Abstract
Objective
To assess the efficacy of the FIGO 2018 classification system for nodal-specific classifications for early-stage cervical cancer; specifically, to examine the impact of nodal metastasis on survival and the effect of postoperative treatments, according to histological subtypes.
Methods
This society-based retrospective observational study in Japan examined 16,539 women with the 2009 FIGO stage IB1 cervical cancer who underwent primary surgical treatment from 2004 to 2015. Associations of cause-specific survival (CSS) with nodal metastasis and postoperative adjuvant therapy were examined according to histology type (squamous cell carcinoma [SCC], n=10,315; and non-SCC, n=6,224).
Results
The nodal metastasis rate for SCC was higher than that for non-SCC (10.7% vs. 8.3%, p<0.001). In multivariable analysis, the impact of nodal metastasis on CSS was greater for non-SCC tumors (adjusted-hazard ratio [HR], 3.11; 95% confidence interval [CI], 2.40–4.02) than for SCC tumors (adjusted-HR, 2.20; 95% CI, 1.70–2.84; p<0.001). Propensity score matching analysis showed significantly lower CSS rates for women with pelvic nodal metastasis from non-SCC tumors than from SCC tumors (5-year CSS rate, 75.4% vs. 90.3%, p<0.001). The CSS rates for women with nodal metastasis in SCC histology were similar between the postoperative concurrent chemoradiotherapy/radiotherapy and chemotherapy groups (89.2% vs. 86.1%, p=0.42), whereas those in non-SCC histology who received postoperative chemotherapy improved the CSS (74.1% vs. 67.7%, p=0.043).
Conclusion
The node-specific staging system in the 2018 FIGO cervical cancer classification is applicable to both non-SCC tumors and SCC tumors; however, the prognostic significance of nodal metastases and efficacy of postoperative therapies vary according to histology.
Keywords: Uterine Cervical Neoplasms, Neoplasm Staging, Histological Type of Neoplasm, Lymph Node Metastasis, Survival
Synopsis
· For early-stage cervical cancer, the impact of nodal metastasis for survival outcome varied by histological subtypes.
· The postoperative practice pattern in Japan has been shifted for chemotherapy and concurrent chemoradiotherapy.
· Survival benefit of postoperative therapies appears to differ by histological subtype.
INTRODUCTION
Cervical cancer remains the most common gynecologic malignancy worldwide, and the incidence is increasing in Japan [1,2]. The National Cancer Center in Japan has estimated that approximately 10,900 new invasive cervical cancer cases were diagnosed in 2020 [3]. While early-stage cervical cancer generally has a favorable prognosis for women who undergo appropriate surgical and/or radiological treatments, tumors exhibiting certain characteristics, such as nodal metastasis, large tumor size, and specific histological subtypes, show an increased risk of recurrence [4,5].
When the standard surgical management of early-stage cervical cancer includes hysterectomy with lymph node evaluation, a recognized risk is the presence of nodal metastasis (2%–35%) [6,7,8]. Cervical cancer cases with nodal metastasis are upstaged (stage IIIC disease). Additionally, in 2018, they were re-characterized as an independent stage category in the cervical cancer staging system, according to the International Federation of Gynecology and Obstetrics (FIGO) [9]. Pathological nodal metastasis is indicated for stage IIIC cervical cancer, which is subdivided into IIIC1 for pelvic nodes only and IIIC2 for para-aortic nodal metastasis.
While nodal metastasis alters cancer staging and can affect survival, the significance of node-specific staging systems remains controversial. Recent studies suggest that the 2018 FIGO staging system provides an improved discriminatory ability for stage IB tumors reflecting the tumor size classification, however, stage III, which includes nodal metastasis, results in an extremely heterogeneous and highly variable survival rate [10,11]. To date, supporting evidence for the suitability of the FIGO 2018 classification system for cervical cancer is solely related to tumor size, and there is insufficient evidence regarding nodal-specific classifications [11]. Additionally, the incidence and the survival impact of nodal metastasis may differ according to the histological subtypes of cervical cancer [12].
This study is aimed to examine the survival impact of nodal metastasis according to histological subtypes and assess the efficacy of the FIGO 2018 classification system regarding nodal-specific classifications for early-stage cervical cancer.
MATERIALS AND METHODS
1. Data source
This was a society-based, retrospective observational study utilizing the Gynecologic Tumor Registry database from the Japan Society of Obstetrics and Gynecology (JSOG). This nationwide project was conducted within the scope of the Japan Society of Gynecologic Oncology (JSGO), and the dataset was provided by the Gynecologic Tumor Committee of the JSOG; thus, it was a JSGO-JSOG joint-study. The JSOG database is an organ-based cancer registry for gynecologic malignancies that records comprehensive information regarding cancer types, tumor characteristics, treatment types, and survival outcomes. The registry is maintained by the Gynecologic Tumor Committee of JSOG and comprises 420 local and leading regional hospitals, encompassing approximately 60% of all new patients with gynecological malignancies in Japan [13]. This study was approved by the JSOG Clinical Research Committee (2018-36-67) and the hosting institution, Tokai University School of Medicine (17R-100). Each participating institution reviewed the protocol and obtained approval as needed.
2. Eligibility criteria
Women with 2009 FIGO stage IB cervical cancer who, based on the major histology types (squamous cell carcinoma [SCC], adenocarcinoma, and adenosquamous cell carcinoma), underwent primary hysterectomy with nodal evaluation from 2004 to2015 were eligible for this analysis. Specifically, cases meeting the criteria for the 2009 FIGO stage IB1, with pathological stages T0-2, N0-2, and M0 [14], were examined. The exclusion criteria included other subgroups of stage IB2 disease, stages II-IV, unknown stages, cervical cancer other than the above three types, unknown histology, non-surgical management, and unknown nodal status.
3. Clinical information
Patient demographics, tumor characteristics, and treatment types were obtained from the database. The patient demographic data collected included age (<40, 40–49, 50–59, ≥60), year (2004–2015), and registry area (North, East, Central, West, and South). The tumor characteristics included histology type (SCC, non-SCC), pathological cancer stage, and pathological nodal metastasis (no metastasis [N0], pelvic nodal metastasis [N1], and para-aortic metastasis [N2]). The treatment types included postoperative adjuvant therapy (concurrent chemoradiotherapy [CCRT], chemotherapy only, and radiotherapy [RT] only). The survival outcomes included follow-up time, vital status, and cause of death.
4. Study definition
The clinical demographics were classified based on a previous study [15] and Statistics Bureau, Ministry of Internal Affairs and Communications [16]. Cause-specific survival (CSS) was defined as the time interval between the cervical cancer diagnosis and the patient’s death from cervical cancer. Cases without a survival event or those that were lost to follow-up were censored at the last visit with known vital status. According to the 2018 FIGO staging system, stage IIIC1 disease (N1) was defined as the involvement of pelvic lymph node metastasis only, irrespective of tumor size and extent, and stage IIIC2 disease (N2) was defined as the presence of para-aortic lymph node metastasis [9]. The definitions of the T, N and M categories corresponded to the TNM stages and were based on the pathology of diagnosis [14].
5. Study aim
The primary objective of this study was to examine the incidence and prognostic impact of nodal metastasis for apparent stage IB1 cervical cancer, stratified by histology subtypes. The secondary objective was to assess the efficacy and trend of postoperative treatments for nodal metastasis.
6. Statistical analysis
The normality of the continuous variables was assessed using the Kolmogorov–Smirnov test, and the data are expressed as the mean and standard deviation or the median and interquartile range, as appropriate. In univariable analysis, the statistical differences were assessed using the Student’s t test, one-way ANOVA test, Mann-Whitney U test, or Kruskal-Wallis H test, as appropriate. For the categorical or ordinal variables, the statistical differences were assessed using the χ2 test. The Kaplan-Meier method was used to construct survival curves, and the differences between the curves were assessed using the log-rank test. Using multivariable models, the association between nodal metastasis and CSS was assessed for each histology type by adjusting for age, year, registry area, pathological cancer stage, and postoperative adjuvant therapy. A Cox proportional hazard regression model was used for the analysis, and the magnitude of the statistical significance is expressed with a hazard ratio (HR) and 95% confidence interval (CI).
Propensity score matching was used to corroborate the background differences between the SCC and non-SCC groups [17]. The propensity score was determined using a multivariable logistic regression model. The propensity score model included age, registry area, year of disease diagnosis, pathological stage (tumor, nodal, and metastasis status), and postoperative treatment. An automated algorithm was used for 1-to-1 propensity score matching, and the optimal caliper width for estimating the differences was equal to 0.2 of the standard deviation of the logit of the propensity score [18]. In the matched model, the standardized difference was assessed to evaluate the effect size between the two groups. p-value ≤0.05 was considered to indicate a good balance between the groups.
The effect of postoperative adjuvant treatment on survival and the postoperative treatment trends were assessed for the nodal metastasis. This analysis was based on the rationale that high-risk patients exhibiting nodal metastasis are recommended to receive postoperative CCRT, as per the current guidelines [19,20], while the actual benefits of the other postoperative adjuvant treatments remain understudied for women with nodal metastasis. Additionally, temporal trends, analyzed using linear segmented regression, were assessed using The Joinpoint Regression Program (version 4.7.0.0), which was provided by the National Cancer Institute (Bethesda, MD, USA) [21]. A log transformation was subsequently performed to determine the annual percent change (APC) in the slope with a 95% CI.
All the statistical analyses were based on a two-sided hypothesis, and a p value <0.05 was considered statistically significant. The Statistical Package for Social Sciences (SPSS; version 26.0, Armonk, NY, USA) was used for all the analyses. The STROBE guidelines were consulted to display the results of the observational cohort study [22].
RESULTS
1. Patient demographics
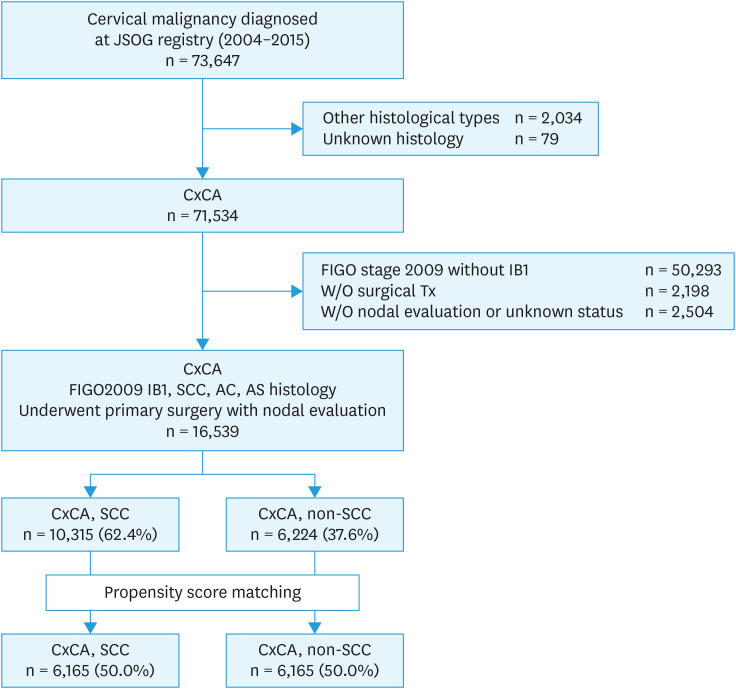
Among the 73,647 cases of cervical malignancy in the database (Fig. 1), there were 71,534 cases of the three major cervical cancer histological subtypes. Of those, 16,539 women with stage IB1 disease who had primary surgical treatments with nodal evaluation met the inclusion criteria for this study. The most common histology type was SCC (n=10,315, 62.4%), followed by adenocarcinoma (n=5,089, 30.8%), and the adenosquamous (n=1,135, 6.8%) subtype.
Fig. 1. Case selection criteria.
AC, adenocarcinoma; AS, adenosquamous cell carcinoma; CxCA, cervical cancer; JSOG, Japan Society of Obstetrics and Gynecology; SCC, squamous cell carcinoma; Tx, treatment.
The patient demographics according to histology type are shown in Table 1. Women with SCC histologies were of an age similar to those with non-SCC histologies (median 46.8 versus 47.2, respectively; p=0.08). The cohort majorly comprised women who had been diagnosed recently (2012–2015). Women with SCC histologies were registered more frequently in the Western (29.1% vs. 27.9%) and Southern (14.1% vs. 12.7%) regions of Japan, had higher rate of up-stage pathological tumor classification (pT1b2 and T2: 12.7% vs. 9.7%), nodal metastasis (10.7% vs. 8.4%) rates, and postoperative CCRT use (12.3% vs. 9.0%) than those with non-SCC histologies (all, p<0.001). Among women with nodal metastasis, the rates of pelvic nodal metastasis were higher with SCC tumors than with non-SCC tumors (9.7% vs. 7.4%, p<0.001), while the rates of para-aortic nodal metastasis were similar between SCC and non-SCC tumors (1.0% vs. 0.9%), Women with non-SCC histologies had a higher rate of postoperative chemotherapy than those with SCC histologies (19.0% vs. 13.7%, respectively; p<0.001).
Table 1. Patient demographics and clinical characteristics of the FIGO2009 IB1 cervical cancer (n=16,539).
| Characteristics | Before PS matching | After PS matching | |||||
|---|---|---|---|---|---|---|---|
| SCC | Non-SCC | p-value | SCC | Non-SCC | p-value | ||
| n=10,315 (62.4) | n=6,224 (37.6) | n=6,165 (50.0) | n=6,165 (50.0) | ||||
| Age (yr) | 46.8±12.7 | 47.2±12.1 | 0.081 | 47.0±12.4 | 47.1±12.1 | 0.684 | |
| ≥70 | 478 (4.6) | 299 (4.8) | 304 (4.9) | 297 (4.8) | |||
| 60–69 | 1,549 (15.0) | 855 (13.7) | 846 (13.7) | 851 (13.8) | |||
| 50–59 | 1,894 (18.4) | 1,164 (18.7) | 1,128 (18.3) | 1,151 (18.7) | |||
| 40–49 | 2,824 (27.8) | 1,976 (31.7) | 1,998 (32.4) | 1,937 (31.4) | |||
| <40 | 3,468 (34.2) | 1,930 (31.0) | 1,889 (30.6) | 1,929 (31.3) | |||
| Registry area | 0.001 | 0.718 | |||||
| North | 762 (7.4) | 418 (6.7) | 401 (6.5) | 417 (6.8) | |||
| East | 3,525 (34.2) | 2,326 (37.4) | 2,341 (38.0) | 2,290 (37.1) | |||
| Central | 1,580 (15.3) | 952 (15.3) | 896 (14.5) | 940 (15.2) | |||
| West | 2,998 (29.1) | 1,739 (27.9) | 1,751 (28.4) | 1,732 (28.1) | |||
| South | 1,450 (14.1) | 789 (12.7) | 776 (12.6) | 786 (12.7) | |||
| Year at diagnosis | 0.286 | 0.597 | |||||
| 2004 | 608 (5.9) | 345 (5.5) | 359 (5.8) | 341 (5.5) | |||
| 2005 | 613 (5.9) | 355 (5.7) | 339 (5.5) | 350 (5.7) | |||
| 2006 | 592 (5.7) | 377 (6.1) | 349 (5.7) | 376 (6.1) | |||
| 2007 | 713 (6.9) | 409 (6.6) | 420 (6.8) | 406 (6.6) | |||
| 2008 | 738 (7.2) | 454 (7.3) | 425 (6.9) | 452 (7.3) | |||
| 2009 | 853 (8.3) | 462 (7.4) | 516 (8.4) | 459 (7.4) | |||
| 2010 | 902 (8.7) | 553 (8.9) | 521 (8.5) | 551 (8.9) | |||
| 2011 | 980 (9.5) | 557 (8.9) | 571 (9.3) | 555 (9.0) | |||
| 2012 | 1,048 (10.2) | 658 (10.6) | 640 (10.4) | 649 (10.5) | |||
| 2013 | 1,112 (10.8) | 661 (10.6) | 683 (11.1) | 652 (10.6) | |||
| 2014 | 1,097 (10.6) | 686 (11.0) | 687 (11.1) | 677 (11.0) | |||
| 2015 | 1,059 (10.3) | 707 (11.4) | 655 (10.6) | 697 (11.3) | |||
| pT | <0.001 | 0.997 | |||||
| T1a | 280 (2.7) | 189 (3.0) | 179 (2.9) | 189 (3.1) | |||
| T1b1 | 7,296 (70.7) | 4,599 (73.9) | 4,550 (73.8) | 4,545 (73.7) | |||
| T1b2 | 396 (3.8) | 195 (3.1) | 193 (3.1) | 195 (3.2) | |||
| T1NOS | 1,415 (13.7) | 830 (13.3) | 836 (13.6) | 827 (13.4) | |||
| T2a | 366 (3.5) | 155 (2.5) | 152 (2.5) | 154 (2.5) | |||
| T2b | 562 (5.4) | 256 (4.1) | 255 (4.1) | 255 (4.1) | |||
| pN | <0.001 | 0.956 | |||||
| N0 | 9,205 (89.2) | 5,703 (91.6) | 5,640 (91.5) | 5,646 (91.6) | |||
| N1 (FIGO 2018 IIIC1) | 1,005 (9.7) | 463 (7.4) | 465 (7.5) | 462 (7.5) | |||
| N2 (FIGO 2018 IIIC2) | 105 (1.0) | 58 (0.9) | 60 (1.0) | 57 (0.9) | |||
| Histology | n.a | n.a | |||||
| SCC | 10,315 (100) | 0 | 6,165 (100) | 0 | |||
| Adenocarcinoma | 0 | 5,089 (81.8) | 0 | 5,042 (81.8) | |||
| Adenosquamous | 0 | 1,135 (18.2) | 0 | 1,125 (18.2) | |||
| Adjuvant Tx | <0.001 | 0.744 | |||||
| No adjuvant Tx | 6,444 (62.5) | 4,137 (66.5) | 4,179 (67.8) | 4,137 (67.1) | |||
| CCRT | 1,270 (12.3) | 561 (9.0) | 545 (8.8) | 561 (9.1) | |||
| RT only | 1,192 (11.6) | 346 (5.6) | 356 (5.8) | 345 (5.6) | |||
| Chemo only | 1,409 (13.7) | 1,180 (19.0) | 1,085 (17.6) | 1,122 (18.2) | |||
Data are presented as mean ± SD or number (%). The χ2 test was used to analyzed p-values. The significant p-values are emboldened.
Chemo, chemotherapy; CCRT, concurrent chemoradiotherapy; FIGO, International Federation of Gynecology and Obstetrics; NOS, not otherwise specified; pN, pathological nodal metastasis; pT, pathological T stage; RT, radiation therapy; SCC, squamous cell carcinoma.
In the entire cohort, there were 1,631 (9.9%; 95% CI, 9.4–10.3) cases of nodal metastasis, and the incidence of pelvic nodal metastasis increased as the pathological tumor stage progressed (Fig. S1). Compared to those with non-SCC histology, women with SCC histology with a pathological stage of T1b1 had a high rate of pelvic nodal metastasis (8.5% vs. 5.7%, both p<0.001). Conversely, women with an SCC histology exhibiting a pathologically up-staged tumor (pT1b2, T2a, and T2b) had a lower rate of pelvic nodal metastasis (pT1b2, 16.7% vs. 17.9%; T2a, 19.1% vs. 20.0%; and T2b, 25.8% vs. 29.7%, all p<0.05). The proportion of para-aortic nodal metastasis cases was similar between the SCC and non-SCC histologies for all the pathological tumor stages (all, p>0.05).
2. Histological type-specific survival
Survival analyses were conducted for the entire cohort. The median follow-up time was 5.2 (interquartile range, 3.8–5.7) years, and there were 671 deaths from ovarian cancer during the follow-up period. On univariable analysis, nodal metastasis was significantly associated with decreased CSS for women with SCC tumor (5-year CSS rates: without metastasis, pelvic metastasis, and para-aortic metastasis: 96.3%, 90.6%, and 73.2%, respectively, p<0.001; Fig. 2A); and these for non-SCC tumor types were (95.0%, 75.4%, and 62.4%, respectively, p<0.001; Fig. 2B). Similarly, after propensity score matching (Fig. S2), the negative impact of CSS for women with nodal metastasis was observed for both SCC and non-SCC types (all, p<0.001).
Fig. 2. Survival curves by histological subtypes.
SCC, squamous cell carcinoma.
On multivariable analysis (Table 2), pathological nodal metastasis (pN1-2), and parametrial involvement (pT2) were independent risk factors for CSS across the histological subtypes (both SCC and non-SCC; all, p<0.001). The impact of nodal metastasis was greater for non-SCC tumors (adjusted-HR, 3.11; 95% CI, 2.40–4.02) than for SCC tumors (adjusted-HR, 2.20; 95% CI, 1.70–2.84). Similar results were observed using the propensity score matched model (Fig. S3). The impact of CSS for women with nodal metastasis was greater for non-SCC histology type than for SCC histology type (pelvic metastasis: 5-year rates 75.4% vs. 90.3%; HR, 2.55; 95% CI, 1.71–3.81; p<0.001); however, it was not significant for para-aortic metastasis cases (62.4% vs. 76.3%; HR, 1.74; 95% CI, 0.82–3.68; p=0.14).
Table 2. Multivariate analysis of cause-specific survival by histological subtypes (n=16,539).
| Characteristics | Type of carcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SCC (n=10,315) | Non-SCC (n=6,143) | ||||||||
| Survival rate | Multivariable | Survival rate | Multivariable | ||||||
| No. | 5-yr (%) | HR (95% CI) | p-value | No. | 5-yr (%) | HR (95% CI) | p-value | ||
| Age (yr) | |||||||||
| ≥60 | 1,864 | 95.5 | 1 | 1,069 | 88.6 | 1 | |||
| 50–59 | 1,737 | 95.3 | 0.91 (0.65–1.27) | 0.567 | 1,081 | 92.3 | 0.64 (0.47–0.87) | 0.005 | |
| 40–49 | 2,612 | 95.7 | 1.00 (0.74–1.37) | 0.979 | 1,837 | 95.5 | 0.46 (0.34–0.63) | <0.001 | |
| <40 | 3,319 | 95.5 | 1.09 (0.81–1.47) | 0.563 | 1,804 | 94.6 | 0.57 (0.43–0.78) | <0.001 | |
| Registry area | |||||||||
| North | 695 | 96.4 | 0.83 (0.52–1.33) | 0.434 | 390 | 92.2 | 1.63 (1.06–2.50) | 0.025 | |
| East | 3,196 | 95.4 | 1 | 2,144 | 94.3 | 1 | |||
| Central | 1,472 | 95.4 | 0.94 (0.68–1.29) | 0.692 | 884 | 93.9 | 0.86 (0.60–1.23) | 0.399 | |
| West | 2,806 | 95.7 | 0.87 (0.66–1.13) | 0.294 | 1,633 | 93.0 | 1.16 (0.88–1.54) | 0.298 | |
| South | 1,363 | 95.1 | 1.13 (0.82–1.55) | 0.448 | 740 | 91.4 | 1.23 (0.88–1.74) | 0.231 | |
| Year at diagnosis | 10,315 | 0.94 (0.91–0.97) | <0.001 | 6,143 | 0.94 (0.91–0.98) | <0.001 | |||
| pT | |||||||||
| T1a | 266 | 99.2 | 0.34 (0.09–1.38) | 0.132 | 183 | 95.0 | 0.43 (0.11–1.78) | 0.249 | |
| T1b1 | 6,790 | 96.2 | 1 | 4,290 | 98.8 | 1 | |||
| T1b2 | 369 | 94.2 | 1.29 (0.81–2.05) | 0.284 | 181 | 91.0 | 1.32 (0.77–2.26) | 0.315 | |
| T1NOS | 1,237 | 94.9 | 1.33 (0.97–1.83) | 0.076 | 381 | 94.8 | 1.32 (0.92–1.90) | 0.129 | |
| T2 | 870 | 90.2 | 1.66 (1.25–2.21) | <0.001 | 756 | 71.8 | 2.76 (2.09–3.65) | <0.001 | |
| pN | |||||||||
| N0 | 4,163 | 96.3 | 1 | 5,320 | 95.0 | 1 | |||
| N1-2 | 726 | 88.9 | 2.20 (1.70–2.84) | <0.001 | 471 | 74.1 | 3.11 (2.40–4.02) | <0.001 | |
| Postoperative Tx | |||||||||
| No adjuvant Tx | 5,946 | 97.5 | 1 | 3,867 | 97.2 | 1 | |||
| CCRT | 1,152 | 89.3 | 3.06 (2.27–4.12) | <0.001 | 520 | 78.8 | 4.54 (3.27–6.30) | <0.001 | |
| RT only | 1,114 | 93.4 | 2.09 (1.52–2.88) | <0.001 | 332 | 87.6 | 2.86 (1.92–4.28) | <0.001 | |
| Chemo only | 1,320 | 93.8 | 2.10 (1.53–2.84) | <0.001 | 1,072 | 88.6 | 2.58 (1.89–3.52) | <0.001 | |
A Cox proportional hazard regression model for multivariable analysis adjusted for collected covariates. Significant p-values are emboldened.
5-yr, 5-year proportion; CCRT, concurrent chemoradiotherapy; Chemo, chemotherapy; CI, confidence interval; HR, hazard ratio; NOS, not otherwise specified; pN, pathological nodal metastasis; pT, pathological T stage; RT, radiation therapy; SCC, squamous cell carcinoma.
3. Patient demographics with postoperative therapies
In the entire cohort, there were 5,958 (36.0%; 95% CI, 35.3–36.8) cases of postoperative adjuvant therapy (Table S1). Compared to those who received CCRT/RT, women who underwent postoperative chemotherapy were younger (median 47.0 vs. 48.0/50.3 years) and were more frequently registered in the Eastern regions of Japan (37.5% vs. 28.7/32.9%) (all, p<0.001). There was a significant increase in chemotherapy use during the study period (p<0.001). Women with postoperative chemotherapy had a tumor with less parametrial involvement (T2, 14.1% vs. 25.8%), less nodal metastasis (N1-2, 18.9% vs. 32.4%), and non-SCC histology (56.5% vs. 36.4%) than those who received CCRT (all, p<0.001).
Among women with nodal metastases who underwent postoperative treatment (n=1,313, Table S2), 823 (62.7%; 95% CI, 60.1–65.7) received CCRT/RT, and 490 received chemotherapy (37.3%). Women with SCC tumor exhibited a higher rate of postoperative CCRT/RT use than those with non-SCC tumor (68.4% vs. 50.1%, p<0.001). Conversely, women with non-SCC tumor exhibited a higher rate of postoperative chemotherapy use than those with SCC tumor (49.9% vs. 31.6%, p<0.001). In addition, there was a significant increase in chemotherapy use (from 18.6% to 42.3%) among women with nodal metastasis during the study period (APC, 3.06; 95% CI, 1.45–6.06; p=0.041), whereas the utilization of CCRT remained stable (median, 43.6%; APC, 2.01; 95% CI, 0.45–4.74; p=0.10, Fig. 3).
Fig. 3. Trends in the utilization of postoperative adjuvant treatment in nodal metastasis.
4. CSS for nodal metastasis and postoperative therapies
On multivariable analysis for CSS (Table 3), the survival of postoperative adjuvant therapy for women who had SCC tumor with nodal metastasis was similar between CCRT/RT and chemotherapy (5-year rates: 89.2% vs. 86.1%; p=0.42). In contrast, women who had non-SCC tumor with nodal metastasis and received postoperative chemotherapy had an improved CSS compared to those who received CCRT/RT (chemotherapy vs. CCRT/RT, 74.1% vs. 67.7%; adjusted-HR, 0.65; 95% CI, 0.43–0.99; p=0.043).
Table 3. Multivariate analysis of cause-specific survival for nodal metastasis cases by histological subtypes (n=1,313).
| Characteristics | Type of carcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SCC (n=902) | Non-SCC (n=411) | ||||||||
| Survival rate | Multivariable | Survival rate | Multivariable | ||||||
| No. | 5-yr (%) | HR (95% CI) | p-value | No. | 5-yr (%) | HR (95% CI) | p-value | ||
| Age (yr) | |||||||||
| ≥60 | 148 | 91.5 | 1 | 80 | 65.6 | 1 | |||
| 50–59 | 182 | 91.4 | 0.84 (0.37–1.91) | 0.676 | 94 | 70.0 | 0.64 (0.35–1.18) | 0.152 | |
| 40–49 | 266 | 83.9 | 1.74 (0.87–3.46) | 0.117 | 124 | 71.2 | 0.55 (0.30–1.00) | 0.051 | |
| <40 | 306 | 85.5 | 1.78 (0.90–3.51) | 0.676 | 113 | 69.8 | 0.76 (0.42–1.35) | 0.441 | |
| Registry area | |||||||||
| East | 289 | 87.2 | 0.96 (0.62–1.49) | 0.854 | 142 | 76.7 | 0.76 (0.47–1.23) | 0.260 | |
| Other area | 613 | 87.0 | 1 | 269 | 67.4 | 1 | |||
| Year at diagnosis | 902 | 0.92 (0.87–0.98) | 0.011 | 411 | 0.99 (0.93–1.06) | 0.855 | |||
| pT | |||||||||
| T1 | 670 | 88.6 | 1 | 298 | 76.2 | 1 | |||
| T2 | 232 | 82.9 | 1.85 (1.20–2.89) | 0.006 | 113 | 55.6 | 2.21 (1.44–3.40) | <0.001 | |
| Postoperative Tx | |||||||||
| CCRT/RT | 617 | 86.1 | 1 | 206 | 67.7 | 1 | |||
| Chemo only | 285 | 89.2 | 0.83 (0.51–1.32) | 0.420 | 205 | 74.1 | 0.65 (0.43–0.99) | 0.043 | |
A Cox proportional hazard regression model for multivariable analysis adjusted for collected covariates. Significant p-values are emboldened.
5-yr, 5-year proportion; CCRT, concurrent chemoradiotherapy; Chemo, chemotherapy; CI, confidence interval; HR, hazard ratio; NOS, not otherwise specified; pT, pathological T stage; RT, radiation therapy; SCC, squamous cell carcinoma.
DISCUSSION
The main findings of the study were that nodal metastasis contributes to the risk of CSS for early-stage cervical cancer; however, the prognostic impact of nodal metastasis varies across histology types. SCC histology was more likely to progress through the pathological stage and develop nodal metastasis, whereas non-SCC with nodal metastasis exhibited a greater impact on cervical cancer death than SCC tumors. Additionally, the survival benefits from postoperative adjuvant therapies following nodal metastasis seem to vary across the histology types. Recently, postoperative chemotherapy use for high-risk women who have cervical cancer with nodal metastasis has been increasing in Japan.
Presently, radical hysterectomy is performed as the primary treatment for women with early-stage cervical cancer at more than 80% of the cancer centers in Japan [23]. Women with nodal metastasis, recognized to be at high-risk for recurrence, are recommended to undergo postoperative CCRT, according to the JSOG and NCCN guidelines [19,20]. However, in our study, approximately 40% of women with nodal metastases underwent postoperative chemotherapy. Moreover, all women with para-aortic nodal metastases underwent postoperative chemotherapy. The use of postoperative CCRT for high-risk patients has been established by randomized trials [5]; however, the role of postoperative chemotherapy for these patients lacks a consensus due to inconsistent results obtained in prior studies [24,25,26,27].
Postoperative CCRT can provide a survival benefit and localized control for high-risk patients. Additionally, cisplatin-based postoperative chemotherapy may be associated with a survival benefit via the reduction of distant metastases because chemotherapy is more effective when the tumor burden is low [24]. Data from the SWOG8797 trial showed that women who underwent postoperative CCRT for high-risk cervical cancer experienced an overall survival benefit (81%); however, the authors also reported a recurrence of distant metastases (10%) during the 4-year follow-up [5]. Therefore, in Japan treatment for women with early-stage cervical cancer majorly involves radical hysterectomies with systematic lymphadenectomy to achieve localized control [28], and if they had nodal metastasis, those procedures are followed by cisplatin-based postoperative chemotherapy to prevent distant metastasis [29].
Additionally, therapeutic resistance to RT for cervical adenocarcinoma is well-known [12]. Moreover, postoperative adjuvant chemotherapy was more utilized for women with non-SCC tumor in our study, and was associated with improved survival for high-risk patient with nodal metastasis. However, the effect of postoperative adjuvant chemotherapy on cancer survival seemed to differ according to histological subtypes, and further strategy regarding postoperative treatment according to histological subtypes is needed among high-risk cases with nodal metastasis in early-stage cervical cancer.
In our study, the use of postoperative RT decreased, the use of postoperative CCRT and chemotherapy increased, and most of the postoperative treatments were chemotherapy. These suggest that gynecologic oncologists in Japan tend to perform postoperative chemotherapy to avoid the radiation-specific-adverse events associated with CCRT. Because the majority of women in Japan are relatively small and have lower body mass indexes than women in Europe and the United States of America [30,31], acute and long-term side effects of postoperative CCRT, including gastrointestinal and genitourinary toxicities, can be severe for Japanese women [32]. Long-term patterns from case studies show that grade 3-4 complications were observed in 4%–17% of women with early-stage cervical cancer who underwent radical hysterectomies following adjuvant CCRT [5,33]. Conversely, in other published reports, the majority of the complications from postoperative chemotherapy were acute-term side effects, which were reported for 3%–11% of the women studied [27,32,34]. An understanding of the potential side effects of postoperative adjuvant treatments and efforts to prevent them are important for the patients’ quality of life and survival issues relating to post-treatments.
Data from a previous Japanese multi-center phase II trial showed that women with stage IB-IIA cervical cancer with pelvic lymph node metastasis who underwent postoperative chemotherapy had a 5-year disease-free survival rate of 86.5%, suggesting that the postoperative management had a high efficacy and was feasible [25]. Currently, the Japanese Gynecologic Oncology Group (JGOG), is examining the efficacy of postoperative chemotherapy for early-stage cervical cancer (JGOG-1082) in a phase III randomized controlled trial [23] which will ultimately address the validity of postoperative chemotherapy for this disease.
The strengths of this study include the relatively large sample size that was analyzed. Additionally, the histology-specific analysis will provide useful information to clinicians, as even common tumor types display characteristic and outcome variability. The sensitivity analyses and propensity score matching also enrich the robustness of this study.
There are also several limitations to this study. First, as it is inherent to retrospective studies, there were unmeasured biases that potentially confounded the analysis. For instance, information regarding the type of hysterectomy (class II, III, and IV) [35] and mode of surgery (minimally invasive versus laparotomy), surgeon type and experience (gynecologic oncologist versus gynecologist), CCRT or chemotherapy details (regimen, number of cycles, and dose), patients’ comorbidity, and tumor characteristics (tumor size, deep stromal invasion, and parametrium invasion), were unavailable. Similarly, the decision-making process to determine the requirement of performing para-aortic lymphadenectomy and administrating postoperative CCRT or chemotherapy was lacking. Thus, it is unknown if the choice of postoperative chemotherapy was due to the patient’s intention or based on the gynecologic oncologist’s discretion. The lack of this information hindered us from analyzing the chemotherapy type-specific benefits to the patients. Additionally, information regarding disease recurrence was unavailable.
The study results have several clinical utilities; however, awareness of high-risk nodal metastasis groups, particularly non-SCC tumor with increasing pathological stages, may be useful in the perioperative treatment planning phase. If preoperative imaging and intraoperative findings suggest the presence of a parametrium invasion and/or a large tumor that is visible to the naked eye, surgeons should be aware of the statistics regarding the substantially high likelihood of nodal metastasis. Intraoperatively, if surgeons intend to perform nodal evaluations for high-risk groups, an appropriate systemic nodal evaluation, rather than nodal mapping, will be necessary to evaluate nodal metastases more accurately [36]. To date, there are pros and cons regarding the potential survival benefits of systematic nodal resection for early-stage cervical cancer [8,37,38]; however, new FIGO staging systems demand nodal evaluation, including pelvic and para-aortic nodes. Nevertheless, further study is warranted to gain a better clinical understanding of the diagnostic and therapeutic benefits of lymphadenectomies.
In conclusion, the results of our study, in which we examined more than 16,000 women with early-stage cervical cancer, shows that non-SCC histology with nodal metastasis has a negative impact on disease survival compared to an SCC histology. Furthermore, our study demonstrates that non-SCC histologies, including adenocarcinoma and adenosquamous histologies, confer a poor survival rate compared to SCC histologies, regardless of nodal status. These results are similar to those previously reported [38]. Moreover, our results indicate that the prognostic significance of nodal metastases and efficacy of postoperative therapies vary according to histology type. This suggests that novel therapeutic strategies are warranted for women with cervical cancer exhibiting this dismal histology with nodal metastasis. Through our nodal evaluations and examination of histology types, the study results provide more meaningful information regarding the significance of nodal metastasis and the validity of the FIGO2018 classification for early-stage cervical cancer.
ACKNOWLEDGEMENTS
The authors would like to thank the member institutions of the JSOG for their cooperation in providing the data for patients with gynecological tumors. Also, the authors would like to thank all the members of the Committee on Gynecological Oncology at JSOG, as well as Dr. Matsuo M.D, PhD for his scientific input.
Footnotes
Conflicts of Interest: Honorarium, Chugai, textbook editorial expense, Springer, and investigator meeting attendance expense, VBL therapeutics (K.M.) and none for others.
- Conceptualization: M.H., M.K., Y.W., M.M.*.
- Data curation: M.H., M.K., K.Y., M.M.†, T.F., T.T., K.E., Y.W., E.Y., K.M., N.S., M.M.*.
- Formal analysis: M.H., T.F.
- Investigation: M.H., M.K., K.Y., M.M.†, T.F., T.T., K.E., Y.W., E.Y., K.M., N.S., M.M.*.
- Methodology: M.K., M.M.*.
- Project administration: M.M.*.
- Resources: M.H., M.K., K.Y., M.M.†, T.F., K.E., Y.W., E.Y., K.M., N.S., M.M.*.
- Software: M.H.
- Supervision: M.M.*.
- Validation: M.H., M.K.
- Visualization: M.H. .
- Writing - original draft: M.H., M.K., K.Y., M.M.†, T.F., T.T., K.E., Y.W., E.Y., K.M., N.S., M.M.*.
- Writing - review & editing: M.H., M.K., K.Y., M.M.†, T.F., T.T., K.E., Y.W., E.Y., K.M., N.S., M.M.*.
M.M.*, Mikio Mikami; M.M.†, Mai Momomura.
SUPPLEMENTARY MATERIALS
Patient demographics for cervical cancer with postoperative therapies (n=5,958)
Patient demographics for nodal metastasis and postoperative therapies (n=1,313)
Incidence of nodal metastasis by histological subtypes.
Survival curves by histological subtypes after propensity score matching.
Survival curves by nodal status after propensity score matching.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Nagase S, Ohta T, Takahashi F, Enomoto T 2017 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology. Annual report of the committee on gynecologic oncology, the Japan Society of Obstetrics and Gynecology: Annual patients report for 2015 and annual treatment report for 2010. J Obstet Gynaecol Res. 2019;45:289–298. doi: 10.1111/jog.13863. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Center Japan. Cancer statistics prediction [Internet] Tokyo: National Cancer Center Japan; 2021. [2021 Oct 4]. Available from: https://ganjoho.jp/reg_stat/statistics/stat/short_pred.html. [Google Scholar]
- 4.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 5.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 6.Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999;85:1547–1554. doi: 10.1002/(sici)1097-0142(19990401)85:7<1547::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Liu J, Li Y, Wan T, Feng Y, Li Z, et al. Metastasis to deep obturator and para-aortic lymph nodes in 649 patients with cervical carcinoma. Eur J Surg Oncol. 2011;37:978–983. doi: 10.1016/j.ejso.2011.08.128. [DOI] [PubMed] [Google Scholar]
- 8.Sakuragi N. Up-to-date management of lymph node metastasis and the role of tailored lymphadenectomy in cervical cancer. Int J Clin Oncol. 2007;12:165–175. doi: 10.1007/s10147-007-0661-2. [DOI] [PubMed] [Google Scholar]
- 9.Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36. doi: 10.1002/ijgo.12611. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152:87–93. doi: 10.1016/j.ygyno.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright JD, Matsuo K, Huang Y, Tergas AI, Hou JY, Khoury-Collado F, et al. Prognostic performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet Gynecol. 2019;134:49–57. doi: 10.1097/AOG.0000000000003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabuchi S, Okazawa M, Matsuo K, Kawano M, Suzuki O, Miyatake T, et al. Impact of histological subtype on survival of patients with surgically-treated stage IA2-IIB cervical cancer: adenocarcinoma versus squamous cell carcinoma. Gynecol Oncol. 2012;127:114–120. doi: 10.1016/j.ygyno.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Japan Society of Obstetrics and Gynecology. Online registration screen [Internet] Tokyo: Japan Society of Obstetrics and Gynecology; 2021. [2021 Oct 4]. Available from: http://plaza.umin.ac.jp/~jsog-go/ [Google Scholar]
- 14.World Health Organization (WHO) Female genital tumours. WHO classification of tumours. 5th ed. Volume 4. Lyon: International Agency for Research on Cancer; 2020. [Google Scholar]
- 15.Machida H, Matsuo K, Yamagami W, Ebina Y, Kobayashi Y, Tabata T, et al. Trends and characteristics of epithelial ovarian cancer in Japan between 2002 and 2015: A JSGO-JSOG joint study. Gynecol Oncol. 2019;153:589–596. doi: 10.1016/j.ygyno.2019.03.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Statistics Bureau of Japan. Classification of geographical areas [Internet] Tokyo: Ministry of Internal Affairs and Communications; 2021. [2021 Jul 27]. Available from: https://www.stat.go.jp/english/data/zensho/1999/6.html#03. [Google Scholar]
- 17.Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol. 2012;30:4215–4222. doi: 10.1200/JCO.2012.41.6701. [DOI] [PubMed] [Google Scholar]
- 18.Zhao XH, Zhang T, Xu M, Yao JH. Effects of physically effective fiber on chewing activity, ruminal fermentation, and digestibility in goats. J Anim Sci. 2011;89:501–509. doi: 10.2527/jas.2010-3013. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Cervical cancer [Internet] Plymouth Meeting, PA: National Comprehensive Cancer Network; 2021. [2021 Oct 4]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1426. [Google Scholar]
- 20.Ebina Y, Mikami M, Nagase S, Tabata T, Kaneuchi M, Tashiro H, et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int J Clin Oncol. 2019;24:1–19. doi: 10.1007/s10147-018-1351-y. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Lee S, Choi JI, Cho H. Binary genetic algorithm for optimal joinpoint detection: Application to cancer trend analysis. Stat Med. 2021;40:799–822. doi: 10.1002/sim.8803. [DOI] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furusawa A, Takekuma M, Mori K, Usami T, Kondo E, Nishio S, et al. A randomized phase III trial of adjuvant chemotherapy versus concurrent chemoradiotherapy (CCRT) for postoperative cervical cancer: Japanese Gynecologic Oncology Group study (JGOG1082) Int J Gynecol Cancer. 2021;31:623–626. doi: 10.1136/ijgc-2020-002344. [DOI] [PubMed] [Google Scholar]
- 24.Monk BJ, Wang J, Im S, Stock RJ, Peters WA, 3rd, Liu PY, et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol Oncol. 2005;96:721–728. doi: 10.1016/j.ygyno.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Takeshima N, Umayahara K, Fujiwara K, Hirai Y, Takizawa K, Hasumi K. Treatment results of adjuvant chemotherapy after radical hysterectomy for intermediate- and high-risk stage IB-IIA cervical cancer. Gynecol Oncol. 2006;103:618–622. doi: 10.1016/j.ygyno.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Takekuma M, Shimokawa M, Nishio S, Omi H, Tabata T, Takei Y, et al. Phase II study of adjuvant chemotherapy with paclitaxel and nedaplatin for uterine cervical cancer with lymph node metastasis. Cancer Sci. 2018;109:1602–1608. doi: 10.1111/cas.13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matoda M, Takeshima N, Michimae H, Iwata T, Yokota H, Torii Y, et al. Postoperative chemotherapy for node-positive cervical cancer: results of a multicenter phase II trial (JGOG1067) Gynecol Oncol. 2018;149:513–519. doi: 10.1016/j.ygyno.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Mikami M, Aoki Y, Sakamoto M, Shimada M, Takeshima N, Fujiwara H, et al. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (Bulky Tumors) in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer. 2014;24:1333–1340. doi: 10.1097/IGC.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda Y, Furusawa A, Kitagawa R, Tokinaga A, Ito F, Ukita M, et al. Practice patterns of adjuvant therapy for intermediate/high recurrence risk cervical cancer patients in Japan. J Gynecol Oncol. 2016;27:e29. doi: 10.3802/jgo.2016.27.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds SL, Hagedorn A, Yeom J, Saito Y, Yokoyama E, Crimmins EM. A tale of two countries--the United States and Japan: are differences in health due to differences in overweight? J Epidemiol. 2008;18:280–290. doi: 10.2188/jea.JE2008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 32.Machida H, Matsuo K, Furusawa A, Kita T, Kitagawa R, Mikami M. Profile of treatment-related complications in women with clinical stage IB-IIB cervical cancer: a nationwide cohort study in Japan. PLoS One. 2019;14:e0210125. doi: 10.1371/journal.pone.0210125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SW, Chun M, Ryu HS, Chang SJ, Kong TW, Oh YT, et al. Long-term results of early adjuvant concurrent chemoradiotherapy for high-risk, early stage uterine cervical cancer patients after radical hysterectomy. BMC Cancer. 2017;17:297. doi: 10.1186/s12885-017-3299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosaka M, Watari H, Kato T, Odagiri T, Konno Y, Endo D, et al. Clinical efficacy of paclitaxel/cisplatin as an adjuvant chemotherapy for patients with cervical cancer who underwent radical hysterectomy and systematic lymphadenectomy. J Surg Oncol. 2012;105:612–616. doi: 10.1002/jso.22136. [DOI] [PubMed] [Google Scholar]
- 35.Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. 1974;44:265–272. [PubMed] [Google Scholar]
- 36.Altgassen C, Hertel H, Brandstädt A, Köhler C, Dürst M, Schneider A, et al. Multicenter validation study of the sentinel lymph node concept in cervical cancer: AGO Study Group. J Clin Oncol. 2008;26:2943–2951. doi: 10.1200/JCO.2007.13.8933. [DOI] [PubMed] [Google Scholar]
- 37.Shah M, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ, et al. Therapeutic role of lymphadenectomy for cervical cancer. Cancer. 2011;117:310–317. doi: 10.1002/cncr.25408. [DOI] [PubMed] [Google Scholar]
- 38.Macdonald OK, Chen J, Dodson M, Lee CM, Gaffney DK. Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix. Am J Clin Oncol. 2009;32:411–416. doi: 10.1097/COC.0b013e31819142dc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient demographics for cervical cancer with postoperative therapies (n=5,958)
Patient demographics for nodal metastasis and postoperative therapies (n=1,313)
Incidence of nodal metastasis by histological subtypes.
Survival curves by histological subtypes after propensity score matching.
Survival curves by nodal status after propensity score matching.