Abstract
Breast cancer is a multifaceted disease that currently represents a leading cause of death in women worldwide. Over the past two decades (1998–2020), the National Health Laboratory Service’s Human Genetics Laboratory in central South Africa screened more than 2,974 breast and/or ovarian cancer patients for abnormalities characteristic of the widely known familial breast cancer genes, Breast Cancer gene 1 (BRCA1) and Breast Cancer gene 2 (BRCA2). Patients were stratified according to the presence of family history, age at onset, stage of the disease, ethnicity and mutation status relative to BRCA1/2. Collectively, 481 actionable (likely-to pathogenic) variants were detected in this cohort among the different ethnic/racial groups. A combination of old (pre-2014) and new (post-2014) laboratory techniques was used to identify these variants. Additionally, targeted genotyping was performed as translational research revealed the first three recurrent South African pathogenic variants, namely BRCA1 c.1374del (legacy name 1493delC), BRCA1 c.2641G>T (legacy name E881X) and BRCA2 c.7934del (legacy name 8162delG). This initial flagship study resulted in a cost-effective diagnostic test that enabled screening of a particular ethnic group for these variants. Since then, various non-Afrikaner frequent variants were identified that were proven to represent recurrent variants. These include BRCA2 c.5771_5774del (legacy name 5999del4) and BRCA2 c.582G>A, both Black African founder mutations. By performing innovative translational research, medical science in South Africa can adopt first-world technologies into its healthcare context as a developing country. Over the past two decades, the progress made in the public sector enabled a pivotal shift away from population-directed genetic testing to the screening of potentially all breast and ovarian cancer patients, irrespective of ethnicity, family history or immunohistochemical status. The modifications over the years complied with international standards and guidelines aimed at universal healthcare for all. This article shares all the cohort stratifications and the likely-to pathogenic variants detected.
Keywords: familial breast and ovarian cancer, predisposing genes BRCA1/2 , central South Africa, National Health Laboratory Service, public sector
1 Introduction
The two familial breast cancer (BC) genes, Breast Cancer gene 1 (BRCA1) and Breast Cancer gene 2 (BRCA2), are highly penetrant and contribute to various cellular events ranging from the response to DNA damage to control of the cell cycle and apoptosis (Yoshida and Miki, 2004). Germline pathogenic variants in these genes create genetic instability impacting their capacity to repair the damage. Likely- to pathogenic variants in these genes are associated with hypersensitivity in BC patients. Their presence results in potentially severe radiotherapy complications during treatment due to spontaneous and enhanced radio-sensitivity (Chistiakov et al., 2008; Kan and Zhang, 2015). Disruptive BRCA1/2 variants are associated with a predisposition to breast and ovarian cancer, and although at a lower frequency, prostate, pancreas and other cancer types are also linked to pathogenic BRCA1/2 variants. Although these two high-impact cancer-predisposing genes were discovered more than two decades ago, they have dominated the field of BC genetics ever since.
BC is the most common cancer and the leading cause of cancer-related death in females worldwide (Bray et al., 2018). Most cases are sporadic. However, 5%–10% can be attributed to a hereditary component (Larsen et al., 2014). The disease was mostly considered an illness of the affluent; however, the incidence in developing countries, such as South Africa (SA), is rapidly increasing (Joffe et al., 2018). The age-standardized annual BC incidence rate (ASR) per 100,000 ranges between 52.92 and 79.3 for Asian, Caucasian and mixed-race SA women, compared to 29.1 for Black women. The average ASR, however, is currently 1 in 32 for a SA female to develop the disease (Francies et al., 2015).
The complex history of sub-Saharan Africa has highlighted the diverse populations of SA regarding the field of medical and population genetics (Oosthuizen et al., 2021). Although SA harbors over 60 million people, each of its main population groups has a unique origin. This diversity resulted from various migration events from all over the globe, such as European colonialism from predominantly north-western Europe, which gave rise to the Afrikaner with its Anglo-European descent (Attlee, 1947). Simultaneously, the indigenous expansion of Black Africans to the southern tip of the African continent resulted in approximately 80% of the entire SA population being neither culturally, linguistically, nor genetically homogenous (Van der Merwe et al., 2012).
Additional genetic lineages were introduced by laborers arriving from south Asia. Their arrival resulted in admixture between various groups already residing in SA, including the indigenous African people such as the Khoikhoi, the San, and the African Xhosa tribe (Attlee, 1947). These groups were eventually absorbed into the mixed ancestry group (Oosthuizen et al., 2021). Finally, the last major grouping (Asian population) originated from admixture of individuals from mainland India, neighboring countries such as Bangladesh, and the Mixed Ancestry population of SA (Vishnu and Morrell, 1991). Therefore, the modern-day Asian (specifically the SA Indian) population comprises mostly of people who migrated from mainland India to SA over 300 years, with admixture involving countries from Eurasia and Africa (Mesthrie, 2006; Isaacs et al., 2013). As our genomes reflect a record of historical events, so too does the genetic diversity in the field of hereditary breast and ovarian cancer (HBOC) reflect the complexity of the SA population (Van der Merwe et al., 2020; Combrink et al., 2021; Oosthuizen et al., 2021).
Patients with likely- to pathogenic germline variants in these high-risk genes have an increased predisposition to develop BC and/or ovarian cancer (OVC) throughout their lifetime. According to global statistics, the cumulative risk for BRCA1 and BRCA2 mutation carriers to develop BC before 80 years of age is 40%–87% and 27%–84%, respectively. The associated risk for OVC varies from 16%–68% and 11%–30%, respectively (Kuchenbaecker et al., 2017). The etiology related to hereditary BC and OVC in SA derived great benefit from population-based genetic research (Reeves et al., 2004; Agenbag, 2005; Van der Merwe and van Rensburg, 2009; Sluiter and Van Rensburg, 2011; Van der Merwe et al., 2012; Peter, 2014; Chen, 2015; Francies et al., 2015; Combrink, 2016; Moeti, 2016; Oosthuizen, 2016; Van der Merwe et al., 2020; Combrink et al., 2021; Mampunye et al., 2021; Oosthuizen et al., 2021), resulting in the identification of five founder variants representative of three of the four major ethnic groups in the country (BRCA1 c.1374del [rs397508862], BRCA1 c.2641G>T [rs39750888] (Reeves et al., 2004); BRCA2 c.7934del [rs80359688] (Van der Merwe and van Rensburg, 2009); BRCA2 c.5771_5774del [80359535] (Van der Merwe et al., 2012); BRCA2 c.582G>A [rs80358810] (Oosthuizen et al., 2021)).
The SA studies performed to date reflect substantial variation in the yield of actionable (likely-pathogenic and pathogenic) germline variants identified in the country. As only 4.5% of the SA total budget is allocated to healthcare expenditure, it burdens an already stressed public sector to seek cost-effective alternatives for routine diagnostic testing of familial breast and ovarian cancer patients. This single-institution public sector study aimed to determine the range and positive mutation percentage of BRCA1 and BRCA2 actionable variants in an unselected large cohort of BC and OVC patients. These patients were screened using various technologies ranging from targeted genotyping to comprehensive screening, employing both older and new technologies. Our results prompted us to contemplate the most appropriate workflow for SA state-owned pathology laboratories to provide cost-effective genetic assessment in a financially constraint health sector.
2 Materials and Methods
2.1 Study Population
A total of 2,975 BC and/or OVC patients were consulted at the National Health Laboratory Service (NHLS) Human Genetics Laboratory in Bloemfontein between 01/01/1998 and 31/12/2020, of whom 81 patients had incomplete data and were excluded from the analysis. The remaining 2,894 BC and/or OVC patients (2,733 females and 161 males) were examined by targeted genotyping or comprehensive screening of BRCA1/2 (Figure 1). These BC and/or OVC patients attended their closest genetic clinic at a regional or provincial hospital, where they were referred for diagnostic genetic testing through their local genetic counselor or attending physician. Indications for testing included BC diagnosed at age 45 years or less or a significant family history (at least one first-degree family member with premenopausal BC or OVC, or multiple second-degree family members with premenopausal BC and/or OVC, or males with BC at any age).
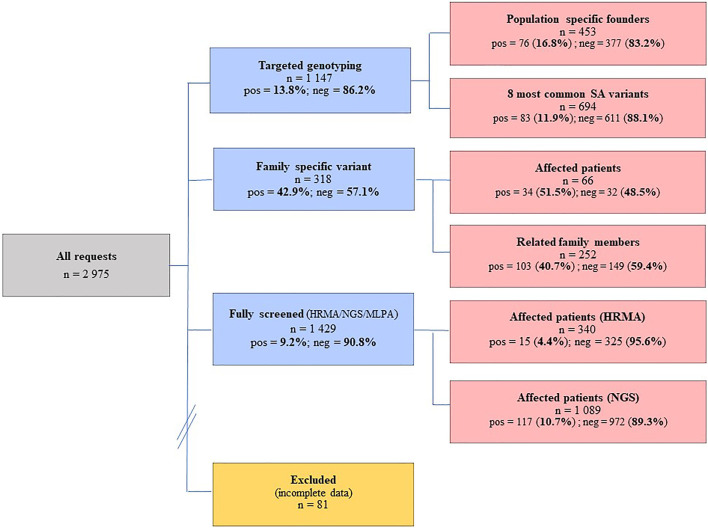
FIGURE 1.
Individuals screened at the NHLS Human Genetics laboratory in Bloemfontein between 1998 and 2020, using a combination of investigative methods ranging from targeted genotyping to comprehensive screening. The numbers screened together with the success rates are indicated. Pos, positives; neg, negatives; HRMA, high resolution melting analysis; NGS, next-generation sequencing; MLPA, multiplex ligation-dependent probe amplification.
Ethnicity was determined by patients’ self-identification at the time of consultation. The major ethnic group classifications have sub-classifications, some contextualized specifically to the SA demographic profile (Table 1). The majority (n = 975) was Caucasian, followed by the Black African (n = 753) and mixed ancestry groups (n = 669), with the Asian group being the minority (n = 297). The ethnicities for the remainder of patients (n = 200) were either not indicated or unknown. The distribution of the patients across the various ethnic groups reflected the demographic profile of the breast clinic only and did not reflect BC incidence within each of these groups. Additionally, patient reports generated over the years were retrospectively analyzed to collect information on epidemiological characteristics, which specifically focused on 1) presence of a family history of BC and/or OVC; 2) age at onset; 3) stage of the disease; 4) ethnicity; and 5) mutation status.
TABLE 1.
Self-identified ethnicity of consecutive BC and/or OVC patients included in the study (n = 2,896).
| Major ethnic classification | Sub-ethnic classification | Count |
|---|---|---|
| Asian | South African Indian, Malay, Pakistani, Chinese, Japanese, Bengali, Mongolian | 297 |
| Black African | All Black African nationalities | 753 |
| Caucasian | South African Afrikaner, British, and European descent | 975 |
| Mixed ancestry | A South African subgroup of people that comprise a mixture of any of the abovementioned ethnic classifications | 669 |
| Unknown | Not specified | 200 |
Majority of individuals included in this cohort were diagnosed with BC and/or OVC. All the individuals had received pre- and post-test counseling at their respective referral centers. Information regarding personal and family history, as well as written informed consent for DNA testing, were obtained. The cohort included patients with a positive family history (two or more affected family members) for BC and/or OVC, with most representing low-risk patients who had no prior knowledge of a family history of either condition but were diagnosed at an early age of onset (<40 years). The Health Sciences Research Ethics Committee (HSREC) of the University of the Free State in Bloemfontein, SA, approved all study protocols submitted since 1995 (ETOVS 31/95; ECUFS 31B/95; ECUFS 31C/95; ETOVS 49/06; ETOVS 65/08; ECUFS 107/2014; ECUFS 108/2014; UFS-HSD2019/1835/2910 and UFS-HSD2020/0194/3006). The NHLS permitted the use of the data (reference PR2110611).
2.2 Laboratory Methods
DNA was extracted from peripheral blood using a standard extraction method. The concentration and purity were determined using spectrophotometry. Targeted genotyping for the founder and recurrent actionable variants was performed using the LightCycler® 480 Genotyping Master Mix (Roche Diagnostics, Germany) on the LightCycler® 480 II real-time instrument (Roche Diagnostics, Germany). These assays employ hybridization and simple probe technology described by Oosthuizen (Oosthuizen, 2016). Targeted genotyping was performed using a standard real-time PCR regime, followed by melt curve analysis. Conventional mutation screening for single nucleotide variants (SNVs) and smaller indels was initially performed for a subset of patients described previously (Combrink, 2016; Oosthuizen, 2016). This approach involved a combination of older technology-based techniques including High-Resolution Melt Analysis (HRMA), the protein truncation test (PTT) and Sanger sequencing (Van der Merwe et al., 2020). A total of 340 patients were screened using a combination of these older techniques (Figure 1).
Individuals screened using the conventional approach were subjected to copy number variants (CNVs) analysis using Multiplex Ligation-dependent Probe Amplification (MLPA). The assays used included the SALSA® MLPA® P002-C1 and SALSA® MLPA® P002-D1 for BRCA1, with SALSA® MLPA® P045-B3 used for BRCA2 (MRC-Holland, Amsterdam, Netherlands). The products were run together with a size standard on an ABI 3130XL Genetic analyzer (Applied Biosystems, Carlsbad, CA, United States). MLPA-positive results, especially in the case of single exon deletions, were corroborated using an alternative confirmation assay for each of the genes, namely SALSA® MLPA® P087-C1 for BRCA1 and SALSA® MLPA® P077-A3 for BRCA2. All the data were analyzed using GeneMarker® software version 2.6.4 (SoftGenetics, LLC, State College, PA, United States). The CNVs were named according to the Human Genome Variation Society (http://www.HGVS.org/varnomen) guidelines and classified using the adapted recommendations of the American Society of Medical Genetics and Genomics (ACMG) for the interpretation and reporting of single-gene CNVs (Brandt et al., 2020).
Once introduced, next-generation sequencing (NGS) was performed to screen the remainder of samples (n = 1,089), using the Oncomine™ BRCA Research Assay (Life Technologies, Carlsbad, CA, United States). The primer pools targeted both genes’ entire coding region and splice-site junctions. Multiplexed primer pools were used to construct the amplicon library using PCR-based targeted amplification. Sequencing was performed on the Ion Proton and S5 Platforms (Life Technologies, Carlsbad, CA, United States), and the Ion Reporter™ Software was used to filter out artifacts and annotate the variants. Raw signal data were analyzed as described by Van der Merwe et al. (2020). The average read-depths obtained were 581×, ranging between 148 and 1,965×. Using the depth per read, quartile statistics were applied to calculate average depth distribution around the mean across the NGS samples. Samples that were located within the 2nd and 3rd quartiles were selected to construct a CNV baseline with the Ion Reporter CNV VCIB 4.0.0.1 algorithm. CNV detection was performed using an algorithm that normalized depth coverage across amplicons to predict the copy number or ploidy states. The computed baseline included a minimum of 100 control samples (each with an average of 24 million bases called and a read count of 215,000), using regions with known ploidy states (https://assets.thermofisher.com/TFS-Assets/LSG/brochures/CNV-Detection-by-Ion.pdf). MLPA was performed to confirm all CNVs detected using NGS. Novel or complex sequence variants were confirmed by means of Sanger DNA sequencing (ABI Prism BigDye® Terminator v3.1 cycle sequencing kit, Foster City, CA, United States), using an Applied Biosystems 3130 automated sequencer (Life Technologies, Foster City, CA, United States).
The clinical significance of variants was determined based on the American College of Medical Genetics guidelines (ACMG, classification confirmed on 02/12/2021) (Richards et al., 2015) and evaluated from freely accessible public databases such as ClinVar and the genomic search engine VarSome (Kopanos et al., 2019). The variant nomenclature was used according to Human Genome Variation Society (HGVS) recommendations (http://www.hgvs.org/rec.html). BRCA1/2 variants were numbered and annotated using the National Center for Biotechnology Information (NCBI) chromosomes and transcript reference sequences (NC_000017.11, NM_007294.4 and NC_000013.11, NM_000059.3), respectively. To prevent potential biases between the different laboratory techniques utilized for mutation screening throughout the years, all potential nucleotide changes were confirmed using Sanger sequencing. The analyses were confirmed using the same mutation detection databases and reference sequences.
3 Results
3.1 Composition of Cohort
The influence of various SA founder variants is reflected in the relatively high mutation-positive rates observed for targeted genotyping (Figure 1), with 16.8% observed using population-directed targeted genotyping (n = 453). Although the initial screening was based on the individual’s ethnicity, the positive mutation rate decreased when cases were screened using a broader approach that included all of the commonly occurring SA variants (11.9%, n = 694). Of the 1,429 patients comprehensively screened, 137 (9.2%) carried a likely- to pathogenic BRCA1/2 variant. Although the majority represented SNV changes consisting predominantly of substitutions and deletions, eight CNVs were identified. Regarding the SA mutation spectrum, the Afrikaner founder variant BRCA2 c.7934del (rs80359688) was the most common, followed by the Black African founder variant BRCA2 c.5771_5774del (rs80359535).
An inherited susceptibility was confirmed in 51.5% of affected family members tested (n = 66), that carried the actionable variant segregating in the family (Figure 1). A relatively low number of unaffected family members (n = 252) were genotyped for various family-specific variants. The positive mutation rate for this group was high (40.7%). By knowing their mutation status, these patients were included in various cancer screening programs to facilitate earlier detection and a potentially better prognosis in case of a cancer diagnosis.
3.2 Epidemiology
The epidemiological data were analyzed according to the five variables highlighted, namely a family history of BC and/or OVC, age at onset, stage of the disease, ethnicity and mutation status. Regarding the age at onset, the majority of patients tested fell in the 40–49 age group (collectively 27.7%), followed by the 50–59 age group (21.2%) (Table 2). These two age intervals also delivered the highest percentage mutation-positive results compared to the other age groups, namely 4.5% and 3.8%, respectively. Only three (0.5%) of the patients identified with a BRCA1/2 actionable variant in the 20–29 age group were observed. These positive patients represented approximately one-third of patients tested in this age group. These patients either represented high-risk BC/OVC families or were diagnosed with aggressive disease early and were therefore genetically screened (Table 2).
TABLE 2.
Comparison between the number of mutation-positive versus mutation-negative patients (reflected in percentages) observed per ten-year intervals.
| Age group | Mutation negative (n = 2,413) | Mutation positive (n = 481) | Total (%) |
|---|---|---|---|
| 0–19 (n = 3) | 0.10 | 0.00 | 0.10 |
| 20–29 (n = 62) | 1.66 | 0.48 | 2.14 |
| 30–39 (n = 480) | 13.96 | 2.63 | 16.59 |
| 40–49 (n = 800) | 23.15 | 4.49 | 27.64 |
| 50–59 (n = 616) | 17.45 | 3.84 | 21.29 |
| 60–69 (n = 467) | 13.41 | 2.73 | 16.14 |
| 70–79 (n = 278) | 8.05 | 1.55 | 9.61 |
| 80–89 (n = 108) | 3.25 | 0.48 | 3.73 |
| 90+ (n = 21) | 0.59 | 0.14 | 0.73 |
| Unknown (n = 59) | 1.76 | 0.28 | 2.04 |
| Total (%) | 83.38 | 16.62 | 100.00 |
Of the 481 mutation carriers identified, the majority were Caucasian. However, the data for this grouping were skewed due to the translational research performed before 1998, revealing the presence of three common founder variants in the Caucasian sub-category Afrikaner group (Table 1). This research initially involved mostly Afrikaner patients with a positive family history of BC and/or OVC being screened. This group served as the ideal research group due to proven extended high linkage disequilibrium with various founder effects reported. This SA group is considered a fruitful “hunting ground” for pathogenic variants associated with disease (Hall et al., 2002; Van der Merwe et al., 2012). The presence of these BRCA1/2 founder variants increased the mutation positivity rate to a remarkable 8.12%, delivering the highest positivity rate for Caucasians, namely 24.1%. The mutation positivity rate is an indicator used as a proxy for the relative percentage of patients that tested positive out of the total sample population (Table 3).
TABLE 3.
Illustration of the mutation detection and positivity rate per major population group (reflected in percentages) observed for the major groups.
| Ethnic group | Mutation positive (n = 481) | Mutation negative (n = 2,413) (%) | Mutation positivity rate (%) | Total (%) |
|---|---|---|---|---|
| Asian (n = 297) | 1.62 | 8.64 | 15.8 | 10.26 |
| Black African (n = 753) | 3.77 | 22.25 | 14.5 | 26.02 |
| Caucasian (n = 975) | 8.12 | 25.57 | 24.1 | 33.69 |
| Mixed ancestry (n = 669) | 2.70 | 20.42 | 11.7 | 23.12 |
| Unknown (n = 200) | 0.41 | 6.50 | 6.0 | 6.91 |
| Total (%) | 16.62 | 83.38 | 16.6 | 100.00 |
Information regarding the presence or absence of a family history of BC and/or OVC was available for most patients (Supplementary Table S1), with 5.8% designated as unknown (n = 141). Patients adopted as children contributed to the unknown category, as they had no prior family information. More than 80% of patients (n = 392) carrying an actionable BRCA1/2 variant reported family members affected with BC and other cancer types. Although the information regarding family structure varied from being limited (lacking maternal or paternal lineages and ages at diagnoses) to extensive (three-generation pedigrees with both paternal and maternal lineages indicated), the percentage highlights the consistent importance of determining the family history as an effective selection criterion for BRCA1/2 genetic testing. Despite reporting a positive family history of BC and/OVC, no actionable variants were detected for 59.9% (n = 1,445) of patients screened (Supplementary Table S1).
As the African continent has previously been associated with more aggressive breast disease and higher mortality rates due to late-stage presentation, the stage at diagnosis was compared between the ethnicities for 455 patients for whom the relevant information was available (Supplementary Table S2). The stage of disease at diagnosis for two groups (the Caucasians and individuals of mixed ancestry) was similar, with most patients diagnosed with Stage 2 disease. These two groups had the highest percentage of Stage 1 BC (10.6%), indicating increased community awareness and successful BC screening programs in the public health sector. These groups also exhibited a low number of patients diagnosed with Stage 4 disease (8.5% and 6.1%, respectively; Supplementary Table S2). This pattern was similar to that for the Black African group, except Stage 3 disease being the most prevalent. This finding still hints towards a later stage at presentation. However, it has improved significantly as a mere 7.6% of patients had Stage 4 disease at diagnosis. The Asian population of SA was the most alarming of the four ethnic groups due to the high percentage of patients diagnosed with Stage 4 disease (16.2%). However, this percentage could have been skewed due to the small sample size of this particular group (n = 37).
Patients affected with BC and/or OVC were divided according to unilateral and bilateral disease related to the presence or absence of a BRCA1/2 variant (Supplementary Table S3). The majority of patients presented with unilateral BC, with only 220 cases affected with bilateral disease and a further 91 affected with OVC. A small number of patients (n = 62) were also diagnosed with a secondary cancer type not specified here (Supplementary Table S3). The mutation-positive rates in this cohort varied between the two BC groups, with each group exhibiting similar success rates. For the OVC cases, the detection rate was considerably higher (21.9%), as 20 patients in total carried BRCA1/2 actionable variants (9 in BRCA1 and 11 in BRCA2).
3.3 Mutation Spectrum
Apart from targeted genotyping, mutation screening of 1,429 patients revealed a wide range of variants across the SA population groups. The data were generated from 340 patients screened using older mutation screening techniques, with a further 1,089 assessed by NGS. The data of the two sets were incorporated and are presented in Table 4. A total of 132 (9.2%) patients representing 73 likely- to pathogenic variants were identified (n = 117 for NGS and n = 15 using older technologies), with 57.6% (76/132) representing BRCA2. Twenty-two of these actionable variants were classified as splice-site variants, mainly located in the intronic splice site boundaries. Various CNVs were detected, ranging from single exon to complete gene deletions. These CNVs have been previously described by Van der Merwe et al. (2020). Across the genes, NGS detected 344 variants, with only 14% present in a homozygous state. Unique variation in the SA population resulted in 196 variants identified with a minor allele frequency (MAF) below 0.01, with 47 consequently being classified as variants of unknown clinical significance (VUS), predominantly in BRCA2. Of these 47 variants, 30 were completely novel and not detected in international databases used for variant interpretation (Table 4).
TABLE 4.
Summary of variants detected during comprehensive screening of 1,429 patients.
| Variant category | Patients (n) | Variants (n) | Homozygous variants (n) | Heterozygous variants (n) |
|---|---|---|---|---|
| BRCA1 actionable variants | 55 | 34 | 0 | 34 |
| BRCA2 actionable variants | 76 | 39 | 0 | 39 |
| Total # of BRCA1 variants | na | 146 | 22 | 124 |
| Total # of BRCA2 variants | na | 198 | 26 | 172 |
| Total # of VUSes in BRCA1 | 30 | 17 | 0 | 17 |
| Total # of VUSes in BRCA2 | 96 | 30 | 0 | 30 |
| BRCA1 variants with MAF <0.01 | na | 84 | 1 | 83 |
| BRCA2 variants with MAF <0.01 | na | 112 | 0 | 112 |
| Splice-site variants in BRCA1 | na | 11 | 0 | 11 |
| Splice-site variants in BRCA2 | na | 11 | 0 | 11 |
| Novel variants in BRCA1 | na | 13 | 1 | 12 |
| Novel variants in BRCA2 | na | 17 | 0 | 17 |
| Copy number variants in BRCA1 | 13 | 6 | 1 | 6 |
| Copy number variants in BRCA2 | 2 | 2 | 0 | 2 |
VUS, variant of unknown clinical significance; na, not applicable.
The mutation positivity rates for the main SA ethnicities varied, with 9.2% reported for the Black African group (44/479), 6.6% for the Asians (12/180), 18.1% for the Caucasians (25/138), 13.2% for the mixed ancestry group (22/167), with 11.8% allocated to the group of unknown ethnicity (14/118). From the 59 actionable variants detected across the populations using NGS, 23 were detected in the Black African group, 10 in the Asian group, 12 in the Caucasian group, 13 in the mixed ancestry group, with 12 falling into the group of unknown ethnicity. Only seven of the 59 variants were detected in two separate populations, with a single pathogenic variant (BRCA2 c.582G>A [rs80358810]) observed across all four main ethnic groups. However, the remainder of the actionable variants (51/59) were restricted to a single population group. Recurrence of these likely- to pathogenic variants was low in the NGS cohort with 66.1% (39/59) observed for a single patient. Another 18.6% of the variants (11/59) were detected twice, followed by 6.8% (4/59) identified in three patients each. A small percentage (8.5%) was common and represented the three most common founder variants, namely BRCA2 c.5771_5774del (detected in eight patients), BRCA2 c.582G>A (10 patients) and BRCA2 c.7934del (detected 17 times in the NGS cohort alone).
3.4 Genomic Consequence
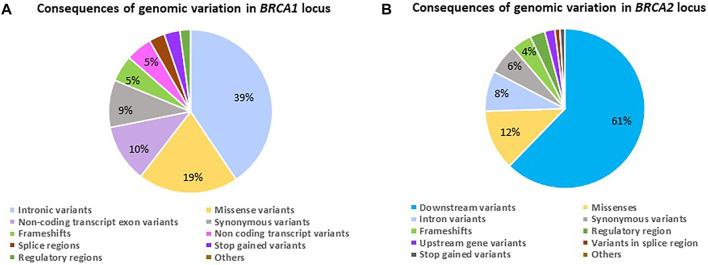
When investigating the consequences of the variation observed, it varied for the two genes (Figures 2A,B). The genomic variant frequencies and associated consequences were only based on the NGS data. Although the bulk of variation for BRCA1 was represented by non-coding transcript variants primarily present in the intronic regions (Figure 2A), the consequence of these variants has the potential to affect both overlapping genes, namely BRCA1 (NM_000294.4 [43,044 295–43,125 364] and Homo sapiens Rho family GTPase 2 (RND2—NM_005440.5 [43,025 231–43,032 041]), involving a total of eight overlapping transcripts and five regulatory features. For BRCA2, the variation was present in the form of downstream changes observed in the 3′ untranslated region (Figure 2B). In comparison, the genomic variation observed in this section of chromosome 13 has the potential to affect three overlapping genes, namely BRCA2 (NM_000059.4 [32,315 508–32,400 268]), Homo sapiens zygote arrest 1 like (ZAR1L—NM_001136571.2 [32,303 699–32,315 363]) and NEDD4 Binding Protein 2 Like 2 (N4BP2L2—NM_001,387,001.1 [32,432 485–32,538 795]), encompassing 28 overlapping transcripts and four regulatory features.
FIGURE 2.
Diagrams indicating the genomic variant frequencies for the BRCA1 and BRCA2 loci amongst 1,089 patients screened using NGS. (A) The consequence ratios relative to variant frequencies observed for the BRCA1 locus. (B) The consequence ratios relative to variant frequencies observed for the BRCA2 locus.
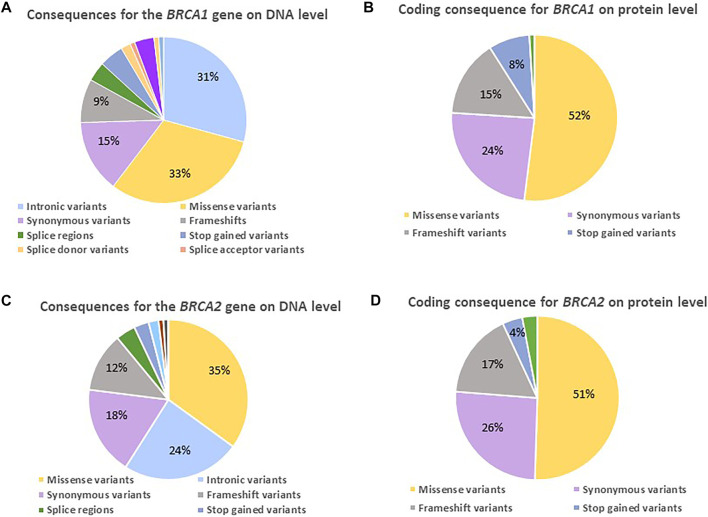
On DNA level, only a small percentage of the observed BRCA1 changes represented variants potentially having an impact, namely frameshift (9%), splice- (3%), and stop-gained variants (5%) (Figure 3A). Their contribution increased on protein level (Figure 3B), directly impacting the protein and consequently efficient DNA repair, as 23% resulted in a prematurely truncated peptide (15% frameshift and 8% stop-gained variants) together with 5% missense variants. The majority of the actionable variants were detected in BRCA1 exon 10. Approximately half of the variants observed at protein level represented missense variants, of which 14/55 changes were classified as VUSes. The missense variants were distributed throughout the gene, with the majority located outside of functional or disordered domains (Table 4).
FIGURE 3.
Diagrams indicating the variants detected for the two familial BC genes on DNA and protein level amongst 1,089 BC and/or OVC patients screened using NGS. (A) The consequence ratios (in percentages) predicted for BRCA1 on a DNA level. (B) The consequence ratios predicted for BRCA1 on a protein level. (C) The consequence ratios predicted for BRCA2 on a DNA level. (D) The consequence ratios predicted for BRCA2 on a protein level.
Although there was an enrichment of actionable BRCA2 variants in our populations (Table 4), the profile regarding the composition and impact of the variants on DNA and protein level corresponded between the two genes (Figures 3A–D), with the exception that the majority missenses of unknown significance were detected in a functional domain or disordered region. BRCA2 did reveal a smaller margin of intronic variants (Figure 3C), as the bulk of non-coding variation was in the 3′ untranslated region. The high percentage of missense variants in the heterozygous form illustrated the unique composition of the SA populations, contributing to the rate of VUSes identified due to a lack of African data in the international context (Table 4).
4 Discussion
4.1 Targeted Genotyping
Translational research performed at the University of Free State in collaboration with Professor L Jansen van Rensburg at the University of Pretoria resulted in the identification of the first two recurrent SA pathogenic variants in BRCA1, namely c.1374del (legacy name 1493delC) and c.2641G>T (legacy name E881X) observed for the Afrikaner population. Based on this research, BRCA1 c.2641G>T was the first SA pathogenic variant to receive founder status, proven by haplotype analysis that indicated a single mutational event (Reeves et al., 2004). This variant was soon followed with founder status for BRCA1 c.1374del and BRCA2 c.7934del (legacy 8162delG). These three founder variants (with BRCA2 c.7934del being the most common) were initially restricted to familial BC families with an Afrikaner heritage. Their founder status was corroborated with genealogical evidence dating back over 13 generations. For each variant, a single founding couple was identified based on genealogical findings traced back to France (BRCA1 c.2641G>T), the Netherlands (BRCA2 c.7934del) and Belgium (BRCA1 c.1374del) (Reeves et al., 2004; Van der Merwe and van Rensburg, 2009).
The research resulted in the first diagnostic test (entailing screening for the three Afrikaner founder variants) offered to patients in the public sector in 1998. Diagnostic testing was initially restricted to Afrikaner individuals as translational research was performed in parallel to identify pathogenic variants in the other SA population groups (Agenbag, 2005; Sluiter and Van Rensburg, 2011; Van der Merwe et al., 2012; Peter, 2014; Chen, 2015; Combrink, 2016; Moeti, 2016; Oosthuizen, 2016). With time, various non-Afrikaner recurrent variants had been identified that were proven to represent recurrent variants, which include BRCA2 c.5771_5774del (legacy 5999del4) and BRCA2 c.582G>A, both Black African founder variants (Van der Merwe et al., 2012; Oosthuizen et al., 2021).
As the demand for diagnostic testing increased over the years, patients were collectively tested for the eight most commonly occurring SA variants, irrespective of ethnicity. This mutation set included the three Ashkenazi Jewish/European founder variants based on the African Lemba tribe’s affinity with the Ashkenazi Jews (Spurdle and Jenkins, 1996) and the SA Indian community to mainland India and Europe (Combrink, 2016). This targeted genotyping assay served as a first-tier test for all patients that proved justified, because with time, the admixture of the SA populations became evident based on the genotyping results. The founder variants were no longer restricted to a single ethnicity but were shared among groups. This situation was expected, as recently illustrated by the findings of Hollfelder et al. (2020). With the use of five million genome-wide markers, 77 Afrikaner individuals were genotyped. Although the bulk of the genetic information represented Europeans, the study indicated a contribution of 1.7% from South Asia or India, 1.3% from the Khoikhoi and the San and 0.8% representing West and East Africa (Hollfelder et al., 2020).
The results obtained from this series indicate that not all variants currently included in the first-tier genotyping assay are worthy of inclusion due to their low prevalence. This applies to the three Ashkenazi Jewish founder/European variants and BRCA1 c.1374del, the rarest Afrikaner founder variant. The maximal financial benefit will be obtained once the first-tier assay has been re-designed to include the most commonly occurring variants in the SA population. It will aid the financially depleted healthcare system by first screening patients for the most common BRCA1/2 variants in the population before advancing to more costly NGS (Oosthuizen et al., 2021).
4.2 Epidemiology
The epidemiology data highlighted three important aspects to be considered for the SA population. The first entailed the relatively high percentage of young individuals (20–29 years) identified with an actionable variant (0.5%, Table 2). Fifty-six percent of the group (n = 62) was unaffected and was referred for carrier testing only. For 22.8% of these individuals, targeted genotyping revealed a positive test result. By knowing their mutation status so early in life, these women were empowered by informed screening decisions and potentially alternative management options. Maximum benefit was therefore gained to reduce their risk of developing cancer types associated with BRCA1/2 pathogenic variants. Asymptomatic young mutation carriers can reduce their risk of BC mortality by being regularly monitored and taking up interventions such as preventive surgery and/or chemoprevention (McCarthy and Armstrong, 2014).
The remainder (44%) of the 20–29 group was affected with early-onset BC (n = 27), with ages at onset ranging from 20 to 29 (average 26.7) years. The patients represented the Black African (n = 15), mixed ancestry (n = 5) and Caucasian (n = 6) ethnicities. Twenty-two percent (n = 6) of these patients received a positive test result (average age at onset 22.5 years), with the majority self-identified as Black African females. Half of these mutation-positive patients presented with a family history of BC and other cancer types, such as prostate cancer, melanoma and pancreatic cancer. Although these females were diagnosed at such a young age, knowing their BRCA1/2 status promoted informed decision-making regarding treatment options and potential surgery. With a familial BRCA variant identified so early, cascade testing of related family members can proceed to assist with risk-reducing interventions themselves.
According to the latest clinical guidelines released for BC control and management by the SA Department of Health in 2018 (National Department of Health of the Republic of South Africa, 2018), all these patients qualified for genetic services, yet the numbers genetically screened are meager (Table 2). With a positive detection rate between 6% and 24%, attention should be drawn to genetic screening, as the benefits would outweigh the financial costs by far (Table 3).
The epidemiological evidence highlighted the ever-important value of a family history as a potential predictor of a positive test result (Supplementary Table S1), as 90% of patients with an actionable BRCA1/2 variant reported family members affected with BC and other cancer types. Identifying an actionable BRCA1/2 variant in an index acts as a key to the entire family. Once a mutation carrier has been identified, the benefits of genetic testing can be magnified through sharing positive test results with at-risk related family members. Doing so will ensure that they benefit from this information and secure their access to predictive testing according to the latest guidelines. This approach is of tremendous importance from a public health perspective, pushing for improved survival and quality of life through earlier detection and optimal management.
Conley et al. (2020) recently investigated the family disclosure patterns of BRCA genetic test results among young Black women with invasive BC in the United States of America. The study examined whether a disclosure to relatives was made, and if so, to whom it was disclosed. The authors reported that the most significant benefit of genetic testing, namely to inform family members of a hereditary predisposition, is not being realized in Black American families. Their findings revealed a reluctance of mutation-positive patients to disclose a positive test result even to their daughters (Conley et al., 2020).
The reluctance to share genetic test results with family members is also evident in SA. Here it is not restricted to the Black African population but clearly occurs among all ethnic groups in SA apart from the Caucasians. Despite 481 individuals receiving a positive test result, a mere 318 predictive or carrier tests had been performed over 24 years. The majority was performed before 2003 as a post-research initiative involving Afrikaner families. From the epidemiological results, it appeared that Caucasians tended to value and discussed the outcome of genetic testing to a greater extent than the other main ethnic groups (Table 1). This trend was also noted by Armstrong et al. (2003), who found that women pursuing BRCA1/2 genetic testing in the United States were significantly more likely to be Caucasian.
This issue has been investigated internationally and although the public attitudes towards genetic testing for the risk of diseases, including cancer, are generally positive, various studies highlighted areas of concern. These include factors such as language barriers, fear of discrimination against those with a genetic predisposition for illness (Haga et al., 2013), being labeled as an individual or family with “good” or “bad” genes (Henneman et al., 2013) and fatalistic views of cancer (Allford et al., 2014). In SA, Schoeman et al. (2013) previously reported a low level of awareness regarding genetic testing for BC and other cancer types. Despite genetic testing being available since 1998, recognizing the value of a predictive test remains low. SA, therefore, needs to investigate innovative approaches to increase awareness among patients and communities, starting with related family members of mutation carriers. As the workforce of genetic providers is extremely low both in SA and globally, the international genetics profession has attempted to adapt to the situation by offering genetic counseling via alternative new methods, including service delivery via telephone, telegenetics and group genetic counseling. Although a face-to-face consultation is always the ideal model to strive for, innovative service delivery models such as group counseling can improve access and contribute to community awareness.
In the paper by Mampunye et al. (2021), one such innovation was described for SA, which investigated the clinical value of rapid point-of-care (POC) genetic testing performed in combination with genetic counseling. As the ParaDNA workflow involves an integrated system from sample collection to report generation, prospective validation using a non-invasive cheek swab or saliva as input DNA is warranted (Mampunye et al., 2021). This approach would be ideal for use as a first-tier test performed by trained healthcare providers in parallel with genetic counseling in rural primary health clinics. This will provide the opportunity to improve the care process by delivering on-demand psychosocial support directly to the patient and indirectly to the community where it is needed. This approach could have many benefits: 1) reducing the lack of early healthcare-seeking due to limited financial resources and transport difficulties; 2) increasing community knowledge and cancer awareness resulting in less stigmatization; 3) creating an opportunity to explain the benefits of knowing your BRCA1/2 genetic status for evidence-based cancer treatment options; and 4) highlighting the benefits of testing for at-risk family members in the case of a positive test result, thereby increasing preventative actions and early detection (Mampunye et al., 2021).
Unfortunately, the burden of BC-related death is ever increasing due to persisting misconceptions surrounding the disease and various other socio-economic factors such as poverty, cultural and religious beliefs (Van der Merwe et al., 2020; Mampunye et al., 2021; Oosthuizen et al., 2021). Despite the efforts of the SA Department of Health’s BC development plan establishing multiple Regional Breast Units (RBUs, 28 distributed throughout the various provinces) at primary and secondary state hospitals, and 14 additional Specialized Breast Units (SBUs) located in mostly tertiary hospitals (National Department of Health of the Republic of South Africa, 2018), the uptake of breast screening remains low in women 30 years and older (Phaswana-Mafuya and Peltzer, 2018). The authors of this large study (n = 10,831) estimated a low prevalence of BC screening similar to that obtained in a 2008 survey involving older SA adults, 50 years and older (Peltzer and Phaswana-Mafuya, 2014). Their estimation corresponded with the prevalence observed in international low-income countries such as Thailand (Mukem et al., 2016) and Turkey (Sözmen et al., 2016), but was lower than that of Brazil (Theme Filha et al., 2016). For SA to advance in this battle and attack BC energetically and effectively, we have to invest in community-health educational out-reaches performed in parallel with highly specialized science to achieve the ultimate goals set for the country.
The epidemiological data finally emphasized the value of screening all OVC patients for actionable variants in BRCA1/2, as screening resulted in a mutation-positive rate of 28.2% (Supplementary Table S3). Nine (45.0%) of the 20 OVC mutation carriers exhibited one of the SA founder variants, with the remainder of actionable variants being family-specific. These founder variants could easily have been identified cost-effectively using first-tier targeted genotyping, before proceeding to comprehensive genetic analysis. A high number of these OVC BRCA1/2 mutation carriers, however, was referred for identification of a rare family-specific variant. Although patients affected with OVC before the age of 60 are included in the national clinical guidelines for the control and management of BC (National Department of Health of the Republic of South Africa, 2018), only 91 OVC patients were received between 1998–2021. This number accounts for 3.9 patients screened per year in the public sector. Currently, the age-standardized incidence rate for OVC in southern Africa is 3.9 per 100,000 (The Council for Medical Schemes, 2019). Based on these statistics, the majority of OVC patients are currently not referred for any BRCA1/2 screening.
Screening these patients could have a two-fold advantage. International ongoing clinical trials involving poly (ADP-ribose) polymerase (PARP) inhibitors have recently resulted in the approval of various inhibitors by the Food and Drug Administration within the US Department of Health and Human Services for clinical use in specifically epithelial OVC patients (Loizzi et al., 2020). This new therapeutic approach for the management of OVC has been suggested in particular for patients with assessed defects in the homologous recombination DNA repair process such as BRCA1/2 (Sunada et al., 2018; Elias et al., 2018). By knowing their mutation status, patients will be appropriately selected for this new revolutionary treatment option. Unfortunately, the use of PARP inhibitors is not yet registered by the South African Health Products Regulatory Authority and their use in treatment requires Section 21 approval for the private sector (The Council for Medical Schemes, 2019). It is currently not available for the clinical treatment of patients in the public sector.
4.3 Mutation Spectrum
We aimed to describe clinically relevant germline BRCA1/2 variants and their distribution across ethnicities in the most extensive unselected African series to date for the public sector. The data represent the current status after 23 years of BRCA testing within our state laboratory. Although testing commenced with a tiered approach in the form of research-based translational targeted genotyping, the total number of test requests and the number of variants identified soared within the past decade. This could be attributed to a heightened public awareness after the Angelina Jolie revelations in 2013 (Troiano et al., 2017) and the incorporation of NGS into our diagnostic workflow, which enabled a more rapid throughput and resulted in more effective variant discovery.
A total of 481 individuals were identified as high-risk BRCA1/2 mutation carriers, with 69.6% (n = 335) representing patients affected by BC and/or OVC, accounting for 9.2% of patients comprehensively screened (132/1,429). The data revealed an extreme distribution for each gene, with only a few frequent pathogenic variants identified. The majority of variants were extremely rare and primarily family-specific (Table 5). Although the proven founder or recurrent variants for the Ashkenazi Jewish, Afrikaner and Black African/mixed ancestry variants were the most common variants observed in terms of the highest frequencies, they only represented <10% of the variants identified (4/55 for BRCA1 and 5/57 for BRCA2, Table 5).
TABLE 5.
Actionable BRCA1/2 variants (likely- to pathogenic) identified for the entire SA cohort (n = 2,896).
| Variant | Protein | Cancer type in index or family | Exon | #Of families | rs number |
|---|---|---|---|---|---|
| BRCA1 | |||||
| NC_000017.11:g.(?_43045584)_(43125327_?)del | BC | 1 – 23 | 1 | no rs | |
| NC_000017.11:g.(?_43123946)_(43125327_?)del | BC & OVC | 1 – 2 | 5 | no rs | |
| NC_000017.11:g.(?_43104032)_(43106675_?)del | BC & OVC | 4 – 6 | 1 | no rs | |
| NC_000017.11:g.(?_43082330)_(43082599_?)dup | BC & OVC | 12 | 2 | no rs | |
| NC_000017.11:g.(?_43063781)_(43064034_?)del | BC & OVC | 17 | 3 | no rs | |
| NC_000017.11:g.(?_43048992)_(43049260_?)del | BC & OVC | 21 | 1 | no rs | |
| NM_007294.4(BRCA1): c.45dup | NP_009225.1: p.Asn16Ter | BC | 2 | 4 | rs730881457 |
| NM_007294.4(BRCA1): c.66dup | NP_009225.1: p.Glu23ArgfsTer18 | BC | 2 | 5 | rs80357783 |
| NM_007294.4(BRCA1): c.68_69del | NP_009225.1: p.Glu23ValfsTer17 | BC & Gastric ca | 2 | 8 | rs80357914 |
| NM_007294.4(BRCA1): c.71G>C | NP_009225.1: p.Cys24Ser | BC | 3 | 2 | no rs |
| NM_007294.4(BRCA1): c.110C>A | NP_009225.1: p.Thr37Lys | BC & OVC | 3 | 1 | rs80356880 |
| NM_007294.4(BRCA1): c.135-1G>T | Splicing defect | BC | 4 | 1 | rs80358158 |
| NM_007294.4(BRCA1): c.181T>G | NP_009225.1: p.Cys61Gly | BC & OVC | 5 | 2 | no rs |
| NM_007294.4(BRCA1): c.191G>A | NP_009225.1: p.Cys64Tyr | BC | 5 | 2 | rs55851803 |
| NM_007294.4(BRCA1): c.212G>A | NP_009225.1: p.Arg71Lys (Splicing defect) | BC | 5 | 1 | rs80356913 |
| NM_007294.4(BRCA1): c.212+1G>A | Splicing defect | BC | 5 | 1 | rs80356913 |
| NM_007294.4(BRCA1): c.415C>T | NP_009225.1: p.Gln139Ter | BC | 6 | 2 | rs80357372 |
| NM_007294.4(BRCA1): c.431dup | NP_009225.1: p.Asn144LysfsTer15 | BC | 6 | 3 | rs397509162 |
| NM_007294.4(BRCA1): c.1016dup | NP_009225.1: p.Val340LysfsTer6 | BC | 10 | 1 | rs80357569 |
| NM_007294.4(BRCA1): c.1360_1361delAG | NP_009225.1: p.Ser454Ter | BC & OVC | 10 | 5 | rs80357969 |
| NM_007294.4(BRCA1): c.1374del | NP_009225.1: p.Asp458GlufsTer17 | BC | 10 | 9 | rs397508862 |
| NM_007294.4(BRCA1): c.1504_1508delTTAAA | NP_009225.1: p.Leu502AlafsTer2 | BC & colon ca | 10 | 3 | rs80357888 |
| NM_007294.4(BRCA1): c.2008G>T | NP_009225.1: p.Glu670Ter | BC & OVC | 10 | 2 | no rs |
| NM_007294.4(BRCA1): c.2070_2073delAAGA | NP_009225.1: p.Lys690AsnfsTer10 | BC | 10 | 1 | no rs |
| NM_007294.4(BRCA1): c.2568T>G | NP_009225.1: p.Tyr856Ter | BC | 10 | 1 | rs80356832 |
| NM_007294.4(BRCA1): c.2597G>A | NP_009225.1: p.Arg866His | BC | 10 | 1 | rs80356911 |
| NM_007294.4(BRCA1): c.2599G>T | NP_009225.1: p.Gln867Ter | BC | 10 | 1 | rs886038001 |
| NM_007294.4(BRCA1): c.2641G>T | NP_009225.1: p.Glu881Ter | BC & OVC | 10 | 28 | rs397508988 |
| NM_007294.4(BRCA1): c.3108del | NP_009225.1: p.Phe1036LeufsTer12 | BC | 10 | 1 | rs80357841 |
| NM_007294.4(BRCA1): c.3228_3229delAG | NP_009225.1: p.Gly1077AlafsTer8 | BC | 10 | 3 | rs80357635 |
| NM_007294.4(BRCA1): c.3288_3289delAA | NP_009225.1: p.Leu1098SerfsTer4 | BC | 10 | 1 | rs80357686 |
| NM_007294.4(BRCA1): c.3331_3334delCAAG | NP_009225.1: p.Gln1111AsnfsTer5 | BC | 10 | 1 | rs80357701 |
| NM_007294.4(BRCA1): c.3400G>T | NP_009225.1: p.Glu1134Ter | BC | 10 | 1 | no rs |
| NM_007294.4(BRCA1): c.3496_3497insT | NP_009225.1: p.Ala1166ValfsTer2 | BC | 10 | 1 | no rs |
| NM_007294.4(BRCA1): c.3549_3550delAGinsT | NP_009225.1: p.Lys1183AsnfsTer27 | Male BC | 10 | 1 | rs273899709 |
| NM_007294.4(BRCA1): c.3593T>A | NP_009225.1: p.Leu1198Ter | Male BC | 10 | 1 | rs397509095 |
| NM_007294.4(BRCA1): c.3732_3733delTA | NP_009225.1: p.His1244GlnfsTer10 | BC | 10 | 1 | no rs |
| NM_007294.4(BRCA1): c.3756_3759delGTCT | NP_009225.1: p.Ser1253ArgfsTer10 | BC & Melanoma | 10 | 2 | rs80357868 |
| NM_007294.4(BRCA1): c.3947_3950delTCTT | NP_009225.1: p.Phe1316Ter | BC | 10 | 2 | rs886040177 |
| NM_007294.4(BRCA1): c.4308_4309delTT | NP_009225.1: p.Ser1437CysfsTer3 | BC | 12 | 2 | no rs |
| NM_007294.4(BRCA1): c.4327C>T | NP_009225.1: p.Arg1443Ter | BC | 12 | 1 | rs41293455 |
| NM_007294.4(BRCA1): c.4524G>A | NP_009225.1: p.Trp1508Ter | BC | 15 | 1 | rs80356885 |
| NM_007294.4(BRCA1): c.4838_4839insC | NP_009225.1: p.Pro1614SerfsTer8 | BC | 15 | 2 | rs397509200 |
| NM_007294.4(BRCA1): c.4868C>T | NP_009225.1: p.Ala1623Val | BC | 15 | 1 | no rs |
| NM_007294.4(BRCA1): c.4987-5T>A | Splicing defect | BC | 16 | 1 | rs397509214 |
| NM_007294.4(BRCA1): c.5095C>T | NP_009225.1: p.Arg1699Trp | BC | 17 | 2 | rs55770810 |
| NM_007294.4(BRCA1): c.5096G>A | NP_009225.1: p.Arg1699Gln | BC | 17 | 3 | rs41293459 |
| NM_007294.4(BRCA1): c.5177_5180delGAAA | NP_009225.1: p.Arg1726LysfsTer3 | BC | 19 | 1 | rs80357867 |
| NM_007294.4(BRCA1): c.5229_5230delAA | NP_009225.1: p.Arg1744LysfsTer85 | BC | 19 | 4 | rs80357852 |
| NM_007294.4(BRCA1): c.5240_5243delGAAA | NP_009225.1: p.Arg1747LysfsTer3 | BC | 19 | 1 | no rs |
| NM_007294.4(BRCA1): c.5266dup | NP_009225.1: p.Gln1756ProfsTer74 | BC & OVC | 19 | 6 | rs80357906 |
| NM_007294.4(BRCA1): c.5332+1G>C | Splicing defect | BC | 21 | 1 | rs80358041 |
| NM_007294.4(BRCA1): c.5365_5366delGCinsA | NP_009225.1: p.Ala1789IlefsTer4 | BC | 21 | 1 | no rs |
| NM_007294.4(BRCA1): c.5467+2T>G | Splicing defect | BC | 21 | 3 | rs80358009 |
| NM_007294.4(BRCA1): c.5468-1G>A | Splicing defect | BC | 23 | 1 | rs80358048 |
| Total | 143 | ||||
| BRCA2 | |||||
| NC_000013.11:g.(?_32313776)_(32398795_?)del | BC | 1 – 27 | 1 | no rs | |
| NC_000013.11:g.(?_32370334)_(32371115_?)del | BC & OVC | 19 – 20 | 1 | no rs | |
| NM_000059.4(BRCA2): c.67+3A>G | Splicing defect | BC, Fanconi anemia | 2 | 1 | rs1593880835 |
| NM_000059.4(BRCA2): c.93G>A | NP_000050.3: p.Trp31Ter | BC | 3 | 1 | rs80359214 |
| NM_000059.4(BRCA2): c.516G>A | NP_000050.3: p.Lys172= | BC | 6 | 2 | rs80359790 |
| NM_000059.4(BRCA2): c.582G>A | NP_000050.3: p.Trp194Ter | BC | 7 | 13 | rs80358810 |
| NM_000059.4(BRCA2): c.771_775delTCAAA | NP_000050.3: p.Asn257LysfsTer17 | BC | 9 | 1 | rs80359671 |
| NM_000059.4(BRCA2): c.1261C>T | NP_000050.3: p.Gln421Ter | BC | 10 | 1 | rs80358419 |
| NM_000059.4(BRCA2): c.1813dup | NP_000050.3: p.Ile605AsnfsTer11 | Male BC | 10 | 1 | rs80359308 |
| NM_000059.4(BRCA2): c.2636_2637delCT | NP_000050.3: p.Ser879Ter | BC | 11 | 1 | rs276174826 |
| NM_000059.4(BRCA2): c.2806_2809delAAAC | NP_000050.3: p.Ala938ProfsTer21 | BC | 11 | 1 | rs80359351 |
| NM_000059.4(BRCA2): c.2826_2829delAATT | NP_000050.3: p.Ile943LysfsTer16 | BC | 11 | 1 | rs397507643 |
| NM_000059.4(BRCA2): c.2828_2831delTTAA | NP_000050.3: p.Ile943LysfsTer16 | BC | 11 | 1 | rs397507643 |
| NM_000059.4(BRCA2): c.3553dup | NP_000050.3: p.Thr1185AsnfsTer3 | BC | 11 | 1 | no rs |
| NM_000059.4(BRCA2): c.3723del | NP_000050.3: p.Phe1241LeufsTer18 | BC | 11 | 1 | rs886040491 |
| NM_000059.4(BRCA2): c.3847del | NP_000050.3: p.Val1283LysfsTer2 | BC | 11 | 1 | rs80359405 |
| NM_000059.4(BRCA2): c.3881T>A | NP_000050.3: p.Leu1294Ter | BC | 11 | 1 | rs80358632 |
| NM_000059.4(BRCA2): c.4003G>T | NP_000050.3: p.Glu1335Ter | BC | 11 | 3 | rs747070579 |
| NM_000059.4(BRCA2): c.4456del | NP_000050.3: p.Val1486LeufsTer6 | BC | 11 | 1 | no rs |
| NM_000059.4(BRCA2): c.4482_4483insAAAG | NP_000050.3: p.Ser1494LysfsTer20 | BC & OVC | 11 | 1 | no rs |
| NM_000059.4(BRCA2): c.4568del | NP_000050.3: p.Gly1523ValfsTer20 | BC | 11 | 1 | no rs |
| NM_000059.4(BRCA2): c.4936G>T | NP_000050.3: p.Glu1646Ter | BC | 11 | 1 | rs886038111 |
| NM_000059.4(BRCA2): c.5082dup | NP_000050.3: p.Glu1695ArgfsTer5 | BC | 11 | 1 | no rs |
| NM_000059.4(BRCA2): c.5213_5216delCTTA | NP_000050.3: p.Thr1738IlefsTer2 | BC & OVC | 11 | 2 | rs80359493 |
| NM_000059.4(BRCA2): c.5279C>G | NP_000050.3: p.Ser1760Ter | BC | 11 | 1 | rs80358751 |
| NM_000059.4(BRCA2): c.5344C>T | NP_000050.3: p.Gln1782Ter | BC & OVC | 11 | 1 | rs80358757 |
| NM_000059.4(BRCA2): c.5564C>G | NP_000050.3: p.Ser1855Ter | OVC | 11 | 1 | no rs |
| NM_000059.4(BRCA2): c.5771_5774delTTCA | NP_000050.3: p.Ile1924ArgfsTer38 | Male BC, BC, OVC, prostate ca, endometrial ca, Fanconi aneamia | 11 | 61 | rs80359535 |
| NM_000059.4(BRCA2): c.5946del | NP_000050.3: p.Ser1982ArgfsTer22 | BC | 11 | 5 | rs80359550 |
| NM_000059.4(BRCA2): c.6082_6086delGAAGA | NP_000050.3: p.Glu2028LysfsTer19 | BC | 11 | 1 | rs80359558 |
| NM_000059.4(BRCA2): c.6228del | NP_000050.3: p.Lys2077ArgfsTer4 | BC | 11 | 1 | no rs |
| NM_000059.4(BRCA2): c.6393_6396delATTA | NP_000050.3: p.Lys2131AsnfsTer5 | BC | 11 | 1 | rs397507849 |
| NM_000059.4(BRCA2): c.6393del | NP_000050.3: p.Lys2131AsnfsTer6 | BC | 11 | 1 | rs886038145 |
| NM_000059.4(BRCA2): c.6447_6448dupTA | NP_000050.3: p.Lys2150IlefsTer19 | BC & OVC | 11 | 5 | rs397507858 |
| NM_000059.4(BRCA2): c.6623del | NP_000050.3: p.Asn2208IlefsTer2 | BC | 11 | 1 | rs886038150 |
| NM_000059.4(BRCA2): c.6937+2delT | Splicing defect | BC | 12 | 1 | no rs |
| NM_000059.4(BRCA2): c.7934del | NP_000050.3: p.Arg2645AsnfsTer3 | Male BC, BC, OVC, prostate cancer, melanoma | 17 | 176 | rs80359688 |
| NM_000059.4(BRCA2): c.7955T>G | NP_000050.3: p.Val2952Gly | BC | 17 | 2 | rs1555286868 |
| NM_000059.4(BRCA2): c.8067T>A | NP_000050.3: p.Cys2689Ter | BC | 18 | 4 | rs80359046 |
| NM_000059.4(BRCA2): c.8165C>G | NP_000050.3: p.Thr2722Arg | BC | 18 | 1 | rs80359062 |
| NM_000059.4(BRCA2): c.8167G>C | NP_000050.3: p.Asp2723His | BC | 18 | 3 | rs41293511 |
| NM_000059.4(BRCA2): c.8168A>T | NP_000050.3: p.Asp2723Val | BC | 18 | 1 | rs41293513 |
| NM_000059.4(BRCA2): c.8331+2T>C | Splicing defect | BC | 18 | 1 | rs309122602 |
| NM_000059.4(BRCA2): c.8504C>G | NP_000050.3: p.Ser2835Ter | BC | 18 | 1 | rs80359102 |
| NM_000059.4(BRCA2): c.8686del | NP_000050.3: p.Arg2896ValfsTer13 | BC | 21 | 2 | no rs |
| NM_000059.4(BRCA2): c.8696_8712del17 | NP_000050.3: p.Gln2899LeufsTer2 | BC & OVC | 21 | 1 | no rs |
| NM_000059.4(BRCA2): c.8754+1G>A | Splicing defect | BC | 21 | 4 | rs397508006 |
| NM_000059.4(BRCA2): c.8954-2A>C | Splicing defect | BC | 23 | 1 | rs1135401928 |
| NM_000059.4(BRCA2): c.8961_8964delGAGT | NP_000050.3: p.Ser2988PhefsTer12 | BC | 23 | 2 | rs80359734 |
| NM_000059.4(BRCA2): c.9105T>G | NP_000050.3: p.Tyr3035Ter | BC | 23 | 3 | rs886040819 |
| NM_000059.4(BRCA2): c.9117G>A | NP_000050.3: p.Pro3039= (Splicing defect) | BC | 23 | 1 | rs28897756 |
| NM_000059.4(BRCA2): c.9138del | NP_000050.3: p.Gln3047ArgfsTer15 | BC | 24 | 3 | no rs |
| NM_000059.4(BRCA2): c.9154C>T | NP_000050.3: p.Arg3052Trp | BC | 24 | 1 | rs45580035 |
| NM_000059.4(BRCA2): c.9351del | NP_000050.3: p.His3117GlnfsTer3 | BC | 25 | 8 | no rs |
| NM_000059.4(BRCA2): c.9382C>T | NP_000050.3: p.Arg3128Ter | BC | 25 | 1 | no rs |
| NM_000059.4(BRCA2): c.9833_9842del | NP_000050.3: p.Pro3278HisfsTer32 | BC | 25 | 1 | no rs |
| NM_000059.4(BRCA2): c.9435_9436delGT | NP_000050.3: p.Ser3147CysfsTer2 | BC & OVC | 25 | 5 | rs80359763 |
| Total | 342 | ||||
Both the number of variants and their mutation spectrum differed for the various population groups. From the NGS data, it seemed as if the Black African group (n = 479) exhibited the largest diversity in both actionable and novel variants (44/479), as approximately double the number of variants were observed compared to the other groups (Asians 12/180; Caucasians 25/138; mixed ancestry 22/167 and individuals of unknown ethnicity 14/118). These numbers, however, do not accurately reflect the contribution of pathogenic variants to this group, as considerably more Black African patients were tested (at a ratio of approximately 3:1). Despite their higher diversity of pathogenic variants, the Black African group had the second-lowest positive detection rate (9.2%), apart from the Asian population with 6.6%. This can partly be attributed to patients being referred for genetic testing based on an early age at diagnosis alone, as most Black African patients were unaware of the accumulation of cancer occurrences in their families. In contrast, the Caucasian population exhibited the highest detection rate, namely 18.1%, despite a much lower number of patients tested using NGS. These patients seemed to be more appropriately selected as the majority of patients reported an intermediate to strong family history of BC and/or OVC. As the majority of mutation-positive patients carried one of the Afrikaner founder variants, the contribution of the Afrikaner founder variants to this group was evident.
The Caucasian and Black African detection rates declined from 18.1% to 9.2% to an overall 10% and 6.6%, respectively, once the founder variants detected during NGS were excluded. This finding indicates an ultimate positive mutation detection rate below 10% for NGS, which is currently not cost-effective. If these patients were screened using the first-tier targeted genotyping assay, costs could have been reduced by excluding these patients prior to NGS analysis. The difference in the positive detection rate between these two ethnic groups with well-characterized variants iterates the importance of family history and genetic cancer registries. By updating these registries, testing centers can keep track of related family members carrying actionable familial BRCA1/2 variants, with the sole purpose to identify at-risk symptomatic-free family members.
This study attempted to report the mutation detection rates over the past two decades from a single institutional series’ perspective, with some biases due to: 1) the various techniques used; 2) disproportionate numbers of multiple ethnic groups studied; and 3) the minimum clinical criteria for BRCA testing changing. Therefore, the mutation detection rates presented per ethnic group does not accurately represent the positive predictive value of each technique and the national mutational burden of the respective groups. It merely reflects the frequencies of actionable variants detected at the time and within the performance specifications regarding the sensitivity and specificity of each mutation screening technique. The genotyping approach only identified selected pathogenic variants and did not enable the reporting of VUSes. Compared to NGS, screening for pathogenic variants using HRMA could have missed pathogenic variants and VUSes due to possible inadequate sensitivity during melt curve analysis in suboptimal PCR conditions (despite rigorous optimization and running each reaction in duplicate) (Combrink, 2016; Oosthuizen, 2016). In addition to a potential reduction in sensitivity of HRMA, the largest exons namely exon 10 of BRCA1 and exons 10 and 11 of BRCA2 were screened for protein-truncating variants only using PTT. The technique would therefore have missed various missense, synonymous or splice-site variants which could have represented actionable variants.
Sanger sequencing was utilized only for the confirmation of variants detected using the various mutation screening techniques. It was therefore not employed in this series for sequencing entire coding regions and splice site boundaries of samples. Moreover, biases in the detection frequencies of ethnic groups could have been introduced due to the disproportionate number of individuals in each group being screened with NGS, the most modern and sensitive technique in the test repertoire. Lastly, the criteria for BRCA1/2 screening broadened with time and became more inclusive throughout the decades with more individuals currently meeting criteria than did a decade or two ago.
This series represents the most extensive report involving the BRCA1/2 mutation spectrum on the African continent, surpassing the Nigerian study involving 1,136 patients (Supplementary Table S4). The positive mutation rate, however, was similar (9.2% versus a collective 11.1% in Nigeria), although the contribution of the genes was reversed, with BRCA2 being more prevalent in SA (Supplementary Table S4). This observation can be attributed to the prevalence of three SA founder variants in BRCA2, representing three of the four SA ethnic groups (Black African, Caucasian and mixed ancestry groups). Although some African countries reported extremely high BRCA1/2 mutation-positive rates above 15% (such as Egypt, Morocco, SA, Sudan and Tunisia), the majority of African studies involved small sample sizes based on very strict selection criteria.
Twenty-seven of the actionable variants were novel, with no unique identifier listed. The total number of novel variants increased when the complete variant list was considered (including benign variants to VUSes), with most present in a heterozygous form (Table 4). These novel variants were mostly observed for the Black African group, which was expected due to the high degree of variation evident in the African genome when compared to that of the Asian, African-American and European genomes. Recent genomic studies (Choudhury et al., 2020) revealed the presence of more than 3 million previously undescribed variants and predicted that only a fraction of the genetic diversity among individuals on the African continent has thus far been uncovered. This study exposed complex patterns of ancestral admixture, as both intra- and inter-population variations were observed. Although the authors did not observe a multitude of pathogenic variants in medically relevant genes, a significant number of variants denoted as likely-pathogenic in other genes were present in the ClinVar database (Choudhury et al., 2020). Such a high degree of genomic variation complicates the general approach of Mendelian classifications for variant interpretation, as for many variants, the MAF is either not known or very low, immediately classifying them as a rare variant, possibly also absent from population databases such as GnomAD and others.
This complexity highlights the necessity of functional assays performed in parallel with haplotype analysis. Haplotype inference for SA based on NGS data was performed by Oosthuizen et al. (2021). The authors reported several variants at low frequency to be in linkage disequilibrium in specific SA population groups, which emphasized the importance of long-range PCR confirmation for phasing. Suspected benign missense variants co-segregating with pathogenic variants or SNV-based VUSes, albeit at a low frequency, could act as potential modifiers regarding disease penetrance. Even subtle influences such as these can possibly contribute to the value of risk scores unique to population groups.
Collectively, these factors contribute to variants not being fully classified as actionable due to a lack of evidence (using the ACMG classification criteria). This resulted in potentially actionable variants currently classified as VUSes because of a paucity of evidence in the international literature. Finally, despite the increase in throughput and the extended scope for BRCA1/2 variant discovery, less than 10% of patients with a personal history of cancer diagnosed at an early age, or who had a positive family history, received a positive test result, leaving the remainder of patients still in the dark regarding alternative management and therapeutic options involving poly (ADP-ribose) (PARP) inhibitors. As various other genes associated with BRCA1/2 have been indicated to contribute to homologous recombination and DNA repair (such as ATM Serine/Threonine Kinase [ATM], BRCA1 Interacting Helicase I [BRIP1], Checkpoint Kinase 2 [CHEK2], RAD51 Paralog C [RAD51C] and Partner And Localizer of BRCA2 [PALB2]), the search for actionable variants responsible for hereditary BC and/or OVC needs to be expanded. With NGS already implemented, we propose moving towards multigene panel testing in the future. It could result in the identification of additional role players contributing to the disease burden in SA. By performing multipanel testing, we will be able to identify deleterious variants in multiple cancer susceptibility genes, which will allow us to identify eligible patients and related family members for clinical interventions, surveillance screening, targeted therapy and potential prevention strategies, according to the National Comprehensive Cancer Network (NCCN) guidelines.
5 Conclusion
The vision of better health systems for African countries is encompassed in the health-related sustainable development goals set by the World Health Organization (WHO) for Africa in 2018 (World Health Organization Africa, 2021). The SA Department of Health has recognized this initiative and pledged to reform this critical sector by releasing updated clinical guidelines for BC management and control during the same year. With the advances made in genetic testing for familial BC and OVC in state laboratories in SA, it has the potential to contribute immensely to the identification of high-risk BRCA1/2 and non-BRCA germline actionable variants in patients. Given the magnitude of the disease, knowing a patient’s mutation status can aid in the individualization of their treatment, which is of great benefit for the attending physician as it contributes to the patient’s overall survival and will be of importance for related family members.
Due to the potential and far-reaching impact genetic testing has on augmenting the risk in a family with a positive family history of BC and/or OVC or in a patient diagnosed at an early age, it is imperative that the search is broadened to include other non-BRCA genes. Together, all these genes play an integral role in multiple signaling pathways inside the cell, with crosstalk between the associated proteins. If one of these signaling molecules becomes nonfunctional, the balance could be disturbed and may contribute to the progression of carcinogenesis.
The large number of novel and the abundance of heterozygous variants detected in this series reflect a high degree of genomic diversity. This highlights the existence of an immense gap in available naturally occurring population-specific knowledge due to a lack of African genomes in public genetic archives. Many diagnostic laboratories rely heavily on MAF and in silico predictions for variant interpretation and classification. In SA, as in many other African countries, this gap results in an unfavorable amount of VUSes classified. The lack of reference genomes increases the struggle to keep up with the rapid evolution in genetic variant screening for the confirmation of diagnosis. Although major strides have been made in the past decade in an attempt to catch up with first-world countries, uptake of genetic diagnostic services will not reach its full potential unless it becomes more affordable and a substantial number of African genomes is available to assist with variant interpretation and classification.
The SA scientific community is therefore compelled to continue with translational research in order to adopt first-world technologies into its healthcare context as a developing country. The vast progress made over the past two decades enabled a vital shift away from population-directed genetic testing to potentially comprehensive screening for all BC and OVC cancer patients. Consequently, the medical and scientific community in SA will continuously strive to comply with international standards and guidelines aimed at universal healthcare for all patients regardless of ethnicity, financial status or continent of birth. For centuries, the people of Africa have been marginalized and disadvantaged in many aspects, including optimal health care. With the WHO focusing on Africa, the health and well-being of its people are improving, resulting in the people of Africa currently sharing a vision for the future that is filled with optimism and hope.
Acknowledgments
The authors acknowledge all previous postgraduate students and medical scientists that participated in the translational research phase of the project or who were directly involved with the testing, namely N. Peter (MMedSc and NHLS Intern Medical Scientist), P. Moeti (MMedSc), C. Schultz, B. Dajee and A. Diedericks. Dr. Daleen Struwig, medical writer/editor, Faculty of Health Sciences, University of the Free State, for the final language editing of the manuscript.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Health Sciences Research Ethics Committee Faculty of Health Sciences University of the Free State. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
NM, JO, and HC made substantial contributions to the conception, design, and completion of this publication. NM obtained the ethics approvals and selected the data for inclusion. JO, HC, KN, and NM performed the genetic studies together with previous medical scientists involved in BRCA testing in this NHLS laboratory over the years. NM, JO and HC were responsible for the optimization of various techniques utilized. KN reviewed all the request forms for the collation of data and assisted with the population of an in-house database designed by HC. NM framed the study in relation to past achievements. All authors contributed to the article and approved the submitted version.
Funding
The translational research performed between 1995–2020 was supported by the South African Medical Research Council (SAMRC—self-initiated research grant 2012), with funds also received from the National Health Laboratory Service Research Trust (NHLSRT—2008, 2011, 2013, 2016, 2017) and the National Research Foundation (NRF—98291, 92192).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.834265/full#supplementary-material
References
- AbdelHamid S. G., Zekri A.-R. N., AbdelAziz H. M., El-Mesallamy H. O. (2021). BRCA1 and BRCA2 Truncating Mutations and Variants of Unknown Significance in Egyptian Female Breast Cancer Patients. Clinica Chim. Acta 512, 66–73. 10.1016/j.cca.2020.11.023 [DOI] [PubMed] [Google Scholar]
- Adedokun B., Zheng Y., Ndom P., Gakwaya A., Makumbi T., Zhou A. Y., et al. (2020). Prevalence of Inherited Mutations in Breast Cancer Predisposition Genes Among Women in Uganda and Cameroon. Cancer Epidemiol. Biomarkers Prev. 29, 359–367. 10.1158/1055-9965.EPI-19-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agenbag G. (2005). Molecular Genetic Analysis of Familial Breast Cancer in South Africa. Stellenbosch: MSc dissertationUniversity of Stellenbosch. Available at: https://scholar.sun.ac.za/handle/10019.1/1521 (Accessed November 16, 2021). [Google Scholar]
- Allford A., Qureshi N., Barwell J., Lewis C., Kai J. (2014). What Hinders Minority Ethnic Access to Cancer Genetics Services and what May Help? Eur. J. Hum. Genet. 22, 866–874. 10.1038/ejhg.2013.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong K., Weber B., Stopfer J., Calzone K., Putt M., Coyne J., et al. (2003). Early Use of Clinical BRCA1/2 Testing: Associations with Race and Breast Cancer Risk. Am. J. Med. Genet. 117A, 154–160. 10.1002/ajmg.a.10928 [DOI] [PubMed] [Google Scholar]
- Ashour M., Ezzat Shafik H. (2019). Frequency of Germline Mutations in BRCA1 and BRCA2 in Ovarian Cancer Patients and Their Effect on Treatment Outcome. Cmar Vol. 11, 6275–6284. 10.2147/CMAR.S206817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attlee M. (1947). The Coloured People of South Africa. Afr. Aff. 46, 148–151. 10.1093/oxfordjournals.afraf.a093570 [DOI] [Google Scholar]
- Awadelkarim K. D., Aceto G., Veschi S., Elhaj A., Morgano A., Mohamedani A. A., et al. (2007). BRCA1 and BRCA2 Status in a Central Sudanese Series of Breast Cancer Patients: Interactions with Genetic, Ethnic and Reproductive Factors. Breast Cancer Res. Treat. 102, 189–199. 10.1007/s10549-006-9303-z [DOI] [PubMed] [Google Scholar]
- Bakkach J., Mansouri M., Derkaoui T., Loudiyi A., El Fahime E., Barakat A., et al. (2020). Contribution of BRCA1 and BRCA2 Germline Mutations to Early Onset Breast Cancer: a Series from north of Morocco. BMC Cancer 20, 859. 10.1186/s12885-020-07352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ayed-Guerfali D., Ben Kridis-Rejab W., Ammous-Boukhris N., Ayadi W., Charfi S., Khanfir A., et al. (2021). Novel and Recurrent BRCA1/BRCA2 Germline Mutations in Patients with Breast/ovarian Cancer: a Series from the South of Tunisia. J. Transl Med. 19, 108. 10.1186/s12967-021-02772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensam M., Hafez E., Awad D., El-Saadani M., Balbaa M. (2014). Detection of New Point Mutations of BRCA1 and BRCA2 in Breast Cancer Patients. Biochem. Genet. 52, 15–28. 10.1007/s10528-013-9623-810.1007/s10528-013-9623-8 [DOI] [PubMed] [Google Scholar]
- Biancolella M., Ouédraogo N. L. M., Zongo N., Zohoncon T. M., Testa B., Rizzacasa B., et al. (2021). Breast Cancer in West Africa: Molecular Analysis of BRCA Genes in Early-Onset Breast Cancer Patients in Burkina Faso. Hum. Genomics 15, 65. 10.1186/s40246-021-00365-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenouar A. C. S., Coulet F., Bendiab F. M. T., Boudinar F. Z., Senhadji R. (2018). BRCA1 and BRCA2 Germline Mutation Screening in Western Algeria Using High Resolution Melting Analysis (HRM). Gulf J. Oncolog 1, 31–37. [PubMed] [Google Scholar]
- Brandt T., Sack L. M., Arjona D., Tan D., Mei H., Cui H., et al. (2020). Adapting ACMG/AMP Sequence Variant Classification Guidelines for Single-Gene Copy Number Variants. Genet. Med. 22, 336–344. 10.1038/s41436-019-0655-2 [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 countriesErratum in. Ca: A. Cancer J. CliniciansCA Cancer J Clin 6870, 394313–394424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Chen W. (2015). The Molecular Aetiology of Inherited Breast Cancer in the South African Black populationMScMed Dissertation. Johannesburg: University of the Witwatersrand. Available at: http://hdl.handle.net/10539/19755 (Accessed September 11, 2021). [Google Scholar]
- Cherbal F., Bakour R., Adane S., Boualga K., Benais-Pont G., Maillet P. (2010). BRCA1andBRCA2Germline Mutations Screening in Algerian Breast/Ovarian Cancer Families. Dis. Markers 28, 377–384. 10.1155/2010/585278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov D. A., Voronova N. V., Chistiakov P. A. (2008). Genetic Variations in DNA Repair Genes, Radiosensitivity to Cancer and Susceptibility to Acute Tissue Reactions in Radiotherapy-Treated Cancer Patients. Acta Oncologica 47, 809–824. 10.1080/02841860801885969 [DOI] [PubMed] [Google Scholar]
- Choudhury A., Aron S., Botigué L. R., Sengupta D., Botha G., Bensellak T., et al. (2020). High-depth African Genomes Inform Human Migration and Health. Nature 586, 741–748. 10.1038/s41586-020-2859-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combrink H. (2016). Molecular Screening of the South African Indian Population for BRCA1 and BRCA2 Using High-Resolution Melting Analysis. M.Med.Sc. Dissertation. Bloemfontein: University of the Free State; . http://hdl.handle.net/11660/4563 (Accessed September 14, 2021). [Google Scholar]
- Combrink H. M., Oosthuizen J., Visser B., Chabilal N., Buccimazza I., Foulkes W. D., et al. (2021). Mutations in BRCA-Related Breast and Ovarian Cancer in the South African Indian Population: a Descriptive Study. Cancer Genet. 258-259, 1–6. 10.1016/j.cancergen.2021.06.002 [DOI] [PubMed] [Google Scholar]
- Conley C. C., Ketcher D., Reblin M., Kasting M. L., Cragun D., Kim J., et al. (2020). The Big Reveal: Family Disclosure Patterns of BRCA Genetic Test Results Among Young Black Women with Invasive Breast Cancer. Jrnl of Gene Coun 29, 410–422. 10.1002/jgc4.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ansari F. Z., Jouali F., Marchoudi N., Bennani M. M., Ghailani N. N., Barakat A., et al. (2020). Screening of BRCA1/2 Genes Mutations and Copy Number Variations in Patients with High Risk for Hereditary Breast and Ovarian Cancer Syndrome (HBOC). BMC Cancer 20, 747. 10.1186/s12885-020-07250-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khachibi M., Diakite B., Hamzi K., Badou A., Senhaji M. A., Bakhchane A., et al. (2015). Screening of Exon 11 of BRCA1 Gene Using the High Resolution Melting Approach for Diagnosis in Moroccan Breast Cancer Patients. BMC Cancer 15, 81. 10.1186/s12885-015-1040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias K. M., Guo J., Bast R. C., Jr (2018). Early Detection of Ovarian Cancer. Hematology/Oncology Clin. North America 32, 903–914. 10.1016/j.hoc.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnour A. M., Elderdery A. Y., Mills J., Mohammed B. A., ElbietAbdelaal D., Mohamed A. O. (2012). BRCA 1 and 2 Mutations in Sudanese Secondary School Girls with Known Breast Cancer in Their Families. Ijhs 6 (1), 63–71. 10.12816/0005974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourati A., Louchez M.-M., Fournier J., Gamoudi A., Rahal K., El May M.-V., et al. (2014). Screening for Common Mutations in BRCA1 and BRCA2 Genes: Interest in Genetic Testing of Tunisian Families with Breast And/or Ovarian Cancer. Bull. du Cancer 101, E36–E40. 10.1684/bdc.2014.2049 [DOI] [PubMed] [Google Scholar]
- Francies F. Z., WainsteinDe Leeneer T., De Leeneer K., Cairns A., Murdoch M., Nietz S., et al. (2015). BRCA1, BRCA2 and PALB2 Mutations and CHEK2 c.1100delC in Different South African Ethnic Groups Diagnosed with Premenopausal And/or Triple Negative Breast Cancer. BMC Cancer 15, 912–921. 10.1186/s12885-015-1913-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa Mogahed S. H., Hamed Y. S., Ibrahim Moursy Y. E., Mahomoud Saied M. H. (2020). Analysis of Heterozygous BRCA1 5382ins Founder Mutation in a Cohort of Egyptian Breast Cancer Female Patients Using Pyrosequencing Technique. Asian Pac. J. Cancer Prev. 21, 431–438. 10.31557/APJCP.2020.21.2.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadiji-Abbes N., Trifa F., Choura M., Khabir A., Sellami-Boudawara T., Frikha M., et al. (2015). A Novel BRCA2 in Frame Deletion in a Tunisian Woman with Early Onset Sporadic Breast Cancer. Pathologie Biologie 63, 185–189. 10.1016/j.patbio.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Haga S. B., Barry W. T., Mills R., Ginsburg G. S., Svetkey L., Sullivan J., et al. (2013). Public Knowledge of and Attitudes toward Genetics and Genetic Testing. Genet. Test. Mol. Biomarkers 17, 327–335. 10.1089/gtmb.2012.0350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D., Wijsman E. M., Roos J. L., Gogos J. A., Karayiorgou M. (2002). Extended Intermarker Linkage Disequilibrium in the Afrikaners. Genome Res. 12, 956–961. 10.1101/gr.136202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi Y., Mighri N., Boujemaa M., Mejri N., Nasr S. N., Rekaya M. B., et al. (2021). Identification of Eleven Novel BRCA Mutations in Tunisia: Impact on the Clinical Management of BRCA Related Cancers. Res. Sq. 10.3389/fonc.2021.674965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman L., Vermeulen E., van El C. G., Claassen L., Timmermans D. R. M., Cornel M. C. (2013). Public Attitudes towards Genetic Testing Revisited: Comparing Opinions between 2002 and 2010. Eur. J. Hum. Genet. 21, 793–799. 10.1038/ejhg.2012.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henouda S., Bensalem A., Reggad R., Serrar N., Rouabah L., 1 Pujo P. (2016). Contribution of BRCA1 and BRCA2 Germline Mutations to Early Algerian Breast Cancer. Dis. Markers. 10.1155/2016/7869095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollfelder N., Erasmus J. C., Hammaren R., Vicente M., Jakobsson M., Greeff J. M., et al. (2020). Patterns of African and Asian Admixture in the Afrikaner Population of South Africa. BMC Biol. 18, 1–16. 10.1186/s12915-020-0746-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs S., Geduld-Ullah T., Benjeddou M. (2013). Reconstruction of Major Maternal and Paternal Lineages of the Cape Muslim Population. Genet. Mol. Biol. 36, 167–176. 10.1590/S1415-47572013005000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe M., Ayeni O., Norris S. A., McCormack V. A., Ruff P., Das I., et al. (2018). Barriers to Early Presentation of Breast Cancer Among Women in Soweto, South Africa. PLoS ONE 13, e0192071. 10.1371/journal.pone.0192071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouali F., Laarabi F.-Z., Marchoudi N., Ratbi I., Elalaoui S. C., Rhaissi H., et al. (2016). First Application of Next-Generation Sequencing in Moroccan Breast/ovarian Cancer Families and Report of a Novel Frameshift Mutation of the BRCA1 Gene. Oncol. Lett. 12, 1192–1196. 10.3892/ol.2016.4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan C., Zhang J. (2015). BRCA1 Mutation: a Predictive Marker for Radiation Therapy? Int. J. Radiat. Oncology*Biology*Physics 93, 281–293. 10.1016/j.ijrobp.2015.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopanos C., Tsiolkas V., Kouris A., Chapple C. E., Albarca Aguilera M., Meyer R., et al. (2019). VarSome: the Human Genomic Variant Search Engine. Bioinformatics 35, 1978–1980. 10.1093/bioinformatics/bty897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchenbaecker K. B., Hopper J. L., Barnes D. R., Phillips K.-A., Mooij T. M., Roos-Blom M.-J., et al. (2017). Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 317, 2402–2416. 10.1001/jama.2017.7112 [DOI] [PubMed] [Google Scholar]
- Laarabi F.-Z., Ratbi I., Elalaoui S. C., Mezzouar L., Doubaj Y., Bouguenouch L., et al. (2017). High Frequency of the Recurrent c.1310_1313delAAGA BRCA2 Mutation in the North-East of Morocco and Implication for Hereditary Breast-Ovarian Cancer Prevention and Control. BMC Res. Notes 10, 188. 10.1186/s13104-017-2511-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraqui A., Uhrhammer N., Lahlou-Amine I., Rhaffouli H. E., Baghdadi J. E., Dehayni M., et al. (2013). Mutation Screening of the BRCA1 Gene in Early Onset and Familial Breast/Ovarian Cancer in Moroccan Population. Int. J. Med. Sci. 10, 60–67. 10.7150/ijms.5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M. J., Thomassen M., Gerdes A-M., Kruse T. A. (2014). Breast Cancer: Basic Clin Res. 10.4137/bcbcr.s18715 Hereditary Breast Cancer: Clinical, Pathological and Molecular Characteristics) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizzi V., Ranieri G., Laforgia M., Gadaleta C., Gargano G., Kardhashi A., et al. (2020). PARP Inhibitors and Epithelial Ovarian Cancer: Molecular Mechanisms, Clinical Development and Future Prospective (Review). Oncol. Lett. 20, 1. 10.3892/ol.2020.11951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfoudh W., Bouaouina N., Ahmed S. B., Gabbouj S., Shan J., Mathew R., et al. (2012). Hereditary Breast Cancer in Middle Eastern and North African (MENA) Populations: Identification of Novel, Recurrent and Founder BRCA1 Mutations in the Tunisian Population. Mol. Biol. Rep. 39, 1037–1046. 10.1007/s11033-011-0829-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mampunye L., van der Merwe N. C., Grant K. A., Peeters A. V., Torrorey-Sawe R., French D. J., et al. (2021). Pioneering BRCA1/2 Point-Of-Care Testing for Integration of Germline and Tumor Genetics in Breast Cancer Risk Management: A Vision for the Future of Translational Pharmacogenomics. Front. Oncol. 11, 619817. 10.3389/fonc.2021.619817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M., Derkaoui T., Bakkach J., Loudiyi A., Ghailani Nourouti N., Barakat A., et al. (2020). Screening of BRCA1 and BRCA2 Germline Mutations in Unselected Triple‐negative Breast Cancer Patients: A Series from north of Morocco. Precision Med. Sci. 9, 43–48. 10.1002/prm2.12009 [DOI] [Google Scholar]
- McCarthy A. M., Armstrong K. (2014). The Role of Testing forBRCA1andBRCA2Mutations in Cancer Prevention. JAMA Intern. Med. 174 (7), 1023–1024. 10.1001/jamainternmed.2014.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehemmai C., Cherbal F., Hamdi Y., Guedioura A., Benbrahim W., Bakour R., et al. (2020). BRCA1 and BRCA2 Germline Mutation Analysis in Hereditary Breast/ovarian Cancer Families from the Aures Region (Eastern Algeria): First Report. Pathol. Oncol. Res. 26, 715–726. 10.1007/s12253-019-00586-4 [DOI] [PubMed] [Google Scholar]
- Mesthrie R. (2006). World Englishes and the Multilingual History of English. World Englishes 25, 381–390. 10.1111/j.1467-971x.2006.00477.x [DOI] [Google Scholar]
- Mestiri S., Monastiri K., Bouaouina N., Presnea N., Bignon Y. J., Khairi H., et al. (2000). Mutational Analysis of Breast/ovarian Cancer Hereditary Predisposition Gene BRCA1 in Tunisian Women, 77. Arch Institut Pasteur Tunis, 11–15. [PubMed] [Google Scholar]
- Mighri N., Hamdi Y., Boujemaa M., Othman H., Nasr S. B., El Benna H., et al. (2020). Identification of Novel BRCA1 and RAD50 Mutations Associated with Breast Cancer Predisposition in Tunisian Patients. Front. Genet. 10.3389/fgene.2020.552971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeti P. J. (2016). Molecular Screening for the Presence of Large Deletions or Duplications in BRCA Using Multiplex Ligation-dependent Probe Amplification in South Africa. M.Med.Sc. Dissertation.. Bloemfontein: University of the Free State. Available at: https://scholar.ufs.ac.za/handle/11660/5392 (Accessed September 14, 2021). [Google Scholar]
- Msolly A., Asma K. (2015). BRCA1 and BRCA2 Mutations Are They Related to Breast Cancer in a Sample of Tunisian Population? Ctoij 1. 10.19080/CTOIJ.2015.01.555551 [DOI] [Google Scholar]
- Mukem S., Meng Q., Sriplung H., Tangcharoensathien V. (2016). Low Coverage and Disparities of Breast and Cervical Cancer Screening in Thai Women: Analysis of National Representative Household Surveys. Asian Pac. J. Cancer Prev. 16, 8541–8551. 10.7314/apjcp.2015.16.18.8541 [DOI] [PubMed] [Google Scholar]
- National Department of Health of the Republic of South Africa (2018). Clinical Guidelines for Breast Cancer Control and Management. Pretoria, South Africa: Department of Health, 1–123. Available at: https://cansa.org.za/files/2019/08/DOH-Breast-Cancer-Guidelines-Final.pdf . [Google Scholar]
- Ndiaye R., Diop J. P. D., Bourdon-Huguenin V., Dem A., Diouf D., Dieng M. M., et al. (2020). Evidence for an Ancient BRCA1 Pathogenic Variant in Inherited Breast Cancer Patients from Senegal. Npj Genom. Med. 5, 8. 10.1038/s41525-020-0114-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen J. (2016). Molecular Screening of Coloured South African Breast Cancer Patients for the Presence of BRCA Mutations Using High Resolution Melting analysis. MMedSc Dissertation. Bloemfontein: University of the Free State. Available at: https://scholar.ufs.ac.za/handle/11660/6426 (Accessed September 13, 2021). [Google Scholar]
- Oosthuizen J., Kotze M. J., van der Merwe N., Myburgh E. J., Bester P., van der Merwe N. C. (2021). Globally Rare BRCA2 Variants with Founder Haplotypes in the South African Population: Implications for point-of-care Testing Based on a Single-Institution BRCA1/2 Next-Generation Sequencing Study. Front. Oncol. 10. 10.3389/fonc.2020.619469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opolade O. I., Gao Q., Adebamowo C. A., Fackenthal J., Das S., Sveen L., et al. (2000). Protein Truncating BRCA1 and BRCA2 Mutations in African Women with Pre-menopausal Breast Cancer. Hum. Genet. 107, 192–194. 10.1007/s004390000342 [DOI] [PubMed] [Google Scholar]
- Peltzer K., Phaswana-Mafuya N. (2014). Breast and Cervical Cancer Screening and Associated Factors Among Older Adult Women in South Africa. Asian Pac. J. Cancer Prev. 15, 2473–2476. 10.7314/apjcp.2014.15.6.2473 [DOI] [PubMed] [Google Scholar]
- Peter N. (2014). Screening of Young And/or Familial African Breast Cancer Patients for the Presence of BRCA Mutations. M.Med.Sc. Dissertation. Bloemfontein: University of the Free State. Available at: https://scholar.ufs.ac.za/handle/11660/1613 (Accessed September 14, 2021) [Google Scholar]
- Phaswana-Mafuya N., Peltzer K. (2018). Breast and Cervical Cancer Screening Prevalence and Associated Factors Among Women in the South African General Population. Asian Pac. J. Cancer Prev. 19, 1465–1470. 10.22034/apjcp.2018.19.6.1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. D., Yawitch T. M., Van der Merwe N. C., Van den Berg H. J., Dreyer G., Van Rensburg E. J. (2004). BRCA1 Mutations in South African Breast And/or Ovarian Cancer Families: Evidence of a Novel Founder Mutation in Afrikaner Families. Int. J. Cancer 110, 677–682. 10.1002/ijc.20186 [DOI] [PubMed] [Google Scholar]
- Riahi A., Ghourabi M. e., Fourati A., Chaabouni-Bouhamed H. (2016). Family History Predictors of BRCA1/BRCA2 Mutation Status Among Tunisian Breast/ovarian Cancer Families. Breast Cancer 24, 238–244. 10.1007/s12282-016-0693-4 [DOI] [PubMed] [Google Scholar]
- Riahi A., Kharrat M., Ghourabi M. E., Khomsi F., Gamoudi A., Lariani I., et al. (2014). Mutation Spectrum and Prevalence ofBRCA1 andBRCA2 Genes in Patients with Familial and Early-Onset Breast/ovarian Cancer from Tunisia. Clin. Genet. 87, 155–160. 10.1111/cge.12337 [DOI] [PubMed] [Google Scholar]
- Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., et al. (2015). Standards and Guidelines for the Interpretation of Sequence Variants: a Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi F., Maachi F., Tazzite A., Aboutaib R., Fekkak J., Azeddoug H., et al. (2021). Next-generation Sequencing of BRCA1 and BRCA2 Genes in Moroccan Prostate Cancer Patients with Positive Family History. PLoS ONE 16, e0254101. 10.1371/journal.pone.0254101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlebusch C. M., Dreyer G., Sluiter M. D., Yawitch T. M., van den Berg H. J., van Rensburg E. J. (2010). Cancer Prevalence in 129 Breast-Ovarian Cancer Families Tested for BRCA1 and BRCA2 Mutations. S Afr. Med. J. 100, 113–117. 10.7196/samj.3235 [DOI] [PubMed] [Google Scholar]
- Schoeman M., Apffelstaedt J. P., Baatjes K., Urban M. (2013). Implementation of a Breast Cancer Genetic Service in South Africa - Lessons Learned. S Afr. Med. J. 103, 529–533. 10.7196/SAMJ.6814 [DOI] [PubMed] [Google Scholar]
- Seymour H. J., Wainstein T., Macaulay S. S., Haw T., Krause A. (2016). Breast Cancer in High-Risk Afrikaner Families: Is BRCA Founder Mutation Testing Sufficient? S Afr. Med. J. 106, 264–267. 10.7196/SAMJ.2016.v106i3.10285 [DOI] [PubMed] [Google Scholar]
- Sluiter M. D., Van Rensburg E. J. (2011). Large Genomic Rearrangements of the BRCA1 and BRCA2 Genes: Review of the Literature and Report of a Novel BRCA1 Mutation. Breast Cancer Res. Treat. 125, 325–349. 10.1007/s10549-010-0817-z [DOI] [PubMed] [Google Scholar]
- Sözmen K., Unal B., Sakarya S., Dinc G., Yardim N., Keskinkilic B., et al. (2016). Determinants of Breast and Cervical Cancer Screening Uptake Among Women in Turkey. Asia Pac. J. Public Health 28, 528–538. 10.1177/1010539516654541 [DOI] [PubMed] [Google Scholar]
- Spurdle A. B., Jenkins T. (1996). The Origins of the Lemba "Black Jews" of Southern Africa: Evidence from p12F2 and Other Y-Chromosome Markers. Am. J. Hum. Genet. 59, 1126–1133. [PMC free article] [PubMed] [Google Scholar]
- Sunada S., Nakanishi A., Miki Y. (2018). Crosstalk of DNA Double‐strand Break Repair Pathways in poly(ADP‐ribose) Polymerase Inhibitor Treatment of Breast Cancer Susceptibility Gene 1/2‐mutated Cancer. Cancer Sci. 109, 893–899. 10.1111/cas.13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzite A., Jouhadi H., Nadifi S., Aretini P., Falaschi E., Collavoli A., et al. (2012). BRCA1 and BRCA2 Germline Mutations in Moroccan Breast/ovarian Cancer Families: Novel Mutations and Unclassified Variants. Gynecol. Oncol. 125, 687–692. 10.1016/j.ygyno.2012.03.007 [DOI] [PubMed] [Google Scholar]
- The Council for Medical Schemes (2019). Final PMB Definition Guidelines for Ovarian Cancer. Pretoria, South Africa: Council for Medical Schemes, 1–27. Available at: https://www.medicalschemes.com/files/PMB/Project/FinalPMBdefinitionguidelineforovariancancer.pdf . [Google Scholar]
- Theme Filha M. M., Leal M. d. C., Oliveira E. F. V. d., Esteves-Pereira A. P., Gama S. G. N. d. (2016). Regional and Social Inequalities in the Performance of Pap Test and Screening Mammography and Their Correlation with Lifestyle: Brazilian National Health Survey, 2013. Int. J. Equity Health 15, 136. 10.1186/s12939-016-0430-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano G., Nante N., Cozzolino M. (2017). The Angelina Jolie Effect - Impact on Breast and Ovarian Cancer Prevention A Systematic Review of Effects after the Public Announcement in May 2013. Health Edu. J. 76, 707–715. 10.1177/0017896917712300 [DOI] [Google Scholar]
- Troudi W., Loueslati B., Baccar A., Ben Ayed F., Ben Ammar El Gaaied A. (2009). Penetrance of BRCA1 Gene Mutation and DNA Mitochondrial in Tunisian Breast Cancer Occurrence. Tunis Med. 87, 494–498. [PubMed] [Google Scholar]
- Troudi W., Uhrhammer N., Sibille C., Dahan C., Mahfoudh W., Bouchlaka Souissi C., et al. (2007). Contribution of the BRCA1 and BRCA2 Mutations to Breast Cancer in Tunisia. J. Hum. Genet. 52, 915–920. 10.1007/s10038-007-0195-5 [DOI] [PubMed] [Google Scholar]
- Van der Merwe N. C., van Rensburg E. J. (2009). Hereditary Breast/ovarian Cancer and BRCA Mutations: A South African Perspective. Curr. Oncol. 16, 91. [Google Scholar]
- Van der Merwe N. C., Oosthuizen J., Theron M., Chong G., Foulkes W. D. (2020). The Contribution of Large Genomic Rearrangements in BRCA1 and BRCA2 to South African Familial Breast Cancer. BMC Cancer 20, 391. 10.1186/s12885-020-06917-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Merwe N., Hamel N., Schneider S.-R., Apffelstaedt J., Wijnen J., Foulkes W. (2012). A Founder BRCA2 Mutation in Non-afrikaner Breast Cancer Patients of the Western Cape of South Africa. Clin. Genet. 81, 179–184. 10.1111/j.1399-0004.2010.01617.x [DOI] [PubMed] [Google Scholar]
- Vishnu P., Morrell R. (1991). Indian Merchants and Dukawallahs in the Natal Economy, C1875-1914. J. South. Afr. Stud. 17, 71–102. https://www.jstor.org/stable/2637287. [Google Scholar]
- World Health Organization Africa The State of Health in the WHO African Region: An Analysis of the Status of Health. Available at: https://reliefweb.int/report/world/state-heatlh-who-african-region-analysis-status-health-services-and-health (Accessed November 14, 2021). [Google Scholar]
- Yawitch T. M. (2005). Inherited Predisposition to Breast/ovarian Cancer: The Frequency and Nature of BRCA1 Gene Mutations in South African familiesMScMed Dissertation. Pretoria: University of Pretoria. Available at: https://repository.up.ac.za/handle/2263/30470 (Accessed September 14, 2021). [Google Scholar]
- Yoshida K., Miki Y. (2004). Role of BRCA1 and BRCA2 as Regulators of DNA Repair, Transcription, and Cell Cycle in Response to DNA Damage. Cancer Sci. 95, 866–871. 10.1111/j.1349-7006.2004.tb0219510.1111/j.1349-7006.2004.tb02195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Fackenthal J. D., Niu Q., Huo D., Sveen W. E., AdebamowoDeMarco C. A., et al. (2009). Evidence for an Ancient BRCA1 Mutation in Breast Cancer Patients of Yoruban Ancestry. Fam. Cancer 8, 15–22. 10.1007/s10689-008-9205-9 [DOI] [PubMed] [Google Scholar]
- Zhang J., Fackenthal J. D., Zheng Y., Huo D., Hou N., Niu Q., et al. (2012). Recurrent BRCA1 and BRCA2 Mutations in Breast Cancer Patients of African Ancestry. Breast Cancer Res. Treat. 134, 889–894. 10.1007/s10549-012-2136-z [DOI] [PubMed] [Google Scholar]
- Zheng Y., Walsh T., Gulsuner S., Casadei S., Lee M. K., Ogundiran T. O., et al. (2018). Inherited Breast Cancer in Nigerian Women. Jco 36, 2820–2825. 10.1200/JCO.2018.78.3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.