Abstract
A 23-year-old female presented with a 3-day history of bilateral (OU) diminution of vision 3 weeks after COVID-19 infection. Best corrected visual acuity (BCVA) was 20/30 in right eye and 20/40 in left eye. Anterior segment showed OU 1+ cells in anterior chamber and anterior vitreous face. Fundus OU showed disc hyperemia and multiple pockets of subretinal fluid (SRF), confirmed on optical coherence tomography. Fundus fluorescein angiography showed multiple pin point leaks suggestive of Vogt Koyanagi Harada disease. Oral corticosteroids 1 mg/kg/day were started. At 2-months’ follow-up, her BCVA improved to 20/25 OU with complete resolution of SRF.
Keywords: Bacillary layer detachment, COVID-19, subretinal fluid, VKH
Vogt Koyanagi Harada disease (VKH) is an autoimmune condition, where T-cell-mediated immune response is triggered against melanocyte rich tissues in our body namely eye, ear, skin, and brain. In eye, VKH mainly manifests as chronic, bilateral, granulomatous panuveitis.[1] The global pandemic caused by COVID-19 is caused by severe acute respiratory syndrome coronavirus-2 virus (SARS-CoV-2). It is a multifaceted disease and various ocular conditions have been reported in association with COVID-19 infection like follicular conjunctivitis, acute anterior uveitis, panuveitis, optic neuritis, and vascular occlusions.[2,3] However, various infective triggers for VKH like Epstein–barr virus, cytomegalovirus, influenza A, and mycoplasma pneumonia have been reported earlier in literature.[1,4] We report a case of a young female with features of VKH, probably triggered by SARS-CoV-2 virus, post COVID-19 infection, which, to the best of our knowledge, is not previously reported in India.
Case Report
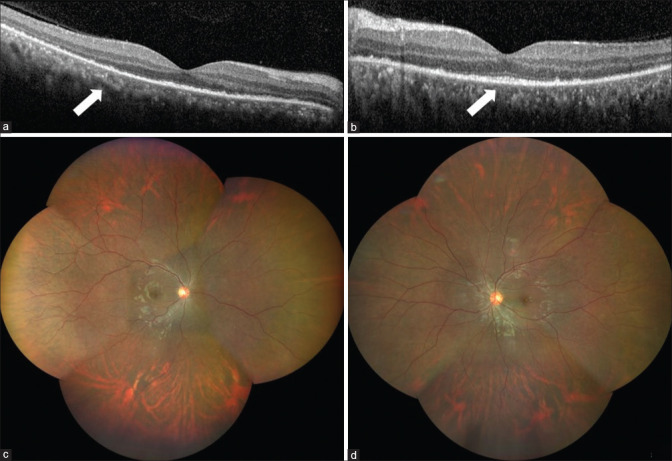
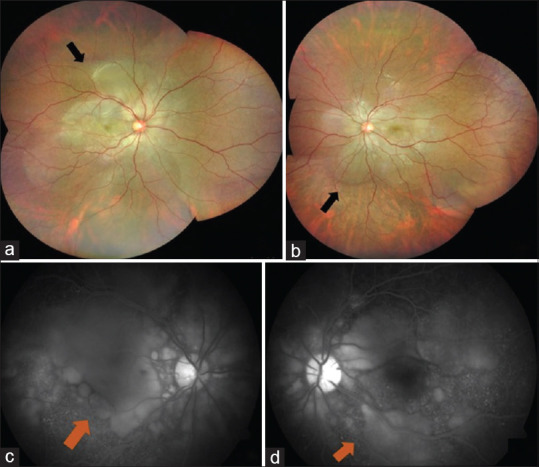
A 23-year-old female presented with headache and bilateral diminution of vision for 3 days. She had no history of tinnitus. She was infected with COVID-19 3 weeks before, confirmed with a positive reverse transcriptase-polymerase chain reaction (RT-PCR) of nasopharyngeal swab. At the onset of COVID -19 infection, she had fever for 2 days with sore throat and dry cough that persisted for a week. Anosmia started on the fourth day and lasted for 20 days. She was tested RT-PCR positive for COVID-19 on the fourth day from the onset of fever. She was advised home quarantine and received a short course of oral azithromycin along with oral corticosteroids and vitamin supplements. Her D-dimer wasn’t done and her ferritin levels and platelet counts were normal. Erythrocyte sedimentation rate (ESR) and C- reactive protein (CRP) were elevated and documented as 28 mm and 7.1, respectively. Chest imaging was not done at the time of COVID-19 infection. She received a short course of oral corticosteroids for a week, which was stopped a week prior to the onset of her ocular symptoms. There was no history of previous ocular trauma. When she presented to us, her best corrected visual acuity (BCVA) was 20/30 in right eye and 20/40 in left eye. Slit lamp examination showed circumciliary congestion and 1+ cells in anterior chamber and anterior vitreous face both eyes (OU). Fundus OU showed multiple pockets of subretinal fluid (SRF) and disc hyperaemia. [Fig. 1a and b]. Fundus fluorescein angiography (FFA) OU showed multiple pin head RPE leaks with disc staining and late pooling of dye in SRF, which were suggestive of VKH. [Fig. 1c and d]. Both eyes’ optical coherence tomography (OCT) revealed SRF with intraretinal edema, septations and bacillary layer detachment (BLD) [Fig. 2a-c]. Anterior segment inflammation was treated with topical corticosteroids. Base line blood investigations were within normal limits except for elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). High resolution computed tomography (HRCT) of chest was normal at this stage and her repeat RT-PCR for COVID-19 was negative. As she already had a positive RT-PCR report for COVID -19 done at the time of COVID-19 infection, SARS-COV-2 antibody test wasn’t done. All other infectious etiologies were ruled out. She was diagnosed as probable VKH, as she didn’t have any neurological or integumentary findings. The patient was started on oral prednisolone 1 mg/kg/day with weekly tapering. At 1-month’s follow-up, her BCVA improved to 20/25 OU, with complete resolution of SRF. At 2-months’ follow-up, her BCVA was stable with no recurrence of inflammation on OCT [Fig. 3a and b] and fundus examination [Fig. 3c and d].
Figure 1.
Fundus photo and fluorescein angiography at initial presentation. Montage of right eye (a) and left eye (b) showing multiple SRF pockets at the posterior pole (black arrow); Right eye (c) and left eye (d) FFA showing disc staining and late pooling of the dye (orange arrow) at posterior pole
Figure 2.
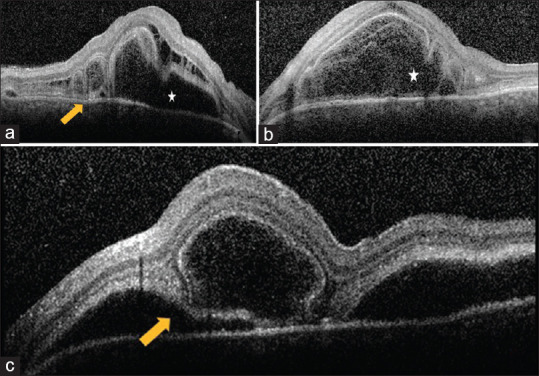
Optical coherence tomography at initial presentation. (a) Right eye OCT showing intraretinal edema, SRF (white star) with bacillary layer detachment (yellow arrow); Left eye OCT at fovea showing intraretinal edema and SRF (white star; b) and inferior to fovea with bacillary layer detachment (yellow arrow; c)
Figure 3.
Optical coherence tomography at 2-months’ follow-up. Right eye (a) and left eye (b) optical coherence tomography showing complete resolution of SRF post treatment (white arrow). Fundus montage of right eye (c) and left eye (d) showing complete resolution of SRF
Discussion
We report a unique case demonstrating SARS-CoV-2 virus as a possible trigger for VKH. VKH is characterized by bilateral serous retinal detachment and disc hyperemia.[1,5] Our patient had a recent COVID-19 infection, following which she developed VKH, with characteristic clinical and imaging features and showed a good response to oral corticosteroids. She was classified as probable VKH as per revised international diagnostic criteria for VKH, as she didn’t have neurological or integumentary findings.[6] VKH is known autoimmune inflammatory condition targeting the melanocyte-rich antigen particularly tyrosinase peptide, through a CD4+ T-cell-mediated response, in genetically susceptible individuals. Expression of HLA-DQA1*0301 and HLA-DQB1*0401 has been identified as a predisposing factor for genetic susceptibility for VKH.[7] Autoimmune response elicited by COVID-19 viral antigen is supported by elevated acute phase reactants like ESR and CRP. Various viral triggers have been reported in causation of VKH especially in genetically susceptible individuals.[1] The onset and exacerbation of VKH by infective triggers like Epstein–barr virus, cytomegalovirus, influenza A, and mycoplasma pneumonia due to molecular mimicry and cross reactivity have been reported earlier.[1,4]
Expression of HLA DRB1*0405, HLA-DQA1*0301 and HLA-DQB1*0401 also makes an individual genetically susceptible to develop autoimmune inflammations following exposure to viral triggers.[7,8] These viral triggers exhibit molecular mimicry, which induces autoimmune response mediated by mononuclear cells including the T cells that cross reacts with melanocyte self-antigens. Homology between the viral antigen peptides and melanocyte self-antigen peptides is responsible for the cross reactivity and molecular mimicry involved in pathogenesis of VKH.[9] Strong association of HLA-DR4 in the pathogenesis of VKH post-viral infections has been demonstrated. HLA-DR4 acts by facilitating antigen presentation to T cells.[1] Post-viral infections, microbial antigens also hypothesized to initiate an innate immune response through toll-like receptors identified in the uveal tact.[10]
So far, two cases of VKH developing post-COVID-19 infection have been reported in literature and SARS-CoV-2 has been identified as a possible trigger factor for VKH. Inflammatory events in these reported cases occurred 2 weeks following COVID-19 infection [Table 1].[10,11]
Table 1.
Comparison of clinical profile and management between our case and other similar case reports[10,11]
| Our case | VKH post COVID-19 Santamaria et al.[10] | VKH post COVID-19 Saraceno et al.[11] | VKH post COVID-19 Vaccıne Saraceno et al.[11] | |
|---|---|---|---|---|
| Age (years) | 22 | 32 | 37 | 62 |
| Sex | Female | Female | Female | Females |
| Duration between COVID-19 infection/vaccine and onset of VKH symptoms | 21 days | 14 days | 14 days | 2 days |
| Treatment | Oral and topical steroids | Oral steroids and immunosuppressive | Oral steroids | Oral steroids |
COVID-19 infection is known to trigger several autoimmune and inflammatory disorders, usually in pediatric age group and rarely in adults.[12] In pediatric age group, autoimmune disorders like Kawasaki disease has been reported in 2–15-year-old children between 18 and 79 days post-COVID-19 infection.[12] In adults, autoimmune diseases like Guillian-–Barre syndrome, idiopathic thrombocytopenic purpura, and autoimmune hemolytic anemia have been reported early in course, usually 4–13 days from the onset of COVID-19 symptoms.[12] Our patient developed ocular symptoms after 3 weeks following COVID -19 infection. To the best of our knowledge, incidence of VKH following a recent COVID-19 infection has not been reported in the Indian population yet.
Conclusion
COVID-19 infection could be a possible trigger factor for VKH. However, further studies are needed to unveil the exact pathogenesis and association. Early initiation of treatment with corticosteroids helps in faster visual recovery in these patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yoshino N, Kawamura A, Ishii A, Yoshida K, Watanabe T, Yamashita T, et al. Vogt-Koyanagi-Harada disease associated with influenza A virus infection. Intern Med. 2018;57:1661–5. doi: 10.2169/internalmedicine.9819-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benito-Pascual B, Gegúndez JA, Díaz-Valle D, Arriola-Villalobos P, Carreño E, Culebras E, et al. Panuveitis and optic neuritis as a possible initial presentation of the Novel coronavirus disease 2019 (COVID-19) Ocul Immunol Inflamm. 2020;28:922–5. doi: 10.1080/09273948.2020.1792512. [DOI] [PubMed] [Google Scholar]
- 3.Walinjkar JA, Makhija SC, Sharma HR, Morekar SR, Natarajan S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J Ophthalmol. 2020;68:2572–4. doi: 10.4103/ijo.IJO_2575_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wade CI, Earley KE, Justin GA, Weber ML. Vogt-Koyanagi-Harada disease presenting secondary to a post-infectious Mycoplasma pneumoniae autoimmune response. Am J Ophthalmol Case Rep. 2020;19:100793. doi: 10.1016/j.ajoc.2020.100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal A, Freund KB, Kumar A, Aggarwal K, Sharma D, Katoch D, et al. OCTA Study Group. Bacillary layer detachment in acute Vogt-Koyanagi-Harada disease:A novel swept-source optical coherence tomography analysis. Retina. 2021;41:774–83. doi: 10.1097/IAE.0000000000002914. [DOI] [PubMed] [Google Scholar]
- 6.Rao NA, Sukavatcharin S, Tsai JH. Vogt-Koyanagi-Harada disease diagnostic criteria. Int Ophthalmol. 2007;27:195–9. doi: 10.1007/s10792-006-9021-x. [DOI] [PubMed] [Google Scholar]
- 7.Liu B, Deng T, Zhu L, Zhong J. Association of human leukocyte antigen (HLA)-DQ and HLA-DQA1/DQB1 alleles with Vogt-Koyanagi-Harada disease:A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e9914. doi: 10.1097/MD.0000000000009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavezzo MM, Sakata VM, Morita C, Rodriguez EE, Abdallah SF, da Silva FT, et al. Vogt-Koyanagi-Harada disease:Review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis. 2016;11:29. doi: 10.1186/s13023-016-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugita S, Takase H, Kawaguchi T, Taguchi C, Mochizuki M. Cross-reaction between tyrosinase peptides and cytomegalovirus antigen by T cells from patients with Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2007;27:87–95. doi: 10.1007/s10792-006-9020-y. [DOI] [PubMed] [Google Scholar]
- 10.Santamaria A, Chang J, Savarain C. SARS-CoV-2 among the potential viral triggers for Vogt-Konayagi-Harada disease:First case report and literature review. Ocul Immunol Inflamm. 2021:1–7. doi: 10.1080/09273948.2021.1966052. doi:10.1080/09273948.2021.1966052. [DOI] [PubMed] [Google Scholar]
- 11.Saraceno JJF, Souza GM, Dos Santos Finamor LP, Nascimento HM, Belfort R., Jr Vogt-Koyanagi-Harada syndrome following COVID-19 and ChAdOx1 nCoV-19 (AZD1222) vaccine. Int J Retina Vitreous. 2021;7:49. doi: 10.1186/s40942-021-00319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16:413–4. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]