Abstract
With the upgrade and development of the high-throughput sequencing technology, multi-omics data can be obtained at a low cost. However, mapping tools that existed for microbial multi-omics data analysis cannot satisfy the needs of data description and result in high learning costs, complex dependencies, and high fees for researchers in experimental biology fields. Therefore, developing a toolkit for multi-omics data is essential for microbiologists to save effort. In this work, we developed MicrobioSee, a real-time interactive visualization tool based on web technologies, which could visualize microbial multi-omics data. It includes 17 modules surrounding the major omics data of microorganisms such as the transcriptome, metagenome, and proteome. With MicrobioSee, methods for plotting are simplified in multi-omics studies, such as visualization of diversity, ROC, and enrichment pathways for DEGs. Subsequently, three case studies were chosen to represent the functional application of MicrobioSee. Overall, we provided a concise toolkit along with user-friendly, time-saving, cross-platform, and source-opening for researchers, especially microbiologists without coding experience. MicrobioSee is freely available at https://microbiosee.gxu.edu.cn.
Keywords: MicrobioSee, metagenome, integration analysis, visualization toolkit, stacked column chart
Introduction
Microorganisms are ubiquitous on earth and play a prominent role in the material cycle, climate change, and human health (Lynch and Pedersen, 2016; Crowther et al., 2019; Jansson and Hofmockel, 2020; Keohane et al., 2020). In the last 2 decades, the development of high-throughput velocimetry allowed us to observe the structure of microbial communities, in which the Earth Microbiome Project (EMP) and the Human Microbiome Project (HMP) have achieved fruitful results (Turnbaugh et al., 2007; Human Microbiome Project, 2012; Gilbert et al., 2014; Thompson et al., 2017). In the last few years, a lot of studies on the interaction between various community microorganisms and their hosts have emerged (Lundberg et al., 2012; Ren et al., 2021). Numerous studies have discovered that the loss of gut microbiota homeostasis exerts a significantly negative impact on Alzheimer’s disease, obesity, cancer, and depression. (Dao et al., 2016; Zheng et al., 2016; Flemer et al., 2017; Kim et al., 2020; Morais et al., 2021). In addition to the metagenomics technique, the multi-omics technique has been used to explore specific interactions between microorganisms and hosts in a greater depth (Lloyd-Price et al., 2019). The Integrative Human Microbiome Project (iHMP) has conducted a series of integration studies to explore human microbial–host interactions from the multi-omics data in three physiological or pathological states of human prematurity, inflammatory bowel disease, and prediabetes (Integrative, 2019).
In multi-omics studies, there are two major challenges for researchers. One is that data integration and analysis consume considerable time and effort of researchers because of the complex usage of combining tools (Lin et al., 2020), and the other is that the existing drawing tools cannot satisfy the demands for describing high-dimensional data, which poses stress for the researchers to represent the analysis results of microbiome data (Sinha et al., 2015; Ramirez et al., 2018). Visualizing the results of data obtained from multi-omics studies is a huge burden for the researchers (Lin et al., 2020).
Currently, various tools have emerged and were used to analyze multi-omics data and visualize omics results. The main steps of the metagenome analysis are clustering or denoising the raw data to obtain abundance tables. In the analysis phase, QIIME2 (Hall and Beiko, 2018; Knight et al., 2018; Rai et al., 2021) performs better than other tools (Straub et al., 2020). However, users have to use dedicated tools to view graph files generated from QIIME2, which adds burden for the users to observe the results (Min et al., 2021).
For big data obtained from the multi-omics studies, visualizing the analysis results requires easier tools (Chen et al., 2020). Traditional graphing tools exhibited excellent graphing capabilities and convenience, especially client tools that are user-friendly but have problems such as low system compatibility and high costs. For example, OriginPro and GraphPad Prism v9 are two drawing tools for the general public, with rich styles of charts but of high economic costs. Command line-based tools, such as the Matplotlib package in Python and the ggplot2 package in R, provide novel graphing methods and graph styles with excellent plotting capabilities and are usually used for the secondary development (Skidmore et al., 2016; Wagih, 2017; Tareen and Kinney, 2020; Liu et al., 2021; Xu et al., 2021). A lot of command line-based tools which are cross-systems and open sources have been developed by bioinformaticians to exhibit the results of the multi-omics data (Ito and Murphy, 2013). However, those command line-based tools require users to spend considerable time learning a programming language, which decreases the efficiency for scientific researchers in non-computer fields, especially doctors and experimenters.
For these issues, we developed MicrobioSee, a web-based toolkit for multi-omics visualization, which is a cross-platform, user-friendly, time-saving, and an open source (Figure 1). The idea of this tool originated from the fact that conventional charts and tools cannot satisfy the demands of visualization for researchers without programming experience in their multi-omics studies. MicrobioSee is an efficient toolkit for visualization that eliminates high costs for users without programming experience.
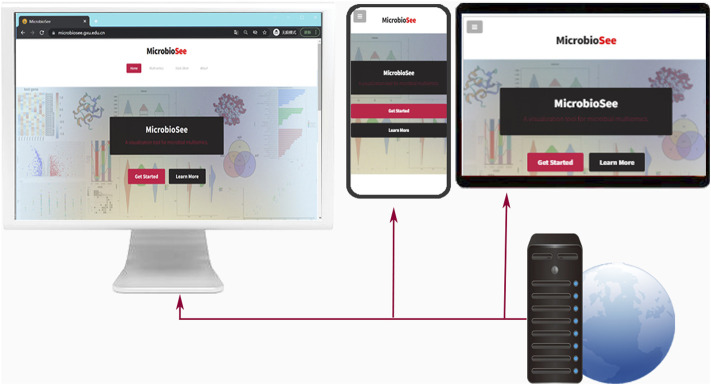
FIGURE 1.
Presentation of index pages of MicrobioSee on different devices, including PC, Pad, and smartphone. The capabilities of the cross-platform are built by a webserver. The index page was designed with responsive web layouts, which enables users to get the best visual effects on different devices.
Methods
MicrobioSee was developed for microbiome multi-omics data, such as the metagenome, proteome, and transcriptome (Figure 2). The whole website was divided into front-end and back-end. In the front-end, the VUE technology is used to render the interface. In the back-end, the R program was used for responding to users’ interactions and built-in drawing commands. In addition, most of the tools in MicrobioSee were built with shiny services to achieve real-time interactive plotting. A series of graphics could be plotted interactively by MicrobioSee, including the rose plot, heat map, box plot, upset plot, Venn diagram, and so on, which could be applied to multi-omics studies (Figure 3). The options for height, width, and resolution in each drawing module were provided for plotting.
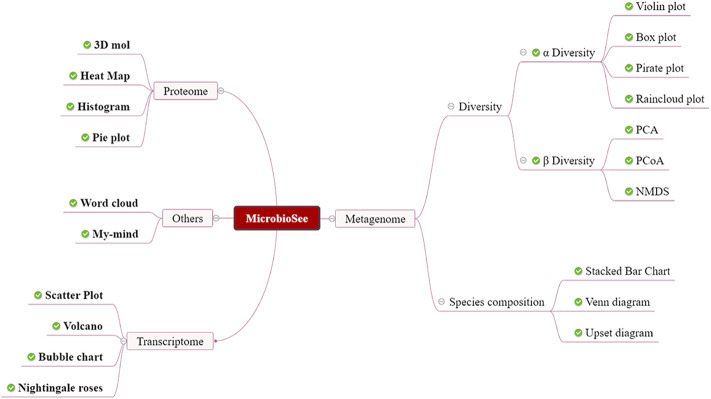
FIGURE 2.
Structure of MicrobioSee. MicrobioSee comprises four modules: metagenome, proteome, transcriptome, and others. In total, 20 graph styles could be plotted into by MicrobioSee so far.
FIGURE 3.
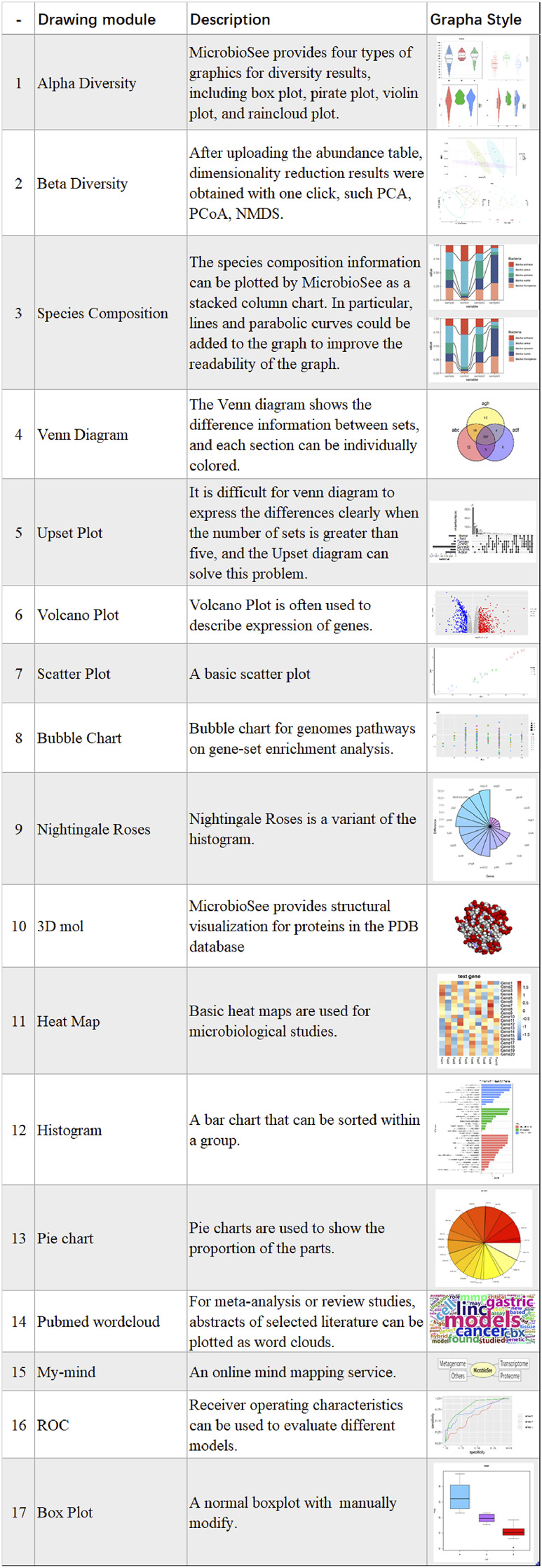
Seventeen drawing modules and related introduction.
Main Function
Alpha diversity that describes the microbial community composition is a critical index of the metagenome (Walters and Martiny, 2020). The results of alpha diversity from the USEARCH program or vegan package (Oksanen et al., 2013) could be plotted into four graph styles by MicrobioSee, including the box plot, raincloud plot, and violin plot. The pirate plot (Phillips, 2017) and the raincloud plot (Allen et al., 2019), composed of various graph styles, are more intuitive than others in aesthetics. The analysis of variance (ANOVA) and Kruskal–Wallis test were used to determine the difference between the groups for alpha diversity. The nonparametric statistical tests were realized by the aov function and the Kruskal–Wallis test function in R. After clicking the start button, users can obtain the selected graph style and test.
Beta diversity is used to describe the variability in species composition between the groups (Whittaker, 1960). Plenty of indicators are used to measure beta diversity, and the Bray–Curtis similarity index is the main one (Bray and Curtis, 1957; Tuomisto, 2010). The distance matrix, generated from operational taxonomic units (OTUs), abundance table, or exact sequence variant tables, is calculated by the vegan package in MicrobioSee. After the dimensionality reduction, results would be plotted with the vegan package. There are three methods for the dimensionality reduction of the distance, including principal component analysis (PCA), principal coordinates analysis (PCoA), and non-metric multidimensional scaling (NMDS). Analysis of similarities (ANOSIM) was used to determine the similarity among the groups in the PCoA plots from MicrobioSee.
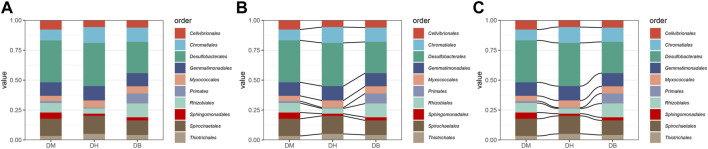
The species composition of the microbial community, one of the most cardinal factors to determine the nature of the community, is the basic characteristic to identify the different community types (Bell et al., 2005; Burrows et al., 2019; Jones et al., 2021). The structure of the species composition is usually plotted into a basic stacked column chart, but it cannot visually represent small differences for the adjacent groups. In MicrobioSee, lines could be added to the graphs among the numerical points of the adjacent groups in a stacked column chart. In addition, the curves could be added to the stacked column charts in a way that parabolic functions are generated by the relations between the taxon in the adjacent groups, which makes the stacked column charts intuitive and elegant. Each parabola would be calculated from the vertex of units and the midpoint of the adjacent units. Although the lines or curves added do not contain any scientific meaning, it could be valuable for users to visualize their results as a stacked column chart with lines or curves.
The screening literature is usually performed at the beginning of integrating data for target subjects. The metadata information of the selected literature, such as titles, abstracts, and keywords, could be accessed and downloaded easily by the crawler technology in MicrobioSee. The metadata would be automatically plotted into word clouds by the ggplot2 package, which describes the characteristics of the integrated literature. MicrobioSee could help researchers identify hot methods of research relating to target keywords.
The metagenome integrated data belong to the big data, and building models for classification and prediction is the most popular application in big data techniques, such as machine learning techniques (Cammarota et al., 2020; Namkung, 2020). For binary classification models, such as in sickness and in health, the receiver operating characteristic (ROC) curves are usually used to evaluate the quality of the models. The area under the curve (AUC), an important feature of the ROC, is one of the most commonly used metrics (Wang and Guo, 2020). The abundance tables of multiple taxonomic levels and metadata were used for constructing models by machine learning software (Yuan et al., 2020). In MicrobioSee, multiple ROC curves from various models were rendered by the pROC package (Robin et al., 2011) in R, which could identify the qualities for the better models. According to the specificity and sensitivity in graphs generated from MicrobioSee, users could evaluate and choose the models of various species classification levels or model methods.
Case Studies
To display the utility of MicrobioSee, three case studies were chosen and visualized by MicrobioSee. For brevity, we cannot explore all the functions of MicrobioSee but focus on the visualization of the most common scientific questions. Relevant data in case studies can be made available in the supplementary files.
Case 1
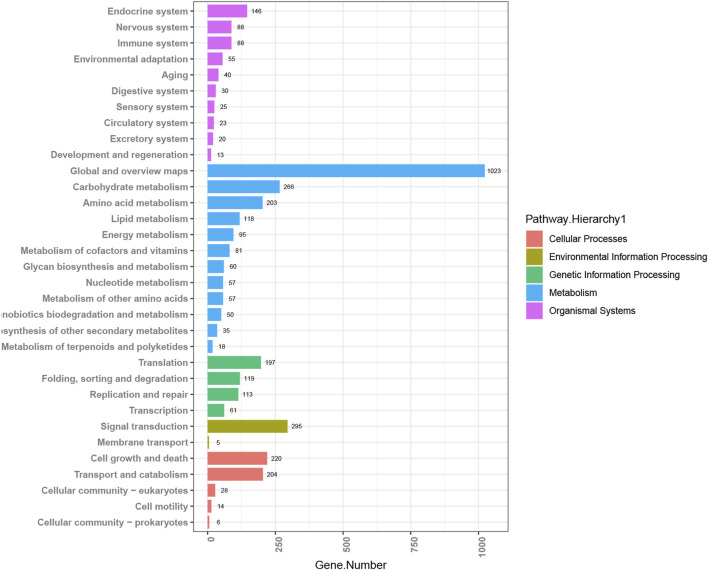
By MicrobioSee, users can plot a histogram easily with axis transposition and group sorting with one click. The data for this example are from the study of copper tolerance in Meyerozyma guilliermondii GXDK6, which was screened from the mangrove sediments (Bu et al., 2021). Multi-omics techniques were used to explore the tolerance mechanisms of the target strain with different copper ion concentrations. After annotating with the KEGG database, the enrichment pathways of differentially expressed genes (DEGs) from the GXDK6 transcriptomics analysis at 600 ppm copper concentration were plotted into a histogram with an order by MicrobioSee (Figure 4). The histogram generated by MicrobioSee can be sorted within the groups, which is more intuitive for users to observe the ranking of the annotation.
FIGURE 4.
Visualization of KEGG annotations from case study 1 by MicrobioSee. The data in the enrichment pathway of the differentially expressed genes (DEGs) among the groups were plotted into a histogram with an order in each group by MicrobioSee.
Case 2
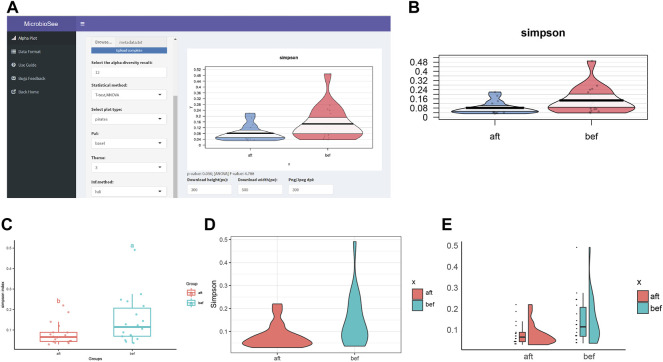
The samples were selected from the study of fecal microbiota transplantation (FMT) (Zhang et al., 2021). The study successfully collected 16S sequencing data of 18 constipated patients before and after FMT. Based on the raw data and analysis methods provided in the literature, we obtained the diversity results and plotted the Simpson index into the four graph styles by MicrobioSee (Figure 5). According to the statistical analysis from MicrobioSee, the Simpson index increased significantly after FMT.
FIGURE 5.
Process and visualization of the diversity analysis from case study 2 by MicrobioSee. (A) Panel of the alpha plot with a pirate plot in MicrobioSee. (B) Pirate plot was generated from case study 2 by MicrobioSee. (C) Box plot was generated from case study 2 by MicrobioSee. (D) Violin plot was generated from case study 2 by MicrobioSee. (E) Raincloud plot was generated from case study 2 by MicrobioSee.
Case 3
Parabolic curves or straight lines were added to the stacked column charts, and the differences in the relative abundance of the species among groups would be observed clearly. The sample chosen was collected from the National Shankou Natural Reserve of Mangrove in the Beibu Gulf of China (Nie et al., 2021). The relative abundance of the top 10 orders in the dry season was plotted into the stacked column charts with lines and curves by MicrobioSee (Figure 6). In the M and H regions, the relative abundance of Desulfobacterales is similar and greater than B in the dry season.
FIGURE 6.
Relative abundance of the top 10 species in case study 3 was plotted with the three stacked column charts by MicrobioSee. (A) Stacked column chart without lines. (B) Stacked column chart with straight lines. (C) Stacked column chart with curves.
Results and Discussion
In this work, we developed MicrobioSee, a web-based toolkit for the multi-omics studies, which contains plenty of computer technologies. The burden of plotting the result of the multi-omics studies would be eased with MicrobioSee. The pirate plot and raincloud plot are utilized as complements to the existing graph styles for visualization of alpha diversity results. Benefiting from the abundant R package resources, 17 plotting modules were developed for MicrobioSee. MicrobioSee also offers a few innovations in aesthetics. For example, elegant parabolic curves were added to the stacked column charts which would make them more aesthetically pleasing in the visualization of species composition.
The functions and advantages of the eight tools were summarized (Table 1). The vegan package (Oksanen et al., 2013) and phyloseq package (McMurdie and Holmes, 2013) are recognized by many researchers, but it is not friendly for researchers because of lacking interfaces. In the studies of the amplicon, QIIME2 (Hall and Beiko, 2018) and USEARCH (Edgar, 2010) are popular for high-speed analysis, but it is rarely used in the visualization of results on account of the insufficient number of their graphic styles. TBtools (Chen et al., 2020) has been popular with Windows users since it was developed, but it is disappointing for users of other platforms. Web-based tools could be used directly through a modern browser without platform limits. Animalcules (Zhao et al., 2021) provides an interface by Shiny technology, but it is a localized service with complex dependencies and could be installed with errors. Metaviz (Wagner et al., 2018) provides a web application for interactive visualization of the microbiome, but the methods of metaviz are not comprehensive or specific for 16S rRNA, metagenomic, or transcriptomic data. MicrobiomeAnalyst (Dhariwal et al., 2017; Chong et al., 2020), an excellent web toolkit in the field of downstream microbiome analysis, provides analysis and visualization, but few visual graphic styles are available. MicrobioSee was developed for interactive visualization of the microbiome, and microbiologists could use it for free and install it for free after short learning. The results in various omics studies can be visualized by MicrobioSee besides 16S rRNA and shotgun sequencing microbiome data. Due to its flexibility, it can also be applied in other fields. As long as uploading is in the same format as the sample data, the images of the corresponding graphic style would be obtained.
TABLE 1.
Comparisons of MicrobioSee and other popular visualization tools.
| Vegan | USEARCH11 64-bit | Phyloseq | Metaviz | Qiime2 | OriginPro | GraphPad Prism v9 | Animalcules | TBtools | MicrobiomeAnalyst | MicrobioSee | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Literature integration | ✔ | ||||||||||
| 3D molecular structure | ✔ | ||||||||||
| Installation-free | ✔ | ✔ | |||||||||
| Interface | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Interactive visualization | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Diversity visualization | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Free use | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Language/platform | R | Linux | R | R | Linux | Windows | Windows/macOS | R | Windows | Modern browsers | Modern browsers |
The features are assessed using the symbol “✔” for “present.”
For a more convenient operation, the tool would be continuously updated. Compared with client tools, it is unnecessary to be reinstalled when a new version is released, which is user-friendly. Inconveniently, web tools are highly dependent on the web environment (Chen et al., 2020) and so is MicrobioSee. When the number of users increases to a certain extent, the servers and bandwidth for MicrobioSee need to be expanded. The servers of MicrobioSee may suffer from attacking for the global open access, and the firewalls need to be constantly upgraded.
Conclusion
In total, 17 plotting modules have been built for MicrobioSee so far, such as the violin plot, box plot, rose plot, heat map, box plot, upset plot, and Venn diagram. Although most functions are not unique to MicrobioSee, they were combined, optimized, and interfaced for researchers with limited coding experience. MicrobioSee simplifies the methods for users without programming experience.
Acknowledgments
This study is particularly grateful to the Information Network Center of Guangxi University for providing the server.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
Corresponding author CJ and JL conceived the article, JHL, SZ, and YS designed, developed the website, and wrote the manuscript, SM, ZZ, CJ, and JL tested the tool and debugged key bugs, SH, XL, GS, JL, and CJ proofread and corrected the manuscript, and all authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 31760437), the Funding Project of Chinese Central Government Guiding to the Guangxi Local Science and Technology Development (Grant No. GUIKEZY21195021), the Natural Science Fund for Distinguished Young Scholars of Guangxi Zhuang Autonomous Region of China (Grant No. 2019GXNSFFA245011), the China-ASEAN International Innovative Center for Health Industry of Traditional Chinese Medicine (AD20297142), the Guangxi Key Laboratory of Beibu Gulf Marine Biodiversity Conservation, Beibu Gulf University (Grant No. 2022KA02), and the Innovation Project of Guangxi Graduate Education (Grant Nos. YCBZ2021012 and YCSW2021064).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.853612/full#supplementary-material
References
- Allen M., Poggiali D., Whitaker K., Marshall T. R., Kievit R. A. (2019). Raincloud Plots: a Multi-Platform Tool for Robust Data Visualization. Wellcome Open Res. 4, 63. 10.12688/wellcomeopenres.15191.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T., Newman J. A., Silverman B. W., Turner S. L., Lilley A. K. (2005). The Contribution of Species Richness and Composition to Bacterial Services. Nature 436 (7054), 1157–1160. 10.1038/nature03891 [DOI] [PubMed] [Google Scholar]
- Bray J. R., Curtis J. T. (1957). An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 27 (4), 325–349. 10.2307/1942268 [DOI] [Google Scholar]
- Bu R., Yan B., Sun H., Zhou M., Bai H., Cai X., et al. (2021). Copper Tolerance Mechanism of the Novel Marine Multi-Stress Tolerant Yeast Meyerozyma Guilliermondii GXDK6 as Revealed by Integrated Omics Analysis. Front. Microbiol. 12 (3445), 771878. 10.3389/fmicb.2021.771878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows M. T., Hawkins S. J., Moore J. J., Adams L., Sugden H., Firth L., et al. (2020). Global‐scale Species Distributions Predict Temperature‐related Changes in Species Composition of Rocky Shore Communities in Britain. Glob. Change Biol. 26, 2093–2105. 10.1111/gcb.14968 [DOI] [PubMed] [Google Scholar]
- Cammarota G., Ianiro G., Ahern A., Carbone C., Temko A., Claesson M. J., et al. (2020). Gut Microbiome, Big Data and Machine Learning to Promote Precision Medicine for Cancer. Nat. Rev. Gastroenterol. Hepatol. 17 (10), 635–648. 10.1038/s41575-020-0327-3 [DOI] [PubMed] [Google Scholar]
- Chen C., Chen H., Zhang Y., Thomas H. R., Frank M. H., He Y., et al. (2020). TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 13 (8), 1194–1202. 10.1016/j.molp.2020.06.009 [DOI] [PubMed] [Google Scholar]
- Chong J., Liu P., Zhou G., Xia J. (2020). Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 15 (3), 799–821. 10.1038/s41596-019-0264-1 [DOI] [PubMed] [Google Scholar]
- Crowther T. W., van den Hoogen J., Wan J., Mayes M. A., Keiser A. D., Mo L., et al. (2019). The Global Soil Community and its Influence on Biogeochemistry. Science 365 (6455). 10.1126/science.aav0550 [DOI] [PubMed] [Google Scholar]
- Dao M. C., Everard A., Aron-Wisnewsky J., Sokolovska N., Prifti E., Verger E. O., et al. (2016). Akkermansia Muciniphilaand Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 65 (3), 426–436. 10.1136/gutjnl-2014-308778 [DOI] [PubMed] [Google Scholar]
- Dhariwal A., Chong J., Habib S., King I. L., Agellon L. B., Xia J. (2017). MicrobiomeAnalyst: a Web-Based Tool for Comprehensive Statistical, Visual and Meta-Analysis of Microbiome Data. Nucleic Acids Res. 45 (W1), W180–W188. 10.1093/nar/gkx295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. (2010). Search and Clustering Orders of Magnitude Faster Than BLAST. Bioinformatics 26 (19), 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Flemer B., Lynch D. B., Brown J. M. R., Jeffery I. B., Ryan F. J., Claesson M. J., et al. (2017). Tumour-associated and Non-tumour-associated Microbiota in Colorectal Cancer. Gut 66 (4), 633–643. 10.1136/gutjnl-2015-309595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. A., Jansson J. K., Knight R. (2014). The Earth Microbiome Project: Successes and Aspirations. BMC Biol. 12, 69. 10.1186/s12915-014-0069-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M., Beiko R. G. (2018). 16S rRNA Gene Analysis with QIIME2. Methods Mol. Biol. 1849, 113–129. 10.1007/978-1-4939-8728-3_8 [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project C. (2012). A Framework for Human Microbiome Research. Nature 486 (7402), 215–221. 10.1038/nature11209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrative (2019). The Integrative Human Microbiome Project. Nature 569 (7758), 641–648. 10.1038/s41586-019-1238-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Murphy D. (2013). Application of Ggplot2 to Pharmacometric Graphics. CPT: Pharmacometrics Syst. Pharmacol. 2, 79. 10.1038/psp.2013.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson J. K., Hofmockel K. S. (2020). Soil Microbiomes and Climate Change. Nat. Rev. Microbiol. 18 (1), 35–46. 10.1038/s41579-019-0265-7 [DOI] [PubMed] [Google Scholar]
- Jones M. L., Rivett D. W., Pascual-García A., Bell T. (2021). Relationships between Community Composition, Productivity and Invasion Resistance in Semi-natural Bacterial Microcosms. Elife 10, 71811. 10.7554/eLife.71811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane D. M., Ghosh T. S., Jeffery I. B., Molloy M. G., O’Toole P. W., Shanahan F. (2020). Microbiome and Health Implications for Ethnic Minorities after Enforced Lifestyle Changes. Nat. Med. 26 (7), 1089–1095. 10.1038/s41591-020-0963-8 [DOI] [PubMed] [Google Scholar]
- Kim M.-S., Kim Y., Choi H., Kim W., Park S., Lee D., et al. (2020). Transfer of a Healthy Microbiota Reduces Amyloid and Tau Pathology in an Alzheimer's Disease Animal Model. Gut 69 (2), 283–294. 10.1136/gutjnl-2018-317431 [DOI] [PubMed] [Google Scholar]
- Knight R., Vrbanac A., Taylor B. C., Aksenov A., Callewaert C., Debelius J., et al. (2018). Best Practices for Analysing Microbiomes. Nat. Rev. Microbiol. 16 (7), 410–422. 10.1038/s41579-018-0029-9 [DOI] [PubMed] [Google Scholar]
- Lin D., Yalamanchili H. B., Zhang X., Lewis N. E., Alves C. S., Groot J., et al. (2020). CHOmics: A Web-Based Tool for Multi-Omics Data Analysis and Interactive Visualization in CHO Cell Lines. Plos Comput. Biol. 16 (12), e1008498. 10.1371/journal.pcbi.1008498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Bai Z., Liu B., Li Z. (2021). Crosslink: An R Package for Network Visualization of Grouped Nodes. Front. Genet. 12, 706854. 10.3389/fgene.2021.706854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J., Arze C., Arze C., Ananthakrishnan A. N., Schirmer M., Avila-Pacheco J., et al. (2019). Multi-omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature 569 (7758), 655–662. 10.1038/s41586-019-1237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg D. S., Lebeis S. L., Paredes S. H., Yourstone S., Gehring J., Malfatti S., et al. (2012). Defining the Core Arabidopsis thaliana Root Microbiome. Nature 488 (7409), 86–90. 10.1038/nature11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. V., Pedersen O. (2016). The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 375 (24), 2369–2379. 10.1056/NEJMra1600266 [DOI] [PubMed] [Google Scholar]
- McMurdie P. J., Holmes S. (2013). Phyloseq: an R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS One 8 (4), e61217. 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min D., Doxey A. C., Neufeld J. D. (2021). AXIOME3: Automation, eXtension, and Integration of Microbial Ecology. Gigascience 10. 10.1093/gigascience/giab006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais L. H., Schreiber H. L., Mazmanian S. K. (2021). The Gut Microbiota-Brain axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 19 (4), 241–255. 10.1038/s41579-020-00460-0 [DOI] [PubMed] [Google Scholar]
- Namkung J. (2020). Machine Learning Methods for Microbiome Studies. J. Microbiol. 58 (3), 206–216. 10.1007/s12275-020-0066-8 [DOI] [PubMed] [Google Scholar]
- Nie S., Zhang Z., Mo S., Li J., He S., Kashif M., et al. (2021). Desulfobacterales Stimulates Nitrate Reduction in the Mangrove Ecosystem of a Subtropical Gulf. Sci. Total Environ. 769, 144562. 10.1016/j.scitotenv.2020.144562 [DOI] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F. G., Kindt R., Legendre P., Minchin P. R., O’Hara R. (2013). Package ‘vegan’. Community Ecology Package. Version 2. Available at: http://CRAN.R-project.org/package=vegan .
- Phillips N. D. (2017). YaRrr! the Pirate’s Guide to R. Available at: https://www.psychologicalscience.org/observer/yarrr-the-pirates-guide-to-r .
- Rai S. N., Qian C., Pan J., Rai J. P., Song M., Bagaitkar J., et al. (2021). Microbiome Data Analysis with Applications to Pre-clinical Studies Using QIIME2: Statistical Considerations. Genes Dis. 8 (2), 215–223. 10.1016/j.gendis.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K. S., Knight C. G., de Hollander M., Brearley F. Q., Constantinides B., Cotton A., et al. (2018). Detecting Macroecological Patterns in Bacterial Communities across Independent Studies of Global Soils. Nat. Microbiol. 3 (2), 189–196. 10.1038/s41564-017-0062-x [DOI] [PubMed] [Google Scholar]
- Ren L., Wang Y., Zhong J., Li X., Xiao Y., Li J., et al. (2021). Dynamics of the Upper Respiratory Tract Microbiota and its Association with Mortality in COVID-19. Am. J. Respir. Crit. Care Med. 204 (12), 1379–1390. 10.1164/rccm.202103-0814OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.-C., et al. (2011). pROC: an Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinformatics 12 (1), 77. 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Abnet C. C., White O., Knight R., Huttenhower C. (2015). The Microbiome Quality Control Project: Baseline Study Design and Future Directions. Genome Biol. 16 (1), 276. 10.1186/s13059-015-0841-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore Z. L., Wagner A. H., Lesurf R., Campbell K. M., Kunisaki J., Griffith O. L., et al. (2016). GenVisR: Genomic Visualizations in R. Bioinformatics 32 (19), 3012–3014. 10.1093/bioinformatics/btw325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub D., Blackwell N., Langarica-Fuentes A., Peltzer A., Nahnsen S., Kleindienst S. (2020). Interpretations of Environmental Microbial Community Studies Are Biased by the Selected 16S rRNA (Gene) Amplicon Sequencing Pipeline. Front. Microbiol. 11, 550420. 10.3389/fmicb.2020.550420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareen A., Kinney J. B. (2020). Logomaker: Beautiful Sequence Logos in Python. Bioinformatics 36 (7), 2272–2274. 10.1093/bioinformatics/btz921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. R., Sanders J. G., Sanders J. G., McDonald D., Amir A., Ladau J., et al. (2017). A Communal Catalogue Reveals Earth's Multiscale Microbial Diversity. Nature 551 (7681), 457–463. 10.1038/nature24621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomisto H. (2010). A Diversity of Beta Diversities: Straightening up a Concept Gone Awry. Part 1. Defining Beta Diversity as a Function of Alpha and Gamma Diversity. Ecography 33 (1), 2–22. 10.1111/j.1600-0587.2009.05880.x [DOI] [Google Scholar]
- Turnbaugh P. J., Ley R. E., Hamady M., Fraser-Liggett C. M., Knight R., Gordon J. I. (2007). The Human Microbiome Project. Nature 449 (7164), 804–810. 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagih O. (2017). Ggseqlogo: a Versatile R Package for Drawing Sequence Logos. Bioinformatics 33 (22), 3645–3647. 10.1093/bioinformatics/btx469 [DOI] [PubMed] [Google Scholar]
- Wagner J., Chelaru F., Kancherla J., Paulson J. N., Zhang A., Felix V., et al. (2018). Metaviz: Interactive Statistical and Visual Analysis of Metagenomic Data. Nucleic Acids Res. 46 (6), 2777–2787. 10.1093/nar/gky136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters K. E., Martiny J. B. H. (2020). Alpha-, Beta-, and Gamma-Diversity of Bacteria Varies across Habitats. PLoS One 15 (9), e0233872. 10.1371/journal.pone.0233872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Guo A. (2020). An Efficient Variance Estimator of AUC and its Applications to Binary Classification. Stat. Med. 39 (28), 4281–4300. 10.1002/sim.8725 [DOI] [PubMed] [Google Scholar]
- Whittaker R. H. (1960). Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 30 (3), 279–338. 10.2307/1943563 [DOI] [Google Scholar]
- Xu S., Dai Z., Guo P., Fu X., Liu S., Zhou L., et al. (2021). ggtreeExtra: Compact Visualization of Richly Annotated Phylogenetic Data. Mol. Biol. Evol. 38 (9), 4039–4042. 10.1093/molbev/msab166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Wen T., Zhang H., Zhao M., Penton C. R., Thomashow L. S., et al. (2020). Predicting Disease Occurrence with High Accuracy Based on Soil Macroecological Patterns of Fusarium Wilt. ISME J. 14 (12), 2936–2950. 10.1038/s41396-020-0720-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Li N., Chen Q., Qin H. (2021). Fecal Microbiota Transplantation Modulates the Gut Flora Favoring Patients with Functional Constipation. Front. Microbiol. 12 (2674), 700718. 10.3389/fmicb.2021.700718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Federico A., Faits T., Manimaran S., Segrè D., Monti S., et al. (2021). Animalcules: Interactive Microbiome Analytics and Visualization in R. Microbiome 9 (1), 76. 10.1186/s40168-021-01013-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., et al. (2016). Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host's Metabolism. Mol. Psychiatry 21 (6), 786–796. 10.1038/mp.2016.44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.