Abstract
Introduction
Rheumatoid arthritis (RA) due to systemic inflammation and insulin resistance increases the risk of cardiovascular disease and reduces life expectancy. In order to develop cardiac death prevention strategies, it is necessary to estimate the prevalence of metabolic syndrome (MetS) in these patients.
Methods
This systematic review and meta-analysis was performed to estimate the prevalence of MetS among patients with RA. International databases (i.e., Scopus, PubMed, Web of Science, and Google Scholar) were searched during the period of October 1 and October 10, 20121. Heterogeneity among the included studies was assessed through the Cochrane Q test statistics and I2 test. Finally, a random-effects meta-analysis model was computed to estimate the pooled prevalence of MetS.
Results
Sixty-one articles with 96 groups and a sample size of 13,644 people were analyzed. The pooled prevalence of MetS was 32% (95% CI: 29.6–34.4). The highest prevalence of MetS is related to studies conducted in Asia (32.7%, 95% CI: 29–36.3) and Europe (32.7%, 95% CI: 27.5.37.9) and the lowest Prevalence was also related to studies conducted in Africa (28%, 95% CI: 28.8–32.2). The prevalence of MetS in men was 33% (95% CI: 26–39) and 34% (95% CI: 29–40) in women. Findings by diagnostic criteria showed that the highest and lowest prevalence of MetS was related to ATP III (37.5%, 95% CI: 30.9–44.2) and EGIR (14.4%, 95% CI: 10.5–18.5), respectively.
Conclusions
MetS is highly prevalent in patients with RA and identification of high-risk patients is necessary to prevent cardiovascular mortality.
Keywords: metabolic syndrome, rheumatoid arthritis, prevalence, systematic review, meta-analysis
Introduction
Rheumatoid arthritis is a chronic inflammatory disease of unknown etiology characterized by systemic symptoms, especially joint involvement and deformity (1). Patients with rheumatoid arthritis are at high risk for cardiovascular disease and premature death due to systemic inflammation, which reduces their life expectancy by 5 to 10 years (2, 3). Rheumatoid arthritis is associated with insulin resistance, dyslipidemia, and changes in adipokines profiles that are components of the metabolic syndrome (MetS) (4). Insulin resistance is a constant risk factor for cardiovascular disease and the central mechanism in metabolic syndrome, which is present in 70% of patients with RA (5, 6).
MetS, also known as syndrome X and insulin resistance syndrome, refers to a set of cardiovascular risk factors (obesity, glucose intolerance, dyslipidemia, and high blood pressure) that can lead to cardiovascular disease (7). MetS increases cardiovascular outcomes and mortality by 2 and 1.5 times, respectively (8, 9). The increased risk of cardiovascular disease in patients with rheumatoid arthritis has been well established, so that the European League Against Rheumatism (EULAR) recommends that screening and management of cardiovascular risk in these patients be performed immediately (10, 11).
Various studies have shown that the prevalence of metabolic syndrome in these patients varies between 10 and 56% (12, 13). In this systematic review and meta-analysis, the cumulative prevalence of metabolic syndrome in patients with rheumatoid arthritis has been estimated.
Methods
Search Strategy
The present systematic review and meta-analysis study was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (14). To access articles examining the prevalence of metabolic syndrome in patients with rheumatoid arthritis, a comprehensive search with no data limit was performed in the following databases: PubMed/MEDLINE, Scopus, Web of Science, and Google Scholar. The search was conducted between October 1 and October 10, 2021. All article published until August 30, 2021 were included. Articles were searched with keywords (“Metabolic Syndrome”[Mesh] OR “Metabolic Syndrome*”[tiab] OR “Insulin Resistance Syndrome*”[tiab] OR “Metabolic X Syndrome*”[tiab] OR “Dysmetabolic Syndrome*”[tiab] OR “Reaven Syndrome*”[tiab] OR “Metabolic Cardiovascular Syndrome*”[tiab]) AND (“rheumatic diseases”[Mesh] OR “Arthritis, Rheumatoid”[Mesh] OR “Rheumatic disease*”[tiab] OR “Rheumatism*”[tiab] OR “Rheumatoid Arthritis”[tiab] OR “Rheumatic symptom*”[tiab]) AND (“Prevalence”[Mesh] OR “Prevalence*”[tiab] OR “Period Prevalence*”[tiab] OR “Point Prevalence*”[tiab]). The reference lists of the included articles were also reviewed to find other eligible articles.
Selection of Studies and Data Extraction
All observational studies published in English that reported the prevalence or frequency of metabolic syndrome in patients with rheumatoid arthritis were analyzed. Interventional, review, and replication studies, as well as studies investigating the prevalence of metabolic syndrome in other rheumatic diseases, were excluded. According to the inclusion and exclusion criteria, the titles and abstracts of the articles were independently reviewed by two researchers and the required information such as first author, year of publication, country of study, sample size, prevalence or frequency of metabolic syndrome in patients with rheumatoid arthritis were extracted and recorded in a pre-prepared form. To evaluate the quality of articles, the modified Newcastle-Ottawa Scale (NOS) was used, which has three main sections. The first part, rated on a scale of one to five stars, focuses on the methodological quality of each study (i.e., sample size, response rate, and sampling technique). The second section considers the comparability of the study cases or cohorts with a possibility of two stars to be gained. The last section is concerned with the outcomes and statistical analysis of the original study with a possibility of three stars to be gained. Two authors extracted the information and evaluated the methodological quality of the articles, independently. Any disagreements between the two reviewers were resolved consensus (15, 16).
Statistical Analysis
Point estimation and 95% confidence interval (CI) of metabolic syndrome due to binomial distribution formula and heterogeneity between studies was evaluated by Cochran Q test with a significance level of less than 0.1 and I2 index. The degree of heterogeneity was assessed using the I2 index. Heterogeneities were divided into three categories: less than 25% (low heterogeneity), 25 to 75% (moderate heterogeneity) and more than 75% (high heterogeneity). Pooled prevalence was estimated using a random-effects model. Subgroup analysis was performed based on diagnostic criteria and continent. To investigate the potential publication bias, funnel plot based on Egger's regression test was used. Univariate meta-regression was used to investigate the relationship between the prevalence of metabolic syndrome and the year of study and the mean age of patients. Data analysis was performed using Stata software version 16.
Results
In the initial search, 938 potentially relevant articles were retrieved. Of these articles, 431 articles were excluded due to duplications and removing duplicate articles, 507 articles remained. The titles and abstracts of the remaining articles were reviewed and 411 irrelevant articles were removed. Of the remaining 96 articles, 34 articles were deleted for not reporting the prevalence of MetS (Figure 1).
Figure 1.
Study selection following PRISMA guidelines.
Study Characteristics
In this study, 62 articles with a sample size of 13,644 people were analyzed, the characteristics of which are listed in Table 1. Most studies were performed in Morocco (n = 9) and Iran (n = 9). Most studies were based on NCEP/ATP III (n = 42) and IDF (n = 21) diagnostic criteria. Thirty-nine studies were conducted in Asia, 25 in Europe, 18 in the United States and 14 in Africa. All selected articles had good methodological quality.
Table 1.
Characteristics of included articles.
| First author | Year | Country | Sample size | Diagnostic criteria | Mean age | RA patients (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Total | M/F | Total | Male | Female | |||||
| Turgunova et al. (17) | 2021 | Kazakhstan | 101 | 31/70 | IDF | - | 40.5 | - | - |
| Hee et al. (18) | 2021 | Singapore | 561 | 0/561 | NCEP/ATP III | - | 44.9 | - | - |
| JC 2009 | - | 49.4 | - | - | |||||
| Giraud et al. (19) | 2021 | France | 75 | 20/55 | WHO | 59.2 | 28 | - | - |
| Kong et al. (20) | 2021 | China | 717 | 152/565 | CDS | 61 | 31.2 | - | - |
| Cioffi et al. (21) | 2021 | Italy | 228 | - | IDF | 58 | 15 | - | - |
| Mobini et al. (13) | 2020 | Iran | 200 | - | NCEP/ATP III | - | 54.5 | - | - |
| IDF | 56 | - | - | ||||||
| Garcia-Chagollan et al. (4) | 2020 | Mexico | 216 | 22/194 | NCEP/ATP III | 46 | 30.6 | - | - |
| ALAD | 28.7 | - | - | ||||||
| Xu et al. (22) | 2020 | Korea | 247 | 48/199 | NCEP/ATP III | 58 | 15 | - | - |
| Shaikh et al. (23) | 2020 | Pakistan | 104 | 10/94 | NCEP/ATP III | 33.4 | 32.7 | - | - |
| Ozkul et al. (24) | 2019 | Turkey | 50 | 11/39 | IDF | 56.9 | 36 | - | - |
| Mulumba et al. (3) | 2019 | Congo | 75 | 15/60 | NCEP/ATP III | 51.8 | 25.3 | - | - |
| Ene et al. (25) | 2019 | Romania | 120 | 31/89 | IDF-NCEP/ATP III | 52.7 | 39.2 | 45.2 | 37.1 |
| Naidu et al. (26) | 2019 | India | 114 | 21/93 | NCEP/ATP III | 44.8 | 31.6 | - | - |
| Kuriya et al. (27) | 2019 | USA | 1543 | 443/1100 | WHO | 54 | 30.8 | 42 | 26 |
| Akbal et al. (28) | 2019 | Turkey | 53 | 12/41 | ATP III | 51 | 47.1 | - | - |
| Aleksic et al. (29) | 2019 | Serbia | 81 | 19/62 | IDF | 59.7 | 54.3 | - | - |
| Mobini et al. (30) | 2018 | Iran | 140 | 25/115 | NCEP/ATP III | 44.7 | 31.4 | - | - |
| IDF | 35 | - | - | ||||||
| Gomes et al. (7) | 2018 | Brazil | 338 | 31/307 | NCEP/ATP III | 53.5 | 51.3 | - | - |
| Burggraaf et al. (31) | 2017 | Netherland | 212 | 65/147 | NCEP/ATP III | 54 | 40.1 | - | - |
| Slimani et al. (32) | 2017 | Algeria | 249 | 36/213 | NCEP/ATP III | 50.1 | 13.9 | 14.3 | 13.8 |
| Pandey et al. (33) | 2017 | India | 84 | 18/66 | ATP III 2004 | 44.8 | 39.2 | - | - |
| Ostojic et al. (34) | 2016 | Serbia | 36 | 6/30 | - | 36 | 30.6 | - | - |
| Lee et al. (35) | 2016 | Korea | 598 | 110/488 | AHA/NHLBI | 63.6 | 36.4 | 34.5 | 36.9 |
| Hugo et al. (36) | 2016 | France | 57 | 15/42 | IDF | 57.6 | 24 | 25 | 24 |
| Zafar et al. (37) | 2016 | Pakistan | 384 | 97/277 | NCEP/ATP III | 43.8 | 31.3 | 18.5 | 35.5 |
| Oliveira et al. (38) | 2016 | Brazil | 107 | 0/107 | NCEP/ATP III | 55.5 | 51.4 | - | 51.4 |
| IDF | 53.4 | - | 53.4 | ||||||
| Muller et al. (39) | 2016 | Estonia | 91 | 66/25 | NCEP/ATP III | 51.6 | 35 | - | - |
| Dihingia et al. (40) | 2016 | India | 72 | 6/66 | NCEP/ATP III | 41.5 | 16.7 | - | - |
| Ghazaly et al. (41) | 2015 | Egypt | 80 | 13/67 | ATP III | 40.7 | 50 | 53 | 49.2 |
| Salamon et al. (42) | 2015 | Croatia | 583 | 100/483 | ATP III | 59 | 43.1 | 40 | 43.7 |
| Tantayakom et al. (43) | 2015 | Thailand | 267 | 31/236 | NCEP/ATP III | 59 | 16.1 | 12.9 | 16.5 |
| Parra-Salcedo et al. (44) | 2015 | Mexico | 160 | 18/142 | AHA/NHLBI | 38.1 | 28 | - | - |
| IDF | 18 | - | - | ||||||
| NCEP/ATP III | 24 | - | - | ||||||
| Craciun et al. (12) | 2014 | Romania | 51 | 7/44 | IDF-AHA | 55.2 | 19 | 10.5 | 82.4 |
| NCEP/ATP III | 23 | - | - | ||||||
| IDF | 18 | - | - | ||||||
| AHA | 14 | - | - | ||||||
| Bilecik et al. (45) | 2014 | Turkey | 100 | 0/100 | IDF | 52 | 33 | - | 33 |
| NCEP/ATP III | 27 | - | 27 | ||||||
| Ozmen et al. (46) | 2014 | Turkey | 52 | 15/37 | NCEP/ATP III | 51 | 17.3 | - | - |
| WHO | 28.8 | - | - | ||||||
| Kumar et al. (47) | 2014 | India | 54 | 6/48 | IDF | 46 | 29 | - | - |
| NCEP/ATP III | 31 | - | - | ||||||
| Abourazzak et al. (48) | 2014 | Morocco | 179 | 22/157 | IDF | 49 | 30.7 | - | - |
| NCEP/ATP III | 29 | - | - | ||||||
| AACE 2003 | 24 | - | - | ||||||
| Salinas et al. (49) | 2013 | Argentina | 409 | 69/340 | ATP III | 55.5 | 30 | 62 | 23.8 |
| IDF | 35 | - | - | ||||||
| Abdul-Qaharr et al. (50) | 2013 | Iraq | 203 | 41/162 | NCEP/ATP III | 46.9 | 51.2 | 12 | 92 |
| Rostom et al. (51) | 2013 | Morocco | 120 | 10/110 | NCEP/ATP III 2004 | 49 | 30.8 | 10 | 32.7 |
| NCEP/ATP III 2001 | 24.6 | - | - | ||||||
| WHO | 20 | - | - | ||||||
| IDF | 48.6 | - | - | ||||||
| EGIR | 18 | - | - | ||||||
| JC 2009 | 32.3 | - | - | ||||||
| Lee et al. (52) | 2013 | Korea | 84 | 0/84 | NCEP/ATP III | 50.6 | 19 | - | 19 |
| Ormseth et al. (53) | 2013 | USA | 162 | 18/144 | ATP III | 54 | 26 | - | - |
| Karakoc et al. (1) | 2012 | Turkey | 54 | 7/47 | IDF | 49.8 | 42.6 | - | - |
| Manka et al. (54) | 2012 | Slovakia | 87 | 4/83 | IDF | 58.8 | 48.3 | - | - |
| NCEP/ATP III | 44.8 | - | - | ||||||
| AHA/NHLBI | 47.1 | - | - | ||||||
| Da Cunha et al. (55) | 2012 | Brazil | 283 | 50/233 | NCEP/ATP III | 56.8 | 39.2 | - | - |
| Goshayeshi et al. (56) | 2012 | Iran | 120 | 14/106 | NCEP/ATP III | 45.5 | 45.2 | - | - |
| Baker et al. (57) | 2012 | USA | 499 | 83/416 | IDF | 49.5 | 10.6 | - | - |
| Crowson et al. (58) | 2011 | USA | 232 | 58/174 | NCEP/ATP III | 58.8 | 33 | 36 | 32 |
| Sahebari et al. (59) | 2011 | Iran | 120 | 14/106 | IDF | 45.5 | 30.8 | 28.6 | 41.5 |
| NCEP/ATP III | 45.2 | 28.6 | 37.7 | ||||||
| Karimi et al. (60) | 2011 | Iran | 92 | 0/92 | NCEP | 48.3 | 27.2 | - | 27.2 |
| WHO | 19.6 | - | 19.6 | ||||||
| Mok et al. (61) | 2011 | Hong Kong | 699 | 133/566 | JS 2009 | 53.3 | 20 | - | - |
| Dao et al. (62) | 2010 | Vietnam | 105 | 0/105 | IDF | 56.3 | 40.9 | - | - |
| NCEP/ATP III 2004 | 32.4 | - | - | ||||||
| NCEP/ATP III 2001 | 24.7 | - | - | ||||||
| JS 2009 | 32.4 | - | - | ||||||
| WHO | 19 | - | - | ||||||
| EGIR | 16.2 | - | - | ||||||
| Raterman et al. (63) | 2010 | Netherland | 236 | 79/157 | NCEP | 62.1 | 19.9 | - | - |
| Solomon et al. (64) | 2010 | South Africa | 291 | 32/259 | NCEP/ATP III | 27.2 | 31.3 | - | - |
| 335 | 65/270 | NCEP/ATP III | 27.2 | 20.3 | - | - | |||
| Giles et al. (65) | 2010 | USA | 131 | 51/80 | NCEP/ATP III | 61 | 36 | - | - |
| Santos et al. (66) | 2010 | Portugal | 98 | 0/98 | ATP III | 49.2 | 25.5 | - | - |
| Toms et al. (67) | 2009 | UK | 387 | 105/282 | IDF | 63.1 | 45.3 | 52.7 | 42.6 |
| NCEP/ATP III 2004 | 40.1 | 42.5 | 39.2 | ||||||
| NCEP/ATP III 2001 | 38.3 | 40 | 37.7 | ||||||
| WHO | 19.4 | 25.5 | 17.2 | ||||||
| EGIR | 12.1 | 22.6 | 8.2 | ||||||
| Chung et al. (2) | 2008 | USA | 66 | 18/48 | WHO | 59 | 42 | - | - |
| Zonana-Nacach et al. (68) | 2008 | Mexico | 107 | - | NCEP/ATP III | 42.9 | 18.7 | - | - |
| Karvounaris et al. (69) | 2007 | Greece | 200 | 53/147 | ATP III | 63 | 44 | 39.6 | 45.6 |
| Montagna et al. (70) | 2007 | Italy | 45 | 3/42 | NCEP/ATP III | 53.8 | 55.5 | - | - |
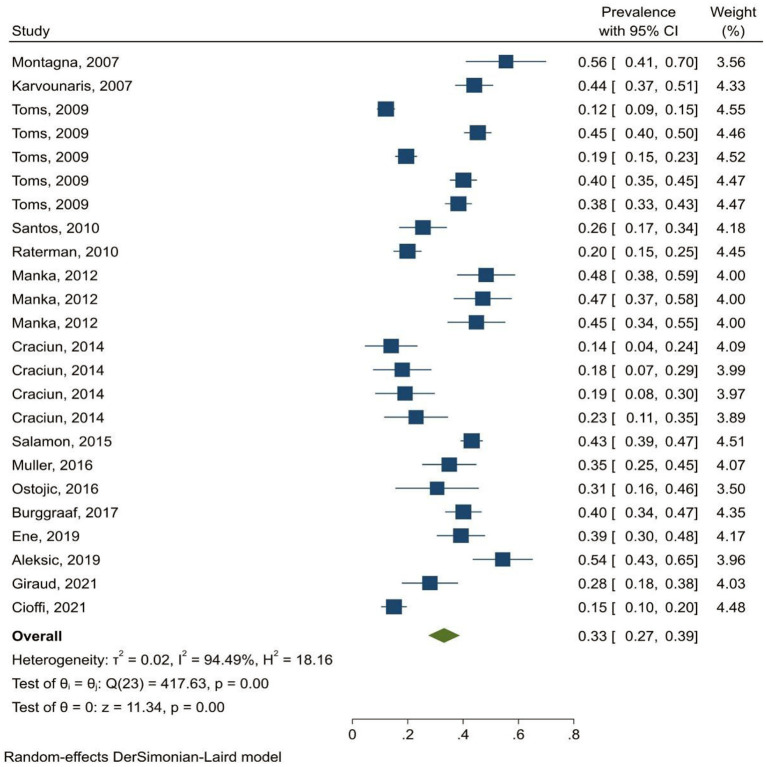
The prevalence of MetS in patients with rheumatoid arthritis was 32% (95% CI: 29.6–34.4%). The prevalence of metabolic syndrome was 33% (95% CI: 26–39%) in men and 34% (95% CI: 29–40%)in women. The findings demonstrated that the highest prevalence of MetS was related to studies in Asia (32.7%, 95% CI: 29–36.3%) and Europe (32.7%, 95% CI: 27.5–37.9%) and the lowest prevalence was related to studies in Africa (28%, 95% CI: 22.8–33.2%) (Figure 2). Findings by diagnostic criteria of metabolic syndrome showed that the highest and lowest prevalence were related to ATP III (37.5%, 95% CI: 30.9–44.2%) and EGIR (14.4%, 95% CI: 10.5–18.5%) criteria, respectively (Table 2).
Figure 2.
Forest plot of the pooled prevalence of MetS in patients with RA in Europe.
Table 2.
Subgroup prevalence of MetS among patients with RA.
| Subgroups | Number of studies | Prevalence (95% CI) | Between studies | Subgroup | |||
|---|---|---|---|---|---|---|---|
| Pheterogeneity | Q | Q | Pheterogeneity | I2 | |||
| Continent | |||||||
| Asia | 39 | 32.7 (29–36.3) | 91.25% | 0.001 | 505.13 | 2.39 | 0.495 |
| Europe | 25 | 32.7 (27.5–38) | 93.37% | 0.001 | 418.57 | ||
| America | 18 | 32.3 (27–37.5) | 94.66% | 0.001 | 345.11 | ||
| Africa | 14 | 28 (22.8–33.2) | 88.24% | 0.001 | 155.11 | ||
| Criteria | |||||||
| WHO | 8 | 25.2 (20–30.4) | 81% | 0.004 | 42.19 | 79.69 | 0.001 |
| IDF | 21 | 35.2 (29.4–41.1) | 93.1% | 0.017 | 482.13 | ||
| JS | 4 | 33.5 (21–46) | 95.6% | 0.015 | 128.65 | ||
| NCEP/ATP III | 42 | 32 (28.5–35.5) | 91.2% | 0.012 | 518.62 | ||
| ATP III | 8 | 37.5 (31–44) | 85.9% | 0.007 | 47.09 | ||
| AACE | 4 | 26.2 (17.3–35.2) | 87.8% | 0.007 | 25.17 | ||
| EGIR | 3 | 14.4 (10.5–18.4) | 36.75 | 0.001 | 2.92 | ||
WHO, World Health Organization; IDF, International Diabetes Federation; EGIR, European Group against Insulin Resistance; NCEP ATPIII, National Cholesterol Education Program Adult Treatment Panel; AACE, American Association of Clinical Endocrinologists; AHA/NHLBI, The American Heart Association / National Heart, Lung, and Blood Institute; JS, Joint Statement.
Meta-Regression
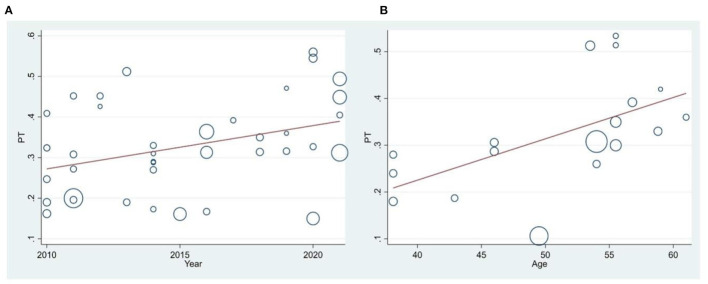
The results of meta-regression showed that the prevalence of MetShad increased significantly with increasing age (in studies in the Americas) (p = 0.006) (Figure 3). Also, the prevalence of MetS over time in studies in Asia was significantly increased (p = 0.024). Also, publication bias was not significant in the analyzed studies (p = 0.569).
Figure 3.
Meta-regression of the prevalence of MetS among patients with RA based on year of study (A) and mean age (B). Circles indicate the weight of studies.
Discussion
The results of this study showed that one third of patients with RA have MetS. The results of a previous meta-analysis of 38 articles (with 70 groups) between 2007 and 2016 showed that the prevalence of MetS in patients with RA was 30.65%, which is almost consistent with the results of the present study (71). The reason for the high prevalence of metabolic syndrome in these patients can be attributed to traditional risk factors such as smoking, body mass index, gender, dyslipidemia and hypertension, although the role of continuous inflammation and activation of endothelial cells cannot be ignored (41). Inflammatory cytokines such as TNFα also reduce insulin function and facilitate insulin resistance (2). On the other hand, these patients use non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids to control the disease, which can cause metabolic disorders such as high blood pressure, obesity and diabetes (27). Serum levels of some biomarkers associated with metabolic syndrome, adipokines such as adiponectin, and biomarkers of endothelial cell activation and inflammation may appear to be useful in predicting cardiovascular risk in patients with RA (72).
The highest prevalence of metabolic syndrome was related to studies in Asia and Europe and the lowest prevalence was related to studies in Africa. Given that nutritional, ethnic and sociodemographic status are the determinants of the prevalence of metabolic syndrome, the reason for this finding can be attributed to these differences in these communities.
In a study by Park et al. (73) the prevalence of metabolic syndrome in Korean and American adults was compared, and the results showed that the prevalence of metabolic syndrome and all its components (except low high density lipoprotein-cholesterol) was higher in American adults than in Korean. The two groups were not different in terms of blood pressure (73). The results of our study differ from those of Park et al. (73); in that they examined the prevalence of metabolic syndrome among patients with rheumatoid arthritis, not the general population. Therefore, further studies in this regard seem necessary.
The highest and lowest prevalence of metabolic syndrome were related to ATP III and EGIR criteria, respectively. In all diagnostic criteria, blood pressure, triglycerides, HDL cholesterol and fasting glucose are measured, and the difference between them is in the selection of the cut-off points and the measure of obesity. In WHO and EGIR criteria, the presence of hyperinsulinemia as an indicator of insulin resistance is the starting point, while in ATP III, the number of abnormalities is considered (69). These differences have led to different prevalence being reported in a group of patients (same patients) based on different criteria, so appropriate standards should be used to diagnose MetS in different regions. In a meta-analysis performed to estimate the prevalence of metabolic syndrome in postmenopausal women, the highest prevalence of metabolic syndrome was based on the ATP III screening criterion (74). The prevalence of metabolic syndrome increased significantly with age (in studies in the Americas). The prevalence of metabolic syndrome in the general population also increases with age (27), which can be due to redistribution of adipose tissue, weight gain, insulin resistance, and lipid changes (75).
Given that the prevalence of metabolic syndrome in patients with rheumatoid arthritis has not been studied in some countries and therefore has not been analyzed, the findings of this study should be generalized with caution worldwide.
Conclusion
Metabolic syndrome is so common in patients with RA that one-third of these patients have MetS, so identifying at-risk patients is essential to prevent cardiovascular events.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
WC: concept, design, and drafting of the manuscript. WC, MP, and XT: acquisition, analysis, or interpretation of data. XT: critical revision of the manuscript for important intellectual content. MP: statistical analysis. All authors gave their final approval of this version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Karakoc M, Batmaz I, Sariyildiz MA, Tahtasiz M, Cevik R, Tekbas E, et al. The relationship of metabolic syndrome with disease activity and the functional status in patients with rheumatoid arthritis. J Clin Med Res. (2012) 4:279. 10.4021/jocmr1001w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung C, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. (2008) 196:756–63. 10.1016/j.atherosclerosis.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 3.Mulumba C, Lebughe P, Mbuyi-Muamba J-M, Makulo J-R, Lepira F, Mukaya J, et al. Prevalence and associated factors of subclinical atherosclerosis in rheumatoid arthritis at the university hospital of Kinshasa. BMC rheumatology. (2019) 3:1–8. 10.1186/s41927-019-0085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Chagollán M, Hernández-Martínez SE, Rojas-Romero AE, Muñoz-Valle JF, Sigala-Arellano R, Cerpa-Cruz S, et al. Metabolic syndrome in rheumatoid arthritis patients: relationship among its clinical components. J Clin Lab Anal. (2021) 35:e23666. 10.1002/jcla.23666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheumatism. (2006) 54:2765–75. 10.1002/art.22053 [DOI] [PubMed] [Google Scholar]
- 6.Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. (2010) 43:661–5. 10.1016/j.clinbiochem.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 7.Gomes KWP, Luz AJP, Felipe MRdB, Beltrão LA, Sampaio AXC, Rodrigues CEM. Prevalence of metabolic syndrome in rheumatoid arthritis patients from Northeastern Brazil: association with disease activity. Mod Rheumatol. (2018) 28:258–63. 10.1080/14397595.2017.1316813 [DOI] [PubMed] [Google Scholar]
- 8.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 56:1113–32. 10.1016/j.jacc.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 9.Ferraz-Amaro I, González-Juanatey C, López-Mejias R, Riancho-Zarrabeitia L, González-Gay MA. Metabolic syndrome in rheumatoid arthritis. Med Inflam. (2013) 2013. 10.1155/2013/710928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters M, Symmons D, McCarey D, Dijkmans B, Nicola P, Kvien T, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. (2010) 69:325–31. 10.1136/ard.2009.113696 [DOI] [PubMed] [Google Scholar]
- 11.Agca R, Heslinga S, Rollefstad S, Heslinga M, McInnes I, Peters M, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. (2017) 76:17–28. 10.1136/annrheumdis-2016-209775 [DOI] [PubMed] [Google Scholar]
- 12.Crăciun L, Crăciun P, Buicu F. Prevalence of metabolic syndrome in psoriatic arthritis and rheumatoid arthritis. Acta Med Marisien. (2014) 60. 10.2478/amma-2014-004134076348 [DOI] [Google Scholar]
- 13.Mobini M, Niksolat F, Bahar A, Mohammadpour R, Karimi M. Metabolic syndrome and its components in patients with rheumatoid arthritis, and their association with disease activity and duration. J Clin Diagn Res. (2020) 14. 10.7860/JCDR/2020/43180.1348231559763 [DOI] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–e34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Hermont AP, Oliveira PA, Martins CC, Paiva SM, Pordeus IA, Auad SM. Tooth erosion and eating disorders: a systematic review and meta-analysis. PLoS ONE. (2014) 9:e111123. 10.1371/journal.pone.0111123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. (2014) 14:1–5. 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turgunova LG, Shalygina AA, Ibrayeva LK, Turmuhambetova AA. Metabolic syndrome as a factor affecting on intima-media thickness in patients with rheumatoid arthritis. Open Access Macedon J Med Sci. (2021) 9:411–6. 10.3889/oamjms.2021.5943 [DOI] [Google Scholar]
- 18.Hee JY, Protani MM, Koh ET, Leong KP. Metabolic syndrome and its effect on the outcomes of rheumatoid arthritis in a multi-ethnic cohort in Singapore. Clin Rheumatol. (2021) 41:649–60. 10.1007/s10067-021-05945-8 [DOI] [PubMed] [Google Scholar]
- 19.Giraud C, Lambert C, Dutheil F, Pereira B, Soubrier M, Tournadre A. The relationship between weight status and metabolic syndrome in patients with rheumatoid arthritis and spondyloarthritis. Joint Bone Spine. (2021) 88:105059. 10.1016/j.jbspin.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 20.Kong Cy, Wang Cl, Niu Kj, Qi W. Prevalence of metabolic syndrome in patients with rheumatoid arthritis in eastern China—A hospital based study. Int J Rheum Dis. (2021) 24:1121–6. 10.1111/1756-185X.14148 [DOI] [PubMed] [Google Scholar]
- 21.Cioffi G, Viapiana O, Tarantini L, Orsolini G, Idolazzi L, Sonographer FO, et al. Clinical profile and outcome of patients with chronic inflammatory arthritis and metabolic syndrome. Internal Emerg Med. (2020) 16:863–74. 10.1007/s11739-020-02520-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Choi S-E, Kang J-K, Park D-J, Lee J-K, Lee S-S. Basal metabolic rate and Charlson Comorbidity Index are independent predictors of metabolic syndrome in patients with rheumatoid arthritis. Joint bone spine. (2020) 87:455–60. 10.1016/j.jbspin.2020.03.015 [DOI] [PubMed] [Google Scholar]
- 23.Shaikh S, Dahani A, Arain SR, Khan F. Metabolic syndrome in young rheumatoid arthritis patients. J Ayub Med Coll Abbottabad: JAMC. (2020) 32:318–22. [PubMed] [Google Scholar]
- 24.Özkul Ö, Yazici A, Aktürk AS, Karadag DT, Işik ÖO, Tekeoglu S, et al. Are there any differences among psoriasis, psoriatic arthritis and rheumatoid arthritis in terms of metabolic syndrome and cardiovascular risk factors? Eur J Rheumatol. (2019) 6:174. 10.5152/eurjrheum.2019.19029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ene CG, Mitroi M, Vladu IM, Radu L, Cojan TST, Predescu AM, et al. Metabolic syndrome among patients with rheumatoid arthritis and the correlation with disease activity. Revista De Chimie. (2019) 70:2108–11. 10.37358/RC.19.6.728528395950 [DOI] [Google Scholar]
- 26.Naidu G, Bhilave N, Sharma K, Verma I, Sharma A. Prevalence of metabolic syndrome in rheumatoid arthritis patients: a case control study from a tertiary care centre in North India. J Assoc Phys India. (2019) 67:22–4. [PubMed] [Google Scholar]
- 27.Kuriya B, Schieir O, Valois M, Pope J, Boire G, Bessette L, et al. Prevalence and characteristics of metabolic syndrome differ in men and women with early rheumatoid arthritis. ACR Open Rheumatol. (2019) 1:535–41. 10.1002/acr2.11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbal N, Aydin K, Tezcan M. Metabolic syndrome is not uncommon in treatment-naïve rheumatoid arthritis patients. Neth J Med. (2019) 77:204. [PubMed] [Google Scholar]
- 29.Aleksić I, Stojanović S, Tasić I, Stamenković B. The impact of disease activity in patients with rheumatoid arthritis on metabolic syndrome and cardiovascular risk assessment. Acta Facult Med Naissensis. (2019) 36:177–87. 10.5937/afmnai1903177A24467375 [DOI] [Google Scholar]
- 30.Mobini M, Niksolat F, Mohammadpour RA, Sadr S, Dashti Dargahloo S. Comparing the prevalence of metabolic syndrome in systemic lupus erythematosus, rheumatoid arthritis and psoriatic arthritis: a cross sectional study. Rheumatol Res. (2018) 3:69–75. 10.22631/rr.2018.69997.1044 [DOI] [Google Scholar]
- 31.Burggraaf B, de Vries MA, Klop B, van Zeben J, van de Geijn G-JM, van der Meulen N, et al. Progression of subclinical atherosclerosis in subjects with rheumatoid arthritis and the metabolic syndrome. Atherosclerosis. (2018) 271:84–91. 10.1016/j.atherosclerosis.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 32.Slimani S, Abbas A, Ammar AB, Rahal F, Khider I, Khelif K, et al. Prevalence of metabolic syndrome in Algerian rheumatoid arthritis patients. Clin Res Rev. (2017) 11:S425–S7. 10.1016/j.dsx.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 33.Pandey PK, Swami A, Biswas TK, Thakuria R. Prevalence of metabolic syndrome in treatment naive rheumatoid arthritis and correlation with disease parameters. Arch Rheumatol. (2017) 32:46. 10.5606/ArchRheumatol.2017.5949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostojic P, Bartolovic D. Disease activity, obesity, functional disability, and depression in patients with rheumatoid arthritis. Zeitschrift für Rheumatol. (2016) 75:716–22. 10.1007/s00393-015-1661-7 [DOI] [PubMed] [Google Scholar]
- 35.Lee S-H, Choi H, Cho B-L, An A-R, Seo Y-G, Jin H-S, et al. Relationship between metabolic syndrome and rheumatoid arthritis. Korean J Fam Med. (2016) 37:44. 10.4082/kjfm.2016.37.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hugo M, Mehsen-Cetre N, Pierreisnard A, Schaeverbeke T, Gin H, Rigalleau V. Energy expenditure and nutritional complications of metabolic syndrome and rheumatoid cachexia in rheumatoid arthritis: an observational study using calorimetry and actimetry. Rheumatol Int. (2016) 55:1202–9. 10.1093/rheumatology/kew038 [DOI] [PubMed] [Google Scholar]
- 37.Zafar ZA, Mahmud TH, Rasheed A, Wagan AA. Frequency of metabolic syndrome in Pakistani cohort of patients with rheumatoid arthritis. J Pak Med Assoc. (2016) 66:671–6. [PubMed] [Google Scholar]
- 38.Oliveira BMGBd, Medeiros MMdC, Cerqueira JVMd, Quixadá RTdS, Oliveira ÍMAXd. Metabolic syndrome in patients with rheumatoid arthritis followed at a University Hospital in Northeastern Brazil. Rev Brasileira de Reumatol. (2016) 56:117–25. 10.1016/j.rbre.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 39.Müller R, Kull M, Põlluste K, Aart A, Eglit T, Lember M, et al. The metabolic profile in early rheumatoid arthritis: a high prevalence of metabolic obesity. Rheumatol Int. (2017) 37:21–7. 10.1007/s00296-016-3464-9 [DOI] [PubMed] [Google Scholar]
- 40.Dihingia P, Das D, Chakraborty A, Debbarma M, Kakati S. Increase frequency of metabolic syndrome among the cases of rheumatoid arthritis: a case control study. J Evol Med Dental Sci. (2016) 5:221–5. 10.14260/jemds/2016/4720890982 [DOI] [Google Scholar]
- 41.Ghazaly Ahah, El-Moez KM, El Shorbagy MS, El-Nahrery EM. Angiopoietin-2 as a biomarker for metabolic syndrome and disease activity in rheumatoid arthritis patients. Egypt Rheumatol. (2016) 38:9–13. 10.1016/j.ejr.2015.03.00125295265 [DOI] [Google Scholar]
- 42.Šalamon L, Morović-Vergles J, Marasović-Krstulović D, Kehler T, Šakić D, Badovinac O, et al. Differences in the prevalence and characteristics of metabolic syndrome in rheumatoid arthritis and osteoarthritis: a multicentric study. Rheumatol Int. (2015) 35:2047–57. 10.1007/s00296-015-3307-0 [DOI] [PubMed] [Google Scholar]
- 43.Tantayakom P, Koolvisoot A, Arromdee E, Chiowchanwisawakit P, Muangchan C, Katchamart W. Metabolic syndrome is associated with disease activity in patients with rheumatoid arthritis. Joint Bone Spine. (2016) 83:563–7. 10.1016/j.jbspin.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 44.Parra-Salcedo F, Contreras-Yáñez I, Elías-López D, Aguilar-Salinas CA, Pascual-Ramos VJ. Prevalence, incidence and characteristics of the metabolic syndrome (MetS) in a cohort of Mexican Mestizo early rheumatoid arthritis patients treated with conventional disease modifying anti-rheumatic drugs: the complex relationship between MetS and disease activity. Arthritis Res Ther. (2015) 17:1–11. 10.1186/s13075-015-0549-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bilecik NA, Tuna S, Samanci N, Balci N, Akbaş H. Prevalence of metabolic syndrome in women with rheumatoid arthritis and effective factors. Int J Clin Exper Med. (2014) 7:2258. [PMC free article] [PubMed] [Google Scholar]
- 46.Özmen M, Yersal Ö, Öztürk S, Soysal D, Köseeoglu MH. Prevalence of the metabolic syndrome in rheumatoid arthritis. Eur J Rheumatol. (2014) 1:1. 10.5152/eurjrheum.2014.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar BS, Naik GS, Mohan A, Kumar DP, Suresh V, Sarma K, et al. Prevalence of thyroid disorders and metabolic syndrome in adult patients with rheumatoid arthritis. J Clin Sci Res. (2014) 3:97–105. 10.15380/2277-5706.JCSR.14.005 [DOI] [Google Scholar]
- 48.Abourazzak FE, Mansouri S, Najdi A, Tahiri L, Nejjari C, Harzy T. Prevalence of metabolic syndrome in patients with rheumatoid arthritis in Morocco: a cross-sectional study of 179 cases. Clin Rheumatol. (2014) 33:1549–55. 10.1007/s10067-014-2570-x [DOI] [PubMed] [Google Scholar]
- 49.Salinas MJH, Bertoli AM, Lema L, Saucedo C, Rosa J, Quintana R, et al. Prevalence and correlates of metabolic syndrome in patients with rheumatoid arthritis in Argentina. JCR. (2013) 19:439–43. 10.1097/RHU.0000000000000039 [DOI] [PubMed] [Google Scholar]
- 50.Abdul-Qaharr Z, Al-Osami MH. Prevalence of metabolic syndrome in iraqi patients with rheumatoid arthritis. IOSR J Dental Med Sci. (2013) 1:69–72. 10.9790/0853-1116972 [DOI] [Google Scholar]
- 51.Rostom S, Mengat M, Lahlou R, Hari A, Bahiri R, Hajjaj-Hassouni N. Metabolic syndrome in rheumatoid arthritis: case control study. BMC Musculoskelet Disord. (2013) 14:1–8. 10.1186/1471-2474-14-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S-G, Kim J-M, Lee S-H, Kim K-H, Kim J-H, Yi J-W, et al. Is the frequency of metabolic syndrome higher in South Korean women with rheumatoid arthritis than in healthy subjects? Korean J Intern Med. (2013) 28:206. 10.3904/kjim.2013.28.2.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ormseth MJ, Lipson A, Alexopoulos N, Hartlage GR, Oeser AM, Bian A, et al. Association of epicardial adipose tissue with cardiometabolic risk and metabolic syndrome in patients with rheumatoid arthritis. Arthr Care Res. (2013) 65:1410–5. 10.1002/acr.22027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manka V, Galajda P, Sagova I, Klimentova A, Kantarova D, Stancik M, et al. Metabolic syndrome in rheumatoid arthritis. Acta Med Martiniana. (2012) 12. 10.2478/v10201-011-0040-6 [DOI] [Google Scholar]
- 55.Da Cunha V, Brenol C, Brenol J, Fuchs S, Arlindo E, Melo I, et al. Metabolic syndrome prevalence is increased in rheumatoid arthritis patients and is associated with disease activity. Scand J Rheumatol. (2012) 41:186–91. 10.3109/03009742.2011.626443 [DOI] [PubMed] [Google Scholar]
- 56.Goshayeshi L, Saber H, Sahebari M, Rezaieyazdi Z, Rafatpanah H, Esmaily H, et al. Association between metabolic syndrome, BMI, and serum vitamin D concentrations in rheumatoid arthritis. Clin Rheumatol. (2012) 31:1197–203. 10.1007/s10067-012-1995-3 [DOI] [PubMed] [Google Scholar]
- 57.Baker JF, Mehta NN, Baker DG, Toedter G, Shults J, Von Feldt JM, et al. Vitamin D, metabolic dyslipidemia, and metabolic syndrome in rheumatoid arthritis. Am J Med. (2012) 125:1036.e9–e15. 10.1016/j.amjmed.2012.01.025 [DOI] [PubMed] [Google Scholar]
- 58.Crowson CS, Myasoedova E, Davis JM, Matteson EL, Roger VL, Therneau TM, et al. Increased prevalence of metabolic syndrome associated with rheumatoid arthritis in patients without clinical cardiovascular disease. J Rheumatol. (2011) 38:29–35. 10.3899/jrheum.100346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahebari M, Goshayeshi L, Mirfeizi Z, Rezaieyazdi Z, Hatef MR, Ghayour-Mobarhan M, et al. Investigation of the association between metabolic syndrome and disease activity in rheumatoid arthritis. Sci World J. (2011) 11:1195–205. 10.1100/tsw.2011.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karimi M, Mazloomzadeh S, Kafan S, Amirmoghadami H. The frequency of metabolic syndrome in women with rheumatoid arthritis and in controls. Int J Rheum Dis. (2011) 14:248–54. 10.1111/j.1756-185X.2011.01595.x [DOI] [PubMed] [Google Scholar]
- 61.Mok CC, Ko GTC, Ho LY Yu KL, Chan PT, To CH. Prevalence of atherosclerotic risk factors and the metabolic syndrome in patients with chronic inflammatory arthritis. Arthr Care Res. (2011) 63:195–202. 10.1002/acr.20363 [DOI] [PubMed] [Google Scholar]
- 62.Dao H-H, Do Q-T, Sakamoto J. Increased frequency of metabolic syndrome among Vietnamese women with early rheumatoid arthritis: a cross-sectional study. Arthr Res Ther. (2010) 12:1–10. 10.1186/ar3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raterman H, Van Eijk I, Voskuyl A, Peters M, Dijkmans B, Van Halm V, et al. The metabolic syndrome is amplified in hypothyroid rheumatoid arthritis patients: a cross-sectional study. Ann Rheum Dis. (2010) 69:39–42. 10.1136/ard.2008.100776 [DOI] [PubMed] [Google Scholar]
- 64.Solomon A, Christian BF, Norton GR, Woodiwiss AJ, Dessein PH. Risk factor profiles for atherosclerotic cardiovascular disease in black and other Africans with established rheumatoid arthritis. J Rheumatol. (2010) 37:953–60. 10.3899/jrheum.091032 [DOI] [PubMed] [Google Scholar]
- 65.Giles JT, Allison M, Blumenthal RS, Post W, Gelber AC, Petri M, et al. Abdominal adiposity in rheumatoid arthritis: association with cardiometabolic risk factors and disease characteristics. Arthritis Rheumatism. (2010) 62:3173–82. 10.1002/art.27629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santos MJ, Vinagre F, Silva J, Gil V, Fonseca J. Cardiovascular risk profile in systemic lupus erythematosus and rheumatoid arthritis: a comparative study of female patients. Acta Reumatol Port. (2010) 35:325–32. [PubMed] [Google Scholar]
- 67.Toms TE, Panoulas VF, John H, Douglas KM, Kitas GD. Methotrexate therapy associates with reduced prevalence of the metabolic syndrome in rheumatoid arthritis patients over the age of 60-more than just an anti-inflammatory effect? A cross sectional study. Arthr Res Ther. (2009) 11:1–10. 10.1186/ar2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zonana-Nacach A, Santana-Sahagun E, Jimenez-Bal deras FJ, Camargo-Coronel A. Prevalence and factors associated with metabolic syndrome in patients with rheumatoid arthritis and systemic lupus erythematous. J Clin Rheumatol I. (2008) 4:74–7. 10.1097/RHU.0b013e31816b2faa [DOI] [PubMed] [Google Scholar]
- 69.Karvounaris SA, Sidiropoulos PI, Papadakis JA, Spanakis EK, Bertsias GK, Kritikos HD, et al. Metabolic syndrome is common among middle-to-older aged Mediterranean patients with rheumatoid arthritis and correlates with disease activity: a retrospective, cross-sectional, controlled, study. Ann Rheum Dis. (2007) 66:28–33. 10.1136/ard.2006.053488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montagna GL, Cacciapuoti F, Buono R, Manzella D, Mennillo GA, Arciello A, et al. Insulin resistance is an independent risk factor for atherosclerosis in rheumatoid arthritis. Diab Vasc Dis Res. (2007) 4:130–5. 10.3132/dvdr.2007.031 [DOI] [PubMed] [Google Scholar]
- 71.Hallajzadeh J, Safiri S, Mansournia MA, Khoramdad M, Izadi N, Almasi-Hashiani A, et al. Metabolic syndrome and its components among rheumatoid arthritis patients: a comprehensive updated systematic review and meta-analysis. PLoS ONE. (2017) 12:e0170361. 10.1371/journal.pone.0170361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.López-Mejías R, Castañeda S, González-Juanatey C, Corrales A, Ferraz-Amaro I, Genre F, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis: the relevance of clinical, genetic and serological markers. Autoimmun Rev. (2016) 15:1013–30. 10.1016/j.autrev.2016.07.026 [DOI] [PubMed] [Google Scholar]
- 73.Park J, Mendoza JA, Carol E, Hilmers DC, Liu Y, Nicklas TA, et al. comparison of the prevalence of the metabolic syndrome in the United States (US) and Korea in young adults aged 20 to 39 years. Asia Pac J Clin Nutr. (2008) 17:471–82. [PubMed] [Google Scholar]
- 74.Ebtekar F, Dalvand S, Gheshlagh RG. The prevalence of metabolic syndrome in postmenopausal women: a systematic review and meta-analysis in Iran. Diab Metab Synd Clin Res Rev. (2018) 12:955–60. 10.1016/j.dsx.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 75.Hallajzadeh J, Khoramdad M, Izadi N, Karamzad N, Almasi-Hashiani A, Ayubi E, et al. Metabolic syndrome and its components in premenopausal and postmenopausal women: a comprehensive systematic review and meta-analysis on observational studies. Menopause. (2018) 25:1155–64. 10.1097/GME.0000000000001136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.