Abstract
Corona virus disease 2019 (COVID-19) has been documented to have a spectrum of neuro-ophthalmic manifestations. However, bilateral non-arteritic anterior ischemic optic neuropathy (NAION) post-COVID-19 has not been reported in the literature. We studied the case of a 45-year-old male who presented to our outpatient department (OPD) with bilateral blurring of vision following an episode of COVID-19, 1 month back. Examination and investigations were conclusive of a bilateral NAION. The patient was given a trial of oral steroids. However, the vision loss could not be recovered. Thus, through this case report, we would like to highlight the importance of a close follow-up of patients following COVID-19 infection to detect any sequelae.
Keywords: Bilateral Non-arteritic Anterior Ischemic Optic Neuropathy (NAION), COVID-19, neuro-ophthalmic manifestation
The common ocular manifestations reported post-corona virus disease (post-COVID) are conjunctivitis (0.8%), retinal vascular occlusions resulting secondary to direct infection of the ocular surface with the virus, and abnormal coagulability.[1,2]
The principle neuro-ophthalmic manifestations reported so far have been very few, mainly including ptosis, external ophthalmoplegia, ocular pain, diplopia, nystagmus, incomplete left third nerve palsy and pupillary involvement, bilateral sixth nerve palsy (Miller Fisher syndrome), isolated sixth nerve palsy, internuclear ophthalmoparesis, esotropia, fixation nystagmus (polyneuritis cranialis), and optic neuritis.[3]
There are reports of unilateral non-arteritic anterior ischemic optic neuropathy (NAION) developing 10 days following COVID-19.[4,5] We herein report a case of bilateral sequential NAION post-COVID-19 that developed 1 month following the onset of symptoms of COVID-19 infection.
Case Report
A 45-year-old male presented with acute-onset blurring of vision in both the eyes. He noticed it in his right eye first; 2 weeks later, he developed similar complaints in the left eye. The diminution of vision was non-progressive and majorly involved the inferior visual field. He was diabetic and hypertensive, well controlled on treatment since past 4 years. Patient gave history of COVID-19 infection 1 month prior to the onset of visual symptoms. He had received oral treatment with hydroxychloroquine(400 mg twice daily for 1 day, then 400mg once daily for 4 days), azithromycin (500 mg once daily)for three days and, paracetamol (650 mg three times a day), Zinc 50 mg OD.
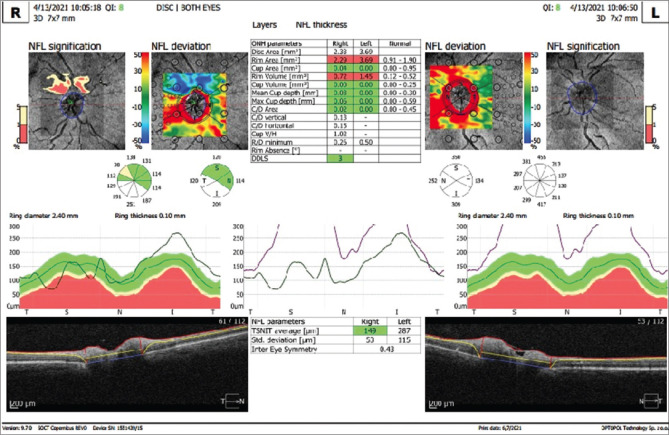
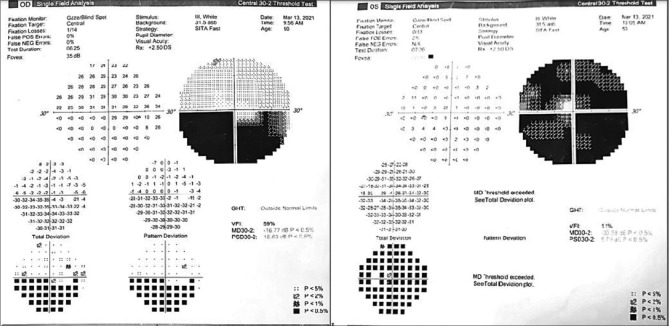
On examination, the best corrected visual acuity was 6/6 in his right eye and 6/24 in his left eye. Anterior segment examination was unremarkable. Relative afferent pupillary defect was noted in the left eye. Fundus examination revealed hyperemic optic disk with blurred margins (OD) and pale edematous disk (OS) [Fig. 1]. Ocular coherence tomography-Retinal nerve fiber layer analysis(OCT-RNFL) centered on the disk showed disk edema in both eyes (left > right) with increased RNFL thickness (left >> right) [Fig. 2]. Color vision (37/38 OD, 13/38 OS) on Ishihara plates and contrast sensitivity was significantly reduced in LE( 1.2 OD, 0.45 OS) on charts. Central 30-2 HVF(Humphrey visual field analysis) showed inferior field defect in the right eye and superior and inferior field defects in the left eye [Fig. 3]. Visually evoked potential (VEP) showed delayed latency of P100 wave (120 ms OD, 225 ms OS) and reduced amplitude ((6 mV OD, 1.7 mV OS) in both eyes (OD > OS).
Figure 1.
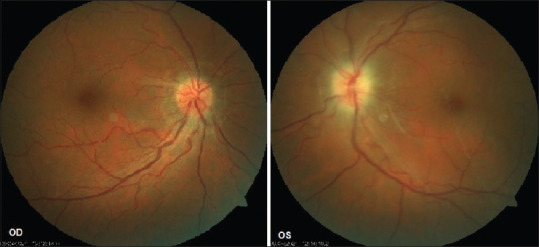
Fundus photograph showing blurring of disk margins OS > OD
Figure 2.
OCT RNFL centered on the disk showing disk edema in both eyes (left > right) with increased RNFL thickness (left >> right)
Figure 3.
Central 30-2 HVF showing inferior field defect in the right eye and superior and inferior field defects in the left eye
Magnetic resonance imaging (MRI) of the brain and orbit revealed normal study. Results of complete blood count, erythrocyte sedimentation rate (ESR), hemoglobin A1c (HbA1c), complete metabolic panel, prothrombin, partial thromboplastin time, antithrombin, protein C, protein S, lupus anticoagulant, and lipid profile were unremarkable. ESR was 40 mm at the end of first hour (normal 0–15); other findings were: hematocrit 54.3% (normal 42%–50%), D-dimer 0.3 mg/ml (normal 0.0–0.5 mg/ml), C-reactive protein 3.18 mg/l (normal <10 mg/l), ferritin 826.14 mg/ml (normal 24–336 mg/ml), and HbA1c 6.7% (normal 4%–6%).
Patient received oral methylprednisolone (1 mg/kg) over 6 weeks (tapering doses every 1 week) after clearance from physician. On follow-up after a month, the visual field defects persisted and disk pallor had set in with gradual resolution of edema in both the eyes.
Discussion
The pathogenesis of COVID-19 remains poorly understood, although there is increasing evidence that inflammatory cytokine storm and viral evasion of cellular immune responses play a fundamental role in disease severity and progression.[6] COVID-19 may predispose patients to thromboembolic events, both in the arterial and venous circulations, due to excessive inflammation, endothelial dysfunction, platelet activation, and stasis.[7] The hypercoagulability induced by COVID-19 could contribute (in addition to other causes) to the development of pulmonary embolism, cerebrovascular accidents (CVAs), deep vein thrombosis, and myocardial infarction.[8,9,10] In the eye, this state of hypercoagulability can lead to formation of thrombi leading to ischemia and compromised vascular circulation, resulting in central retinal vein occlusion or ischemic optic neuropathies.[11]
Ischemic optic neuropathy and other sight-threatening ophthalmic disorders (ocular surface disease, acute angle closure, orbital compartment syndrome and vascular occlusions) have also been documented to have been potentiated by proning, as prolonged proning causes periorbital edema with elevation of orbital venous pressures, exacerbated with high-volume fluid resuscitation.[12,13] However, in our case, proning was not done as the patient had mild symptoms; so, it cannot be considered a causative factor.
Neuroretinitis, optic neuritis, and panuveitis as posterior segment involvements due to viral infections are well known. The proposed mechanisms for such ocular manifestations in viral entities include direct viral involvement or a delayed immune response (type 3 hypersensitivity) to the viral antigen.[4] It is usually seen 1–4 weeks following the onset of fever in viral infections.[4] A post-infectious hyperinflammatory disease has been described as an additional outcome after SARS-CoV-2 (Severe Acute Respiratory Distress Syndrome Coronavirus 2) infection following the involvement of a dysregulated adaptive immune system.[14,15] There is scientific evidence that in COVID-19 vasculitis a life-threatening escalation from type 2 T-helper immune response (humoral immunity) to type 3 hypersensitivity (immune complex disease) takes place leading to deposition of immune complexes inside the vascular walls, inducing a severe inflammatory state and a cytokine release syndrome causing ischemic complications.[16]
In our case, a middle-aged man presented with disk edema and altitudinal visual field defect after a time lag of a month between acute infection and retinal manifestation, which suggests an immune-mediated pathogenesis post-viral infection which led to bilateral NAION. To the best of our knowledge, this is the first reported case of bilateral NAION following COVID-19 infection. Thus, a close follow-up is needed for patients appearing to have recovered fully from COVID-19, especially those with preexisting comorbidities.
Conclusion
Thus, we report a case of bilateral NAION post-COVID-19 in a middle-aged male secondary to immune-mediated mechanisms compromising the perfusion of the optic nerve. It is imperative to note that the patients recovered from COVID-19 should be followed up closely for COVID-19-related sequelae.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanjay S, Mutalik D, Gowda S, Mahendradas P, Kawali A, Shetty R. Post coronavirus disease (COVID-19) reactivation of a quiescent unilateral anterior uveitis. SN Compr Clin Med. 2021:1–5. doi: 10.1007/s42399-021-00985-2. doi:10.1007/s42399-021-00985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luís ME, Hipólito-Fernandes D, Mota C, Maleita D, Xavier C, Maio T, et al. A Review of neuro-ophthalmological manifestations of human coronavirus infection. Eye Brain. 2020;12:129–37. doi: 10.2147/EB.S268828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A, Kudchadkar US, Shirodkar R, Usgaonkar UPS, Naik A. Unilateral inferior altitudinal visual field defect related to COVID-19. Indian J Ophthalmol. 2021;69:989–91. doi: 10.4103/ijo.IJO_3666_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tohamy D, Sharaf M, Abdelazeem K, Saleh MGA, Rateb MF, Soliman W, et al. Ocular manifestations of post-acute COVID-19 syndrome, upper Egypt early report. J Multidiscip Healthc. 2021;14:1935–44. doi: 10.2147/JMDH.S323582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margallo LN, Diaz M, Lim PP. Novel coronavirus pandemic:what do we know? S D Med. 2019;73:262–4. [PubMed] [Google Scholar]
- 7.ikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19, and thrombotic or thromboembolic disease:Implications for prevention, antithrombotic therapy and follow-up. J Am Coll Cardiol. 2020;75:2950–73. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit:A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–42. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Zhou Y, Chang J, Xian Y, Mao L, Hong C, et al. Acute, cerebrovascular disease following COVID-19:A single center retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–84. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China:A retrospective case series study. JAMA Neurol. 2020;77:683–90. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yahalomi T, Pikkel J, Arnon R, Pessach Y. Central retinal vein occlusion in a young healthy COVID-19 patient:A case report. Am J Ophthalmol Case Rep. 2020;20:100992. doi: 10.1016/j.ajoc.2020.100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanghi P, Malik M, Hossain IT, Manzouri B. Ocular complications in the prone position in the critical care setting:The COVID-19 pandemic. J Intensive Care Med. 2021;36:361–72. doi: 10.1177/0885066620959031. [DOI] [PubMed] [Google Scholar]
- 13.Goyal A, Elminawy M, Alvi MA, Long TR, Chen JJ, Bradley E, et al. Ischemic Optic Neuropathy Following Spine Surgery:Case Control Analysis and Systematic Review of the Literature. Spine (Phila Pa 1976) 2019;44:1087–96. doi: 10.1097/BRS.0000000000003010. [DOI] [PubMed] [Google Scholar]
- 14.Yong SJ. Long COVID or post-COVID-19 syndrome:Putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021;53:737–54. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 16.Roncati L, Ligabue G, Fabbiani L, Malagoli C, Gallo G, Lusenti B, et al. Type 3 hypersensitivity in COVID-19 vasculitis. Clin Immunol. 2020;217:108487. doi: 10.1016/j.clim.2020.108487. [DOI] [PMC free article] [PubMed] [Google Scholar]