Abstract
Background:
Efficacy and safety of inebilizumab for treatment of neuromyelitis optica spectrum disorder in adults seropositive for aquaporin-4 (AQP4)–immunoglobulin (Ig) G were demonstrated in the 28-week randomized controlled period of the N-MOmentum study.
Objective:
To assess efficacy and safety of long-term inebilizumab treatment.
Methods:
Post hoc analysis was performed in 75 AQP4–IgG–seropositive participants receiving inebilizumab for ⩾4 years in the randomized controlled period and open-label extension of the N-MOmentum study.
Results:
Eighteen attacks occurred in 13 participants during inebilizumab treatment (annualized attack rate, 0.052 attacks/person-year). Twelve attacks occurred during the first year of treatment, and two each occurred in years 2–4. Disability scores remained stable throughout ⩾4 years of treatment. Inebilizumab was well tolerated, with two (2.7%) serious treatment-emergent adverse events related to inebilizumab and no deaths. Immunoglobulin G levels decreased over time; however, correlation between severe infections and low IgG levels could not be determined because of their small numbers.
Conclusion:
These results from the N-MOmentum study continue to support use of inebilizumab for treatment of neuromyelitis optica spectrum disorder. Furthermore, the findings suggest that efficacy of inebilizumab may be enhanced after the first year of treatment, warranting additional long-term investigation.
Keywords: Anti-CD19 monoclonal antibody, optic neuritis, transverse myelitis, neuromyelitis optica spectrum disorder
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory disease of the central nervous system thought to be predominantly mediated by B cells.1,2 Immunoglobulin (Ig) G antibodies against the astrocyte water channel aquaporin-4 (AQP4) are highly specific and detected in the serum of most persons with NMOSD.1,2 Inebilizumab is an afucosylated humanized anti-CD19 IgG1 κ monoclonal antibody that binds to and depletes a wide range of B cells, including plasmablasts and plasma cells.3–5 The efficacy and safety of inebilizumab for treatment of NMOSD were demonstrated during the 28-week randomized controlled period of the pivotal N-MOmentum trial. 4 In 213 participants who were AQP4–IgG seropositive, inebilizumab was associated with a significant reduction in attacks versus placebo (11% vs 42%; p < 0.001). 4 Because of the limited number of AQP4–IgG–seronegative participants (n = 17), efficacy of inebilizumab could not be determined in this cohort. 4
The randomized period of the N-MOmentum trial was limited to 28 weeks to reduce the risk of placebo exposure; 6 therefore, long-term data are necessary to inform the efficacy and safety of inebilizumab. After the randomized controlled period, 201 APQ4–IgG–seropositive participants from both treatment groups continued into an open-label extension and received inebilizumab every 26 weeks. 4 Herein, we present the efficacy and safety outcomes in the subset of AQP4–IgG–seropositive participants who received inebilizumab for ⩾4 years (n = 75).
Materials and methods
Detailed methods of N-MOmentum (NCT02200770) were previously published. 4 Participants were randomized to receive inebilizumab or placebo during the 28-week randomized controlled period followed by an open-label extension, during which all participants received inebilizumab. During the randomized controlled period, inebilizumab or placebo were administered on days 1 and 15. Participants randomized to inebilizumab in the randomized controlled period received inebilizumab 300 mg on day 1 of the open-label extension and placebo on day 15 (to maintain masking). Participants randomized to placebo in the randomized controlled period received inebilizumab 300 mg on days 1 and 15 of the open-label extension to establish B-cell depletion. Subsequently, inebilizumab 300 mg was administered intravenously every 26 weeks to maintain B-cell depletion. Attacks were assessed and confirmed by an independent adjudication committee; the same adjudication criteria and process were used in the randomized controlled period and open-label extension.
Post hoc analyses of efficacy and safety outcomes were performed in participants who were AQP4–IgG seropositive and receiving inebilizumab treatment for ⩾4 years. The NMOSD attack-free probability was estimated using the Kaplan–Meier estimator. Annualized attack rates (AARs) were estimated using a negative binomial regression model. Additional outcomes assessed in this population were summarized with descriptive statistics and included change from baseline in CD20-positive B-cell counts; change from baseline in Expanded Disability Status Scale (EDSS); incidence of treatment-emergent adverse events (TEAEs) and adverse events of special interest (AESIs), which included infusion-related reactions, anaphylactic reactions, hypersensitivity, hepatic function abnormality, cytopenia, infections/opportunistic infections, and malignancies; and change from baseline in IgG, IgM, IgA, and IgE levels.
Results
Participant disposition and baseline characteristics
Of the 213 AQP4–IgG–seropositive participants enrolled in the randomized controlled period, 201 entered the open-label extension. At the time of this analysis, 75 participants in N-MOmentum who were AQP4–IgG seropositive had received inebilizumab treatment for ⩾4 years, including 10 participants who originally received placebo during the randomized controlled period (Table 1). Most participants (93%) were women, and 74 (99%) participants were aged <65 years, with a mean age of 42.7 years. Mean baseline EDSS score was 3.89. Mean (standard deviation (SD)) AAR during the 24 months before the first inebilizumab dose was 1.8 (1.3) attacks/person-year. Participants had a mean (SD) baseline disease duration of 2.5 (3.2) years. Prior steroid treatment was common, with 51 (68%) participants having been treated with methylprednisolone, 25 (33%) with prednisolone, 20 (27%) with methylprednisolone sodium succinate, and 16 (21%) with prednisone. Participants had also been treated with other immunosuppressants (including azathioprine (n = 30; 40%), mycophenolate (n = 11; 15%), cyclophosphamide (n = 3; 4%), and tacrolimus (n = 2; 3%)) and biologics (including rituximab (n = 6; 8%) and eculizumab (n = 1; 1%)). There were also 29 (39%) participants who received prior plasmapheresis, with an average of 8.4 sessions each.
Table 1.
Baseline demographics and characteristics in AQP4–IgG–seropositive participants receiving inebilizumab for ⩾4 years.
| Parameter | Participants (n = 75) |
|---|---|
| Age, mean (SD), years | 42.7 (11.1) |
| Female, n (%) | 70 (93.3) |
| Race, n (%) a | |
| American Indian/Alaskan Native | 5 (6.7) |
| Asian | 19 (25.3) |
| Black/African American | 7 (9.3) |
| White | 39 (52.0) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 10 (13.3) |
| Not Hispanic/Latino | 65 (86.7) |
| Weight, mean (SD), kg | 68.45 (17.91) |
| Body mass index, mean (SD), kg/m2 | 25.36 (5.30) |
| Height, mean (SD), cm | 163.62 (7.75) |
| Baseline EDSS | |
| Mean (SD) | 3.89 (1.64) |
| Median (range) | 3.50 (1.0–7.5) |
| Duration of inebilizumab, years | |
| Mean | 4.60 |
| Median (range) | 4.54 (4.01–5.53) |
| Assigned group in randomized controlled period, n (%) | |
| Inebilizumab | 65 (86.7) |
| Placebo | 10 (13.3) |
AQP4; aquaporin-4; Ig, immunoglobulin; SD: standard deviation; EDSS: Expanded Disability Status Scale.
Each category counts participants who selected only that demographic.
Efficacy
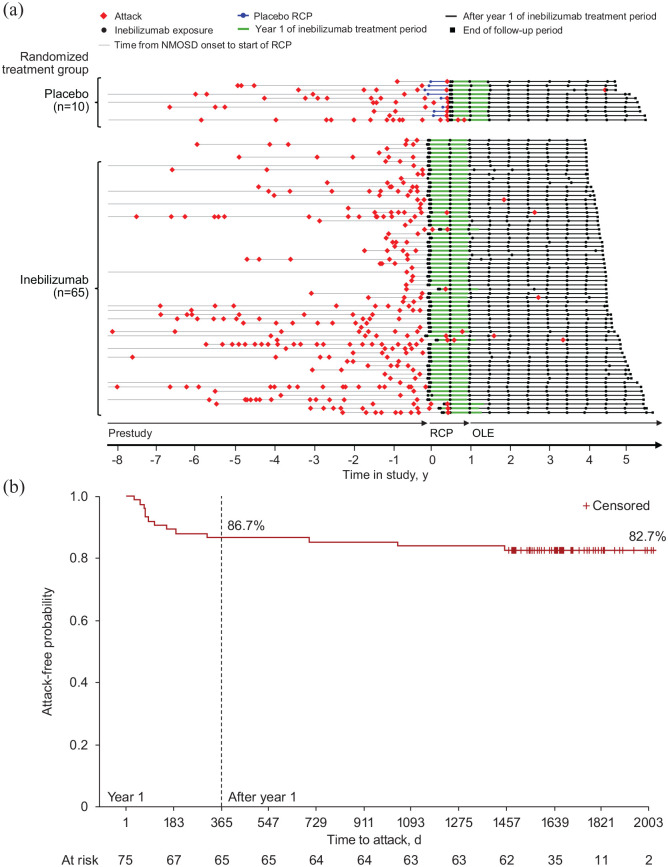
A total of 26 adjudicated attacks occurred in this cohort, 8 of which occurred during the randomized controlled period in the placebo group (before initiation of inebilizumab; Figure 1(a)). Eighteen attacks occurred after initiation of inebilizumab, resulting in an AAR (95% confidence interval (CI)) of 0.052 (0.029–0.092) attacks/person-year. Five attacks (28%) were rated as major in severity, and all but one of the major attacks occurred during year 1. Of the remaining attacks, 11 (61%) were minor, whereas severity was not captured for the other 2 (11%). These 18 attacks occurred in 13 (17%) participants, with 4 participants experiencing multiple adjudicated attacks (2 attacks (n = 3) and 3 attacks (n = 1)). Most attacks that occurred after initiation of inebilizumab occurred in the first year of treatment (12 (67%)); 10 participants experienced ⩾1 attack during year 1. Six (33%) attacks occurred in subsequent years (two attacks each in years 2–4), with one attack each in six participants. Sixty-two (83%) participants were attack free throughout ⩾4 years of inebilizumab treatment. After receiving inebilizumab for 1 year, 69 (92%) participants were attack free during the remainder of the follow-up period. Three participants experienced a first attack after receiving inebilizumab for >1 year, with one first attack each in years 2–4. The Kaplan–Meier estimate of attack-free probability was 87% at year 1 and remained stable through subsequent years (Figure 1(b)).
Figure 1.
Adjudicated attacks in AQP4–IgG–seropositive participants receiving inebilizumab for ⩾4 years. (a) Timeline of adjudicated attacks for AQP4–IgG–seropositive participants with ⩾4 years of inebilizumab treatment. (b) Kaplan–Meier plot of attack-free probability after initiation of inebilizumab.
AQP4: aquaporin-4; Ig: immunoglobulin; NMOSD: neuromyelitis optica spectrum disorder; OLE: open-label extension; RCP: randomized controlled period.
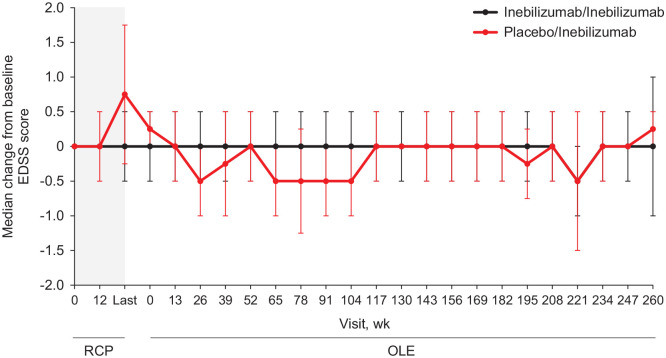
Inebilizumab treatment resulted in a robust depletion of CD20-positive B cells that was maintained throughout ⩾4 years, regardless of the original study group during the randomized controlled period (Supplemental Figure 1). Disability by EDSS score remained stable throughout ⩾4 years after initiation of inebilizumab (Figure 2). Median change from baseline in EDSS score was ⩽0.5 after initiation of inebilizumab throughout the follow-up period.
Figure 2.
Median change from baseline in EDSS score by visit and initial randomized treatment group in AQP4–IgG–seropositive participants receiving inebilizumab for ⩾4 years.
AQP4: aquaporin-4; EDSS: Expanded Disability Status Scale; Ig: immunoglobulin; OLE: open-label extension; RCP: randomized controlled period.
The RCP is indicated in gray. All participants received inebilizumab during the OLE. Bars represent the median absolute deviation.
Safety
A total of 70 (93%) participants experienced a TEAE during treatment (Table 2); 29 (39%) participants had ⩾1 TEAE considered related to inebilizumab. Serious TEAEs occurred in seven (9%) participants; two (3%) participants had a serious TEAE considered related to study treatment. Four (5%) participants had ⩾1 event leading to dose interruption. No TEAEs leading to discontinuation were reported and no deaths occurred.
Table 2.
TEAEs, serious AEs, and AESIs in AQP4–IgG–seropositive participants receiving inebilizumab for ⩾4 years.
| Event | Participants (n = 75) |
|---|---|
| TEAE, n (%) | |
| Any TEAE | 70 (93.3) |
| Related to inebilizumab | 29 (38.7) |
| Grade ⩾3 | 16 (21.3) |
| Leading to treatment discontinuation | 0 |
| Leading to dose interruption a | 4 (5.3) |
| Death | 0 |
| Serious b | 7 (9.3) |
| Serious b and related to inebilizumab | 2 (2.7) |
| AESI, n (%) | |
| Any AESI | 62 (82.7) |
| Infusion-related reaction | 11 (14.7) |
| Anaphylactic reaction | 0 |
| Hypersensitivity | 0 |
| Infection | 59 (78.7) |
| Hepatic function abnormality | 4 (5.3) |
| Cytopenia | 4 (5.3) |
| Opportunistic infection | 0 |
| Progressive multifocal leukoencephalopathy | 0 |
| Treatment-emergent AESI rate after inebilizumab, incidence per 100 PY (95% CI) | |
| Any AESI | 88.8 (79.1–99.3) |
| Infusion-related reaction | 13.9 (10.3–18.5) |
| Infection | 71.4 (62.7–80.9) |
| Treatment-emergent infection rate after inebilizumab, incidence per 100 PY (95% CI) | |
| Overall | 71.4 (62.7–80.9) |
| Year 1 | 112.0 (89.3–138.7) |
| Year 2 | 69.3 (51.8–90.9) |
| Year 3 | 56 (40.4–75.7) |
| Year 4 | 56 (40.4–75.7) |
AE: adverse event; AESI: adverse event of special interest; AQP4: aquaporin-4; Ig: immunoglobulin; PY: person-year; TEAE: treatment-emergent AE; CI: confidence interval.
Three dose interruptions were related to infusion-related reactions: one participant received a full dose in 101 minutes; one received a full dose in 92 minutes; and one received a partial dose for 18 minutes.
Serious AE criteria include death, life-threatening, required inpatient hospital stay, prolongation of existing hospital stay, persistent or significant disability/incapacity, important medical event, and congenital anomaly/birth defect in the offspring of the participant.
Sixty-two (83%) participants experienced an AESI (Table 2); infusion-related reactions and infections were the most commonly reported AESIs. Of 866 total infusions administered, 27 (3%) infusion-related adverse events (AEs) occurred, with infusion-related reactions occurring at a rate of 13.9 events per 100 person-years. No infusion-related reactions were grade ⩾3. Dose interruptions due to infusion-related reactions occurred in three participants. In the first participant, the dose was interrupted on week 234 of the open-label extension because of a grade 2 reaction (swelling), but treatment was continued on that day and the full dose was received over a period of 101 minutes. In the second participant, a grade 2 reaction (dysgeusia, vomiting, headache, dyspnea, and feeling hot) occurred on day 1 of the open-label extension. Infusion was stopped after 18 minutes and the full dose was not received during that treatment session. In the third participant, although the dose was interrupted, the dose was completed within 92 minutes. Only one dose interruption occurred due to a noninfusion-related AE.
Infections occurred in 59 (79%) participants, with an infection incidence of 71.4 events per 100 person-years. The infection rate in participants receiving inebilizumab ⩾4 years did not increase over time on treatment; infection rates in years 1–4 were 112.0, 69.3, 56.0, and 56.0 events per 100 person-years, respectively. Most infections were mild (grade 1) or moderate (grade 2) in severity, and the severity of infections did not appear to increase over time (Table 3). Infections that were serious or grade ⩾3 appear in Supplemental Table 1. Pneumonia was the only serious/severe (grade 3) infection to occur in >1 participant (n = 2 (3%)). Life-threatening (grade 4) pneumonia and urinary tract infection occurred in 1 (1%) participant. No opportunistic infections were reported, nor were any cases of progressive multifocal leukoencephalopathy (PML).
Table 3.
Number of AQP4–IgG–seropositive participants receiving inebilizumab for ⩾4 years by infection grade and year.
| Year after inebilizumab initiation | Grade, n | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1 | 29 | 14 | 2 | 0 |
| 2 | 19 | 13 | 3 | 0 |
| 3 | 19 | 11 | 1 | 0 |
| 4 | 15 | 9 | 2 | 0 |
| ⩾ 5 | 8 | 6 | 2 | 1 |
AQP4: aquaporin-4; Ig: immunoglobulin.
Concentrations of IgG, IgM, IgA, and IgE decreased with inebilizumab treatment (Figure 3; Supplemental Figure 2). Most participants (57 (76%)) maintained normal IgG levels, and the lowest reported IgG categories did not appear to be associated with severe infections, although the number of these participants was small (Table 4). Three (4%) participants had a lowest IgG titer value of <300 mg/dL. No participants required intravenous Ig for hypogammaglobulinemia.
Figure 3.
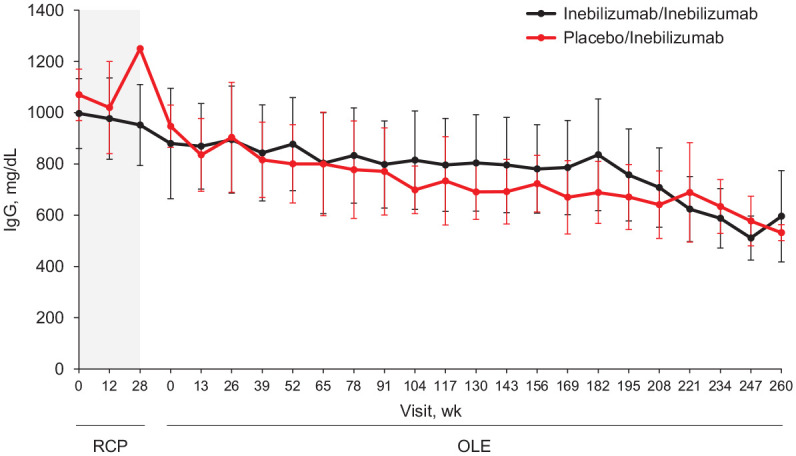
Median IgG titers by visit and initial randomized treatment group in AQP4–IgG–seropositive participants receiving inebilizumab for ⩾4 years.
AQP4: aquaporin-4; Ig: immunoglobulin; OLE: open-label extension; RCP: randomized controlled period.
The RCP is indicated in gray. All participants received inebilizumab during the OLE. Bars represent the median absolute deviation.
Table 4.
Infection status by the lowest IgG titer in AQP4–IgG–seropositive participants receiving inebilizumab for ⩾4 years.
| Titer, n (%) | Infection status a | Total (n = 75) | |
|---|---|---|---|
| Yes (n = 60) | No (n = 15) | ||
| Normal (⩾700 mg/dL) | 26 (43.3) | 9 (60.0) | 35 (46.7) |
| Mild (500 to <700 mg/dL) | 20 (33.3) | 2 (13.3) | 22 (29.3) |
| Moderate (300 to <500 mg/dL) | 12 (20.0) | 3 (20.0) | 15 (20.0) |
| Severe (<300 mg/dL) | 2 (3.3) | 1 (6.7) | 3 (4.0) |
AQP4: aquaporin-4; Ig: immunoglobulin.
p = 0.3479 (Fisher’s exact test).
Median lymphocyte counts were consistent throughout ⩾4 years of follow-up (Supplemental Figure 3A). Neutrophil counts decreased after the first dose of inebilizumab and then gradually increased over ⩾4 years of follow-up (Supplemental Figure 3B). Maximum toxicity grades in lymphocyte and neutrophil counts appear in Supplemental Table 2. Lymphocyte-related AEs were reported in four participants (grade 1 lymphocyte count decreased (n = 1), grade 1 lymphopenia (n = 1), and grade 2 lymphopenia (n = 2)). Three participants reported a neutrophil-related AE (grade 1 neutropenia (n = 1), grade 2 neutropenia (n = 1), and grade 1 neutrophil count increased (n = 1)).
Discussion
NMOSD is a chronic disease, and patients with NMOSD require lifelong maintenance therapy; therefore, long-term data are critical for assessing the safety and efficacy of any new treatment. The randomized controlled period of the N-MOmentum study was relatively short (28 weeks) to reduce the risk of placebo exposure. 6 The post hoc analysis presented here of 75 AQP4–IgG–seropositive participants who received inebilizumab treatment for ⩾4 years provides important information on long-term treatment outcomes. Inebilizumab treatment was associated with a low AAR (0.052 attacks/person-year) in this cohort, and an estimated 83% of participants were attack free at year 4. Most attacks (67%) occurred within the first year of inebilizumab treatment, and nearly all (92%) participants were attack free in subsequent years. This observation may suggest that long-term treatment with inebilizumab is associated with a continuous reduced risk of NMOSD attacks. Potential reasons for this phenomenon are unclear and may be related to the depth and extent of B-cell depletion over time. Additional long-term pharmacodynamic investigations are warranted to provide additional support for this hypothesis. Disability by EDSS scores remained stable throughout ⩾4 years of inebilizumab treatment, a finding consistent with results from the randomized controlled period, which demonstrated reduced odds of worsening disability versus placebo. Furthermore, these current findings support that inebilizumab may be effective in preventing long-term worsening disability associated with NMOSD.
The safety profile of inebilizumab for a treatment period of ⩾4 years did not raise new or unexpected concerns. Inebilizumab was generally well tolerated in participants with NMOSD, with few treatment-related dose interruptions and no treatment discontinuations. Although B-cell depleting therapies have been reported to be associated with an increased risk of fatal infusion reactions, hepatitis B virus reactivation, and PML,7,8 these were not observed in this group of participants with NMOSD. Note that before enrollment in the N-MOmentum trial, all participants were screened for hepatitis B and hepatitis C, and a positive test was exclusionary. Infection rates decreased after the first year of treatment and then remained relatively stable thereafter. Immunoglobulin levels decreased over time with inebilizumab treatment. Although the lowest categories of IgG levels reported among participants were not significantly correlated with infection risk, the rate of severe infections was low and may not have been sufficient to rule out correlation. Further follow-up of patients receiving long-term inebilizumab treatment is required to draw conclusions on the association of Ig levels and severe infections, as longer durations of treatment may reveal a relationship. No participants required Ig replacement.
The long-term efficacy and safety of the complement inhibitor eculizumab and the interleukin-6 receptor antagonist satralizumab have also been assessed in NMOSD. In a long-term analysis of the phase 3 PREVENT study, an estimated 94% of AQP4–IgG–seropositive participants receiving eculizumab, which is administered intravenously every 2 weeks, remained relapse free after 96 and 192 weeks of treatment. 9 The package insert for eculizumab contains a black box warning for serious meningococcal infections, 10 although no cases were observed in the long-term analysis of the PREVENT trial. 9 In a phase 3 trial of satralizumab, which is administered subcutaneously every 4 weeks, 74% of participants remained relapse free after >3 years on treatment. 11 Additional analyses of two phase 3 studies of satralizumab observed that rates of infections were ~100–150 events/person-year and did not increase over 5 years. 12 Lack of head-to-head studies and differences in the study design of the pivotal phase 3 trials (e.g. concomitant therapies, enrollment criteria, adjudication of attacks) limit the ability to make direct comparisons between therapies.
Assessing the relative efficacy of inebilizumab compared with the CD20 inhibitor rituximab is complicated by the lack of head-to-head data and varying attack criteria used in studies. Although clinical outcomes of CD19 versus CD20 inhibition in NMOSD require further investigation, the variable expression of B cells may account for potential differences between these two treatments. Because CD19 is expressed on a greater subset of B cells, with the marker appearing earlier and persisting longer during B-cell development and differentiation compared with CD20, inebilizumab may have a greater treatment effect than rituximab. 13 Furthermore, studies have shown that most antibody-secreting B cells in the periphery are CD19 positive and CD20 negative, whereas antibodies to previously encountered antigens (e.g. from vaccination) are primarily produced by long-lived CD19-negative plasma cells. This suggests that anti-CD19 therapies like inebilizumab may specifically target cells producing pathogenic antibodies while minimizing immunosuppressive effects (although data on AQP4-antibody depletion have not yet been analyzed for this study and merit attention in future publications). 13 In contrast, there is more evidence for the impact of rituximab on other cell lines in addition to CD20-positive B cells, possibly leading to neutropenia, thrombocytopenia, and T-cell hyporesponsiveness. 14
Strengths of the current analysis include the long duration of inebilizumab exposure and a relatively large number of participants analyzed, representing a substantial proportion of the AQP4–IgG–seropositive N-MOmentum study population. This analysis did not include AQP4–IgG–seronegative participants because of the small number of participants in this group. 4 Overall, these results from the N-MOmentum trial in AQP4–IgG–seropositive participants receiving ⩾4 years of inebilizumab treatment continue to support the efficacy and safety of inebilizumab as a disease-modifying therapy for NMOSD.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585211047223 for Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin-4–immunoglobulin G–seropositive participants taking inebilizumab for ≽4 years in the N-MOmentum trial by Mary Rensel, Aram Zabeti, Maureen A Mealy, Daniel Cimbora, Dewei She, Jorn Drappa and Eliezer Katz in Multiple Sclerosis Journal
Supplemental material, sj-eps-1-msj-10.1177_13524585211047223 for Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin-4–immunoglobulin G–seropositive participants taking inebilizumab for ≽4 years in the N-MOmentum trial by Mary Rensel, Aram Zabeti, Maureen A Mealy, Daniel Cimbora, Dewei She, Jorn Drappa and Eliezer Katz in Multiple Sclerosis Journal
Supplemental material, sj-eps-2-msj-10.1177_13524585211047223 for Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin-4–immunoglobulin G–seropositive participants taking inebilizumab for ≽4 years in the N-MOmentum trial by Mary Rensel, Aram Zabeti, Maureen A Mealy, Daniel Cimbora, Dewei She, Jorn Drappa and Eliezer Katz in Multiple Sclerosis Journal
Supplemental material, sj-eps-3-msj-10.1177_13524585211047223 for Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin-4–immunoglobulin G–seropositive participants taking inebilizumab for ≽4 years in the N-MOmentum trial by Mary Rensel, Aram Zabeti, Maureen A Mealy, Daniel Cimbora, Dewei She, Jorn Drappa and Eliezer Katz in Multiple Sclerosis Journal
Acknowledgments
Medical writing and editorial assistance were funded by the Horizon Therapeutics and provided under the direction of the authors by Elizabeth A Harvie, PhD, CMPP, ELS, Sara Gibson, PhD, and Sherri Damlo, ELS, of MedThink SciCom.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.R. has received research funding from Genentech, NMSS, Novartis, and PPD; has received patient-education funds from Genzyme; has served on the DSMC for Biogen; has served as a speaker or consultant for Genentech, Improve Consulting, Kijia, MSAA, Novartis, and Serono; and is the owner or co-owner of Brain Fresh and Brain Ops Group. A.Z. has received research funding from Alexion, BEAT-MS (NIH), DELIVER (PICORI), Genentech-Roche, and Novartis; and has served as a speaker or on advisory boards for Alexion, Biogen, Genentech-Roche, Horizon Therapeutics, Novartis, and Sanofi-Genzyme. M.A.M., D.C., D.S., J.D., and E.K. are the employees of Horizon Therapeutics and may hold stock and/or stock options in the company.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Horizon Therapeutics (formerly known as Viela Bio).
ORCID iDs: Mary Rensel  https://orcid.org/0000-0001-9613-8394
https://orcid.org/0000-0001-9613-8394
Maureen A Mealy  https://orcid.org/0000-0001-8967-6338
https://orcid.org/0000-0001-8967-6338
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Mary Rensel, Mellen Center for Multiple Sclerosis, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA; Mellen Center for Multiple Sclerosis, Cleveland Clinic, Cleveland, OH, USA.
Aram Zabeti, University of Cincinnati, Cincinnati, OH, USA.
Maureen A Mealy, Horizon Therapeutics plc, Deerfield, IL, USA; (known as Viela Bio at the time of study conduct).
Daniel Cimbora, Horizon Therapeutics plc, Deerfield, IL, USA; (known as Viela Bio at the time of study conduct).
Dewei She, Horizon Therapeutics plc, Deerfield, IL, USA; (known as Viela Bio at the time of study conduct).
Jorn Drappa, Horizon Therapeutics plc, Deerfield, IL, USA; (known as Viela Bio at the time of study conduct).
Eliezer Katz, Horizon Therapeutics plc, Deerfield, IL, USA; (known as Viela Bio at the time of study conduct).
References
- 1. Bennett JL, O’Connor KC, Bar-Or A, et al. B lymphocytes in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm 2015; 2(3): e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Viela Bio. Uplizna [package insert]. Gaithersburg, MD: Viela Bio, 2020. [Google Scholar]
- 4. Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): A double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019; 394: 1352–1363. [DOI] [PubMed] [Google Scholar]
- 5. Chen D, Gallagher S, Monson NL, et al. Inebilizumab, a B cell-depleting anti-CD19 antibody for the treatment of autoimmune neurological diseases: Insights from preclinical studies. J Clin Med 2016; 5: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cree BAC, Bennett JL, Sheehan M, et al. Placebo-controlled study in neuromyelitis optica: Ethical and design considerations. Mult Scler 2016; 22(7): 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Genentech Inc. Rituxan [package insert]. San Francisco, CA: Genentech Inc, 2020. [Google Scholar]
- 8. Genentech Inc. Ocrevus [package insert]. San Francisco, CA: Genentech Inc, 2020. [Google Scholar]
- 9. Wingerchuk DM, Fujihara K, Palace J, et al. Long-term safety and efficacy of eculizumab in aquaporin-4 IgG-positive NMOSD. Ann Neurol 2021; 89(6): 1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alexion Pharmaceuticals Inc. Soliris [package insert]. Cheshire, CT: Alexion Pharmaceuticals Inc, 2011. [Google Scholar]
- 11. Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med 2019; 381: 2114–2124. [DOI] [PubMed] [Google Scholar]
- 12. Lucas E, Weinshenker B, Blondeau K, et al. Infection rates with satralizumab in patients with neuromyelitis optica spectrum disorder (NMOSD): Results from the phase 3 SAkura studies. In: MSVirtual 2020 (Virtual meeting), 11–13 September 2020. [Google Scholar]
- 13. Forsthuber TG, Cimbora DM, Ratchford JN, et al. B cell-based therapies in CNS autoimmunity: Differentiating CD19 and CD20 as therapeutic targets. Ther Adv Neurol Disord 2018; 11: 761697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Focosi D, Tuccori M, Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: What do we know after 20 years of rituximab. Rev Med Virol 2019; 29(6): e2077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585211047223 for Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin-4–immunoglobulin G–seropositive participants taking inebilizumab for ≽4 years in the N-MOmentum trial by Mary Rensel, Aram Zabeti, Maureen A Mealy, Daniel Cimbora, Dewei She, Jorn Drappa and Eliezer Katz in Multiple Sclerosis Journal
Supplemental material, sj-eps-1-msj-10.1177_13524585211047223 for Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin-4–immunoglobulin G–seropositive participants taking inebilizumab for ≽4 years in the N-MOmentum trial by Mary Rensel, Aram Zabeti, Maureen A Mealy, Daniel Cimbora, Dewei She, Jorn Drappa and Eliezer Katz in Multiple Sclerosis Journal
Supplemental material, sj-eps-2-msj-10.1177_13524585211047223 for Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin-4–immunoglobulin G–seropositive participants taking inebilizumab for ≽4 years in the N-MOmentum trial by Mary Rensel, Aram Zabeti, Maureen A Mealy, Daniel Cimbora, Dewei She, Jorn Drappa and Eliezer Katz in Multiple Sclerosis Journal
Supplemental material, sj-eps-3-msj-10.1177_13524585211047223 for Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin-4–immunoglobulin G–seropositive participants taking inebilizumab for ≽4 years in the N-MOmentum trial by Mary Rensel, Aram Zabeti, Maureen A Mealy, Daniel Cimbora, Dewei She, Jorn Drappa and Eliezer Katz in Multiple Sclerosis Journal