Abstract
Objective
Vaccine efficacy among previously exposed, but currently uninfected women, i.e., those who have serological evidence of a prior human papillomavirus (HPV) infection without corresponding detectable HPV DNA, remains incompletely defined. This meta-analysis assessed the serotype-specific efficacy of prophylactic HPV vaccination against HPV16/18 persistent infection (PI) and cervical intraepithelial neoplasia (CIN) among seropositive, DNA negative (SPDN) women enrolled to randomized controlled trials (RCTs) of HPV L1-based vaccines.
Methods
Searches were conducted on 08/16/20 on MEDLINE, Embase, Scopus and CENTRAL. RCTs of L1-based prophylactic bivalent or quadrivalent HPV vaccines, reporting serotype-specific clinical efficacy endpoints in the HPV16/18 seropositive, DNA-negative populations were included. Relative risks (RRs) of 6-month PI (6mPI), 12-month PI (12mPI), CIN1+ and CIN2+ were pooled using a random-effects model.
Results
A total of 1,727 citations were reviewed. 8 studies, with a total of 9,569 SPDN participants, met all eligibility criteria. The RR of 6mPI (RR=0.22; 95% confidence interval [CI]=0.08–0.61; p=0.018), 12mPI (RR=0.20; 95% CI=0.05–0.80; p=0.035), CIN1+ (RR=0.13; 95% CI=0.05–0.30; p=0.003) and CIN2+ (RR=0.15; 95% CI=0.04–0.59; p=0.022) was significantly reduced in the vaccinated compared to the unvaccinated group.
Conclusion
Our findings suggest high serotype-specific efficacy for HPV vaccination among cohorts of women with evidence of prior HPV16/18 infections, including 87% efficacy (95% CI=70%–95%; p=0.003) against HPV16/18 cervical dysplasia. HPV vaccination is highly effective among uninfected women, regardless of prior exposure history.
Trial Registration
PROSPERO Identifier: CRD42020206888
Keywords: Uterine Cervical Neoplasms, Human Papillomavirus 16, Human Papillomavirus 18, Vaccination
INTRODUCTION
Despite significant progress in the understanding and treatment of human papillomavirus (HPV)-associated malignancies, improvements in cancer screening [1,2], and the advent of prophylactic vaccines, cervical cancer remains a leading cause of cancer related morbidity and mortality worldwide, particularly affecting women in low-to-middle income countries [3]. Persistent infection with high-risk HPV (hrHPV) is responsible for virtually all [4] of the approximately 570,000 global cases of cervical cancer and 311,000 global deaths annually [3].
Three prophylactic HPV vaccines, Cervarix® (GlaxoSmithKline, Brentford, UK), Gardasil® (Merck, Kenilworth, NJ, USA) and Gardasil9® (Merck) are in widespread clinical use [5]; the principal use of these vaccines is in the prevention of cervical intraepithelial neoplasia (CIN) 3, AIS, or invasive cervical malignancy requiring surgical or multimodal treatment. Prophylactic HPV vaccines consist of recombinant HPV L1 capsid protein virus-like particles [5], and have been demonstrated to be highly immunogenic, clinically effective, and safe in preventing incident cervical infection with vaccine-type HPV strains and the cytopathological sequelae of such infections [6,7,8,9,10]. Over one hundred countries have adopted HPV L1-based vaccination as part of national vaccine programs [11], and epidemiological studies demonstrate a falling incidence of cervical cancer, cervical dysplasia, and other non-malignant HPV-associated diseases such as genital warts, among vaccinated populations [10,12,13,14]. Non-L1 based, and therapeutic vaccines, have not demonstrated sufficient efficacy to justify adoption in routine clinical practice, despite promising research in this area [15].
The incidence of HPV infection rises rapidly after sexual debut [16,17,18]. The majority of hrHPV infections are characterised by indolent transient infection followed by spontaneous clinical clearance (DNA non-detectability), within two years [19,20,21]. Consistent evidence demonstrates that efficacy of L1-based vaccines is greatest when administered in adolescence or to women who are naïve to HPV [6]. In established, DNA detectable infection, vaccination does not reduce progression to cervical dysplasia [6,22,23,24,25], although some moderate-level evidence supports the use of vaccination when administered prior to, or following, ablative surgical procedures [26,27].
Accurate estimates of vaccine efficacy (VE) among seropositive, DNA negative (SPDN) populations is of particular relevance in the context of primary HPV screening modalities which provide HPV DNA status in routine clinical practice [1,2]. HPV-based screening may present physicians with uncertainty as to how to advise individual women with a personal history of HPV infection, whose screening no longer detects HPV DNA. Although prophylactic HPV vaccination is approved on an individual basis up to the age of 45 by the FDA [28], efficacy among such previously exposed, currently uninfected, subjects (defined by seropositivity to type-specific HPV IgG without corresponding DNA positivity, i.e., SPDN), remains incompletely defined.
This paper summarises published data regarding the efficacy of L1-based HPV16/18 vaccination against 6-month persistent infection (6mPI), 12-month persistent infection (12mPI), HPV16/18 associated CIN 1 or worse (CIN1+), and HPV16/18 associated CIN 2 or worse (CIN2+) among populations of SPDN women enrolled to randomised controlled trials of HPV L1-based vaccines. All CIN endpoint determinations included assessment of the causative HPV serotype. Data was collected from published materials, including primary study subgroup analyses, clinical study reports, publication supplements, and from a prior post-trial cohort study. To our knowledge, this paper represents the first meta-analysis of published data for VE among HPV SPDN populations.
METHODS
This study was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA). The PRISMA checklist can be found in the Data S1. The protocol for this systematic review was prospectively registered on PROSPERO (CRD42020206888) and can be found in the Data S1.
1. Search strategy
A search was carried out on MEDLINE (PubMed), Embase, Scopus, Cochrane Central Register of Controlled Trials for studies published between 1 January 2000 and August 2020. The search strategy included medical subject heading, free text words and synonyms covering ‘HPV’, ‘vaccine’ and ‘trials’, and was restricted to studies in English and conducted in humans. The full search strategy is available in the Data S1. References from other relevant systematic reviews [6,29] were hand-searched for additional studies. Duplicate records were removed using the Covidence systematic review manager.
2. Screening and article selection
Two independent authors (RP & CME) reviewed titles and abstracts to identify relevant studies. Full-text manuscripts and relevant study supplements (Data S1) were assessed independently by 2 reviewers (RP and CME) against predefined inclusion criteria: randomised controlled trials of prophylactic HPV vaccination versus placebo/other non-HPV vaccine comparator, reporting efficacy among patients that were seropositive to HPV16 and/or HPV18 but cervical DNA-negative to the corresponding serotype(s) at enrolment.
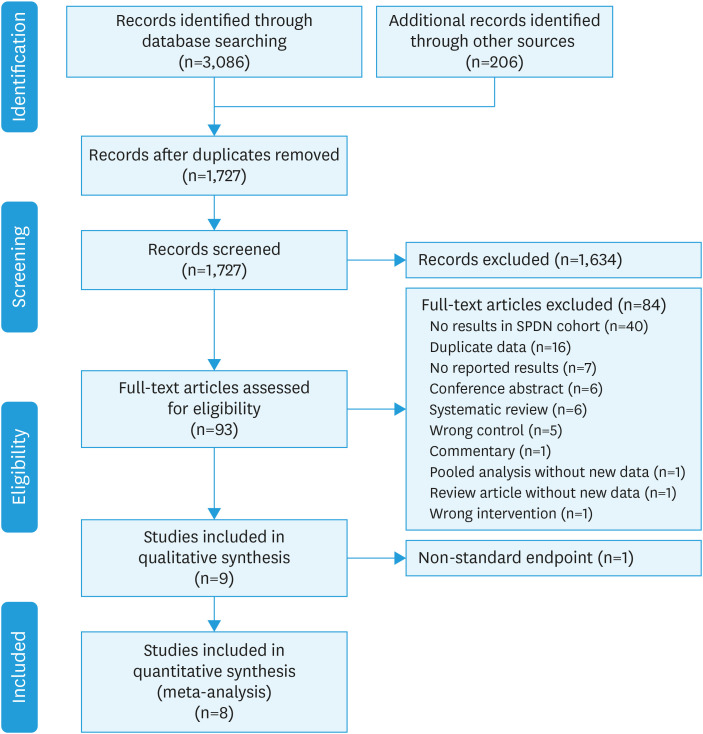
Trials that included either bivalent (HPV16/18, Cervarix®) or quadrivalent (HPV16/18/6/11, Gardasil®) were included. The single large RCT of the nonavalent Gardasil9® vaccine [30] was excluded as it was assessed against a quadrivalent vaccine comparator, precluding assessment of HPV16/18 endpoints. Case reports, case series, observational studies, conference abstracts, commentaries, and editorials were excluded. Trials that reported efficacy only in men and HIV positive cohorts, those with endpoints other than cervical dysplasia, and those that concerned therapeutic HPV vaccination or non-L1 based vaccines were excluded. Any disagreement between reviewers was resolved by discussion until consensus was reached. The study selection procedure is presented using a PRISMA flowchart (Fig. 1). Where efficacy of the seropositive, DNA-negative cohort was not reported in the manuscript or the Data S1, clinical study reports, where available, were reviewed.
Fig. 1. PRISMA flowchart of search strategy.
3. Data extraction
Two authors (RP & CME) independently extracted data. Detailed references to included data are provided in the Data S2 as a spreadsheet file. Conflicts were resolved by discussion, and findings were reported in accordance with PRISMA guidelines. Data were extracted using Microsoft Excel in a standardised proforma under the following headings: Sponsor ID; clinicaltrials.gov trial number; year of publication; study location; study period; inclusion criteria including age range, pap smear at enrolment and number of sexual partners; definition of HPV seropositivity including laboratory technique and titres threshold; definition of HPV DNA negativity; intervention; control; whether total vaccinated cohort or according to protocol analyses were used; total enrolment; total individuals that were seropositive and DNA negative to HPV16/18. The number of events in the intervention and control arm was extracted for endpoints including HPV16/18-associated 6mPI); 12mPI; CIN1+ and CIN2+. HPV-16 and HPV-18 endpoints were independently assessed in all included studies.
4. Assessment of bias
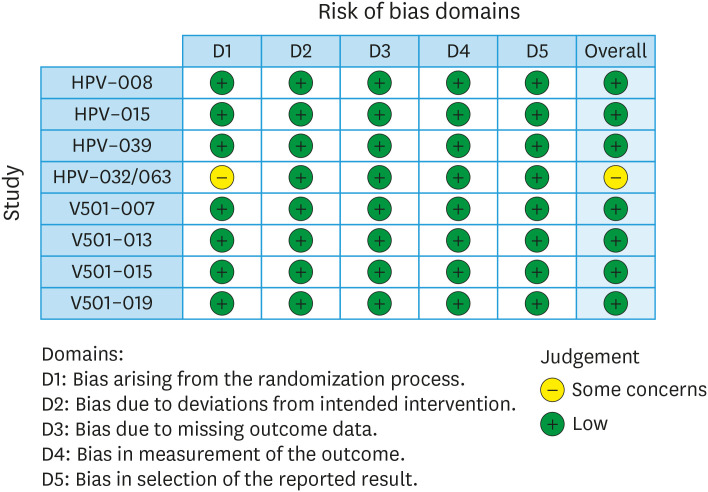
The risk of bias in included RCTs was assessed using Cochrane's Risk of Bias Tool and the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions [31]. This included assessment of the randomisation process, deviations from intended intervention, missing outcome data, measurement of outcome, and selection of the reported result. Results are presented in both a risk of bias chart and a risk of bias summary.
5. Statistical analysis
Relative risk (RR) for 6-moPI, 12-moPI, CIN1+ and CIN2+ in the intervention (SPDN-vaccinated) versus the control arm (SPDN-unvaccinated), were calculated along with 95% confidence intervals (95% CIs). As the true effect was expected to vary between populations, the results were pooled using a random-effect model, employing the Mantel-Haenszel method [32]. VE was estimated using the formula: VE = 1 – RR. The number needed to vaccinate (NNV) was calculated using the formula NNV = 1/Risk Difference [31]. Heterogeneity was assessed both graphically using Forest plots, as well as Cochran’s Q test and the I2 statistic [33]. Prespecified subgroup analyses using the type of vaccine as an independent variable were carried out to explore sources of heterogeneity. Sensitivity analyses were undertaken to explore the effect of studies with a higher risk of bias and the effect of each individual study on the overall pooled estimate. Statistical analysis was carried out using R version 4.0.2 packages meta, metafor, and dmetar.
RESULTS
1. Identified studies
Study design and participants
Our original search identified 3,086 studies. 206 studies were added from the references of two other systematic reviews [6,29]. After removal of duplicates, 1,727 studies remained; 1,634 of these were excluded following title and abstract screening (Fig. 1). Of the 93 remaining articles, 40 did not separately report efficacy in the SPDN subgroup in either the manuscript, Data S1 or publicly available clinical study report. Otherwise, 16 articles represented duplicate data, 7 did not report results, 6 were conference abstracts, 6 were systematic reviews, 5 did not have a placebo control arm, 1 was a commentary, 1 was a pooled analysis with no new data, 1 was a review, and 1 had the wrong intervention. One study had a non-standard endpoint of 4-year point prevalence, and was included for discussion in the narrative synthesis, but not in the meta-analysis [34].
A summary of study characteristics can be found in Table 1. A total of eight studies matched all the eligibility criteria for inclusion in the meta-analysis. Four studies evaluated the bivalent HPV16/18 vaccine (Cervarix®) while four studies evaluated the quadrivalent HPV6/11/16/18 vaccine (Gardasil®). All studies were randomised control trials; six were phase III trials, one was a phase II trial, and one was a phase II/III trial. Most included studies were international, but there was one trial from each of China and Japan. Six trials recruited younger women aged from mid-teens to 23–26. Two trials recruited older women in mid-twenties and above.
Table 1. Included studies.
| Study name | V501–007 | V501–013 | V501–015 | V501–019 | HPV–008 | HPV–009 | HPV–015 | HPV–039 | HPV–032/063 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Trial number | NCT00365716 | NCT00092521 | NCT00092534 | NCT00090220 | NCT00122681 | NCT00128661 | NCT00294047 | NCT00779766 | NCT00316693 | |
| Year | 2000–2004 | 2001–2007 | 2002–2007 | 2004–2009 | 2005–2010 | 2005–2012 | 2006–2012 | 2008–2014 | 2009–2013 | |
| Phase | III | III | III | III | III | III | III | II/III | II | |
| Intervention | Gardasil | Gardasil | Gardasil | Gardasil | Cervarix | Cervarix | Cervarix | Cervarix | Cervarix | |
| Comparator | Placebo | Placebo | Placebo | Placebo | Hep A vaccine | Hep A vaccine | Placebo | Placebo | Hep A vaccine | |
| Country | International | International | International | International | International | Costa Rica | International | China | Japan | |
| Key inclusion criteria | ||||||||||
| Age | 16–23 | 16–23 | 15–26 | 24–45 | 15–25 | 18–25 | ≥26 | 18–25 | 20–25 | |
| Lifetime partners | 0–4 | 0–4 | 0–4 | Any | 0–6 | Any | Any | NR | NR | |
| Endpoints | ||||||||||
| Point prevalence at 4 years | x | |||||||||
| 6-mo persistent infection | x | x | x | x | ||||||
| 12-mo persistent infection | x | x | x | x | ||||||
| CIN1+ | x | x | x | x | x | x | x | |||
| CIN2+ | x | x | x | x | x | x | ||||
| Participants | ||||||||||
| Total vaccine | 9,087 | 2,723 | 6,087 | 1,910 | 9,319 | 3,727 | 2,877 | 3,026 | 375 | |
| Total control | 9,087 | 2,732 | 6,080 | 1,907 | 9,325 | 3,729 | 2,870 | 3,025 | 377 | |
| SPDN vaccine | 1,298 | 377 | 498 | 496 | 1,710 | 986 | 2,870 | 286 | 117 | |
| SPDN control | 1,319 | 379 | 524 | 505 | 1,777 | 980 | 2,908 | 270 | 90 | |
CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; NR, not reported; SPDN, seropositive, DNA negative.
2. Risk of bias
Risk of bias was assessed in all 8 trials (Fig. 2). All trials were randomised, double blinded trials with prespecified outcomes. Randomisation sequence was adequately described in all 8 studies. 7 studies adequately described allocation concealment. One study did not adequately describe allocation concealment, and therefore we had ‘Some Concerns’ about Domain 1 and in the overall assessment. There was one pooled analysis that reported the pooled results of three trials in the SPDN subgroup. This pooled analysis was used for the quantitative synthesis. The overall risk of bias was deemed “Low” in seven trials, and “Some Concerns” in one trial (HPV-032/063).
Fig. 2. Risk of bias plot for studies included in the meta-analysis.
HPV, human papillomavirus.
EFFICACY AMONG SPDN POPULATIONS
1. Risk of CIN
A total of 8 trials, comprising 9,513 SPDN patients (4,732 in the vaccine arm; 4,781 in the placebo arm) reported CIN1+ as an endpoint. One trial (V501–019) with 506 patients in the vaccine arm and 513 patients in the control arm reported no cases of CIN1+, and was therefore not included in the pooled analysis, in line with Cochrane guidelines [31].
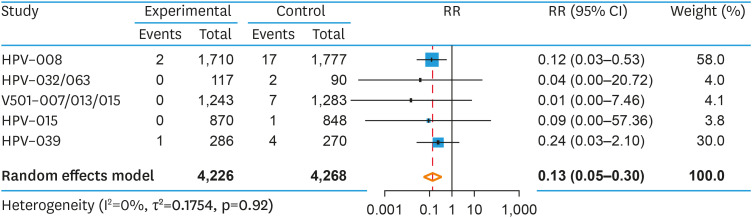
2. CIN1+
The pooled RR of histologically confirmed CIN1+ was 0.13 (95% CI=0.05–0.30; p=0.003), with a corresponding VE of 87% (95% CI=70%–95%; p=0.003) (Fig. 3). Heterogeneity was low (I2=0, p=0.92). A prespecified subgroup analysis found no difference in RR of CIN1+ between the bivalent and quadrivalent HPV vaccine (Q=0.51; p=0.477). The NNV to prevent one case of CIN1+ was 152 (95% CI=107–256). As there were fewer than 10 studies in this pooled analysis, assessing publication bias was not appropriate [31].
Fig. 3. Pooled RR of CIN1+ among HPV seropositive, DNA negative women that were prophylactically vaccinated against HPV16/18 versus placebo.
CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; RR, relative risk.
3. CIN2+
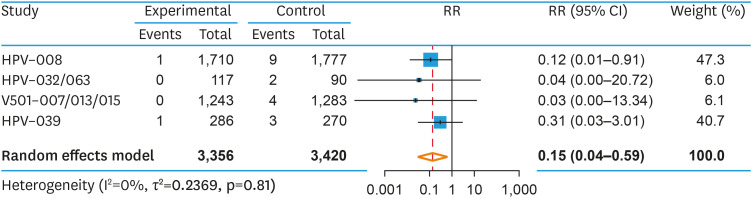
Six studies, comprising 6,776 SPDN patients (3,356 patients in the vaccine arm; 3,420 patients in the placebo arm), reported CIN2+ as an endpoint. The pooled risk of developing CIN2+ was reduced in the vaccinated versus the unvaccinated group (RR=0.15; 95% CI=0.04–0.59; p=0.022), with a corresponding VE of 85% (95% CI=41%–96%, p=0.022) (Fig. 4). Heterogeneity was low (I2=0%; p=0.81). A prespecified subgroup analysis found no difference in RR of CIN2+ between the bivalent and quadrivalent HPV vaccine (Q=0.34, p=0.559). The NNV to prevent one case of CIN2+ was 208 (95% CI=135–476). As above, investigation of publication bias was not appropriate [31].
Fig. 4. Pooled RR of CIN2+ among HPV seropositive, DNA negative women that were prophylactically vaccinated against HPV16/18 versus placebo.
CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; RR, relative risk.
4. Risk of persistent infection
6mPI
Four studies reported serotype specific 6mPI, totalling 6,257 SPDN patients (3,097 in the vaccine arm; 3,160 in the control arm). The pooled risk of 6mPI was reduced in the group that received the vaccine (RR=0.22; 95% CI=0.08–0.61; p=0.018), with a corresponding VE of 78% (95% CI=39%–92%; p=0.018) (Fig. S1).
12mPI
Four studies reported serotype specific 12mPI. This included 2,872 patients that received vaccine and 2,877 that received placebo. The pooled RR of 12mPI in the vaccinated versus the unvaccinated group was 0.20 (95% CI=0.05–0.80; p=0.035), with a corresponding VE of 80% (95% CI=20%–95%; p=0.035) (Fig. S2).
5. Sensitivity analyses
A prespecified sensitivity analysis was conducted, wherein studies with a high or unclear risk of bias were excluded from the pooled estimate. Excluding these studies, the pooled RR of CIN1+ was 0.14 (95% CI=0.05–0.39; p=0.009, Fig. S3) among vaccinated SPDN women. This was not substantially different to the original pooled result. However, pooled RR of CIN2+, although similar to the original analysis, (RR=0.16; 95% CI=0.02–1.32; p=0.065, Fig. S4), was no longer statistically significant.
In another a priori sensitivity analysis, when a named study was omitted, the pooled estimate remained close to the observed overall estimate. This suggests that no individual study had a large influence on the pooled estimate. The plot for the analysis estimates for CIN1+ and CIN2+ is provided in Figs. S5 and S6.
DISCUSSION
This paper represents the first meta-analysis of published data describing L1-based HPV VE among seropositive DNA negative women. We report statistically significant and clinically meaningful efficacy in the prespecified, IARC validated [35] endpoints of (6mPI, 12mPI, CIN1+, and CIN2+). These findings strengthen the case for vaccination of all DNA negative women aged between 15–45, regardless of serostatus, on the basis that DNA negative women must either be seropositive or seronegative, and efficacy among women defined as DNA negative seronegative (such as sexually naive women) has already been conclusively established [6].
Efficacy of L1 vaccination among SPDN women has been described before (Table 2) [6,25,34,36,37,38]. However, most published estimates either lack statistical power, or use non-standard (or composite) endpoints, which have unclear surrogacy for risk of progression to cervical cancer, thus limiting their clinical utility.
Table 2. Published estimates of HPV vaccine efficacy among SPDN cohorts.
| Year | Author | Study | Endpoint | Efficacy | 95% CI | No. (SPDN) |
|---|---|---|---|---|---|---|
| 2009 | Olsson et al. [36] | V501–007/FUTURE I/II | HPV-6/11/16/18 related CIN1+ | VE 100% | 28.7%–100% | 2,526 |
| 2011 | Castellsagué et al. [37] | FUTURE III | HPV6/11/16/18 infection or CIN | VE 66.9% | 4.3%–90.6% | 1,019 |
| 2012 | Szarewski et al. [25] | PATRICIA | 6mPI | VE 72.3% | 53.0%–84.5% | 3,421 |
| PATRICIA | CIN1+ | VE 67.2% | 10.9%–89.9% | 3,487 | ||
| PATRICIA | CIN2+ | VE 68.8% | −28.3%–95.0% | 3,487 | ||
| 2014 | Skinner et al. [38] | VIVIANE | HPV16/18 associated 6mPI/CIN1+ | VE 86.4% | 30.1%–99.0% | NR |
| 2016 | Beachler et al. [34] | Costa Rica Vaccine Study | Cervical HPV point prevalence at 4 years | VE 76.5% | 54.6%–88.8% | 1,384 |
| 2018 | Arbyn et al. [6] | Future II/PATRICIA | CIN2+ | RR 0.19 | 0.09–0.77 | 4,506 |
6mPI, 6-month persistent infection; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; NR, not reported; RR, relative risk; SPDN, seropositive, DNA negative; VE, vaccine efficacy.
Persistent uncertainty regarding the true lifecycle of HPV [39] means that the mechanism of the observed effect remains unclear. Immunosuppression models both in animals [40,41] and humans [42,43,44,45,46,47], as well as epidemiological evidence [48,49,50,51,52,53] support the concept that HPV forms latent infections in basal epithelial stem cells [54,55], and that clinical clearance of HPV infection represents T-cell mediated [39] suppression of the virus to levels below the threshold of detectability: i.e. ‘cryptic, persistent, and reversible infection of cells’ [56], rather than true eradication [54,57,58]. Data regarding immunity following such ‘cleared’ natural infections is inconsistent [50,59,60,61,62], but it is likely that natural infection, perhaps particularly among younger women [63], offers a modest degree of protection against type-specific, clinically apparent or DNA-detectable, reinfection [64].
Conceptually, SPDN women may fall into a number of clinically indistinguishable subcategories, across which vaccine efficacy, risk of new infection, and risk of those infections progressing to cytopathological changes, might differ [58]. Assuming a model of latency following initial infection, individual women may be, variously, immune to clinically detectable reinfection or relapse as a result of a competent immune response to the original infection [64]; at risk of exposural reinfection; or at risk of autologous reinfection without re-exposure [65]. It is conceivable that vaccine efficacy might differ between these clinically indistinguishable groups, or indeed that individual women may move between these groups over time during periods of relative immunosuppression [43,44,46,66], as a consequence of changes in the vaginal microbiome [67,68], or as immune responsiveness to HPV infections naturally wanes [69]. These factors may account to some extent for the incomplete efficacy observed in this group as a whole.
It is also unclear why vaccine efficacy should differ so substantially between SPDN women (and indeed among women who have undergone ablative surgical procedures [26,27]) compared to women with detectable DNA infection, among whom vaccination has been demonstrated to be ineffective [6,22–25], but whose classification as DNA positive would, under the supposition of latency, simply reflect an isolated measurement in an infection whose natural history is characterised by oscillation between periods of active viral shedding and periods of latency/immune control [70,71,72]. One possible answer may be that the immune response observed with L1-based vaccination differs both quantitatively [38], and qualitatively [73], to immune responses occurring as a consequence of natural HPV infection, particularly with respect to the generation of highly-avid neutralising antibodies [73,74]. Such differences may be sufficient to explain the maintenance or prolongation of periods of viral suppression among subjects who have already mounted a competent immune response, or to provide greater protection against HPV infections occurring as a consequence of re-exposure, despite being insufficient to control or reverse active infections.
It should be highlighted that the relative risk and NNV figures presented above should not be understood to represent those of a population-level programme which intended to vaccinate large cohorts of DNA negative women irregardless of serostatus (for example, in the setting of HPV catch-up programs), among whom the efficacy and clinical benefit of vaccination would likely be greater, for a number of reasons which bear mentioning.
Firstly, we do not adjust for the already lower relative risk of infection among our comparator population (SPDN-unvaccinated), relative to the population of all DNA-negative women (reported elsewhere as RR=0.65; 95% CI=0.50–0.80 for HPV-16; RR=0.70; 95% CI=0.43–0.98 for HPV-18 [64]). Secondly, we do not account for the likely composition of any such DNA-negative vaccination cohort, which would comprise not only SPDN women, but also DNA-negative, seronegative women, among whom NNV is substantially lower [6]. Finally, women may also derive benefit from vaccination against vaccine strains with which they have never been infected [75,76], and through cross-protection against non-vaccine serotypes [77,78,79], although the clinical significance of these benefits are uncertain.
There is ongoing debate [80,81,82,83] regarding the cost-effectiveness of population-level HPV catch-up programmes up to age 45, with most models [81,82,83], excluding those supported by industry [80], concluding that the expansion of population-level vaccination programs to include older women represents inefficient healthcare expenditure and poor value for money. Our findings identify a discrete and increasingly identifiable cohort which may differentially benefit from such programs. Although detailed pharmacoeconomic analysis is beyond the scope of this paper, it is reasonable to expect that approaches which sought to enrich such catchup programs with HrDNA-negative (i.e., “high-efficacy”) cohorts, would result in greater cost-effectiveness through the exclusion of women in whom vaccination has been demonstrated to be ineffective [6,22,23,24,25].
1. Relevant excluded/missing studies
A number of studies which examined efficacy among SPDN cohorts could not be included in this metaanalysis. Six potentially relevant studies conducted among women were identified for which no published SPDN data regarding cervical 6mPI, 12mPI, CIN1+ or CIN2+ could be identified: HPV-001 [84], including followup cohorts [85,86], HPV-009 (discussed above), V501-005 [87], V501-027 [88], V501-041 [89], and NCT02296255 [90]. Data describing persistent infection and/or cytopathological endpoints was available for 76.6% of the identified eligible population across all identified studies (n=51,767/n=67,614). Furthermore, we did not identify any studies or data which failed to corroborate a finding of efficacy among SPDN populations.
2. Limitations
This analysis has a number of important limitations.
It is known that HPV infection does not always induce a measurable immune response, and that antibody detectability wanes over time [20,37,91,92,93]. Negative serological testing does not therefore universally exclude prior infection; this, along with false negative DNA results, and false positive serology results, potentially resulted in the misclassification of some subjects as SPDN in this analysis. This limitation is mitigated by the fact that patients misclassified this way (i.e. classified as Sero-/DNA-, despite being truly Sero+/DNA-), would have been included in the primary ATP analyses of naïve cohorts, among whom efficacy is also well established.
HPV is known to cause infection at anatomical sites not routinely assessed by many of the included studies [4]. The contribution of primary extragenital infections to the prevalence of enrolment seropositivity among study participants could not be determined by this analysis. Similarly, the possibility of protection at extragenital sites afforded by vaccination among SPDN women could not be assessed using available data.
This study analysed vaccine efficacy against HPV16 and HPV18 independently, but did not assess other strains of hrHPV covered by the nonavalent vaccine in widespread clinical use. The single large efficacy RCT of nonavalent vaccine was conducted against a comparator quadrivalent vaccine rather than placebo, which precluded analysis of HPV16/18 outcomes from this trial [30].
Data in this analysis is extracted from RCTs predominantly enrolling women aged between 15–26. Prevalence of SPDN status increases with age [25], and the risk of acquiring new infections (either through reexposure or through relapse of latent infection), as well as the likelihood of such infections progressing to CIN may differ from those among younger women [94,95,96]. Similarly, the immunogenicity of L1-based vaccines among older women, particularly against HPV18, may differ from that seen among younger women [97].
The above limitations may have implications for pharmacoeconomic modelling of the cost-effectiveness of widespread vaccination among SPDN cohorts. Our findings should not be understood to justify population-level vaccination of DNA negative women in the absence of such modelling, which is beyond the scope of this paper.
CONCLUSION
To our knowledge, this paper represents the first systematic review of published data describing vaccine efficacy among seropositive DNA negative women to date (Appendix 1). Notwithstanding the limitations outlined above, we provide strong evidence of the serotype-specific efficacy of HPV L1-based vaccination against validated endpoints of cervical cancer risk, among women previously infected with HPV16/18, including robust efficacy against cervical dysplasia (CIN1+) of 87% (95% CI=70%–95%; p=0.003). These findings are of increasing clinical relevance in the context of a transition towards HPV-based cervical screening [1,2,98,99], and the consequent availability of such results in routine clinical practice. On an individual level, these results may be of use in the context of shared decision making and individualised risk assessment discussions with HPV16/18 DNA negative women aged up to 45, in accordance with ACIP guidelines[100].
Appendix 1
Declarations

The authors would like to thank Prof. Maeve Lowery, St James’s Hospital Dublin, for her mentorship with this project.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: C.M.E.
- Data curation: C.M.E., R.P.
- Formal analysis: C.M.E., R.P., I.P.
- Investigation: C.M.E., R.P.
- Methodology: C.M.E., R.P.
- Project administration: C.M.E., R.P., D.B.
- Resources: C.M.E.
- Supervision: C.M.E., I.P., D.B.
- Validation: C.M.E., I.P., D.B.
- Visualization: C.M.E., R.P.
- Writing - original draft: C.M.E., R.P.
- Writing - review & editing: C.M.E., D.B.
SUPPLEMENTARY MATERIALS
Supplementary data
Dataset
Pooled RR of 6-month persistent infection among HPV seropositive, DNA negative women that were prophylactically vaccinated against HPV16/18 versus placebo.
Pooled RR of 12-month persistent infection among HPV seropositive, DNA negative women that were prophylactically vaccinated against HPV16/18 versus placebo.
Bias assessment, CIN 1+ Endpoint.
Bias assessment, CIN 2+ Endpoint.
Analysis Estimate: CIN1+ Endpoint.
Analysis Estimate: CIN2+ Endpoint.
References
- 1.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, et al. Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69:184–210. doi: 10.3322/caac.21557. [DOI] [PubMed] [Google Scholar]
- 2.von Karsa L, Arbyn M, De Vuyst H, Dillner J, Dillner L, Franceschi S, et al. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res. 2015;1:22–31. [Google Scholar]
- 3.Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 5.Harper DM, DeMars LR. HPV vaccines - a review of the first decade. Gynecol Oncol. 2017;146:196–204. doi: 10.1016/j.ygyno.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavanagh K, Pollock KG, Cuschieri K, Palmer T, Cameron RL, Watt C, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis. 2017;17:1293–1302. doi: 10.1016/S1473-3099(17)30468-1. [DOI] [PubMed] [Google Scholar]
- 8.Palmer T, Wallace L, Pollock KG, Cuschieri K, Robertson C, Kavanagh K, et al. Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12–13 in Scotland: retrospective population study. BMJ. 2019;365:l1161. doi: 10.1136/bmj.l1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabrizi SN, Brotherton JML, Kaldor JM, Skinner SR, Liu B, Bateson D, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14:958–966. doi: 10.1016/S1473-3099(14)70841-2. [DOI] [PubMed] [Google Scholar]
- 10.Dehlendorff C, Baandrup L, Kjaer SK. Real-world effectiveness of Human Papillomavirus vaccination against vulvovaginal high-grade precancerous lesions and cancers. J Natl Cancer Inst. 2021;113:869–874. doi: 10.1093/jnci/djaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Major milestone reached as 100 countries have introduced HPV vaccine into national schedule [Internet] Geneva: World Health Organization; c2019. [cited 2021 Jan 13]. Available from: https://www.who.int/news/item/31-10-2019-major-milestone-reached-as-100-countries-have-introduced-hpv-vaccine-into-national-schedule. [Google Scholar]
- 12.Luostarinen T, Apter D, Dillner J, Eriksson T, Harjula K, Natunen K, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142:2186–2187. doi: 10.1002/ijc.31231. [DOI] [PubMed] [Google Scholar]
- 13.Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young U.S. females after human papillomavirus vaccine introduction. Am J Prev Med. 2018;55:197–204. doi: 10.1016/j.amepre.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith LM, Strumpf EC, Kaufman JS, Lofters A, Schwandt M, Lévesque LE. The early benefits of human papillomavirus vaccination on cervical dysplasia and anogenital warts. Pediatrics. 2015;135:e1131–e1140. doi: 10.1542/peds.2014-2961. [DOI] [PubMed] [Google Scholar]
- 15.Hancock G, Hellner K, Dorrell L. Therapeutic HPV vaccines. Best Pract Res Clin Obstet Gynaecol. 2018;47:59–72. doi: 10.1016/j.bpobgyn.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Kjaer SK, Chackerian B, van den Brule AJ, Svare EI, Paull G, Walbomers JM, et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse) Cancer Epidemiol Biomarkers Prev. 2001;10:101–106. [PubMed] [Google Scholar]
- 17.Winer RL, Feng Q, Hughes JP, O’Reilly S, Kiviat NB, Koutsky LA. Risk of female human papillomavirus acquisition associated with first male sex partner. J Infect Dis. 2008;197:279–282. doi: 10.1086/524875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellsagué X, Paavonen J, Jaisamrarn U, Wheeler CM, Skinner SR, Lehtinen M, et al. Risk of first cervical HPV infection and pre-cancerous lesions after onset of sexual activity: analysis of women in the control arm of the randomized, controlled PATRICIA trial. BMC Infect Dis. 2014;14:551. doi: 10.1186/s12879-014-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, et al. Human papillomavirus infection is transient in young women: a population-based cohort study. J Infect Dis. 1995;171:1026–1030. doi: 10.1093/infdis/171.4.1026. [DOI] [PubMed] [Google Scholar]
- 20.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–S22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 23.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 24.Haupt RM, Wheeler CM, Brown DR, Garland SM, Ferris DG, Paavonen JA, et al. Impact of an HPV6/11/16/18 L1 virus-like particle vaccine on progression to cervical intraepithelial neoplasia in seropositive women with HPV16/18 infection. Int J Cancer. 2011;129:2632–2642. doi: 10.1002/ijc.25940. [DOI] [PubMed] [Google Scholar]
- 25.Szarewski A, Poppe WA, Skinner SR, Wheeler CM, Paavonen J, Naud P, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer. 2012;131:106–116. doi: 10.1002/ijc.26362. [DOI] [PubMed] [Google Scholar]
- 26.Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344:e1401. doi: 10.1136/bmj.e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartels HC, Postle J, Rogers AC, Brennan D. Prophylactic human papillomavirus vaccination to prevent recurrence of cervical intraepithelial neoplasia: a meta-analysis. Int J Gynecol Cancer. 2020;30:777–782. doi: 10.1136/ijgc-2020-001197. [DOI] [PubMed] [Google Scholar]
- 28.Center for Biologics Evaluation and Research. Gardasil 9 [Internet] Silver Spring, MD: U.S. Food and Drug Administration; c2020. [cited 2021 Jun 1]. Available from: https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9. [Google Scholar]
- 29.Jørgensen L, Gøtzsche PC, Jefferson T. Index of the human papillomavirus (HPV) vaccine industry clinical study programmes and non-industry funded studies: a necessary basis to address reporting bias in a systematic review. Syst Rev. 2018;7:8. doi: 10.1186/s13643-018-0675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 32.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 33.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 34.Beachler DC, Kreimer AR, Schiffman M, Herrero R, Wacholder S, Rodriguez AC, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV infection. J Natl Cancer Inst. 2015;108:djv302. doi: 10.1093/jnci/djv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IARC HPV Working Group. Primary end-points for prophylactic HPV vaccine trials. Lyon: International Agency for Research on Cancer; 2015. [PubMed] [Google Scholar]
- 36.Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5:696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 37.Castellsagué X, Muñoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011;105:28–37. doi: 10.1038/bjc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skinner SR, Szarewski A, Romanowski B, Garland SM, Lazcano-Ponce E, Salmerón J, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet. 2014;384:2213–2227. doi: 10.1016/S0140-6736(14)60920-X. [DOI] [PubMed] [Google Scholar]
- 39.Gravitt PE. Evidence and impact of human papillomavirus latency. Open Virol J. 2012;6:198–203. doi: 10.2174/1874357901206010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amella CA, Lofgren LA, Ronn AM, Nouri M, Shikowitz MJ, Steinberg BM. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency. Am J Pathol. 1994;144:1167–1171. [PMC free article] [PubMed] [Google Scholar]
- 41.Maglennon GA, McIntosh PB, Doorbar J. Immunosuppression facilitates the reactivation of latent papillomavirus infections. J Virol. 2014;88:710–716. doi: 10.1128/JVI.02589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozsaran AA, Ateş T, Dikmen Y, Zeytinoglu A, Terek C, Erhan Y, et al. Evaluation of the risk of cervical intraepithelial neoplasia and human papilloma virus infection in renal transplant patients receiving immunosuppressive therapy. Eur J Gynaecol Oncol. 1999;20:127–130. [PubMed] [Google Scholar]
- 43.Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 44.Paternoster DM, Cester M, Resente C, Pascoli I, Nanhorngue K, Marchini F, et al. Human papilloma virus infection and cervical intraepithelial neoplasia in transplanted patients. Transplant Proc. 2008;40:1877–1880. doi: 10.1016/j.transproceed.2008.05.074. [DOI] [PubMed] [Google Scholar]
- 45.Nowak RG, Gravitt PE, Morrison CS, Gange SJ, Kwok C, Oliver AE, et al. Increases in human papillomavirus detection during early HIV infection among women in Zimbabwe. J Infect Dis. 2011;203:1182–1191. doi: 10.1093/infdis/jiq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinten F, Hilbrands LB, Meeuwis KA, IntHout J, Quint WG, Hoitsma AJ, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563–1573. doi: 10.1111/ajt.14181. [DOI] [PubMed] [Google Scholar]
- 47.Kelly H, Weiss HA, Benavente Y, de Sanjose S, Mayaud P, Qiao Y, et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV. 2018;5:e45–e58. doi: 10.1016/S2352-3018(17)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xi LF, Demers GW, Koutsky LA, Kiviat NB, Kuypers J, Watts DH, et al. Analysis of human papillomavirus type 16 variants indicates establishment of persistent infection. J Infect Dis. 1995;172:747–755. doi: 10.1093/infdis/172.3.747. [DOI] [PubMed] [Google Scholar]
- 49.Xi LF, Koutsky LA, Castle PE, Edelstein ZR, Hulbert A, Schiffman M, et al. Human papillomavirus type 16 variants in paired enrollment and follow-up cervical samples: implications for a proper understanding of type-specific persistent infections. J Infect Dis. 2010;202:1667–1670. doi: 10.1086/657083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trottier H, Ferreira S, Thomann P, Costa MC, Sobrinho JS, Prado JC, et al. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70:8569–8577. doi: 10.1158/0008-5472.CAN-10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Low risk of type-specific carcinogenic HPV re-appearance with subsequent cervical intraepithelial neoplasia grade 2/3. Int J Cancer. 2012;131:1874–1881. doi: 10.1002/ijc.27418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rositch AF, Burke AE, Viscidi RP, Silver MI, Chang K, Gravitt PE. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: acquisition and reactivation in older women. Cancer Res. 2012;72:6183–6190. doi: 10.1158/0008-5472.CAN-12-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul P, Hammer A, Rositch AF, Burke AE, Viscidi RP, Silver MI, et al. Rates of new human papillomavirus detection and loss of detection in middle-aged women by recent and past sexual behavior. J J Infect Dis. 2021;223:1423–1432. doi: 10.1093/infdis/jiaa557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414:153–163. doi: 10.1016/j.virol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 56.Speck SH, Ganem D. Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe. 2010;8:100–115. doi: 10.1016/j.chom.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Broker TR, Jin G, Croom-Rivers A, Bragg SM, Richardson M, Chow LT, et al. Viral latency--the papillomavirus model. Dev Biol. 2001;106:443–451. [PubMed] [Google Scholar]
- 58.Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121:4593–4599. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102:1653–1662. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malik ZA, Hailpern SM, Burk RD. Persistent antibodies to HPV virus-like particles following natural infection are protective against subsequent cervicovaginal infection with related and unrelated HPV. Viral Immunol. 2009;22:445–449. doi: 10.1089/vim.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castellsagué X, Naud P, Chow SN, Wheeler CM, Germar MJ, Lehtinen M, et al. Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired human papillomavirus type 16/18 antibodies: analysis of the control arm of PATRICIA. J Infect Dis. 2014;210:517–534. doi: 10.1093/infdis/jiu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosillon D, Baril L, Del Rosario-Raymundo MR, Wheeler CM, Skinner SR, Garland SM, et al. Risk of newly detected infections and cervical abnormalities in adult women seropositive or seronegative for naturally acquired HPV-16/18 antibodies. Cancer Med. 2019;8:4938–4953. doi: 10.1002/cam4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Velicer C, Zhu X, Vuocolo S, Liaw KL, Saah A. Prevalence and incidence of HPV genital infection in women. Sex Transm Dis. 2009;36:696–703. doi: 10.1097/OLQ.0b013e3181ad25ff. [DOI] [PubMed] [Google Scholar]
- 64.Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N. Natural acquired immunity against subsequent genital human papillomavirus infection: a systematic review and meta-analysis. J Infect Dis. 2016;213:1444–1454. doi: 10.1093/infdis/jiv753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson L, Pawlita M, Castle PE, Waterboer T, Sahasrabuddhe V, Gravitt PE, et al. Seroprevalence of 8 oncogenic human papillomavirus genotypes and acquired immunity against reinfection. J Infect Dis. 2014;210:448–455. doi: 10.1093/infdis/jiu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moscicki AB, Flowers L, Huchko MJ, Long ME, MacLaughlin KL, Murphy J, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Genit Tract Dis. 2019;23:87–101. doi: 10.1097/LGT.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 67.Gillet E, Meys JF, Verstraelen H, Bosire C, De Sutter P, Temmerman M, et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis. BMC Infect Dis. 2011;11:10. doi: 10.1186/1471-2334-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis. 2014;210:1723–1733. doi: 10.1093/infdis/jiu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.García-Piñeres AJ, Hildesheim A, Herrero R, Trivett M, Williams M, Atmetlla I, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006;66:11070–11076. doi: 10.1158/0008-5472.CAN-06-2034. [DOI] [PubMed] [Google Scholar]
- 70.Wheeler CM, Greer CE, Becker TM, Hunt WC, Anderson SM, Manos MM. Short-term fluctuations in the detection of cervical human papillomavirus DNA. Obstet Gynecol. 1996;88:261–268. doi: 10.1016/0029-7844(96)00120-2. [DOI] [PubMed] [Google Scholar]
- 71.Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182–192. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu SH, Cummings DAT, Zenilman JM, Gravitt PE, Brotman RM. Characterizing the temporal dynamics of human papillomavirus DNA detectability using short-interval sampling. Cancer Epidemiol Biomarkers Prev. 2014;23:200–208. doi: 10.1158/1055-9965.EPI-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scherer EM, Smith RA, Gallego DF, Carter JJ, Wipf GC, Hoyos M, et al. A single human papillomavirus vaccine dose improves B cell memory in previously infected subjects. EBioMedicine. 2016;10:55–64. doi: 10.1016/j.ebiom.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schiller JT. The potential benefits of HPV vaccination in previously infected women. EBioMedicine. 2016;10:5–6. doi: 10.1016/j.ebiom.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castellsagué X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol Oncol. 2009;115:S15–S23. doi: 10.1016/j.ygyno.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 76.Bosch FX, Robles C, Díaz M, Arbyn M, Baussano I, Clavel C, et al. HPV-FASTER: broadening the scope for prevention of HPV-related cancer. Nat Rev Clin Oncol. 2016;13:119–132. doi: 10.1038/nrclinonc.2015.146. [DOI] [PubMed] [Google Scholar]
- 77.Herrero R. Human papillomavirus (HPV) vaccines: limited cross-protection against additional HPV types. J Infect Dis. 2009;199:919–922. doi: 10.1086/597308. [DOI] [PubMed] [Google Scholar]
- 78.Malagón T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–789. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 79.Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016;16:1154–1168. doi: 10.1016/S1473-3099(16)30120-7. [DOI] [PubMed] [Google Scholar]
- 80.Daniels V, Prabhu VS, Palmer C, Samant S, Kothari S, Roberts C, et al. Public health impact and cost-effectiveness of catch-up 9-valent HPV vaccination of individuals through age 45 years in the United States. Hum Vaccin Immunother. 2021;17:1943–1951. doi: 10.1080/21645515.2020.1852870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JJ, Simms KT, Killen J, Smith MA, Burger EA, Sy S, et al. Human papillomavirus vaccination for adults aged 30 to 45 years in the United States: a cost-effectiveness analysis. PLoS Med. 2021;18:e1003534. doi: 10.1371/journal.pmed.1003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laprise JF, Chesson HW, Markowitz LE, Drolet M, Martin D, Bénard É, et al. Effectiveness and cost-effectiveness of human papillomavirus vaccination through age 45 years in the United States. Ann Intern Med. 2020;172:22–29. doi: 10.7326/M19-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chesson HW, Meites E, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of HPV vaccination for adults through age 45 years in the United States: Estimates from a simplified transmission model. Vaccine. 2020;38:8032–8039. doi: 10.1016/j.vaccine.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Efficacy study of HPV-16/18 vaccine (GSK 580299) to prevent HPV-16 and/or -18 cervical infection in young healthy women [Internet] Bethesda, MD: National Library of Medicine; c2016. [cited 2021 Apr 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT00689741. [Google Scholar]
- 85.Follow-up study of GSK biologicals’ human papilloma virus (HPV) vaccine to prevent cervical infection in young adults [Internet] Bethesda, MD: National Library of Medicine; c2016. [cited 2021 Apr 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT00120848?term=NCT00120848&draw=2&rank=1. [Google Scholar]
- 86.Follow-up study to evaluate the long-term efficacy of the HPV vaccine (580299) in healthy young adult women in Brazil [Internet] Bethesda, MD: National Library of Medicine; c2016. [cited 2021 Apr 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT00518336?term=NCT00518336&draw=2&rank=1. [Google Scholar]
- 87.Study of human papillomavirus (HPV) 16 vaccine in the prevention of HPV 16 infection in 16- to 23-year-old females (V501–005) [Internet] Bethesda, MD: National Library of Medicine; c2015. [cited 2021 Apr 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT00365378?term=NCT00365378&draw=2&rank=1. [Google Scholar]
- 88.V501 efficacy study in women aged 18 to 26 (V501–027) [Internet] Bethesda, MD: National Library of Medicine; c2017. [cited 2021 Apr 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT00378560?term=NCT00378560&draw=2&rank=1. [Google Scholar]
- 89.Prevention of human papillomavirus (HPV) in 20 to 45 year old Chinese women (V501–041) [Internet] Bethesda, MD: National Library of Medicine; c2019. [cited 2021 Apr 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT00834106?term=NCT00834106&draw=2&rank=1. [Google Scholar]
- 90.Effect of HPV vaccination on women aged 25 years [Internet] Bethesda, MD: National Library of Medicine; c2014. [cited 2021 Apr 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT02296255?term=NCT02296255&draw=2&rank=1. [Google Scholar]
- 91.Carter JJ, Koutsky LA, Wipf GC, Christensen ND, Lee SK, Kuypers J, et al. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis. 1996;174:927–936. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- 92.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 93.Ho GYF, Studentsov YY, Bierman R, Burk RD. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev. 2004;13:110–116. doi: 10.1158/1055-9965.epi-03-0191. [DOI] [PubMed] [Google Scholar]
- 94.Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–1816. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 95.Kjaer S, Høgdall E, Frederiksen K, Munk C, van den Brule A, Svare E, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630–10636. doi: 10.1158/0008-5472.CAN-06-1057. [DOI] [PubMed] [Google Scholar]
- 96.Rodríguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102:315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwarz TF, Galaj A, Spaczynski M, Wysocki J, Kaufmann AM, Poncelet S, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723–2731. doi: 10.1002/cam4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fontham ETH, Wolf AMD, Church TR, Etzioni R, Flowers CR, Herzig A, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321–346. doi: 10.3322/caac.21628. [DOI] [PubMed] [Google Scholar]
- 99.Public Health England. Cervical screening: programme overview [Internet] London: Public Health England; c2015. [cited 2021 Apr 6]. Available from: https://www.gov.uk/guidance/cervical-screening-programme-overview. [Google Scholar]
- 100.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68:698–702. doi: 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Dataset
Pooled RR of 6-month persistent infection among HPV seropositive, DNA negative women that were prophylactically vaccinated against HPV16/18 versus placebo.
Pooled RR of 12-month persistent infection among HPV seropositive, DNA negative women that were prophylactically vaccinated against HPV16/18 versus placebo.
Bias assessment, CIN 1+ Endpoint.
Bias assessment, CIN 2+ Endpoint.
Analysis Estimate: CIN1+ Endpoint.
Analysis Estimate: CIN2+ Endpoint.