Abstract
Vaginal microbiota plays a central role in women’s health and reproduction. Vaginal microbiota is dynamic and shaped by hormonal shifts in each stage of a woman’s life from pre-puberty to postmenopause. Current research has mainly focused on vaginal bacterial and fungal members of the community and emphasized their role in disease. However, the impact of balanced vaginal microbiota on health and its interaction with the host is yet poorly understood. High abundance of vaginal lactobacilli is most strongly associated with health, but the concept of health may vary as vaginal dysbiosis may be asymptomatic. Furthermore, there is a lot of variation between ethnic groups in terms of dominating vaginal bacteria. Probiotic lactobacilli could be a safe and natural means to balance and maintain healthy vaginal microbiota. Research evidence is accumulating on their role in supporting women’s health throughout life. This review describes the current literature on vaginal microbiota, the major factors affecting its composition, and how the communities change in different life stages. Furthermore, we focused on reviewing available literature on probiotics and their impact on vaginal microbiota and health.
Keywords: vaginal, microbiota, mycobiota, lactobacilli, probiotics, health, dysbiosis
Introduction
In the past decade, exploration of human microbiota has increasingly focused on vaginal microbiota (VMB) composition, diversity, and its impact on health, reproduction, and disease. VMB is known to consist of various bacteria, viruses, archaea, fungi, and protozoa (Ursell et al., 2012; Dekaboruah et al., 2020). Nevertheless, the research has mainly focused on bacterial and fungal members in the vaginal community and their association with women’s health. Typically, VMB is described in the context of vaginal infections or dysbiosis, which occurs, e.g., via antibiotic use or lifestyle changes. Most common infections and conditions include vulvovaginal candidiasis (VVC) and bacterial vaginosis (BV), respectively [reviewed by van de Wijgert and Jespers (2017), De Bernardis et al. (2018)]. BV is in turn associated with urinary tract infections (UTIs), increased risk of infertility, fallopian tube (uterine tube) inflammation, adverse pregnancy outcomes, and preterm birth (PTB) [Hootan et al., 1989; Hay et al., 1994; Hillier et al., 1995; Koumans et al., 1999; Hillerbrand et al., 2002; reviewed by van Oostrum et al. (2013)]. Moreover, dysbiosis in the vaginal tract is associated with sexually transmitted infections such as human immune deficiency virus (HIV), human papillomaviruses (HPV), herpes, chlamydia, and gonorrhea [reviewed by Gupta et al. (2019)].
In contrast to the gut (Eisenstein, 2020), defining the characteristics of healthy microbiota in the vaginal tract seems to be less challenging, as one of the hallmarks of a healthy vaginal tract is low bacterial diversity and high abundance of vaginal lactobacilli. These lactobacilli play a fundamental role in interacting with the host innate immune system, and research shows that low abundance of lactobacilli increases the risk of several vaginal infections (Amabebe and Anumba, 2018b). Lactobacilli for instance produce lactic acid which creates an acidic microenvironment and thus prevents the overgrowth of potentially harmful bacteria. However, the definition of healthy VMB is not straightforward. For example, vaginal dysbiosis is not always presented with symptoms and not all species of vaginal lactobacilli are equally protective (Mitchell et al., 2017; Smith and Ravel, 2017). VMB varies among ethnic groups, where healthy/balanced state is maintained with more diverse VMB composition (e.g., among Black or African American) (Zhou et al., 2007). Furthermore, hormonal fluctuations shape the microbial community even on a daily or monthly basis (Eschenbach et al., 2000). In addition, the ways on how the healthy VMB is shaped in women at different stages of life have not been comprehensively characterized.
Vaginal dysbiosis and associated infections affect all women during their lifetime by causing discomfort and are the most common reasons why women seek medical care [reviewed by van de Wijgert and Jespers (2017), Peebles et al. (2019)]. Unfortunately, antibiotics and antifungal medicines to treat BV and candidiasis are not always optimal in efficacy as bacteria and fungi are able to resist these treatments by forming biofilms or they have naturally acquired resistance (Swidsinski et al., 2008, 2015; Bhattacharya et al., 2016, 2020). Antibiotics may also disrupt endogenous lactobacilli promoting BV and potentially candidiasis recurrence (Bradshaw and Sobel, 2016; Verwijs et al., 2020). Therefore, rigorous research is underway to identify more effective and natural solutions for balancing VMB and maintaining healthy vaginal environment. Well-characterized probiotic bacteria, which are typically lactobacilli, could be applied for maintaining healthy VMB or restoring normal microbiota after antibiotic treatment. Indeed, several systematic reviews and meta-analyses suggest that probiotic use could be potential, e.g., in helping to relieve BV (Li et al., 2019; Wang et al., 2019) or VVC (Xie et al., 2017; Jeng et al., 2020).
The main objectives of this review were to provide first an overview of VMB and major factors affecting the composition. Secondly, we focused on identifying and describing the key changes in VMB throughout different life stages of women and discussed the impact of these changes in health. As probiotics are targeted for maintaining and promoting health and healthy microbiota, we also explored the probiotic’s role on VMB and women’s health across the lifespan.
Overview of Vaginal Microbiota and Factors Affecting the Composition
Advancements in the next-generation sequencing technologies and bioinformatic tools have rapidly increased our understanding of all members in the VMB community—bacteria, viruses, archaea, fungi, and protozoa, of which identification was previously difficult with other methods, such as culturing (Hong et al., 2016; Ceccarani et al., 2019). However, if there are no cultured isolates for a bacterium identified with molecular techniques, full understanding of the potential impact/mechanism of action of how the specific bacterium may influence vaginal health and/or disease may not be fully comprehended. In these cases, culturomics approach, using dozens of different culture media and conditions, could prove useful (Lagier et al., 2012). Moreover, advances in culturomics have enabled isolation and identification of hundreds of new human-associated bacteria. In practice, the number of cultured human-associated bacterial species increased from 2,776 in 2018, to 3,253 in 2020 (Diakite et al., 2021). Within these new genera/species were also many that originated from vaginal isolates, such as Vaginimicrobium propionicum, a novel propionic acid bacterium isolated from a healthy human’s vaginal discharge (Diop et al., 2020) and Janibacter massiliensis isolated from vaginal discharge of a bacterial vaginosis patient (Maaloum et al., 2019).
Thus, what was previously regarded as relatively simple microbial community has now been revealed to be in fact dynamic and complex. Each woman has her own unique VMB composition, which fluctuates over time and is affected, e.g., by diet, lifestyle, hormones, genetics, and age [reviewed by Farage and Maibach (2006); Gupta et al. (2019)]. Here we focused on the bacterial and fungal members of the community as these are the most prevalent microbes associated with vaginal health.
Bacteria and Fungi
Vaginal microbiota composition in humans is relatively unique when compared with other mammals, including non-human primates. For instance, lactobacilli typically dominate 70% of the human VMB community, while in other mammals, lactobacilli comprise only low percentages of the total VMB composition (Stumpf et al., 2013; Miller et al., 2016). This in turn is associated with low glycogen and lactic acid levels. Consequently, other mammals have vaginal pH closer to neutral.
Advanced molecular detection methods utilizing 16S rRNA gene sequencing have enabled clustering of the human vaginal bacterial community into specific community state types (CSTs) (Smith and Ravel, 2017; France et al., 2020). CST I, II, III, and V are dominated by Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii, respectively. CST IV includes higher abundance of strictly anaerobic bacteria such as species from genera Prevotella, Dialister, Atopobium, Gardnerella, Megasphaera, Peptoniphilus, Sneathia, Eggerthella, Aerococcus, Finegoldia, and Mobiluncus and is characterized by higher diversity and evenness (Ravel et al., 2011; Gajer et al., 2012; France et al., 2020). CST IV can be further divided into subgroups: CST IV-A containing a proportion of L. iners and strict anaerobes, and CST IV-B containing BV-associated bacterial species (BVAB) (bacteria in the order Clostridiales) (Anahtar et al., 2018; France et al., 2020). CST IV-C is characterized by the abundance of a diverse array of facultative and strictly anaerobic bacteria and can be further divided into five sub-categories (France et al., 2020). Overall, CST I, II, and V are most often associated with health, whereas predominance of CST IV can manifest clinically as BV or aerobic vaginitis (AV). Interestingly, CST III has been associated with both health and dysbiosis, and often with BV.
The role and impact of fungi overall are much less understood compared to bacteria. Although fungi have an important role in maintaining homeostasis in the human body, and fungi contribute to our immune defense, much less is known about the role of fungi in healthy vagina throughout the lifespan. However, knowledge on the fungal community structure and distributions is rapidly increasing (Nash et al., 2017; Chee et al., 2020). High diversity is observed within and across individuals (Nash et al., 2017; Forbes et al., 2019), but this diversity is significantly lower than the bacterial diversity (Nash et al., 2017) as well as greater unevenness when compared to the bacterial communities (Raimondi et al., 2019). However, fungi comprise a smaller portion of human microbial sequences compared to bacteria (Kong and Morris, 2017). The fungal community, including yeasts and filamentous fungi colonizing the lower female reproductive tract, is referred to as vaginal mycobiota (Bradford and Ravel, 2017). Vaginal fungi mainly belong to Ascomycota and Basidiomycota (Drell et al., 2013; Bradford and Ravel, 2017; Chee et al., 2020). The predominant fungal genera in the genitourinary tract (vagina) include Candida, Cladosporium, Pichia, Aspergillus, and Rhodotorula (Drell et al., 2013; Lai et al., 2019). Notably, many studies reported unspecified fungal sequences which were not taxonomically identified during that time (Drell et al., 2013). In total, approximately 390 different fungi have been associated with human skin, vagina, oral cavity, and gut samples so far (Gouba and Drancourt, 2015). Changes in bacterial communities due to the dysbiosis is known to increase opportunities for fungal growth and for opportunistic pathogens.
Candida is the predominant genus in the vaginal mycobiota, and several species have been detected including Candida albicans, C. alimentaria, C. dubliensis, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis (Drell et al., 2013; Ward et al., 2018; Chee et al., 2020). Interestingly, vaginal colonization with Candida spp. seems to occur more commonly in women with a lactobacilli-dominated VMB than in women with dysbiosis (van de Wijgert et al., 2014). The research so far has been mainly focused on C. albicans, which is the best-known member of the vaginal mycobiota (Bradford and Ravel, 2017). It is an opportunistic pathogen causing VVC. However, C. albicans has been reported to be the dominant fungal species in asymptomatic non-pregnant women and it colonizes 20% of women (Drell et al., 2013; Ameen et al., 2017; Chee et al., 2020). Our recent study supported the previous findings that C. albicans and/or other Candida spp. were detectable in approximately 17% of samples derived from healthy women (Lehtoranta et al., 2021). However, a lot of variation exists between the studies as Drell et al. (2013) reported that Candida spp. could be detected in 65% of asymptomatic healthy women.
Major Factors Affecting the Composition
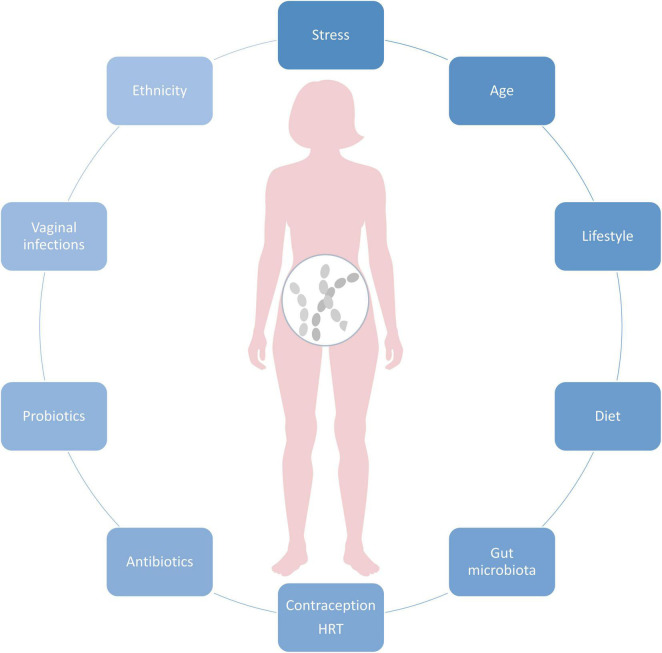
Vaginal microbiota composition is highly dynamic and affected by age, ethnicity, and physiological factors, such as monthly hormonal fluctuations and the immune system. Vaginal infections, medications, as well as probiotics shape the VMB, as well as lifestyle and diet (Figure 1). In the below chapters we focus on major known modulators of VMB.
FIGURE 1.
Key factors affecting vaginal microbiota composition.
Ethnicity
Genetic heritage plays a role determining VMB composition as the CSTs are known to vary among ethnic groups. In general, lactobacilli dominate the VMB among the Asian and White women, whereas Black or African-American and Hispanic women are more likely to be dominated with diverse VMB, containing several BV-associated species, such as Gardnerella vaginalis, BVAB1, BVAB2, Atopobium vaginae, Megasphaera spp., Sneathia spp., and Prevotella spp. (Ravel et al., 2011; Fettweis et al., 2014; Borgdorff et al., 2017; Gupta et al., 2017) and CST IV-A or B (France et al., 2020). Women with black ethnicity are considerably more likely to be diagnosed with BV and PTB (Fettweis et al., 2014). Interestingly, L. iners-dominated VMB (CST III) is prevalent in VMB of women in all ethnic groups [reviewed by Petrova et al. (2017)]. Limited evidence exists for vaginal Candida species and their prevalence among ethnic groups (Wei et al., 2010).
Menstrual Cycle
Reproductive hormones and especially estrogen play a key role in shaping the VMB composition throughout life from the onset of puberty, during reproductive years toward menopause, and postmenopause when the estrogen levels decline [reviewed by Moosa et al. (2020)].
In the vaginal epithelial cells, estrogen increases glycogen storage, which is an important energy source for vaginal lactobacilli affecting their ability to colonize the vaginal tract and produce lactic acid, and which further promotes the ability to grow and thrive in acidic conditions. Therefore, it is evident that estrogen fluctuations throughout menstrual cycle modulates VMB composition (Eschenbach et al., 2000; Srinivasan et al., 2010; Gajer et al., 2012). The highest estrogen levels occur in the follicular phase, which correlates with dominance of lactobacilli and low bacterial diversity (Kaur et al., 2020). Gajer et al. (2012) investigated temporal dynamics of the vaginal bacterial communities in 32 women at reproductive age over 16 weeks (including 4–5 menstrual cycles). The study revealed variation across women in terms of how communities changed over short periods of time. For instance, women with CST IV-B often transitioned to CST III. Likewise, women with CST I (L. crispatus) most often transitioned to CST III (L. iners), or CST IV-A. Interestingly, CST II (L. gasseri) rarely underwent transitioned to other CSTs. The bacterial communities seemed to be less stable during menses and to have low abundance of lactobacilli, although diversity was not necessarily increased. Similarly, in another study with 14 healthy women, Srinivasan et al. (2010) showed that vaginal lactobacilli fluctuated over time. However, at the onset and during menses, the dominance of L. crispatus and L. jensenii significantly decreased, whereas G. vaginalis and L. iners increased and subsequently decreased with the end of menstruation. This finding may be potentially explained by the fact that G. vaginalis requires iron for growth and could acquire iron from menstrual blood by lysing erythrocytes with vaginolysin (Jarosik et al., 1998; Srinivasan et al., 2010). L. iners is also able to grow well in blood agar (Falsen et al., 1999), but not in MRS or Rogosa agar, indicating the potential requirement of iron of this species for growth and thus may explain why the strains favor co-occurrence during menses. Another longitudinal study investigating VMB composition throughout two menstrual cycles reported that L. crispatus was the least affected by the menses (Lopes Dos Santos Santiago et al., 2011). With regard to the effects of menstrual cycle on the mycobiota, studies suggest that the estrogen peak during ovulation stimulates the growth of C. albicans resulting in higher occurrence of symptoms of C. albicans later in the luteal phase (Eschenbach et al., 2000).
Despite the widespread use of hormonal contraceptives during reproductive years (United Nations, Department of Economic and Social Affairs, Population Division, 2019), their effects on VMB communities and the dynamics have not been fully elucidated (Achilles and Hillier, 2013; Anahtar et al., 2018) and there are inconsistent results due to variable contraceptive methods used. However, it seems that there is an association between oral contraceptives and reduced BV (Achilles and Hillier, 2013; Brooks et al., 2017; Anahtar et al., 2018). For yeasts, there is no consensus whether hormonal contraceptives increase or reduce vaginal yeast colonization or their infections (Achilles and Hillier, 2013). A study by Song et al. showed that healthy women not using hormonal contraceptives and women using combined contraceptives had similar periodic fluctuations of VMB that corresponded to stages of the menstrual cycle and high Lactobacillus abundance. Then, women with progestin-only contraceptives showed altered periodic fluctuations of VMB and low average abundance of Lactobacillus (Song et al., 2020).
Lifestyle
Similar to the gut microbiota, different lifestyle factors influence VMB composition [reviewed by Bradford and Ravel (2017); Moosa et al. (2020)]. Here we focus on the most documented factors as well as emerging factors influencing VMB that are a direct result of modernization of the society. Intuitively, personal hygiene practices (i.e., vaginal douching, use of soaps, type of underwear, menstrual protection, and sprays) are the most direct ways to affect VMB composition. Among these, research suggests that vaginal douching seems to be most strongly associated with increased risk of BV/vaginal dysbiosis (Klebanoff et al., 2010; Lewis et al., 2017). In contrast, for the occurrence of Candida infection type in VVC, vaginal douching seems to have a low impact (Shaaban et al., 2015). Regarding sexual habits, multiple sexual partners are also a known risk factor for BV/lactobacilli depletion (Beigi et al., 2005; Schwebke and Desmond, 2005).
In addition, smoking is a well-known factor to increase the risk of vaginal dysbiosis and BV (Hellberg et al., 2000; Cherpes et al., 2008; Brotman et al., 2014a) by, e.g., affecting estrogen production (Westhoff et al., 1996) and altering vaginal metabolite production profile, particularly by increasing levels of nicotine and derivatives as well as biogenic amines (Nelson et al., 2018). Similarly, alcohol use is associated with increased BV cases (Francis et al., 2016).
Interestingly, new emerging research indicates that the modernization of the society related to, e.g., increased psychological stress, consumption of processed food rich in fat and carbohydrates, and urbanization has an impact on VMB [Neggers et al., 2007; Thoma et al., 2011b; reviewed by Amabebe and Anumba (2018a); Vargas-Robles et al. (2020)]. Women experience more stress than men (American Psychological Association, 2018) and the effects of stress seem to extend to the vaginal tract. More specifically, research implies that chronic psychosocial stress can influence the balance in the vaginal lactobacilli, potentially through dysregulated immune system and elevated cortisol levels, which further correlate with reduced vaginal glycogen, lower abundance of lactobacilli, elevated vaginal pH, and increased proinflammatory response [reviewed by Amabebe and Anumba (2018a)].
Diet does not only shape the gut microbiota, but the effects are known to extend to the vaginal tract. More specifically, research suggests that healthy diet rich in nutrients and with low glycemic index and lower fat intake could reduce the risk of BV (Neggers et al., 2007; Thoma et al., 2011b). Furthermore, micronutrient intake, particularly increased folate, vitamin A, and calcium could decrease BV risk (Neggers et al., 2007). In addition, diet rich in betaine is associated with higher vaginal Lactobacillus spp. abundance (Tuddenham et al., 2019). Interestingly, a recent study by Song et al. (2020) reported that overall vaginal microbial diversity was higher among vegetarian women than non-vegetarians, although the sample size was small. Brookheart et al. (2019) reported that independent of ethnicity, overweight and obese women suffer more frequently from BV than lean women. Potential underlying reasons include obesity-associated dysfunction/disturbances in the host metabolism, hormonal, and immune system regulation which may affect vaginal environment. Furthermore, obesity is associated with dysbiotic gut microbiota composition which can further affect VMB composition by serving as an “extravaginal source” for vaginal bacteria (Marrazzo et al., 2012). However, larger studies exploring the role of various diets and/or body mass index (BMI) on specific CSTs are lacking.
Urbanization may also play a role in affecting the VMB composition by increasing diversity, but the data is yet limited to a few ethnic groups (Vargas-Robles et al., 2020). Among the socioeconomic factors, there is an indication that the education level seems to be associated with VMB composition. A study by Virtanen et al. (2019) showed that Finnish women aged 25–45 with higher education level had more often Lactobacillus-dominated VMB community (especially L. crispatus).
Immune System
Vaginal mucosal immune system both interacts with and regulates VMB composition [e.g., reviewed by Smith and Ravel (2017); Villa et al. (2020)]. The interplay between the immune system and the VMB is complex and involves variable factors such as epithelial and immune cells, antimicrobial peptides, pro/anti-inflammatory cytokines/chemokines, as well as secretory antibodies (Wira et al., 2010; Yarbrough et al., 2015). Epithelial and immune cells (dendritic cells) in the cervicovaginal mucosa maintain homeostasis with the VMB and simultaneously survey for pathogens. These cells detect microbial structures (antigens) via pattern recognition receptors such as Toll-like receptors (TLRs), which induces production of antimicrobial peptides as well immunomodulatory cytokines/chemokines. Dendritic cells further function as an integral link between innate and adaptive immune system by presenting antigens to other immune cells such as macrophages, NK-cells, neutrophils, and T- and B-cells (White et al., 1997; Duluc et al., 2013). In general, when epithelial cells encounter endogenous vaginal lactobacilli, epithelial cells produce low levels of antimicrobial peptides/cytokines resulting in mucosal homeostasis. Depletion of the endogenous lactobacilli and the dominance of BV-associated community results in higher levels and a different secretion profile of antimicrobial peptides as well as inflammatory cytokines/chemokines [reviewed by Smith and Ravel (2017), Yarbrough et al. (2015)]. Specifically, there are indications that vaginal lactobacilli assigned to CSTs affect differently the type and magnitude of innate immune response. For instance, there are reports showing that CST-IV induces higher pro-inflammatory response (IL-1α, IL-1β, TNF- α, IFN-γ, IL-4, IL-8, IL-12p70) than CST-I or CST-II, whereas CST-III induces an intermediate response (IL-8) (Rose et al., 2012; Anahtar et al., 2015). However, more studies are needed to understand in-depth how different CSTs regulate the immune system in a healthy state and transition into dysbiosis.
Vaginal Infections and Disorders/Dysbiosis and Antimicrobial Therapy
Bacterial vaginosis is the most common vaginal disorder/dysbiosis of women affecting at least 30% of women in reproductive age annually (Koumans et al., 2007; Bradshaw et al., 2013). BV is characterized by a decrease in lactobacilli and increase in atypical anaerobic bacteria (van de Wijgert and Jespers, 2017). BV increases the risk of the acquisition of sexually transmitted diseases, such as Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, herpes simplex virus type 2 (HSV-2), HPV, and HIV (Cherpes et al., 2003; Wiesenfeld et al., 2003; Myer et al., 2005; Allsworth et al., 2008; Brotman et al., 2010; Liang et al., 2019). BV is also a risk factor for other infections such as pelvic inflammatory disease, endometritis, chorioamnionitis, and amniotic fluid infection (Hay et al., 1994; Hillier et al., 1995; Koumans et al., 1999). C. albicans-associated VVC is one of the most common mucosal infections in the female genital tract (De Bernardis et al., 2018). Estimations show that majority of women (>75%) suffer from Candida infections at least once in their lifetime (Bongomin et al., 2017). Some women are more susceptible to VVC and recurrence of VVC is common (Blostein et al., 2017). C. glabrata is the second most common type causing 15% of the infections (Shaaban et al., 2013).
The major modulators of VMB composition are antibiotics and antifungal medicines targeted against vaginal infections. Metronidazole and clindamycin are the first-line antibiotic regimens for BV. The short-term cure rates are approximately 80%, with 50% recurrence rate within 6–12 months (Bradshaw and Sobel, 2016). Biofilm formation as well as antibiotic resistance of BV-associated bacteria such as G. vaginalis may be determinant factors for persistence and recurrence (Bradshaw and Sobel, 2016; Verwijs et al., 2020). The same applies for C. albicans which is effective in biofilm formation (Chandra et al., 2001). The hyphal (mycelial) form contributes to adherence and mucosal invasion is characteristic of symptomatic disease (Pappas et al., 2018). Biofilm provides increased virulence as well as resistance to host immune responses’ antimicrobial agents and may lead to recurrent candidiasis and reduced impact of antifungal treatments (Bradford and Ravel, 2017; Kalia et al., 2020). With regard to endogenous lactobacilli, some trials show that use of metronizadole, doxycycline, azithromycin, clotrimazole, and fluconazole has no substantial impact on overall vaginal Lactobacillus spp. colonization (Agnew and Hillier, 1995; Nyirjesy et al., 2006). Specific vaginal lactobacilli tolerate metronidazole up to 1000 μg/ml in vitro (Simoes et al., 2001). Furthermore, in women with diagnosed BV, few studies show that VMB seems to recover from metronidazole treatment (metronidazole) within a few days (Mayer et al., 2015; Lehtoranta et al., 2020), and several studies show that L. iners dominates the community in transition to the recovery (Macklaim et al., 2015; Mayer et al., 2015; Deng et al., 2018; Lehtoranta et al., 2020).
Probiotics
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014). As probiotics are commonly lactobacilli, their role in vaginal health has been extensively investigated especially in the context of vaginal infections in premenopausal women (Borges et al., 2014; Petrova et al., 2015). Increasing evidence show that specific probiotic strains or their combinations elevate vaginal lactobacilli counts in healthy women or women with BV and/or VVC and support natural VMB during/after recovery from antibiotics/antifungal treatment (Xie et al., 2017; Li et al., 2019). However, there is high heterogeneity among the studies due to variation in study designs and probiotic strains used. Probiotics may elicit beneficial effects in the vaginal tract in several ways, i.e., by producing lactic acid and hydrogen peroxide to lower the vaginal pH. Furthermore, probiotics may produce antimicrobial compounds and stimulate the immune system to help to maintain bacterial balance in the vaginal tract. By adhering to vaginal epithelia, probiotics may inhibit attachment of pathogenic bacteria and utilize the same nutrients as pathogens and thereby restrict their growth (Petrova et al., 2015; Chee et al., 2020).
Probiotics targeted for vaginal health are widely available as dietary supplements or vaginal capsules/suppositories. In vaginal applications, probiotics are applied directly at the site of action, whereas orally supplemented probiotics need to first passage through the gastrointestinal tract before migrating to the vaginal tract. Interestingly, research shows that both application routes are efficacious (Li et al., 2019; Wang et al., 2019). Orally administered probiotics, however, may provide additional beneficial effects to vaginal health via so called “gut-vagina axis” by balancing gut microbiota and inhibiting/preventing ascension of urogenital pathogens from the rectum to vaginal tract as well as stimulating the gut and systemic immune system.
Vaginal Microbiota Changes Across the Life Span and Impact in Health
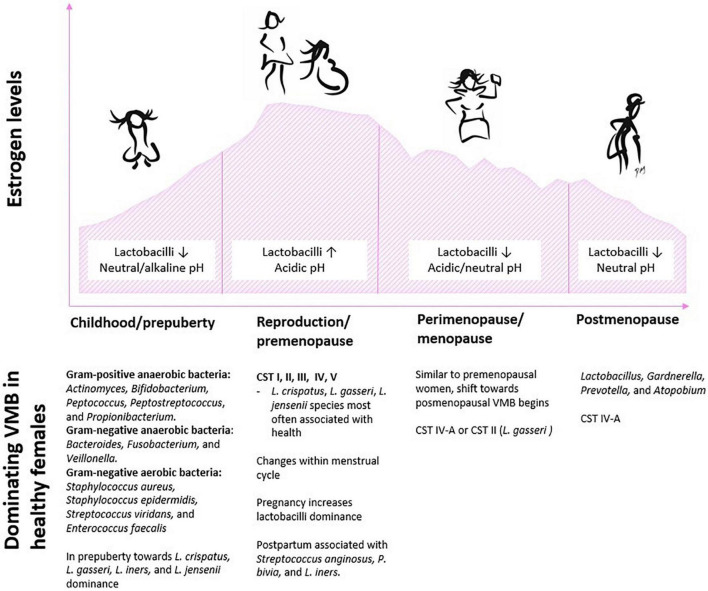
The VMB composition changes considerably across the female life span from childhood to the onset of puberty, during reproduction and pregnancy, as well over the transition period to menopause and after menopause (Figure 2). Below we take a closer look at the VMB composition at each lifestage and review available probiotic intervention studies conducted at each stage and summarize the key findings in Table 1. The primary focus here is on bacteria, since very little is known about the development of healthy vaginal mycobiota throughout life.
FIGURE 2.
Schematic illustration of typical characteristics and an overview of dominating bacteria in the vaginal environment in context with fluctuating estrogen levels across the female life span. Abbreviations: CST, community state type; VMB, vaginal microbiota.
TABLE 1.
Summary of potential benefits of probiotic intervention studies on vaginal microbiota (VMB) throughout female life span.
| Childhood/puberty/adolescence | Fertility | Pregnancy | Menopause/postmenopause |
| - Lack of probiotic studies to conclude potential benefits in these age groups | - Potential to balance VMB composition, but findings yet inconclusive | - No significant changes in overall VMB composition reported - Some benefits in Group B Streptococcus colonization - No safety concerns - Additional potential benefits include - Balancing gut microbiota - Supporting metabolic health - Postpartum well-being |
- Potential to balance and maintain healthy VMB composition as evidenced by - Increase in vaginal lactobacilli levels after consumption/application - Nugent score between 0 and 3 - Reduction of the risk of vaginal infections |
Childhood and Puberty/Adolescence
During perinatal development, residual maternal estrogen induces thickening of the vaginal epithelium and glycogen deposit in the epithelial cells. When epithelial cells exfoliate, glycogen is released, which favors glucose-fermenting micro-organisms. After birth, the maternal estrogen is metabolized, resulting in a thinning of the mucosa and reduction in glycogen- and glucose-fermenting microorganisms, and leads to dominance of wide range of aerobes and facultative anaerobes [reviewed by Younes et al. (2018)]. In childhood, VMB is dominated by Gram-positive anaerobic bacteria (including species from genera Actinomyces, Bifidobacterium, Peptococcus, Peptostreptococcus, and Propionibacterium), Gram-negative anaerobic bacteria (such as species from genera Bacteroides, Fusobacterium, and Veillonella), as well as aerobic bacteria (such as Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus viridans, and Enterococcus faecalis) (Hammerschlag et al., 1978a,b; Myhre et al., 2002; Ranđeloviė et al., 2012). The vaginal pH of a young girl changes from birth until pre-puberty to become neutral or slightly alkaline [reviewed by Farage and Maibach (2006)].
Prepubertal girls have low abundance of lactobacilli, G. vaginalis, and Prevotella bivia in the vaginal tract (Myhre et al., 2002; Ranđeloviė et al., 2012). In the onset of puberty, when estrogen levels increase, the vaginal epithelium thickens, which selectively favors glucose-fermenting microorganisms. The microbiome of adolescent girls resembles VMB of adult women and is dominated by L. crispatus, L. gasseri, L. iners, and L. jensenii (Yamamoto et al., 2009; Thoma et al., 2011a; Hickey et al., 2015). Furthermore, the role of genetic heritage on VMB composition has been addressed by collecting vaginal samples from the mothers (Hickey et al., 2015). However, no consistent correlation exists in the VMB composition similarity between mothers and their daughters. Therefore, the role of genetic component in VMB composition remains unclear.
The most common cause of gynecological complaints in children and young girls is vulvovaginitis [reviewed by Beyitler and Kavukcu (2017)]. Risk factors include hypoestrogenism, anatomical proximity of rectum, and delicate vulvar skin and vaginal mucosa. Research suggests that the main causative agents for prepubertal vulvovaginitis are of fecal origin, such as Escherichia coli and E. faecalis (Bumbulienė et al., 2014) or Group A beta-hemolytic streptococci (Gerstner et al., 1982; Ranđeloviė et al., 2012; Bumbulienė et al., 2014). The clinical features, however, drive the interpretation whether an isolated microorganism is part of the normal VMB or is the cause of symptomatic vulvovaginitis. In contrast in puberty, C. albicans seems to be the most prevalent micro-organism isolated in vulvovaginitis infections (Yilmaz et al., 2012). As elevated levels of estrogen concentrations and subsequent glucose in the vaginal epithelium during the pubertal period increase susceptibility to C. albicans, it is logical that C. albicans vaginal infections are less prevalent in pre-puberty.
Investigation of VMB in adolescents may provide insight into the complexity and variability of VMB in adults and may help to understand whether the VMB development during puberty is associated with vaginal health in adulthood. Most likely, physiologic changes occurring in puberty and initiation of cyclic menstruation have profound effects on VMB, but research during these stages is very limited.
Probiotics
Thus far, no reports exist on probiotics and their impact on VMB in pre-puberty or puberty. However, it is important to acknowledge the role of estrogen in driving the VMB composition in pre-puberty and puberty, and this should be taken into consideration when designing probiotic studies in this age group for supporting vaginal health.
Reproduction and Fertility
Fertility
Infertility is a global concern affecting millions of people of reproductive age (Mascarenhas et al., 2012). In women, multiple factors can cause infertility, such as disorders concerning fallopian tubes, uterus, ovaries, as well as the endocrine system (Walker and Tobler, 2021). Knowledge on the role of VMB in fertility is increasing. BV affects, on average 30% of women in reproductive age and is a known risk factor for infertility [Koumans et al., 2007; reviewed by van Oostrum et al. (2013)]. Research shows that, particularly, the depletion of vaginal lactobacilli coupled with increased presence of G. vaginalis, A. vaginae, Ureaplasma parvum, Ureaplasma urealyticum, as well as Candida spp. are associated with issues in fertility [reviewed by Koedooder et al. (2019a)]. Vaginal dysbiosis is also linked with increased risk of sexually transmitted diseases (STDs) and may promote ascension of bacterial pathogens to fallopian tubes affecting reproduction (García-Velasco et al., 2017, 2020). Low abundance of vaginal lactobacilli is associated with infertility and vice versa (García-Velasco et al., 2020; Hong et al., 2020). Furthermore, composition of VMB can impact the success of fertility treatment/artificial reproductive technologies. For instance, high dominance of vaginal lactobacilli and in particular L. crispatus seems to affect positively on a successful outcome of the in vitro fertilization/embryo transfer (Hyman et al., 2012; Koedooder et al., 2019b).
Probiotics
Probiotics represent an attractive means to balance microbiota and counteract dysbiosis of the reproductive tract thus positively influencing fertility. Probiotic lactobacilli could be used to “prime” the VMB in the pre- and peri-conceptual periods and to prevent pathogenic infections associated with fertility and PTB. However, current research on the role of probiotics in fertility and/or infertility is yet scarce and inconclusive (Corbett et al., 2021). In an open label clinical trial with 117 women, a single dose of a vaginally administered probiotic containing Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum at the time of egg retrieval for in vitro fertilization (IVF) was not associated with an increase in vaginal Lactobacillus colonization or with improved pregnancy outcomes (Gilboa et al., 2005). In another study, Ligilactobacillus salivarius CECT5713 (formerly Lactobacillus salivarius) was administered daily for 6 months to 44 pregnant women either with a history of recurrent miscarriages or infertility with abnormal VMB and vaginal pH at baseline. The intervention resulted in 56% of successful pregnancy rate in these women and those with full-term pregnancy had significant increase in vaginal lactobacilli, decrease in vaginal pH, and Nugent score after intervention, suggesting probiotic’s ability to balance VMB and support healthy pregnancy in women with fertility issues (Fernández et al., 2021). Women with unexplained infertility diagnosis and receiving a 4-week probiotic supplementation of L. crispatus LbV 88, L. rhamnosus LbV 96, L. jensenii LbV 116, and L. gasseri LbV 150N had no change in overall VMB diversity or composition when compared with controls (Schenk et al., 2021). Interestingly, after the intervention, the relative abundance of Ureaplasma parvum, a pathogen associated with infertility and reproduction issues, was significantly lower in the probiotic group (0.77% vs. 3.52%, probiotic vs. control).
In the future, carefully designed and adequately powered clinical trials with well-characterized probiotic strains and treatment regimens are necessary to evaluate the effect of probiotics on fertility outcomes (Mohapatra et al., 2019; Balaghi et al., 2020; Cozzolino et al., 2020).
Pregnancy
In normal healthy pregnancy, studies comparing VMB composition between pregnant and non-pregnant women show that lactobacilli dominance increases throughout pregnancy along with increased stability and decreased dominance of bacteria associated with BV (e.g., Romero et al., 2014; Serrano et al., 2019). Notably, VMB change and increase in lactobacilli, especially L. iners, throughout pregnancy is most pronounced in women of African ancestry (Serrano et al., 2019). Among dominating lactobacilli, L. crispatus, seems to be the most stable species across pregnancy (Serrano et al., 2019). These findings are supported by several studies [reviewed by Anahtar et al. (2018)]. After delivery in the postpartum period, the composition of VMB shifts to less diverse and is characterized by lower abundance of Lactobacillus spp. (CST I, II, CST V) and significantly higher proportions of Streptococcus anginosus, P. bivia, and L. iners (Nunn et al., 2021).
It is likely that both estrogen and progesterone contribute to the increased dominance of lactobacilli during pregnancy, which stimulate glycogen accumulation in the vaginal epithelial cells favoring Lactobacillus spp. colonization (Anahtar et al., 2018). During pregnancy, the function of the mucosal barrier decreases due to congestion and edema of vaginal mucosa. Furthermore, pregnancy increases immune system modulation, which may lead to differential responses against pathogens (Mor and Cardenas, 2010). Microbial dysbiosis is linked with negative reproductive outcomes, such as prelabor rupture of membranes and PTB (Elovitz et al., 2019). Fettweis and colleagues assessed how VMB changes across pregnancies correlate with the risk of PTB by comparing data from 45 preterm and 90 term birth controls in a cohort of women of predominantly African ancestry (Fettweis et al., 2019). Those women who delivered preterm significantly exhibited lower vaginal levels of L. crispatus and, e.g., higher levels of BVAB1, Sneathia amnii, and Prevotella spp. Early pregnancy, such as the first 8 weeks of gestation, is a critical time for successful pregnancy as most miscarriages occur early in the first trimester (Preisler et al., 2015). A study by Al-Memar et al. (2020) investigating 161 pregnancies reported that in miscarriages occurring during the first trimester, the vaginal bacterial composition was less abundant with Lactobacillus spp., and a significantly higher proportion of these cases were dominated by CST IV.
Probiotics
Probiotics could provide natural means to support healthy and balanced VMB composition throughout pregnancy and reduce the risk of microbial dysbiosis. Thus far, clinical studies in pregnant women targeting VMB have examined either probiotic effects on overall VMB composition or specifically on Group B Streptococcus (GBS) colonization.
The studies assessing probiotic effects on VMB composition or changes throughout pregnancy have used either oral or vaginal probiotics. Oral administration of Lacticaseibacillus rhamnosus GR-1 and Limosilactobacillus reuteri RC-14 either from 9 to 14 weeks of gestation to delivery (Husain et al., 2020) or for 8 weeks in mid-pregnancy (Gille et al., 2016) did not have a significant impact on VMB as assessed by Nugent score when compared with placebo (Gille et al., 2016; Husain et al., 2020). Similarly, supplementation with the combination of species of Bifidobacterium longum, Lactobacillus delbrueckii subsp. bulgaricus, and Streptococcus thermophilus from 32 weeks of gestation until delivery had no significant changes in VMB composition when analyzed by 16S and compared with controls (Liang et al., 2021). In a study by Stojanović et al. (2012), the effect of vaginal application containing L. rhamnosus BMX 54 once a week for 12 weeks was assessed in pregnant women enrolled at second trimester of their pregnancy. The women receiving vaginal probiotic had more stable VMB and did not experience significant changes over the course of the study, whereas women without probiotics had a significant trend toward increase in the presence of vaginal pathogenic microorganisms, especially C. albicans over the course of the study.
Maternal GBS colonization is a predominant risk factor for both early- and late-onset neonatal sepsis. Regardless of intrapartum antibiotic prophylaxis (IAP), infants of GBS-colonized and antibiotic-treated mothers are at some risk of presenting with sepsis [reviewed by Patras and Nizet (2018)]. Probiotics with their antimicrobial potential could be used to support healthy gut and VMB and thus reduce GBS colonization. No impact on GBS colonization was found with consumption of four-strain probiotic blend L. jensenii Lbv116, L. crispatus Lbv88, L. rhamnosus Lbv96, and L. gasseri Lbv150 when consumed for 2 weeks in GBS-positive women with 33–37 weeks of gestation (Farr et al., 2020). Furthermore, no significant impact on GBS colonization was observed in an open-label trial of 10 women with a probiotic combination containing L. acidophilus strains La-14 and NCFM, Bifidobacterium animalis subsp. lactis strains HN019 and Bi-07, and B. longum subsp. longum Bl-05 either, when taken from 28 weeks until 36 weeks of pregnancy (Hanson et al., 2014). The efficacy of vaginal isolate L. salivarius CECT 9145 against intestinal and vaginal GBS eradication was tested in a pilot trial involving 57 healthy GBS-positive pregnant women from week 26 to week 38 (Martín et al., 2019). At the end of the intervention, there was reduction in the rate of GBS-colonized women receiving L. salivarius which further decreased the number of women receiving IAP during delivery. Olsen et al. showed that oral probiotics L. rhamnosus GR-1 and L. reuteri RC-1 when taken either at mid-pregnancy or 3 weeks prior to delivery had no significant impact on GBS rates when compared with controls (Olsen et al., 2018; Sharpe et al., 2019). However, in another study, the same combination was taken at 35–37 weeks of pregnancy until delivery showed that 42.9% of the GBS-positive women in the probiotic group compared with 18.0% in the placebo group achieved a negative GBS culture at the time of delivery (Ho et al., 2016).
Taken together, the above studies indicate that the VMB of women in healthy pregnancy is relatively stable and probiotics as such may not induce significant changes on the VMB composition. Larger and well-controlled trials are needed to make further conclusions on their efficacy. Nevertheless, probiotics do not negatively impact VMB, and no safety concerns have been raised with their use in pregnancy (Sheyholislami and Connor, 2021). Furthermore, probiotics may have other beneficial effects in pregnant women, such as balancing the gut microbiota and having a beneficial effect on metabolic outcomes, such as gestational diabetes mellitus (Lv et al., 2018) and postpartum depression/anxiety (Slykerman et al., 2017). It should be noted that probiotic effects are strain-specific and the dose, stability, length of intervention, delivery vehicle/matrix, and survival in the gut and vaginal tract are essential for their ability to exert positive health effects.
Menopause and Postmenopause
As the hormonal balance shifts significantly during menopausal transition, changes are also evident in the VMB of postmenopausal women. The most dominant vaginal bacterial species in healthy postmenopausal women are those from genera Lactobacillus, Gardnerella, Prevotella, and Atopobium (Shen et al., 2016). The percentage of Lactobacillus spp. has shown to be lower after menopause compared to pre- and perimenopausal period (Hillier and Lau, 1997; Brotman et al., 2014b; Kim et al., 2021). A cross-sectional study by Brotman et al. (2014b) demonstrated that the abundance of Lactobacillus spp. in the vagina of pre- and perimenopausal women was 83%, whereas the abundance of those in postmenopausal women was 54%. In the same study, it was shown that menopausal status and CST strictly correlate with each other: perimenopausal women were most often classified to have type CST IV-A or the L. gasseri CST, whereas postmenopausal women were typically CST IV-A, a type with large abundance of anaerobic bacteria from the genera Anaerococcus, Peptoniphilus, Prevotella, and Streptococcus (as compared to premenopausal women with CSTs dominated by L. crispatus and L. iners).
The use of hormone replacement therapy (HT) has been shown to affect VMB in postmenopausal women (Pabich et al., 2003; Devillard et al., 2004; Gliniewicz et al., 2019). A study by Gliniewicz et al. (2019) including both pre- and postmenopausal women showed that the vaginal CST of postmenopausal women under HT was more likely to resemble that of premenopausal women than that of postmenopausal women without HT. These findings emphasize the role of estrogen in defining the VMB composition. However, the association between clinical vulvovaginal symptoms and Lactobacillus levels is not so clear. According to Mitchell et al. (2017), despite the Lactobacillus spp. dominance, the number of vaginal symptoms was not any less, whereas other studies have reported that vaginal lactobacilli abundance and the symptoms are associated inversely in postmenopausal women (Shen et al., 2016). Furthermore, another study by Mitchell et al. (2018) showed some indications (not reaching the level of significance) that the potential to improve genitourinary symptoms is more pronounced with higher lactobacilli abundance.
The decrease in the vaginal Lactobacillus abundance in postmenopausal women can lead to higher microbial diversity and higher vaginal pH increasing the risks for infections (Ginkel et al., 1993; Gliniewicz et al., 2019).
Menopause-related decline in estrogen (17β-estradiol) levels is associated with changes in VMB and subsequently in the (vulvo)vaginal lining. Vulvovaginal atrophy (VVA) is a common condition, affecting 25–50% of postmenopausal women, causing various symptoms such as itching, discharge, bleeding, burning sensations, and difficulties in intercourse (Bachmann and Nevadunsky, 2000). Brotman et al. (2014b) demonstrated that VMB is associated with the signs of VVA: postmenopausal women with type CST IV-A VMB were most prone to VVA compared to pre- and perimenopausal women. Shen et al. (2016) demonstrated similarly that postmenopausal women with atrophic vaginitis (AV) have lower abundance in lactobacilli than healthy postmenopausal women. According to their results, genera Gardnerella and Atopobium were related to AV after menopause.
The increased vaginal pH along with declining ovarian estrogen during menopause allows the growth of harmful microbes such as Escherichia coli, Candida spp., and Gardnerella spp. leading to increased risk of BV and VVC [reviewed by Kim and Park (2017)]. The diagnosis of BV and thus defining VMB composition among postmenopausal women has been considered challenging, since many of the presumptions related to the testing procedures are only valid for premenopausal/fertile women (Cauci et al., 2002). Firstly, vaginal pH is commonly increased at menopause, whereas during fertile years, it is an indication of BV (according to Amsel criteria). Secondly, what comes to Nugent scoring method, it assumes that normal VMB is highly abundant with lactobacilli, which is not the case among postmenopausal women. In a study including pre-, peri-, and postmenopausal women, Cauci et al. (2002) showed, that in postmenopausal women with low lactobacilli abundance, BV prevalence was lower compared to fertile or perimenopausal women. It has been suggested that instead being a sign of BV, higher vaginal pH, in the absence of pathogenic bacteria, is an indication of menopause. To support this theory, it has been shown that serum estradiol levels are negatively associated with the vaginal pH (Caillouette et al., 1997).
Vulvovaginal candidiasis is considered to be strongly associated with estrogens; therefore, the prevalence of Candida spp. seems to decrease with aging (Hoffmann et al., 2014). However, VVC as a possible adverse effect of estrogen-based HT is a common finding (Nwokolo and Boag, 2000; Fischer and Bradford, 2011). Furthermore, postmenopausal women with uncontrolled chronic conditions such as diabetes or immunosuppressive disorders, are specifically prone for recurrent complicated VVCs (Nwokolo and Boag, 2000). This susceptibility is mainly due to already compromised immune status and possible excessive use of antibiotics creating opportunities for Candida spp. invasion (Low and Rotstein, 2011).
Probiotics
As mentioned above, Nugent score is one of the key diagnostic tools for BV. Intermediate Nugent score (4–6) is of particular interest as it is seen as a risk for developing BV (Guedou et al., 2012; Amegashie et al., 2017). A study by Petricevic et al. (2008) showed that VMB can be balanced in postmenopausal women (n = 72) with intermediate Nugent score by 14-day supplementation of probiotics L. rhamnosus GR-1 and L. reuteri RC-14. In this clinical trial, Nugent score improved in 60% of the women in the probiotic group, whereas the improvement was only 16% in the placebo group. Another study with the same strains demonstrated that 3-day vaginal administration of the probiotics did not change the Nugent score in postmenopausal women (n = 14) with intermediate Nugent scores (Bisanz et al., 2014). However, the 16S sequencing analyses showed that there was a significant increase in the vaginal abundance of Lactobacillus spp. in the probiotic group, indicating a potential health benefit from the microbiota perspective.
Promising results have been received from a clinical trial performed in 22 menopausal breast cancer patients under chemotherapy (Marschalek et al., 2017). The study participants had vaginal atrophy and intermediate Nugent Score at the baseline and received either combination of four probiotics (L. crispatus LbV 88, L. rhamnosus LbV 96, L. jensenii LbV 116, L. gasseri LbV 150N) or placebo twice a day for 2 weeks. Vaginal swabs were collected at baseline, 1 day after the treatment, and 1 week after the treatment. Improvement toward healthy Nugent Score (0–3) was detected in 63% women in the probiotic group and in 36% in the placebo group. Nugent Score was reduced at Day 1 and after 1 week in the probiotic group. In addition, a pilot placebo-controlled clinical trial by Kwak et al. (2017) investigated the vaginal colonization of topically applied L. gasseri LN40, Limosilactobacillus fermentum LN99, and L. rhamnosus LN113 product for 10 days in postmenopausal women (n = 18). Potential vaginal persistence was shown for LN99 and LN113 either at the end or after the intervention, but not for LN40. Another study in postmenopausal women with symptoms of vaginal atrophy and taking vaginal tablets containing low dose of estriol (0.03 mg) and L. acidophilus KS400 showed benefits in improving vaginal maturation index and related clinical symptoms (Jaisamrarn et al., 2013).
Furthermore, there are multiple studies on BV and probiotic benefits on VMB, including women with the age range varying from 18 to 70 years [reviewed by Hanson et al. (2016); Li et al. (2019), Wang et al. (2019); van de Wijgert and Verwijs (2020)]. In most of the studies there is no clear differentiation between pre- and postmenopausal women. An observational pilot study in women with history of recurrent BV and undergoing surgical menopause showed that when L. rhamnosus BMX 54 was administered as vaginal tablets altogether for 6 months, women experienced less BV recurrences (Parma et al., 2013). However, due to relatively low number of studies focusing on menopausal/postmenopausal population only, more studies on probiotics’ effects on VMB focusing on this population are warranted.
Conclusion and Future Perspectives
As research evidence accumulates, it is apparent that VMB and especially vaginal lactobacilli play a crucial role in women’s health throughout life. In healthy women, estrogen levels are one of the major factors shaping the VMB from the onset of puberty to menopause and postmenopause (Figure 2) as well as across menstrual cycle and pregnancy. Furthermore, ethnicity and variable external factors such as lifestyle and antimicrobial medication are well-known to determine and influence the composition and dysbiosis/homeostasis state. Through rapid technology development, research is starting to unravel the interactions between the VMB and the human host and how the microbial balance shifts from homeostasis to dysbiosis and progresses to disease. Nevertheless, the impact of balanced vaginal bacterial as well fungal community on health is not fully characterized, and research has mainly focused on these organisms’ associations with disease. Especially, the role of vaginal fungi has been neglected although evidence indicates that mycobiota is important and, for example, gut-associated fungi can modulate host immune responses (Underhill and Iliev, 2014; Wheeler et al., 2016; Raimondi et al., 2019). Symbiosis exists between yeast and bacteria in different environmental niches, which applies also in the vaginal tract. Polymicrobial interactions (Krüger et al., 2019) and factors turning a normal fungal inhabitant as a pathogenic organism (Sam et al., 2017) have received attention in the past years. However, more research is necessary to elucidate bacteria–fungi and fungi–fungi relationships and interactions. Understanding of these interactions as well as signaling and immunological responses together with metabolites produced by bacteria and fungi in vaginal health and disease will open new means for microbiota management. For example, for BV, alternative options for its management are much needed, as the pathology is still not well-understood, and the current treatment options are inadequate in efficacy and the recurrence rate is high.
Probiotics are considered as natural and safe means to balance the VMB composition and support during antimicrobial treatment or help in recovery from dysbiosis. However, although safety of probiotics has been well-established, not all clinical studies have been successful in terms of efficacy, and large variation exists between the studies in terms of probiotic strains/combinations used, the target population by ethnicity and age/life stage, and study designs. Furthermore, there is an increasing interest to understand how probiotics support women’s health throughout life and how to take these specific conditions into account (and especially the hormonal fluctuations), when designing probiotic products and studies for women. Research should also focus on identifying the most optimal probiotic species/strains for vaginal health and VMB composition and their mechanisms of actions on VMB and the host. In the future, indigenous vaginal lactobacilli consortia and/or transplantations of healthy women may be more effective solutions for women suffering from vaginal dysbiosis and associated infections/adversities. For example, fecal microbiota transplantation has been successfully applied to treat Clostridioides difficile infections. Indeed, a similar approach has been applied to treat recurrent intractable BV (Lev-Sagie et al., 2019), where promising results were reported in terms of achieving long-term remission of BV after 5–12 months of VMB transplantation from healthy donors.
Author Contributions
All authors wrote the original draft of the manuscript, contributed to the article, and approved the submitted version.
Conflict of Interest
LL, RA-J, AL, and JM are current or previous workers of International Flavors and Fragrances, a company that manufactures probiotics.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This study was fully funded by Danisco Sweeteners Oy, part of IFF Health & Biosciences.
References
- Achilles S. L., Hillier S. L. (2013). The complexity of contraceptives: understanding their impact on genital immune cells and vaginal microbiota. AIDS 27(Suppl. 1) S5–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew K. J., Hillier S. L. (1995). The effect of treatment regimens for vaginitis and cervicitis on vaginal colonization by lactobacilli. Sex. Transm. Dis. 22 269–273. 10.1097/00007435-199509000-00001 [DOI] [PubMed] [Google Scholar]
- Allsworth J. E., Lewis V. A., Peipert J. F. (2008). Viral sexually transmitted infections and bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Sex. Transm. Dis. 35 791–796. 10.1097/OLQ.0b013e3181788301 [DOI] [PubMed] [Google Scholar]
- Al-Memar M., Bobdiwala S., Fourie H., Mannino R., Lee Y. S., Smith A., et al. (2020). The association between vaginal bacterial composition and miscarriage: a nested case-control study. BJOG 127 264–274. 10.1111/1471-0528.15972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabebe E., Anumba D. O. C. (2018a). Psychosocial stress, cortisol levels, and maintenance of vaginal health. Front. Endocrinol. (Lausanne) 9:568. 10.3389/fendo.2018.00568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabebe E., Anumba D. O. C. (2018b). The vaginal microenvironment: the physiologic role of lactobacilli. Front. Med. 5:181. 10.3389/fmed.2018.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen F., Moslem M., Al Tami M., Al-Ajlan H., Al-Qahtani N. (2017). Identification of Candida species in vaginal flora using conventional and molecular methods. J. Mycol. Med. 27 364–368. 10.1016/j.mycmed.2017.04.105 [DOI] [PubMed] [Google Scholar]
- Amegashie C. P., Gilbert N. M., Peipert J. F., Allsworth J. E., Lewis W. G., Lewis A. L. (2017). Relationship between nugent score and vaginal epithelial exfoliation. PLoS One 12:e0177797. 10.1371/journal.pone.0177797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association (2018). Stress in America: Generation Z. Stress in America™ Survey. Washington, DC: American Psychological Association (APA). [Google Scholar]
- Anahtar M. N., Byrne E. H., Doherty K. E., Bowman B. A., Yamamoto H. S., Soumillon M., et al. (2015). Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42 965–976. 10.1016/j.immuni.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anahtar M. N., Gootenberg D. B., Mitchell C. M., Kwon D. S. (2018). Cervicovaginal microbiota and reproductive health: the virtue of simplicity. Cell Host Microbe 23 159–168. 10.1016/j.chom.2018.01.013 [DOI] [PubMed] [Google Scholar]
- Bachmann G. A., Nevadunsky N. S. (2000). Diagnosis and treatment of atrophic vaginitis. Am. Fam. Physician 61 3090–3096. [PubMed] [Google Scholar]
- Balaghi Z., Azima S., Motamedifar M., Kaviani M., Poordast T., Zare N. (2020). The effect of lactofem oral probiotic capsule on lactobacilli colonization and some vaginal health parameters. Gynecol. Obstet. Invest. 85 245–251. 10.1159/000506802 [DOI] [PubMed] [Google Scholar]
- Beigi R. H., Wiesenfeld H. C., Hillier S. L., Straw T., Krohn M. A. (2005). Factors associated with absence of H2O2-producing Lactobacillus among women with bacterial vaginosis. J. Infect. Dis. 191 924–929. 10.1086/428288 [DOI] [PubMed] [Google Scholar]
- Beyitler İ, Kavukcu S. (2017). Clinical presentation, diagnosis and treatment of vulvovaginitis in girls: a current approach and review of the literature. World J. Pediatr. 13 101–105. 10.1007/s12519-016-0078-y [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Sae-Tia S., Fries B. C. (2020). Candidiasis and mechanisms of antifungal resistance. Antibiotics (Basel) 9:312. 10.3390/antibiotics9060312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S., Sobel J. D., White T. C. (2016). A combination fluorescence assay demonstrates increased efflux pump activity as a resistance mechanism in azole-resistant vaginal Candida albicans isolates. Antimicrob. Agents Chemother. 60 5858–5866. 10.1128/AAC.01252-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisanz J. E., Seney S., Mcmillan A., Vongsa R., Koenig D., Wong L., et al. (2014). A systems biology approach investigating the effect of probiotics on the vaginal microbiome and host responses in a double blind, placebo-controlled clinical trial of post-menopausal women. PLoS One 9:e104511. 10.1371/journal.pone.0104511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blostein F., Levin-Sparenberg E., Wagner J., Foxman B. (2017). Recurrent vulvovaginal candidiasis. Ann. Epidemiol. 27 575–582.e573. [DOI] [PubMed] [Google Scholar]
- Bongomin F., Gago S., Oladele R. O., Denning D. W. (2017). Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi 3:57. 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgdorff H., Van Der Veer C., Van Houdt R., Alberts C. J., De Vries H. J., Bruisten S. M., et al. (2017). The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS One 12:e0181135. 10.1371/journal.pone.0181135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges S., Silva J., Teixeira P. (2014). The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 289 479–489. 10.1007/s00404-013-3064-9 [DOI] [PubMed] [Google Scholar]
- Bradford L. L., Ravel J. (2017). The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence 8 342–351. 10.1080/21505594.2016.1237332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C. S., Sobel J. D. (2016). Current treatment of bacterial vaginosis-limitations and need for innovation. J. Infect. Dis. 214(Suppl. 1) S14–S20. 10.1093/infdis/jiw159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C. S., Walker J., Fairley C. K., Chen M. Y., Tabrizi S. N., Donovan B., et al. (2013). Prevalent and incident bacterial vaginosis are associated with sexual and contraceptive behaviours in young Australian women. PLoS One 8:e57688. 10.1371/journal.pone.0057688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookheart R. T., Lewis W. G., Peipert J. F., Lewis A. L., Allsworth J. E. (2019). Association between obesity and bacterial vaginosis as assessed by Nugent score. Am. J. Obstet. Gynecol. 220 476.e1–476.e11. 10.1016/j.ajog.2019.01.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. P., Edwards D. J., Blithe D. L., Fettweis J. M., Serrano M. G., Sheth N. U., et al. (2017). Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on the vaginal microbiome. Contraception 95 405–413. 10.1016/j.contraception.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman R. M., He X., Gajer P., Fadrosh D., Sharma E., Mongodin E. F., et al. (2014a). Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect. Dis. 14:471. 10.1186/1471-2334-14-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman R. M., Klebanoff M. A., Nansel T. R., Yu K. F., Andrews W. W., Zhang J., et al. (2010). Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J. Infect. Dis. 202 1907–1915. 10.1086/657320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman R. M., Shardell M. D., Gajer P., Fadrosh D., Chang K., Silver M. I., et al. (2014b). Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 21 450–458. 10.1097/gme.0b013e3182a4690b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumbulienė Ž, Venclavičiūtė K., Ramašauskaitė D., Arlauskienė A., Bumbul E., Drąsutienė G. (2014). Microbiological findings of vulvovaginitis in prepubertal girls. Postgrad. Med. J. 90 8–12. 10.1136/postgradmedj-2013-131959 [DOI] [PubMed] [Google Scholar]
- Caillouette J. C., Sharp C. F., Jr., Zimmerman G. J., Roy S. (1997). Vaginal pH as a marker for bacterial pathogens and menopausal status. Am. J. Obstet. Gynecol. 176 1270–1275; discussion 1275–1277. 10.1016/s0002-9378(97)70345-4 [DOI] [PubMed] [Google Scholar]
- Cauci S., Driussi S., De Santo D., Penacchioni P., Iannicelli T., Lanzafame P., et al. (2002). Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J. Clin. Microbiol. 40 2147–2152. 10.1128/JCM.40.6.2147-2152.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarani C., Foschi C., Parolin C., D’Antuono A., Gaspari V., Consolandi C., et al. (2019). Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 9:14095. 10.1038/s41598-019-50410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra J., Kuhn D. M., Mukherjee P. K., Hoyer L. L., McCormick T., Ghannoum M. A. (2001). Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183 5385–5394. 10.1128/JB.183.18.5385-5394.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee W. J. Y., Chew S. Y., Than L. T. L. (2020). Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 19:203. 10.1186/s12934-020-01464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherpes T. L., Hillier S. L., Meyn L. A., Busch J. L., Krohn M. A. (2008). A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex. Transm. Dis. 35 78–83. 10.1097/OLQ.0b013e318156a5d0 [DOI] [PubMed] [Google Scholar]
- Cherpes T. L., Meyn L. A., Krohn M. A., Lurie J. G., Hillier S. L. (2003). Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin. Infect. Dis. 37 319–325. 10.1086/375819 [DOI] [PubMed] [Google Scholar]
- Corbett G. A., Crosby D. A., Mcauliffe F. M. (2021). Probiotic therapy in couples with infertility: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 256 95–100. 10.1016/j.ejogrb.2020.10.054 [DOI] [PubMed] [Google Scholar]
- Cozzolino M., Vitagliano A., Pellegrini L., Chiurazzi M., Andriasani A., Ambrosini G., et al. (2020). Therapy with probiotics and synbiotics for polycystic ovarian syndrome: a systematic review and meta-analysis. Eur. J. Nutr. 59 2841–2856. 10.1007/s00394-020-02233-0 [DOI] [PubMed] [Google Scholar]
- De Bernardis F., Graziani S., Tirelli F., Antonopoulou S. (2018). Candida vaginitis: virulence, host response and vaccine prospects. Med. Mycol. 56 26–31. 10.1093/mmy/myx139 [DOI] [PubMed] [Google Scholar]
- Dekaboruah E., Suryavanshi M. V., Chettri D., Verma A. K. (2020). Human microbiome: an academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 202 2147–2167. 10.1007/s00203-020-01931-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z.-L., Gottschick C., Bhuju S., Masur C., Abels C., Wagner-Döbler I. (2018). Metatranscriptome analysis of the vaginal microbiota reveals potential mechanisms for protection against metronidazole in bacterial vaginosis. mSphere 3:e00262-18. 10.1128/mSphereDirect.00262-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillard E., Burton J. P., Hammond J.-A., Lam D., Reid G. (2004). Novel insight into the vaginal microflora in postmenopausal women under hormone replacement therapy as analyzed by PCR-denaturing gradient gel electrophoresis. Eur. J. Obstet. Gynecol. Reprod. Biol. 10 76–81. 10.1016/j.ejogrb.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Diakite A., Dubourg G., Raoult D. (2021). Updating the repertoire of cultured bacteria from the human being. Microb. Pathog. 150:104698. 10.1016/j.micpath.2020.104698 [DOI] [PubMed] [Google Scholar]
- Diop K., Cadoret F., Nguyen T. T., Baudoin J.-P., Armstrong N., Raoult D., et al. (2020). Vaginimicrobium propionicum gen. nov., sp. nov., a novel propionic acid bacterium derived from human vaginal discharge. Int. J. Syst. Evol. Microbiol. 70 4091–4097. 10.1099/ijsem.0.004106 [DOI] [PubMed] [Google Scholar]
- Drell T., Lillsaar T., Tummeleht L., Simm J., Aaspõllu A., Väin E., et al. (2013). Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS One 8:e54379. 10.1371/journal.pone.0054379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc D., Gannevat J., Anguiano E., Zurawski S., Carley M., Boreham M., et al. (2013). Functional diversity of human vaginal APC subsets in directing T-cell responses. Mucosal Immunol. 6 626–638. 10.1038/mi.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein M. (2020). The hunt for a healthy microbiome. Nature 577 S6–S8. 10.1038/d41586-020-00193-3 [DOI] [PubMed] [Google Scholar]
- Elovitz M. A., Gajer P., Riis V., Brown A. G., Humphrys M. S., Holm J. B., et al. (2019). Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat. Commun. 10:1305. 10.1038/s41467-019-09285-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbach D. A., Thwin S. S., Patton D. L., Hooton T. M., Stapleton A. E., Agnew K., et al. (2000). Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis. 30 901–907. 10.1086/313818 [DOI] [PubMed] [Google Scholar]
- Falsen E., Pascual C., Sjödén B., Ohlén M., Collins M. D. (1999). Phenotypic and phylogenetic characterization of a novel Lactobacillus species from human sources: description of Lactobacillus iners sp. nov. Int. J. Syst. Evol. Microbiol. 49 217–221. 10.1099/00207713-49-1-217 [DOI] [PubMed] [Google Scholar]
- Farage M., Maibach H. (2006). Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 273 195–202. 10.1007/s00404-005-0079-x [DOI] [PubMed] [Google Scholar]
- Farr A., Sustr V., Kiss H., Rosicky I., Graf A., Makristathis A., et al. (2020). Oral probiotics to reduce vaginal group B streptococcal colonization in late pregnancy. Sci. Rep. 10:19745. 10.1038/s41598-020-76896-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández L., Castro I., Arroyo R., Alba C., Beltrán D., Rodríguez J. M. (2021). Application of Ligilactobacillus salivarius CECT5713 to achieve term pregnancies in women with repetitive abortion or infertility of unknown origin by microbiological and immunological modulation of the vaginal ecosystem. Nutrients 13:162. 10.3390/nu13010162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettweis J. M., Brooks J. P., Serrano M. G., Sheth N. U., Girerd P. H., Edwards D. J., et al. (2014). Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading, England) 160 2272–2282. 10.1099/mic.0.081034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettweis J. M., Serrano M. G., Brooks J. P., Edwards D. J., Girerd P. H., Parikh H. I., et al. (2019). The vaginal microbiome and preterm birth. Nat. Med. 25 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G., Bradford J. (2011). Vulvovaginal candidiasis in postmenopausal women: the role of hormone replacement therapy. J. Low. Genit. Tract. Dis. 15 263–267. 10.1097/LGT.0b013e3182241f1a [DOI] [PubMed] [Google Scholar]
- Forbes J. D., Bernstein C. N., Tremlett H., Van Domselaar G., Knox N. C. (2019). A fungal world: could the gut mycobiome be involved in neurological disease? Front. Microbiol. 9:3249. 10.3389/fmicb.2018.03249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- France M. T., Ma B., Gajer P., Brown S., Humphrys M. S., Holm J. B., et al. (2020). VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8:166. 10.1186/s40168-020-00934-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S. C., Looker C., Vandepitte J., Bukenya J., Mayanja Y., Nakubulwa S., et al. (2016). Bacterial vaginosis among women at high risk for HIV in Uganda: high rate of recurrent diagnosis despite treatment. Sex. Trans. Infect. 92 142–148. 10.1136/sextrans-2015-052160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajer P., Brotman R. M., Bai G., Sakamoto J., Schütte U. M., Zhong X., et al. (2012). Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4 132–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Velasco J. A., Budding D., Campe H., Malfertheiner S. F., Hamamah S., Santjohanser C., et al. (2020). The reproductive microbiome – clinical practice recommendations for fertility specialists. Reprod. Biomed. Online 41 443–453. 10.1016/j.rbmo.2020.06.014 [DOI] [PubMed] [Google Scholar]
- García-Velasco J. A., Menabrito M., Catalán I. B. (2017). What fertility specialists should know about the vaginal microbiome: a review. Reprod. Biomed. Online 35 103–112. 10.1016/j.rbmo.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Gerstner G. J., Grünberger W., Boschitsch E., Rotter M. (1982). Vaginal organisms in prepubertal children with and without vulvovaginitis. A vaginoscopic study. Arch. Gynecol. 231 247–252. 10.1007/BF02110125 [DOI] [PubMed] [Google Scholar]
- Gilboa Y., Bar-Hava I., Fisch B., Ashkenazi J., Voliovitch I., Borkowski T., et al. (2005). Does intravaginal probiotic supplementation increase the pregnancy rate in IVF-embryo transfer cycles? Reprod. Biomed. Online 11 71–75. 10.1016/s1472-6483(10)61301-6 [DOI] [PubMed] [Google Scholar]
- Gille C., Böer B., Marschal M., Urschitz M. S., Heinecke V., Hund V., et al. (2016). Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am. J. Obstet. Gynecol. 215 608.e1–608.e7. 10.1016/j.ajog.2016.06.021 [DOI] [PubMed] [Google Scholar]
- Ginkel P. D., Soper D. E., Bump R. C., Dalton H. P. (1993). Vaginal flora in postmenopausal women: the effect of estrogen replacement. Infect. Dis. Obstet. Gynecol. 1 94–97. 10.1155/S1064744993000225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliniewicz K., Schneider G. M., Ridenhour B. J., Williams C. J., Song Y., Farage M. A., et al. (2019). Comparison of the vaginal microbiomes of premenopausal and postmenopausal women. Front. Microbiol. 10:193. 10.3389/fmicb.2019.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouba N., Drancourt M. (2015). Digestive tract mycobiota: A source of infection. Méd. Mal. Infect. 45 9–16. 10.1016/j.medmal.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Guedou F. A., Van Damme L., Mirembe F., Solomon S., Becker M., Deese J., et al. (2012). Intermediate vaginal flora is associated with HIV prevalence as strongly as bacterial vaginosis in a cross-sectional study of participants screened for a randomised controlled trial. Sex. Transm. Infect. 88 545–551. 10.1136/sextrans-2011-050319 [DOI] [PubMed] [Google Scholar]
- Gupta S., Kakkar V., Bhushan I. (2019). Crosstalk between vaginal microbiome and female health: a review. Microb. Pathog. 136:103696. 10.1016/j.micpath.2019.103696 [DOI] [PubMed] [Google Scholar]
- Gupta V. K., Paul S., Dutta C. (2017). Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 8:1162. 10.3389/fmicb.2017.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag M. R., Alpert S., Onderdonk A. B., Thurston P., Drude E., Mccormack W. M., et al. (1978a). Anaerobic microflora of the vagina in children. Am. J. Obstet. Gynecol. 131 853–856. 10.1016/s0002-9378(16)33130-1 [DOI] [PubMed] [Google Scholar]
- Hammerschlag M. R., Alpert S., Rosner I., Thurston P., Semine D., Mccomb D., et al. (1978b). Microbiology of the vagina in children: normal and potentially pathogenic organisms. Pediatrics 62 57–62. 10.1542/peds.62.1.57 [DOI] [PubMed] [Google Scholar]
- Hanson L., Vandevusse L., Duster M., Warrack S., Safdar N. (2014). Feasibility of oral prenatal probiotics against maternal group B Streptococcus vaginal and rectal colonization. J. Obstet. Gynecol. Neonatal Nurs. 43 294–304. 10.1111/1552-6909.12308 [DOI] [PubMed] [Google Scholar]
- Hanson L., VandeVusse L., Jermé M., Abad C. L., Safdar N. (2016). Probiotics for treatment and prevention of urogenital infections in women: a systematic review. J. Midwifery Womens Health 61 339–355. 10.1111/jmwh.12472 [DOI] [PubMed] [Google Scholar]
- Hay P. E., Lamont R. F., Taylor-Robinson D., Morgan D. J., Ison C., Pearson J. (1994). Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ 308 295–298. 10.1136/bmj.308.6924.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg D., Nilsson S., Mårdh P. A. (2000). Bacterial vaginosis and smoking. Int. J. STD AIDS 11 603–606. 10.1258/0956462001916461 [DOI] [PubMed] [Google Scholar]
- Hickey R. J., Zhou X., Settles M. L., Erb J., Malone K., Hansmann M. A., et al. (2015). Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio 6:e00097-15. 10.1128/mBio.00097-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G. R., Merenstein D. J., Pot B., et al. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11 506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Hillerbrand L., Harmanli O. H., Whiteman V. (2002). Urinary tract infections in pregnant women with bacterial vaginosis. Am. J. Obstet. Gynecol. 186 916–917. 10.1067/mob.2002.123987 [DOI] [PubMed] [Google Scholar]
- Hillier S. L., Lau R. J. (1997). Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin. Infect. Dis. 25(Suppl. 2) S123–S126. 10.1086/516221 [DOI] [PubMed] [Google Scholar]
- Hillier S. L., Nugent R. P., Eschenbach D. A., Krohn M. A., Gibbs R. S., Martin D. H., et al. (1995). Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 333 1737–1742. 10.1056/NEJM199512283332604 [DOI] [PubMed] [Google Scholar]
- Ho M., Chang Y. Y., Chang W. C., Lin H. C., Wang M. H., Lin W. C., et al. (2016). Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial. Taiwan. J. Obstet. Gynecol. 55 515–518. 10.1016/j.tjog.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Hoffmann J. N., You H. M., Hedberg E. C., Jordan J. A., Mcclintock M. K. (2014). Prevalence of bacterial vaginosis and Candida among postmenopausal women in the United States. J. Gerontol. B Psychol. Sci. Soc. Sci. 69(Suppl. 2) S205–S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K. H., Hong S. K., Cho S. I., Ra E., Han K. H., Kang S. B., et al. (2016). Analysis of the vaginal microbiome by next-generation sequencing and evaluation of its performance as a clinical diagnostic tool in vaginitis. Ann. Lab. Med. 36 441–449. 10.3343/alm.2016.36.5.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X., Ma J., Yin J., Fang S., Geng J., Zhao H., et al. (2020). The association between vaginal microbiota and female infertility: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 302 569–578. 10.1007/s00404-020-05675-3 [DOI] [PubMed] [Google Scholar]
- Hootan T. M., Fihn S. D., Johnson C., Roberts P. L., Stamm W. E. (1989). Association between bacterial vaginosis and acute cystitis in women using diaphragms. Arch. Intern. Med. 149 1932–1936. 10.1001/archinte.149.9.1932 [DOI] [PubMed] [Google Scholar]
- Husain S., Allotey J., Drymoussi Z., Wilks M., Fernandez-Felix B. M., Whiley A., et al. (2020). Effects of oral probiotic supplements on vaginal microbiota during pregnancy: a randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG 127 275–284. 10.1111/1471-0528.15675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R. W., Herndon C. N., Jiang H., Palm C., Fukushima M., Bernstein D., et al. (2012). The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J. Assist. Reprod. Genet. 29 105–115. 10.1007/s10815-011-9694-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaisamrarn U., Triratanachat S., Chaikittisilpa S., Grob P., Prasauskas V., Taechakraichana N. (2013). Ultra-low-dose estriol and lactobacilli in the local treatment of postmenopausal vaginal atrophy. Climacteric 16 347–355. 10.3109/13697137.2013.769097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosik G. P., Land C. B., Duhon P., Chandler R., Jr., Mercer T. (1998). Acquisition of iron by Gardnerella vaginalis. Infect. Immun. 66 5041–5047. 10.1128/iai.66.10.5041-5047.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng H. S., Yan T. R., Chen J. Y. (2020). Treating vaginitis with probiotics in non-pregnant females: A systematic review and meta-analysis. Exp. Ther. Med. 20 3749–3765. 10.3892/etm.2020.9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia N., Singh J., Kaur M. (2020). Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann. Clin. Microbiol. Antimicrob. 19:5. 10.1186/s12941-020-0347-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Merchant M., Haque M. M., Mande S. S. (2020). Crosstalk between female gonadal hormones and vaginal microbiota across various phases of women’s gynecological lifecycle. Front. Microbiol. 11:551. 10.3389/fmicb.2020.00551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. M., Park Y. J. (2017). Probiotics in the prevention and treatment of postmenopausal vaginal infections: review article. J. Menopausal Med. 23 139–145. 10.6118/jmm.2017.23.3.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Seo H., Rahim M. A., Lee S., Kim Y. S., Song H. Y. (2021). Changes in the microbiome of vaginal fluid after menopause in Korean women. J. Microbiol. Biotechnol. 31 1490–1500. 10.4014/jmb.2106.06022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff M. A., Nansel T. R., Brotman R. M., Zhang J., Yu K. F., Schwebke J. R., et al. (2010). Personal hygienic behaviors and bacterial vaginosis. Sex. Transm. Dis. 37 94–99. 10.1097/OLQ.0b013e3181bc063c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedooder R., Mackens S., Budding A., Fares D., Blockeel C., Laven J., et al. (2019a). Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum. Reprod. Update 25 298–325. 10.1093/humupd/dmy048 [DOI] [PubMed] [Google Scholar]
- Koedooder R., Singer M., Schoenmakers S., Savelkoul P. H. M., Morré S. A., De Jonge J. D., et al. (2019b). The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum. Reprod. 34 1042–1054. 10.1093/humrep/dez065 [DOI] [PubMed] [Google Scholar]
- Kong H. H., Morris A. (2017). The emerging importance and challenges of the human mycobiome. Virulence 8 310–312. 10.1080/21505594.2017.1279780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumans E. H., Markowitz L. E., Berman S. M., St Louis M. E. (1999). A public health approach to adverse outcomes of pregnancy associated with bacterial vaginosis. Int. J. Gynaecol. Obstet. 67 S29–S33. 10.1016/s0020-7292(99)00136-8 [DOI] [PubMed] [Google Scholar]
- Koumans E. H., Sternberg M., Bruce C., McQuillan G., Kendrick J., Sutton M., et al. (2007). The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex. Transm. Dis. 34 864–869. 10.1097/OLQ.0b013e318074e565 [DOI] [PubMed] [Google Scholar]
- Krüger W., Vielreicher S., Kapitan M., Jacobsen I. D., Niemiec M. J. (2019). Fungal-bacterial interactions in health and disease. Pathogens 8:70. 10.3390/pathogens8020070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y. K., Daroczy K., Colque P., Kuhn I., Mollby R., Kopp Kallner H. (2017). Persistence of lactobacilli in postmenopausal women – a double-blind, randomized, pilot study. Gynecol. Obstet. Invest. 82 144–150. 10.1159/000446946 [DOI] [PubMed] [Google Scholar]
- Lagier J.-C., Armougom F., Million M., Hugon P., Pagnier I., Robert C., et al. (2012). Microbial culturomics: paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 18 1185–1193. 10.1111/1469-0691.12023 [DOI] [PubMed] [Google Scholar]
- Lai G. C., Tan T. G., Pavelka N. (2019). The mammalian mycobiome: A complex system in a dynamic relationship with the host. Wiley Interdiscip. Rev. Syst. Biol. Med. 11:e1438. 10.1002/wsbm.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtoranta L., Hibberd A. A., Reimari J., Junnila J., Yeung N., Maukonen J., et al. (2020). Recovery of vaginal microbiota after standard treatment for bacterial vaginosis infection: an observational study. Microorganisms 8:875. 10.3390/microorganisms8060875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtoranta L., Hibberd A. A., Yeung N., Laitila A., Maukonen J., Ouwehand A. C. (2021). Short communication: Characterization of vaginal fungal communities in healthy women and women with bacterial vaginosis (BV); a pilot study. Microb. Pathog. 161:105055. 10.1016/j.micpath.2021.105055 [DOI] [PubMed] [Google Scholar]
- Lev-Sagie A., Goldman-Wohl D., Cohen Y., Dori-Bachash M., Leshem A., Mor U., et al. (2019). Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 25 1500–1504. 10.1038/s41591-019-0600-6 [DOI] [PubMed] [Google Scholar]
- Lewis F. M. T., Bernstein K. T., Aral S. O. (2017). Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet. Gynecol. 129 643–654. 10.1097/AOG.0000000000001932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang T., Li Y., Zhang T., Wang Q., He J., et al. (2019). Probiotics for the treatment of women with bacterial vaginosis: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Pharmacol. 864:172660. 10.1016/j.ejphar.2019.172660 [DOI] [PubMed] [Google Scholar]
- Liang X., Li Z., Tye K. D., Chen Y., Luo H., Xiao X. (2021). The effect of probiotic supplementation during pregnancy on the interaction network of vaginal microbiome. J. Obstet. Gynaecol. Res. 47 103–113. 10.1111/jog.14434 [DOI] [PubMed] [Google Scholar]
- Liang Y., Chen M., Qin L., Wan B., Wang H. (2019). A meta-analysis of the relationship between vaginal microecology, human papillomavirus infection and cervical intraepithelial neoplasia. Infect. Agents Cancer 14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes Dos Santos Santiago G., Cools P., Verstraelen H., Trog M., Missine G., Aila N. E., et al. (2011). Longitudinal Study of the dynamics of vaginal microflora during two consecutive menstrual cycles. PLoS One 6:e28180. 10.1371/journal.pone.0028180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. Y., Rotstein C. (2011). Emerging fungal infections in immunocompromised patients. F1000 Med. Rep. 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y., Yan Z., Zhao X., Gang X., He G., Sun L., et al. (2018). The effects of gut microbiota on metabolic outcomes in pregnant women and their offspring. Food Funct. 9 4537–4547. 10.1039/c8fo00601f [DOI] [PubMed] [Google Scholar]
- Maaloum M., Diop K., Diop A., Anani H., Tomei E., Richez M., et al. (2019). Description of Janibacter massiliensis sp. nov., cultured from the vaginal discharge of a patient with bacterial vaginosis. Antonie Van Leeuwenhoek 112 1147–1159. 10.1007/s10482-019-01247-x [DOI] [PubMed] [Google Scholar]
- Macklaim J. M., Clemente J. C., Knight R., Gloor G. B., Reid G. (2015). Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 26 27799–27799. 10.3402/mehd.v26.27799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrazzo J. M., Fiedler T. L., Srinivasan S., Thomas K. K., Liu C., Ko D., et al. (2012). Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J. Infect. Dis. 205 1580–1588. 10.1093/infdis/jis242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschalek J., Farr A., Marschalek M. L., Domig K. J., Kneifel W., Singer C. F., et al. (2017). Influence of orally administered probiotic Lactobacillus strains on vaginal microbiota in women with breast cancer during chemotherapy: a randomized placebo-controlled double-blinded pilot study. Breast Care (Basel) 12 335–339. 10.1159/000478994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín V., Cárdenas N., Ocaña S., Marín M., Arroyo R., Beltrán D., et al. (2019). Rectal and vaginal eradication of Streptococcus agalactiae (GBS) in pregnant women by using Lactobacillus salivarius CECT 9145, a target-specific probiotic strain. Nutrients 11:810. 10.3390/nu11040810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas M. N., Flaxman S. R., Boerma T., Vanderpoel S., Stevens G. A. (2012). National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 9:e1001356. 10.1371/journal.pmed.1001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. T., Srinivasan S., Fiedler T. L., Marrazzo J. M., Fredricks D. N., Schiffer J. T. (2015). Rapid and profound shifts in the vaginal microbiota following antibiotic treatment for bacterial vaginosis. J. Infect. Dis. 212 793–802. 10.1093/infdis/jiv079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. A., Beasley D. E., Dunn R. R., Archie E. A. (2016). Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front. Microbiol. 7:1936. 10.3389/fmicb.2016.01936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. M., Srinivasan S., Plantinga A., Wu M. C., Reed S. D., Guthrie K. A., et al. (2018). Associations between improvement in genitourinary symptoms of menopause and changes in the vaginal ecosystem. Menopause 25 500–507. 10.1097/GME.0000000000001037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. M., Srinivasan S., Zhan X., Wu M. C., Reed S. D., Guthrie K. A., et al. (2017). Vaginal microbiota and genitourinary menopausal symptoms: a cross-sectional analysis. Menopause 24 1160–1166. 10.1097/GME.0000000000000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. K., Ranvir S., Nikam P., David J. (2019). Alleviation of fertility disorders based on probiotics induced immune system improvement: a review. Crit. Rev. Biomed. Eng. 47 427–436. 10.1615/CritRevBiomedEng.2019031055 [DOI] [PubMed] [Google Scholar]
- Moosa Y., Kwon D., De Oliveira T., Wong E. B. (2020). Determinants of vaginal microbiota composition. Front. Cell. Infect. Microbiol. 10:467. 10.3389/fcimb.2020.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G., Cardenas I. (2010). The immune system in pregnancy: a unique complexity. Am. J. Reprod. Immunol. (New York, N.Y. 1989) 63 425–433. 10.1111/j.1600-0897.2010.00836.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer L., Kuhn L., Stein Z. A., Wright T. C., Jr., Denny L. (2005). Intravaginal practices, bacterial vaginosis, and women’s susceptibility to HIV infection: epidemiological evidence and biological mechanisms. Lancet Infect. Dis. 5 786–794. 10.1016/S1473-3099(05)70298-X [DOI] [PubMed] [Google Scholar]
- Myhre A. K., Bevanger L. S., Berntzen K., Bratlid D. (2002). Anogenital bacteriology in non-abused preschool children: a descriptive study of the aerobic genital flora and the isolation of anogenital Gardnerella vaginalis. Acta Paediatr. 91 885–891. 10.1080/080352502760148586 [DOI] [PubMed] [Google Scholar]
- Nash A. K., Auchtung T. A., Wong M. C., Smith D. P., Gesell J. R., Ross M. C., et al. (2017). The gut mycobiome of the human microbiome project healthy cohort. Microbiome 5:153. 10.1186/s40168-017-0373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers Y. H., Nansel T. R., Andrews W. W., Schwebke J. R., Yu K. F., Goldenberg R. L., et al. (2007). Dietary intake of selected nutrients affects bacterial vaginosis in women. J. Nutr. 137 2128–2133. 10.1093/jn/137.9.2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T. M., Borgogna J. C., Michalek R. D., Roberts D. W., Rath J. M., Glover E. D., et al. (2018). Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Sci. Rep. 8:852. 10.1038/s41598-017-14943-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn K. L., Witkin S. S., Schneider G. M., Boester A., Nasioudis D., Minis E., et al. (2021). Changes in the vaginal microbiome during the pregnancy to postpartum transition. Reprod. Sci. 28 1996–2005. 10.1007/s43032-020-00438-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwokolo N. C., Boag F. C. (2000). Chronic vaginal candidiasis. Management in the postmenopausal patient. Drugs Aging 16 335–339. 10.2165/00002512-200016050-00003 [DOI] [PubMed] [Google Scholar]
- Nyirjesy P., Mcintosh M. J., Gattermeir D. J., Schumacher R. J., Steinmetz J. I., Joffrion J. L. (2006). The effects of intravaginal clindamycin and metronidazole therapy on vaginal lactobacilli in patients with bacterial vaginosis. Am. J. Obstet. Gynecol. 194 1277–1282. 10.1016/j.ajog.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Olsen P., Williamson M., Traynor V., Georgiou C. (2018). The impact of oral probiotics on vaginal Group B streptococcal colonisation rates in pregnant women: A pilot randomised control study. Women Birth 31 31–37. 10.1016/j.wombi.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Pabich W. L., Fihn S. D., Stamm W. E., Scholes D., Boyko E. J., Gupta K. (2003). Prevalence and determinants of vaginal flora alterations in postmenopausal women. J. Infect. Dis. 188 1054–1058. 10.1086/378203 [DOI] [PubMed] [Google Scholar]
- Pappas P. G., Lionakis M. S., Arendrup M. C., Ostrosky-Zeichner L., Kullberg B. J. (2018). Invasive candidiasis. Nat. Rev. Dis. Primers 4:18026. [DOI] [PubMed] [Google Scholar]
- Parma M., Dindelli M., Caputo L., Redaelli A., Quaranta L., Candiani M. (2013). The role of vaginal Lactobacillus rhamnosus (Normogin®) in preventing Bacterial Vaginosis in women with history of recurrences, undergoing surgical menopause: a prospective pilot study. Eur. Rev. Med. Pharmacol. Sci. 17 1399–1403. [PubMed] [Google Scholar]
- Patras K. A., Nizet V. (2018). Group B streptococcal maternal colonization and neonatal disease: molecular mechanisms and preventative approaches. Front. Pediatr. 6:27. 10.3389/fped.2018.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles K., Velloza J., Balkus J. E., McClelland R. S., Barnabas R. V. (2019). High global burden and costs of bacterial vaginosis: a systematic review and meta-Analysis. Sex. Transm. Dis. 46 304–311. 10.1097/OLQ.0000000000000972 [DOI] [PubMed] [Google Scholar]
- Petricevic L., Unger F. M., Viernstein H., Kiss H. (2008). Randomized, double-blind, placebo-controlled study of oral lactobacilli to improve the vaginal flora of postmenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 141 54–57. 10.1016/j.ejogrb.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Petrova M. I., Lievens E., Malik S., Imholz N., Lebeer S. (2015). Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 6:81. 10.3389/fphys.2015.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova M. I., Reid G., Vaneechoutte M., Lebeer S. (2017). Lactobacillus iners: Friend or Foe? Trends Microbiol. 25 182–191. 10.1016/j.tim.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Preisler J., Kopeika J., Ismail L., Vathanan V., Farren J., Abdallah Y., et al. (2015). Defining safe criteria to diagnose miscarriage: prospective observational multicentre study. BMJ 351:h4579. 10.1136/bmj.h4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S., Amaretti A., Gozzoli C., Simone M., Righini L., Candeliere F., et al. (2019). Longitudinal survey of fungi in the human gut: ITS profiling, phenotyping, and colonization. Front. Microbiol. 10:1575. 10.3389/fmicb.2019.01575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranđeloviė G., Mladenović V., Ristić L., Otašević S., Branković S., Mladenović-Antić S., et al. (2012). Microbiological aspects of vulvovaginitis in prepubertal girls. Eur. J. Pediatr. 171 1203–1208. 10.1007/s00431-012-1705-9 [DOI] [PubMed] [Google Scholar]
- Ravel J., Gajer P., Abdo Z., Schneider G. M., Koenig S. S., Mcculle S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl. 1) 4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R., Hassan S. S., Gajer P., Tarca A. L., Fadrosh D. W., Nikita L., et al. (2014). The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Erratum in: Microbiome 2014;2:4. doi: 10.1186/2049-2618-2-4. Microbiome 2:10. 10.1186/2049-2618-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose W. A., Mcgowin C. L., Spagnuolo R. A., Eaves-Pyles T. D., Popov V. L., Pyles R. B. (2012). Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One 7:e32728. 10.1371/journal.pone.0032728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam Q. H., Chang M., Chai L. (2017). The fungal mycobiome and its interaction with gut bacteria in the host. Int. J. Mol. Sci. 18:330. 10.3390/ijms18020330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk M., Grumet L., Sternat J., Reinschissler N., Weiss G. (2021). Effect of probiotics on vaginal Ureaplasma parvum in women suffering from unexplained infertility. Reprod. Biomed. Online 43 503–514. 10.1016/j.rbmo.2021.06.004 [DOI] [PubMed] [Google Scholar]
- Schwebke J. R., Desmond R. (2005). Risk factors for bacterial vaginosis in women at high risk for sexually transmitted diseases. Sex. Transm. Dis. 32 654–658. 10.1097/01.olq.0000175396.10304.62 [DOI] [PubMed] [Google Scholar]
- Serrano M. G., Parikh H. I., Brooks J. P., Edwards D. J., Arodz T. J., Edupuganti L., et al. (2019). Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 25 1001–1011. 10.1038/s41591-019-0465-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban O. M., Abbas A. M., Moharram A. M., Farhan M. M., Hassanen I. H. (2015). Does vaginal douching affect the type of candidal vulvovaginal infection? Med. Mycol. 53 817–827. 10.1093/mmy/myv042 [DOI] [PubMed] [Google Scholar]
- Shaaban O. M., Youssef A. E., Khodry M. M., Mostafa S. A. (2013). Vaginal douching by women with vulvovaginitis and relation to reproductive health hazards. BMC Womens Health 13:23. 10.1186/1472-6874-13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M., Shah V., Freire-Lizama T., Cates E. C., Mcgrath K., David I., et al. (2019). Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: a midwifery-led double-blind randomized controlled pilot trial. J. Matern. Fetal Neonatal Med. 34 1814–1821. 10.1080/14767058.2019.1650907 [DOI] [PubMed] [Google Scholar]
- Shen J., Song N., Williams C. J., Brown C. J., Yan Z., Xu C., et al. (2016). Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci. Rep. 6:24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheyholislami H., Connor K. L. (2021). Are probiotics and prebiotics safe for use during pregnancy and lactation? A systematic review and meta-analysis. Nutrients 13:2382. 10.3390/nu13072382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes J. A., Aroutcheva A. A., Shott S., Faro S. (2001). Effect of metronidazole on the growth of vaginal lactobacilli in vitro. Infect. Dis. Obstet. Gynecol. 9 41–45. 10.1155/S1064744901000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slykerman R. F., Hood F., Wickens K., Thompson J. M. D., Barthow C., Murphy R., et al. (2017). Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine 24 159–165. 10.1016/j.ebiom.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. B., Ravel J. (2017). The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 595 451–463. 10.1113/JP271694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. D., Acharya K. D., Zhu J. E., Deveney C. M., Walther-Antonio M. R. S., Tetel M. J., et al. (2020). Daily vaginal microbiota fluctuations associated with natural hormonal cycle, contraceptives, diet, and exercise. mSphere 5:e00593-20. 10.1128/mSphere.00593-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Liu C., Mitchell C. M., Fiedler T. L., Thomas K. K., Agnew K. J., et al. (2010). Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 5:e10197. 10.1371/journal.pone.0010197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanović N., Plećaš D., Plešinac S. (2012). Normal vaginal flora, disorders and application of probiotics in pregnancy. Arch. Gynecol. Obstet. 286 325–332. 10.1007/s00404-012-2293-7 [DOI] [PubMed] [Google Scholar]
- Stumpf R. M., Wilson B. A., Rivera A., Yildirim S., Yeoman C. J., Polk J. D., et al. (2013). The primate vaginal microbiome: comparative context and implications for human health and disease. Am. J. Phys. Anthropol. 152(Suppl. 57) 119–134. 10.1002/ajpa.22395 [DOI] [PubMed] [Google Scholar]
- Swidsinski A., Loening-Baucke V., Swidsinski S., Verstraelen H. (2015). Polymicrobial Gardnerella biofilm resists repeated intravaginal antiseptic treatment in a subset of women with bacterial vaginosis: a preliminary report. Arch. Gynecol. Obstet. 291 605–609. 10.1007/s00404-014-3484-1 [DOI] [PubMed] [Google Scholar]
- Swidsinski A., Mendling W., Loening-Baucke V., Swidsinski S., Dörffel Y., Scholze J., et al. (2008). An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol. 198 97e1–e6. 10.1016/j.ajog.2007.06.039 [DOI] [PubMed] [Google Scholar]
- Thoma M. E., Gray R. H., Kiwanuka N., Aluma S., Wang M. C., Sewankambo N., et al. (2011a). Longitudinal changes in vaginal microbiota composition assessed by gram stain among never sexually active pre- and postmenarcheal adolescents in Rakai, Uganda. J. Pediatr. Adolesc. Gynecol. 24 42–47. 10.1016/j.jpag.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma M. E., Klebanoff M. A., Rovner A. J., Nansel T. R., Neggers Y., Andrews W. W., et al. (2011b). Bacterial vaginosis is associated with variation in dietary indices. J. Nutr. 141 1698–1704. 10.3945/jn.111.140541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuddenham S., Ghanem K. G., Caulfield L. E., Rovner A. J., Robinson C., Shivakoti R., et al. (2019). Associations between dietary micronutrient intake and molecular-Bacterial Vaginosis. Reprod. Health 16:151. 10.1186/s12978-019-0814-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill D. M., Iliev I. D. (2014). The mycobiota: interactions between commensal fungi and the host immune system. Nat. Rev. Immunol. 14 405–416. 10.1038/nri3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division (2019). Contraceptive Use by Method 2019: Data Booklet. ST/ESA/SER.A/435. New York, NY: Department of Economic and Social Affairs, Population Division. [Google Scholar]
- Ursell L. K., Metcalf J. L., Parfrey L. W., Knight R. (2012). Defining the human microbiome. Nutr. Rev. 70(Suppl. 1) S38–S44. 10.1111/j.1753-4887.2012.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wijgert J., Jespers V. (2017). The global health impact of vaginal dysbiosis. Res. Microbiol. 168 859–864. 10.1016/j.resmic.2017.02.003 [DOI] [PubMed] [Google Scholar]
- van de Wijgert J., Verwijs M. C. (2020). Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG Int. J. Obstet. Gynaecol. 127 287–299. 10.1111/1471-0528.15870 [DOI] [PubMed] [Google Scholar]
- van de Wijgert J., Borgdorff H., Verhelst R., Crucitti T., Francis S., Verstraelen H., et al. (2014). The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One 9:e105998. 10.1371/journal.pone.0105998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostrum N., De Sutter P., Meys J., Verstraelen H. (2013). Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum. Reprod. 28 1809–1815. 10.1093/humrep/det096 [DOI] [PubMed] [Google Scholar]
- Vargas-Robles D., Morales N., Rodríguez I., Nieves T., Godoy-Vitorino F., Alcaraz L. D., et al. (2020). Changes in the vaginal microbiota across a gradient of urbanization. Sci. Rep. 10:12487. 10.1038/s41598-020-69111-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwijs M. C., Agaba S. K., Darby A. C., Van De Wijgert J. (2020). Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am. J. Obstet. Gynecol. 222 157.e1–157.e13. 10.1016/j.ajog.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P., Cipolla C., D’ippolito S., Amar I. D., Shachor M., Ingravalle F., et al. (2020). The interplay between immune system and microbiota in gynecological diseases: a narrative review. Eur. Rev. Med. Pharmacol. Sci. 24 5676–5690. 10.26355/eurrev_202005_21359 [DOI] [PubMed] [Google Scholar]
- Virtanen S., Rantsi T., Virtanen A., Kervinen K., Nieminen P., Kalliala I., et al. (2019). Vaginal microbiota composition correlates between pap smear microscopy and next generation sequencing and associates to socioeconomic status. Sci. Rep. 9:7750. 10.1038/s41598-019-44157-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. H., Tobler K. J. (2021). “Female Infertility,” in StatPearls. Treasure Island, FL: StatPearls Publishing. [Google Scholar]
- Wang Z., He Y., Zheng Y. (2019). Probiotics for the treatment of bacterial vaginosis: a meta-analysis. Int. J. Environ. Res. Public Health 16:3859. 10.3390/ijerph16203859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward T. L., Dominguez-Bello M. G., Heisel T., Al-Ghalith G., Knights D., Gale C. A. (2018). Development of the human mycobiome over the first month of life and across body sites. mSystems 3:e00140-17. 10.1128/mSystems.00140-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y. P., Feng J., Luo Z. C. (2010). Isolation and genotyping of vaginal non-albicans Candida spp. in women from two different ethnic groups in Lanzhou, China. Int. J. Gynaecol. Obstet. 110 227–230. 10.1016/j.ijgo.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Westhoff C., Gentile G., Lee J., Zacur H., Helbig D. (1996). Predictors of ovarian steroid secretion in reproductive-age women. Am. J. Epidemiol. 144 381–388. 10.1093/oxfordjournals.aje.a008939 [DOI] [PubMed] [Google Scholar]
- Wheeler M. L., Limon J. J., Bar A. S., Leal C. A., Gargus M., Tang J., et al. (2016). Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe 19 865–873. 10.1016/j.chom.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. D., Yeaman G. R., Givan A. L., Wira C. R. (1997). Mucosal immunity in the human female reproductive tract: cytotoxic T lymphocyte function in the cervix and vagina of premenopausal and postmenopausal women. Am. J. Reprod. Immunol. 1 30–38. 10.1111/j.1600-0897.1997.tb00190.x [DOI] [PubMed] [Google Scholar]
- Wiesenfeld H. C., Hillier S. L., Krohn M. A., Landers D. V., Sweet R. L. (2003). Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin. Infect. Dis. 36 663–668. [DOI] [PubMed] [Google Scholar]
- Wira C. R., Fahey J. V., Ghosh M., Patel M. V., Hickey D. K., Ochiel D. O. (2010). Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am. J. Reprod. Immunol. (New York, N.Y. 1989) 63 544–565. 10.1111/j.1600-0897.2010.00842.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H. Y., Feng D., Wei D. M., Mei L., Chen H., Wang X., et al. (2017). Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst. Rev. 11:Cd010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Zhou X., Williams C. J., Hochwalt A., Forney L. J. (2009). Bacterial populations in the vaginas of healthy adolescent women. J. Pediatr. Adolesc. Gynecol. 22 11–18. 10.1016/j.jpag.2008.01.073 [DOI] [PubMed] [Google Scholar]
- Yarbrough V. L., Winkle S., Herbst-Kralovetz M. M. (2015). Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Update 21 353–377. 10.1093/humupd/dmu065 [DOI] [PubMed] [Google Scholar]
- Yilmaz A. E., Celik N., Soylu G., Donmez A., Yuksel C. (2012). Comparison of clinical and microbiological features of vulvovaginitis in prepubertal and pubertal girls. J. Formos. Med. Assoc. 111 392–396. 10.1016/j.jfma.2011.05.013 [DOI] [PubMed] [Google Scholar]
- Younes J. A., Lievens E., Hummelen R., Van Der Westen R., Reid G., Petrova M. I. (2018). Women and their microbes: the unexpected friendship. Trends Microbiol. 26 16–32. 10.1016/j.tim.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Zhou X., Brown C. J., Abdo Z., Davis C. C., Hansmann M. A., Joyce P., et al. (2007). Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 1 121–133. 10.1038/ismej.2007.12 [DOI] [PubMed] [Google Scholar]