Abstract
The use of PARP inhibitors (PARPi) in patients with epithelial ovarian cancer is expanding, with the transition from use in recurrent disease to the first-line setting. This is accompanied with an increasing population of patients who develop acquired PARPi resistance. Coupled with those patients with primary PARPi resistance, there is an urgent need to better understand mechanisms of resistance and identify means to overcome this resistance. Combination therapy offers the potential to overcome innate and acquired resistance, by either working synergistically with PARPi or by restoring homologous recombination deficiency, targeting the homologous recombination repair pathway through an alternate strategy. We discuss mechanisms of PARPi resistance and data on novel combinations which may restore PARPi sensitivity.
Keywords: Poly(ADP-ribose) Polymerase Inhibitors; Drug Resistance, Neoplasm; Carcinoma, Ovarian Epithelial; Drug Therapy, Combination
INTRODUCTION
The introduction of PARP inhibitors (PARPi) has transformed the management of patients with high-grade serous/endometroid (HGS) ovarian, primary peritoneal or fallopian-tube cancer (subsequently referred to as high-grade serous/endometroid ovarian cancer [HGSC]), in both relapsed and first-line settings (Table 1) [1,2,3,4,5,6,7,8]. Initially, approved for the maintenance treatment of recurrent platinum sensitive BRCA1 or BRCA2 (BRCA1/2) mutant epithelial ovarian cancer (EOC), subsequent data demonstrated a benefit beyond those with a BRCA1/2 mutation. The key to this sensitivity is believed to be homologous recombination deficiency (HRD), which is present in up to 50% of all HGSC [9,10]. Most commonly, this is characterized by the lack of a functional copy of either BRCA1 or BRCA2. However, BRCA1/2 genes can be inactivated by non-mutational process and there are other proteins involved in homologous recombination repair (HRR), whose loss can also confer an HRD phenotype [11]. HRD cancers exhibit genomic instability manifested by abnormal copy-number profiles and thousands of somatic mutations. Whilst many diverse HRD assays have been proposed, the only HRD assays that have been validated in clinical trials to date are based on next-generation sequencing of DNA from tumor tissue. These assays detect genomic ‘scars’ and measure levels of loss of heterozygosity, telomeric allelic imbalance and large scale transition [11].
Table 1. Pivotal trials leading to PARPi approval.
| Trial name (NCT number) | Patient population | PARPi | Number | Primary outcomes | PFS PARPi vs. placebo (mo) | HR (95% CI) | |
|---|---|---|---|---|---|---|---|
| First-line maintenance therapy | |||||||
| SOLO1 (NCT01844986) [1] | BRCA1/2mut | Olaparib | 391 | PFS in ITT population | BRCA1/2mut: NR vs. 13.8 | 0.30 (0.23–0.41) | |
| PAOLA-1 (NCT02477644) [2] | All comers | Olaparib plus bevacizumab | 806 | PFS in ITT population | All patients: 22.1 vs. 16.6 | 0.59 (0.49–0.72) | |
| HRD: 37.2 vs. 17.7 | 0.33 (0.25–0.45) | ||||||
| PRIMA (NCT02655016) [3] | All comers | Niraparib | 733 | PFS in ITT and HRD | All patients: 13.8 vs. 8.2 | 0.62 (0.50–0.76) | |
| HRD: 21.9 vs. 10.4 | 0.43 (0.31–0.59) | ||||||
| VELIA (NCT0247058) [4] | All comers | Veliparib in combination with chemotherapy and as maintenance therapy | 1,140 | PFS in veliparib throughout group vs. control group in ITT, BRCA1/2mut and HRD | All patients: 23.5 vs. 17.3 | 0.68 (0.56–0.83) | |
| BRCA1/2mut: 34.7 vs. 22 | 0.44 (0.28–0.68) | ||||||
| HRD: 31.9 vs. 20.5 | 0.57 (0.43–0.76) | ||||||
| Maintenance therapy in platinum sensitive recurrent HGOC | |||||||
| Study19 (NCT00753545) [5] | All comers | Olaparib | 265 | PFS in ITT and BRCA1/2 status | All patients: 10.8 vs. 5.4 | 0.35 (0.25–0.49) | |
| BRCA1/2mut: 11.2 vs. 4.3 | 0.18 (0.34–0.85) | ||||||
| BRCA1/2wt 7.4 vs. 5.5 | 0.54 (0.34–0.85) | ||||||
| NOVA (NCT01847274) [6] | All comers | Niraparib | 553 | PFS according to BRCA1/2 and HRD status | gBRCA1/2mut: 21 vs. 5.5 | 0.27 (0.17–0.41) | |
| gBRCA1/2wt: 9.3 vs. 3.9 | 0.45 (0.34–0.61) | ||||||
| HRD & BRCA1/2wt: 12.9 vs. 3.8 | 0.38 (0.24–0.59) | ||||||
| SOLO2 (NCT01874353) [7] | BRCA1/2mut | Olaparib | 295 | PFS | BRCA1/2mut: 19.1 vs. 5.5 | 0.33 (0.24–0.44) | |
| ARIEL3 (NCT01968213) [8] | All comers | Rucaparib | 564 | PFS in ITT, HRD and BRCA1/2mut group | All patients: 10.8 vs. 5.4 | 0.36 (0.30–0.45) | |
| BRCA1/2mut: 16.6 vs. 5.4 | 0.23 (0.16–0.34) | ||||||
| HRD: 13.6 vs. 5.4 | 0.32 (0.24–0.42) | ||||||
CI, confidence interval; gBRCA1/2, germline BRCA1/2; HR, hazard ratio; HRD, homologous recombination deficient; ITT, intention to treat; mut, mutant; NR, not reached; PARPi, PARP inhibitor; PFS, progression free survival; wt, wild-type.
Many of the pivotal PARPi studies incorporated HRD testing, albeit often performed retrospectively, and a common theme emerges with an incremental reduction in benefit observed, from BRCA1/2 mutant tumors to BRCA1/2 wild-type/HRD to homologous recombination proficient (HRP) tumors [11]. Indeed, the most recent PARPi approval for olaparib in combination with bevacizumab was only granted for HRD cancers (defined by the presence of a BRCA1/2 mutation and/or a high genomic instability score; GIS) [12,13]. This is based on results from the results of the PAOLA1 trial where no benefit was observed from the addition of olaparib to bevacizumab in HRP tumors, as defined by a low GIS score [2,12,13]. In addition to tumors classified as HRP, not all patients with BRCA1/2 mutant or HRD tumors respond to treatment and in most patients, resistance eventually develops. There is a need to overcome both innate and acquired PARPi resistance to optimize clinical benefit from these drugs. Combination strategies is one means to overcome resistance. This review will discuss mechanism of PARPi resistance and data on the most promising combination strategies.
PARPi RESISTANCE
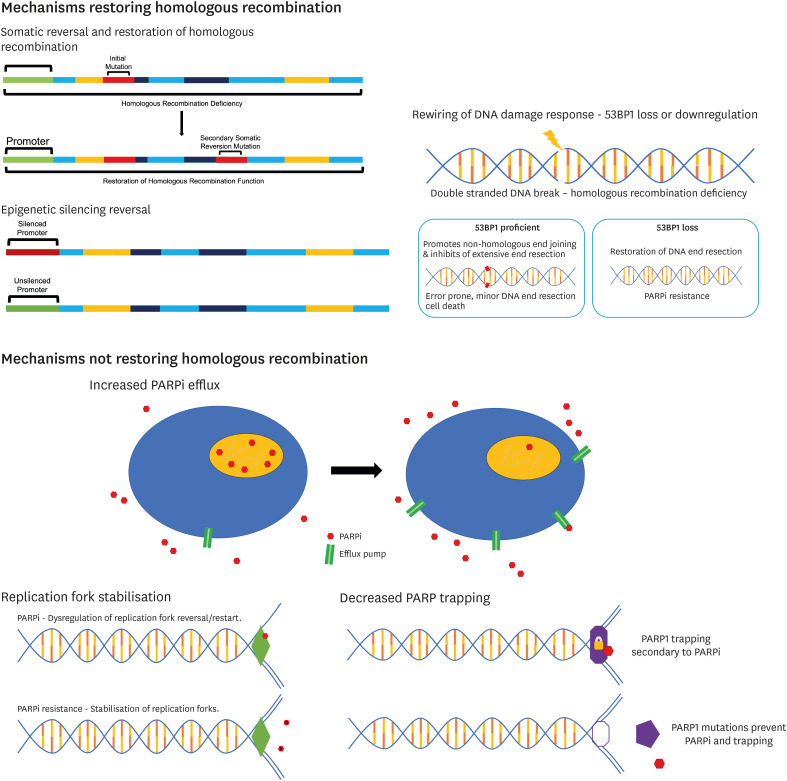
The use of PARPi in patients with EOC is increasing, particularly as approvals move into the first line setting. Despite unprecedented benefit observed in some groups, not all patients benefit, and treatment failure is common due to either de novo or acquired resistance. As the population of PARPi resistant patients increases there is an urgent need to better understand and clinically validate proposed acquired resistance mechanisms. This will determine the most rational PARPi combinations and post progression strategies. These strategies may also be applicable to HRP cancers, where only minimal benefit is seen with single agent PARPi. Numerous mechanisms of acquired resistance to PARPi have been described and these can broadly be separated into 2 main groups. The first involves changes that restore HRR, either through re-expression of a gene that was mutationally or epigenetically silenced, or through rewiring of the DNA damage response. The second group of resistance mechanisms do not result in restoration of HRR and includes processes such as reduction in PARP trapping, [14,15] replication fork protection [16,17] and increased drug efflux (Fig. 1) [18].
Fig. 1. Mechanisms of PARPi resistance.
PARPi, PARP inhibitor.
RESTORATION OF HRR FUNCTION
1. Reversion mutations
A common mechanism of resistance to PARPi is the restoration of at least partial homologous recombination (HR) capabilities. This is through either restoration of a HR protein or alteration of an inhibitory process facilitating double stranded DNA (dsDNA) repair breaks, decreasing genomic instability and replication stress. Perhaps the best described example is restoration of HR function through somatic reversion mutations. Here, secondary mutations restore the open reading frame of HR repair genes (BRCA1/2, PALB2, RAD51C/D) in tumors with frameshift or nonsense mutations, resulting in a functional HR protein [19,20,21,22]. Following PARPi treatment, multiple parallel reversion mutations may develop, representing multiple subclones and may promote clinically heterogenous intra-patient progression of disease [20,21,22,23,24]. The presence of HR reversion mutations in either pre-treatment cell-free DNA (cfDNA) or tumor biopsies is associated with reduced response to PARPi in clinical studies [25,26]. These reversion mutations are identified in only a small subset (up to 25%) of patients with PARPi resistance [27]. Prospective studies which incorporate liquid or tumor biopsy sampling before and during treatment, and at the time of disease progression, are needed to better understand the prevalence and influence of these reversion mutations in the context of PARPi resistance.
2. HR gene promotor alterations
While epigenetic silencing of BRCA1 and RAD51C by promoter region hypermethylation results in sensitivity to PARPi, demethylation is associated with mRNA re-expression and development of resistance [28,29,30]. For example, demethylation of BRCA1 has been shown to restore BRCA1 expression, reinstate HR machinery, and result in PARPi resistance in BRCA1-mutated and BRCA1-methylated patient-derived xenograft (PDX) models [29]. Similarly, in the phase 2 study of rucaparib treatment for platinum sensitive, relapsed EOC, analysis of pre- and post-treatment biopsy samples demonstrated that heterozygous demethylation of BRCA1 resulted in PARPi resistance [30]. The zygosity of BRCA1 methylation appears important for PARPi sensitivity or resistance, with heterozygous methylation or expression of functional hypomorphic variants correlating with PARPi resistance in PDX [30,31,32,33].
3. 53BP1 regulation
In the absence of HR gene reversion, hyperactivation of non-homologous end-joining (NHEJ) may contributor to PARPi resistance. Unlike HR, NHEJ involves only minor resection of DNA ends at sites of dsDNA breaks and is typically error-prone [34]. Normally regulated by BRCA1, the TP53 binding protein 1 (53BP1) maintains the balance between HR and NHEJ, which shifts to NHEJ in BRCA1-mutated and HRD tumors [35]. 53BP1 promotes NHEJ by inhibiting the extensive DNA end-resection required for HR repair, therefore loss of 53BP1 function (by mutation or downregulation) facilitates BRCA1-independent end-resection and conveys PARPi resistance [36]. In vitro studies demonstrated that loss of 53BP1 partially restores HR function in BRCA1 deficient cancers, reversing PARPi sensitivity [35]. The restoration of HR due to the loss of 53BP1 appears specific to BRCA1 but not BRCA2 deficiency, with the type of BRCA1 mutation dictating the extent to which HR is restored [37].
REPLICATION FORK PROTECTION
In addition to their role in HRR, BRCA1 and BRCA2 function to protect replication forks under replication stress conditions by stabilizing RAD51 nucleofilaments and preventing excessive processing of forks by nucleases. As such, one mechanism of PARPi mediated cytotoxicity is via dysregulation of replication fork reversal and/or restart. Therefore, stabilisation of replication forks may result in PARPi resistance [38]. Fork remodelling is controlled by several chromatin remodelling proteins, which when depleted increase fork stability and reduce replication stress-induced DNA damage and chromosomal instability, ultimately leading to olaparib resistance in BRCA1/2 deficient cells [17].
PARP EXPRESSION
Preclinical data suggests that mutations in PARP1 which alter PARP trapping, or allow PARP1 to maintain endogenous functions, may result in PARPi resistance. For example, in vitro studies with Crispr-cas9 mutagenesis screen identified in-frame mutations within the DNA-binding zinc-finger domain of PARP1, impacting the ability of PARP1 to bind sites of DNA damage and leading to PARPi resistance [15]. There have been anecdotal case reports supporting this hypothesis; a patient with EOC and primary resistance to olaparib was found to have a PARP1 mutation, affecting a critical region for communication between DNA-binding and catalytic domains. Whilst the resultant PARP1 protein retained DNA-binding capacity, it was unable to become trapped in response to PARPi [15]. The frequency of PARP modulation as a mechanism of PARPi resistance in not yet established in large clinical datasets.
PARPi EFFLUX
Upregulation of drug efflux pumps is a well described mechanism of resistance to PARPi, as well as other chemotherapeutic and targeted drugs. Mutations in the ABCB1 gene, which encodes the multi-drug efflux pump MDR1 (also known as also known as p-glycoprotein), leads to increased expression of ABCB1 [10,39]. In Brca1-null and p53-null mouse mammary gland tumors, several p-glycoproteins (Abcb1a, Abcb1b, Abcc1, Abcg2) were upregulated in response to both treatment and maintenance dosing of olaparib [40]. Approximately 8% of HGSC specimens, taken at the time of post-PARPi recurrence, were found to have upregulation of ABCB1 via fusions and translocations [10]. Whilst treatment with the p- glycoprotein inhibitor, tariquidar, re-sensitised olaparib resistant tumors to olaparib in vitro [40], clinical use of p-glycoprotein inhibitors is limited by toxicity and lack of specificity.
PARPi COMBINATION STRATEGIES
The use of combination strategies has the potential to overcome innate and acquired PARPi resistance. Several agents have shown promise in this area, either by working synergistically with PARPi or by targeting the HRR pathway through an alternate strategy. Treatment with agents that induce an HRD-like phenotype are proposed to re-sensitize resistant cells to PARPi, or to induce PARPi sensitivity in EOC that is HRP at baseline.
Although combining PARPi with DNA-damaging chemotherapy is appealing due to potential synergy, overlapping toxicity (especially myelosuppression) limits this combination [41,42]. More appealing is the combination of PARPi with inhibitors of angiogenesis, DNA damage repair (DDR), and the cell cycle, as well as immune checkpoint inhibitors (ICI) and tyrosine kinase inhibitors. These combinations have the potential to increase clinical synthetic lethality, or alternatively act by independent mechanisms without overlapping toxicity. Except for PARPi with anti-angiogenesis, most combination strategies are only in the pre-clinical or early phase trial stage, and the lack of comparator arms limits the ability to fully evaluate these combinations at this stage.
MOLECULARLY TARGETED AGENTS
1. PARPi and anti-angiogenesis
PARPi with anti-angiogenic therapy is probably the most evaluated combination to date in EOC. Angiogenesis is a key hallmark of cancer and plays a critical role in ovarian cancer pathogenesis. Pre-clinical data supports synergy between PARPi and anti-angiogenics, although the underlying mechanism(s) of these combinations are still not fully understood, may vary with antiangiogenic agent, and have not yet been proven in clinical trials. The vascular endothelial growth factor (VEGF) protein family consist of growth factors that promote increased vascularity and angiogenesis, in response to hypoxic conditions. For example, the PARP-1 pathway regulates gene expression, controlling angiogenesis through hypoxia inducible factors leading to VEGF-A upregulation [43]. Induction of hypoxia with drugs such as cediranib modifies HRR through a variety of mechanisms including downregulation of BRCA1/2 and RAD51 [44,45] which has the potential to sensitise to PARPi, particularly in BRCA1/2 wild-type patients or those with HR reversion mutations. Two angiogenesis inhibitors, cediranib and bevacizumab, with distinct mechanisms of action, have demonstrated antitumor activity as single agents, with response rates of up to 20% in patients with advanced EOC [46,47]. Bevacizumab has also been shown to improve progression-free survival (PFS) when combined with chemotherapy, and then as maintenance therapy, for both newly diagnosed and recurrent EOC [48,49,50].
Several studies have evaluated the combination of olaparib and cediranib in patients with recurrent EOC. In a randomized phase 2 trial comparing olaparib against olaparib/cediranib in platinum-sensitive recurrent HGSC patients (NCT01116648), an overall PFS of 17.7 months in the combination group and 9.0 months in the olaparib monotherapy group was observed [51]. Subsequent exploratory analysis by BRCA1/2 status demonstrated that the combination was active in both BRCA1/2 mutant and wild-type cohorts. A greater benefit from the addition of cediranib was observed in the BRCA1/2 wild-type cohort, increasing median PFS from 5.7 months with olaparib monotherapy to 16.5 months (p=0.008) with combination therapy. In comparison, in the BRCA1/2 mutant cohort the median PFS was 16.5 months with olaparib vs. 19.4 months (p=0.16) with the combination [51].
Although the recent GY004 phase III (NCT02446600) study demonstrated similar activity with cediranib plus olaparib compared to standard-of-care (SOC) chemotherapy in platinum sensitive recurrent EOC, the study did not meet the primary endpoint of improved PFS with median PFS of 10.3, 8.2 and 10.4 months for SOC chemotherapy, olaparib monotherapy and cediranib/olaparib combination respectively [52]. The combination of olaparib and cediranib has also been evaluated in platinum resistant disease in the BAROCCO (NCT03314740) and OCTOVA (NCT03117933) and AMBITION (NCT03699449) clinical trials [53,54,55]. Whilst no significant improvement in PFS was observed with either continuous or intermittent cediranib with olaparib compared with weekly paclitaxel within BAROCCO, continuous administration demonstrated a trend for improved PFS compared to chemotherapy (5.8 months vs. 3.1 months, hazard ratio [HR]=0.76; 90% confidence interval [CI]=0.50–1.14) [53]. In the OCTOVA trial, cediranib and olaparib treatment significantly increased PFS compared to olaparib monotherapy (median PFS 5.4 vs. 3.7 months, HR=0.70; 95% CI=0.57–0.86) and was numerically superior to weekly paclitaxel (median PFS 3.9 months), although the trial was not designed to compare these treatment arms directly [55]. The improved PFS observed with the olaparib/cediranib combination compared to olaparib monotherapy was independent of BRCA1/2 status, or prior PARP or anti-angiogenic use [55]. In the AMBITION trial, cediranib and olaparib showed 50% (95% CI=24.7–75.4) overall response rate (ORR) in platinum resistant patients with HRR gene mutations [54].
The combination of cediranib plus olaparib following progression on prior PARPi monotherapy was evaluated in the EVOLVE study (NCT02681237). Clinical benefit was observed in a small number of patients [26]. Importantly, paired biopsies were obtained, and at the time of PARPi progression the following genomic alterations were observed; HR gene reversion mutations (BRCA1, BRCA2, or RAD51B) (19%); CCNE1 amplification (16%); ABCB1 upregulation (15%); and SLFN11 downregulation (7%). Interestingly, translational findings suggest that patients with HR gene reversion mutations, or upregulated ABCB1 at trial baseline, had poor outcomes and did not benefit from cediranib/olaparib combination [26]. This preliminary data highlights that different means of acquired PARPi resistance will require different treatment approaches and are likely to respond differently to both PARP rechallenge and combination therapy.
Bevacizumab, a monoclonal antibody that binds to circulating VEGF and prevents it binding to its receptors, has also been investigated in combination with PARPi. The combination of niraparib and bevacizumab as treatment (rather than maintenance therapy) significantly improved response rate compared to niraparib alone in the ANANOVA2 trial (NCT02354131, 60% vs. 27%), regardless of HRD status [56].
These studies suggest as treatment, PARPi combination with antiangiogenic agents demonstrates efficacy based on objective response rates and PFS [53,55,56]. Whilst combination therapy has not been shown to be superior to SOC chemotherapy, based on toxicity and patient preferences it may provide a viable option as a chemotherapy sparing regime. Further biomarker analysis from these trials will hopefully help elucidate which patients benefit most from this combination and which group of PARPi resistant patients will benefit.
The phase III PAOLA-1 study (NCT02477644) first evaluated a PARPi and antiangiogenic combination as maintenance treatment in the first-line setting [2]. Patients with advanced EOC, regardless of BRCA1/2 status, who had a response following platinum-based chemotherapy and bevacizumab, received olaparib plus bevacizumab or placebo plus bevacizumab. A statistically significant improvement in median PFS for olaparib plus bevacizumab vs. bevacizumab alone (22.1 vs. 16.6 months, HR=0.59; 95% CI=0.49–0.72) was observed in the overall population. In the predefined subgroups, substantial PFS benefit was observed with the combination treatment vs. bevacizumab in the HRD population (including BRCA1/2 mutant; 37.2 vs. 17.7 months; HR=0.33; 95% CI=0.25–0.45) but not in the HRP population, leading to Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval of the combination as maintenance therapy for HRD-positive patients only [2,13]. Due to the lack of an olaparib-only maintenance arm, it is not possible to determine whether olaparib alone is driving improved efficacy or whether the combination has synergistic effects.
Whether the combination of anti-angiogenesis and PARPi is superior to PARP monotherapy as maintenance treatment in patients with platinum sensitive recurrent ovarian cancer will be addressed in the ongoing ICON9 trial, which is comparing olaparib/cediranib with olaparib monotherapy (NCT03278717) [57], and in the ongoing NIRVANA-1 trial, which is comparing niraparib/bevacizumab with niraparib monotherapy (NCT05183984). Overall, current data do not answer whether maintenance therapy with PARPi and angio-angiogenesis is superior to PARPi monotherapy and further data is warranted.
OReO/ENGOT Ov-38 (NCT03106987) is a randomized, double-blind phase III study to evaluate PARPi rechallenge in platinum sensitive relapsed ovarian cancer patients with one prior line of PARPi maintenance and were in response to their most recent platinum based chemotherapy [58]. PARPi rechallenge showed a significant improvement in PFS vs. placebo, irrespective of BRCA mutation status. Recently, NIRVANA-R (NCT04734665) is ongoing to determine the efficacy of niraparib with bevacizumab with platinum sensitive recurrent ovarian cancer patients previously treated with a PARPi [59].
2. PARPi and PI3K/AKT/mTOR signalling pathway
The phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT/mTOR pathway is frequently dysregulated in cancers, however, to date, limited activity has been observed with single agent PI3K/AKT inhibitors [60]. Pre-clinical data suggests synergism between PARPi and inhibitors of the PI3K pathway, via multi-mechanisms which centre on inducing an HRD phenotype. For example, PI3K inhibitors can downregulate BRCA1/2 and induce HRD [61] and mTOR inhibitors induce suppression of the DNA double strand break (DSB) repair protein SUV39H1, with suppression of HRR gene expression [62].
A phase I trial of buparlisib (pan-Pi3K inhibitor) with olaparib in patients with breast and ovarian cancer showed approximately 30% response rate for the combination, with responses observed regardless of BRCA1/2 status [63]. However due to poor tolerability of buparlisib. this combination has not been perused. The more specific PI3Kalpha inhibitor, alpelisib, was combined with olaparib in patients with recurrent and predominately platinum resistant HGSC. There was an ORR of 36%, independent of germline HRR gene mutation status [64]. These data, demonstrating response regardless of HR status, suggest that PI3K inhibitors may induce HRD in patients with baseline HRP and thereby sensitise to the effects of PARPi. EPIK-O/ENGOT-ov61 (NCT04729387) is ongoing to compare the efficacy and safety of alpelisib plus olaparib vs. single-agent chemotherapy in patients without a germline BRCA1/2 mutation, platinum-resistant/refractory HGSC. Two phase one studies have explored the combination of capivasertib, an AKT inhibitor, with olaparib. The ComPAKT trial (NCT02338622) evaluated the combination in patients with advanced solid tumors, of the evaluable patients, 44.6% achieved clinical benefit (RECIST complete/partial response or stable disease ≥4 months), including BRCA1/2 mutant cancers and BRCA1/2 wildtype cancers with or without DDR and PI3K/AKT pathway alterations [65]. A second study in patients with endometrial, ovarian, and triple-negative breast cancers demonstrated a similar clinical benefit rate of 41% [66]. Provisional analysis of tumor samples from this study determined that markers of DNA damage checkpoint activation and decreased mTOR activity were associated with response, whereas resistance to the combination was associated with high receptor tyrosine kinase activity levels and mTOR activation [66]. The authors conclude that these putative biomarkers could improve patient selection for this combination, although it is unclear whether these markers of resistance were dependent on prior PARPi exposure or varied between malignancies. Nevertheless, these data provide a suggestion of a molecularly defined population who may be more likely to benefit, and provide greater insight to additional resistance mechanisms, although further randomised trials are warranted to confirm.
3. PARPi and RAS/RAF/MEK pathway
Data suggests that the MAPK pathway may be a relevant target for PARPi re-sensitisation, as PARPi resistance is associated with upregulation of the RAS/MAPK pathway [67]. In vitro and in vivo data suggests that combined MEK and PARP inhibition induces more DNA damage, has the potential to induce cell death and increase the magnitude, duration, and spectrum of PARPi activity [67,68]. Whether this combination is beneficial in patients is under investigation in the ongoing phase I/II trial of olaparib and selumetinib (MEK inhibitor, NCT03162627), which includes an expansion cohort of PARPi-resistant EOC.
4. PARPi and BET inhibitors
Bromodomain containing 4 is a member of the BET protein family with roles in epigenetic gene regulation, which promotes oncogene transcription. BET inhibitors have been shown to suppress DDR genes, induce an HRD phenotype in HRP cell lines, and re-sensitise to PARPi [69,70,71]. The combination of BET and PARPi showed increased tumor cytotoxicity compared to cells treated with either agent alone [69]. This combination was effective in a number of cell lines with varied genomic backgrounds, representing a number of established mechanisms of PARPi resistance including BRCA1/2 wild-type, 53BP1 deficient, and PARP1 deficient [69]. Cumulatively, these findings suggest that BET inhibitors in combination with PARPi may have clinical application, particularly in HRP and PARPi resistant tumors; early phase trials are underway (e.g., NCT03205176 combination of olaparib and AZD5153 [BET inhibitor] in advanced solid tumors, including EOC, with prior PARPi exposure allowed).
PARPi AND ICI
The rationale for this combination is based on 2 hypotheses. Firstly, HRD cancers have a higher tumor mutational burden leading to elevated neo-antigen loads, which is thought to stimulate an increased anti-tumor immune response [72,73]. Secondly, treatment with PARPi upregulates PD-L1 expression in vivo and in vitro [74], and in the absence of a functional BRCA pathway there is activation of the innate immune response via the STING/TKB1/IRF3 response [75], which may enhance the antitumor effect of the combination.
Preliminary results from early-phase single-arm trials demonstrate activity for the combination of PARPi with ICI, with an ORR in HGSC between 18%–72%, depending on the patient subgroup examined [76,77]. One of the largest studies to date in the recurrent setting, is the phase I/II TOPACIO (NCT02657889) trial which explored the combination of niraparib and the PD-1 inhibitor, pembrolizumab, in a predominately platinum-resistant (76%) population. The combination was tolerable with an ORR of 18% (90% CI=11%–29%), and a disease control rate (DCR) of 65% (90% CI=54%–75%), with similar ORR regardless of HRD or BRCA1/2 mutation status [77].
A second phase I/II ongoing trial MEDIOLA (NCT02734004) is evaluating olaparib and durvalumab (PD-L1 inhibitor), as a chemotherapy sparing regimen for platinum-sensitive recurrent disease in both BRCA1/2 mutant and wild-type populations. Within the BRCA1/2 mutant cohort, interim results suggest an ORR of 71.9% (95% CI=53–86) [76]. In the germline BRCA1/2 wild-type group, patients were allocated in a non-randomized fashion to receive either olaparib and durvalumab, or triplet therapy with olaparib, durvalumab, and bevacizumab [78]. This triplet combination was well tolerated with an ORR of 77.4% (95% CI=59–90), compared with the doublet ORR of 31.3% (95% CI=16–50) [78]. OPEB-01 (NCT04361370) trial, is evaluating another triplet combination with olaparib, pembrolizumab, and bevacizumab as maintenance therapy in BRCA1/2 wild-type platinum-sensitive recurrent ovarian cancer [79]. The results of interim analysis reported a DCR of 86.4% from 22 patients [80]. None of these studies were randomised and the difference in ORR observed reflects the markedly different patient population with regards to established predictive factors such as platinum and HRD/BRCA1/2 status and number of lines of prior therapies, including prior PARP exposure. These factors, along with markers of PARPi resistance will need to be interrogated in ongoing combination studies with PARPi and ICI. Several larger randomised phase 3 trials with PARPi and ICI in the first line setting are underway (Table 2). To date, the addition of ICI to SOC chemotherapy +/− bevacizumab, and then as maintenance treatment in the first-line EOC setting, has been disappointing with negative results from both the JAVELIN Ovarian 200 (avelumab, NCT02580058) and IMagyn050 (atezolizumab, NCT03038100) studies. It is hoped that due to the aforementioned synergy observed in pre-clinical models, the combination of PARPi and ICI will yield greater patient benefit. Furthermore, translational work performed alongside these trials may allow better context specificity with regards to potential predictive biomarkers.
Table 2. Ongoing first line PARPi and immune checkpoint inhibitor combination trials (first-line maintenance therapy).
| Trial name (NCT number) | PARPi | Combination | Comparator | Indication | Patient population |
|---|---|---|---|---|---|
| ATHENA (NCT03522246) | Rucaparib | Nivolumab | Maintenance therapy: rucaparib/nivolumab vs. rucaparib/placebo vs. placebo/nivolumab vs. placebo/placebo | Maintenance following first line chemotherapy | Any BRCA mutation or HRD status |
| DUO-O (NCT03737643) | Olaparib | Durvalumab | Maintenance therapy: bevacizumab/placebo/placebo vs. bevacizumab/durvalumab/placebo vs. bevacizumba/durvalumab/olaparib | Durvalumab/placebo with concurrent chemotherapy and bevacizumab followed by maintenance therapy first line | Any BRCA mutation or HRD status |
| FIRST (NCT03602859) | Niraparib | Dostarlimab | Maintenance therapy: placebo/placebo vs. niraparib/placebo vs. niraparib/dostarlimab +/− bevacizumab as SOC | Dostarlimab/placebo with concurrent chemotherapy followed by maintenance therapy first line | Any BRCA mutation or HRD status |
| KEYLYNK-001 (NCT03740165) | Olaparib | Pembrolizumab | Maintenance therapy: bevacizumab/placebo/placebo vs. bevacizumab/pembrolizumab/placebo vs. bevacizumba/pembrolizumab//olaparib | Pembrolizumab/placebo with concurrent chemotherapy followed by maintenance therapy first line | BRCA1/2 wild-type |
HRD, homologous recombination deficient; PARPi, PARP inhibitor; SOC, standard-of-care.
PARPi AND OTHER INHIBITORS OF DNA DAMAGE RESPONSE
To ensure cellular survival in the context of DNA damage, cells activate cell cycle checkpoints to halt the cell cycle and stimulate DDR mechanisms to repair single-strand DNA breaks, and accumulated DSBs through PARPi and replication fork collapse in S phase. As the S-phase checkpoint is reliant on p53, tumors with p53 mutations, which is almost ubiquitous in EOC, are more reliant on the G2 checkpoint for DDR [81,82]. Combining PARPi with other modulators of DDR maximises the accumulation of damage during G1 and S phases of the cell cycle, as well as inhibiting repair during G2 by minimizing the time to repair. Inhibition of the ATR/Chk1/Wee1 axis restores HR and replication fork stability, re-sensitising BRCA1/2 deficient tumors to PARPi [83]. Pre-clinical studies suggest synergistic anti-tumor effect of combination PARPi and ATR inhibitors [82], and early phase clinical trials are ongoing (Table 3). Similarly, several ongoing studies are evaluating the combination of PARPi with Wee1 inhibitors in ovarian cancer and PARPi resistance (Table 3). For example, the EFFORT study (NCT03579316) combining olaparib and the Wee1 inhibitor adavosertib have demonstrated benefit in PARPi resistant EOC, with an ORR for the combination of 29% compared to 23% for adavosertib monotherapy [84]. However, this combination was associated with high levels of grade 3 or 4 toxicity (76%), particularly haematological, requiring dose interruptions and reductions [84]. Animal studies demonstrated that sequential administration of PARPi and adavosertib was as effective as a concurrent administration, but with fewer haematological effects [85], suggesting that this dosing schedule could be considered for future clinical studies. Prexasertib, a Chk1 inhibitor, induces HRD and synergises with olaparib in vitro [86]; early clinical trials have demonstrated evidence of clinical effectiveness in combination with olaparib, including patients with PARPi resistant, BRCA1 mutant HGSC [87]. Results from the completed phase 2 study investigating prexasertib monotherapy in platinum and PARPi resistant patients are awaited (NCT03414047).
Table 3. Ongoing PARPi and DDR combination trials.
| Trial name (NCT number) | Phase | Comparator | PARPi | DDR agent | Patient cohorts |
|---|---|---|---|---|---|
| CAPRI (NCT03462342) | II | Single arm | Olaparib | Ceralasertib (ATRi) | Platinum sensitive/recurrent |
| PARPi resistant | |||||
| HRD/BRCA or regardless of biomarker | |||||
| ATARI (NCT04065269) | II | AZD6738 monotherapy | Olaparib | AZD6738 (ATRi) | ARID1A proficient gyncologic cancer |
| ARID1A deficient gynecologic cancer | |||||
| NCT04267939 | Ib | Single arm | Niraparib | Bay 1895344 (ATRi) | HRD solid tumors |
| Platinum-resistant, HRD, PARPi naïve HGSC | |||||
| PARPi-resistant HGSC | |||||
| EFFORT (NCT03579316) | II | Adavosertib monotherapy | Olaparib | Adavosertib (WEE1i) | EOC who have progressed on prior PARPi |
| STAR (NCT04197713) | I | Sequential rather than combination treatment | Olaparib | Adavosertib (WEE1i) | BRCA1/2 mutant primary PARPi resistant solid tumors |
| Prior PARP with pre-defined DDR mutation solid tumors | |||||
| OLAPCO (NCT02576444) | II | Olaparib monotherapy | Olaparib | Ceralasertib (ATRi) | Solid tumors with mutation in HRR gene |
| Adavosertib (WEE1i) | Solid tumors with TP53 or KRAS mutation | ||||
| Capivasertib (AKTi) | Solid tumors with mutations or other molecular aberrations leading to dysregulation of the PI3K/AKT pathway |
ATRi, ATR inhibitor; AKTi, AKT inhibitor; DDR, DNA damage repair; EOC, epithelial ovarian cancer; HGSC, high-grade serous cancer; HRD, homologous recombination deficient; PARPi, PARP inhibitor; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; WEE1i, WEE1 inhibitor.
SUMMARY
Although the introduction of PARPi has transformed the outcomes for many patients with HGSC, the impressive gain in clinical benefit is mostly limited to the tumors characterized by BRCA1/2 mutations or HRD. Even in these subgroups, the development of resistance is common. Combination therapy offers a potential strategy to overcome acquired PARPi resistance and may also have a role for tumors with de novo resistance such as HRP tumors. Several classes of drugs have shown promise in this area by either targeting HRR and restoring HRD, or by working synergistically with PARPi to increase response rates. Whilst there is pre- clinical evidence to support numerous PARPi combination with other agents such as anti-angiogenics, immune-checkpoint inhibitors, DDR, PI3K/AKT, EGFR, and BET inhibitors, underlying mechanisms remain poorly understood and have not been well validated in clinical trial samples. Parallel translational research with collection of tumor biopsies and/or cfDNA are required with clinical studies to better define predictive biomarkers and facilitate patient stratification for combination therapy. An additional consideration is the risk of adverse events with combination therapy, and treatment dose and scheduling needs to be optimized to maximize the overall risk-benefit ratio. As clinical data from combination studies matures, we will gain a better understanding of which patients derived the greatest benefit. This will allow us to increase the number of patients who benefit from PARPi, beyond the impressive gains observed to date with monotherapy in molecularly defined sub-groups.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: M.R.E., E.S.K.H., L.J.Y.
- Writing - original draft: M.R.E., E.S.K.H., L.J.Y.
- Writing - review & editing: M.R.E., E.S.K.H., L.J.Y.
References
- 1.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 2.Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 3.González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 6.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 7.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 8.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller RE, Leary A, Scott CL, Serra V, Lord CJ, Bowtell D, et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol. 2020;31:1606–1622. doi: 10.1016/j.annonc.2020.08.2102. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration (FDA) FDA approves olaparib plus bevacizumab as maintenance treatment for ovarian, fallopian tube, or primary peritoneal cancers [Internet] Silver Spring, MD: FDA; 2020. [cited 2020 May 18]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-plus-bevacizumab-maintenance-treatment-ovarian-fallopian-tube-or-primary. [Google Scholar]
- 13.European Medicines Agency (EMA) Olaparib [Internet] Amsterdam: EMA; 2021. [cited 2022 Feb 28]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/lynparza. [Google Scholar]
- 14.Herzog M, Puddu F, Coates J, Geisler N, Forment JV, Jackson SP. Detection of functional protein domains by unbiased genome-wide forward genetic screening. Sci Rep. 2018;8:6161. doi: 10.1038/s41598-018-24400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettitt SJ, Krastev DB, Brandsma I, Dréan A, Song F, Aleksandrov R, et al. Genome-wide and high-density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat Commun. 2018;9:1849. doi: 10.1038/s41467-018-03917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rondinelli B, Gogola E, Yücel H, Duarte AA, van de Ven M, van der Sluijs R, et al. EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat Cell Biol. 2017;19:1371–1378. doi: 10.1038/ncb3626. [DOI] [PubMed] [Google Scholar]
- 17.Taglialatela A, Alvarez S, Leuzzi G, Sannino V, Ranjha L, Huang JW, et al. Restoration of replication fork stability in BRCA1- and BRCA2-deficient cells by inactivation of SNF2-family fork remodelers. Mol Cell. 2017;68:414–430.e8. doi: 10.1016/j.molcel.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaidyanathan A, Sawers L, Gannon AL, Chakravarty P, Scott AL, Bray SE, et al. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br J Cancer. 2016;115:431–441. doi: 10.1038/bjc.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondrashova O, Nguyen M, Shield-Artin K, Tinker AV, Teng NN, Harrell MI, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2017;7:984–998. doi: 10.1158/2159-8290.CD-17-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodall J, Mateo J, Yuan W, Mossop H, Porta N, Miranda S, et al. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. 2017;7:1006–1017. doi: 10.1158/2159-8290.CD-17-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229:422–429. doi: 10.1002/path.4140. [DOI] [PubMed] [Google Scholar]
- 22.Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29:3008–3015. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 24.Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin KK, Harrell MI, Oza AM, Oaknin A, Ray-Coquard I, Tinker AV, et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019;9:210–219. doi: 10.1158/2159-8290.CD-18-0715. [DOI] [PubMed] [Google Scholar]
- 26.Lheureux S, Oaknin A, Garg S, Bruce JP, Madariaga A, Dhani NC, et al. EVOLVE: a multicenter open-label single-arm clinical and translational phase II trial of cediranib plus olaparib for ovarian cancer after PARP inhibition progression. Clin Cancer Res. 2020;26:4206–4215. doi: 10.1158/1078-0432.CCR-19-4121. [DOI] [PubMed] [Google Scholar]
- 27.Domchek SM. Reversion mutations with clinical use of parp inhibitors: many genes, many versions. Cancer Discov. 2017;7:937–939. doi: 10.1158/2159-8290.CD-17-0734. [DOI] [PubMed] [Google Scholar]
- 28.Castroviejo-Bermejo M, Cruz C, Llop-Guevara A, Gutiérrez-Enríquez S, Ducy M, Ibrahim YH, et al. A RAD51 assay feasible in routine tumor samples calls PARP inhibitor response beyond BRCA mutation. EMBO Mol Med. 2018;10:e9172. doi: 10.15252/emmm.201809172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ter Brugge P, Kristel P, van der Burg E, Boon U, de Maaker M, Lips E, et al. Mechanisms of therapy resistance in patient-derived xenograft models of BRCA1-deficient breast cancer. J Natl Cancer Inst. 2016;108:djw148. doi: 10.1093/jnci/djw148. [DOI] [PubMed] [Google Scholar]
- 30.Kondrashova O, Topp M, Nesic K, Lieschke E, Ho GY, Harrell MI, et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun. 2018;9:3970. doi: 10.1038/s41467-018-05564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S, Llop-Guevara A, Ibrahim YH, Gris-Oliver A, et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol. 2018;29:1203–1210. doi: 10.1093/annonc/mdy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drost R, Dhillon KK, van der Gulden H, van der Heijden I, Brandsma I, Cruz C, et al. BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J Clin Invest. 2016;126:2903–2918. doi: 10.1172/JCI70196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Bernhardy AJ, Cruz C, Krais JJ, Nacson J, Nicolas E, et al. The BRCA1-Δ11q alternative splice isoform bypasses germline mutations and promotes therapeutic resistance to PARP inhibition and cisplatin. Cancer Res. 2016;76:2778–2790. doi: 10.1158/0008-5472.CAN-16-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang HH, Pannunzio NR, Adachi N, Lieber MR. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18:495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bunting SF, Callén E, Wong N, Chen HT, Polato F, Gunn A, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makvandi M, Xu K, Lieberman BP, Anderson RC, Effron SS, Winters HD, et al. A radiotracer strategy to quantify PARP-1 expression in vivo provides a biomarker that can enable patient selection for PARP inhibitor therapy. Cancer Res. 2016;76:4516–4524. doi: 10.1158/0008-5472.CAN-16-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouwman P, Aly A, Escandell JM, Pieterse M, Bartkova J, van der Gulden H, et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol. 2010;17:688–695. doi: 10.1038/nsmb.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray Chaudhuri A, Callen E, Ding X, Gogola E, Duarte AA, Lee JE, et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature. 2016;535:382–387. doi: 10.1038/nature18325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christie EL, Pattnaik S, Beach J, Copeland A, Rashoo N, Fereday S, et al. Multiple ABCB1 transcriptional fusions in drug resistant high-grade serous ovarian and breast cancer. Nat Commun. 2019;10:1295. doi: 10.1038/s41467-019-09312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Conte G, Sessa C, von Moos R, Viganò L, Digena T, Locatelli A, et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer. 2014;111:651–659. doi: 10.1038/bjc.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Noll R, Ang JE, Jager A, Marchetti S, Mergui-Roelvink M, De BonoMartijn Lolkema JS, et al. Phase I study of olaparib in combination with carboplatin and/or paclitaxel in patients with advanced solid tumors. J Clin Oncol. 2013;31:2579. [Google Scholar]
- 43.Martin-Oliva D, Aguilar-Quesada R, O’valle F, Muñoz-Gámez JA, Martínez-Romero R, García Del Moral R, et al. Inhibition of poly(ADP-ribose) polymerase modulates tumor-related gene expression, including hypoxia-inducible factor-1 activation, during skin carcinogenesis. Cancer Res. 2006;66:5744–5756. doi: 10.1158/0008-5472.CAN-05-3050. [DOI] [PubMed] [Google Scholar]
- 44.Bindra RS, Schaffer PJ, Meng A, Woo J, Måseide K, Roth ME, et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24:8504–8518. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim JJ, Yang K, Taylor-Harding B, Wiedemeyer WR, Buckanovich RJ. VEGFR3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of BRCA1 and BRCA2. Neoplasia. 2014;16:343–353.e1-2. doi: 10.1016/j.neo.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 47.Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27:5601–5606. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 50.Poveda AM, Selle F, Hilpert F, Reuss A, Savarese A, Vergote I, et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol. 2015;33:3836–3838. doi: 10.1200/JCO.2015.63.1408. [DOI] [PubMed] [Google Scholar]
- 51.Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, et al. A phase III study comparing single-agent olaparib or the combination of cediranib and olaparib to standard platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer. J Clin Oncol. 2020;38:6003. [Google Scholar]
- 53.Colombo N, Tomao F, Benedetti Panici P, Nicoletto MO, Tognon G, Bologna A, et al. Randomized phase II trial of weekly paclitaxel vs. cediranib-olaparib (continuous or intermittent schedule) in platinum-resistant high-grade epithelial ovarian cancer. Gynecol Oncol. 2022;164:505–513. doi: 10.1016/j.ygyno.2022.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Lee JY, Yi JY, Kim HS, Lim J, Kim S, Nam BH, et al. An umbrella study of biomarker-driven targeted therapy in patients with platinum-resistant recurrent ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG 3045), AMBITION. Jpn J Clin Oncol. 2019;49:789–792. doi: 10.1093/jjco/hyz085. [DOI] [PubMed] [Google Scholar]
- 55.Nicum SH, McGregor N, Dunn R, Collins L, Kaye S, McNeish I, et al. Randomised phase II trial of olaparib compared to weekly paclitaxel or olaparib plus cediranib in patients with platinum-resistant ovarian cancer (OCTOVA) Ann Oncol. 2021;32:S725–S726. [Google Scholar]
- 56.Mirza MR, Åvall Lundqvist E, Birrer MJ, dePont Christensen R, Nyvang GB, Malander S, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019;20:1409–1419. doi: 10.1016/S1470-2045(19)30515-7. [DOI] [PubMed] [Google Scholar]
- 57.Elyashiv O, Ledermann J, Parmar G, Farrelly L, Counsell N, Feeney A, et al. ICON 9-an international phase III randomized study to evaluate the efficacy of maintenance therapy with olaparib and cediranib or olaparib alone in patients with relapsed platinum-sensitive ovarian cancer following a response to platinum-based chemotherapy. Int J Gynecol Cancer. 2021;31:134–138. doi: 10.1136/ijgc-2020-002073. [DOI] [PubMed] [Google Scholar]
- 58.Pujade-Lauraine ES, Scambia G, Asselain B, Marme F, Lindermann K, Colombo N, et al. LBA33 Maintenance olaparib rechallenge in patients (pts) with ovarian carcinoma (OC) previously treated with a PARP inhibitor (PARPi): Phase IIIb OReO/ENGOT Ov-38 trial. Ann Oncol. 2021;32:S1308–S1309. doi: 10.1016/j.annonc.2023.09.3110. [DOI] [PubMed] [Google Scholar]
- 59.Park J, Lim MC, Lee JK, Jeong DH, Kim SI, Choi MC, et al. A single-arm, phase II study of niraparib and bevacizumab maintenance therapy in platinum-sensitive, recurrent ovarian cancer patients previously treated with a PARP inhibitor: Korean Gynecologic Oncology Group (KGOG 3056)/NIRVANA-R trial. J Gynecol Oncol. 2022;33:e12. doi: 10.3802/jgo.2022.33.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodon J, Braña I, Siu LL, De Jonge MJ, Homji N, Mills D, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32:670–681. doi: 10.1007/s10637-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 61.Ibrahim YH, García-García C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2:1036–1047. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mo W, Liu Q, Lin CC, Dai H, Peng Y, Liang Y, et al. mTOR inhibitors suppress homologous recombination repair and synergize with PARP inhibitors via regulating SUV39H1 in BRCA-proficient triple-negative breast cancer. Clin Cancer Res. 2016;22:1699–1712. doi: 10.1158/1078-0432.CCR-15-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matulonis UA, Wulf GM, Barry WT, Birrer M, Westin SN, Farooq S, et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann Oncol. 2017;28:512–518. doi: 10.1093/annonc/mdw672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konstantinopoulos PA, Barry WT, Birrer M, Westin SN, Cadoo KA, Shapiro GI, et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 2019;20:570–580. doi: 10.1016/S1470-2045(18)30905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yap TA, Kristeleit R, Michalarea V, Pettitt SJ, Lim JS, Carreira S, et al. Phase I trial of the PARP inhibitor olaparib and AKT inhibitor capivasertib in patients with BRCA1/2- and non-BRCA1/2-mutant cancers. Cancer Discov. 2020;10:1528–1543. doi: 10.1158/2159-8290.CD-20-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westin SN, Labrie M, Litton JK, Blucher A, Fang Y, Vellano CP, et al. Phase 1b dose expansion and translational analyses of olaparib in combination with capivasertib in recurrent endometrial, triple negative breast, and ovarian cancer. Clin Cancer Res. 2021;27:6354–6365. doi: 10.1158/1078-0432.CCR-21-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun C, Fang Y, Yin J, Chen J, Ju Z, Zhang D, et al. Rational combination therapy with PARP and MEK inhibitors capitalizes on therapeutic liabilities in RAS mutant cancers. Sci Transl Med. 2017;9:eaal5148. doi: 10.1126/scitranslmed.aal5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vena F, Jia R, Esfandiari A, Garcia-Gomez JJ, Rodriguez-Justo M, Ma J, et al. MEK inhibition leads to BRCA2 downregulation and sensitization to DNA damaging agents in pancreas and ovarian cancer models. Oncotarget. 2018;9:11592–11603. doi: 10.18632/oncotarget.24294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun C, Yin J, Fang Y, Chen J, Jeong KJ, Chen X, et al. BRD4 inhibition is synthetic lethal with PARP inhibitors through the induction of homologous recombination deficiency. Cancer Cell. 2018;33:401–416.e8. doi: 10.1016/j.ccell.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang L, Zhang Y, Shan W, Hu Z, Yuan J, Pi J, et al. Repression of BET activity sensitizes homologous recombination-proficient cancers to PARP inhibition. Sci Transl Med. 2017;9:eaal1645. doi: 10.1126/scitranslmed.aal1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karakashev S, Zhu H, Yokoyama Y, Zhao B, Fatkhutdinov N, Kossenkov AV, et al. BET bromodomain inhibition synergizes with PARP inhibitor in epithelial ovarian cancer. Cell Reports. 2017;21:3398–3405. doi: 10.1016/j.celrep.2017.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higuchi T, Flies DB, Marjon NA, Mantia-Smaldone G, Ronner L, Gimotty PA, et al. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol Res. 2015;3:1257–1268. doi: 10.1158/2326-6066.CIR-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang J, Wang L, Cong Z, Amoozgar Z, Kiner E, Xing D, et al. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1-/- murine model of ovarian cancer. Biochem Biophys Res Commun. 2015;463:551–556. doi: 10.1016/j.bbrc.2015.05.083. [DOI] [PubMed] [Google Scholar]
- 74.Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23:3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parkes EE, Walker SM, Taggart LE, McCabe N, Knight LA, Wilkinson R, et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst. 2016;109:djw199. doi: 10.1093/jnci/djw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drew Y, de Jonge M, Hong SH, Park YH, Wolfer A, Brown J, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): results in germline BRCA-mutated (gBRCAm) platinum-sensitive relapsed (PSR) ovarian cancer (OC) Gynecol Oncol. 2018;149:246–247. [Google Scholar]
- 77.Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5:1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drew YP, O’Malley DM, Kim JW, Zimmermann S, Roxburgh P, Sohn J, et al. 814MO Phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): Initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC) Ann Oncol. 2020;31:S615–S616. [Google Scholar]
- 79.Lee YJ, Lim MC, Kim BG, Ngoi NY, Choi CH, Park SY, et al. A single-arm phase II study of olaparib maintenance with pembrolizumab and bevacizumab in BRCA non-mutated patients with platinum-sensitive recurrent ovarian cancer (OPEB-01) J Gynecol Oncol. 2021;32:e31. doi: 10.3802/jgo.2021.32.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.The 7th Biennial Meeting of Asian Society of Gynecologic Oncology, November 25-27, 2021, Thailand. J Gynecol Oncol. 2021;32:S3–87. doi: 10.3802/jgo.2021.32.S1. [DOI] [PubMed] [Google Scholar]
- 81.Rundle S, Bradbury A, Drew Y, Curtin NJ. Targeting the ATR-CHK1 axis in cancer therapy. Cancers (Basel) 2017;9:41. doi: 10.3390/cancers9050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee EK, Matulonis UA. PARP inhibitor resistance mechanisms and implications for post-progression combination therapies. Cancers (Basel) 2020;12:2054. doi: 10.3390/cancers12082054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haynes B, Murai J, Lee JM. Restored replication fork stabilization, a mechanism of PARP inhibitor resistance, can be overcome by cell cycle checkpoint inhibition. Cancer Treat Rev. 2018;71:1–7. doi: 10.1016/j.ctrv.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Westin SN, Coleman RL, Fellman BM, Yuan Y, Sood AK, Soliman PT, et al. EFFORT: EFFicacy Of adavosertib in parp ResisTance: a randomized two-arm non-comparative phase II study of adavosertib with or without olaparib in women with PARP-resistant ovarian cancer. J Clin Oncol. 2021;39:5505. [Google Scholar]
- 85.Fang Y, McGrail DJ, Sun C, Labrie M, Chen X, Zhang D, et al. Sequential therapy with PARP and WEE1 inhibitors minimizes toxicity while maintaining efficacy. Cancer Cell. 2019;35:851–867.e7. doi: 10.1016/j.ccell.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mani C, Jonnalagadda S, Lingareddy J, Awasthi S, Gmeiner WH, Palle K. Prexasertib treatment induces homologous recombination deficiency and synergizes with olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2019;21:104. doi: 10.1186/s13058-019-1192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parmar K, Kochupurakkal BS, Lazaro JB, Wang ZC, Palakurthi S, Kirschmeier PT, et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin Cancer Res. 2019;25:6127–6140. doi: 10.1158/1078-0432.CCR-19-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]