Abstract
Background:
Pulmonary arterial hypertension (PAH) is a rare disease leading to right ventricular (RV) failure and manifests in decreasing exercise tolerance. Our study aimed to assess the usefulness of electrocardiographic parameters reflecting right heart hypertrophy as predictors of clinical status in PAH.
Methods:
The retrospective analysis included 26 patients, mean 49 ± 17 years of age, diagnosed with PAH, and eligible to undergo cardiopulmonary exercise test (CPET). The relations between ECG values and parameters obtained in procedures such as six-minute walk test (6-MWT), echocardiography, right heart catheterization (RHC), and CPET were analyzed.
Results:
P-wave amplitude in lead II correlated positively with CPET parameter of respiratory response: minute ventilation to carbon dioxide production slope (VE/VCO2 slope; r = 0.436, p = 0.029) and echocardiographic estimated RA pressure (RAP; r = 0.504, p = 0.02). RV Sokolow-Lyon index (RVSLI) positively correlated with echocardiographic parameters reflecting RV function, overload, and afterload–tricuspid regurgitation pressure gradient (TRPG; r = 0.788, p < 0.001), RV free wall thickness (r = 0.738, p < 0.001), and mean pulmonary arterial pressure (mPAPECHO; r = 0.62, p = 0.0016), respectively, as well as VE/VCO2 slope (r = 0.593, p = 0.001) and mPAP assessed directly in RHC (mPAPRHC; r = 0.469, p = 0.0497). R-wave in lead aVR correlated positively with TRPG (r = 0.719, p < 0.001), mPAPECHO (r = 0.446, p = 0.033), and several hemodynamic criteria of PAH diagnosis: positively with mPAPRHC (r = 0.505, p = 0.033) and pulmonary vascular resistance (r = 0.554, p = 0.026) and negatively with pulmonary capillary wedge pressure (r = −0.646, p = 0.004). QRS duration correlated positively with estimated RAP (r = 0.589, p = 0.004), vena cava inferior diameter (r = 0.506, p = 0.016), and RA area (r = 0.679, p = 0.002) and negatively with parameters of exercise capacity: peak VO2 (r = −0.486, p = 0.012), CPET maximum load (r = − 0.439, p = 0.025), and 6-MWT distance (r = −0.430, p = 0.046). ROC curves to detect intermediate/high 1-year mortality risk (based on ESC criteria) indicate RVSLI (cut-off point: 1.57 mV, AUC: 0.771) and QRS duration (cut-off points: 0.09 s, AUC: 703 and 0.1 s, AUC: 0.759) as relevant predictors.
Conclusion:
Electrocardiography appears to be an important and underappreciated tool in PAH assessment. ECG corresponds with clinical parameters reflecting PAH severity.
Keywords: cardiopulmonary exercise test, electrocardiogram, pulmonary arterial hypertension, right heart catheterization, right ventricle
Introduction
Pulmonary arterial hypertension (PAH), a pre-capillary subtype of pulmonary hypertension (PH), is a rare disease characterized by remodeling in the pulmonary circulation leading to increased pulmonary vascular resistance (PVR) and pulmonary artery pressure (PAP). Consequently, the enlargement and hypertrophy of right ventricle (RV) as well as right atrial (RA) enlargement occur as secondary remodeling and further lead to right heart failure (HF). 1 According to current guidelines, PAH is defined as mean pulmonary arterial pressure (mPAP) ⩾25 mmHg, pulmonary capillary wedge pressure (PCWP) ⩽15 mmHg, and PVR >3 Wood units (WU) in a direct hemodynamic measurement during right heart catheterization (RHC), which is the gold standard in PAH diagnosis. 2 In addition to RHC, there is a wide range of non-invasive tests in PAH patient assessment.3,4 However, early PAH diagnosis remains a challenge. 5 In Europe, the PAH prevalence is 15–60 cases per million and incidence is 5–10 cases per million per year. In Poland, the PAH population has reached around 970 cases, which were enrolled in the Data Base of Pulmonary Hypertension in the Polish Population (BNP-PL) Registry. 6
Structural changes and deterioration of myocardial function are related to the patient’s clinical condition.7 –9 Major importance is assigned to the remaining non-invasive and relatively easy available methods, including echocardiography, laboratory tests, six-minute walk test (6-MWT), and cardiopulmonary exercise test (CPET).10 –15 The six-minute walking test distance (6-MWD), peak oxygen uptake (VO2 peak), and minute ventilation to carbon dioxide production slope (VE/VCO2 slope) are established parameters reflecting patients’ clinical condition and prognosis. 2
The evidence from the literature about the usefulness of electrocardiogram (ECG) in an investigation of patients with PAH is still inconclusive. It possesses only a supportive role in diagnostics due to low sensitivity and specificity. 16 Nevertheless, particular abnormalities such as right ventricular (RV) and right atrial (RA) hypertrophy and overload are observed more often in this disease. 17 ECG as a non-invasive tool has the potential implication in the assessment of the treatment and the clinical condition of the patient without the necessity of stress testing. 18 Tonelli et al. compared the parameters of the ECG performed at initial diagnosis, during the course of the disease, and close to the time of death and revealed gradually occurring abnormalities. 19 Kukla et al. showed the potential role of electrocardiographic signs of RV strain in prognostication of patients with intermediate and high-risk forms of acute pulmonary embolism. 20 However, the role of ECG in PAH patient assessment and risk stratification remains not fully investigated.
The study aimed to assess the usefulness of ECG in the assessment of clinical status of PAH patients in relation to invasive and non-invasive methods.
Materials and methods
The retrospective analysis included 26 patients (14 females), mean 49 ± 17 years of age, and body mass index (BMI) mean 25.16 ± 5.51 kg/m2, treated with PAH-specific pharmacotherapy. The inclusion criteria for the study included PAH confirmed according to the hemodynamic criteria 2 in patients who were able to perform CPET. One patient was qualified in World Health Organization (WHO) functional class I, 10 patients in WHO class II, and 15 patients in WHO class III. Specific treatment strategies applied in the study group are demonstrated in Table 1. The standard diagnostic procedures at baseline hospitalization were as follows: physical examination, ECG, laboratory parameters, 6-MWT, and echocardiography. Moreover, they had CPET and RHC. Analyzing the clinical status and obtained values of additional tests, patients were assigned to the individual 1-year mortality risk groups based on the European Society of Cardiology (ESC) criteria. 2 To precisely categorize patients as low, intermediate, or high risk, the stratification strategy suggested by Kylhammar et al. 21 was used. Thereby, the specific cut-off values established in the guidelines were graded from 1 to 3 (1: low risk, 2: intermediate risk, and 3: high risk). For every individual case, the sum of all points was divided by the number of included variables and rounded to the nearest integer to indicate the appropriate risk group.
Table 1.
A summary of the different treatment strategies applied in the analyzed study group.
| Treatment strategy | Patients |
|---|---|
| Sildenafil + endothelin receptor antagonist | 14 |
| Sildenafil + treprostinil | 3 |
| Sildenafil + endothelin receptor antagonist + treprostinil | 3 |
| Sildenafil in monotherapy | 2 |
| Endothelin receptor antagonist in monotherapy | 2 |
| Sildenafil + iloprost | 1 |
| Sildenafil + riociguat | 1 |
Electrocardiography
The standard 12-lead ECG (SCHILLER Cardiovit CS-200) was performed at rest on a paper speed of 25 mm/s and a sensitivity of 1 mV = 10 mm. The following parameters were analyzed: heart rhythm, rate, heart axis, intraventricular conduction abnormalities, RA hypertrophy parameter: P-wave amplitude in lead II (P-pulmonale criteria >0.25 mV), RV hypertrophy: RV Sokolow-Lyon index (RVSLI) estimated as R in V1 + deepest S in V5 or V6 (positive for values >1.05 mV), amplitude of R-wave in lead aVR (positive⩾0.7 mV), and QRS duration.
Laboratory tests
The basic element of diagnostics was the implementation of a panel of laboratory tests: blood count, coagulation, and biochemical profile including NT-proBNP (N-terminal prohormone of brain natriuretic peptide) concentration. All tests were carried out at the local medical laboratory.
Six-minute walking test
This exercise test was conducted following the standard methodology applied at the Department, as described previously. 22 The obtained parameters, including the results of 6-MWD, were recorded in the study protocol. 23
Echocardiography
Each patient underwent diagnostic transthoracic two-dimensional echocardiography with Doppler technology (Philips EPIQ) to assess the morphology and function of the heart. Direct measurements of RV dimension in four-chamber projection, RV free wall thickness, RA area, tricuspid annular plane systolic excursion (TAPSE), vena cava inferior (VCI) diameter, and acceleration time (ACT) of pulmonary artery were assessed. Furthermore, the following parameters were estimated: tricuspid regurgitation pressure gradient (TRPG) based on the tricuspid regurgitation peak velocity (TRV) using the Bernoulli equation TRPG = 4(TRV) 2 , RA pressure (RAP) based on the inferior vena cava (IVC) respiratory collapsibility, and systolic PAP (sPAP) using the formula sPAP = 4(TRV) 2 + RAP.
Right heart catheterization
The test assessing hemodynamic parameters of the heart was performed with local anesthesia. A Swan-Gantz catheter was inserted through the internal jugular vein into the right heart cavities to assess the following parameters: systolic, diastolic, and mean PAP values as well as PCWP and subsequently to estimate PVR, cardiac output (CO), and cardiac index (CI) using both methods: thermodilution: injecting 10 ml of 0.9% NaCl solution at 0°C–5°C to RA, and Fick’s: with blood samples from the pulmonary artery and radial or femoral artery taken simultaneously. 24
Cardiopulmonary exercise test
All patients had CPET on a treadmill, with the use of Raise, Activate, Mobilize, Potentiate (RAMP) protocol (SCHILLER Cardiovit CS-200). During the test, patients were constantly monitored with ECG for arrhythmias or changes of the ST segment. The procedure was terminated after completing the protocol or when the patient was unable to continue: the maximum load in MET (metabolic equivalent) has been noted (MET = 3.5 ml/min/kg).
Statistical analysis
The descriptive statistics of analyzed parameters was performed and presented as mean and standard deviation or median with interquartile range, according to data distribution. To assess the normality and homogeneity of variance, the Shapiro–Wilk test and Levene’s test were performed, respectively. Pearson’s ‘r’ linear correlation coefficient was used to appraise the relationship between ECG and other test parameters. Receiver operating characteristic (ROC) curves were constructed at the variable values of ECG markers to estimate the optimal cut-off points for both specificity and sensitivity to detect patients with increased 1-year mortality risk based on ESC criteria. 2 The statistical package Statistica 13 (StatSoft Inc., USA) was used for data analysis, and individual p value <0.05 was considered statistically significant. Considering multiple test correction, we applied the conservative Bonferroni method and the additional statistical significance threshold for these statistics calculated as 0.05 divided by 26 (i.e. p value <0.0019), as we related each ECG parameter with another 26 different clinical evaluation values. Due to the preliminary, informative nature of the study and to reduce the type II testing error, the results were presented taking into account both significance levels.
The study complies with the Declaration of Helsinki.
Results
The descriptive characteristics of the analyzed study group are presented in Table 2.
Table 2.
Characteristics of the study group: additional test parameters divided into particular procedures.
| Parameter | Median | Interquartile range |
|---|---|---|
| Electrocardiography | ||
| P-wave amplitude in lead II (mV) | 0.226 | 0.162–0.273 |
| R-wave amplitude in lead aVR (mV) | 0.538 | 0.314–0.795 |
| QRS duration (s) | 0.100 | 0.090–0.120 |
| RVSLI (mV) | 1.550 | 0.842–1.952 |
| Cardiopulmonary exercise test | ||
| VO2 peak (ml/kg/min) | 13.70 | 11.00–16.80 |
| VO2 AT (ml/kg/min) | 11.55 | 9.40–14.50 |
| VE/VCO2 slope (ml/kg/min) | 40.94 | 35.45–47.86 |
| Maximum load (MET) | 3.70 | 3.00–4.70 |
| Right heart catheterization | ||
| mPAPRHC (mmHg) | 42.25 | 36.00–56.00 |
| PCWP (mmHg) | 10.00 | 8.50–12.00 |
| PVR (thermodilution method; WU–wood unit) | 8.46 | 3.91–10.23 |
| CO (thermodilution method; l/min) | 4.60 | 4.13–5.57 |
| CI (thermodilution method; l/min × m2) | 2.61 | 2.29–3.04 |
| PVR (Fick’s method; WU) | 5.21 | 3.07–7.57 |
| CO (Fick’s method; l/min) | 5.48 | 4.65–6.79 |
| CI (Fick’s method; l/min × m2) | 2.96 | 2.50–3.72 |
| Echocardiography | ||
| LVEF vis. (%) | 60.00 | 55.00–60.00 |
| VCI diameter (mm) | 16.50 | 14.00–18.00 |
| TRPG (mmHg) | 61.00 | 40.50–89.00 |
| Estimated RAP (mmHg) | 5.00 | 3.00–5.00 |
| mPAPECHO (mmHg) | 44.09 | 28.23–61.17 |
| TAPSE (mm) | 20.00 | 17.00–22.00 |
| ACT (ms) | 97.00 | 90.00–107.50 |
| RV free wall thickness (mm) | 8.00 | 8.00–10.00 |
| RV four-chamber projection (cm) | 4.70 | 3.90–5.30 |
| RA area (cm 2 ) | 21.50 | 16.00–30.00 |
| Laboratory test | ||
| NT-proBNP (pg/ml) | 187.50 | 115.50–982.10 |
| Six-minute walk test | ||
| 6-MWD (m) | 480.00 | 430.00–540.00 |
6-MWD, six-minute walk distance; ACT, acceleration time of pulmonary flow; CI, cardiac index; CO, cardiac output; LVEF vis., left ventricle ejection fraction visually; mPAPECHO, mean pulmonary arterial pressure estimated by echocardiography; mPAPRHC, mean pulmonary arterial pressure obtained by RHC; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA; right atrium; RAP, right atrium pressure; RHC, right heart catheterization; RV, right ventricle; RVSLI, right ventricle Sokolow-Lyon index; TAPSE, tricuspid annular plane systolic excursion; TRPG, tricuspid regurgitation pressure gradient; VCI, vena cava inferior; VE/VCO2 slope, minute ventilation to carbon dioxide production slope; VO2 AT, oxygen uptake at anaerobic threshold; VO2 peak, peak oxygen uptake.
Electrocardiogram
ECG was evaluated in all 26 patients. In this group, 25 patients had sinus rhythm and 1 had persistent atrial fibrillation. The mean heart rate was 85.08 ± 15.76 beats per minute. The ECG axis was assessed as follows: normal: 7 cases, right deviation: 9, left deviation: 5, and indeterminate: 5. The following intraventricular conduction abnormalities were found: right bundle branch block (RBBB): 7, incomplete RBBB: 6, left bundle branch block (LBBB): 1. The RA hypertrophy marker P-pulmonale was assessed as positive in nine cases. RV hypertrophy criteria were met in 19 patients: 19 patients had a positive RVSLI, 8 of which have increased R in aVR. The analysis of the data from ECG and other procedures revealed numerous significant correlations, which are presented in Table 3. Notably, there were no statistically significant correlations with WHO functional class score and laboratory marker of HF: NT-proBNP. An extended summary of all correlations (including statistically insignificant) is provided in Supplementary Table 1.
Table 3.
Statistically significant correlations between ECG and other tests useful in evaluation of patients with PAH.
| Correlated parameter | r | p |
|---|---|---|
| P-wave amplitude in lead II | ||
| VE/VCO2 slope | 0.436 | 0.029 |
| Estimated RAP | 0.504 | 0.02 |
| RVSLI | ||
| VE/VCO2 slope | 0.593 | 0.001* |
| TRPG | 0.788 | <0.001* |
| RV free wall thickness | 0.738 | <0.001* |
| mPAPECHO | 0.62 | 0.0016* |
| mPAPRHC | 0.469 | 0.0497 |
| R-wave amplitude in lead aVR | ||
| TRPG | 0.719 | <0.001* |
| mPAPECHO | 0.446 | 0.033 |
| mPAPRHC | 0.505 | 0.033 |
| PVR (thermodilution method) | 0.554 | 0.026 |
| PCWP | –0.646 | 0.004 |
| QRS duration | ||
| VCI diameter | 0.506 | 0.016 |
| estimated RAP | 0.589 | 0.004 |
| RA area | 0.679 | 0.002 |
| 6-MWD | –0.430 | 0.046 |
| VO2 peak | –0.486 | 0.012 |
| Maximum load in CPET | –0.439 | 0.025 |
6-MWD, six-minute walk distance; CPET, cardiopulmonary exercise test; ECG, electrocardiogram; mPAPECHO, mean pulmonary arterial pressure estimated by echocardiography; mPAPRHC, mean pulmonary arterial pressure obtained by RHC; p, statistical significance; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; r, correlation coefficient; RA, right atrium; RAP, right atrium pressure; RHC, right heart catheterization; RV, right ventricle; RVSLI, right ventricle Sokolow-Lyon index; TRPG, tricuspid regurgitation pressure gradient; VCI, vena cava inferior; VE/VCO2 slope, minute ventilation to carbon dioxide production slope; VO2 peak, peak oxygen uptake; VR, vascular resistance.
Every presented correlation achieved the individual significance statistical significance (p < 0.05). The Bonferroni-corrected statistical significance (p < 0.0019) was achieved by parameters marked with ‘*’.
P-wave in lead II
The first parameter evaluated, P-wave in lead II, presented only two significant, positive correlations with respiratory response measurement: VE/VCO2 slope assessed in CPET, and with echocardiographic parameter of RV pressure overload–estimated RAP.
RV Sokolow-Lyon index
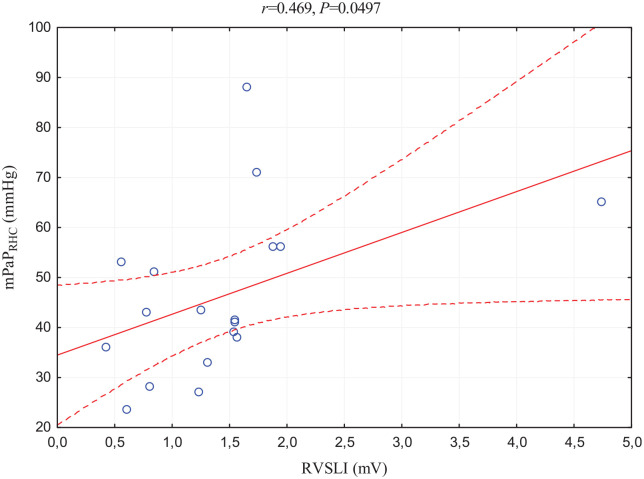
RVSLI positively correlated with echocardiographic parameters of RV pressure overload, TRPG and RV free wall thickness; afterload, mPaPECHO; CPET parameter of respiratory response, VE/VCO2 slope; and RHC hemodynamic marker of RV afterload, mPAPRHC (Figure 1). With respect to the last-mentioned correlation, one of the values was distinctly outlier, thereby the additional statistic omitting this case was prepared. The novel correlation was statistically insignificant (p = 0.057). Importantly, correlations with TRPG, RV free wall thickness, VE/VCO2, and mPAPRHC also achieved the Bonferroni-corrected statistical significance (Table 3).
Figure 1.
Scatterplot of the correlation between RVSLI and mPAPRHC.
mPAPRHC, mean pulmonary arterial pressure obtained by RHC; p, statistical significance; r, correlation coefficient.
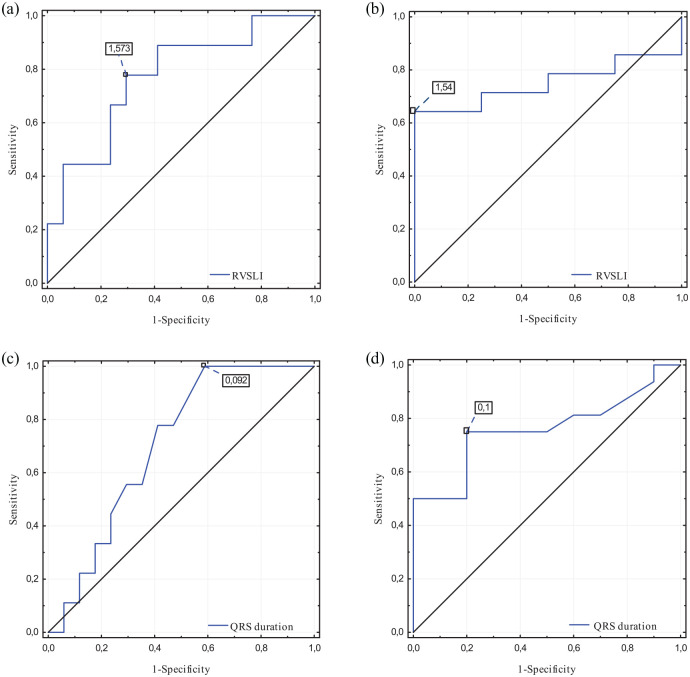
The ROC curve analysis confirmed relation of RVSLI values to the following parameters: VE/VCO2 slope ⩾45 ml/kg/min [Figure 5(a); area under curve (AUC): 0.771, 95% confidence interval (CI): 0.579–0.964, p = 0.006] and mPAPRHC >35 mmHg [Figure 5(b); AUC: 0.750, 95% CI: 0.531–0.969, p = 0.025].
Figure 5.
Receiver operating characteristic curves constructed for detection of (a) increased VE/VCO2 slope (⩾45 ml/kg/min) by RVSLI, (b) severe PAH diagnosis (defined as mPAPRHC >35 mmHg) by RVSLI, (c) increased VE/VCO2 slope (⩾ 45 ml/kg/min) by QRS duration, and (d) decreased VO2 peak (⩽15 ml/kg/min) by QRS duration.
R-wave in lead aVR
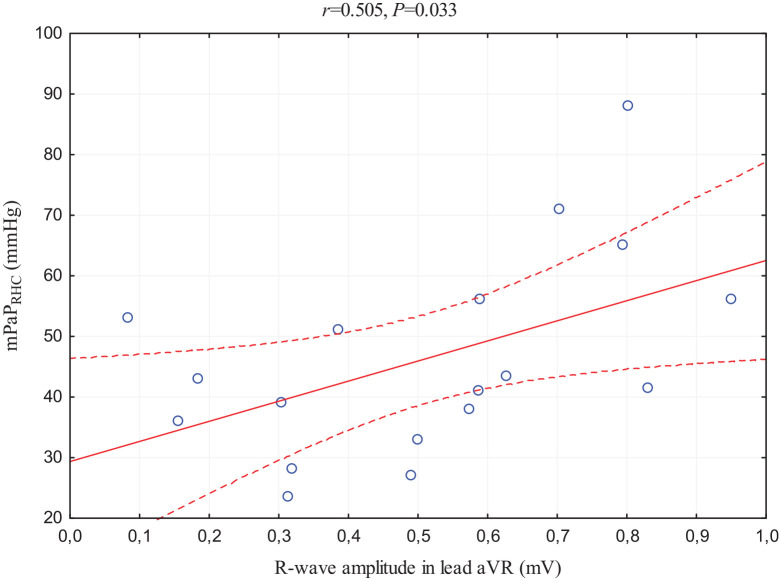
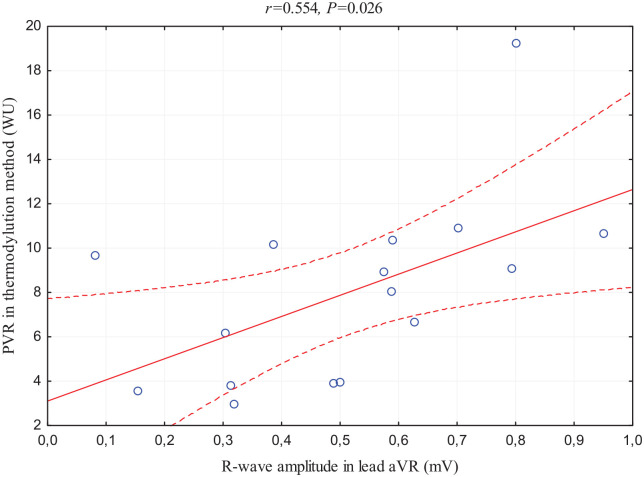
Another aspect was the strong and positive relationship between R-wave in lead aVR and echocardiographic assessment of TRPG (this correlation achieved Bonferroni-corrected statistical significance) and relatively poor correlation with mPAPECHO. In addition, this ECG parameter has correlations with all three RHC standard measurements in PAH diagnosis: positive with mPAPRHC (Figure 2), PVR assessed in thermodilution method (Figure 3), and negative with PCWP.
Figure 2.
Scatterplot of the correlation between R-wave amplitude in lead aVR and mPAPRHC.
mPAPRHC, mean pulmonary arterial pressure obtained by RHC; p, statistical significance; r, correlation coefficient.
Figure 3.
Scatterplot of the correlation between R-wave amplitude in lead aVR and PVR measured by thermodilution method.
p, statistical significance; PVR, pulmonary vascular resistance; r, correlation coefficient; WU, Wood units.
QRS duration
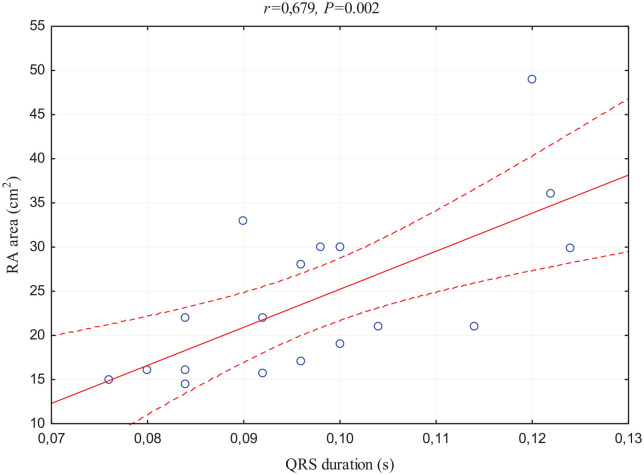
First, QRS duration was revealed to possess a wide amount of positive correlations with echocardiographic parameters of RV pressure overload, namely, estimated RAP as well as enlargement; RA area (Figure 4); and VCI diameter. These findings were accompanied by negative correlations with markers assessing exercise capacity: 6-MWD, VO2 peak, and maximum load in CPET.
Figure 4.
Scatterplot of the correlation between QRS duration and RA area measured by echocardiography.
p, statistical significance; r, correlation coefficient; RA, right atrium.
The ROC curves analysis confirmed relations between QRS complex extension and CPET parameters reflecting worse clinical condition: high VE/VCO2 slope [Figure 5(c); as ⩾45 ml/kg/min; AUC: 0.703, 95% CI: 0.505–0.900, p = 0.045] and low peak VO2 [Figure 5(d); as ⩽15 ml/kg/min; AUC: 0.759, 95% CI: 0.573–0.945, p = 0.006].
Mortality risk stratification
According to the ESC criteria of 1-year mortality risk, patients were qualified to the following categories: low (<5%): 11 patients, intermediate (5–10%): 11 patients, and high (>10%): 4 patients. Furthermore, the analysis of presented ROC curves indicated statistically significant results of ECG parameters in detecting patients with increased 1-year mortality risk: RVSLI to detect high- versus low- + intermediate-risk patients (Figure 5(a)), and QRS duration to detect high- versus low- + intermediate-risk patients (Figure 5(c)) as well as intermediate- + high- versus low-risk patients (Figure 5(d)).
Discussion
The ECG is one of the methods that might be helpful in PH diagnosis, treatment response, and survival prediction.4,25 –29 The presented retrospective study purposed to expand the available knowledge considering the relevance of ECG parameters in comparison with the other standard invasive and non-invasive tests in the clinical assessment of PAH patients.
Our analysis revealed the relation of electrocardiographic RV hypertrophy parameters with the hemodynamic measurement of RV afterload reflected by mPAP assessment in RHC, which positively correlates with both RVSLI and R-wave in lead aVR. Interestingly, the analysis showed that R-wave in lead aVR, a less commonly used parameter of RV hypertrophy, has more and stronger correlations with RHC markers used in PAH diagnosis. To confirm, mPAPRHC correlates stronger with this index than with RVSLI, and also R-wave in lead aVR is the only one that showed correlations with the parameters differentiating PH subgroups: positive with PVR and negative with PWCP. This fact confirms the impact of increased PAP on right heart hypertrophy and subsequent ECG changes, especially R-wave in lead aVR. 30
The analysis of the comparability of ECG parameters as clinical status predictors is more complicated. In our study, the ECG parameters have stronger correlations with echocardiographic markers of RV enlargement and overload over functional and hemodynamic assessment tests such as CPET and RHC.
A questionable role may be attributed to the RA enlargement criterion, increased P-wave in lead II, which manifests only two and poor correlations with VE/VCO2 slope and estimated RAP. The analysis of this parameter should be extended because Bossone et al. in his study linked P-pulmonale presence with a decrease in patient survival. 31 On the other hand, Seyyedi et al. wrote about the increased incidence of P-pulmonale in patients with severe RV dysfunction, whereas in the same article they showed the lack of correlation between P-wave amplitude and NT-proBNP concentration as well as 6-MWD, 32 which was observed in our study.
The presented study revealed that RVSLI is a reliable parameter in the clinical assessment of PAH patients. This is demonstrated by correlations with echocardiographic parameters: TRPG, RV free wall thickness, mPaPECHO, and with VE/VCO2 slope assessed in CPET. It follows that the hypertrophy of the right heart walls has a significant impact on the decrease in cardiopulmonary function. Similarly, Kramer et al. described a strong relationship between the RVSLI and the severity of the disease in children suffering from idiopathic PAH. 33 He showed a significant increase in the risk of cardiac events for patients with RVSLI > 2.1 mV. Similar conclusions arise from our analysis of ROC curves constructed to detect PAH patients with high 1-year mortality risk (over 10%). According to the optimal cut-off point derived from this analysis, a worse survival prognosis may be expected for patients with RVSLI above 1.57 mV. Besides, a close value of 1.54 mV was obtained from the ROC curve performed to detect severe PAH [Figure 5(b); defined as mPAPRHC >35 mmHg] by this index in our study group.
Our study revealed that R-wave in lead aVR reflecting RV hypertrophy seems to be a weak predictor of the clinical status; however, this might be a result of small number of patients included in the study. However, in risk stratification, Cheng et al. 34 indicate R-wave in lead aVR >0.40 mV as an independent predictor of mortality.
The last parameter, QRS duration, showed numerous relationships with imaging and exercise tests. The study confirms that changes in the heart structure secondary to an increase of PAP lead to intraventricular conduction disturbances detected in the ECG as an prolongation of the QRS complex.35,36 As a consequence of this process, the hemodynamics of the heart is impaired, which is reflected in the deterioration of the established markers of the patient’s clinical condition. In addition to our results, Sun et al. confirmed that QRS prolongation is associated with the worse WHO functional class and correlated negatively with 6-MWD. 37 Furthermore, he showed the importance of prolonged QRS as a predictor of mortality and found 2.5-fold increased risk of death in idiopathic PAH for QRS duration of 0.12 s or higher.
Regarding the mortality risk evaluation, it should be emphasized that in the presented study the electrocardiographic parameters were compared with the established risk parameters in PAH. These factors, including WHO functional class, 6-MWD, VO2 peak, VE/VCO2 slope, NT-proBNP, RA area, and CI, are used rather integrally with other parameters than as individual predictors. 2 Therefore, our findings suggest that RVSLI and QRS duration may be the parameters useful in the assessment of clinical condition as well as prognosis in PAH patients. Adding these ECG values to the risk stratification scores such as COMPERA or REVEAL 2.0 may improve their prognostic accuracy.38,39 However, it has to be confirmed in a subsequent, dedicated study with a sufficient size of a study group and a wide range of disease severity, which was not the purpose and the design of this study.
Limitations of the study
The limitations of the study are caused by the limited number of patients and data collected retrospectively from a single center.
Conclusions
Electrocardiography is an important, underestimated tool in the evaluation of patients suffering from PAH. ECG parameters reflecting right heart hypertrophy and overload, such as P-wave in lead II, RVSLI, R-wave in lead aVR, and QRS duration, significantly correspond with clinical parameters reflecting PAH severity.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666221087846 for ECG in the clinical and prognostic evaluation of patients with pulmonary arterial hypertension: an underestimated value by Tomasz Adam Michalski, Joanna Pszczola, Anna Lisowska, Malgorzata Knapp, Bozena Sobkowicz, Karol Kaminski and Katarzyna Ptaszynska-Kopczynska in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors thank Lisane Łuczaj for language corrections.
Footnotes
Author contributions: Tomasz Adam Michalski: Conceptualization; Data curation; Methodology; Project administration; Resources; Software; Visualization; Writing – original draft.
Joanna Pszczola: Conceptualization; Data curation; Methodology; Resources; Software; Visualization; Writing – original draft.
Anna Lisowska: Formal analysis; Investigation; Supervision; Validation; Writing – review & editing.
Malgorzata Knapp: Formal analysis; Investigation; Supervision; Validation; Writing – review & editing.
Bozena Sobkowicz: Formal analysis; Investigation; Supervision; Validation; Writing – review & editing.
Karol Kaminski: Formal analysis; Investigation; Supervision; Validation; Writing – review & editing.
Katarzyna Ptaszynska-Kopczynska: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Resources; Software; Supervision; Validation; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by the statutory funding granted to KPK (Medical University of Bialystok, Poland).
ORCID iD: Tomasz Adam Michalski  https://orcid.org/0000-0003-2799-3965
https://orcid.org/0000-0003-2799-3965
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tomasz Adam Michalski, Students’ Scientific Society, Department of Cardiology, Medical University of Bialystok, Bialystok, Poland1st Department of Cardiology, Medical University of Gdansk, Gdansk, Poland.
Joanna Pszczola, Students’ Scientific Society, Department of Cardiology, Medical University of Bialystok, Bialystok, Poland.
Anna Lisowska, Department of Cardiology, Medical University of Bialystok, Bialystok, Poland.
Malgorzata Knapp, Department of Cardiology, Medical University of Bialystok, Bialystok, Poland.
Bozena Sobkowicz, Department of Cardiology, Medical University of Bialystok, Bialystok, Poland.
Karol Kaminski, Department of Cardiology, Medical University of Bialystok, Bialystok, Poland; Department of Population Medicine and Lifestyle Diseases Prevention, Medical University of Bialystok, Bialystok, Poland.
Katarzyna Ptaszynska-Kopczynska, Department of Cardiology, Medical University of Bialystok, M. Sklodowskiej-Curie 24A Street, 15-276 Bialystok, Poland.
References
- 1. Chemla D, Lau EM, Papelier Y, et al. Pulmonary vascular resistance and compliance relationship in pulmonary hypertension. Eur Respir J 2015; 46: 1178–1189. [DOI] [PubMed] [Google Scholar]
- 2. Galie N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 3. Grignola JC. Hemodynamic assessment of pulmonary hypertension. World J Cardiol 2011; 3: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43: 40–47. [DOI] [PubMed] [Google Scholar]
- 5. Sahay S. Evaluation and classification of pulmonary arterial hypertension. J Thorac Dis 2019; 11(Suppl. 14): S1789–S1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kopeć G, Kurzyna M, Mroczek E, et al. Characterization of patients with pulmonary arterial hypertension: data from the polish registry of pulmonary hypertension (BNP-PL). J Clin Med 2020; 9: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoeper MM, Simon R, Gibbs J. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev 2014; 23: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delcroix M, Howard L. Pulmonary arterial hypertension: the burden of disease and impact on quality of life. Eur Respir Rev 2015; 24: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Badesch DB, Champion HC, Sanchez MAG. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: 55–66. [DOI] [PubMed] [Google Scholar]
- 10. Howard LS, Grapsa J, Dawson D, et al. Echocardiographic assessment of pulmonary hypertension: standard operating procedure. Eur Respir Rev 2012; 21: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiong PY, Jaff Z, D’Arsigny CL, et al. Evaluation of the impact of an echocardiographic diagnosis of pulmonary hypertension on patient outcomes. CJC Open 2020; 2: 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gan CT, McCann GP, Marcus JT, et al. NT-proBNP reflects right ventricular structure and function in pulmonary hypertension. Eur Respir J 2006; 28: 1190–1194. [DOI] [PubMed] [Google Scholar]
- 13. Querejeta Roca G, Campbell P, Claggett B, et al. Right atrial function in pulmonary arterial hypertension. Circ Cardiovasc Imaging 2015; 8: e003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Souza R, Channick R, Delcroix M, et al. Association between six-minute walk distance and long-term outcomes in patients with pulmonary arterial hypertension: data from the randomized SERAPHIN trial. PLoS One 2018; 13: e0193226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farina S, Correale M, Bruno N, et al. The role of cardiopulmonary exercise tests in pulmonary arterial hypertension. Eur Respir Rev 2018; 27: 170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 2004; 126(1 Suppl): 14S–34S. [DOI] [PubMed] [Google Scholar]
- 17. Bonderman D, Wexberg P, Martischnig AM, et al. A noninvasive algorithm to exclude pre-capillary pulmonary hypertension. Eur Respir J 2011; 37: 1096–1103. [DOI] [PubMed] [Google Scholar]
- 18. Piłka M, Darocha S, Banaszkiewicz M, et al. Assessment of electrocardiographic markers of acute and long-term hemodynamic improvement in patients with pulmonary hypertension. Ann Noninvasive Electrocardiol 2020; 25: e12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tonelli AR, Baumgartner M, Alkukhun L, et al. Electrocardiography at diagnosis and close to the time of death in pulmonary arterial hypertension. Ann Noninvasive Electrocardiol 2014; 19: 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kukla P, Kosior DA, Tomaszewski A, et al. Correlations between electrocardiogram and biomarkers in acute pulmonary embolism: Analysis of ZATPOL-2 Registry. Ann Noninvasive Electrocardiol 2017; 22: e12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kylhammar D, Kjellström B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. [DOI] [PubMed] [Google Scholar]
- 22. Kazimierczyk R, Szumowski P, Nekolla SG, et al. Prognostic role of PET/MRI hybrid imaging in patients with pulmonary arterial hypertension. Heart 2021; 107: 54–60. [DOI] [PubMed] [Google Scholar]
- 23. Demir R, Küçükoğlu MS. Six-minute walk test in pulmonary arterial hypertension. Anatol J Cardiol 2015; 15: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ptaszyńska-Kopczyńska K, Krentowska A, Sawicka E, et al. The strengths and weaknesses of non-invasive parameters obtained by echocardiography and cardiopulmonary exercise testing in comparison with the hemodynamic assessment by the right heart catheterization in patients with pulmonary hypertension. Adv Med Sci 2017; 62: 39–44. [DOI] [PubMed] [Google Scholar]
- 25. Al-Naamani K, Hijal T, Nguyen V, et al. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int J Cardiol 2008; 127: 214–218. [DOI] [PubMed] [Google Scholar]
- 26. Waligóra M, Tyrka A, Podolec P, et al. ECG markers of hemodynamic improvement in patients with pulmonary hypertension. Biomed Res Int 2018; 2018: 4606053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henkens IR, Gan CT-J, Van Wolferen SA, et al. ECG monitoring of treatment response in pulmonary arterial hypertension patients. Chest 2008; 134: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 28. Bandorski D, Bogossian H, Ecke A, et al. Evaluation of the prognostic value of electrocardiography parameters and heart rhythm in patients with pulmonary hypertension. Cardiol J 2016; 23: 465–472. [DOI] [PubMed] [Google Scholar]
- 29. Sato S, Ogawa A, Matsubara H. Change in R wave in lead V1 predicts survival of patients with pulmonary arterial hypertension. Pulm Circ 2018; 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kopec G, Anna Tyrka A, Miszalski-Jamka T, et al. Electrocardiogram for the diagnosis of right ventricular hypertrophy and dilation in idiopathic pulmonary arterial hypertension. Circ J 2012; 76: 1744–1749. [DOI] [PubMed] [Google Scholar]
- 31. Bossone E, Paciocco G, Iarussi D, et al. The prognostic role of the ECG in primary pulmonary hypertension. Chest 2002; 121: 513–518. [DOI] [PubMed] [Google Scholar]
- 32. Seyyedi SR, Sharif-Kashani B, Sadr M, et al. The relationship between electrocardiographic changes and prognostic factors in severely symptomatic pulmonary hypertension. Tanaffos 2019; 18: 34–40. [PMC free article] [PubMed] [Google Scholar]
- 33. Krämer J, Kreuzer F, Kaestner M, et al. Impact of the right ventricular sokolow–lyon index in children with idiopathic pulmonary arterial hypertension. Pediatr Cardiol 2018; 39: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 34. Cheng XL, He JG, Liu ZH, et al. The value of the electrocardiogram for evaluating prognosis in patients with idiopathic pulmonary arterial hypertension. Lung 2017; 195: 139–146. [DOI] [PubMed] [Google Scholar]
- 35. Cirulis MM, Ryan JJ, Archer SL. Pathophysiology, incidence, management, and consequences of cardiac arrhythmia in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Pulm Circ 2019; 9: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Badagliacca R, Reali M, Poscia R, et al. Right intraventricular dyssynchrony in idiopathic, heritable, and anorexigen-induced pulmonary arterial hypertension: clinical impact and reversibility. JACC Cardiovasc Imaging 2015; 8: 642–652. [DOI] [PubMed] [Google Scholar]
- 37. Sun P-Y, Jiang X, Gomberg-Maitland M, et al. Prolonged QRS duration: a new predictor of adverse outcome in idiopathic pulmonary arterial hypertension. Chest 2012; 141: 374–380. [DOI] [PubMed] [Google Scholar]
- 38. Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 39. Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666221087846 for ECG in the clinical and prognostic evaluation of patients with pulmonary arterial hypertension: an underestimated value by Tomasz Adam Michalski, Joanna Pszczola, Anna Lisowska, Malgorzata Knapp, Bozena Sobkowicz, Karol Kaminski and Katarzyna Ptaszynska-Kopczynska in Therapeutic Advances in Respiratory Disease