Abstract
The whole world waiting for the elimination of COVID-19. This is a short series of three cases that presented with optic neuritis. On further inquiry, all had received the Covishield vaccine within 5–12 days just before the presentation, with no history of COVID-19 positive RT-PCR. The range of age was 27–48 years. All patients improved after pulse steroid therapy and are still under follow-up. After being plagued by COVID-19 for nearly 2 years, the whole world wishes for little more than complete eradication of the disease. Our country commenced the much-awaited vaccination drive from Jan 2021. Ophthalmic manifestations have appeared in many forms post-COVID-19, among which neuro-ophthalmic manifestations are infrequent. To the best of our knowledge, this is the first report of a short case series from our country presenting with optic neuritis after COVID-19 vaccination, without any sign of active infection.
Keywords: COVID-19, Covishield vaccine, postvaccine optic neuritis
Case Reports
Case 1
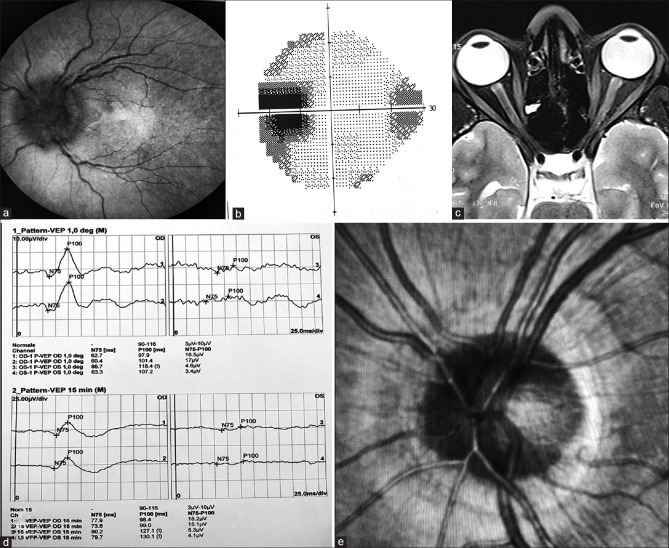
A 27 year nondiabetic, nonhypertensive female presented with a sudden progressive blurring of vision in the left eye (LE), following mild pain over the periocular region for 5 days. The best-corrected visual acuity (BCVA) was 20/20 in the right eye (RE) and 20/200 in LE. There was RAPD and color desaturation in LE. Dilated LE fundus revealed diffuse swelling of the optic nerve head [Fig. 1a]. Automated perimetry (AP) showed an enlarged blind spot [Fig. 1b]. On inquiry, she had received her first dose of Covishield vaccine 9 days before presentation. MRI brain and orbit (T2) revealed enhancement of left optic nerve head just behind the disc [Fig. 1c]. VEP showed a flat wave in LE compared to RE [Fig. 1d]. Diagnosis of optic neuritis (ON) was made in LE, and a neurologist opinion was requested. ESR, CRP was within normal limit, and antibody titer (Ab): ANA, ANCA, MOG, and NMO (Aquaporin4) was negative. Intravenous methylprednisolone pulse therapy was started for 3 days followed by the oral steroid. BCVA improved to 20/40 in LE, and fundus revealed decreased swelling of optic nerve head [Fig. 1e].
Figure 1.
(a) Fundus showing swollen optic disc with blurred margins in LE. (b) AP showing enlarged blind spot. (c) MRI brain and orbit showing enhancement of the left optic nerve. (d) VEP showing flat waves in LE. (e) Fundus picture showing reduced swelling of the disc on follow-up
Case 2
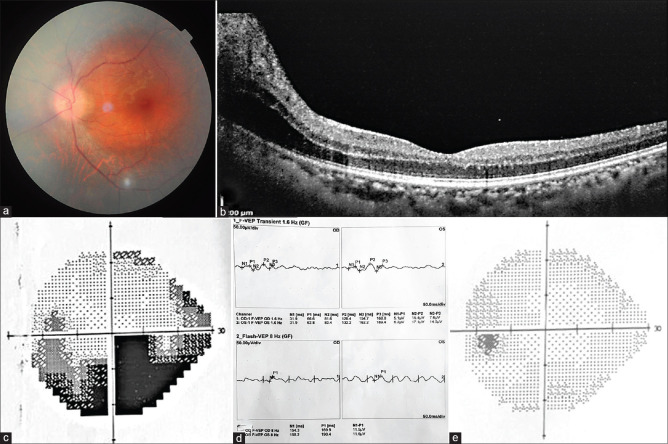
A 48-year-old woman presented with the painless gradual diminution of vision in LE for 3 days. BCVA was 20/30 in RE and 20/80 in LE with RAPD. Dilated fundus revealed swollen optic disc with blurred margins [Fig. 2a]. OCT showed peripapillary swelling of the retina [Fig. 2b]. Visual field revealed an inferior arcuate defect [Fig. 2c], and VEP showed delayed latency and decreased amplitude in LE [Fig. 2d]. On further inquiry, she received the second dose of Covishield vaccine 5 days before presentation and had no history of preceding fever. Diagnosis of ON was made in LE. ESR, CRP, MRI brain, and orbit were within normal limits. Intravenous methylprednisolone pulse therapy had been started after neurologist consultation. On follow-up, BCVA improved to 20/30 in LE and AP revealed marked improvement [Fig. 2e].
Figure 2.
(a) Fundus showing swollen optic disc with blurred margin in LE. (b) OCT showing peripapillary swelling of the retina. (c) AP revealing an arcuate defect in the inferior hemifield. (d) VEP showing delayed latency and decreased amplitude. (e) Follow-up AP showing improvement
Case 3
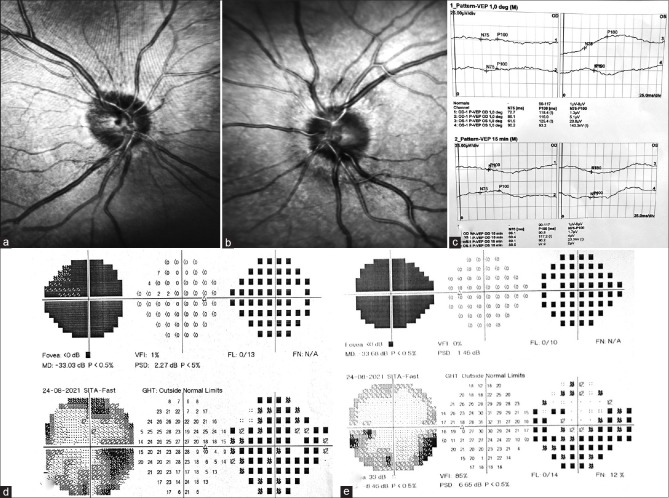
A 40-year-old male presented with a sudden blurring of vision in both eyes (BE), associated with peri-ocular pain for 7 days. He had received the first dose of the Covishield vaccine 12 days before presentation. BCVA was 20/200 with sluggishly reacting pupil in BE. Dilated fundus revealed bilaterally blurred and swollen optic disc margin [Fig. 3a and b]. AP revealed generalized depression of the visual fields in BE [Fig. 3d and e]. VEP showed BE flat waves [Fig. 3c]. Diagnosis of bilateral ON was made. ESR and CRP were within normal range. BCVA improved to 20/30 in RE and 20/40 in LE after steroid therapy. Serial AP showed improvement of the field with inferior arcuate defect [Fig. 3d and e]. MRI brain and orbit requested on follow-up.
Figure 3.
(a and b) Fundus photo showing swollen optic disc with blurred margin in both eyes. (c) VEP showing bilateral flat waves. (d and e) AP showing bilateral generalized depression of field on presentation, with improvement on follow-up
Discussion
We are living amid a pandemic, where along with various systems, COVID-19 also involves the eye. Starting from the anterior segment in the form of conjunctivitis, episcleritis to posterior segments like vascular occlusion, maculopathy, and neuroophthalmic involvement like ON, tonic pupil, and even orbital involvement.[1] COVID-19 vaccines form a crucial step in controlling the pandemic, with over a hundred million vaccines administered since the commencement of the mass vaccination program in early Dec 2020. The COVID-19 vaccine, like any other, can cause side effects mostly low-grade fever or muscle aches, and rarely neurological ones.[2] Pichi et al.[3] reported ocular adverse events after Sinopharm COVID-19 vaccination in nine eyes of seven patients, which were mostly retinal including one case of paracentral acute middle maculopathy, two acute macular neuro-retinopathy, one subretinal fluid. In this case series, all patients presented with ON, which developed within 5–12 days (mean 8.6 days) of COVID-19 vaccination. Postvaccine ON is a known but uncommon side effect where the exact mechanism remains elusive, mostly believed that the vaccine activates the host immune system, leading to T cells activation which damages the myelin sheath of the optic nerve.[4] VAERS (vaccine adverse event reporting system) is a passive surveillance system collecting reports of adverse effects following vaccination from health care providers, vaccine manufacturers, and affected individuals. Among 537 cases of vaccine-related ON, which have been reported, 229 were isolated ones.[5,6] Postinfluenza vaccine ON was most commonly reported, followed by post-HPV, HBV vaccine.[7] ON developing as early as 24 h post-MMR vaccination, had also been reported.[8] Sawalha et al.[9] reported bilateral ON followed within a week of COVID-19 symptoms. Similarly, another case had been reported within few days of COVID-19 by Zhou et al.[10] Although ON following vaccination is an uncommon side effect, safety concerns are required. Recently two cases of bilateral arteritic anterior ischemic optic neuropathy (AAION) and acute zonal occult outer retinopathy (AZOOR) had been reported following COVID-19 m-RNA vaccination.[11] There was a report of acute reduction of visual acuity and visual field after the second dose of Pfizer-BioNTech vaccine.[12] To date, the largest multinational report after vaccination against SARS-CoV-2 is from Alvarez et al. and Leber et al. [Table 1][13,14] (pre-print version). Among 55 patients diagnosed with ON in this report with three different vaccines, majority of cases (38/55) were associated with AstraZeneca vaccine, mostly with a negative history for neuroinflammation. There was also a recent case report of development of acute thyroiditis and bilateral ON following CoronaVac vaccine [Table 1].[13,14]
Table 1.
Review of literature of development of optic neuritis following COVID-19 vaccination
| Study | Type | Number of cases | Presentation | Duration between development of ON and vaccination | Vaccine | Age |
|---|---|---|---|---|---|---|
| Alvarez et al. (pre-print) | Observational study, Cohort | 55 | 27 papillitis | Median - 18 days (range: 1-69) | 38/55- AstraZeneca 13- Pfizer-BioNTech 4 - Sinovac | Median -45 years (range: 18-75) |
| Leber et al. | Case report | 1 | 14- MOG + Acute thyroiditis, Bilateral ON | CoronaVac |
This is probably the first series of ON following COVID-19 vaccination without active infection from India. All three patients received the approved Covishield vaccine (Recombinant m-RNA, Serum Institute of India). Among them, two patients developed ON after the first dose and one after the second dose. None of the patients had RT-PCR positivity. Although a review of safety has shown that the vaccine is generally well tolerated, possibility of ON should be kept in mind. A way forward can be to ask to report any new visual symptoms following vaccination early. In our series, all three patients responded well with steroids. Although it is hard to determine, whether post-COVID-19 vaccine ON is a coincidence or cause. This series highlights the importance of taking the history of recent vaccination, especially in patients presenting with ON in COVID-19 pandemic era.
Conclusion
Although this is a small case series, any further cases should be reported to build a foundation for whether post-COVID-19 vaccine ON is a consequence or coincidence. In a presentation of ON, one should proceed with heightened suspicion not only for a preceding COVID-19 infection but also for a recent history of COVID-19 vaccination, as per the established connection. Long-term follow-up is needed to detect early demyelinating disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and eye:A review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol. 2021;69:488–509. doi: 10.4103/ijo.IJO_297_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu L, Xiong W, Mu J, Zhang Q, Zhang H, Zou L, et al. Neurological side effects of COVID-19 vaccines are rare. Acta Neurol Scand. 2021;144:111–2. doi: 10.1111/ane.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139:1131–5. doi: 10.1001/jamaophthalmol.2021.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shams PN, Plant GT. Optic neuritis:A review. Int MS J. 2009;16:82–9. [PubMed] [Google Scholar]
- 5.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine adverse event reporting system (VAERS) Vaccine. 2015;33:4398–405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roszkiewicz J, Shoenfeld Y. Vaccines and Optic Neuritis:Consequence or Coincidence? Immunome Research. 2021;17:1–2. [Google Scholar]
- 7.Michael ND, Jaffar TN, Hussein A, Hitam WH. Simultaneous bilateral optic neuritis following human papillomavirus vaccination in a young child. Cureus. 2018;10:e3352. doi: 10.7759/cureus.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moradian S, Ahmadieh H. Early onset optic neuritis following measles-rubella vaccination. J Ophthalmic Vis Res. 2008;3:118–22. [PMC free article] [PubMed] [Google Scholar]
- 9.Sawalha K, Adeodokun S, Kamoga GR. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep. 2020;8:2324709620976018. doi: 10.1177/2324709620976018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. 2020;40:398–402. doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maleki A, Look-Why S, Manhapra A, Foster CS. COVID-19 recombinant mRNA vaccines and serious ocular inflammatory side effects:Real or coincidence? J Ophthalmic Vis Res. 2021;16:490–501. doi: 10.18502/jovr.v16i3.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santovito LS, Pinna G. Acute reduction of visual acuity and visual field after Pfizer-BioNTech COVID-19 vaccine 2nd dose:A case report. Inflamm Res. 2021;70:931–3. doi: 10.1007/s00011-021-01476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez LM, Ning Neo Y, Davagnanam I, Ashenhurst M, Acheson J, Abdel-Hay A, et al. Post vaccination optic neuritis:Observations from the SARS-CoV-2 pandemic. SSRN Electron J. 2021 DOI:10.2139/ssrn. 3889990. [Google Scholar]
- 14.Leber HM, Sant'Ana L, Konichi da Silva NR, Raio MC, Mazzeo TJ, Endo CM, et al. Acute thyroiditis and bilateral optic neuritis following SARS-CoV-2 vaccination with coronavac:A case report. Ocul Immunol Inflamm. 2021:1–7. doi: 10.1080/09273948.2021.1961815. doi:10.1080/09273948.2021.1961815. Online ahead of print. [DOI] [PubMed] [Google Scholar]