HAE and effects of COVID-19 vaccines on patients with HAE. To date, there are minimal data available to answer these questions and address these concerns. Initial data from Brazil, France, and Turkey showed no significant increase in HAE-1/2 activity during or after COVID-19, although some patients who were only using on-demand therapy did report increased HAE activity.380, 381, 382, 383 A manuscript, in press, using the database of the HAE-A demonstrated no evidence that HAE patients were at greater risk for infection or for serious infection, nor adverse effects to the vaccine. In a recent survey from the Netherlands, following 111 COVID-19 vaccine doses administered, 11 attacks were reported, 6 arose more than 48 h after vaccination. Seven attacks were reported to be mild, 4 were assessed as moderate, and most were treated with on-demand medication. The majority of the attacks occurred in patients whose disease was not well controlled (AECT <10).384 These initial data underscore the importance of optimal control of HAE-1/2 disease, particularly during the pandemic, first to minimize the attack rate and second to reduce the need to visit emergency treatment facilities due to the potentially increased risk of COVID-19 infection.
Family screening in HAE
HAE-1/2 is a genetic disorder with autosomal dominant transmission. Family members including grandparents, parents, siblings, children, and grandchildren of HAE-1/2 patients should be screened for C1–INH function, C1–INH protein, and C4 plasma levels (Recommendation 28).4,176,297,355,385, 386, 387 Delayed diagnosis leads to morbidity and decreased quality of life due to delayed introduction of appropriate therapy. There is a risk that the first HAE attack may affect the airway or the abdomen and could cause asphyxia or unnecessary surgery.97 Once HAE is diagnosed, on-demand therapy should be prescribed to be available for the first and subsequent attacks, even if attacks have not yet occurred.
| RECOMMENDATION 28 |
| We recommend screening family members of patients for HAE. |
| 100% agreement, evidence level D |
Notes on use/Disclaimer
This is an updated version of the international HAE guideline. It is based on the update and revision of this guideline published in 2018: Maurer, M., Magerl, M., Ansotegui, I., Aygören-Pürsün, E., Betschel, S., Bork, K., Bowen, T., Balle Boysen, H., Farkas, H., Grumach, A., Hide, M., Katelaris, C., Lockey, R., Longhurst, H., Lumry, W., Martinez-Saguer, I., Moldovan, D., Nast, A., Pawankar, R., Potter, P., Riedl, M., Ritchie, B., Rosenwasser, L., Sánchez-Borges, M., Zhi, X., Zuraw, B., and Craig, T.: The international WAO/EAACI guideline for the management of hereditary angioedema – the 2017 revision and update. World Allergy Organization Journal 2018: 11; 5 and Allergy 2018: 73; 1575–1596. This article is co-published with permission in Allergy and the World Allergy Organization Journal.
Endorsing societies
Founder societies
WAO (World Allergy Organization), EAACI (European Academy of Allergy and Clinical Immunology).
Organizations represented by delegates to the expert panel and author group
Angioedema Centers of Reference and Excel-lence (ACARE).
Association of Allergy, Asthma and Immunology Buenos Aires (AAIBA) Argentina.
Chinese College of Allergy and Asthma (CCAA).
Danish Dermatological Society (DDS).
Dutch Society for Internal Medicine (NIV).
German Society for Angioedema Research (DGA/GSAR).
German Society for Internal Medicine (DGIM).
Global Allergy and Asthma European Network (GA2LEN).
HAE International (HAEi).
HAE UK.
Hungarian Society of Immunology (IUIS).
Japanese Dermatological Association.
Latin American Association of HAE (ALaeh).
Swedish Physicians Association for Immunode-ficiency (SLIPI).
urticaria network e.V. (unev).
WAO Member Societies represented by delegates to the expert panel and author group
Allergy Society of South Africa (ALLSA).
American College of Allergy Asthma and Immunology (ACAAI).
Australasian Society of Clinical Immunology and Allergy (ASCIA).
Austrian Society of Allergology and Immunology (OEGAI).
Brazilian Association of Allergy and Immunology (ASBAI).
Canadian Society of Allergy and Clinical Immunology (CSACI).
Chinese Society of Allergy (CSA).
European Academy of Allergy and Clinical Immunology (EAACI).
French Society of Allergology (SFA).
Global Allergy and Asthma European Network (GA2LEN).
Hungarian Society of Allergology and Clinical Immunology (MAKIT).
Korean Academy of Asthma, Allergy, and Clinical Immunology (KAAACI).
Japanese Society of Allergology (JSA).
Mexican College of Clinical Immunology and Allergy (CMICA).
Polish Society of Allergology (PSA).
Romanian Society of Allergy and Clinical Immunology (SRAIC).
Saudi Allergy, Asthma, and Immunology Society (SAAIS).
Spanish Society of Allergy & Clinical Immunology (SEAIC).
Funding and support
Funding and support of the development of this update and revision of the guideline came from WAO and EAACI. This revision and update of the guideline benefitted from the help and support of the GA2LEN/HAEi network of Angioedema Centers of Reference and Excellence (ACARE, https://acare-network.com).
Ethics statement
Not applicable.
Availability of materials
Not applicable.
Author contributions
The work on this guideline was coordinated by the members of the steering committee (MMau, MMag, SB, TC). MMau, MMag, SB, TC, IA, EAP, ABa, NBa, IBG, KB, LB, HB, NBr, PB, ABy, TC, AC, DCo, DCs, HF, MG, RG, AG, GGF, MH, HK, AK, CK, WLe, RL, HL, WLu, AMG, AM, IMS, JM, AN, DN, SN, JP, NP, AR, MR, BR, FS, WS, PS, MS, ET, LV, AZ, YZ, and BZ were the members of one of 4 taskforces to critically review the wording of the recommendations. The WELPHI process was developed and monitored by the methodologists AN and KW, supported by the steering committee members. MMau, MMag, SB, TC, IA, EAP, ABa, NBa, IBG, KB, LB, HB, NBr, PB, ABy, TC, DCo, DCs, HF, MG, RG, AG, GGF, MH, HK, AK, CK, WLe, RL, HL, WLu, AMG, AM, IMS, JM, AN, DN, SN, JP, NP, AR, MR, BR, FS, WS, PS, MS, ET, LV, AZ, YZ, BZ, WA, SKA, and GP were the voting members of the DELPHI panel. The initial draft of the paper was written by MMau and MMag and reviewed by SB and TC. The raw version of the manuscript was then revised for critically important intellectual content by all authors. All feedback was summarized by the steering committee members in the final version of the manuscript. All authors read and approved the final manuscript.
Consent for publication
All authors agreed to the publication of this work.
Declaration of competing interest
Dr. Maurer reports personal fees from Alnylam, grants and personal fees from BioCryst, grants from Centogene, grants and personal fees from CSL Behring, grants from Dyax, grants and personal fees from Kalvista, grants from Pharming, personal fees from Pharvaris, grants and personal fees from Shire/Takeda, outside the submitted work.
Dr. Magerl reports personal fees and non-financial support from Shire/Takeda, personal fees and non-financial support from CSL Behring, personal fees from Pharming, personal fees and non-financial support from Biocryst, during the conduct of the study; personal fees and non-financial support from Shire/Takeda, personal fees and non-financial support from CSL Behring, personal fees from Pharming, personal fees and non-financial support from Biocryst, personal fees from Kalvista, personal fees from Octapharma, personal fees from Novartis, outside the submitted work.
Dr Betschel has received fees for advisory boards, presentations and research from CSL and Takeda. Advisor fees from Biocryst and Kalvista, Octapharma, Pharming, outside of the submitted work.
Dr. Aberer has nothing to disclose.
Dr. Ansotegui has nothing to disclose.
Dr. Aygören-Pürsün reports personal fees from Biocryst, personal fees from Biomarin, personal fees from Centogene, grants and personal fees from CSL Behring, personal fees from Kalvista, personal fees from Pharming, personal fees from Pharvaris, grants and personal fees from Shire/Takeda, outside the submitted work.
Dr. Banerji reports grants from Takeda, Biocryst, other from Takeda, Biocryst, Pharming, CSL, Kalvista, Pharvaris, outside the submitted work.
Dr. Bara reports personal fees from commercial sponsor, outside the submitted work.
Dr. Boccon-gibod reports grants, personal fees and non-financial support from Takeda, grants, personal fees and non-financial support from Biocryst Pharmaceutical, non-financial support from Pharming, outside the submitted work.
Dr. Bork reports grants and personal fees from CSL Behring, personal fees from Shire/Takeda, personal fees from Pharvaris, outside the submitted work.
Dr. Bouillet reports grants and personal fees from Takeda, Biocryst, Novartis, and GSK.
H. B. Boysen has nothing to disclose.
Dr. Brodski has nothing relevant to disclose.
Dr. Busse reports grants and personal fees from CSL Behring, BioCryst, Takeda; personal fees from Medscape, CVS Health, Novartis, Regeneron
Dr. Bygum reports grants and personal fees from CSL Behring, grants from BioCryst, personal fees from Takeda/Shire outside the submitted work.
Dr. Caballero reports grants, personal fees and other from CSL-BEHRING, grants, personal fees and other from TAKEDA, personal fees and other from PHARMING NV, personal fees from OCTAPHARMA, personal fees and other from BIOCRYST, personal fees and other from NOVARTIS, outside the submitted work.
Dr. Cancian reports and has received grant research support and/or speaker/consultancy fees from BioCryst, CSL Behring, Kalvista, Pharming, Shire (a Takeda company) and SOBI; has received funding to attend conferences/educational events from CSL Behring, Menarini, MSD, Novartis, Pharming and Shire; is/has been a clinical trial/registry investigator for BioCryst, CSL Behring, Kalvista, Novartis, Pharming, Shire and UCB.
Dr. Castaldo has nothing to disclose.
Dr. Cohn reports personal fees from CSL Behring, personal fees from Shire/Takeda, personal fees from Ionis Pharmaceuticals, Inc, personal fees from KalVista, personal fees from BioCryst, personal fees from Pharming, personal fees from Pharvaris, personal fees from Sanofi/Genzyme Europe, outside the submitted work.
Dr. Csuka has nothing to disclose.
Dr. Farkas reports grants and personal fees from CSL Behring, grants and personal fees from Shire/Takeda, grants and personal fees from Pharming Group NV, personal fees from Biocryst Pharmaceuticals, personal fees from Kalvista, personal fees from ONO Pharmaceuticals, outside the submitted work.
Dr. Gompels reports fee for CSL and conference attendance support, member of the immunology clinical reference group, clinical trial work for Novartis, BioCryst.
Dr. Gower reports Industry funded research/investigator: BioCryst, Kalvista, Pharming, Pharvaris, Takeda/Shire/Dyax Consultant: BioCryst, CSL Behring, Fresenius Kabi, Pharming, Takeda/Shire/Dyax Speakers Bureau, Faculty, Peer Reviewer: BioCryst, Pharming, Takeda/Shire/Dyax Advisory Committee/Board: BioCryst, CSL Behring, Fresenius Kabi, Pharming, Takeda/Shire/Dyax.
Dr Grumach reports grants from Shire/Takeda and personal fees from CSL Behring, Takeda, and Catalyst for educative programs and consulting.
Dr. Guidos-Fogelbach has nothing to disclose.
Dr Hide reports personal fees from BioCryst, CSL Behring, Takeda and Torii.
Dr. Kang has nothing to disclose.
Dr. Kaplan has nothing to disclose.
Dr Katelaris reports grants from CSL Behring, personal fees from CSL Behring, personal fees from Takeda, during the conduct of the study.
Dr. Kiani-Alikhan reports personal fees from BioCryst, personal fees from CSL Behring, personal fees from Takeda, outside the submitted work.
Dr. Lei has nothing to disclose.
Dr. Lockey has nothing to disclose.
Dr. Longhurst has consulted for, acted as speaker for or collaborated in research with the following: Adverum, BioCryst, CSL Behring, GSK, Intellia, Ionis, Kalivista, Pharming, Pharvaris, and Takeda.
Dr. Lumry has consulted for, acted as speaker for or collaborated in research with the following: Adverum, Astra Zeneca, BioCryst, Biomarin, CSL Behring, Fresenius Kabi, GSK, Intellia, Ionis, Kalivista, Pharming, Pharvaris, Sanofi Regeneron, Takeda and Teva.
Dr. MacGinnitie reports personal fees from Biocryst, during the conduct of the study.
Dr. Malbran has nothing to disclose.
Inmaculada Martinez Saguer has received research grant support and/or speaker/consultancy fees from BioCryst, CSL Behring, KalVista, Pharming, and Takeda.
Dr. Matta has nothing to disclose.
Dr. Nast has no conflict of interest in relation to this manuscript.
Dr. Nguyen has nothing to disclose.
Dr. Nieto-Martinez has nothing to disclose.
Dr. Pawankar declares no conflicts of interest in relation to this manuscript.
Dr. Peter has nothing to disclose.
Dr. Porebski is or recently was a speaker and/or advisor for CSL Behring, Takeda, Pharming, and has served as an investigator for clinical trials sponsored by BioCryst Pharmaceuticals.
Dr. Prior reports receipt of research grant support and/or speaker/consultancy fees from CSL Behring, Pharming, and Shire (currently Takeda).
Dr. Reshef Received research grants, speakers and consulting honoraria from CSL Behring, Stallergens, Teva, Pharming, BioCryst, Pharvaris, Takeda (Shire) - not related to the article.
Dr. Marc Riedl has received research grants from BioCryst, CSL Behring, Ionis, Kalvista, Pharvaris, and Takeda outside the submitted work; consultancy fees from Astria, BioCryst, Biomarin, CSL Behring, Cycle Pharma, Fresenius-Kabi, Ipsen, Kalvista, Ono Pharma, Pfizer, Pharming, Pharvaris, RegenexBio, and Takeda outside the submitted work.
Dr. Bruce Ritchie has received Research grants from CSL-Behring, and has participated in clinical trials with Biocrist, CSL-Behring, Ionis, Kalvista, Pharvaris, and Takeda. The University of Alberta has received donations in lieu of consultancy fees to Dr. Ritchie from CSL Behring, Takeda.
Dr. Sheikh reports other from Takeda, grants from CSL Behring, outside the submitted work.
Dr. Smith reports other from BioCryst, during the conduct of the study; personal fees from Takeda/Shire, personal fees from CSL Behring, outside the submitted work.
Dr. Späth reports other from CSL Ltd, outside the submitted work; and Expert in an ongoing litigation.
Dr. Stobiecki reports other from Takeda, Biocryst, Pharming, Kalvista, fees from CSL Behring, Takeda, Biocryst, outside the submitted work.
Dr. Toubi has no conflict of interest in relation to this manuscript.
Dr. Varga has no conflict of interest in relation to this manuscript.
Dr. Weller reports personal fees from Biocryst, CSL Behring, MOXIE and Pharvaris as well as grants and personal fees from Shire/Takeda, all outside the submitted work.
Dr. Zanichelli has no conflict of interest in relation to this manuscript.
Dr. Zhi has nothing to disclose.
Dr. Zuraw has been a paid consultant for Biomarin, CSL Behring, Cycle Pharma, Fresenius-Kabi, and Takeda. He serves on adjudication boards for Novartis and Genentech.
Dr. Craig does research for CSL Behring, Ionis, Takeda, Kalvista, Pharvaris, BioMarin, and Biocryst. He speaks for CSL Behring, Takeda and Grifols. He has a grant to support education from Takeda. He consults with Spark, CSL Behring, Takeda, BioMarin, Pharming and Biocryst.
Acknowledgements
The authors would like to thank to Beate Schinzel and Sofia Dorsano for their fantastic support during the process of the guideline development. They would also like to thank the societies for the support of their delegates.
Footnotes
Important: As this is an international guideline, no information is provided regarding the licensing of the drugs mentioned for the treatment of HAE. It is the duty of the treating physician to adhere to the relevant local regulations.
Full list of author information is available at the end of the article.
Contributor Information
Marcus Maurer, Email: marcus.maurer@charite.de.
Markus Magerl, Email: markus.magerl@charite.de.
References
- 1.Rosen F.S., Pensky J., Donaldson V., Charache P. Hereditary angioneurotic edema: two genetic variants. Science. 1965;148(3672):957–958. doi: 10.1126/science.148.3672.957. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson V.H., Evans R.R. A biochemical abnormality in herediatry angioneurotic edema: absence of serum inhibitor of C' 1-esterase. Am J Med. 1963;35:37–44. doi: 10.1016/0002-9343(63)90162-1. [DOI] [PubMed] [Google Scholar]
- 3.Nussberger J., Cugno M., Amstutz C., Cicardi M., Pellacani A., Agostoni A. Plasma bradykinin in angio-oedema. Lancet. 1998;351(9117):1693–1697. doi: 10.1016/S0140-6736(97)09137-X. [DOI] [PubMed] [Google Scholar]
- 4.Maurer M., Magerl M., Ansotegui I., et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2017 revision and update. Allergy. 2018;73(8):1575–1596. doi: 10.1111/all.13384. [DOI] [PubMed] [Google Scholar]
- 5.Maurer M., Magerl M., Ansotegui I., et al. The international WAO/EAACI guideline for the management of hereditary angioedema – the 2017 revision and update. World Allergy Org J. 2018;11:5. doi: 10.1111/all.13384. [DOI] [PubMed] [Google Scholar]
- 6.Bowen T., Cicardi M., Farkas H., et al. Canadian 2003 international consensus algorithm for the diagnosis, therapy, and management of hereditary angioedema. J Allergy Clin Immunol. 2004;114(3):629–637. doi: 10.1016/j.jaci.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Bowen T., Cicardi M., Bork K., et al. Hereditary angiodema: a current state-of-the-art review, VII: Canadian Hungarian 2007 international consensus algorithm for the diagnosis, therapy, and management of hereditary angioedema. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2008;100(1 Suppl 2):S30–S40. doi: 10.1016/s1081-1206(10)60584-4. [DOI] [PubMed] [Google Scholar]
- 8.Bowen T., Cicardi M., Farkas H., et al. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2010;6(1):24. doi: 10.1186/1710-1492-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longhurst H.J., Farkas H., Craig T., et al. HAE international home therapy consensus document. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2010;6(1):22. doi: 10.1186/1710-1492-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caballero T., Baeza M.L., Cabanas R., et al. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part I. Classification, epidemiology, pathophysiology, genetics, clinical symptoms, and diagnosis. J Investig Allergol Clin Immunol. 2011;21(5):333–347. quiz follow 347. [PubMed] [Google Scholar]
- 11.Caballero T., Baeza M.L., Cabanas R., et al. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part II. Treatment, follow-up, and special situations. J Investig Allergol Clin Immunol. 2011;21(6):422–441. quiz 442-423. [PubMed] [Google Scholar]
- 12.Giavina-Bianchi P., Franca A.T., Grumach A.S., et al. Brazilian guidelines for the diagnosis and treatment of hereditary angioedema. Clinics. 2011;66(9):1627–1636. doi: 10.1590/S1807-59322011000900021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brozek J.L., Akl E.A., Compalati E., et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy. 2011;66(5):588–595. doi: 10.1111/j.1398-9995.2010.02530.x. [DOI] [PubMed] [Google Scholar]
- 14.Jindal A.K., Reshef A., Longhurst H., workgroup G. Mitigating disparity in health-care resources between countries for management of hereditary angioedema. Clin Rev Allergy Immunol. 2021;61(1):84–97. doi: 10.1007/s12016-021-08854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalkey N. Experimental study of group opinion - delphi method. Futures. 1969;1(5):408–426. [Google Scholar]
- 16.Diamond I.R., Grant R.C., Feldman B.M., et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Bork K., Meng G., Staubach P., Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):267–274. doi: 10.1016/j.amjmed.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 18.Cicardi M., Bergamaschini L., Marasini B., Boccassini G., Tucci A., Agostoni A. Hereditary angioedema: an appraisal of 104 cases. Am J Med Sci. 1982;284(1):2–9. doi: 10.1097/00000441-198207000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Dinkelacker E. Kiel: Medicinische Facultät zu Kiel; 1882. Ueber Acutes Oedem. Inaugural-Dissertation. [Google Scholar]
- 20.Rosen F.S., Austen K.F. The "neurotic edema" (hereditary angioedema) N Engl J Med. 1969;280(24):1356–1357. doi: 10.1056/NEJM196906122802414. [DOI] [PubMed] [Google Scholar]
- 21.Zuraw B.L., Christiansen S.C. Pathophysiology of hereditary angioedema. Am J Rhinol Allergy. 2011;25(6):373–378. doi: 10.2500/ajra.2011.25.3661. [DOI] [PubMed] [Google Scholar]
- 22.Cicardi M., Aberer W., Banerji A., et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69(5):602–616. doi: 10.1111/all.12380. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan A.P., Greaves M.W. Angioedema. J Am Acad Dermatol. 2005;53(3):373–388. doi: 10.1016/j.jaad.2004.09.032. quiz 389-392. [DOI] [PubMed] [Google Scholar]
- 24.Maurer M., Magerl M. Differences and similarities in the mechanisms and clinical expression of bradykinin-mediated vs. Mast cell-mediated angioedema. Clin Rev Allergy Immunol. 2021;61(1):40–49. doi: 10.1007/s12016-021-08841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulkes K.J., Van den Elzen M.T., Hack E.C., Otten H.G., Bruijnzeel-Koomen C.A., Knulst A.C. Clinical similarities among bradykinin-mediated and mast cell-mediated subtypes of non-hereditary angioedema: a retrospective study. Clin Transl Allergy. 2015;5(1):5. doi: 10.1186/s13601-015-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Can P.K., Degi Rmentepe E.N., Etikan P., et al. Assessment of disease activity and quality of life in patients with recurrent bradykinin-mediated versus mast cell-mediated angioedema. World Allergy Organ J. 2021;14(7):100554. doi: 10.1016/j.waojou.2021.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obtulowicz K. Bradykinin-mediated angioedema. Pol Arch Med Wewn. 2016;126(1-2):76–85. doi: 10.20452/pamw.3273. [DOI] [PubMed] [Google Scholar]
- 28.Cicardi M., Zuraw B.L. Angioedema due to bradykinin dysregulation. J Allergy Clin Immunol Pract. 2018;6(4):1132–1141. doi: 10.1016/j.jaip.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Lepelley M., Bernardeau C., Defendi F., Crochet J., Mallaret M., Bouillet L. Update on bradykinin-mediated angioedema in 2020. Therapie. 2020;75(2):195–205. doi: 10.1016/j.therap.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Bork K., Barnstedt S.E., Koch P., Traupe H. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet. 2000;356(9225):213–217. doi: 10.1016/S0140-6736(00)02483-1. [DOI] [PubMed] [Google Scholar]
- 31.Dewald G., Bork K. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun. 2006;343(4):1286–1289. doi: 10.1016/j.bbrc.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 32.Bafunno V., Firinu D., D'Apolito M., et al. Mutation of the angiopoietin-1 gene (ANGPT1) associates with a new type of hereditary angioedema. J Allergy Clin Immunol. 2018;141(3):1009–1017. doi: 10.1016/j.jaci.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Bork K., Wulff K., Steinmuller-Magin L., et al. Hereditary angioedema with a mutation in the plasminogen gene. Allergy. 2018;73(2):442–450. doi: 10.1111/all.13270. [DOI] [PubMed] [Google Scholar]
- 34.Bork K., Wulff K., Rossmann H., et al. Hereditary angioedema cosegregating with a novel kininogen 1 gene mutation changing the N-terminal cleavage site of bradykinin. Allergy. 2019;74(12):2479–2481. doi: 10.1111/all.13869. [DOI] [PubMed] [Google Scholar]
- 35.Ariano A., D'Apolito M., Bova M., et al. A myoferlin gain-of-function variant associates with a new type of hereditary angioedema. Allergy. 2020;75(11):2989–2992. doi: 10.1111/all.14454. [DOI] [PubMed] [Google Scholar]
- 36.Bork K., Wulff K., Mohl B.S., et al. Novel hereditary angioedema linked with a heparan sulfate 3-O-sulfotransferase 6 gene mutation. J Allergy Clin Immunol. 2021;148(4):1041–1048. doi: 10.1016/j.jaci.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Bork K., Machnig T., Wulff K., Witzke G., Prusty S., Hardt J. Clinical features of genetically characterized types of hereditary angioedema with normal C1 inhibitor: a systematic review of qualitative evidence. Orphanet J Rare Dis. 2020;15(1):289. doi: 10.1186/s13023-020-01570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magerl M., Germenis A.E., Maas C., Maurer M. Hereditary angioedema with normal C1 inhibitor: update on evaluation and treatment. Immunol Allergy Clin. 2017;37(3):571–584. doi: 10.1016/j.iac.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Sobotkova M., Zachova R., Hakl R., et al. Acquired angioedema with C1 inhibitor deficiency: occurrence, clinical features, and management: a nationwide retrospective study in the Czech republic patients. Int Arch Allergy Immunol. 2021;182(7):642–649. doi: 10.1159/000512933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caldwell J.R., Ruddy S., Schur P.H., Austen K.F. Acquired C1 inhibitor deficiency in lymphosarcoma. Clin Immunol Immunopathol. 1972;1:39–52. [Google Scholar]
- 41.Sabroe R.A., Black A.K. Angiotensin-converting enzyme (ACE) inhibitors and angio-oedema. Br J Dermatol. 1997;136(2):153–158. [PubMed] [Google Scholar]
- 42.Brown T., Gonzalez J., Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377–1382. doi: 10.1111/jch.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasmussen E.R., von Buchwald C., Wadelius M., et al. Assessment of 105 patients with angiotensin converting enzyme-inhibitor induced angioedema. Int J Otolaryngol. 2017;2017:1476402. doi: 10.1155/2017/1476402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanichelli A., Azin G.M., Wu M.A., et al. Diagnosis, course, and management of angioedema in patients with acquired C1-inhibitor deficiency. J Allergy Clin Immunol Pract. 2017;5(5):1307–1313. doi: 10.1016/j.jaip.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 45.Bygum A. Hereditary angio-oedema in Denmark: a nationwide survey. Br J Dermatol. 2009;161(5):1153–1158. doi: 10.1111/j.1365-2133.2009.09366.x. [DOI] [PubMed] [Google Scholar]
- 46.Zanichelli A., Arcoleo F., Barca M.P., et al. A nationwide survey of hereditary angioedema due to C1 inhibitor deficiency in Italy. Orphanet J Rare Dis. 2015;10:11. doi: 10.1186/s13023-015-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aygoren-Pursun E., Magerl M., Maetzel A., Maurer M. Epidemiology of Bradykinin-mediated angioedema: a systematic investigation of epidemiological studies. Orphanet J Rare Dis. 2018;13(1):73. doi: 10.1186/s13023-018-0815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lumry W.R., Settipane R.A. Hereditary angioedema: epidemiology and burden of disease. Allergy Asthma Proc. 2020;41(Suppl 1):S08–S13. doi: 10.2500/aap.2020.41.200050. [DOI] [PubMed] [Google Scholar]
- 49.Germenis A.E., Speletas M. Genetics of hereditary angioedema revisited. Clin Rev Allergy Immunol. 2016;51(2):170–182. doi: 10.1007/s12016-016-8543-x. [DOI] [PubMed] [Google Scholar]
- 50.Ponard D., Gaboriaud C., Charignon D., et al. SERPING1 mutation update: mutation spectrum and C1 Inhibitor phenotypes. Hum Mutat. 2020;41(1):38–57. doi: 10.1002/humu.23917. [DOI] [PubMed] [Google Scholar]
- 51.Pappalardo E., Cicardi M., Duponchel C., et al. Frequent de novo mutations and exon deletions in the C1inhibitor gene of patients with angioedema. J Allergy Clin Immunol. 2000;106(6):1147–1154. doi: 10.1067/mai.2000.110471. [DOI] [PubMed] [Google Scholar]
- 52.Proper S.P., Lavery W.J., Bernstein J.A. Definition and classification of hereditary angioedema. Allergy Asthma Proc. 2020;41(Suppl 1):S03–S07. doi: 10.2500/aap.2020.41.200040. [DOI] [PubMed] [Google Scholar]
- 53.Guryanova I., Suffritti C., Parolin D., et al. Hereditary angioedema due to C1 inhibitor deficiency in Belarus: epidemiology, access to diagnosis and seven novel mutations in SERPING1 gene. Clin Mol Allergy. 2021;19(1):3. doi: 10.1186/s12948-021-00141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaplan A.P., Joseph K. Complement, kinins, and hereditary angioedema: mechanisms of plasma instability when C1 inhibitor is absent. Clin Rev Allergy Immunol. 2016;51(2):207–215. doi: 10.1007/s12016-016-8555-6. [DOI] [PubMed] [Google Scholar]
- 55.de Maat S., Joseph K., Maas C., Kaplan A.P. Blood clotting and the pathogenesis of types I and II hereditary angioedema. Clin Rev Allergy Immunol. 2021;60(3):348–356. doi: 10.1007/s12016-021-08837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Maat S., Hofman Z.L.M., Maas C. Hereditary angioedema: the plasma contact system out of control. J Thromb Haemostasis. 2018;16(9):1674–1685. doi: 10.1111/jth.14209. [DOI] [PubMed] [Google Scholar]
- 57.Donaldson V.H., Rosen F.S., Bing D.H. Kinin generation in hereditary angioneurotic edema (H.A.N.E.) plasma. Adv Exp Med Biol. 1983;156:183–191. [PubMed] [Google Scholar]
- 58.Jacques L., Couture R., Drapeau G., Regoli D. Capillary permeability induced by intravenous neurokinins. Receptor characterization and mechanism of action. Naunyn-Schmiedeberg’s Arch Pharmacol. 1989;340(2):170–179. doi: 10.1007/BF00168965. [DOI] [PubMed] [Google Scholar]
- 59.Whalley E.T., Amure Y.O., Lye R.H. Analysis of the mechanism of action of bradykinin on human basilar artery in vitro. Naunyn-Schmiedeberg’s Arch Pharmacol. 1987;335(4):433–437. doi: 10.1007/BF00165559. [DOI] [PubMed] [Google Scholar]
- 60.Whalley E.T., Nwator I.A., Stewart J.M., Vavrek R.J. Analysis of the receptors mediating vascular actions of bradykinin. Naunyn-Schmiedeberg’s Arch Pharmacol. 1987;336(4):430–433. doi: 10.1007/BF00164878. [DOI] [PubMed] [Google Scholar]
- 61.Maas C., Lopez-Lera A. Hereditary angioedema: insights into inflammation and allergy. Mol Immunol. 2019;112:378–386. doi: 10.1016/j.molimm.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 62.Bork K., Wulff K., Witzke G., Hardt J. Treatment for hereditary angioedema with normal C1-INH and specific mutations in the F12 gene (HAE-FXII) Allergy. 2017;72(2):320–324. doi: 10.1111/all.13076. [DOI] [PubMed] [Google Scholar]
- 63.Bork K., Wulff K., Witzke G., Machnig T., Hardt J. Treatment of patients with hereditary angioedema with the c.988A>G (p.Lys330Glu) variant in the plasminogen gene. Orphanet J Rare Dis. 2020;15(1):52. doi: 10.1186/s13023-020-1334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cicardi M., Zanichelli A. Diagnosing angioedema. Immunol Allergy Clin. 2013;33(4):449–456. doi: 10.1016/j.iac.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Nowicki R.J., Grubska-Suchanek E., Porebski G., et al. Angioedema. Interdisciplinary diagnostic and therapeutic recommendations of the polish dermatological society (PTD) and polish society of allergology (PTA) Postepy Dermatol Alergol. 2020;37(4):445–451. doi: 10.5114/ada.2020.98226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karim Y., Griffiths H., Deacock S. Normal complement C4 values do not exclude hereditary angioedema. J Clin Pathol. 2004;57(2):213–214. doi: 10.1136/jcp.2003.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarzi M.D., Hickey A., Forster T., Mohammadi M., Longhurst H.J. An evaluation of tests used for the diagnosis and monitoring of C1 inhibitor deficiency: normal serum C4 does not exclude hereditary angio-oedema. Clin Exp Immunol. 2007;149(3):513–516. doi: 10.1111/j.1365-2249.2007.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagenaar-Bos I.G., Drouet C., Aygoren-Pursun E., et al. Functional C1-inhibitor diagnostics in hereditary angioedema: assay evaluation and recommendations. J Immunol Methods. 2008;338(1-2):14–20. doi: 10.1016/j.jim.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 69.Aabom A., Bygum A., Koch C. Complement factor C4 activation in patients with hereditary angioedema. Clin Biochem. 2017;50(15):816–821. doi: 10.1016/j.clinbiochem.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Pedrosa M., Phillips-Angles E., Lopez-Lera A., Lopez-Trascasa M., Caballero T. Complement study versus CINH gene testing for the diagnosis of type I hereditary angioedema in children. J Clin Immunol. 2016;36(1):16–18. doi: 10.1007/s10875-015-0222-9. [DOI] [PubMed] [Google Scholar]
- 71.Lai Y., Zhang G., Inhaber N., et al. A robust multiplexed assay to quantify C1-inhibitor, C1q, and C4 proteins for in vitro diagnosis of hereditary angioedema from dried blood spot. J Pharm Biomed Anal. 2021;195:113844. doi: 10.1016/j.jpba.2020.113844. [DOI] [PubMed] [Google Scholar]
- 72.Germenis A.E., Margaglione M., Pesquero J.B., et al. International consensus on the use of genetics in the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2020;8(3):901–911. doi: 10.1016/j.jaip.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 73.Ebo D.G., Van Gasse A.L., Sabato V., et al. Hereditary angioedema in 2 sisters due to paternal gonadal mosaicism. J Allergy Clin Immunol Pract. 2018;6(1):277–279 e271. doi: 10.1016/j.jaip.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Buttgereit T., Maurer M. [Classification and pathophysiology of angioedema] Hautarzt. 2019;70(2):84–91. doi: 10.1007/s00105-018-4318-z. [DOI] [PubMed] [Google Scholar]
- 75.Loules G., Parsopoulou F., Zamanakou M., et al. Deciphering the genetics of primary angioedema with normal levels of C1 inhibitor. J Clin Med. 2020;9(11) doi: 10.3390/jcm9113402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emelyanov A.V., Leshenkova E.V., Kameneva G.A. [Diagnosis and treatment of hereditary angioedema with normal C1-inhibitor level] Ter Arkh. 2020;92(12):86–90. doi: 10.26442/00403660.2020.12.200447. [DOI] [PubMed] [Google Scholar]
- 77.Polai Z., Balla Z., Andrasi N., et al. A follow-up survey of patients with acquired angioedema due to C1-inhibitor deficiency. J Intern Med. 2021;289(4):547–558. doi: 10.1111/joim.13182. [DOI] [PubMed] [Google Scholar]
- 78.Gobert D., Bouillet L., Armengol G., et al. [Acquired angioedema due to C1-inhibitor deficiency: CREAK recommendations for diagnosis and treatment] Rev Med Interne. 2020;41(12):838–842. doi: 10.1016/j.revmed.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Gobert D., Paule R., Ponard D., et al. A nationwide study of acquired C1-inhibitor deficiency in France: characteristics and treatment responses in 92 patients. Medicine (Baltim) 2016;95(33) doi: 10.1097/MD.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bork K., Staubach-Renz P., Hardt J. Angioedema due to acquired C1-inhibitor deficiency: spectrum and treatment with C1-inhibitor concentrate. Orphanet J Rare Dis. 2019;14(1):65. doi: 10.1186/s13023-019-1043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balla Z., Zsilinszky Z., Polai Z., et al. The importance of complement testing in acquired angioedema related to angiotensin-converting enzyme inhibitors. J Allergy Clin Immunol Pract. 2021;9(2):947–955. doi: 10.1016/j.jaip.2020.08.052. [DOI] [PubMed] [Google Scholar]
- 82.Bas M., Greve J., Strassen U., Khosravani F., Hoffmann T.K., Kojda G. Angioedema induced by cardiovascular drugs: new players join old friends. Allergy. 2015;70(10):1196–1200. doi: 10.1111/all.12680. [DOI] [PubMed] [Google Scholar]
- 83.Bouillet L., Boccon-Gibod I., Ponard D., et al. Bradykinin receptor 2 antagonist (icatibant) for hereditary angioedema type III attacks. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2009;103(5):448. doi: 10.1016/S1081-1206(10)60369-9. [DOI] [PubMed] [Google Scholar]
- 84.Bova M., Suffritti C., Bafunno V., et al. Impaired control of the contact system in hereditary angioedema with normal C1-inhibitor. Allergy. 2020;75(6):1394–1403. doi: 10.1111/all.14160. [DOI] [PubMed] [Google Scholar]
- 85.Magerl M., Bader M., Gompel A., et al. Bradykinin in health and disease: proceedings of the bradykinin symposium 2012, berlin 23-24 august 2012. Inflamm Res. 2014;63(3):173–178. doi: 10.1007/s00011-013-0693-1. [DOI] [PubMed] [Google Scholar]
- 86.Zuberbier T., Aberer W., Asero R., et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 87.Rasmussen E.R., de Freitas P.V., Bygum A. Urticaria and prodromal symptoms including erythema marginatum in Danish patients with hereditary angioedema. Acta Derm Venereol. 2016;96(3):373–376. doi: 10.2340/00015555-2233. [DOI] [PubMed] [Google Scholar]
- 88.Tadros S., Hayman G.R. Chronic spontaneous urticaria and angioedema requiring treatment with omalizumab in a patient with hereditary angioedema. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2019;122(6):666–667. doi: 10.1016/j.anai.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 89.Martin L., Renne T., Drouet C. Urticaria as a presenting prodromal manifestation of attacks of hereditary angioedema. Acta Derm Venereol. 2016;96(4):574–575. doi: 10.2340/00015555-2350. [DOI] [PubMed] [Google Scholar]
- 90.Maurer M., Hawro T., Krause K., et al. Diagnosis and treatment of chronic inducible urticaria. Allergy. 2019;74(12):2550–2553. doi: 10.1111/all.13878. [DOI] [PubMed] [Google Scholar]
- 91.Bernstein J.A., Lang D.M., Khan D.A., et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270–1277. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 92.Peveling-Oberhag A., Reimann H., Weyer V., Goloborodko E., Staubach P. High-concentration liquid prednisolone formula: filling a therapeutic niche in severe acute attacks of urticaria and angioedema. Skin Pharmacol Physiol. 2016;29(1):9–12. doi: 10.1159/000439032. [DOI] [PubMed] [Google Scholar]
- 93.Santa C., Valente C.L., Mesquita M., et al. Acute urticaria in children: from pediatric emergency department to allergology consultation at a central hospital. Eur Ann Allergy Clin Immunol. 2021 doi: 10.23822/EurAnnACI.1764-1489.204. [DOI] [PubMed] [Google Scholar]
- 94.Rajan N., Sharma V., Patro S.K., Goyal A. Acute presentation of undiagnosed hereditary angioedema of the larynx: averting death. Turk Arch Otolaryngol. 2020;58(4):279–281. doi: 10.5152/tao.2020.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Piotrowicz-Wojcik K., Porebski G. Life-threatening laryngeal attacks in hereditary angioedema patients. Otolaryngol Pol. 2020;74(2):1–5. doi: 10.5604/01.3001.0014.0619. [DOI] [PubMed] [Google Scholar]
- 96.Moldovan D., Bara N., Nadasan V., Gabos G., Mihaly E. Consequences of misdiagnosed and mismanaged hereditary angioedema laryngeal attacks: an overview of cases from the Romanian registry. Case Rep Emerg Med. 2018;2018:6363787. doi: 10.1155/2018/6363787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bork K., Hardt J., Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol. 2012;130(3):692–697. doi: 10.1016/j.jaci.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 98.Gutierrez M., Veronez C.L., Rodrigues Valle S.O., et al. Unnecessary abdominal surgeries in attacks of hereditary angioedema with normal C1 inhibitor. Clin Rev Allergy Immunol. 2021;61(1):60–65. doi: 10.1007/s12016-021-08852-7. [DOI] [PubMed] [Google Scholar]
- 99.Poza Cordon J., de Maria Pallares P., Caballero Molina T. Ultrasound findings in an abdominal crisis of a patient with hereditary angioedema. Rev Esp Enferm Dig. 2020;112(5):418. doi: 10.17235/reed.2020.6469/2019. [DOI] [PubMed] [Google Scholar]
- 100.Bork K., Staubach P., Eckardt A.J., Hardt J. Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. Am J Gastroenterol. 2006;101(3):619–627. doi: 10.1111/j.1572-0241.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- 101.Kusuma A., Relan A., Knulst A.C., et al. Clinical impact of peripheral attacks in hereditary angioedema patients. Am J Med. 2012;125(9) doi: 10.1016/j.amjmed.2011.12.016. 937 e917-924. [DOI] [PubMed] [Google Scholar]
- 102.Christiansen S.C., Bygum A., Banerji A., et al. Before and after, the impact of available on-demand treatment for HAE. Allergy Asthma Proc. 2015;36(2):145–150. doi: 10.2500/aap.2015.36.3831. [DOI] [PubMed] [Google Scholar]
- 103.Zanichelli A., Mansi M., Azin G.M., et al. Efficacy of on-demand treatment in reducing morbidity in patients with hereditary angioedema due to C1 inhibitor deficiency. Allergy. 2015;70(12):1553–1558. doi: 10.1111/all.12731. [DOI] [PubMed] [Google Scholar]
- 104.Bork K., Barnstedt S.E. Treatment of 193 episodes of laryngeal edema with C1 inhibitor concentrate in patients with hereditary angioedema. Arch Intern Med. 2001;161(5):714–718. doi: 10.1001/archinte.161.5.714. [DOI] [PubMed] [Google Scholar]
- 105.Craig T.J., Wasserman R.L., Levy R.J., et al. Prospective study of rapid relief provided by C1 esterase inhibitor in emergency treatment of acute laryngeal attacks in hereditary angioedema. J Clin Immunol. 2010;30(6):823–829. doi: 10.1007/s10875-010-9442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sheffer A.L., MacGinnitie A.J., Campion M., Stolz L.E., Pullman W.E. Outcomes after ecallantide treatment of laryngeal hereditary angioedema attacks. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2013;110(3):184–188 e182. doi: 10.1016/j.anai.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 107.Banta E., Horn P., Craig T.J. Response to ecallantide treatment of acute attacks of hereditary angioedema based on time to intervention: results from the EDEMA clinical trials. Allergy Asthma Proc. 2011;32(4):319–324. doi: 10.2500/aap.2011.32.3440. [DOI] [PubMed] [Google Scholar]
- 108.Craig T.J., Bewtra A.K., Bahna S.L., et al. C1 esterase inhibitor concentrate in 1085 Hereditary Angioedema attacks--final results of the I.M.P.A.C.T.2 study. Allergy. 2011;66(12):1604–1611. doi: 10.1111/j.1398-9995.2011.02702.x. [DOI] [PubMed] [Google Scholar]
- 109.Craig T.J., Rojavin M.A., Machnig T., Keinecke H.O., Bernstein J.A. Effect of time to treatment on response to C1 esterase inhibitor concentrate for hereditary angioedema attacks. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2013;111(3):211–215. doi: 10.1016/j.anai.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 110.Maurer M., Aberer W., Bouillet L., et al. Hereditary angioedema attacks resolve faster and are shorter after early icatibant treatment. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0053773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hernandez Fernandez de Rojas D., Ibanez E., Longhurst H., et al. Treatment of HAE attacks in the icatibant outcome survey: an analysis of icatibant self-administration versus administration by health care professionals. Int Arch Allergy Immunol. 2015;167(1):21–28. doi: 10.1159/000430864. [DOI] [PubMed] [Google Scholar]
- 112.Leibovich-Nassi I., Golander H., Somech R., Har-Even D., Reshef A. New instrument for the evaluation of prodromes and attacks of hereditary angioedema (HAE-EPA) Clin Rev Allergy Immunol. 2021;61(1):29–39. doi: 10.1007/s12016-021-08843-8. [DOI] [PubMed] [Google Scholar]
- 113.Leibovich-Nassi I., Reshef A. The enigma of prodromes in hereditary angioedema (HAE) Clin Rev Allergy Immunol. 2021;61(1):15–28. doi: 10.1007/s12016-021-08839-4. [DOI] [PubMed] [Google Scholar]
- 114.Magerl M., Doumoulakis G., Kalkounou I., et al. Characterization of prodromal symptoms in a large population of patients with hereditary angio-oedema. Clin Exp Dermatol. 2014;39(3):298–303. doi: 10.1111/ced.12285. [DOI] [PubMed] [Google Scholar]
- 115.Prematta M.J., Bewtra A.K., Levy R.J., et al. Per-attack reporting of prodromal symptoms concurrent with C1-inhibitor treatment of hereditary angioedema attacks. Adv Ther. 2012;29(10):913–922. doi: 10.1007/s12325-012-0053-5. [DOI] [PubMed] [Google Scholar]
- 116.Cinryze Shire. 2021. Prescribing Information. Accessed August 16, 2021. [Google Scholar]
- 117.Behring C. 2021. Berinert 500 Prescribing Information. 2021. August 16, 2021. [Google Scholar]
- 118.Takeda . 2020. Kalbitor Prescribing Information. Accessed August 16, 2021. [Google Scholar]
- 119.PharmingGroup . 2020. Ruconest Prescribing Information. Accessed August 16, 2021. [Google Scholar]
- 120.Takeda . 2021. Prescribing Information Icatibant. [Google Scholar]
- 121.Cicardi M., Banerji A., Bracho F., et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363(6):532–541. doi: 10.1056/NEJMoa0906393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Craig T.J., Levy R.J., Wasserman R.L., et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol. 2009;124(4):801–808. doi: 10.1016/j.jaci.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 123.Levy R.J., Lumry W.R., McNeil D.L., et al. EDEMA4: a phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2010;104(6):523–529. doi: 10.1016/j.anai.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 124.Riedl M.A., Bernstein J.A., Li H., et al. Recombinant human C1-esterase inhibitor relieves symptoms of hereditary angioedema attacks: phase 3, randomized, placebo-controlled trial. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2014;112(2):163–169 e161. doi: 10.1016/j.anai.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 125.Zuraw B., Cicardi M., Levy R.J., et al. Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol. 2010;126(4):821–827 e814. doi: 10.1016/j.jaci.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 126.Zuraw B.L., Busse P.J., White M., et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010;363(6):513–522. doi: 10.1056/NEJMoa0805538. [DOI] [PubMed] [Google Scholar]
- 127.Longhurst H.J. Emergency treatment of acute attacks in hereditary angioedema due to C1 inhibitor deficiency: what is the evidence? Int J Clin Pract. 2005;59(5):594–599. doi: 10.1111/j.1742-1241.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 128.Wentzel N., Panieri A., Ayazi M., et al. Fresh frozen plasma for on-demand hereditary angioedema treatment in South Africa and Iran. World Allergy Organ J. 2019;12(9):100049. doi: 10.1016/j.waojou.2019.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sabeen Ahmed A., Fayyaz S. Novel use of fresh frozen plasma in treating hereditary angioedema: a success story from Pakistan. Cureus. 2020;12(8) doi: 10.7759/cureus.9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zanichelli A., Vacchini R., Badini M., Penna V., Cicardi M. Standard care impact on angioedema because of hereditary C1 inhibitor deficiency: a 21-month prospective study in a cohort of 103 patients. Allergy. 2011;66(2):192–196. doi: 10.1111/j.1398-9995.2010.02433.x. [DOI] [PubMed] [Google Scholar]
- 131.Kunschak M., Engl W., Maritsch F., et al. A randomized, controlled trial to study the efficacy and safety of C1 inhibitor concentrate in treating hereditary angioedema. Transfusion. 1998;38(6):540–549. doi: 10.1046/j.1537-2995.1998.38698326333.x. [DOI] [PubMed] [Google Scholar]
- 132.Waytes A.T., Rosen F.S., Frank M.M. Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. N Engl J Med. 1996;334(25):1630–1634. doi: 10.1056/NEJM199606203342503. [DOI] [PubMed] [Google Scholar]
- 133.Schulz K.H. [Hereditary Quincke's edema. New therapeutic ways] Hautarzt. 1974;25(1):12–16. [PubMed] [Google Scholar]
- 134.Bernstein J.A., Ritchie B., Levy R.J., et al. Population pharmacokinetics of plasma-derived C1 esterase inhibitor concentrate used to treat acute hereditary angioedema attacks. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2010;105(2):149–154. doi: 10.1016/j.anai.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 135.Martinez-Saguer I., Cicardi M., Suffritti C., et al. Pharmacokinetics of plasma-derived C1-esterase inhibitor after subcutaneous versus intravenous administration in subjects with mild or moderate hereditary angioedema: the PASSION study. Transfusion. 2014;54(6):1552–1561. doi: 10.1111/trf.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martinez-Saguer I., Rusicke E., Aygoren-Pursun E., von Hentig N., Klingebiel T., Kreuz W. Pharmacokinetic analysis of human plasma-derived pasteurized C1-inhibitor concentrate in adults and children with hereditary angioedema: a prospective study. Transfusion. 2010;50(2):354–360. doi: 10.1111/j.1537-2995.2009.02394.x. [DOI] [PubMed] [Google Scholar]
- 137.Brackertz D., Isler E., Kueppers F. Half-life of C1INH in hereditary angioneurotic oedema (HAE) Clin Allergy. 1975;5(1):89–94. doi: 10.1111/j.1365-2222.1975.tb01839.x. [DOI] [PubMed] [Google Scholar]
- 138.Hofstra J.J., Budde I.K., van Twuyver E., et al. Treatment of hereditary angioedema with nanofiltered C1-esterase inhibitor concentrate (Cetor(R)): multi-center phase II and III studies to assess pharmacokinetics, clinical efficacy and safety. Clin Immunol. 2012;142(3):280–290. doi: 10.1016/j.clim.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 139.De Serres J., Groner A., Lindner J. Safety and efficacy of pasteurized C1 inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus Apher Sci: Off J World Apher Asso Off J Euro Soc Haemapher. 2003;29(3):247–254. doi: 10.1016/j.transci.2003.08.006. jean.de.serres@aventis.com. [DOI] [PubMed] [Google Scholar]
- 140.Groner A., Nowak T., Schafer W. Pathogen safety of human C1 esterase inhibitor concentrate. Transfusion. 2012;52(10):2104–2112. doi: 10.1111/j.1537-2995.2012.03590.x. [DOI] [PubMed] [Google Scholar]
- 141.Simon T.L., Kalina U., Laske R., Mycroft S., Widmer E., Roth N.J. Manufacturing of plasma-derived C1-inhibitor concentrate for treatment of patients with hereditary angioedema. Allergy Asthma Proc. 2020;41(2):99–107. doi: 10.2500/aap.2020.41.190021. [DOI] [PubMed] [Google Scholar]
- 142.Terpstra F.G., Kleijn M., Koenderman A.H., et al. Viral safety of C1-inhibitor NF. Biologicals. 2007;35(3):173–181. doi: 10.1016/j.biologicals.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 143.Reshef A., Grivcheva-Panovska V., Kessel A., et al. Recombinant human C1 esterase inhibitor treatment for hereditary angioedema attacks in children. Pediatr Allergy Immunol. 2019;30(5):562–568. doi: 10.1111/pai.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Farrell C., Hayes S., Relan A., van Amersfoort E.S., Pijpstra R., Hack C.E. Population pharmacokinetics of recombinant human C1 inhibitor in patients with hereditary angioedema. Br J Clin Pharmacol. 2013;76(6):897–907. doi: 10.1111/bcp.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Doorn M.B., Burggraaf J., van Dam T., et al. A phase I study of recombinant human C1 inhibitor in asymptomatic patients with hereditary angioedema. J Allergy Clin Immunol. 2005;116(4):876–883. doi: 10.1016/j.jaci.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 146.van Veen H.A., Koiter J., Vogelezang C.J., et al. Characterization of recombinant human C1 inhibitor secreted in milk of transgenic rabbits. J Biotechnol. 2012;162(2-3):319–326. doi: 10.1016/j.jbiotec.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 147.Moldovan D., Bernstein J.A., Cicardi M. Recombinant replacement therapy for hereditary angioedema due to C1 inhibitor deficiency. Immunotherapy. 2015;7(7):739–752. doi: 10.2217/imt.15.44. [DOI] [PubMed] [Google Scholar]
- 148.Moldovan D., Reshef A., Fabiani J., et al. Efficacy and safety of recombinant human C1-inhibitor for the treatment of attacks of hereditary angioedema: European open-label extension study. Clin Exp Allergy. 2012;42(6):929–935. doi: 10.1111/j.1365-2222.2012.03984.x. [DOI] [PubMed] [Google Scholar]
- 149.Riedl M. Recombinant human C1 esterase inhibitor in the management of hereditary angioedema. Clin Drug Invest. 2015;35(7):407–417. doi: 10.1007/s40261-015-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.DyaxCorp . 2011. Withdrawal of Kalbitor at EMA. [Google Scholar]
- 151.Cicardi M., Levy R.J., McNeil D.L., et al. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010;363(6):523–531. doi: 10.1056/NEJMoa0905079. [DOI] [PubMed] [Google Scholar]
- 152.Craig T.J., Li H.H., Riedl M., et al. Characterization of anaphylaxis after ecallantide treatment of hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2015;3(2):206–212 e204. doi: 10.1016/j.jaip.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 153.Antonio A., Rocha E.S.M. Coronary vasodilation produced by bradykinin on isolated mammalian heart. Circ Res. 1962;11:910–915. doi: 10.1161/01.res.11.6.910. [DOI] [PubMed] [Google Scholar]
- 154.Erikson U. Peripheral arteriography during bradykinin induced vasodilation. Acta Radiol Diagn (Stockh). 1965;3:193–201. doi: 10.1177/028418516500300301. [DOI] [PubMed] [Google Scholar]
- 155.Rocha E.S.M., Beraldo W.T., Rosenfeld G. Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. Am J Physiol. 1949;156(2):261–273. doi: 10.1152/ajplegacy.1949.156.2.261. [DOI] [PubMed] [Google Scholar]
- 156.Longhurst H.J., Aberer W., Bouillet L., et al. Analysis of characteristics associated with reinjection of icatibant: results from the icatibant outcome survey. Allergy Asthma Proc. 2015;36(5):399–406. doi: 10.2500/aap.2015.36.3892. [DOI] [PubMed] [Google Scholar]
- 157.Bork K., Frank J., Grundt B., Schlattmann P., Nussberger J., Kreuz W. Treatment of acute edema attacks in hereditary angioedema with a bradykinin receptor-2 antagonist (Icatibant) J Allergy Clin Immunol. 2007;119(6):1497–1503. doi: 10.1016/j.jaci.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 158.Farkas H. Icatibant as acute treatment for hereditary angioedema in adults. Expet Rev Clin Pharmacol. 2016;9(6):779–788. doi: 10.1080/17512433.2016.1182425. [DOI] [PubMed] [Google Scholar]
- 159.Farkas H., Kohalmi K.V. Icatibant for the treatment of hereditary angioedema with C1-inhibitor deficiency in adolescents and in children aged over 2 years. Expet Rev Clin Immunol. 2018;14(6):447–460. doi: 10.1080/1744666X.2018.1476851. [DOI] [PubMed] [Google Scholar]
- 160.Bork K., Bernstein J.A., Machnig T., Craig T.J. Efficacy of different medical therapies for the treatment of acute laryngeal attacks of hereditary angioedema due to C1-esterase inhibitor deficiency. J Emerg Med. 2016;50(4):567–580 e561. doi: 10.1016/j.jemermed.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 161.Bork K., Siedlecki K., Bosch S., Schopf R.E., Kreuz W. Asphyxiation by laryngeal edema in patients with hereditary angioedema. Mayo Clin Proc. 2000;75(4):349–354. doi: 10.4065/75.4.349. Mayo Clinic. [DOI] [PubMed] [Google Scholar]
- 162.Javaud N., Gompel A., Bouillet L., et al. Factors associated with hospital admission in hereditary angioedema attacks: a multicenter prospective study. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2015;114(6):499–503. doi: 10.1016/j.anai.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 163.Aygoren-Pursun E., Martinez Saguer I., Kreuz W., Klingebiel T., Schwabe D. Risk of angioedema following invasive or surgical procedures in HAE type I and II--the natural history. Allergy. 2013;68(8):1034–1039. doi: 10.1111/all.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bork K., Barnstedt S.E. Laryngeal edema and death from asphyxiation after tooth extraction in four patients with hereditary angioedema. J Am Dent Assoc. 2003;134(8):1088–1094. doi: 10.14219/jada.archive.2003.0323. [DOI] [PubMed] [Google Scholar]
- 165.Bork K., Hardt J., Staubach-Renz P., Witzke G. Risk of laryngeal edema and facial swellings after tooth extraction in patients with hereditary angioedema with and without prophylaxis with C1 inhibitor concentrate: a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(1):58–64. doi: 10.1016/j.tripleo.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 166.Farkas H., Zotter Z., Csuka D., et al. Short-term prophylaxis in hereditary angioedema due to deficiency of the C1-inhibitor--a long-term survey. Allergy. 2012;67(12):1586–1593. doi: 10.1111/all.12032. [DOI] [PubMed] [Google Scholar]
- 167.Forrest A., Milne N., Soon A. Hereditary angioedema: death after a dental extraction. Aust Dent J. 2017;62(1):107–110. doi: 10.1111/adj.12447. [DOI] [PubMed] [Google Scholar]
- 168.Nanda M.K., Singh U., Wilmot J., Bernstein J.A. A cross-sectional questionnaire assessing patient and physician use of short-term prophylaxis for hereditary angioedema. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2014;113(2):198–203. doi: 10.1016/j.anai.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Farkas H., Gyeney L., Gidofalvy E., Fust G., Varga L. The efficacy of short-term danazol prophylaxis in hereditary angioedema patients undergoing maxillofacial and dental procedures. J Oral Maxillofac Surg: Off J Am Asso Oral Maxillofac Surg. 1999;57(4):404–408. doi: 10.1016/s0278-2391(99)90280-x. [DOI] [PubMed] [Google Scholar]
- 170.Magerl M., Frank M., Lumry W., et al. Short-term prophylactic use of C1-inhibitor concentrate in hereditary angioedema: findings from an international patient registry. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2017;118(1):110–112. doi: 10.1016/j.anai.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 171.Valerieva A., Staevska M., Jesenak M., et al. Recombinant human C1 esterase inhibitor as short-term prophylaxis in patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2020;8(2):799–802. doi: 10.1016/j.jaip.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 172.Bernstein J.A., Coleman S., Bonnin A.J. Successful C1 inhibitor short-term prophylaxis during redo mitral valve replacement in a patient with hereditary angioedema. J Cardiothorac Surg. 2010;5:86. doi: 10.1186/1749-8090-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ajewole O., Lanlokun M., Dimanche S., Craig T. Short-term prophylaxis for children and adolescents with hereditary angioedema. Allergy Asthma Proc. 2021;42(3):205–213. doi: 10.2500/aap.2021.42.210006. [DOI] [PubMed] [Google Scholar]
- 174.Bowen T. Hereditary angioedema consensus 2010. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2010;6(1):13. doi: 10.1186/1710-1492-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gompels M.M., Lock R.J., Abinun M., et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005;139(3):379–394. doi: 10.1111/j.1365-2249.2005.02726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Betschel S., Badiou J., Binkley K., et al. The international/Canadian hereditary angioedema guideline. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2019;15:72. [Google Scholar]
- 177.Busse P.J., Christiansen S.C., Riedl M.A., et al. US HAEA medical advisory board 2020 guidelines for the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9(1):132–150 e133. doi: 10.1016/j.jaip.2020.08.046. [DOI] [PubMed] [Google Scholar]
- 178.Maurer M., Aygoren-Pursun E., Banerji A., et al. Consensus on treatment goals in hereditary angioedema: a global Delphi initiative. J Allergy Clin Immunol. 2021;148(6):1526–1532. doi: 10.1016/j.jaci.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 179.Kuman Tuncel O., Gokmen N.M., Demir E., Gulbahar O., Pirildar S. The impact of hereditary angioedema on quality of life and family planning decisions. Int J Psychiatr Med. 2019;54(6):377–394. doi: 10.1177/0091217419837068. [DOI] [PubMed] [Google Scholar]
- 180.Lumry W.R., Weller K., Magerl M., et al. Impact of lanadelumab on health-related quality of life in patients with hereditary angioedema in the HELP study. Allergy. 2021;76(4):1188–1198. doi: 10.1111/all.14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nicolas A., Launay D., Duprez C., et al. [Impact of disease on daily activities, emotions and quality of life of patients with hereditary angioedema] Rev Med Interne. 2021;42(9):608–615. doi: 10.1016/j.revmed.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 182.Lumry W.R., Zuraw B., Cicardi M., et al. Long-term health-related quality of life in patients treated with subcutaneous C1-inhibitor replacement therapy for the prevention of hereditary angioedema attacks: findings from the COMPACT open-label extension study. Orphanet J Rare Dis. 2021;16(1):86. doi: 10.1186/s13023-020-01658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lumry W.R., Miller D.P., Newcomer S., Fitts D., Dayno J. Quality of life in patients with hereditary angioedema receiving therapy for routine prevention of attacks. Allergy Asthma Proc. 2014;35(5):371–376. doi: 10.2500/aap.2014.35.3783. [DOI] [PubMed] [Google Scholar]
- 184.Brix A.T.H., Boysen H.B., Weller K., Caballero T., Bygum A. Patient-reported outcome measures for angioedema: a literature review. Acta Derm Venereol. 2021;101(5) doi: 10.2340/00015555-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Caballero T., Prior N. Burden of illness and quality-of-life measures in angioedema conditions. Immunol Allergy Clin. 2017;37(3):597–616. doi: 10.1016/j.iac.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 186.Forjaz M.J., Ayala A., Caminoa M., et al. HAE-AS: a specific disease activity scale for hereditary angioedema with C1-inhibitor deficiency. J Investig Allergol Clin Immunol. 2021;31(3):246–252. doi: 10.18176/jiaci.0479. [DOI] [PubMed] [Google Scholar]
- 187.Prior N., Remor E., Gomez-Traseira C., et al. Development of a disease-specific quality of life questionnaire for adult patients with hereditary angioedema due to C1 inhibitor deficiency (HAE-QoL): Spanish multi-centre research project. Health Qual Life Outcome. 2012;10:82. doi: 10.1186/1477-7525-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Prior N., Remor E., Perez-Fernandez E., et al. Psychometric field study of hereditary angioedema quality of life questionnaire for adults: HAE-QoL. J Allergy Clin Immunol Pract. 2016;4(3):464–473 e464. doi: 10.1016/j.jaip.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 189.Weller K., Donoso T., Magerl M., et al. Development of the Angioedema Control Test-A patient-reported outcome measure that assesses disease control in patients with recurrent angioedema. Allergy. 2020;75(5):1165–1177. doi: 10.1111/all.14144. [DOI] [PubMed] [Google Scholar]
- 190.Weller K., Donoso T., Magerl M., et al. Validation of the angioedema control test (AECT)-A patient-reported outcome instrument for assessing angioedema control. J Allergy Clin Immunol Pract. 2020;8(6):2050–2057 e2054. doi: 10.1016/j.jaip.2020.02.038. [DOI] [PubMed] [Google Scholar]
- 191.Weller K., Groffik A., Magerl M., et al. Development and construct validation of the angioedema quality of life questionnaire. Allergy. 2012;67(10):1289–1298. doi: 10.1111/all.12007. [DOI] [PubMed] [Google Scholar]
- 192.Weller K., Groffik A., Magerl M., et al. Development, validation, and initial results of the angioedema activity score. Allergy. 2013;68(9):1185–1192. doi: 10.1111/all.12209. [DOI] [PubMed] [Google Scholar]
- 193.Weller K., Magerl M., Peveling-Oberhag A., Martus P., Staubach P., Maurer M. The Angioedema Quality of Life Questionnaire (AE-QoL) - assessment of sensitivity to change and minimal clinically important difference. Allergy. 2016;71(8):1203–1209. doi: 10.1111/all.12900. [DOI] [PubMed] [Google Scholar]
- 194.Banerji A., Davis K.H., Brown T.M., et al. Patient-reported burden of hereditary angioedema: findings from a patient survey in the United States. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2020;124(6):600–607. doi: 10.1016/j.anai.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 195.Banerji A., Riedl M.A., Bernstein J.A., et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108–2121. doi: 10.1001/jama.2018.16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Buttgereit T., Vera C., Weller K., et al. Lanadelumab efficacy, safety, and injection interval extension in HAE: a real-life study. J Allergy Clin Immunol Pract. 2021;9(10):3744–3751. doi: 10.1016/j.jaip.2021.04.072. [DOI] [PubMed] [Google Scholar]
- 197.Craig T., Zuraw B., Longhurst H., et al. Long-term outcomes with subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2019;7(6):1793–1802 e1792. doi: 10.1016/j.jaip.2019.01.054. [DOI] [PubMed] [Google Scholar]
- 198.Hahn J., Trainotti S., Wigand M.C., Schuler P.J., Hoffmann T.K., Greve J. Prospective analysis in patients with HAE under prophylaxis with lanadelumab: a real-life experience. J Drugs Dermatol JDD. 2020;19(10):978–983. doi: 10.36849/JDD.2020.5269. [DOI] [PubMed] [Google Scholar]
- 199.Henry Li H., Riedl M., Kashkin J. Update on the use of C1-esterase inhibitor replacement therapy in the acute and prophylactic treatment of hereditary angioedema. Clin Rev Allergy Immunol. 2019;56(2):207–218. doi: 10.1007/s12016-018-8684-1. [DOI] [PubMed] [Google Scholar]
- 200.Li H.H., Zuraw B., Longhurst H.J., et al. Subcutaneous C1 inhibitor for prevention of attacks of hereditary angioedema: additional outcomes and subgroup analysis of a placebo-controlled randomized study. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2019;15:49. doi: 10.1186/s13223-019-0362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zarnowski J., Rabe M., Kage P., Simon J.C., Treudler R. Prophylactic treatment in hereditary angioedema is associated with reduced anxiety in patients in leipzig, Germany. Int Arch Allergy Immunol. 2021:1–8. doi: 10.1159/000514973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nicola S., Rolla G., Brussino L. Breakthroughs in hereditary angioedema management: a systematic review of approved drugs and those under research. Drugs Context. 2019;8:212605. doi: 10.7573/dic.212605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Aberer W., Maurer M., Bouillet L., et al. Breakthrough attacks in patients with hereditary angioedema receiving long-term prophylaxis are responsive to icatibant: findings from the Icatibant Outcome Survey. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2017;13:31. doi: 10.1186/s13223-017-0203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Banerji A., Bernstein J.A., Johnston D.T., et al. Long-term prevention of hereditary angioedema attacks with lanadelumab: the HELP OLE Study. Allergy. 2021 doi: 10.1111/all.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Longhurst H., Cicardi M., Craig T., et al. Prevention of hereditary angioedema attacks with a subcutaneous C1 inhibitor. N Engl J Med. 2017;376(12):1131–1140. doi: 10.1056/NEJMoa1613627. [DOI] [PubMed] [Google Scholar]
- 206.Wedner H.J., Aygoren-Pursun E., Bernstein J., et al. Randomized trial of the efficacy and safety of berotralstat (BCX7353) as an oral prophylactic therapy for hereditary angioedema: results of APeX-2 through 48 Weeks (Part 2) J Allergy Clin Immunol Pract. 2021;9(6):2305–2314 e2304. doi: 10.1016/j.jaip.2021.03.057. [DOI] [PubMed] [Google Scholar]
- 207.Zuraw B., Lumry W.R., Johnston D.T., et al. Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: a randomized, double-blind, placebo-controlled phase 3 trial. J Allergy Clin Immunol. 2021;148(1):164–172 e169. doi: 10.1016/j.jaci.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 208.Craig T., Aygoren-Pursun E., Bork K., et al. WAO guideline for the management of hereditary angioedema. World Allergy Organ J. 2012;5(12):182–199. doi: 10.1097/WOX.0b013e318279affa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Frank M.M. Update on preventive therapy (prophylaxis) for hereditary angioedema. Immunol Allergy Clin. 2013;33(4):495–503. doi: 10.1016/j.iac.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 210.Greve J., Strassen U., Gorczyza M., et al. Prophylaxis in hereditary angioedema (HAE) with C1 inhibitor deficiency. J Dtsch Dermatol Ges. 2016;14(3):266–275. doi: 10.1111/ddg.12856. [DOI] [PubMed] [Google Scholar]
- 211.Maurer M., Magerl M. Long-term prophylaxis of hereditary angioedema with androgen derivates: a critical appraisal and potential alternatives. J Dtsch Dermatol Ges. 2011;9(2):99–107. doi: 10.1111/j.1610-0387.2010.07546.x. [DOI] [PubMed] [Google Scholar]
- 212.Betschel S., Badiou J., Binkley K., et al. Canadian hereditary angioedema guideline. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2014;10(1):50. doi: 10.1186/1710-1492-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Aygoren-Pursun E., Soteres D.F., Nieto-Martinez S.A., et al. A randomized trial of human C1 inhibitor prophylaxis in children with hereditary angioedema. Pediatr Allergy Immunol. 2019;30(5):553–561. doi: 10.1111/pai.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lumry W., Manning M.E., Hurewitz D.S., et al. Nanofiltered C1-esterase inhibitor for the acute management and prevention of hereditary angioedema attacks due to C1-inhibitor deficiency in children. J Pediatr. 2013;162(5):1017–1022 e1011. doi: 10.1016/j.jpeds.2012.11.030. 1012. [DOI] [PubMed] [Google Scholar]
- 215.Lumry W.R., Martinez-Saguer I., Yang W.H., et al. Fixed-dose subcutaneous C1-inhibitor liquid for prophylactic treatment of C1-INH-HAE: SAHARA randomized study. J Allergy Clin Immunol Pract. 2019;7(5):1610–1618 e1614. doi: 10.1016/j.jaip.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 216.Zuraw B.L., Cicardi M., Longhurst H.J., et al. Phase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrate. Allergy. 2015;70(10):1319–1328. doi: 10.1111/all.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bernstein J.A., Manning M.E., Li H., et al. Escalating doses of C1 esterase inhibitor (CINRYZE) for prophylaxis in patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2014;2(1):77–84. doi: 10.1016/j.jaip.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 218.Giardino F., Cicardi M., Neri S. Use of subcutaneous-C1 INH for acute therapy and prophylaxis of a child with HAE. Pediatr Allergy Immunol. 2015;26(3):296–297. doi: 10.1111/pai.12364. [DOI] [PubMed] [Google Scholar]
- 219.Kruger R., Dahlinger N., Magerl M., von Bernuth H., Wahn V. Daily subcutaneous administration of human C1 inhibitor in a child with hereditary angioedema type 1. Pediatr Allergy Immunol. 2016;27(2):223–224. doi: 10.1111/pai.12495. [DOI] [PubMed] [Google Scholar]
- 220.Weller K., Kruger R., Maurer M., Magerl M. Subcutaneous self-injections of C1 inhibitor: an effective and safe treatment in a patient with hereditary angio-oedema. Clin Exp Dermatol. 2016;41(1):91–93. doi: 10.1111/ced.12681. [DOI] [PubMed] [Google Scholar]
- 221.Lumry W.R., Craig T., Zuraw B., et al. Health-related quality of life with subcutaneous C1-inhibitor for prevention of attacks of hereditary angioedema. J Allergy Clin Immunol Pract. 2018;6(5):1733–1741 e1733. doi: 10.1016/j.jaip.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 222.Andarawewa S., Aygoren-Pursun E. Subcutaneous C1-Inhibitor Concentrate for prophylaxis during pregnancy and lactation in a patient with C1-INH-HAE. Clin Case Rep. 2021;9(3):1273–1275. doi: 10.1002/ccr3.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Weller K., Maurer M., Fridman M., Supina D., Schranz J., Magerl M. Health-related quality of life with hereditary angioedema following prophylaxis with subcutaneous C1-inhibitor with recombinant hyaluronidase. Allergy Asthma Proc. 2017;38(2):143–151. doi: 10.2500/aap.2017.38.4025. [DOI] [PubMed] [Google Scholar]
- 224.Bernstein J.A., Li H.H., Craig T.J., et al. Indirect comparison of intravenous vs. subcutaneous C1-inhibitor placebo-controlled trials for routine prevention of hereditary angioedema attacks. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2019;15:13. doi: 10.1186/s13223-019-0328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hahn J., Nordmann-Kleiner M., Trainotti S., Hoffmann T.K., Greve J. Successful long-term prophylactic treatment with subcutaneous C1 esterase inhibitor in a patient with hereditary angioedema. J Pharm Pract. 2020;33(6):907–911. doi: 10.1177/0897190019857407. [DOI] [PubMed] [Google Scholar]
- 226.Craig T., Busse P., Gower R.G., et al. Long-term prophylaxis therapy in patients with hereditary angioedema with C1 inhibitor deficiency. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2018;121(6):673–679. doi: 10.1016/j.anai.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 227.Rasmussen E.R., Aagaard L., Bygum A. Real-life experience with long-term prophylactic C1 inhibitor concentrate treatment of patients with hereditary angioedema: effectiveness and cost. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2016;116(5):476–477. doi: 10.1016/j.anai.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 228.Ärzteschaft Add. Schwerwiegende Thrombenbildung nach Berinert® HS. Dtsch Ärztebl. 2000;97(15):1010–1016. [Google Scholar]
- 229.Crowther M., Bauer K.A., Kaplan A.P. The thrombogenicity of C1 esterase inhibitor (human): review of the evidence. Allergy Asthma Proc. 2014;35(6):444–453. doi: 10.2500/aap.2014.35.3799. [DOI] [PubMed] [Google Scholar]
- 230.Farkas H., Kohalmi K.V., Veszeli N., Zotter Z., Varnai K., Varga L. Risk of thromboembolism in patients with hereditary angioedema treated with plasma-derived C1-inhibitor. Allergy Asthma Proc. 2016;37(2):164–170. doi: 10.2500/aap.2016.37.3933. [DOI] [PubMed] [Google Scholar]
- 231.Kalaria S., Craig T. Assessment of hereditary angioedema treatment risks. Allergy Asthma Proc. 2013;34(6):519–522. doi: 10.2500/aap.2013.34.3702. [DOI] [PubMed] [Google Scholar]
- 232.Riedl M.A., Bygum A., Lumry W., et al. Safety and usage of C1-inhibitor in hereditary angioedema: Berinert registry data. J Allergy Clin Immunol Pract. 2016;4(5):963–971. doi: 10.1016/j.jaip.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 233.Burnham K., Reinert J.P. Thromboembolic risk of C1 esterase inhibitors: a systematic review on current evidence. Expet Rev Clin Pharmacol. 2020;13(7):779–786. doi: 10.1080/17512433.2020.1776110. [DOI] [PubMed] [Google Scholar]
- 234.Bork K., Hardt J. Hereditary angioedema: increased number of attacks after frequent treatments with C1 inhibitor concentrate. Am J Med. 2009;122(8):780–783. doi: 10.1016/j.amjmed.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 235.Banerji A., Busse P., Shennak M., et al. Inhibiting plasma kallikrein for hereditary angioedema prophylaxis. N Engl J Med. 2017;376(8):717–728. doi: 10.1056/NEJMoa1605767. [DOI] [PubMed] [Google Scholar]
- 236.Chyung Y., Vince B., Iarrobino R., et al. A phase 1 study investigating DX-2930 in healthy subjects. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2014;113(4):460–466 e462. doi: 10.1016/j.anai.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 237.Riedl M.A., Maurer M., Bernstein J.A., et al. Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy. 2020;75(11):2879–2887. doi: 10.1111/all.14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Takeda . 2021. Prescribing Information Lanadelumab. [Google Scholar]
- 239.Ohsawa I., Honda D., Suzuki Y., et al. Oral berotralstat for the prophylaxis of hereditary angioedema attacks in patients in Japan: a phase 3 randomized trial. Allergy. 2021;76(6):1789–1799. doi: 10.1111/all.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Aygoren-Pursun E., Bygum A., Grivcheva-Panovska V., et al. Oral plasma kallikrein inhibitor for prophylaxis in hereditary angioedema. N Engl J Med. 2018;379(4):352–362. doi: 10.1056/NEJMoa1716995. [DOI] [PubMed] [Google Scholar]
- 241.BioCryst . 2021. Prescribing Information Orladeyo. Accessed August 21, 2021. [Google Scholar]
- 242.Manning M.E., Kashkin J.M. Berotralstat (BCX7353) is a novel oral prophylactic treatment for hereditary angioedema: review of phase II and III studies. Allergy Asthma Proc. 2021;42(4):274–282. doi: 10.2500/aap.2021.42.210034. [DOI] [PubMed] [Google Scholar]
- 243.Farkas H., Stobiecki M., Peter J., et al. Long-term safety and effectiveness of berotralstat for hereditary angioedema: the open-label APeX-S study. Clin Transl Allergy. 2021;11(4) doi: 10.1002/clt2.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Banerji A., Anderson J., Johnston D.T. Optimal management of hereditary angioedema: shared decision-making. J Asthma Allergy. 2021;14:119–125. doi: 10.2147/JAA.S284029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Valerieva A., Caccia S., Cicardi M. Recombinant human C1 esterase inhibitor (Conestat alfa) for prophylaxis to prevent attacks in adult and adolescent patients with hereditary angioedema. Expet Rev Clin Immunol. 2018;14(9):707–718. doi: 10.1080/1744666X.2018.1503055. [DOI] [PubMed] [Google Scholar]
- 246.Castaldo A.J., Jervelund C., Corcoran D., Boysen H.B., Christiansen S.C., Zuraw B.L. Assessing the cost and quality-of-life impact of on-demand-only medications for adults with hereditary angioedema. Allergy Asthma Proc. 2021;42(2):108–117. doi: 10.2500/aap.2021.42.200127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mendivil J., Murphy R., de la Cruz M., et al. Clinical characteristics and burden of illness in patients with hereditary angioedema: findings from a multinational patient survey. Orphanet J Rare Dis. 2021;16(1):94. doi: 10.1186/s13023-021-01717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Agostoni A., Cicardi M., Martignoni G.C., Bergamaschini L., Marasini B. Danazol and stanozolol in long-term prophylactic treatment of hereditary angioedema. J Allergy Clin Immunol. 1980;65(1):75–79. doi: 10.1016/0091-6749(80)90181-5. [DOI] [PubMed] [Google Scholar]
- 249.Blackmore W.P. Danazol in the treatment of hereditary angio-neurotic oedema. J Int Med Res. 1977;5(Suppl 3):38–43. [PubMed] [Google Scholar]
- 250.Bork K., Bygum A., Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2008;100(2):153–161. doi: 10.1016/S1081-1206(10)60424-3. [DOI] [PubMed] [Google Scholar]
- 251.Farkas H., Czaller I., Csuka D., et al. The effect of long-term danazol prophylaxis on liver function in hereditary angioedema-a longitudinal study. Eur J Clin Pharmacol. 2010;66(4):419–426. doi: 10.1007/s00228-009-0771-z. [DOI] [PubMed] [Google Scholar]
- 252.Gelfand J.A., Sherins R.J., Alling D.W., Frank M.M. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med. 1976;295(26):1444–1448. doi: 10.1056/NEJM197612232952602. [DOI] [PubMed] [Google Scholar]
- 253.Fust G., Farkas H., Csuka D., Varga L., Bork K. Long-term efficacy of danazol treatment in hereditary angioedema. Eur J Clin Invest. 2011;41(3):256–262. doi: 10.1111/j.1365-2362.2010.02402.x. [DOI] [PubMed] [Google Scholar]
- 254.Kreuz W., Martinez-Saguer I., Aygoren-Pursun E., Rusicke E., Heller C., Klingebiel T. C1-inhibitor concentrate for individual replacement therapy in patients with severe hereditary angioedema refractory to danazol prophylaxis. Transfusion. 2009;49(9):1987–1995. doi: 10.1111/j.1537-2995.2009.02230.x. [DOI] [PubMed] [Google Scholar]
- 255.Sheffer A.L., Fearon D.T., Austen K.F. Clinical and biochemical effects of stanozolol therapy for hereditary angioedema. J Allergy Clin Immunol. 1981;68(3):181–187. doi: 10.1016/0091-6749(81)90181-0. [DOI] [PubMed] [Google Scholar]
- 256.Zotter Z., Veszeli N., Csuka D., Varga L., Farkas H. Frequency of the virilising effects of attenuated androgens reported by women with hereditary angioedema. Orphanet J Rare Dis. 2014;9:205. doi: 10.1186/s13023-014-0205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bouillet L., Gompel A. Hereditary angioedema in women: specific challenges. Immunol Allergy Clin. 2013;33(4):505–511. doi: 10.1016/j.iac.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 258.Brunskill P.J. The effects of fetal exposure to danazol. Br J Obstet Gynaecol. 1992;99(3):212–215. doi: 10.1111/j.1471-0528.1992.tb14501.x. [DOI] [PubMed] [Google Scholar]
- 259.Wentz A.C. Adverse effects of danazol in pregnancy. Ann Intern Med. 1982;96(5):672–673. doi: 10.7326/0003-4819-96-5-672. [DOI] [PubMed] [Google Scholar]
- 260.Riedl M.A. Critical appraisal of androgen use in hereditary angioedema: a systematic review. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2015;114(4):281–288 e287. doi: 10.1016/j.anai.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 261.Stankovic I., Vlahovic-Stipac A., Putnikovic B., Cvetkovic Z., Neskovic A.N. Concomitant administration of simvastatin and danazol associated with fatal rhabdomyolysis. Clin Therapeut. 2010;32(5):909–914. doi: 10.1016/j.clinthera.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 262.Johnston D.T., Henry Li H., Craig T.J., et al. Androgen use in hereditary angioedema: a critical appraisal and approaches to transitioning from androgens to other therapies. Allergy Asthma Proc. 2021;42(1):22–29. doi: 10.2500/aap.2021.42.200106. [DOI] [PubMed] [Google Scholar]
- 263.Craig T.J. Appraisal of danazol prophylaxis for hereditary angioedema. Allergy Asthma Proc. 2008;29(3):225–231. doi: 10.2500/aap.2008.29.3107. [DOI] [PubMed] [Google Scholar]
- 264.Sheffer A.L., Fearon D.T., Austen K.F. Methyltestosterone therapy in hereditary angioedema. Ann Intern Med. 1977;86(3):306–308. doi: 10.7326/0003-4819-86-3-306. [DOI] [PubMed] [Google Scholar]
- 265.Bork K., Pitton M., Harten P., Koch P. Hepatocellular adenomas in patients taking danazol for hereditary angio-oedema. Lancet. 1999;353(9158):1066–1067. doi: 10.1016/S0140-6736(99)00110-5. [DOI] [PubMed] [Google Scholar]
- 266.Bork K., Schneiders V. Danazol-induced hepatocellular adenoma in patients with hereditary angio-oedema. J Hepatol. 2002;36(5):707–709. doi: 10.1016/s0168-8278(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 267.Cicardi M., Bork K., Caballero T., et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012;67(2):147–157. doi: 10.1111/j.1398-9995.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 268.Hochberg Z., Pacak K., Chrousos G.P. Endocrine withdrawal syndromes. Endocr Rev. 2003;24(4):523–538. doi: 10.1210/er.2001-0014. [DOI] [PubMed] [Google Scholar]
- 269.Wu Y.S., Chen S.D., Li T.H., Liu J.S., Lan M.Y., Chang Y.Y. Intracranial hypertension associated with danazol withdrawal: a case report. Acta Neurol Taiwanica. 2007;16(3):173–176. [PubMed] [Google Scholar]
- 270.Lundh B., Laurell A.B., Wetterqvist H., White T., Granerus G. A case of hereditary angioneurotic oedema, successfully treated with epsilon-aminocaproic acid. Studies on C'1 esterase inhibitor, C'1 activation, plasminogen level and histamine metabolism. Clin Exp Immunol. 1968;3(7):733–745. [PMC free article] [PubMed] [Google Scholar]
- 271.Gwynn C.M. Therapy in hereditary angioneurotic oedema. Arch Dis Child. 1974;49(8):636–640. doi: 10.1136/adc.49.8.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Blohme G. Treatment of hereditary angioneurotic oedema with tranexamic acid. A random double-blind cross-over study. Acta Med Scand. 1972;192(4):293–298. doi: 10.1111/j.0954-6820.1972.tb04818.x. [DOI] [PubMed] [Google Scholar]
- 273.Sheffer A.L., Austen K.F., Rosen F.S. Tranexamic acid therapy in hereditary angioneurotic edema. N Engl J Med. 1972;287(9):452–454. doi: 10.1056/NEJM197208312870907. [DOI] [PubMed] [Google Scholar]
- 274.Lundh B. Tranexamic acid in hereditary angioneurotic edema--a progress report. N Engl J Med. 1973;288(1):53. doi: 10.1056/NEJM197301042880125. [DOI] [PubMed] [Google Scholar]
- 275.Caballero T., Farkas H., Bouillet L., et al. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol. 2012;129(2):308–320. doi: 10.1016/j.jaci.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 276.Farkas H., Harmat G., Fust G., Varga L., Visy B. Clinical management of hereditary angio-oedema in children. Pediatr Allergy Immunol. 2002;13(3):153–161. doi: 10.1034/j.1399-3038.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 277.Katelaris C.H., Lima H., Marsland A., Weller K., Shah A., Waserman S. How to measure disease activity, impact, and control in patients with recurrent wheals, angioedema, or both. J Allergy Clin Immunol Pract. 2021;9(6):2151–2157. doi: 10.1016/j.jaip.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 278.Balla Z., Ignacz B., Varga L., Kohalmi K.V., Farkas H. How angioedema quality of life questionnaire can help physicians in treating C1-inhibitor deficiency patients? Clin Rev Allergy Immunol. 2021;61(1):50–59. doi: 10.1007/s12016-021-08850-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Bork K., Anderson J.T., Caballero T., et al. Assessment and management of disease burden and quality of life in patients with hereditary angioedema: a consensus report. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2021;17(1):40. doi: 10.1186/s13223-021-00537-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kulthanan K., Chularojanamontri L., Rujitharanawong C., Weerasubpong P., Weller K., Maurer M. Angioedema activity score (AAS): a valid and reliable tool to use in asian patients. BioMed Res Int. 2019;2019:9157895. doi: 10.1155/2019/9157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kulthanan K., Chularojanamontri L., Rujitharanawong C., Weerasubpong P., Maurer M., Weller K. Angioedema quality of life questionnaire (AE-QoL) - interpretability and sensitivity to change. Health Qual Life Outcome. 2019;17(1):160. doi: 10.1186/s12955-019-1229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bygum A., Busse P., Caballero T., Maurer M. Disease severity, activity, impact, and control and how to assess them in patients with hereditary angioedema. Front Med (Lausanne) 2017;4:212. doi: 10.3389/fmed.2017.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Nordenfelt P., Nilsson M., Lindfors A., Wahlgren C.F., Bjorkander J. Health-related quality of life in relation to disease activity in adults with hereditary angioedema in Sweden. Allergy Asthma Proc. 2017;38(6):447–455. doi: 10.2500/aap.2017.38.4087. [DOI] [PubMed] [Google Scholar]
- 284.Riedl M.A., Aygoren-Pursun E., Baker J., et al. Evaluation of avoralstat, an oral kallikrein inhibitor, in a Phase 3 hereditary angioedema prophylaxis trial: the OPuS-2 study. Allergy. 2018;73(9):1871–1880. doi: 10.1111/all.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Aygoren-Pursun E., Magerl M., Graff J., et al. Prophylaxis of hereditary angioedema attacks: a randomized trial of oral plasma kallikrein inhibition with avoralstat. J Allergy Clin Immunol. 2016;138(3):934–936 e935. doi: 10.1016/j.jaci.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 286.Fukunaga A., Morita E., Miyagi T., et al. [Efficacy, pharmacokinetics, pharmacodynamics, and safety of intravenous C1 inhibitor for long-term prophylaxis and treatment of breakthrough attacks in Japanese subjects with hereditary angioedema: a phase 3 open-label study] Arerugi. 2020;69(3):192–203. doi: 10.15036/arerugi.69.192. [DOI] [PubMed] [Google Scholar]
- 287.Squeglia V., Barbarino A., Bova M., et al. High attack frequency in patients with angioedema due to C1-inhibitor deficiency is a major determinant in switching to home therapy: a real-life observational study. Orphanet J Rare Dis. 2016;11(1):133. doi: 10.1186/s13023-016-0518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bonnekoh H., Ellrich A., Hawro T., Weller K., Maurer M., Magerl M. vol. 1. Charité - Universitätsmedizin Berlin, Charitéplatz; Berlin: 2016. Hereditary angioedema due to C1-INH deficiency: age of onset and delay in diagnosis in Germany; p. 10117. (Global Forum on Hereditary Angioedema, Warsaw, Poland: Dpt. Of Dermatology and Allergy, Allergie-Centrum-Charité). [Google Scholar]
- 289.Bork K., Hardt J., Schicketanz K.H., Ressel N. Clinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiency. Arch Intern Med. 2003;163(10):1229–1235. doi: 10.1001/archinte.163.10.1229. [DOI] [PubMed] [Google Scholar]
- 290.Christiansen S.C., Davis D.K., Castaldo A.J., Zuraw B.L. Pediatric hereditary angioedema: onset, diagnostic delay, and disease severity. Clin Pediatr (Phila) 2016;55(10):935–942. doi: 10.1177/0009922815616886. [DOI] [PubMed] [Google Scholar]
- 291.Martinez-Saguer I., Graff J., Rusicke E., Aygören-Pürsün E., Klingebiel T., Kreuz W. Does early clinical manifestation of hereditary angioedema (HAE) influence the clinical course of the disease? J Allergy Clin Immunol. 2013;131(2):AB30. [Google Scholar]
- 292.Martinez-Saguer I., Farkas H. Erythema marginatum as an early symptom of hereditary angioedema: case report of 2 newborns. Pediatrics. 2016;137(2) doi: 10.1542/peds.2015-2411. [DOI] [PubMed] [Google Scholar]
- 293.Nguyen A., Zuraw B.L., Christiansen S.C. Contact system activation during erythema marginatum in hereditary angioedema. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2020;124(4):394–395 e391. doi: 10.1016/j.anai.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 294.Boyle R.J., Nikpour M., Tang M.L. Hereditary angio-oedema in children: a management guideline. Pediatr Allergy Immunol. 2005;16(4):288–294. doi: 10.1111/j.1399-3038.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 295.Caballero T. Angio-oedema due to hereditary C1 inhibitor deficiency in children. Allergol Immunopathol (Madr). 2013;41(1):45–53. doi: 10.1016/j.aller.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 296.Farkas H. Pediatric hereditary angioedema due to C1-inhibitor deficiency. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2010;6(1):18. doi: 10.1186/1710-1492-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Farkas H., Martinez-Saguer I., Bork K., et al. International consensus on the diagnosis and management of pediatric patients with hereditary angioedema with C1 inhibitor deficiency. Allergy. 2017;72(2):300–313. doi: 10.1111/all.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Frank M.M., Zuraw B., Banerji A., et al. Management of children with hereditary angioedema due to C1 inhibitor deficiency. Pediatrics. 2016;138(5) doi: 10.1542/peds.2016-0575. [DOI] [PubMed] [Google Scholar]
- 299.Wahn V., Aberer W., Aygoren-Pursun E., et al. Hereditary angioedema in children and adolescents - a consensus update on therapeutic strategies for German-speaking countries. Pediatr Allergy Immunol. 2020;31(8):974–989. doi: 10.1111/pai.13309. [DOI] [PubMed] [Google Scholar]
- 300.Aabom A., Andersen K.E., Fagerberg C., Fisker N., Jakobsen M.A., Bygum A. Clinical characteristics and real-life diagnostic approaches in all Danish children with hereditary angioedema. Orphanet J Rare Dis. 2017;12(1):55. doi: 10.1186/s13023-017-0604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Nielsen E.W., Johansen H.T., Holt J., Mollnes T.E. C1 inhibitor and diagnosis of hereditary angioedema in newborns. Pediatr Res. 1994;35(2):184–187. doi: 10.1203/00006450-199402000-00012. [DOI] [PubMed] [Google Scholar]
- 302.Yokoyama K., Horiuchi T., Hashimura C., Yoshida A. A novel C1 inhibitor gene mutation in a family with hereditary angioedema: use of genetic analysis to facilitate early diagnosis. Allergol Int. 2020;69(1):148–149. doi: 10.1016/j.alit.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 303.Grumach A.S., Ceccon M.E., Rutz R., Fertig A., Kirschfink M. Complement profile in neonates of different gestational ages. Scand J Immunol. 2014;79(4):276–281. doi: 10.1111/sji.12154. [DOI] [PubMed] [Google Scholar]
- 304.Spath P.J., Wuthrich B. Angioedema. A review on the acquired, allergic or non-allergic, and the hereditary forms. Recenti Prog Med. 1990;81(7-8):513–531. [PubMed] [Google Scholar]
- 305.Speletas M., Szilagyi A., Psarros F., et al. Hereditary angioedema: molecular and clinical differences among European populations. J Allergy Clin Immunol. 2015;135(2):570–573. doi: 10.1016/j.jaci.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 306.Pappalardo E., Caccia S., Suffritti C., Tordai A., Zingale L.C., Cicardi M. Mutation screening of C1 inhibitor gene in 108 unrelated families with hereditary angioedema: functional and structural correlates. Mol Immunol. 2008;45(13):3536–3544. doi: 10.1016/j.molimm.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 307.Farkas H., Reshef A., Aberer W., et al. Treatment effect and safety of icatibant in pediatric patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2017;5(6):1671–1678 e1672. doi: 10.1016/j.jaip.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 308.Lumry W., Soteres D., Gower R., et al. Safety and efficacy of C1 esterase inhibitor for acute attacks in children with hereditary angioedema. Pediatr Allergy Immunol. 2015;26(7):674–680. doi: 10.1111/pai.12444. [DOI] [PubMed] [Google Scholar]
- 309.Farkas H., Csuka D., Zotter Z., et al. Treatment of attacks with plasma-derived C1-inhibitor concentrate in pediatric hereditary angioedema patients. J Allergy Clin Immunol. 2013;131(3):909–911. doi: 10.1016/j.jaci.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 310.Farkas H., Csuka D., Zotter Z., Varga L., Fust G. Prophylactic therapy in children with hereditary angioedema. J Allergy Clin Immunol. 2013;131(2) doi: 10.1016/j.jaci.2012.08.001. 579-582 e571-572. [DOI] [PubMed] [Google Scholar]
- 311.Wahn V., Aberer W., Eberl W., et al. Hereditary angioedema (HAE) in children and adolescents-a consensus on therapeutic strategies. Eur J Pediatr. 2012;171(9):1339–1348. doi: 10.1007/s00431-012-1726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Caballero T., Maurer M., Longhurst H.J., et al. Triggers and prodromal symptoms of angioedema attacks in patients with hereditary angioedema. J Investig Allergol Clin Immunol. 2016;26(6):383–386. doi: 10.18176/jiaci.0102. [DOI] [PubMed] [Google Scholar]
- 313.Bouillet L., Longhurst H., Boccon-Gibod I., et al. Disease expression in women with hereditary angioedema. Am J Obstet Gynecol. 2008;199(5):484 e481–484. doi: 10.1016/j.ajog.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 314.Saule C., Boccon-Gibod I., Fain O., et al. Benefits of progestin contraception in non-allergic angioedema. Clin Exp Allergy. 2013;43(4):475–482. doi: 10.1111/cea.12055. [DOI] [PubMed] [Google Scholar]
- 315.Zotter Z., Csuka D., Szabo E., et al. The influence of trigger factors on hereditary angioedema due to C1-inhibitor deficiency. Orphanet J Rare Dis. 2014;9:44. doi: 10.1186/1750-1172-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Farkas H., Varga L., Szeplaki G., Visy B., Harmat G., Bowen T. Management of hereditary angioedema in pediatric patients. Pediatrics. 2007;120(3):e713–e722. doi: 10.1542/peds.2006-3303. [DOI] [PubMed] [Google Scholar]
- 317.Czaller I., Visy B., Csuka D., Fust G., Toth F., Farkas H. The natural history of hereditary angioedema and the impact of treatment with human C1-inhibitor concentrate during pregnancy: a long-term survey. Eur J Obstet Gynecol Reprod Biol. 2010;152(1):44–49. doi: 10.1016/j.ejogrb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 318.Martinez-Saguer I., Rusicke E., Aygoren-Pursun E., Heller C., Klingebiel T., Kreuz W. Characterization of acute hereditary angioedema attacks during pregnancy and breast-feeding and their treatment with C1 inhibitor concentrate. Am J Obstet Gynecol. 2010;203(2):131 e131–137. doi: 10.1016/j.ajog.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 319.Gonzalez-Quevedo T., Larco J.I., Marcos C., et al. Management of pregnancy and delivery in patients with hereditary angioedema due to C1 inhibitor deficiency. J Investig Allergol Clin Immunol. 2016;26(3):161–167. doi: 10.18176/jiaci.0037. [DOI] [PubMed] [Google Scholar]
- 320.Satomura A., Fujita T., Nakayama T. Comparison of the frequency of angioedema attack, before and during pregnancy, in a patient with type I hereditary angioedema. Intern Med. 2018;57(5):751–755. doi: 10.2169/internalmedicine.9407-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sankrithi P., Shah K., Bernabe C.C. Pregnancy-induced exacerbation of hereditary angioedema in a multiparous caucasian female. Cureus. 2020;12(5) doi: 10.7759/cureus.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Caballero T., Canabal J., Rivero-Paparoni D., Cabanas R. Management of hereditary angioedema in pregnant women: a review. Int J Womens Health. 2014;6:839–848. doi: 10.2147/IJWH.S46460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Halbmayer W.M., Hopmeier P., Mannhalter C., et al. C1-esterase inhibitor in uncomplicated pregnancy and mild and moderate preeclampsia. Thromb Haemostasis. 1991;65(2):134–138. [PubMed] [Google Scholar]
- 324.Ogston D., Walker J., Campbell D.M. C1 inactivator level in pregnancy. Thromb Res. 1981;23(4-5):453–455. doi: 10.1016/0049-3848(81)90206-1. [DOI] [PubMed] [Google Scholar]
- 325.Baker J.W., Craig T.J., Riedl M.A., et al. Nanofiltered C1 esterase inhibitor (human) for hereditary angioedema attacks in pregnant women. Allergy Asthma Proc. 2013;34(2):162–169. doi: 10.2500/aap.2013.34.3645. [DOI] [PubMed] [Google Scholar]
- 326.Fox J., Vegh A.B., Martinez-Saguer I., et al. Safety of a C1-inhibitor concentrate in pregnant women with hereditary angioedema. Allergy Asthma Proc. 2017;38(3):216–221. doi: 10.2500/aap.2017.38.4038. [DOI] [PubMed] [Google Scholar]
- 327.Brooks J.P., Radojicic C., Riedl M.A., Newcomer S.D., Banerji A., Hsu F.I. Experience with intravenous plasma-derived C1-inhibitor in pregnant women with hereditary angioedema: a systematic literature review. J Allergy Clin Immunol Pract. 2020;8(6):1875–1880 e1873. doi: 10.1016/j.jaip.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 328.Kardum Z., Prus V., Milas Ahic J., Kardum D. Successful treatment with Cinryze(R) replacement therapy of a pregnant patient with hereditary angioedema: a case report. J Med Case Rep. 2021;15(1):20. doi: 10.1186/s13256-020-02622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Moldovan D., Bernstein J.A., Hakl R., et al. Safety of recombinant human C1 esterase inhibitor for hereditary angioedema attacks during pregnancy. J Allergy Clin Immunol Pract. 2019;7(8):2938–2940. doi: 10.1016/j.jaip.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 330.Kaminsky L.W., Kelbel T., Ansary F., Craig T. Multiple doses of icatibant used during pregnancy. Allergy Rhinol (Providence) 2017;8(3):178–181. doi: 10.2500/ar.2017.8.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Farkas H., Kohalmi K.V., Veszeli N., Toth F., Varga L. First report of icatibant treatment in a pregnant patient with hereditary angioedema. J Obstet Gynaecol Res. 2016;42(8):1026–1028. doi: 10.1111/jog.13003. [DOI] [PubMed] [Google Scholar]
- 332.Zanichelli A., Mansi M., Periti G. Icatibant exposure during pregnancy in a patient with hereditary angioedema. J Investig Allergol Clin Immunol. 2015;25(6):447–449. [PubMed] [Google Scholar]
- 333.Galan H.L., Reedy M.B., Starr J., Knight A.B. Fresh frozen plasma prophylaxis for hereditary angioedema during pregnancy. A case report. J Reprod Med. 1996;41(7):541–544. [PubMed] [Google Scholar]
- 334.Nathani F., Sullivan H., Churchill D. Pregnancy and C1 esterase inhibitor deficiency: a successful outcome. Arch Gynecol Obstet. 2006;274(6):381–384. doi: 10.1007/s00404-006-0183-6. [DOI] [PubMed] [Google Scholar]
- 335.Caliskaner Z., Ozturk S., Gulec M., Dede M., Erel F., Karaayvaz M. A successful pregnancy and uncomplicated labor with C1INH concentrate prophylaxis in a patient with hereditary angioedema. Allergol Immunopathol (Madr) 2007;35(3):117–119. doi: 10.1157/13106781. [DOI] [PubMed] [Google Scholar]
- 336.Gorman P.J. Hereditary angioedema and pregnancy: a successful outcome using C1 esterase inhibitor concentrate. Cana Family Phys Med de Famille Canad. 2008;54(3):365–366. [PMC free article] [PubMed] [Google Scholar]
- 337.Farkas H., Csuka D., Toth F., Koszegi L., Varga L. Successful pregnancy outcome after treatment with C1-inhibitor concentrate in a patient with hereditary angioedema and a history of four miscarriages. Eur J Obstet Gynecol Reprod Biol. 2012;165(2):366–367. doi: 10.1016/j.ejogrb.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 338.Bouillet L., Lehmann A., Gompel A., et al. [Hereditary angioedema treatments: recommendations from the French national center for angioedema (Bordeaux consensus 2014)] Presse Med. 2015;44(5):526–532. doi: 10.1016/j.lpm.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 339.Gilad O., Merlob P., Stahl B., Klinger G. Outcome following tranexamic acid exposure during breastfeeding. Breastfeed Med. 2014;9(8):407–410. doi: 10.1089/bfm.2014.0027. [DOI] [PubMed] [Google Scholar]
- 340.Katelaris C.H. Self-management plans in patients with hereditary angioedema: strategies, outcomes and integration into clinical care. J Asthma Allergy. 2020;13:153–158. doi: 10.2147/JAA.S200900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Longhurst H. Optimum use of acute treatments for hereditary angioedema: evidence-based expert consensus. Front Med (Lausanne). 2017;4:245. doi: 10.3389/fmed.2017.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Paige D., Maina N., Anderson J.T. Hereditary angioedema: comprehensive management plans and patient support. Allergy Asthma Proc. 2020;41(Suppl 1):S38–S42. doi: 10.2500/aap.2020.41.200059. [DOI] [PubMed] [Google Scholar]
- 343.Riedl M.A. Creating a comprehensive treatment plan for hereditary angioedema. Immunol Allergy Clin. 2013;33(4):471–485. doi: 10.1016/j.iac.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 344.Williams A.H., Craig T.J. Perioperative management for patients with hereditary angioedema. Allergy Rhinol (Providence) 2015;6(1):50–55. doi: 10.2500/ar.2015.6.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zuraw B.L. Diagnosis and management of hereditary angioedema: an American approach. Transfus Apher Sci : Off J World Apher Asso: Off J Euro Soc Haemapher. 2003;29(3):239–245. doi: 10.1016/j.transci.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 346.Zuraw B.L., Banerji A., Bernstein J.A., et al. US Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J Allergy Clin Immunol Pract. 2013;1(5):458–467. doi: 10.1016/j.jaip.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 347.Banerji A., Bas M., Bernstein J.A., et al. Expert perspectives on hereditary angioedema: key areas for advancements in care across the patient journey. Allergy Rhinol (Providence) 2016;7(3):172–181. doi: 10.2500/ar.2016.7.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Banerji A., Busse P., Christiansen S.C., et al. Current state of hereditary angioedema management: a patient survey. Allergy Asthma Proc. 2015;36(3):213–217. doi: 10.2500/aap.2015.36.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Boccon-Gibod I. [Hereditary angioedema: treatment and educational therapeutic program] Presse Med. 2015;44(1):78–88. doi: 10.1016/j.lpm.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 350.Nasr I.H., Manson A.L., Al Wahshi H.A., Longhurst H.J. Optimizing hereditary angioedema management through tailored treatment approaches. Expet Rev Clin Immunol. 2016;12(1):19–31. doi: 10.1586/1744666X.2016.1100963. [DOI] [PubMed] [Google Scholar]
- 351.Aygoren-Pursun E., Bork K. [Hereditary angioedema] Internist (Berl) 2019;60(9):987–995. doi: 10.1007/s00108-019-0644-1. [DOI] [PubMed] [Google Scholar]
- 352.Aygoren-Pursun E., Martinez-Saguer I., Rusicke E., Klingebiel T., Kreuz W. On demand treatment and home therapy of hereditary angioedema in Germany - the Frankfurt experience. Allergy Asthma Clin Immunol: Off J Canad Soc Allergy Clin Immunol. 2010;6(1):21. doi: 10.1186/1710-1492-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Gill P., Betschel S.D. The clinical evaluation of angioedema. Immunol Allergy Clin. 2017;37(3):449–466. doi: 10.1016/j.iac.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 354.Manson A.L., Price A., Dempster J., et al. In pursuit of excellence: an integrated care pathway for C1 inhibitor deficiency. Clin Exp Immunol. 2013;173(1):1–7. doi: 10.1111/cei.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Krack A.T., Bernstein J.A., Ruddy R.M. Recognition, evaluation, and management of pediatric hereditary angioedema. Pediatr Emerg Care. 2021;37(4):218–223. doi: 10.1097/PEC.0000000000002402. [DOI] [PubMed] [Google Scholar]
- 356.Maurer M., Aberer W., Agondi R., et al. Definition, aims, and implementation of GA(2) LEN/HAEi angioedema centers of reference and excellence. Allergy. 2020;75(8):2115–2123. doi: 10.1111/all.14293. [DOI] [PubMed] [Google Scholar]
- 357.Serpa F.S., Mansour E., Aun M.V., et al. Hereditary angioedema: how to approach it at the emergency department? Einstein (Sao Paulo) 2021;19 doi: 10.31744/einstein_journal/2021RW5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Pines J.M., Poarch K., Hughes S. Recognition and differential diagnosis of hereditary angioedema in the emergency department. J Emerg Med. 2021;60(1):35–43. doi: 10.1016/j.jemermed.2020.09.044. [DOI] [PubMed] [Google Scholar]
- 359.Hahn J., Bock B., Muth C.M., et al. [The ulm emergency algorithm for the acute treatment of drug-induced, bradykinin-mediated angioedema] Med Klin Intensivmed Notfallmed. 2019;114(8):708–716. doi: 10.1007/s00063-018-0483-1. [DOI] [PubMed] [Google Scholar]
- 360.Long B.J., Koyfman A., Gottlieb M. Evaluation and management of angioedema in the emergency department. West J Emerg Med. 2019;20(4):587–600. doi: 10.5811/westjem.2019.5.42650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Cicardi M., Craig T.J., Martinez-Saguer I., Hebert J., Longhurst H.J. Review of recent guidelines and consensus statements on hereditary angioedema therapy with focus on self-administration. Int Arch Allergy Immunol. 2013;161(Suppl 1):3–9. doi: 10.1159/000351232. [DOI] [PubMed] [Google Scholar]
- 362.Muhlberg H., Ettl N., Magerl M. An analysis of the teaching of intravenous self-administration in patients with hereditary angio-oedema. Clin Exp Dermatol. 2016;41(4):366–371. doi: 10.1111/ced.12806. [DOI] [PubMed] [Google Scholar]
- 363.Zanichelli A., Azin G.M., Cristina F., Vacchini R., Caballero T. Safety, effectiveness, and impact on quality of life of self-administration with plasma-derived nanofiltered C1 inhibitor (Berinert(R)) in patients with hereditary angioedema: the SABHA study. Orphanet J Rare Dis. 2018;13(1):51. doi: 10.1186/s13023-018-0797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Aberer W., Maurer M., Reshef A., et al. Open-label, multicenter study of self-administered icatibant for attacks of hereditary angioedema. Allergy. 2014;69(3):305–314. doi: 10.1111/all.12303. [DOI] [PubMed] [Google Scholar]
- 365.Kreuz W., Rusicke E., Martinez-Saguer I., Aygoren-Pursun E., Heller C., Klingebiel T. Home therapy with intravenous human C1-inhibitor in children and adolescents with hereditary angioedema. Transfusion. 2012;52(1):100–107. doi: 10.1111/j.1537-2995.2011.03240.x. [DOI] [PubMed] [Google Scholar]
- 366.Bernstein J.A., Riedl M., Zacek L., Shapiro R.S. Facilitating home-based treatment of hereditary angioedema. Allergy Asthma Proc. 2015;36(2):92–99. doi: 10.2500/aap.2015.36.3820. [DOI] [PubMed] [Google Scholar]
- 367.Bygum A. Hereditary angioedema - consequences of a new treatment paradigm in Denmark. Acta Derm Venereol. 2014;94(4):436–441. doi: 10.2340/00015555-1743. [DOI] [PubMed] [Google Scholar]
- 368.Li H.H. Self-administered C1 esterase inhibitor concentrates for the management of hereditary angioedema: usability and patient acceptance. Patient Prefer Adherence. 2016;10:1727–1737. doi: 10.2147/PPA.S86379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Tourangeau L.M., Castaldo A.J., Davis D.K., Koziol J., Christiansen S.C., Zuraw B.L. Safety and efficacy of physician-supervised self-managed c1 inhibitor replacement therapy. Int Arch Allergy Immunol. 2012;157(4):417–424. doi: 10.1159/000329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Tuong L.A., Olivieri K., Craig T.J. Barriers to self-administered therapy for hereditary angioedema. Allergy Asthma Proc. 2014;35(3):250–254. doi: 10.2500/aap.2014.35.3753. [DOI] [PubMed] [Google Scholar]
- 371.Symons C., Rossi O., Magerl M., Andritschke K. Practical approach to self-administration of intravenous C1-INH concentrate: a nursing perspective. Int Arch Allergy Immunol. 2013;161(Suppl 1):17–20. doi: 10.1159/000351236. [DOI] [PubMed] [Google Scholar]
- 372.Wilson D.A., Bork K., Shea E.P., Rentz A.M., Blaustein M.B., Pullman W.E. Economic costs associated with acute attacks and long-term management of hereditary angioedema. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2010;104(4):314–320. doi: 10.1016/j.anai.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 373.Zilberberg M.D., Jacobsen T., Tillotson G. The burden of hospitalizations and emergency department visits with hereditary angioedema and angioedema in the United States, 2007. Allergy Asthma Proc. 2010;31(6):511–519. doi: 10.2500/aap.2010.31.3403. [DOI] [PubMed] [Google Scholar]
- 374.Abdel-Karim O., Dizdarevic A., Bygum A. Hereditary angioedema: children should be considered for training in self-administration. Pediatr Dermatol. 2014;31(6):e132–e135. doi: 10.1111/pde.12408. [DOI] [PubMed] [Google Scholar]
- 375.Craig T. Triggers and short-term prophylaxis in patients with hereditary angioedema. Allergy Asthma Proc. 2020;41(Suppl 1):S30–S34. doi: 10.2500/aap.2020.41.200058. [DOI] [PubMed] [Google Scholar]
- 376.Steiner U.C., Kolliker L., Weber-Chrysochoou C., et al. Food as a trigger for abdominal angioedema attacks in patients with hereditary angioedema. Orphanet J Rare Dis. 2018;13(1):90. doi: 10.1186/s13023-018-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Teranishi R., Makino Y., Amano E., Shibuya H., Okada T. Perioperative management of a patient with hereditary angioedema: a case report. Masui. 2015;64(4):441–443. [PubMed] [Google Scholar]
- 378.Visy B., Fust G., Bygum A., et al. Helicobacter pylori infection as a triggering factor of attacks in patients with hereditary angioedema. Helicobacter. 2007;12(3):251–257. doi: 10.1111/j.1523-5378.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 379.Zotter Z., Veszeli N., Kohalmi K.V., et al. Bacteriuria increases the risk of edematous attacks in hereditary angioedema with C1-inhibitor deficiency. Allergy. 2016;71(12):1791–1793. doi: 10.1111/all.13034. [DOI] [PubMed] [Google Scholar]
- 380.Belbezier A., Arnaud M., Boccon-Gibod I., et al. COVID-19 as a trigger of acute attacks in people with hereditary angioedema. Clin Exp Allergy. 2021;51(7):947–950. doi: 10.1111/cea.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Can Bostan O., Tuncay G., Damadoglu E., Karakaya G., Kalyoncu A.F. Effect of COVID-19 on hereditary angioedema activity and quality of life. Allergy Asthma Proc. 2021;42(5):403–408. doi: 10.2500/aap.2021.42.210066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Grumach A.S., Goudouris E., Dortas Junior S., et al. COVID-19 affecting hereditary angioedema patients with and without C1 inhibitor deficiency. J Allergy Clin Immunol Pract. 2021;9(1):508–510. doi: 10.1016/j.jaip.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Mete Gokmen N., Kuman Tuncel O., Bogatekin G., et al. Psychiatric and clinical characteristics of hereditary angioedema patients who experienced attacks during COVID-19. J Investig Allergol Clin Immunol. 2021;31(4):356–357. doi: 10.18176/jiaci.0701. [DOI] [PubMed] [Google Scholar]
- 384.Fijen L.M., Levi M., Cohn D.M. COVID-19 vaccination and the risk of swellings in patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9(11):4156–4158. doi: 10.1016/j.jaip.2021.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Araujo-Simoes J., Boanova A.G.P., Constantino-Silva R.N., et al. The challenges in the follow-up and treatment of Brazilian children with hereditary angioedema. Int Arch Allergy Immunol. 2021;182(7):585–591. doi: 10.1159/000512944. [DOI] [PubMed] [Google Scholar]
- 386.Johnston D.T., Smith R.C. Hereditary angioedema: special considerations in children. Allergy Asthma Proc. 2020;41(Suppl 1):S43–S46. doi: 10.2500/aap.2020.41.200042. [DOI] [PubMed] [Google Scholar]
- 387.Valle S.O.R., Alonso M.L.O., Tortora R.P., Abe A.T., Levy S.A.P., Dortas S.D., Jr. Hereditary angioedema: screening of first-degree blood relatives and earlier diagnosis. Allergy Asthma Proc. 2019;40(4):279–281. doi: 10.2500/aap.2019.40.4213. [DOI] [PubMed] [Google Scholar]