Abstract
The global coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, has disrupted routines in education, work, exercise, and dining habits. To prevent viral spread, communal spaces including offices, schools, restaurants, and gyms have closed or drastically limited their capacity. Additionally, government-mandated lockdown orders have forced people to spend more time at home. Studies have shown that these COVID-19 restrictions have led to unhealthier eating patterns, increased sedentary behaviors, and decreased physical activity, leading to weight gain, dysglycemia, and increased metabolic risk. While strict social distancing measures have been necessary to curb the spread of the SARS-CoV-2 virus, people have been forced to adapt by altering their daily routines. Based on existing literature, a model is proposed for intentionally creating daily routines to ensure healthy habits, minimize weight gain, and prevent worsening dysglycemia.
Keywords: COVID-19, cardiometabolic risk, dysglycemia, obesity, routine
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak began in Wuhan, China, in 2019, and was declared a global pandemic on March 11, 2020. 1 As of January 2022, over 327 million people have been infected by the virus, and over 5.5 million deaths have been reported worldwide. 2 To cope with disease burden and limit viral spread on a global scale, dining limitations, gym closures, mask mandates, remote schooling, and work-from-home protocols were enacted and enforced. Although the introduction of vaccinations halted the spread of the virus in early 2021, 3 the rise of new variants has forced the prolongation and reinstitution of social distancing and quarantine measures. Disruptions in daily routine have altered eating, exercise, and sleep habits, as well as traditional ways of seeking medical care.
The consequences of the COVID-19 pandemic have been felt disproportionately by people of color and individuals from lower socioeconomic backgrounds.4,5 The latter group makes up a significant proportion of the essential workforce, who do not have the option to isolate and work from home. Individuals from lower socioeconomic backgrounds may also live in higher occupancy households, often intergenerational, enabling a more rapid spread of communicable illnesses.6,7 Within the United States, data have shown higher rates of infection, hospitalization, and deaths from COVID-19 in American Indian, Asian, Black/African American, and Latinx persons, compared to Caucasians. 8 It is known that non-white individuals are subjected to overt and subconscious racial biases within the healthcare system,9,10 which may lead to greater negative health outcomes. These health disparities in the United States preceded the COVID-19 pandemic and are reflected in higher rates of diabetes and its complications in people of color.11,12
Type 2 diabetes (T2D) is a chronic disease that begins with the development of insulin resistance. Following a pancreatic beta-cell defect, hyperglycemia (prediabetes and T2D) develops and can cause subsequent complications, including cardiometabolic disease. 13 Management of T2D focuses on lifestyle modifications, such as healthy eating patterns, physical activity, and weight loss. The prevalence of prediabetes and T2D in the United States is 88 and 31 million, respectively 14 ; however, the impact of the pandemic on the development of dysglycemia needs to be further defined.
We propose that maintaining a daily routine during the pandemic, focused on healthy eating and regular activity, can prevent unhealthy dietary habits, sedentary behavior, and worsening dysglycemia. The benefits of routines on healthy lifestyles are well-established, 15 and have been best studied in children, known as the “structured days hypothesis.” This theory purports that consistent structure, routine, and regulation within a day positively shapes children’s obesogenic behaviors.16,17 With the prolonging of social distancing and quarantine measures, the role of medical professionals in helping patients adhere to, or recreate, their routines will become increasingly important.
The Impact of the COVID-19 Pandemic on Diet and Physical Activity
Multiple cross-sectional survey-based studies have demonstrated the negative impact of quarantine and social distancing measures on daily routines. From April 3 to May 3, 2020, Flanagan et al 18 surveyed 7753 adults in Canada, Australia, Ireland, the United Kingdom, and the United States to assess the effects of stay-at-home orders during the pandemic on physical activity, diet, sleep, sedentary behavior, and mental health, compared to before the pandemic. The authors found a significant decrease in daily exercise during quarantine among all participants, regardless of weight classification (obese, overweight, or normal weight). Overall, physical activity decreased during the pandemic by an average of 18.32 ± 4.63 minutes/week and 111.88 ± 22 weekly MET minutes/week, compared to before the pandemic. In addition, time spent engaging in sedentary activities increased by 16.83 ± .84 and 21.25 ± .90 minutes/day on weekend days (P < .001) and weekdays (P < .001), respectively. Weight gain was reported in 27.4% of all participants and was more common in those with obesity (33.4%) compared to those who were overweight (20.5%) or normal weight (24.7%). 18
There were mixed results when subjects were questioned about eating habits, as measured by the shortened rapid eating assessment for participants (REAP-S) survey. The REAP-S survey asks diet-related questions and tallies a score that is compared to a healthy diet as defined by the Dietary Guidelines for Americans, 19 where a higher score indicates a higher quality diet. In this study, the REAP-S score increased by an average of .81 ± .04 points from before to during quarantine (P < .001). 18 When asked about eating habits, subjects reported consuming fewer fast and fried foods, and cooking at home more often than eating take-out from restaurants. This is consistent with prior studies that demonstrate that home cooking contains less fat than food cooked in restaurants. 20 People with obesity had the greatest increase in healthy eating (1.13 ± .08) compared to those who were overweight (.83 ± .08) or normal weight (.67 ± .08). Despite the positive outcome with the REAP-S score, 35.6% of respondents reported eating subjectively less healthy meals during quarantine, compared to 20.7% of subjects who reported eating healthier meals. 18
As a result of quarantine, self-reported anxiety, measured using the generalized anxiety disorder (GAD) 7 questionnaire, increased among subjects by an average of 8.78 ± .21 points (P < .001), with 20% of subjects reporting symptomatic anxiety. 18 Notably, anxiety was significantly higher among the subjects with obesity, with 24% reporting symptomatic anxiety during quarantine, compared to 17% of those with overweight or normal weight individuals (P < .001). Anxiety levels were not significantly different between groups prior to the pandemic, when only 14% reported symptomatic anxiety. 18 This study was crucial in highlighting the adverse consequences of the pandemic on daily activity levels, weight gain, and anxiety, with mixed results on eating habits.
A second study conducted by Ammar et al 21 surveyed 1047 adults located in Europe, Africa, Asia, and a minority in the Americas on their health behaviors in the spring of 2020. They found significant decreases in total physical activity during compared to before quarantine. Total physical activity decreased by an average of 31 minutes, and vigorous activity, moderate activity, and leisurely walking decreased by an average of 12.7 minutes, 10.7 minutes, and 12.6 minutes, respectively (P < .001). Daily sedentary activity increased by 28% from an average of 5.31 hours/day to 8.41 hours/day (P < .001). 21 In this study, subjects reported significantly unhealthier dietary patterns during quarantine. 18.4% of respondents endorsed unhealthy eating habits prior to the COVID-19 pandemic, compared to 23.3% during the pandemic (P < .001). Binge eating increased from 9.7% to 20.4% (P < .001), snacking increased from 13.9% to 24.4% (P < .001), and the number of subjects reporting consumption of more than 3 large meals per day increased from 6.6% to 14% (P < .001). Notably, alcohol consumption also increased significantly during quarantine from 5.4% to 10.1% (P < .001). 21
A third study conducted by Almandoz et al 22 utilized an online questionnaire to investigate health behaviors, anxiety levels, and depression in patients with obesity at a single hospital site during COVID-19 stay-at-home orders. The survey was sent to 123 participants from April 15 to May 3, 2020. During quarantine, the subjects complied with strict stay-at-home orders, and 87% of respondents left the house only for absolute necessities. Of the survey respondents, whose body mass index was 40.2 kg/m2 on average, 69.6% reported trouble achieving their weight-loss goals during the pandemic. This was attributed to a combination of unhealthier eating and less exercise: 61.2% reported stress eating and found adhering to a healthy diet more challenging, 47.9% reported less exercise time, and 55.8% reported decreased exercise intensity during the pandemic. With regard to mood, 72.8% of respondents reported increased anxiety and 83.6% of respondents reported increased feelings of depression during, compared to before, the pandemic. 22 The relationship between exercise, eating habits, weight, and mood are complex and multifactorial.23-25 These studies reinforce the negative impact of routine changes on eating habits, physical activity levels, and mental health.
The COVID-19 pandemic has led to disturbances in normal sleep schedules. In a cross-sectional study, Pérez-Carbonell et al 26 assessed the effect of the pandemic on sleep patterns in 843 adults. 69.4% of the participants noted a change in their sleep pattern, with only 44.7% feeling refreshed from their sleep and 45.6% feeling more tired than before the lockdown. Poorer sleep during the pandemic was reported as disrupted sleep (42.3%), difficulty falling asleep (30.9%), difficulty staying asleep (30.8%), and going to bed later (30.0%). The use of sleep medications increased from 5.2% of subjects before lockdown to 7.4% during lockdown. Only a minority of subjects (1.9%) reported improved sleep during the pandemic. 26
The negative effects of the pandemic on sleep were exacerbated in subjects who were forced to completely self-isolate, either due to suspected COVID-19 or to exposure to the disease. 60.8% of subjects who were self-isolating reported worse sleep compared to 51.7% of subjects who were not self-isolating (P < .05). Compared to subjects not isolating, those in isolation reported insomnia (P = .012), daytime sleepiness (P = .030), abnormal sleep behavior (P = .018), restless legs (P = .01), more sleep disruptions (P = .003), nightmares (P = .008), falling asleep unintentionally (P = .02), and abnormal sleep rhythms (P = .016). 26
There was a significant association between adverse mental health and reported sleep symptoms. Subjects who reported worse mental health had, on average, 5 sleep symptoms, compared to those who did not and reported no sleep symptoms (P < .001). Difficulty falling asleep, sleep disruption, excessive sleepiness, falling asleep unintentionally, and nightmares were all directly correlated to worse mental health (P < .001), possibly due to factors including decreased exercise, decreased exposure to sunlight, increased screen time, and increased alcohol consumption. Interestingly, there were no significant sleep disruptions in subjects who self-identified as “keyworkers” (healthcare workers, supermarket employees, mail delivery personnel, and others required to report to their job sites throughout the pandemic). 26 It is possible that the continuation of pre-pandemic daily routines prevented adverse sleep effects, supporting the hypothesis that daily routines are critical for sustaining healthy habits during the pandemic.
The above studies, summarized in Table 1, have limitations, including selection bias, lack of generalizability, and subjectivity in responses. Respondents hailed from different geographic regions, each with different lockdown measures in place at different times and with distinct cultural challenges. Therefore, the results may not be applicable outside the populations studied. However, they give an important baseline understanding of the effects of social distancing and quarantining on individuals’ loss of daily routine and changes in weight, exercise, sleep, and mental health.
Table 1.
Scientific Evidence of Effects of Routine Change on Cardiometabolic Risk Factors.
| Study | Subjects | Discussion |
|---|---|---|
| Flanagan et al. (2020) 16 | N = 7753 adults Normal weight: 32.2% Overweight: 32.1% Obese: 34.0% Female: 80% White: 89.6% Mean age: 51.2 years Mean BMI: 28.6 ± .09 kg/m2 |
Physical activity and weight Weight gain reported: 27.5% (all subjects); 33.4% (obese subjects) Sedentary leisure activity time increased: +16.83 minutes on weekdays and +21.25 minutes on weekends, P < .001 Physical activity time decreased: −18.32 minutes/week and 111.88 weekly MET minutes Diet Healthy eating increased during the pandemic, based on REAP-S score: +.8, P < .001 20.7% of subjects reported healthier eating; 35.6% reported unhealthier eating Mood Increased anxiety among all participants using GAD questionnaire: +9.52 (obese subjects), + 8.14 (overweight subjects), + 7.88 (normal weight subjects) |
| Ammar et al. (2020) 19 | N = 1047 adults Europe: 21% Africa: 40% Asia: 36% Female: 54% Single: 43.5% Married/coupled: 53.7% Widowed/separated: 2.9% College graduates: 88.2% Employed: 51.4% Self-employed: 7.1% Unemployed: 7.2% Students: 24.7% Retired: 2.2% No CVD/CVD risk factors: 91.3% |
Physical activity and weight Decrease in overall physical activity from 108 min/week before pandemic to 71.8 min during pandemic, P < .001 Diet Increase in unhealthy eating habits from 18.4% before pandemic to 23.3% during pandemic, P < .001 Increase in binge eating from 9.7% before pandemic to 20.4% during pandemic, P < .001 Increase in snacking from 13.9% before pandemic to 24.4% during pandemic, P < .001 Decrease in alcohol binge drinking from 10.1% before pandemic to 5.4% during pandemic, P < .001 |
| Almandoz et al. (2020) 20 | N = 123 adults Female: 87% Mean age: 51.2 years Mean BMI: 40.2 kg/m2 Post-bariatric surgery: 33.1% White: 49.2% Black: 28.7% Hispanic: 16.4% College graduate: 56.1% Under strict quarantine: 91.5% |
Physical activity and weight 69.6% reported it was more difficult to achieve weight loss during quarantine 47.9% reported less time for exercise during quarantine 55.8% reported decreased intensity of exercise during quarantine Diet 61.2% reported healthy eating was challenging during quarantine 61.2% reported stress eating during quarantine Mood 72.8% report anxiety during quarantine 83.6% report depression during quarantine Hispanics were less likely to report anxiety than non-Hispanics (adjusted odds ratio .16), P = .009 |
| Pérez-carbonell et al. (2020) 24 | N = 843 adults Female: 67.4% Mean age: 52 years White: 92.2% Black: 3.8% Asian: 3.8% Mean BMI: 29.4 kg/m2 |
Sleep 69.4% report changes in sleep routine during lockdown 44.7% report refreshing sleep during lockdown 45.6% report feeling sleepier than before lockdown 42.3% report disrupted sleep during lockdown 35.2% report falling asleep unintentionally during lockdown 30.9% report difficulty falling asleep during lockdown 30.8% report difficulty staying asleep during lockdown 30.0% report later bedtimes during lockdown Mood 65.2% reported negative impact on mental health There was a significant association between sleep-related changes and negative mental health outcomes, P < .001 |
Abbreviations: BMI, body mass index; MET, metabolic equivalent of a task; REAP-S, rapid eating assessment for participants-shortened version; GAD, generalized anxiety disorder; CVD, cardiovascular disease.
The Impact of the COVID-19 Pandemic on Dysglycemia
The effects of the COVID-19 pandemic on glycemic control, diabetes management, and cardiometabolic parameters are inconsistent. Karatas et al 27 conducted a prospective cohort study on body mass index (BMI) and metabolic parameters in adults before and 6 months into quarantine. Data were collected in 85 subjects with T2D and 55 subjects without diabetes at a single hospital site in Turkey, from March, 2019 to October, 2020. The investigators found that subjects, regardless of diabetes, experienced significant weight gain over the 6-month study period. The mean weight for subjects without diabetes increased from 85.56 ± 10.83 kg to 86.10 ± 10.48 kg (P < .05), and the mean weight for subjects with T2D increased from 87.83 ± 18.27 kg to 89.75 ± 18.68 kg (P < .05). 27 The extent of the increase in body weight was not significantly different between the 2 groups.
Although changes in weight were similar between the groups, changes in fasting serum glucose and hemoglobin A1c (HbA1c) during lockdown were significantly different in subjects with and without diabetes. In subjects with diabetes, mean fasting glucose levels increased significantly from 184.38 ± 62.92 mg/dl to 224.08 ± 89.01 mg/dl (P < .001), compared to a non-significant decrease in subjects without diabetes, from 94.90 ± 11.41 mg/dl to 94.40 ± 9.30 mg/dl (P = .396). Similarly, mean HbA1c in the group with diabetes increased significantly from 8.54 ± 1.56% to 9.26 ± 1.70% (P < .001), compared to a non-significant mean HbA1c increase in the group without diabetes from 5.46 ± .32% to 5.48 ± .33% (P = .387). 27 Other notable differences included significant increases in mean low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels in the T2D group, compared to the non-diabetic group (P-values of .032, .027, and .044, respectively). 27 This study shows that independent of any weight changes, subjects with T2D experienced worsening glycemic and lipid parameters during the pandemic compared to those without T2D.
The above findings were consistent with another study by Munekawa et al 28 investigating weight and HbA1c changes in Japanese individuals during the COVID-19 pandemic. The authors found that 159 subjects with T2D had an insignificant mean weight gain from 65.6 ± 15.3 kg to 65.8 ± 15.2 kg (P = .126), but a significant mean HbA1c increase from 7.5 ± 1.0% to 7.6 ± 1.0% (P = .001). When stratified, the HbA1c change was greater in those reporting shorter sleep time (P = .034), increased total diet intake (P = .038), and increased snack consumption (P = .077). 28
Sohn et al 29 conducted an observational cohort study investigating changes in cardiometabolic parameters during the COVID-19 pandemic, and compared them to changes in prior years. The study looked at 1485 adults with known cardiometabolic risk factors, defined as impaired glucose metabolism, hypertension, dyslipidemia, and obesity, who visited a single hospital site in South Korea between September 1, 2016, and May 31, 2020. Changes in various parameters were measured during the pandemic, 2019–2020, and compared to prior years: 2016–2017, 2017–2018, and 2018–2019. The investigators found the largest increase in HbA1c values in the 2019–2020 year (.07 ± .93%), compared to −.04 ± .82%, .03 ± .79%, and .05 ± .78% in the 2016–2017, 2017–2018, and 2018–2019 years, respectively (P < .05). Additionally, increases in BMI, systolic and diastolic blood pressures, total cholesterol, TG levels, and LDL-C levels were all greater in the 2019–2020 year compared to earlier years. Using the Framingham Risk Score, the authors determined that the 10-year coronary heart disease risk increased in 2019–2020 by 1.0 ± 6.2%, compared to decreases in the prior years: −.2 ± 5.4% (2016–2017); −.2 ± 5.3% (2017–2018); and −.7 ± 6.0% (2018–2019) (P < .05). 29 These studies demonstrate the negative consequences of social distancing measures on the health of people with cardiometabolic risk factors.
In countries that enacted national lockdowns, interruptions in routine medical appointments severely impacted treatment of noncommunicable diseases, including diabetes, 30 but the data on countries without mandated lockdowns are sparser. Tanji et al 31 conducted a retrospective cohort study of 1009 subjects with T2D to examine changes in HbA1c values during the pandemic amongst patients at a single hospital center in Japan. Data were collected between January 1, 2019, and August 31, 2020. All subjects, except for 30, continued their usual in-person care, due to the absence of government-mandated lockdown measures requiring them to stay home. The investigators found no difference in BMI before and during the pandemic, but found statistically significant elevations in HbA1c during the pandemic in all subjects. When stratified for age, sex, BMI, insulin requirement, and baseline HbA1c levels, significant HbA1c increases were seen in women, older adults (> 65 years of age), overweight individuals (BMI > 25 kg/m2), non-insulin users, and subjects with baseline HbA1c values < 7.0%. In subjects with an initial HbA1c < 7.0%, mean HbA1c increased from 6.4% to 6.6% (P < .0001), while in individuals with initial HbA1c > 7.0, mean HbA1c increased from 7.89% to 7.92% (P = .342). 31 The authors attribute these findings to better and more consistent glycemic control in subjects using insulin, which tends to be subjects with higher HbA1c.
The above findings by Tanji et al. contrast with the findings of a study by Falcetta et. al 32 which showed worsening HbA1c during the pandemic in insulin users compared to non-insulin users. The study compared metabolic parameters in 304 adult subjects with T2D at a single hospital site in Italy after the nation-wide lockdown from March 9, 2020, to May 3, 2020, to values prior to lockdown, on average 6 ½ months earlier. They found that 28.8% of subjects using insulin had higher HbA1c levels after the 6-month period, compared to 16.5% of subjects not using insulin (P = .012). 32 This study had mixed findings with regard to changes in lipid parameters during the pandemic: total cholesterol decreased from 4.2 ± 0.8 mmol/l to 4.0 ± 0.8 mmol/l (P = .021), and LDL-C decreased from 2.2 ± 0.7 mmol/l to 2.1 ± 0.7 mmol/l (P = .006), while HDL-C decreased from 1.3 ± 0.3 mmol/l to 1.2 ± 0.3 mmol/l (P = .008), and TG increased non-significantly from 1.5 ± .9 mmol/l to 1.6 ± .9 mmol/l (P = .379). 32
In contrast to the above studies, there were several studies that found no significant differences—and even improvements—in HbA1c levels during the pandemic. Sankar et al 33 conducted face-to-face interviews with 110 subjects with T2D at a single hospital site in India to examine differences in HbA1c, body weight, lifestyle changes, and psychosocial stressors before and during the pandemic. The investigators found no significant differences in HbA1c or weight. Mean HbA1c levels increased from 8.12 ± 1.6% to 8.2 ± 1.3%, and mean weight increased from 71.5 ± 14.8 kg to 71.8 ± 13.6 kg, neither of which achieved statistical significance. 33 Most individuals (more than 80%) reported no changes in their physical activity levels or dietary patterns. In keeping with known data on the relationship between diet, exercise, and HbA1c levels,34-36 the investigators found a correlation between physical inactivity and an increase in HbA1c (P = .009), as well as between physical inactivity and unhealthy dietary patterns (P = .04) during lockdown. 33 This confirms the need to continue a healthy diet and good exercise habits during the pandemic to prevent worsening dysglycemia.
In agreement with the above data, a study by Ruissen et al 37 found no overall differences in HbA1c and weight in subjects with T2D before and during the pandemic. The investigators conducted an observational cohort study examining 435 adults with type 1 and type 2 diabetes at a single hospital site in the Netherlands. Data were collected via surveys, continuous glucose sensors, and blood samples for HbA1c determinations. Data collected 8–11 weeks after the start of the lockdown on March 15, 2020, were compared to the last known measurements prior to March 15, 2020. While the investigators found significantly improved HbA1c levels during lockdown for subjects with type 1 diabetes (mean HbA1c levels decreased from 7.68 ± 1.2% to 7.52 ± 1.1%, P < .0001), they found no significant HbA1c changes for subjects with T2D. When stratified by baseline HbA1c levels, there was improvement during the pandemic in T2D diabetic subjects with high baseline HbA1c (8.16%–12.72%). In this subgroup, HbA1c decreased by .62% (P = .0036). The authors also found a significant association between psychological stress levels, measured by the perceived stress scale, 38 and poor glycemic control (P = .034). 37
The data, summarized in Table 2, present mixed findings relating to the effect of the COVID-19 pandemic on diabetes and cardiometabolic parameters. The varied data may be attributable to geographic differences among study locations. In less densely populated areas, outdoor physical activity may be easier to do and there may be less anxiety surrounding the possibility of becoming infected with the SARS-CoV-2 virus from other people.
Table 2.
Summary of Changes in Glycemic Parameters During the COVID-19 Pandemic.
| Study | Subjects | Take-Home Points |
|---|---|---|
| Karatas S. et al. (2021) 25 | N = 85 adults with type 2 diabetes in Istanbul, Turkey Mean age: 54.81 years Female: 68.2% Mean BMI: 33.44 kg/m2 Duration of diabetes: 11.71 years Mean HbA1c: 8.52% N = 55 non-diabetic (control) subjects in Istanbul, Turkey Mean age: 52.61 years Female: 56.4% Mean BMI: 31.63 kg/m2 Mean HbA1c: 5.46% |
BMI and Weight Significant weight gain in non-diabetic subjects during lockdown .54 ± .95 kg, P = .001 Significant weight gain in diabetic subjects during lockdown: 1.91 ± 5.48 kg, P = .002 No significant difference in weight gain between non-diabetic and diabetic subjects during lockdown: .54 ± .95 kg gain (non-diabetic), and +1.91 ± 5.48 kg gain (diabetic), P = .069 No significant difference in change in BMI between non-diabetic and diabetic subjects during lockdown: .20 ± .36 kg/m2 (non-diabetic) and .71 ± 2.11 kg/m2 (diabetic), P = .075 Diabetes parameters Significant increase in HbA1c in diabetic subjects: 8.54 ± 1.56% to 9.26 ± 1.70%, P < .001 Non-significant increase in HbA1c in non-diabetic subjects: 5.46 ± .32% to 5.48 ± .33%, P = .387 Significant increase in fasting glucose in diabetic subjects: 184.38 ± 62.92 mg/dl to 224.08 ± 89.01 mg/dl, P < .001 Non-significant decrease in fasting glucose in non-diabetic subjects 94.90 ± 11.41 mg/dl to 94.40 ± 9.30 mg/dl, P = .396 Significant difference in HbA1c change between non-diabetic subjects (.02 ± .19%) and diabetic subjects (.71 ± 1.35%), P = .002 Lipid parameters Non-significant increase in LDL-C in diabetic subjects: 122.07 ± 33.06 mg/dl to 129.68 ± 30.52 mg/dl, P = .077 Non-significant decrease in LDL-C in non-diabetic subjects: 137.88 ± 36.5 mg/dl to 134.35 ± 29.71 mg/dl, P = .089 Significant increase in TG in diabetic subjects: 229.97 ± 162.56 to 288.18 ± 186.78 mg/dl, P = .030 Non-significant decrease in TG in non-diabetic subjects: 143.56 ± 86.44 mg/dl to 137.09 ± 54.68 mg/dl, P = .256 Non-significant increase in HDL-C in diabetic subjects: 43.84 ± 9.19 to 44.94 ± 9.83, P = .220 Significant decrease in HDL-C in non-diabetic subjects: 55.34 ± 12.66 to 53.45 ± 12.31, P = .001 |
| Munekawa C. et al. (2021) 26 | N = 203 adults with T2D Mean age: 67.4 years Female: 37.9% Insulin dependence: 33.5% |
Weight Non-significant mean weight gain from 65.6 ± 15.3 kg before pandemic to 65.8 ± 15.2 kg after, P = .126 Diabetes parameters Significant increase in HbA1c from 7.5 ± 1.0% before pandemic to 7.6 ± 1.0% after, P = .001 |
| Sohn M. et al. (2021) 27 | N = 1485 adults Mean age: 61.8 years Female: 49% |
BMI Significantly more weight gain during the pandemic compared to years prior: −.34 ± 2.18 (2017–2018); −.39 ± 3.03 kg (2018–2019); +.09 ± 1.16 kg (2019–2020), P < .05 Cardiometabolic parameters Significantly larger increase in HbA1c during pandemic compared to years prior: −.04 ± .82 (2016–2017); +.03 ± .79 (2017–2018); +.05 ± .78 (2018–2019); and +.07 ± .93% (2019–2020), P < .05 Significantly larger change in 10-year CHD risk score during the pandemic compared to years prior: −.2 ± 5.4% (2016–2017); −.2 ± 5.3% (2017–2018); −.7 ± 6.0% (2018–2019); and +1.0 ± 6.2% (2019–2020), P < .05 |
| Tanji Y. et al. (2021) 29 | N = 1009 adults with T2D in Japan Female: 34.9% Mean age: 64.0 years Mean BMI: 26.4 ± 11.9% Mean HbA1c: 7.8% Insulin dependent: 31.2% |
BMI No significant changes in BMI before to after the pandemic: 26.4 kg/m2 (before), 26.4 kg/m2 (after), P > .05 Diabetes parameters Significant mean increase in HbA1c in all subjects, from 7.45% before the pandemic to 7.53% during the pandemic, P < .05 When stratified, significant increase in HbA1c for subjects with initial HbA1c < 7.0%: From 6.42% (before) to 6.60% (after), P < .0001, but no significant increase in HbA1c for subjects with initial HbA1c > 7.0%: 7.89% (before) to 7.92% (after), P = .342 When stratified, significant increase in HbA1c from before to after the pandemic for individuals ≥ 65 years, females, BMI ≥ 25 kg/m2, non-insulin users, P < .05 |
| Falcetta P. et al. (2021) 30 | N = 304 adults with T2D in Italy Female: 34.9% Mean age: 69.1 years Mean duration of T2D: 16 years Hypertension: 75.3% Dyslipidemia: 67.4% Insulin dependent: 34.2% |
BMI and weight Significant increase in BMI from before to during lockdown: 29.2 kg/m2 to 29.3 kg/m2, P = .032 Significant increase in weight from before to during lockdown: 81.5 kg to 81.8 kg, P = .023 Significant increase in waist circumference from before to during lockdown: 204.4 ± 12.4 cm to 105 ± 13.9 cm, P = .001 Diabetes parameters No significant difference in fasting plasma glucose before and during lockdown: 8.6 ± 2.1 mmol/l (before) and 8.8 ± 2.5 mmol/l (After), P = .353 No significant difference in A1c before and during lockdown: 7.1 ± .9% (before) and 7.1 ± .9% (during), P = .60 When stratified by insulin use, significantly more insulin users had an A1c increase (28.8%), compared to non-insulin users (16.5%), P = .012 Cardiac parameters Significant decrease in mean Tchol from before to during lockdown: 4.2 ± 0.8 mmol/l to 4.0 ± 0.8 mmol/l, P = .021 Significant decrease in mean HDL from before to during lockdown 1.3 ± .3 mmol/l to 1.2 ± .3 mmol/l, P = .008 Significant decrease in mean LDL from before to during lockdown 2.2 ± .7 mmol/l to 2.1 ± .7 mmol/l, P = .006 Non-significant increase in mean TG from before to during lockdown: 1.5 ± .9 mmol/l to 1.6 ± .9 mmol/l, P = .379 |
| Sankar P. et al. (2020) 31 | N = 110 adults with T2D in Kerala, India Mean age: 58.67 years Female: 61.8% Insulin dependent: 46.4% Heart disease: 10.9% |
Weight No significant change in weight from before the pandemic 71.5 ± 14.8 kg, to during the pandemic, 71.8 ± 13.6 kg Diabetes parameters No significant change in A1c from before the pandemic 8.12 ± 1.6%, to during the pandemic 8.2 ± 1.3% |
| Ruissen M. et al. (2021) 35 | N = 280 adults with T1D in the Netherlands Mean age: 50.1 years Female: 46.1% Mean BMI: 25.9 kg/m2 Mean duration of DM: 27.5 years Higher education: 59.6% Insulin dependence: 99.6% N = 155 adults with T2D in the Netherlands Mean age: 62.5 years Female: 54% Mean BMI: 30.2 kg/m2 Mean duration of DM: 15.8 years Higher education: 57.0% Insulin dependence: 56.0% |
Weight 40.9% of participants reported weight gain during the pandemic, while 12% reported weight loss 10% of participants reported more exercise during the pandemic, while 45.7% reported less exercise Diabetes parameters Decrease in A1c in T1D subjects from 7.68 ± 1.2% before the pandemic to 7.52 ± 1.1% after the pandemic (P < .0001) No improvement in HbA1c in T2D overall In T2D with high baseline HbA1c (> 8.16%), decrease in HbA1c by .62% from before to during the pandemic, P = .0036 |
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; T1DM, type 1 diabetes mellitus; T2D, type 2 diabetes mellitus; Tchol, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; CHD, coronary heart disease.
Conclusion: Creating a Routine During the Pandemic
Restrictions and lockdowns during the COVID-19 pandemic have led to disruptions in daily routines with negative impacts on exercise, diet, weight, sleep, mood, and, in some cases, dysglycemia. This can be especially true in those who are already overweight, obese, or have T2D. We propose that the intentional creation of daily routines that focus on healthy behaviors can prevent weight gain and the worsening of cardiometabolic risk factors.
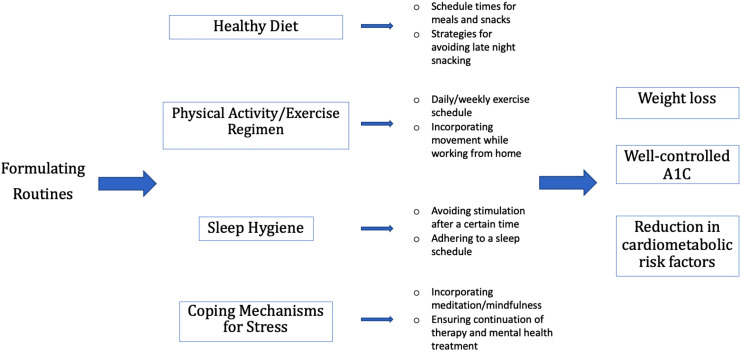
The formation of routines takes anywhere from 18 to 254 days, and requires consistency and repetition. 39 Members of the healthcare team, including physicians, nurses, nurse practitioners, dieticians, and health educators, can assist patients in outlining routines ahead of time, eliminating the real-time need for decision making by the patient. 15 In addition, clinicians can offer guidance on limiting the intake of processed foods, juices, and sodas, and adhering to a low-fat, carbohydrate-controlled diet. 40 Late night cravings can be prevented by establishing a schedule for meals and stopping food intake after a certain hour in the evening, as increased carbohydrate intake later in the day can lead to dysglycemia.41,42 Re-establishing physical activity is also important, with incorporation of daily walks or smaller bouts of movement throughout the day.43,44 Workout routines can be recreated at home with appropriate equipment, even without access to a commercial gym. To cope with stress, individuals can incorporate short periods of meditation or mindfulness, 45 but should continue care with mental health professionals.
Telemedicine can be used by medical professionals to maintain continuity of care and encourage healthy lifestyle behaviors and the creation of daily routines. A meta-analysis conducted before the COVID-19 pandemic found that when used alongside conventional care, telemedicine, including texting, phone calls, internet-based programs, videoconferencing, and electronic physician–patient communication, led to a decrease in HbA1c by an average of .37%, compared to conventional care alone (P < .001). 46 The use of telemedicine has increased since the start of the pandemic, 47 and has shown benefits in diabetes management.48-51 With online data sharing and video appointments, clinicians can review patient-recorded glucose values and adjust insulin doses, view the contents of patients’ refrigerators and pantries as part of their discussion on healthful foods, and visualize space restraints when helping devise exercise regimens. Continued optimization of telemedicine services, limited by the requirement of expensive smart devices and internet access, will help clinicians guide patients in developing healthy habits and reducing metabolic complications.
Limitations to this review include the fact that data are from surveys, which are subjective, non-uniform, and prone to biases. The studies are multinational, and each country has a unique set of COVID-19 restrictions, which have varied through the course of the pandemic. Additionally, data are largely from developed nations, and therefore, conclusions may not be applicable to individuals in developing countries, which have different sets of challenges regarding both healthcare and the COVID-19 pandemic. We acknowledge that specific cultural, socioeconomic, and personal challenges may limit access to medical care and the circumstances required to create consistent routines. For example, recreating a gym space within the home is dependent on having space and resources to purchase various equipment. Telemedicine, too, requires access to internet, which is sparse in certain parts of the world, and devices such as tablets or smartphones, which are costly and not accessible to all individuals.
In summary, this review compiles existing data on how the COVID-19 pandemic has affected daily routine, weight, exercise, and diet. We offer Figure 1 as an example of how physicians and healthcare personal can guide their patients with the formulation of routines. For individuals who are unable to slow the trajectory of weight gain or control glucose levels in the setting of diabetes, maintaining care with a general internist (or other primary care clinician) and/or endocrinologist/obesity medicine specialist is crucial. Further studies are crucial to assess the benefits of daily routines on care of patients with obesity and dysglycemia.
Figure 1.
A model for physician-assisted routine formulation. Figure 1 shows 4 main areas that may have been disrupted and require the purposeful creation of routines during the COVID-19 pandemic, and provides example of how physicians can assist their patients to achieve beneficial outcomes.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Reshmi Srinath https://orcid.org/0000-0001-7051-1365
References
- 1.Ghebreyesus TA. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. Geneva, Switzerland: World Health Organization; 2020. https://www.who.int/ [Google Scholar]
- 2.Johns Hopkins Coronavirus Resource Center. (n.d.) COVID-19 Dashboard. Johns Hopkins University & Medicine. Retrieved February 1, 2020, from https://coronavirus.jhu.edu/map.html. [Google Scholar]
- 3.Christie A, Henley SJ, Mattocks L, et al. Decreases in COVID-19 cases, emergency department visits, hospital admissions, and deaths among older adults following the introduction Of COVID-19 vaccine - United States, September 6, 2020-May 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(23):858-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):703-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Disease. 2020;222(6):890-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkins D. Differential occupational risk For COVID-19 and other infection exposure according to race and ethnicity. Am J Ind Med. 2020;63(9):817-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cromer SJ, Lakhani CM, Wexler DJ, et al. Geospatial analysis of individual and community-level socioeconomic factors impacting SARS-CoV-2 prevalence and outcomes. MedRxiv. 2020. [Google Scholar]
- 8.Center for Disease Control and Prevention (CDC) . Risk for COVID-19 Infection, Hospitalization, and Death by Race/Ethnicity. Geneva, Switzerland: CDC. Updated November 22, 2021. [Google Scholar]
- 9.Marcelin JR, Siraj DS, Victor R, Kotadia S, Maldonado YA. The impact of unconscious bias in healthcare: How to recognize and mitigate it. J Infect Dis. 2019;220:S62-S73. [DOI] [PubMed] [Google Scholar]
- 10.Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine – A time for reckoning with racism. N Engl J Med. 2021;384:474-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canedo JR, Miller ST, Schlundt D, Fadden MK, Sanderson M. Racial/ethnic disparities in diabetes quality of care: The role of healthcare access and socioeconomic status. J Racial Ethn Health Disparities. 2017;5(1):7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haw JS, Shah M, Turbow S, et al. Diabetes complications in racial and ethnic minority populations in the USA. Curr Diab Rep. 2021;21(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mechanick JI, Garber AJ, Grunberger G, Handelsman Y, Garvey WT. Dysglycemia-based chronic disease: An American Association of clinical endocrinologists position statement. Endocr Pract. 2018;24(11):995-1011. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) . National Diabetes Prevention Program. Geneva, Switzerland: CDC; 2021. www.cdc.gov [Google Scholar]
- 15.Arlinghaus KR, Johnston CA. The importance of creating habits and routine. Am J Lifestyle Med. 2019;13(2):142-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brazendale K, Beets MW, Weaver RG, et al. Understanding differences between summer vs. School obesogenic behaviors of children: The structured days hypothesis. Int J Behav Nutr Phys Activ. 2017;1 4(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brazendale K, Beets MW, Armstrong B, et al. Children’s moderate-to-vigorous physical activity on weekdays versus weekend days: A multi-country analysis. Int J Behav Nutr Phys Activ. 2021;18(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan EW, Beyl RA, Fearnbach SN, Altazan AD, Martin CK, Redman LM. The impact Of COVID-19 stay-at-home orders on health behaviors in adults. Obesity. 2021;29(2):438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Department of Agriculture and U.S. Department of Health and Human Services . Dietary Guidelines for Americans, 2020-2025. 9th ed. Washington, DC: USDA; 2020. [Google Scholar]
- 20.Jia X, Liu J, Chen B, et al. Differences in nutrient and energy contents of commonly consumed dishes prepared in restaurants V. At home in hunan province, China. Public Health Nutr. 2018;21(7):1307-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammar A, Brach M, Trabelsi K, et al. Effects Of COVID-19 home confinement on eating behaviour and physical activity: Results of the ECLB-COVID19 international online survey. Nutrients. 2020;12(6):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almandoz JP, Xie L, Schellinger JN, et al. Impact Of COVID-19 stay-at-home orders on weight-related behaviours among patients with obesity. Clin Obes. 2020;10(5):e12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomiyama AJ. Stress and obesity. Annu Rev Psychol. 2019;70:703-718. [DOI] [PubMed] [Google Scholar]
- 24.Annesi JJ. Self-regulation foci and mood affect healthy and unhealthy eating behaviours differently in successful weight-loss treatment participants. Int J Psychol. 2020;55(3):398-404. [DOI] [PubMed] [Google Scholar]
- 25.Mikkelsen K, Stojanovska L, Polenakovic M, Bosevski M, Apostolopoulos V. Exercise and mental health. Maturitas. 2017;106:48-56. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Carbonell L, Meurling IJ, Wassermann D, et al. Impact of the novel coronavirus (COVID-19) pandemic on sleep. J Thorac Dis. 2020;12(suppl 2):S163-S175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karatas S, Yesim T, Beysel S. Impact of lockdown COVID-19 on metabolic control in type 2 diabetes mellitus and healthy people. Prim Care Diabetes. 2021;15(3):424-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munekawa C, Hosomi Y, Hashimoto Y, et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: A cross-section and retrospective cohort study. Endocr J. 2021;68(2):201-210. [DOI] [PubMed] [Google Scholar]
- 29.Sohn M, Koo BK, Yoon HI. Impact Of COVID-19 and associated preventive measures on cardiometabolic risk factors in South Korea. J Obes Metab Syndr. 2021;30:248-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyer O. Covid-19: Pandemic is having ‘‘Severe’’ impact on non-communicable disease care, WHO survey finds. BMJ. 2020;369:m2210. [DOI] [PubMed] [Google Scholar]
- 31.Tanji Y, Sawada S, Watanabe T, et al. Impact Of COVID-19 pandemic on glycemic control among outpatients with type 2 diabetes in Japan: A hospital-based survey from a country without lockdown. Diabetes Res Clin Pract. 2021;176:108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falcetta P, Aragona M, Ciccarone A, et al. Impact Of COVID-19 lockdown on glucose control of elderly people with type 2 diabetes in Italy. Diabetes Res Clin Pract. 2021;174:108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankar P, Ahmed WN, Mariam Koshy V, Jacob R, Sasidharan S. Effects Of COVID-19 lockdown on type 2 diabetes, lifestyle and psychosocial health: A hospital-based cross-sectional survey from South India. Diabetes Metab Syndr. 2020;14(6):1815-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gummesson A, Nyman E, Knutsson M, et al. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(9):1295-1305. [DOI] [PubMed] [Google Scholar]
- 35.Pan B, Ge L, Xun YQ, et al. Exercise training modalities in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Int J Behav Nutr Phys Activ. 2018;15(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amanat S, Ghahri S, Dianatinasab A, et al. Exercise and type 2 diabetes. Adv Exp Med Biol. 2020;1228:91-105. [DOI] [PubMed] [Google Scholar]
- 37.Ruissen MM, Regeer H, Landstra CP, et al. Increased stress, weight gain and less exercise in relation to glycemic control in people with type 1 and type 2 diabetes during the COVID-19 pandemic. BMJ Open Diabetes Res Care. 2021;9(1):e002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396. [PubMed] [Google Scholar]
- 39.Lally P, van Jaarsveld CHM, Potts HWW, Wardle J. How are habits formed: Modelling habit formation in the real world. Eur J Soc Psychol. 2010;40(6):998-1009. [Google Scholar]
- 40.American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(1):S15. [DOI] [PubMed] [Google Scholar]
- 41.Henry CJ, Kaur B, Quek RYC. Chrononutrition in the management of diabetes. Nutr Diabetes. 2020;10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mezitis NHE, Bhatnagar V. Chrononutrition applied to diabetes management: A paradigm shift long delayed. Diabetes Spectr. 2018;31(4):349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mechanick JI, Rosenson RS, Pinney SP, Mancini DM, Narula J, Fuster V. Coronavirus and cardiometabolic syndrome: JACC focus seminar. J Am Coll Cardiol. 2020;76(17):2024-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson CR, Newton TL, Abraham JJ, et al. A meta-analysis of pedometer-based walking interventions and weight loss. Ann Fam Med. 2008;6(1):69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lloyd A, White R, Eames C, Crane R. The utility of home-practice in mindfulness-based group interventions: A systematic review. Mindfulness. 2018;9(3):673-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhai Y-k, Zhu W-j, Cai Y-l, Sun D-x, Zhao J. Clinical- and cost-effectiveness of telemedicine in type 2 diabetes mellitus: A systematic review and meta-analysis. Medicine. 2014;93(28):e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doraiswamy S, Abraham A, Mamtani R, Cheema S. Use of telehealth during the COVID-19 pandemic: Scoping review. J Med Internet Res. 2020;22(12):e24087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lian X, Dalan R, Seow CJ, et al. Diabetes care during COVID-19 pandemic in Singapore using a telehealth strategy. Horm Metab Res. 2021;53(03):191-196. [DOI] [PubMed] [Google Scholar]
- 49.Sayed S. COVID-19 and diabetes; Possible role of polymorphism and rise of telemedicine. Prim Care Diabetes. 2021;15(1):4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodeling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghosh A, Gupta R, Misra A. Telemedicine for diabetes care in India during COVID19 pandemic and national lockdown period: Guidelines for physicians. Diabetes Metab Syndr. 2020;14:273-276. [DOI] [PMC free article] [PubMed] [Google Scholar]