Abstract
Objective:
To evaluate whether oral contraceptive (OC) use is associated with the risk of a second attack and disability accrual in women with a clinically isolated syndrome (CIS) and early multiple sclerosis (MS).
Methods:
Reproductive information from women included in the Barcelona CIS prospective cohort was collected through a self-reported cross-sectional survey. We examined the relationship of OC exposure with the risk of a second attack and confirmed Expanded Disability Status Scale of 3.0 using multivariate Cox regression models, adjusted by age, topography of CIS, oligoclonal bands, baseline brain T2 lesions, body size at menarche, smoking, and disease-modifying treatment (DMT). OC and DMT exposures were considered as time-varying variables. Findings were confirmed with sensitivity analyses using propensity score models.
Results:
A total of 495 women were included, 389 (78.6%) referred to ever use OC and 341 (68.9%) started OC before the CIS. Exposure to OC was not associated with a second attack (adjusted hazard ratio (aHR) = 0.73, 95% confidence interval (CI) = 0.33–1.61) or disability accrual (aHR = 0.81, 95% CI = 0.17–3.76). Sensitivity analyses confirmed these results.
Conclusion:
OC use does not modify the risk of second attack or disability accrual in patients with CIS and early MS, once considered as a time-dependent exposure and adjusted by other potential confounders.
Keywords: Clinically isolated syndrome, multiple sclerosis, oral contraceptives, second relapse, disability, cohort study
Introduction
Hormonal exposures and environmental factors could play a role in multiple sclerosis (MS) risk and prognosis. High doses of estrogen seem to be protective in the animal model of MS and in patients during pregnancy, when the disease activity decreases significantly.1,2 Therefore, other variations in estrogen levels during a woman’s lifetime, such as the use of oral contraceptives (OC), could potentially have an effect on the risk of developing MS and its prognosis, but their role is still conflicting. Different population-based case–control or cohort studies conclude protective,3,4 neutral, 5 or even negative 6 effects of OC exposure on the risk of MS. Studies focusing on patients with confirmed MS report no effect on clinical and magnetic resonance imaging (MRI) activity7–9 and inconsistencies with respect to disability accumulation, with a reduced risk8,10 or an increased risk in patients with progressive onset. 11 The lack of consistency between results could be partially explained by the influence of potential clinical, 12 reproductive, 13 and environmental confounders14,15 that affect disease evolution and also determine the patient’s decision to take OC leading to a possible selection bias. Moreover, there is a lack of evidence on the role of oral contraceptives in the transition to confirmed MS in women with a clinically isolated syndrome (CIS), which represents an important target population for clinical counseling, as the time of first exposure to OC usually overlaps with the average age at CIS. In this study, we assess whether exposure to OC in patients with CIS is associated with the risk of (1) experiencing the clinically definite MS (CDMS) and (2) reaching a confirmed Expanded Disability Status Scale (EDSS) of 3.0 using a time-dependent multivariable approach with relevant confounders. This study is framed within the Barcelona MS&Gender Project aimed at studying the role of hormonal factors in the modulation of MS prognosis.16,17
Methodology
Study design and data collection
The Barcelona MS&Gender Project is an observational study based on an ongoing prospective CIS cohort that includes patients aged below 50 years who exhibit a CIS suggestive of central nervous system demyelination. Patients are seen on a regular basis and demographic, clinical, cerebrospinal fluid (CSF), and MRI information is prospectively collected using a pre-specified protocol that has been previously described. 12 Female patients belonging to this cohort were invited to participate in the Barcelona MS&Gender Project 16 by signing an informed consent and completing a self-administered survey on reproductive information, external hormonal exposures, and other environmental risk factors. The survey was completed from February 2015 until November 2015 during the patient’s regular clinical assessments, using a tablet device or by email. A reminder email was sent up to three times if the patient did not fill out the survey after the first email invitation. Any missing or ambiguous data were confirmed during clinical visits or by telephone call.
Data prospectively collected in the Barcelona CIS cohort include demographics (date of birth and sex), baseline clinical data (age at CIS, topography of the CIS, previous history of neurological abnormalities, disability according to the EDSS) and follow-up clinical information (disease-modifying treatment (DMT) and date of its initiation, relapses, and annual EDSS), presence of oligoclonal bands (OB) in CSF, and MRI information (number and topography of T2 lesions, the presence of contrast gadolinium-enhancing lesions (CEL), and the number of new T2 lesions on follow-up MRIs). The information collected within the MS&Gender questionnaire included exposure to any composition of OCs (estrogen, progestogen, or combination), pregnancy history (number of pregnancies, date, and outcome), smoking habits (recorded as yes/no and year of smoking onset), and body size self-perception (1 of 9 body silhouettes on the Stunkard figure rating scale). 18
We studied the association of OC exposure with the following outcomes: (1) time to MS diagnosis by presence of a second attack (new symptoms suggestive of relapse occurring after an interval of at least 1 month) and (2) time to confirmed EDSS score of 3.0 (EDSS score ⩾ 3.0 in two evaluations over the course of the disease, performed during stable periods).
Data management and statistical analysis
For the purpose of this study, women were categorized with respect to their OC exposure in “never-users” or “ever-users.” The “ever-users” category was also subdivided into those who started the OC exposure before or after their CIS, respectively. For DMT modeling, we considered the first drug exposure of the patient and date of its initiation (any disease-modifying drug). The total number of T2 lesions was categorized in four levels: 0, 1–3, 4–9, and 10 or more and CEL was categorized into 0, 1, or >1 lesions. Body size self-perception was categorized into underweight 1–3, normal weight 4–6, and overweight 7–9.
The association between OC use with the diagnosis and prognosis was evaluated with Cox proportional hazards (PH) regression models in which OC exposure was considered as a dichotomous time-dependent variable (considering the date of OC initiation as the beginning of the exposure). For these models, we selected women who were never exposed to OC and women who were exposed after their CIS. This was done to avoid a potential overrepresentation of exposure to OC in the cohort, as the time-dependent modeling of OC assumes an ongoing exposure during the complete observation period. For those women who start OC early (before the CIS), it is unclear whether they would still be on OCs after disease onset and their exclusion allows minimizing the risk of classification bias. Two main PH Cox models were built for each outcome: (1) univariate model and (2) multivariate model adjusted by the age at CIS, topography of the CIS, presence of OB in CSF, baseline number of brain T2 lesions, CEL at baseline, and DMT initiation. Body size at menarche and smoking at the time of the CIS were also included in the multivariable adjustment. In the latter approach, DMT exposure was modeled as a dichotomous time-dependent covariate.
Additionally, we confirmed our findings by fitting a propensity score model for OC use at any time using inverse probability weighting. These models control for the possible selection bias given that women who decided to use OC could exhibit different prognoses. The weights were estimated using a logistic regression to estimate the probability of starting OC use including age at CIS, parity, displaying ⩾10 T2 brain lesions at baseline, presence of CEL, presence of OB in CSF, presenting an optic neuritis at CIS, and being treated before the CDMS. Standardized mean differences (SMD) were estimated to examine the balance of covariate distribution according to the exposure of OC after weighting.
Finally, a sensitivity analysis was conducted including also the group of women who started OC before the CIS into the univariable and multivariable Cox regression models, in order to confirm the robustness of the previous findings.
In all PH Cox models, the proportionality assumption was diagnosed via a statistical significance of the test of time independence of the Schoenfeld residuals. Analyses were performed using R software (3.6.0 version).
Standard protocol approvals, registrations, and patient consents
This study was performed with the approval of the Ethics Committee of Hospital Universitari Vall d’ Hebron. All patients signed a written informed consent form.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
A total of 1137 patients were enrolled in the prospective CIS cohort up to February 2016, of whom 764 (67.2%) were female and 496 responded the questionnaire (65% response rate). As compared with those who did not participate, patients who filled out the survey had a similar age at CIS (31.6 vs 31.5 years, p = 0.971) but were more prone to exhibit an abnormal baseline MRI (49.4% vs 34.4%, p < 0.001), were more frequently on DMT (54% vs 16%, p < 0.001), and had a longer follow-up (9.7 vs 5.2 years, p < 0.001). One patient was excluded for not providing information regarding the date of OC initiation. From the 495 responders, 389 (78.6%) referred to “ever use” OC of whom 341 (68.9%) started OC before their CIS. Patients who started OC before CIS were older at CIS, had a smaller body size at menarche, and a shorter follow-up. No differences were found in other clinical or radiological characteristics (see Table 1). From the 495 responders, 208 (42%) fulfill McDonald 2017 criteria at baseline and 352 (71.1%) at the time of responding the questionnaire. A total of 266 (54%) women experienced a second attack (CDMS) during their follow-up and 66 (13%) reached confirmed EDSS 3.0. At the time when the questionnaire was administered, 143 (28.9%) women were CIS patients not fulfilling McDonald 2017 criteria. However, the percentage of patients who reported OC use was similar among women who were and were not diagnosed at the time they responded to the questionnaire (77.3% vs 81.8%) and also between those who had and had not reached EDSS 3.0 (77.3% vs 78.8%).
Table 1.
Patients’ characteristics according to oral contraceptive use.
| Never OC | OC before CIS | OC after CIS | |
|---|---|---|---|
| N = 106 | N = 341 | N = 48 | |
| Age at CIS, mean (SD) | 31.0 (7.9) | 32.7 (7.5) | 24.4 (6.8) |
| CIS topography, n (%) | |||
| Optic nerve | 35 (33.0) | 109 (32.0) | 14 (29.2) |
| Brainstem | 34 (32.1) | 82 (24.0) | 20 (41.7) |
| Spinal cord | 29 (27.4) | 105 (30.8) | 9 (18.8) |
| Other | 8 (7.5) | 45 (13.2) | 5 (10.4) |
| Positive OB, n (%) | 56 (61.5) | 189 (64.7) | 25 (64.1) |
| Baseline MRI T2 lesions | |||
| 0 lesions | 20 (19.6) | 74 (22.6) | 10 (21.3) |
| 1–3 lesions | 13 (12.7) | 47 (14.4) | 9 (19.1) |
| 4–9 lesions | 16 (15.7) | 50 (15.3) | 2 (4.3) |
| ⩾10 lesions | 53 (52.0) | 156 (47.7) | 26 (55.3) |
| Baseline MRI CEL lesions | |||
| 0 lesions | 44 (54.3) | 163 (64.7) | 15 (53.6) |
| 1 lesion | 13 (16.0) | 35 (13.9) | 5 (17.9) |
| >1 lesion | 24 (29.6) | 54 (21.4) | 8 (28.6) |
| Body size at CIS, n (%) | |||
| Underweight (1–3) | 57 (53.8) | 199 (58.4) | 21 (43.8) |
| Normal weight (4–6) | 41 (38.7) | 128 (37.5) | 20 (41.7) |
| Overweight (7–9) | 8 (7.5) | 14 (4.1) | 7 (14.6) |
| Parity, n (%) | |||
| 0 | 45 (42.5) | 93 (27.3) | 19 (39.6) |
| ⩾1 | 61 (57.5) | 248 (72.9) | 29 (60.4) |
| Smoking status at CIS | |||
| Smoker | 66 (62.3) | 233 (68.3) | 26 (54.2) |
| EDSS at questionnaire, median (IQR) | 1.5 (1–2) | 1.5 (1–2) | 1 (1–2) |
| Confirmed MS a at questionnaire | 80 (75.5) | 235 (68.9) | 37 (77.1) |
| DMTs at questionnaire | |||
| First line b | 40/45 (88.9) | 102/134 (76.1) | 17/25 (68) |
| Second line c | 5/45 (11.1) | 29/134 (21.6) | 8/25 (32) |
| Follow-up years, mean (SD) | 10.1 (5.6) | 9.2 (5.4) | 12.9 (4.3) |
OC: oral contraceptives; CIS: clinically isolated syndrome; SD: standard deviation; OB: oligoclonal bands; MRI: magnetic resonance imaging; CEL: contrast gadolinium-enhancing lesions; EDSS: Expanded Disability Status Scale; IQR: interquartile range; MS: multiple sclerosis; DMT: disease-modifying treatment.
A total of 3/341 (0.9%) patients starting OC before the CIS were enrolled in a clinical trial at the time of the questionnaire.
According to McDonald 2017 criteria.
First line: interferons, glatiramer acetate, teriflunomide, and dimethyl fumarate.
Second line: natalizumab, S1P receptors, cladribine, alemtuzumab, and anti-CD20.
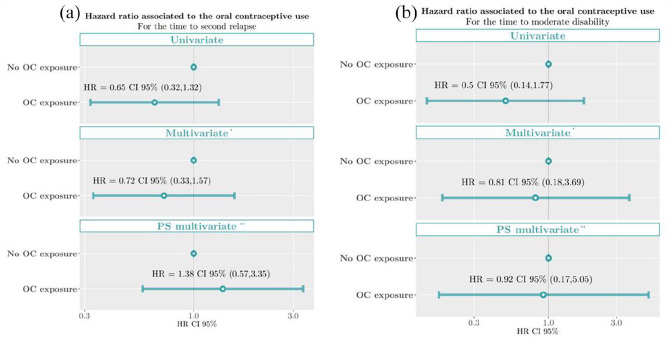
The proportionality assumption was held both for the univariate PH Cox models (p = 0.601 of Schoenfeld residuals test for CDMS; 0.314 for EDSS 3.0) and the multivariate models (p = 0.501 for CDMS; p = 0.480 for EDSS 3.0). The univariate time-dependent analysis showed no association between OC exposure neither with the risk of experiencing CDMS (hazard ratio (HR) = 0.65, 95% confidence interval (CI) = 0.32–1.32) nor for EDSS 3.0 (HR = 0.50, 95% CI = 0.14–1.77). The same association was seen in the multivariate time-dependent analyses, showing no association between OC exposure with the risk of CDMS (adjusted hazard ratio (aHR) = 0.73, 95% CI = 0.33–1.61) or for EDSS 3.0 (aHR = 0.81, 95% CI = 0.17–3.76). The results of the HR estimates for the covariates included in the multivariate time-dependent model are shown in Table 2. This absence of association was supported by the multivariable HR calculated within the propensity score sensitivity analyses (aHR = 1.44, 95% CI = 0.59–3.54 for CDMS and aHR = 0.92, 95% CI = 0.16–5.09 for EDSS3) (Figure 1). Finally, the same results were obtained in the sensitivity analysis when considering the whole study cohort (no OC use, OC use before the CIS, and OC use after the CIS), showing no association between OC use and the risk of second attack (HR: 0.91 (0.69, 1.18), p = 0.471 and aHR: 1.08 (0.82, 1.41), p = 0.5832) or the risk of EDSS 3.0 (HR: 1.09 (0.62, 1.92), p = 0.775 and aHR: 1.13 (0.61, 2.08), p = 0.6969).
Table 2.
HR estimates of the adjusting variables of the multivariate time-dependent Cox models.
| Time to second attack | Time to confirmed EDSS 3.0 | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age at CIS | 0.96 | 0.93–0.99 | 0.0098 | 1.04 | 0.99–1.1 | 0.143 |
| CIS topography | ||||||
| Optic nerve | Ref. | Ref. | ||||
| Brainstem | 0.68 | 0.36–1.26 | 0.2161 | 2.11 | 0.33–13.49 | 0.429 |
| Spinal cord | 1.31 | 0.72–2.39 | 0.3752 | 3.55 | 0.57–22.05 | 0.175 |
| Other | 0.68 | 0.28–1.61 | 0.3785 | 4.64 | 0.53–40.68 | 0.166 |
| OB | ||||||
| Negative | Ref. | Ref. | ||||
| Positive | 1.62 | 0.90–2.91 | 0.1058 | 1.44 | 0.32–6.4 | 0.635 |
| Unknown | 1.01 | 0.48–2.12 | 0.9711 | 3.13 | 0.69–14.12 | 0.138 |
| MRI T2 lesions | ||||||
| <10 lesions | Ref. | Ref. | ||||
| ⩾10 lesions | 2.85 | 1.61–5.02 | 0.0003 | 2.24 | 0.57–8.77 | 0.248 |
| MRI CEL lesions | ||||||
| 0 lesions | Ref. | Ref. | ||||
| >0 lesions | 1.88 | 1.12–3.18 | 0.0178 | 1.6 | 0.45–5.61 | 0.467 |
| Unknown | 0.53 | 0.31–0.91 | 0.0225 | 0.65 | 0.11–3.78 | 0.630 |
| Body size, n (%) | ||||||
| Underweight (1–3) | Ref. | Ref. | ||||
| Normal weight (4–6) | 0.8 | 0.49–1.30 | 0.3708 | 0.63 | 0.18–2.22 | 0.475 |
| Overweight (7–9) | 1.12 | 0.58–2.17 | 0.7449 | 2.47 | 0.31–19.47 | 0.390 |
| Smoking status at CIS | ||||||
| Non-smoker | Ref. | Ref. | ||||
| Smoker | 0.95 | 0.60–1.49 | 0.8156 | 1 | 0.31–3.24 | 0.998 |
| Treatment initiation | ||||||
| No initiation | Ref. | Ref. | ||||
| Treated | 0.48 | 0.25–0.92 | 0.0270 | 0.9 | 0.26–3.17 | 0.875 |
HR: hazard ratio; EDSS: Expanded Disability Status Scale; CI: confidence interval; CIS: clinically isolated syndrome; OB: oligoclonal bands; MRI: magnetic resonance imaging; CEL: contrast gadolinium-enhancing lesions.
The values highlighted in bold are those with statistical significance (p < 0.05).
Figure 1.
Proportional hazard Cox model estimates of the oral contraceptive exposure associated with the risk of (a) second relapse and (b) moderate disability (confirmed EDSS of 3.0). *Adjusted by age at CIS, topography of CIS, presence of OB in CSF, baseline number of T2 lesions, baseline CEL, body size at menarche, and treatment initiation. **Propensity score (PS) model for the OC use at any time using inverse probability weighting.
OC: oral contraceptives; HR: hazard ratio.
Discussion
Exposure to OCs is not associated neither with the risk of second attack or with disability accumulation in CIS patients, once considered as time-dependent covariates and adjusted for other confounders. The absence of effect on MS activity (second attack) would support the idea that low doses of estrogen (alone or in combination with progestin) contained in contemporary OCs might not be high enough to play an anti-inflammatory role in CIS patients. 19 There is not specific data focusing on CIS patients, but this lack of association would be in line with previous studies looking at the potential effects of OC in patients with relapsing-remitting MS (RRMS), indicating no increase in relapse rate. 7 It would also be consistent with the neutral effect of OC exposure on MS risk reported in studies performed in unaffected populations. 5
We did not find a protective effect on disability accumulation as suggested by other authors8,10 possibly due to the different outcomes used. Previous studies focused on the mean EDSS between groups while in the present work we used the time to confirmed EDSS 3.0 which we believe to be a more robust disability outcome. Moreover, in our study, clinical and radiological characteristics were similar between groups at baseline and an additional adjustment was performed analyzing OC and DMT as time-changing exposures. This was not the case in previous studies where women with less severe disease were more frequently exposed to OC after CIS8,9 and the protective effect of OC could be in part confounded by a “healthy user” bias. In this situation, the adjustment for other confounders is crucial.
We found that the use of OC before the CIS was associated with a later age at onset of the disease as compared to women who were never exposed to OC and women who started the exposure after their CIS. This result could be clearly affected by the reverse causality, 20 indicating that women exposed to OC before their CIS were those who had enough time to start using OC given that their CIS occurred at older ages. These results lead us to emphasize the importance of being aware of this phenomenon and to be cautious when considering association and causality in observational studies.
The present study has two major strengths: the study population characteristics and the analytical approach. The patients of this study belong to a large and deeply phenotyped prospective cohort of CIS patients with a long follow-up in which demographic, clinical, MRI, and biological data have been collected under strict protocols and quality controls. This valuable information allows for a multivariable approach to understand the potential impact of OC in the presence of other relevant explanatory variables. The time-dependent approach takes into account that real time when exposure begins rather than assuming it is present since the beginning of the follow-up. In addition, we performed a propensity score analysis for the OC use to address the possible selection bias as women with a milder disease could more likely be engaged in sexual activity and thus exposed to contraceptives as compared to women with a more severe course of the disease.
However, we are also aware of certain limitations. We selected the subgroup of women who were never exposed to OC and women who were exposed after their CIS to avoid a potential overrepresentation of exposure to OC and minimizing the risk of classification bias. The studied cohort is similar in terms of clinical and MRI characteristics to the excluded patients and, therefore, the risk of selection bias is unlikely. This was further confirmed in the sensitivity analysis with consistent results for the whole cohort. However, reducing the study sample can have an impact on the precision of our estimates and larger numbers are needed to confirm our findings. Other potential limitations are the lack of information about the OCs hormonal composition and the duration of the exposure. Nevertheless, none of the previous studies found a dose effect,3,5,6 which favors our results of a non-impact of OC, or a difference in the course of the disease depending on the OC formulation used. 8 Also, a possible recall bias could potentially underestimate the exposure, but the percentage of patients reporting to ever use OC in our cohort is slightly higher than other cohorts of RRMS patients and the numbers are similar regardless of the time when the questionnaire was completed (before or after the outcomes were met). Additionally, being a relatively young cohort can reduce the risk of recall bias. Finally, our study sample has an underrepresentation of patients belonging to the CIS inception cohort with normal baseline MRI who are not on DMT, as they are not regularly seen in the clinic and did not respond the MS&Gender questionnaire despite several email reminders. However, the study cohort does not substantially differ from other available CIS cohorts in terms of baseline clinical and MRI characteristics.21,22 Finally, the resulting cohorts after weighting for the propensity of OC exposure still presented slight imbalances (Supplementary Table 2). Therefore, estimates of the HRs associated with OC exposure were obtained within adjusted models.
This multivariate time-dependent analysis provides evidence that OC use after the CIS is not significantly associated with the risk of second attack or disability accumulation. These findings are relevant for clinical counseling for young women with CIS and/or recent MS diagnosis.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585211053001 for Oral contraceptives do not modify the risk of a second attack and disability accrual in a prospective cohort of women with a clinically isolated syndrome and early multiple sclerosis by Susana Otero-Romero, Pere Carbonell-Mirabent, Luciana Midaglia, María Zuluaga, Ingrid Galán, Alvaro Cobo-Calvo, Jordi Rio, Georgina Arrambide, Angela Vidal-Jordana, Joaquín Castillo, Breogán Rodríguez-Acevedo, Manuel Comabella, Marta Rodríguez, Carmen Tur, Cristina Auger, Alex Rovira, Jaume Sastre-Garriga, Xavier Montalban and Mar Tintoré in Multiple Sclerosis Journal
Footnotes
Author Contributions: S.O.-R. designed and conceptualized the study, analyzed the data, and drafted the manuscript for intellectual content. P.C.-M. played a major role in the analysis of the data, interpreted the data, and revised the manuscript for intellectual content. L.M. played a major role in the acquisition of data, interpreted the data, and revised the manuscript for intellectual content. A.R. played a major role in the acquisition of data and revised the manuscript for intellectual content. M.Z. interpreted the data and revised the manuscript for intellectual content. I.G. played a major role in the acquisition of data. J.R. interpreted the data and revised the manuscript for intellectual content. G.A. interpreted the data and revised the manuscript for intellectual content. A.V.-J., J.C., B.R.-A., M.C., M.R., and C.A. played a major role in the acquisition of data. J.S.-G. interpreted the data and revised the manuscript for intellectual content. X.M. interpreted the data and revised the manuscript for intellectual content. M.T. designed and conceptualized study, analyzed the data, and drafted the manuscript for intellectual content.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.O.-R. has received speaking honoraria and consulting fees from Genzyme, Biogen-Idec, and MSD and research support from Novartis. P.C.-M. declares that there is no conflict of interest. L.M. reports no disclosures relevant to the manuscript. A.R. serves on scientific advisory boards for Biogen-Idec, Novartis, Genzyme, and OLEA Medical, and on the editorial boards of the American Journal of Neuroradiology, Neuroradiology, and European Radiology; has received speaker honoraria from Bayer, Genzyme, Sanofi-Aventis, Bracco, Merck-Serono, Teva Pharmaceutical Industries Ltd., OLEA Medical, Stendhal, Novartis, and Biogen-Idec; and has research agreements with Siemens AG. M.Z. has received compensation for consulting services from Novartis, Stendhal, Genzyme, Merck-Serono, and Bayer. A.C.-C. has received grant from Instituto de Salud Carlos III, Spain; JR19/00007I. I.G. declares that there is no conflict of interest. J.R. has received speaking honoraria and personal compensation for participating on advisory boards from Almirall, Bayer-Schering Healthcare, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Teva, and Sanofi-Aventis. G.A. has received compensation for consulting services from Biogen-Idec, research support from Novartis, and speaking honoraria from Sanofi-Aventis. A.V.-J. has received speaking honoraria and consulting fees from Novartis, Roche, and Sanofi-Aventis. J.C. reports no disclosures relevant to the manuscript. B.R.-A. declares that there is no conflict of interest. M.C. has received compensation for consulting services and speaking honoraria from Bayer Schering Pharma, Merck-Serono, Biogen-Idec, Teva Pharmaceuticals, Sanofi-Aventis, and Novartis. M.R. declares that there is no conflict of interest. C.A. has received speaking honoraria from Novartis, Biogen, and Stendhal. J.S.-G. has received compensation for participating on advisory boards, speaking honoraria, and travel expenses for scientific meetings, consulting services, or research support from Celgene, Novartis, Biogen, Teva, Merck, Almirall, and Genzyme. X.M. has received speaking honoraria and travel expenses for participation in scientific meetings and has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past with Actelion, Almirall, Bayer, Biogen, Celgene, Genzyme, Hoffmann-La Roche, Novartis, Oryzon Genomics, Sanofi-Genzyme, and Teva Pharmaceutical. M.T. has received compensation for consulting services and speaking honoraria from Bayer Schering Pharma, Merck-Serono, Biogen-Idec, Teva Pharmaceuticals, Sanofi-Aventis, Novartis, Almirall, Genzyme, Viela-Bio, and Roche. Go to Neurology. org/N for full disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by FIS PI15/0070 from Ministry of Economy and Competitiveness of Spain.
ORCID iDs: Alvaro Cobo-Calvo  https://orcid.org/0000-0002-2574-0721
https://orcid.org/0000-0002-2574-0721
Jordi Rio  https://orcid.org/0000-0003-4546-7627
https://orcid.org/0000-0003-4546-7627
Georgina Arrambide  https://orcid.org/0000-0002-2657-5510
https://orcid.org/0000-0002-2657-5510
Angela Vidal-Jordana  https://orcid.org/0000-0002-7270-5507
https://orcid.org/0000-0002-7270-5507
Breogán Rodríguez-Acevedo  https://orcid.org/0000-0002-0314-753X
https://orcid.org/0000-0002-0314-753X
Carmen Tur  https://orcid.org/0000-0003-1849-3184
https://orcid.org/0000-0003-1849-3184
Alex Rovira  https://orcid.org/0000-0002-2132-6750
https://orcid.org/0000-0002-2132-6750
Jaume Sastre-Garriga  https://orcid.org/0000-0002-1589-2254
https://orcid.org/0000-0002-1589-2254
Mar Tintoré  https://orcid.org/0000-0001-9999-5359
https://orcid.org/0000-0001-9999-5359
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Susana Otero-Romero, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain/Servicio de Medicina Preventiva y Epidemiología, Antigua Escuela de Enfermeria, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Pere Carbonell-Mirabent, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Luciana Midaglia, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
María Zuluaga, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Ingrid Galán, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Alvaro Cobo-Calvo, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Jordi Rio, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Georgina Arrambide, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Angela Vidal-Jordana, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Joaquín Castillo, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Breogán Rodríguez-Acevedo, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Manuel Comabella, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Marta Rodríguez, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Carmen Tur, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Cristina Auger, Sección de Neuroradiologia, Servei de Radiologia, Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Alex Rovira, Sección de Neuroradiologia, Servei de Radiologia, Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Jaume Sastre-Garriga, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Xavier Montalban, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Mar Tintoré, Centro de Esclerosis Múltiple de Catalunya (Cemcat), Department of Neurology and Neuroimmunology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
References
- 1. Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol 2012; 33(1): 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voskuhl RR, Wang H, Wu TC, et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: A randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15(1): 35–46. [DOI] [PubMed] [Google Scholar]
- 3. Alonso A, Jick SS, Olek MJ, et al. Recent use of oral contraceptives and the risk of multiple sclerosis. Arch Neurol 2005; 62(9): 1362–1365. [DOI] [PubMed] [Google Scholar]
- 4. Holmqvist P, Hammar M, Landtblom AM, et al. Age at onset of multiple sclerosis is correlated to use of combined oral contraceptives and childbirth before diagnosis. Fertil Steril 2010; 94(7): 2835–2837. [DOI] [PubMed] [Google Scholar]
- 5. Hernan MA, Hohol MJ, Olek MJ, et al. Oral contraceptives and the incidence of multiple sclerosis. Neurology 2000; 55(6): 848–854. [DOI] [PubMed] [Google Scholar]
- 6. Hellwig K, Chen LH, Stancyzk FZ, et al. Oral contraceptives and multiple sclerosis/clinically isolated syndrome susceptibility. PLoS ONE 2016; 11(3): e0149094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bove R, Rankin K, Chua AS, et al. Oral contraceptives and MS disease activity in a contemporary real-world cohort. Mult Scler 2018; 24(2): 227–230. [DOI] [PubMed] [Google Scholar]
- 8. Gava G, Bartolomei I, Costantino A, et al. Long-term influence of combined oral contraceptive use on the clinical course of relapsing-remitting multiple sclerosis. Fertil Steril 2014; 102(1): 116–122. [DOI] [PubMed] [Google Scholar]
- 9. Pozzilli C, De Giglio L, Barletta VT, et al. Oral contraceptives combined with interferon beta in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2015; 2(4): e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sena A, Couderc R, Vasconcelos JC, et al. Oral contraceptive use and clinical outcomes in patients with multiple sclerosis. J Neurol Sci 2012; 317(1–2): 47–51. [DOI] [PubMed] [Google Scholar]
- 11. D’Hooghe MB, Haentjens P, Nagels G, et al. Menarche, oral contraceptives, pregnancy and progression of disability in relapsing onset and progressive onset multiple sclerosis. J Neurol 2012; 259(5): 855–861. [DOI] [PubMed] [Google Scholar]
- 12. Tintore M, Rovira A, Rio J, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015; 138(Pt 7): 1863–1874. [DOI] [PubMed] [Google Scholar]
- 13. Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol 2012; 8(5): 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mowry EM, Azevedo CJ, McCulloch CE, et al. Body mass index, but not vitamin D status, is associated with brain volume change in MS. Neurology 2018; 91(24): e2256–e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manouchehrinia A, Tench CR, Maxted J, et al. Tobacco smoking and disability progression in multiple sclerosis: United Kingdom cohort study. Brain 2013; 136(Pt 7): 2298–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zuluaga MI, Otero-Romero S, Rovira A, et al. Menarche, pregnancies, and breastfeeding do not modify long-term prognosis in multiple sclerosis. Neurology 2019; 92(13): e1507–e1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otero-Romero S, Midaglia L, Carbonell-Mirabent P, et al. Menopause does not modify disability trajectories in a longitudinal cohort of women with clinically isolated syndrome and multiple sclerosis followed from disease onset. Eur J Neurol. Epub ahead of print 20 February 2021. DOI: 10.1111/ene.14782. [DOI] [PubMed] [Google Scholar]
- 18. Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 1983; 60: 115–120. [PubMed] [Google Scholar]
- 19. Ysrraelit MC, Correale J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019; 156(1): 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim TJ, von dem Knesebeck O. Income and obesity: What is the direction of the relationship? A systematic review and meta-analysis. BMJ Open 2018; 8: e019862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brex PA, Ciccarelli O, O’Riordan JI, et al. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 2002; 346(3): 158–164. [DOI] [PubMed] [Google Scholar]
- 22. Bove R, Chitnis T, Cree BA, et al. SUMMIT (Serially Unified Multicenter Multiple Sclerosis Investigation): Creating a repository of deeply phenotyped contemporary multiple sclerosis cohorts. Mult Scler 2018; 24(11): 1485–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585211053001 for Oral contraceptives do not modify the risk of a second attack and disability accrual in a prospective cohort of women with a clinically isolated syndrome and early multiple sclerosis by Susana Otero-Romero, Pere Carbonell-Mirabent, Luciana Midaglia, María Zuluaga, Ingrid Galán, Alvaro Cobo-Calvo, Jordi Rio, Georgina Arrambide, Angela Vidal-Jordana, Joaquín Castillo, Breogán Rodríguez-Acevedo, Manuel Comabella, Marta Rodríguez, Carmen Tur, Cristina Auger, Alex Rovira, Jaume Sastre-Garriga, Xavier Montalban and Mar Tintoré in Multiple Sclerosis Journal
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.